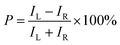Open Access Article
Open Access ArticleBoosting circularly polarized luminescence by optimizing off-centering octahedral distortion in zero-dimensional hybrid indium-antimony halides†
Yulian
Liu
 ,
Yi
Wei
,
Zhishan
Luo
,
Bin
Xu
,
Yi
Wei
,
Zhishan
Luo
,
Bin
Xu
 ,
Meiying
He
,
Peibin
Hong
,
Chen
Li
and
Zewei
Quan
,
Meiying
He
,
Peibin
Hong
,
Chen
Li
and
Zewei
Quan
 *
*
Department of Chemistry, Academy for Advanced Interdisciplinary Studies, Southern University of Science and Technology, China. E-mail: quanzw@sustech.edu.cn
First published on 4th September 2024
Abstract
Chiral zero-dimensional hybrid metal halides (0D HMHs) are being extensively studied as they can directly generate circularly polarized luminescence (CPL) with high photoluminescence quantum yields (PLQYs), yet improving their luminescence dissymmetry factor (glum) remains a challenge. This study proposes a general strategy to boost the glum value of chiral 0D HMHs by optimizing the off-centering distortion  of inorganic octahedra. Accordingly, (R/S-MBA)2(2MA)In0.95Sb0.05Cl6 (MBA = α-methylbenzylammonium, 2MA = dimethylamine) and (R/S-MBA)2(3MA)In0.95Sb0.05Cl6 (3MA = trimethylamine) with near-unity PLQYs are accordingly synthesized. With increasing the
of inorganic octahedra. Accordingly, (R/S-MBA)2(2MA)In0.95Sb0.05Cl6 (MBA = α-methylbenzylammonium, 2MA = dimethylamine) and (R/S-MBA)2(3MA)In0.95Sb0.05Cl6 (3MA = trimethylamine) with near-unity PLQYs are accordingly synthesized. With increasing the 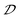 from 0.012 to 0.020, the |glum| is accordingly increased from 7.8 × 10−3 to 2.0 × 10−2. Notably, the |glum| can be further boosted to an impressive value of 3.8 × 10−2 while maintaining near-unity PLQYs by continuously increasing the
from 0.012 to 0.020, the |glum| is accordingly increased from 7.8 × 10−3 to 2.0 × 10−2. Notably, the |glum| can be further boosted to an impressive value of 3.8 × 10−2 while maintaining near-unity PLQYs by continuously increasing the  . Experimental results reveal that the choice of achiral ligands and varied Sb3+ dopant concentrations can modulate the distribution and strength of hydrogen bonds around indium-antimony halogen octahedra, respectively, thus regulating the
. Experimental results reveal that the choice of achiral ligands and varied Sb3+ dopant concentrations can modulate the distribution and strength of hydrogen bonds around indium-antimony halogen octahedra, respectively, thus regulating the  parameter of octahedra in 0D hybrid metal halides. Additionally, light-emitting diodes with a polarization of 1.6% are fabricated. This work sheds light on the relationship between the distortion of inorganic octahedra and the glum value.
parameter of octahedra in 0D hybrid metal halides. Additionally, light-emitting diodes with a polarization of 1.6% are fabricated. This work sheds light on the relationship between the distortion of inorganic octahedra and the glum value.
Introduction
Circularly polarized luminescence (CPL) has attracted tremendous research interest due to its potential applications in a wide variety of fields such as three-dimensional displays, quantum computing and optical information storage.1–6 Novel and efficient devices to generate and detect CPL are thus of great importance. In conventional polarized emitters, additional optical elements such as a linear polarizer and a quarter waveplate are included to obtain polarization control. Although efficient polarization control is realized, it hinders their miniaturization and applicability in integration circuits. In this aspect, chiral hybrid metal halides (HMHs) that combine tunable chirality of organic ligands with excellent optical properties of inorganic units can be used to directly detect and generate circularly polarized light. Since the first report of chiral HMHs, significant progress has been made, and a series of chiral HMHs with obvious CPL signals are successfully synthesized.7–15 Both high photoluminescence quantum yield (PLQY) and large luminescence dissymmetry factor (glum) values are essential to technical applications of CPL-active materials. However, it is still challenging to achieve chiral HMHs with simultaneously high PLQYs and large glum values.16Particularly, zero-dimensional (0D) HMHs, in which inorganic polyhedra are fully separated by organic ligands, usually exhibit tunable emissions with high PLQYs.17–19 Typically, alloying at the In–Sb site is a common strategy to increase the PLQYs of 0D HMHs and also provides a means to modulate the distortion of metal halogen octahedra, thereby improving the glum values. Besides, low-dimensional chiral HMHs should exhibit a higher degree of chirality as a result of strong intermolecular interactions induced by the greater percentage of chiral ligands.20 Chiral 0D HMHs are thus expected to be a class of high performance CPL-active materials with both high PLQYs and large glum values. It has been recently demonstrated that high PLQYs can be readily achieved in chiral 0D HMHs.21–25 Nevertheless, the glum values of reported chiral 0D HMHs are relatively low, most of which are in the magnitude of 10−3.14,23,26 Significant effort has been devoted to enhancing the glum values of chiral HMHs and understanding the chirality induction mechanism.27–30 It is found that the asymmetric hydrogen bonds between organic ligands and inorganic frameworks in two-dimensional (2D) HMHs can enhance their glum values.31,32 Moreover, the chiroptical activity of chiral 2D hybrid perovskites is revealed to originate from the symmetry-breaking helical distortion of the inorganic lattice induced by the asymmetric hydrogen bonding interactions with the inorganic lattice.33,34 Similar phenomena are recently reported in chiral 0D HMHs. The chiroptical activity of chiral 0D lead-tin bromide hybrids is attributed to the asymmetric polyhedra induced by hydrogen bonds.22 By modulating the hydrogen bonding interactions between organic ligands and inorganic units, enhanced CPL performance with large glum values of ±1.0 × 10−2 and near-unity PLQY is achieved in a series of chiral indium-antimony hybrids.25 Right now, the glum values of chiral 0D HMHs need to be further improved to meet the practical applications of chiral HMHs as chiroptical devices.10
In this work, the glum values of chiral 0D HMHs are significantly improved based on a general strategy, that is, optimizing the off-centering distortion of inorganic octahedra by modulating the strength and distribution of hydrogen bonds around indium-antimony halogen octahedra therein. Five pairs of enantiomorphic (R/S-MBA)2(2MA)In0.95Sb0.05Cl6 (R/S-1) (MBA = α-methylbenzylammonium, 2MA = dimethylamine) and (R/S-MBA)2(3MA)In0.95Sb0.05Cl6 (R/S-2) (3MA = trimethylamine), (R/S-MBA)2(3MA)In0.79Sb0.21Cl6 (R/S-2A), (R/S-MBA)2(3MA)In0.72Sb0.28Cl6 (R/S–2B) and (R/S-MBA)2(3MA)In0.59Sb0.41Cl6 (R/S-2C) are synthesized, and all of them exhibit near-unity PLQYs. With adopting different achiral ligands and modifying Sb3+ dopant concentrations, the off-centering distortion of inorganic octahedra is continuously increased from 0.012 to 0.047 and the |gabs|/|glum| is increased from 0.16 × 10−3/7.8 × 10−3 to 1.25 × 10−3/3.8 × 10−2, indicating the crucial role of large off-centering distortion in achieving a high |gabs|/|glum| value. Moreover, the highest glum value of ±3.8 × 10−2 and near-unity PLQY in R/S-2C outperform those of currently reported chiral HMHs. Combined crystal structure and photophysical studies demonstrate that the distribution and strength of hydrogen bonds can be delicately modified to increase the off-centering distortions of inorganic octahedra. With R-2C as the phosphor, light-emitting diodes with a polarization of 1.6% at room temperature are fabricated, demonstrating their potential as CPL light sources in three-dimensional displays and quantum computing.
Results and discussion
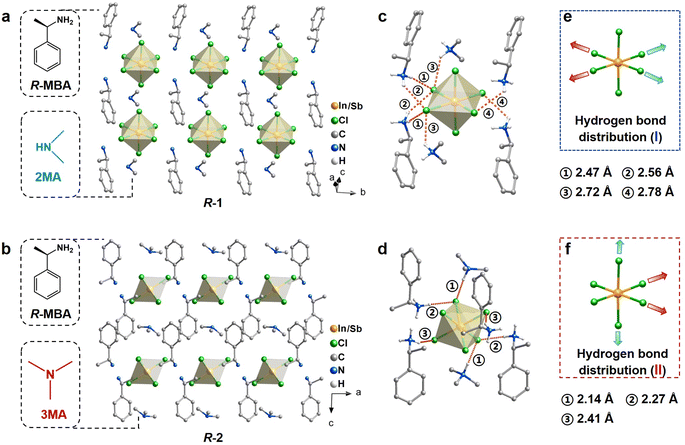
In this work, common chiral R/S-methylbenzylamine (R/S-MBA) ligands are selected as chiral inducers.20,35 Achiral ligands with different sizes and steric hindrance including dimethylamine hydrochloride (2MACl) and trimethylamine hydrochloride (3MACl) are integrated into chiral HMHs to modulate the hydrogen bonds with inorganic octahedra. (R/S-MBA)2(2MA)In0.95Sb0.05Cl6 (R/S-1) are synthesized by cooling the aqueous HCl solution of stoichiometric amounts of R/S-MBA, 2MACl, InCl3 and SbCl3. (R/S-MBA)2(3MA)In0.95Sb0.05Cl6 (R/S-2) are obtained through a similar procedure to R/S-1, except that the achiral ligands are replaced with 3MACl. The synthetic details are provided in the ESI.† The crystal structures of each chiral HMH incorporated with different achiral ligands are determined by single crystal X-ray diffraction (SCXRD). The phase purity of all compounds is examined by powder X-ray diffraction (PXRD) characterization (Fig. S1†). The actual concentration of the Sb3+ dopant is determined by inductively coupled plasma mass spectrometry (ICP-MS), and the results reveal that the actual molar ratio of Sb/In of all compounds is 0.05/0.95 (Table S1†). For convenience, the R-configuration is selected as the representative of each pair of compounds, as the structures of R- and S- enantiomers are identical. As shown in Fig. 1a and b, R-1 and R-2 show typical 0D structural features, where anionic [In/SbCl6]3− octahedra are fully separated by organic ligands.17,18,36,37 Both R-1 and R-2 crystallize in the Sohncke space group of C2 (Table S2†). The R-MBA and achiral ligands in both R-1 and R-2 are arranged around each [In/SbCl6]3− octahedron in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 manner, with the planes of the benzene ring of R-MBA being parallel to the c-axis, and the planes of C–N–C (2MA) and C–C–C (3MA) being perpendicular to the c-axis. The arrangement and molar ratio of organic ligands around inorganic octahedra can also be clearly seen from the a-axis (Fig. S2†).
2 manner, with the planes of the benzene ring of R-MBA being parallel to the c-axis, and the planes of C–N–C (2MA) and C–C–C (3MA) being perpendicular to the c-axis. The arrangement and molar ratio of organic ligands around inorganic octahedra can also be clearly seen from the a-axis (Fig. S2†).
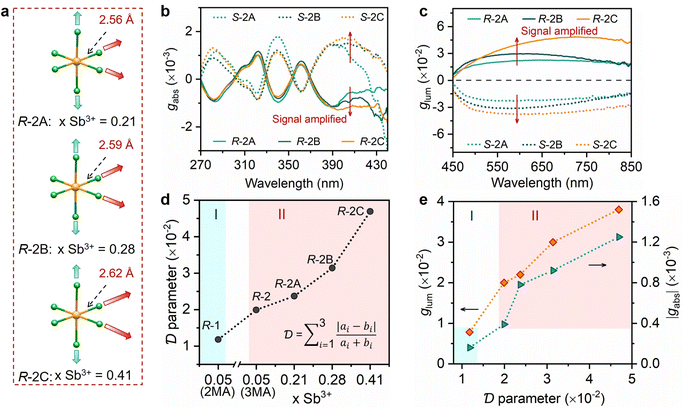
Despite the congruence in the space group, crystal structure, and the molar ratios of chiral and achiral ligands between compounds R-1 and R-2, a significant disparity emerges in the distribution and strength of hydrogen bonds between organic ligands and inorganic octahedra. These deviations originate from the use of different achiral ligands in R-1 and R-2. As shown in Fig. 1c and d, the H⋯Cl distances between organic ligands and inorganic octahedra are measured to be 2.47–2.78 Å for R-1 and 2.14–2.41 Å for R-2, respectively, indicating the stronger hydrogen bonds in R-2. Moreover, the N–H⋯Cl angles are closer to 180° in R-2, further indicating stronger hydrogen bonds (Fig. S3†).38 To clearly visualize the hydrogen bond distribution around the inorganic octahedron, the hydrogen bonds along three different axes are represented by colored arrows. As shown in Fig. 1e, all the strong and weak hydrogen bonds between organic ligands and inorganic octahedra in R-1 are aligned in the equatorial Cl–In/Sb–Cl plane, designated as type I. Consequently, the In/Sb–Cl bonds in these two axes are simultaneously elongated, with the bond lengths of 2.57 and 2.54 Å, respectively. The In/Sb–Cl bonds in the vertical axis without hydrogen bonding interactions have relatively shorter lengths of 2.47 Å. In contrast, the hydrogen bonds in R-2 are present in a distinct distribution, denoted as type II (Fig. 1f). The presence of only one strong hydrogen bond in the two axes in the equatorial Cl–In/Sb–Cl plane (indicated by red arrows) results in the elongation of these two In/Sb–Cl bonds, with the bond lengths of 2.53 and 2.49 Å, respectively. Conversely, the weak hydrogen bonds (denoted by blue arrows) in the vertical axis yield the In/Sb–Cl bond lengths of 2.55 Å. Overall, the distinct distribution and strength of hydrogen bonds lead to varied octahedral distortions. Based on the refined crystal structures, the distortions of inorganic octahedra are quantitatively assessed by adopting an off-centering distortion index  to evaluate their dissymmetry (eqn (1)):39
to evaluate their dissymmetry (eqn (1)):39
 | (1) |
 values of R-1 and R-2 are estimated to be 0.012 and 0.020, respectively, which are primarily determined by the distribution type of hydrogen bonds formed with the octahedra. The larger
values of R-1 and R-2 are estimated to be 0.012 and 0.020, respectively, which are primarily determined by the distribution type of hydrogen bonds formed with the octahedra. The larger  value of the octahedron can be attained when the distribution of hydrogen bonds is asymmetrical, like type II.
value of the octahedron can be attained when the distribution of hydrogen bonds is asymmetrical, like type II.
To examine the effect of the  parameter on chiroptical properties, a series of photophysical characterization studies were conducted. As shown in Fig. 2a, R-1 and R-2 exhibit obvious absorptions in the 270–405 nm range, which are different from the absorption spectra of (R-MBA)2(2MA)InCl6 and (R-MBA)2(3MA)InCl6 in the 270–290 nm range (Fig. S4†). These differences indicate that these absorptions in R-1 and R-2 originate from the optically active antimony halogen octahedra. Under 325 nm excitation, the PL emission spectra of R-1 and R-2 exhibit broad emissions peaked at 600 and 590 nm, respectively (Fig. 2b). The PL excitation (PLE) spectra are located in the range of 270–405 nm, consistent with their corresponding absorption spectra (Fig. S5a†). The remarkable near-unity PLQYs are recorded for R-1 and R-2. To reveal the origins of the broadband emission, excitation-dependent, time-resolved and power-dependent PL spectra are measured. The PL spectra obtained at various excitation wavelengths exhibit identical profiles, confirming that the broad emissions originate from the same excited states (Fig. S5b and c†). The PL lifetimes of R-1 and R-2 are measured to be 3.80 and 3.27 μs, respectively (Fig. S6†). The microsecond PL lifetimes indicate that the emission arises from inorganic modules, rather than from the organic ligands with nanosecond PL lifetimes (Fig. S7†). The linear excitation power-dependent PL intensities rule out the possibility of defect-related emissions (Fig. S8†).40–42 Moreover, these PL spectra and PL lifetimes are obviously different from their pristine counterparts, which exhibit excitation-dependent PL spectra and longer PL lifetimes (Fig. S9 and 10†). Therefore, these broad emissions with high PLQYs are assigned to the Sb3+-related emissions. Such high PLQYs benefit from their intrinsic 0D structure with isolated inorganic octahedra, which exhibit strong quantum confinement effects.36,43 These broadband and large Stokes-shifted PL characteristics of R-1 and R-2 are well consistent with those of recently reported Sb-based HMHs.44–49
parameter on chiroptical properties, a series of photophysical characterization studies were conducted. As shown in Fig. 2a, R-1 and R-2 exhibit obvious absorptions in the 270–405 nm range, which are different from the absorption spectra of (R-MBA)2(2MA)InCl6 and (R-MBA)2(3MA)InCl6 in the 270–290 nm range (Fig. S4†). These differences indicate that these absorptions in R-1 and R-2 originate from the optically active antimony halogen octahedra. Under 325 nm excitation, the PL emission spectra of R-1 and R-2 exhibit broad emissions peaked at 600 and 590 nm, respectively (Fig. 2b). The PL excitation (PLE) spectra are located in the range of 270–405 nm, consistent with their corresponding absorption spectra (Fig. S5a†). The remarkable near-unity PLQYs are recorded for R-1 and R-2. To reveal the origins of the broadband emission, excitation-dependent, time-resolved and power-dependent PL spectra are measured. The PL spectra obtained at various excitation wavelengths exhibit identical profiles, confirming that the broad emissions originate from the same excited states (Fig. S5b and c†). The PL lifetimes of R-1 and R-2 are measured to be 3.80 and 3.27 μs, respectively (Fig. S6†). The microsecond PL lifetimes indicate that the emission arises from inorganic modules, rather than from the organic ligands with nanosecond PL lifetimes (Fig. S7†). The linear excitation power-dependent PL intensities rule out the possibility of defect-related emissions (Fig. S8†).40–42 Moreover, these PL spectra and PL lifetimes are obviously different from their pristine counterparts, which exhibit excitation-dependent PL spectra and longer PL lifetimes (Fig. S9 and 10†). Therefore, these broad emissions with high PLQYs are assigned to the Sb3+-related emissions. Such high PLQYs benefit from their intrinsic 0D structure with isolated inorganic octahedra, which exhibit strong quantum confinement effects.36,43 These broadband and large Stokes-shifted PL characteristics of R-1 and R-2 are well consistent with those of recently reported Sb-based HMHs.44–49
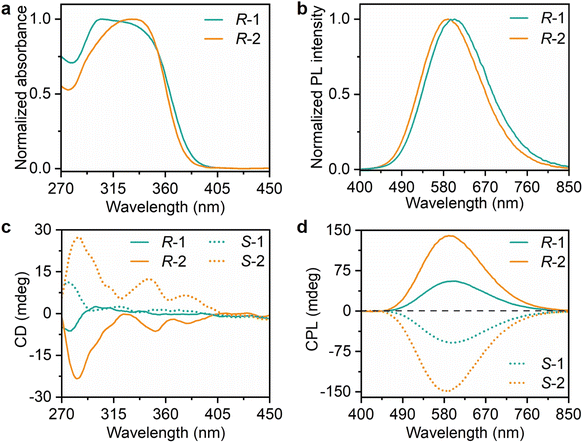 | ||
| Fig. 2 Optical properties of R-1 and R-2. (a) Normalized UV-Vis absorption spectra, (b) normalized PL emission spectra, (c) CD spectra and (d) CPL spectra of R-1 and R-2. | ||
To shed light on the role of structural variations in inorganic octahedra in determining chiroptical activities, solid-state circular dichroism (CD) and CPL spectra of chiral HMHs are measured and compared. As shown in Fig. 2c, the CD spectra of chiral R/S-1 and R/S-2 exhibit obvious Cotton effects in the range of 270–405 nm, which agree well with their corresponding absorption spectra but differ from those of R/S-MBA, implying that the observed CD signals originate from inorganic octahedra.25 All R-configurations of chiral HMHs exhibit negative peaks in the range of 270–405 nm regardless of the type of achiral ligands. As the same amounts of samples are used for CD characterization, R-1 exhibits a small |CD| value (6.57 mdeg), while R-2 reveals a significantly enhanced |CD| signal (23.3 mdeg). To quantitatively assess this difference, the absorption dissymmetry factor gabs is calculated using eqn (2):50
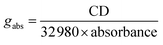 | (2) |
 parameter of inorganic octahedra. At the same time, CPL spectra reflect the excited state properties of chiral systems. As shown in Fig. 2d, both R/S-1 and R/S-2 enantiomers show mirror symmetrical curves in their CPL spectra, which match well with their corresponding PL spectra, suggesting that the CPL signals originate from the inorganic octahedra. The CPL magnitude is quantitively evaluated by the luminescence dissymmetry factor glum = 2(IL − IR)/(IL + IR), where IL and IR represent the intensities of left- and right-handed CPL, respectively.51 The glum values calculated from these CPL spectra are 7.8 × 10−3 for R-1 and 2.0 × 10−2 for R-2, corresponding to a 2.6-fold enhancement from R-1 to R-2 (Fig. S12†). Such CPL analyses reveal that the excited state chirality of these HMHs can be also improved with increasing the
parameter of inorganic octahedra. At the same time, CPL spectra reflect the excited state properties of chiral systems. As shown in Fig. 2d, both R/S-1 and R/S-2 enantiomers show mirror symmetrical curves in their CPL spectra, which match well with their corresponding PL spectra, suggesting that the CPL signals originate from the inorganic octahedra. The CPL magnitude is quantitively evaluated by the luminescence dissymmetry factor glum = 2(IL − IR)/(IL + IR), where IL and IR represent the intensities of left- and right-handed CPL, respectively.51 The glum values calculated from these CPL spectra are 7.8 × 10−3 for R-1 and 2.0 × 10−2 for R-2, corresponding to a 2.6-fold enhancement from R-1 to R-2 (Fig. S12†). Such CPL analyses reveal that the excited state chirality of these HMHs can be also improved with increasing the  parameter.
parameter.
As previously demonstrated, chiroptical activities in HMHs originate from inorganic modules due to the chirality induction via N–H⋯Cl hydrogen bonds.22,31–33 To clearly reveal the relationship between the chiroptical performance and the distortion of inorganic octahedra, detailed structural analyses of inorganic octahedra in R-1 and R-2 are conducted. To quantify the distortion of [In/SbCl6]3− octahedra in R-1 and R-2, both bond length distortion and bond angle distortion are calculated.52,53 As shown in Table S3,† the bond length distortions of R-1 and R-2 are calculated to be 2.76 × 10−4 and 1.1 × 10−4, respectively, and the bond angle distortions of R-1 and R-2 are 3.73 and 1.27, respectively. It is clear that the extent of bond length and bond angle distortion in these 0D HMHs varies inversely with the values of |gabs| and |glum|, although the bond angle distortion of the inorganic framework in 2D HMHs could promote their chiroptical properties.32 As demonstrated above, the values of |gabs| and |glum| increase with larger values of the 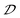 parameter, suggesting that the augmentation in chiroptical activity is correlated with the increased off-centering distortion of inorganic octahedra. To support this claim, a series of control experiments are carried out to elucidate the relationship between the
parameter, suggesting that the augmentation in chiroptical activity is correlated with the increased off-centering distortion of inorganic octahedra. To support this claim, a series of control experiments are carried out to elucidate the relationship between the 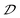 parameter and the magnitudes of |gabs| and |glum|.
parameter and the magnitudes of |gabs| and |glum|.
With continuously increasing the Sb3+ concentration, three pairs of enantiomorphic (R/S-MBA)2(3MA)In0.79Sb0.21Cl6 (R/S-2A), (R/S-MBA)2(3MA)In0.72Sb0.28Cl6 (R/S-2B) and (R/S-MBA)2(3MA)In0.59Sb0.41Cl6 (R/S-2C) are accordingly synthesized. Structural and composition characterization studies demonstrate their crystal structure, phase purity and actual Sb3+ dopant concentration (Fig. S13, 14, Tables S4 and 5†). The lattice constants (b and c axes) and angles (α, β, γ) remain nearly constant with increasing Sb3+ dopant concentration due to the similar ionic radii of In3+ (80 pm) and Sb3+ (76 pm) (Fig. S15†). The a-axis length slightly increases due to the synergetic effect of the similar ionic radii of In3+ and Sb3+ and decreased ionic bond polarity after the substitution of Sb3+.54 Similar to R-2, R-2A, R-2B and R-2C show typical 0D structures with inorganic octahedra fully isolated by organic ligands. The distribution of hydrogen bonds (type II) in these compounds is independent of Sb3+ dopant concentration (Fig. S13†), however, the strength of hydrogen bonds changes with Sb3+ dopant content. Specifically, the strength of hydrogen bonds demonstrates a positive correlation with the concentration of Sb3+ (Fig. S13†), as demonstrated by the shorter distance between N atoms and Cl− ions and increased N–H⋯Cl angles (Fig. S16 and Table S6†). Thus, the In/Sb–Cl bonds involved in the formation of hydrogen bonds are further elongated from 2.56 to 2.62 Å, as the Sb3+ dopant concentration increases (Fig. 3a). Simultaneously, the  parameter changes and follows the trend of R-2A (2.4 × 10−2) < R-2B (3.1 × 10−2) < R-2C (4.7 × 10−2), indicating that the
parameter changes and follows the trend of R-2A (2.4 × 10−2) < R-2B (3.1 × 10−2) < R-2C (4.7 × 10−2), indicating that the  parameter increases with increasing the Sb3+ dopant concentration.
parameter increases with increasing the Sb3+ dopant concentration.
R-2A, R-2B and R-2C all show identical yellow emissions with high PLQYs of near-unity (Fig. S17a†). Moreover, their microsecond PL lifetimes, excitation-independent PL spectra and linearly fitted power-dependent data demonstrate that these bright emissions originate from Sb3+, similar to R-2 (Fig. S17 and 18†). The CD spectra of these three chiral HMHs exhibit obvious Cotton effects in the range of 270–450 nm (Fig. S19†). The magnitude of |gabs| increases from 0.78 × 10−3 (at 400 nm, R-2A) to 1.25 × 10−3 (at 400 nm, R-2C) corresponding to a 1.6-fold enhancement, and follows the trend of R-2A < R-2B < R-2C (Fig. 3b). Intriguingly, the CPL intensities of chiral HMHs are enhanced with increasing Sb3+ concentration (Fig. S20†). The |glum| values also show the trend of R-2A (2.2 × 10−2) < R-2B (3.0 × 10−2) < R-2C (3.8 × 10−2), which is in line with |gabs| (Fig. 3c). By plotting the |gabs| and glum values and corresponding  parameters of all chiral HMHs, it is found that the |gabs| and glum values are positively proportional to the
parameters of all chiral HMHs, it is found that the |gabs| and glum values are positively proportional to the  parameter (Fig. 3d and e). Moreover, the bond length and bond angle distortion exhibit totally different variations compared to
parameter (Fig. 3d and e). Moreover, the bond length and bond angle distortion exhibit totally different variations compared to  parameters (Fig. S21†). These results clearly demonstrate that a large
parameters (Fig. S21†). These results clearly demonstrate that a large 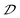 parameter of inorganic octahedra in chiral HMHs is crucial to enhancing the values of |gabs| and |glum|. Furthermore, the glum value (+3.8 × 10−2) and PLQY (near-unity) of R-2C exceed the CPL performances of currently reported chiral HMHs (Table S8†).
parameter of inorganic octahedra in chiral HMHs is crucial to enhancing the values of |gabs| and |glum|. Furthermore, the glum value (+3.8 × 10−2) and PLQY (near-unity) of R-2C exceed the CPL performances of currently reported chiral HMHs (Table S8†).
Based on the above crystallographic and chiroptical spectra studies, a plausible mechanism to enhance the values of |gabs| and |glum| in 0D HMHs is proposed (Fig. 4):
(i) The coexistence of chiral R-MBA and achiral 2MA ligands induces the formation of asymmetric hydrogen bonds between organic ligands and inorganic octahedra [In/SbCl]63−. As strong and weak hydrogen bonds are aligned along the same axis of equatorial Cl–In/Sb–Cl with opposite directions (type I), inorganic octahedra with small off-centering distortion can be observed;
(ii) Substituting 2MA with the 3MA ligand that is a stronger hydrogen bond acceptor and has a greater steric hindrance results in the distribution of strong and weak hydrogen bonds surrounding inorganic octahedra along two distinct axes of equatorial Cl–In/Sb–Cl (type II). Compared with type I, the off-centering distortion of inorganic octahedra in type II is obviously enhanced. Accordingly, the |gabs| and |glum| values of chiral 0D HMHs in type II surpass those of type I, despite having identical crystal structures, inorganic components, and PLQYs;
(iii) The off-centering distortion of inorganic octahedra in type II can be further enhanced by increasing the Sb3+ dopant concentration. The higher electronegativity of Sb3+ compared to In3+ results in reduced distances between N atoms and Cl− ions, thereby reinforcing the strong hydrogen bonds and amplifying the asymmetric stretch of In/Sb–Cl bonds. Consequently, both |gabs| and |glum| values increase with the increase of off-centering distortion of inorganic octahedra.
Such outstanding CPL performances make R/S-2C promising candidates for CPL light sources. R/S-2C are therefore used as the phosphors to emit CPL. R/S-light-emitting diodes (R/S-LEDs) are fabricated by coating R/S-2C polycrystals onto 365 nm ultraviolet GaN LED chips. The ultraviolet emission of the GaN chip is turned on at 3.0 V, which excites the R/S-2C polycrystals to generate CPL. CPL characterization studies of R/S-LEDs are performed on a JASCO CPL-300 through inserting the fabricated LED into the sample chamber and blocking the xenon lamp light source. These R/S-LEDs exhibit bright yellow emissions, and their CPL spectra show a mirror image profile (Fig. 5a). The polarized degree (P) is used to evaluate the CPL performance of R/S-LEDs, which is defined as
Conclusions
In summary, five pairs of enantiomorphic R/S-1, R/S-2, R/S-2A, R/S-2B and R/S-2C chiral 0D indium-antimony HMHs based on achiral ligands 2MA and 3MA and different Sb3+ dopant concentrations are prepared. Among these, R-2C exhibits the largest |glum| value of 3.8 × 10−2, as well as high PLQY of near-unity, outperforming currently reported chiral HMHs. Structural analyses and photophysical characterization studies reveal that the large off-centering distortion of inorganic octahedra, which is determined by the distribution and strength of hydrogen bonds formed around inorganic octahedra, plays critical roles in achieving higher gabs and glum values. Furthermore, R/S-LEDs with ±1.6% polarized degree are prepared, providing a feasible strategy to fabricate an efficient UV-pumped CPL light source. This work not only provides a general strategy to improve gabs/glum values of chiral 0D HMHs, but also sheds light on the relationship between the structure of the inorganic unit and chiroptical activity.Data availability
Additional data supporting the findings described in this paper are available in the ESI.† Crystallographic data for the structures reported in the present article have been deposited at the Cambridge Crystallographic Data Center (CCDC). The accession CCDC numbers for R-1, R-2, R-2A, R-2B and R-2C crystal structure CIFs reported in this paper are: 2344292, 2344293, 2344294, 2344295 and 2344296. These data are provided free of charge by the joint CCDC and Fachinformationszentrum Karlsruhe Access Structures service.Author contributions
Z. Q. and Y. L. proposed and supervised the project; Y. L. designed the experiments, analyzed the data and wrote the paper; Y. W., Z. L., and B. X. participated in discussions and gave much helpful advice; M. H. helped with the PXRD measurements; P. H. helped with power-dependent PL measurements; C. L. helped with the PL measurements of chiral ligands.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (22375084), the Guangdong Education Department (2021ZDZX2049), and the Shenzhen Science and Technology Innovation Committee (RCJC202106091044441068, RCBS20221008093335082, and KCXFZ20211020174805008). The computational resource was supported by the Center for Computational Science and Engineering at the Southern University of Science and Technology (SUSTech).References
- X. Q. Zhan, F. F. Xu, Z. H. Zhou, Y. L. Yan, J. N. A. Yao and Y. S. Zhao, 3D Laser Displays Based on Circularly Polarized Lasing from Cholesteric Liquid Crystal Arrays, Adv. Mater., 2021, 33, 2104418 CrossRef CAS.
- J. F. Sherson, H. Krauter, R. K. Olsson, B. Julsgaard, K. Hammerer, I. Cirac and E. S. Polzik, Quantum Teleportation Between Light and Matter, Nature, 2006, 443, 557–560 CrossRef CAS PubMed.
- Y. Kan, S. K. H. Andersen, F. Ding, S. Kumar, C. Zhao and S. I. Bozhevolnyi, Metasurface-Enabled Generation of Circularly Polarized Single Photons, Adv. Mater., 2020, 32, 1907832 CrossRef CAS PubMed.
- J. Zhang, M. Calame and M. L. Perrin, Contacting Atomically Precise Graphene Nanoribbons for Next-Generation Quantum Electronics, Matter, 2022, 5, 2497–2499 CrossRef CAS.
- M. Zhang, Q. Guo, Z. Li, Y. Zhou, S. Zhao, Z. Tong, Y. Wang, G. Li, S. Jin, M. Zhu, T. Zhuang and S.-H. Yu, Processable Circularly Polarized Luminescence Material Enables Flexible Stereoscopic 3D Imaging, Sci. Adv., 2023, 9, eadi9944 CrossRef CAS.
- H. Z. Zheng, W. R. Li, W. Li, X. J. Wang, Z. Y. Tang, S. X. A. Zhang and Y. Xu, Uncovering the Circular Polarization Potential of Chiral Photonic Cellulose Films for Photonic Applications, Adv. Mater., 2018, 30, 1705948 CrossRef.
- J. Ahn, E. Lee, J. Tan, W. Yang, B. Kim and J. Moon, A New Class of Chiral Semiconductors: Chiral-Organic-Molecule-Incorporating Organic-Inorganic Hybrid Perovskites, Mater. Horiz., 2017, 4, 851–856 RSC.
- J. Q. Ma, C. Fang, C. Chen, L. Jin, J. Q. Wang, S. Wang, J. Tang and D. H. Li, Chiral 2D Perovskites with a High Degree of Circularly Polarized Photoluminescence, ACS Nano, 2019, 13, 3659–3665 CrossRef CAS PubMed.
- J. X. Gao, W. Y. Zhang, Z. G. Wu, Y. X. Zheng and D. W. Fu, Enantiomorphic Perovskite Ferroelectrics with Circularly Polarized Luminescence, J. Am. Chem. Soc., 2020, 142, 4756–4761 CrossRef CAS PubMed.
- H. Duim and M. A. Loi, Chiral Hybrid Organic-Inorganic Metal Halides: A Route Toward Direct Detection and Emission of Polarized Light, Matter, 2021, 4, 3835–3851 CrossRef CAS.
- A. Pietropaolo, A. Mattoni, G. Pica, M. Fortino, G. Schifino and G. Grancini, Rationalizing the Design and Implementation of Chiral Hybrid Perovskites, Chem, 2022, 8, 1231–1253 CAS.
- X. Han, P. Cheng, S. Han, Z. Wang, J. Guan, W. Han, R. Shi, S. Chen, Y. Zheng, J. Xu and X. H. Bu, Multi-stimuli-responsive Luminescence Enabled by Crown Ether Anchored Chiral Antimony Halide Phosphors, Chem. Sci., 2024, 15, 3530–3538 RSC.
- X. Y. Niu, Z. C. Zeng, Z. Y. Wang, H. L. Lu, B. Sun, H. L. Zhang, Y. S. Chen, Y. P. Du and G. K. Long, The First Chiral Cerium Halide Towards Circularly-Polarized Luminescence in The UV Region, Sci. China: Chem., 2024, 67, 1961–1968 CrossRef CAS.
- D. Y. Liu, H. Y. Li, R. P. Han, H. L. Liu and S. Q. Zang, Multiple Stimuli-Responsive Luminescent Chiral Hybrid Antimony Chlorides for Anti-Counterfeiting and Encryption Applications, Angew. Chem., Int. Ed., 2023, 62, e202307875 CrossRef CAS PubMed.
- Q. W. Guan, H. Ye, T. T. Zhu, X. Y. Zhang, S. H. You, J. B. Wu, Y. X. Zheng, X. T. Liu and J. H. Luo, Formamidine Engineering the Lattice Distortion of Chiral Halide Perovskites for Efficient Blue Circularly Polarized Emission, Adv. Opt. Mater., 2023, 11, 2202726 CrossRef CAS.
- Z.-L. Gong, X. Zhu, Z. Zhou, S.-W. Zhang, D. Yang, B. Zhao, Y.-P. Zhang, J. Deng, Y. Cheng, Y.-X. Zheng, S.-Q. Zang, H. Kuang, P. Duan, M. Yuan, C.-F. Chen, Y. S. Zhao, Y.-W. Zhong, B. Z. Tang and M. Liu, Frontiers in Circularly Polarized Luminescence: Molecular Design, Self-Assembly, Nanomaterials, and Applications, Sci. China: Chem., 2021, 64, 2060–2104 CrossRef CAS.
- M. Z. Li and Z. G. Xia, Recent Progress of Zero-Dimensional Luminescent Metal Halides, Chem. Soc. Rev., 2021, 50, 2626–2662 RSC.
- K. M. McCall, V. Morad, B. M. Benin and M. V. Kovalenko, Efficient Lone-Pair-Driven Luminescence: Structure–Property Relationships in Emissive 5s2 Metal Halides, ACS Mater. Lett., 2020, 2, 1218–1232 CrossRef CAS PubMed.
- T. Cai, W. W. Shi, R. Z. Wu, C. Chu, N. Jin, J. Y. Wang, W. W. Zheng, X. Z. Wang and O. Chen, Lanthanide Doping into All-Inorganic Heterometallic Halide Layered Double Perovskite Nanocrystals for Multimodal Visible and Near-Infrared Emission, J. Am. Chem. Soc., 2024, 146, 3200–3209 CrossRef CAS.
- G. K. Long, R. Sabatini, M. I. Saidaminov, G. Lakhwani, A. Rasmita, X. G. Liu, E. H. Sargent and W. B. Gao, Chiral-Perovskite Optoelectronics, Nat. Rev. Mater., 2020, 5, 423–439 CrossRef.
- H. L. Xuan, J. L. Li, L. J. Xu, D. S. Zheng and Z. N. Chen, Circularly Polarized Luminescence based on 0D Lead-Free Antimony (III) Halide Hybrids, Adv. Opt. Mater., 2022, 10, 2200591 CrossRef CAS.
- Y. Wei, C. Li, Y. W. Li, Z. S. Luo, X. Y. Wu, Y. L. Liu, L. M. Zhang, X. He, W. Wang and Z. W. Quan, Circularly Polarized Luminescence from Zero-Dimensional Hybrid Lead-Tin Bromide with Near-Unity Photoluminescence Quantum Yield, Angew. Chem., Int. Ed., 2022, 61, e202212685 CrossRef CAS.
- M. P. Davydova, L. Q. Meng, M. I. Rakhmanova, I. Y. Bagryanskaya, V. S. Sulyaeva, H. Meng and A. V. Artem'ev, Highly Emissive Chiral Mn(II) Bromide Hybrids for UV-Pumped Circularly Polarized LEDs and Scintillator Image Applications, Adv. Opt. Mater., 2023, 11, 2202811 CrossRef CAS.
- J. J. Guan, Y. S. Zheng, P. X. Cheng, W. Q. Han, X. Han, P. H. Wang, M. Y. Xin, R. C. Shi, J. L. Xu and X. H. Bu, Free Halogen Substitution of Chiral Hybrid Metal Halides for Activating the Linear and Nonlinear Chiroptical Properties, J. Am. Chem. Soc., 2023, 145, 26833–26842 CrossRef CAS.
- Y. L. Liu, Z. S. Luo, Y. Wei, C. Li, Y. L. Chen, X. He, X. Y. Chang and Z. W. Quan, Integrating Achiral and Chiral Organic Ligands in Zero-Dimensional Hybrid Metal Halides to Boost Circularly Polarized Luminescence, Angew. Chem., Int. Ed., 2023, 62, e202306821 CrossRef CAS PubMed.
- Y. Deng, F. Li, Z. Zhou, M. Wang, Y. Zhu, J. Zhao, S. Liu and Q. Zhao, Chiral Induction and Sb3+ Doping in Indium Halides to Trigger Second Harmonic Generation and Circularly Polarized Luminescence, Chin. Chem. Lett., 2024, 35, 109085 CrossRef CAS.
- G. K. Long, C. Y. Jiang, R. Sabatini, Z. Y. Yang, M. Y. Wei, L. N. Quan, Q. M. Liang, A. Rasmita, M. Askerka, G. Walters, X. W. Gong, J. Xing, X. L. Wen, R. Quintero-Bermudez, H. F. Yuan, G. C. Xing, X. R. Wang, D. T. Song, O. Voznyy, M. T. Zhang, S. Hoogland, W. B. Gao, Q. H. Xiong and E. H. Sargent, Spin Control in Reduced-Dimensional Chiral Perovskites, Nat. Photonics, 2018, 12, 528–533 CrossRef CAS.
- T. T. Zhu, W. Weng, C. M. Ji, X. Y. Zhang, H. Ye, Y. P. Yao, X. L. Li, J. L. Li, W. X. Lin and J. H. Luo, Chain-to-Layer Dimensionality Engineering of Chiral Hybrid Perovskites to Realize Passive Highly Circular-Polarization-Sensitive Photodetection, J. Am. Chem. Soc., 2022, 144, 18062–18068 CrossRef CAS PubMed.
- Y. S. Zheng, X. Han, P. X. Cheng, X. D. Jia, J. L. Xu and X. H. Bu, Induction of Chiral Hybrid Metal Halides from Achiral Building Blocks, J. Am. Chem. Soc., 2022, 144, 16471–16479 CrossRef CAS.
- W. D. Cai, J. J. Qin, T. Q. Pang, X. Y. Cai, R. X. Jia and F. Gao, Chirality Induced Crystal Structural Difference in Metal Halide Composites, Adv. Opt. Mater., 2022, 10, 2102140 CrossRef CAS.
- S. Ma, Y. K. Jung, J. Ahn, J. Kyhm, J. Tan, H. Lee, G. Jang, C. U. Lee, A. Walsh and J. Moon, Elucidating the Origin Of Chiroptical Activity in Chiral 2D Perovskites through Nano-Confined Growth, Nat. Commun., 2022, 13, 3259 CrossRef CAS PubMed.
- J. Son, S. Ma, Y. K. Jung, J. Tan, G. Jang, H. Lee, C. U. Lee, J. Lee, S. Moon, W. Jeong, A. Walsh and J. Moon, Unraveling Chirality Transfer Mechanism by Structural Isomer-Derived Hydrogen Bonding Interaction in 2D Chiral Perovskite, Nat. Commun., 2023, 14, 3124 CrossRef.
- M. K. Jana, R. Y. Song, H. L. Liu, D. R. Khanal, S. M. Janke, R. D. Zhao, C. Liu, Z. V. Vardeny, V. Blum and D. B. Mitzi, Organic-To-Inorganic Structural Chirality Transfer in a 2D Hybrid Perovskite and Impact on Rashba-Dresselhaus Spin-Orbit Coupling, Nat. Commun., 2020, 11, 4699 CrossRef CAS PubMed.
- K. J. Liu, J. Zhao and Q. L. Liu, Chiral Organic Cations and Distorted Inorganic Polyhedra Composing Metal Halide Nonlinear Optical Materials, Laser Photonics Rev., 2024, 2400345 CrossRef.
- H. P. Lu, Z. V. Vardeny and M. C. Beard, Control of Light, Spin and Charge with Chiral Metal Halide Semiconductors, Nat. Rev. Chem, 2022, 6, 470–485 CrossRef.
- H. R. Lin, C. K. Zhou, Y. Tian, T. Siegrist and B. W. Ma, Low-Dimensional Organometal Halide Perovskites, ACS Energy Lett., 2018, 3, 54–62 CrossRef CAS.
- S. Q. Sun, M. Lu, X. P. Gao, Z. F. Shi, X. Bai, W. W. Yu and Y. Zhang, 0D Perovskites: Unique Properties, Synthesis, and Their Applications, Adv. Sci., 2021, 8, e2102689 CrossRef.
- E. Arunan, G. R. Desiraju, R. A. Klein, J. Sadlej, S. Scheiner, I. Alkorta, D. C. Clary, R. H. Crabtree, J. J. Dannenberg, P. Hobza, H. G. Kjaergaard, A. C. Legon, B. Mennucci and D. J. Nesbitt, Definition of the hydrogen bond (IUPAC Recommendations 2011), Pure Appl. Chem., 2011, 83, 1637–1641 CrossRef CAS.
- X. J. Lü, C. Stoumpos, Q. Y. Hu, X. D. Ma, D. Z. Zhang, S. H. Guo, J. Hoffman, K. J. Bu, X. F. Guo, Y. Q. Wang, C. Ji, H. J. Chen, H. W. Xu, Q. X. Jia, W. G. Yang, M. G. Kanatzidis and H. K. Mao, Regulating Off-Centering Distortion Maximizes Photoluminescence in Halide Perovskites, Natl. Sci. Rev., 2021, 8, nwaa288 CrossRef PubMed.
- G. H. Wu, C. K. Zhou, W. M. Ming, D. Han, S. Y. Chen, D. Yang, T. Besara, J. Neu, T. Siegrist, M. H. Du, B. W. Ma and A. G. Dong, A One-Dimensional Organic Lead Chloride Hybrid with Excitation-Dependent Broadband Emissions, ACS Energy Lett., 2018, 3, 1443–1449 CrossRef CAS.
- T. Schmidt, G. Daniel and K. Lischka, The Excitation Power Dependence of the near Band Edge Photoluminescence of II-VI Semiconductors, J. Cryst. Growth, 1992, 117, 748–752 CrossRef CAS.
- T. Schmidt, K. Lischka and W. Zulehner, Excitation-Power Dependence of The Near-Band-Edge Photoluminescence of Semiconductors, Phys. Rev. B, 1992, 45, 8989–8994 CrossRef PubMed.
- S. R. Li, J. J. Luo, J. Liu and J. Tang, Self-Trapped Excitons in All-Inorganic Halide Perovskites: Fundamentals, Status, and Potential Applications, J. Phys. Chem. Lett., 2019, 10, 1999–2007 CrossRef CAS PubMed.
- Z. P. Wang, Z. Z. Zhang, L. Q. Tao, N. N. Shen, B. Hu, L. K. Gong, J. R. Li, X. P. Chen and X. Y. Huang, Hybrid Chloroantimonates(III): Thermally Induced Triple-Mode Reversible Luminescent Switching and Laser- Printable Rewritable Luminescent Paper, Angew. Chem., Int. Ed., 2019, 58, 9974–9978 CrossRef CAS.
- G. M. Song, M. Z. Li, S. Z. Zhang, N. Z. Wang, P. F. Gong, Z. G. Xia and Z. S. Lin, Enhancing Photoluminescence Quantum Yield in 0D Metal Halides by Introducing Water Molecules, Adv. Funct. Mater., 2020, 30, 2002468 CrossRef CAS.
- L. J. Xu, A. Plaviak, X. Lin, M. Worku, Q. He, M. Chaaban, B. J. Kim and B. Ma, Metal Halide Regulated Photophysical Tuning of Zero-Dimensional Organic Metal Halide Hybrids: From Efficient Phosphorescence to Ultralong Afterglow, Angew. Chem., Int. Ed., 2020, 59, 23067–23071 CrossRef CAS PubMed.
- F. Liu, T. J. Zhang, D. Mondal, S. Y. Teng, Y. Zhang, K. K. Huang, D. Y. Wang, W. S. Yang, P. Mahadevan, Y. S. Zhao, R. G. Xie and N. Pradhan, Light-Emitting Metal-Organic Halide 1D and 2D Structures: Near-Unity Quantum Efficiency, Low-Loss Optical Waveguide and Highly Polarized Emission, Angew. Chem., Int. Ed., 2021, 60, 13548–13553 CrossRef CAS PubMed.
- V. Morad, S. Yakunin, B. M. Benin, Y. Shynkarenko, M. J. Grotevent, I. Shorubalko, S. C. Boehme and M. V. Kovalenko, Hybrid 0D Antimony Halides as Air-Stable Luminophores for High-Spatial-Resolution Remote Thermography, Adv. Mater., 2021, 33, e2007355 CrossRef PubMed.
- H. Liu, T. B. Shonde, F. Gonzalez, O. J. Olasupo, S. J. Lee, D. Luong, X. S. Lin, J. S. R. V. Winfred, E. Lochner, I. Fatima, K. Hanson and B. W. Ma, Efficient Red Light Emitting Diodes Based on a Zero-Dimensional Organic Antimony Halide Hybrid, Adv. Mater., 2023, 35, e2209417 CrossRef.
- N. Berova, L. Di Bari and G. Pescitelli, Application of Electronic Circular Dichroism In Configurational And Conformational Analysis of Organic Compounds, Chem. Soc. Rev., 2007, 36, 914–931 RSC.
- F. S. Richardson and J. P. Riehl, Circularly Polarized Luminescence Spectroscopy, Chem. Rev., 1977, 77, 773–792 CrossRef CAS.
- J. A. Alonso, M. J. Martínez-Lope, M. T. Casais and M. T. Fernández-Díaz, Evolution of the Jahn–Teller Distortion of MnO6 Octahedra in RMnO3 Perovskites (R = Pr, Nd, Dy, Tb, Ho, Er, Y): A Neutron Diffraction Study, Inorg. Chem., 2000, 39, 917–923 CrossRef CAS PubMed.
- M. W. Lufaso and P. M. Woodward, Jahn-Teller Distortions, Cation Ordering and Octahedral Tilting in Perovskites, Acta Crystallogr., Sect. B: Struct. Sci., 2004, 60, 10–20 CrossRef PubMed.
- Y. Y. Jing, Y. Liu, X. X. Jiang, M. S. Molokeev, Z. S. Lin and Z. G. Xia, Sb3+ Dopant and Halogen Substitution Triggered Highly Efficient and Tunable Emission in Lead-Free Metal Halide Single Crystals, Chem. Mater., 2020, 32, 5327–5334 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2344292–2344296. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc04399e |
| This journal is © The Royal Society of Chemistry 2024 |