Open Access Article
Open Access ArticleMetal–organic frameworks with two different-sized aromatic ring-confined nanotraps for benchmark natural gas upgrade†
Shu-Yi
Li
a,
Ying-Ying
Xue
a,
Jia-Wen
Wang
a,
Hai-Peng
Li
a,
Jiao
Lei
a,
Hong-Juan
Lv
a,
Xianhui
Bu
 *b,
Peng
Zhang
a,
Ying
Wang
*b,
Peng
Zhang
a,
Ying
Wang
 a,
Wen-Yu
Yuan
a and
Quan-Guo
Zhai
a,
Wen-Yu
Yuan
a and
Quan-Guo
Zhai
 *a
*a
aKey Laboratory of Applied Surface and Colloid Chemistry, Ministry of Education, School of Chemistry & Chemical Engineering, Shaanxi Normal University, Xi'an, Shaanxi 710062, China. E-mail: zhaiqg@snnu.edu.cn
bDepartment of Chemistry and Biochemistry, California State University, Long Beach, California 90840, USA. E-mail: xianhui.bu@csulb.edu
First published on 4th October 2024
Abstract
Recovery of light alkanes from natural gas is of great significance in petrochemical production. Herein, a promising strategy utilizing two types of size-complementary aromatic ring-confined nanotraps (called bi-nanotraps here) is proposed to efficiently trap ethane (C2H6) and propane (C3H8) selectively at their respective sites. Two isostructural metal–organic frameworks (MOFs, SNNU-185/186), each containing bi-nanotraps decorated with six aromatic rings, are selected to demonstrate the feasibility of this method. The smaller nanotrap acts as adsorption sites tailored for C2H6 while the larger one is optimized in size for C3H8. The separation is further facilitated by the large channels, which serve as mass transfer pathways. These advanced features give rise to multiple C–H⋯π interactions and size/shape-selective interaction sites, enabling SNNU-185/186 to achieve high C2H6 adsorption enthalpy (43.5/48.8 kJ mol−1) and a very large thermodynamic interaction difference between C2H6 and CH4. Benefiting from the bi-nanotrap effect, SNNU-185/186 exhibits benchmark experimental natural gas upgrade performance with top-level CH4 productivity (6.85/6.10 mmol g−1), ultra-high purity and first-class capture capacity for C2H6 (1.23/0.90 mmol g−1) and C3H8 (2.33/2.15 mmol g−1).
Introduction
Natural gas is mainly composed of methane (CH4, 85% by volume) which is an important clean energy source and essential chemical feedstock. However, the presence of ethane (9% C2H6) and propane (3% C3H8) not only reduces the combustion efficiency and conversion rate of CH4, but also affects the safety of CH4 storage.1–5 In addition, C2H6 and C3H8 are valuable petrochemical feedstocks for the manufacture of alkenes and polymers.6–11 Therefore, efficient separation and recovery of C2H6 and C3H8 from natural gas are important for both CH4 upgrading and full energy utilization. The current separation process is mainly based on cryogenic distillation technology which is energy intensive and environmentally unfriendly.12–14 By contrast, adsorption-based separation using solid adsorbents is cost- and energy-efficient.15–20With guest accessible porosity, and a variety of different components contributing to the tunability of pore structures and surface properties, porous coordination polymers (PCPs) or metal–organic frameworks (MOFs) are a promising class of solid adsorbents capable of overcoming the performance bottleneck resulting from imprecise pore control, few structural building units, and a limited number of coordination pathways of traditional adsorbents.21–25 To date, many MOFs have been investigated for C3H8/C2H6/CH4 separation based on the thermodynamic separation mechanism. Generally, creating a polar pore surface (C–H⋯O/N/F hydrogen bonds)26–30 or non-polar pore environments (aromatic C–H⋯π bonds or confined aliphatic C–H⋯C hydrogen bonds), and simultaneously regulating pore size to provide a confined space for enhanced MOF–gas interaction,31–34 are effective strategies. One difficulty is that the performance of MOF materials is limited by the C2H6/CH4 separation step as shown in Zn-BPZ-SA,5 LIFM-ZZ-1,9 BSF-2,14 MIL-101,30 UiO-66-NaPh34 and CFA-1,35 largely due to the greater similarity in molecular size and chemical properties between C2H6 and CH4.4,34 Another often-neglected but crucial reason is the competitive adsorption between C3H8 and C2H6 in the ternary gas separation system.36 C3H8 molecules preferentially occupy adsorption sites to form stronger interaction with the framework due to their larger polarizability and molecular size compared to C2H6, which further increases the difficulty of the C2H6/CH4 separation step. Therefore, the key to improving the performance of C2H6/CH4 separation is to increase the thermodynamic difference between C2H6 and CH4 while simultaneously installing size-selective sites for C2H6 and C3H8 to minimize the competitive adsorption between C2H6 and C3H8.
Fortunately, the difference in molecular polarizability and the number of H-donors between C3H8, C2H6, and CH4 could enable thermodynamic preferential adsorption of C3H8 or C2H6 by creating polar/non-polar pore surfaces. Compared to single adsorption sites, nanotraps or molecular traps that allow for the selective capture of specific gas molecules are more effective and attractive.37–45 With multiple and gas-specific adsorption sites, nanotraps provide stronger binding interactions and recognition capabilities for target molecules, which is promising for widening the thermodynamic gap between C3H8, C2H6 and CH4. However, the construction of nanotraps is rare and challenging for MOFs.
In addition, the combination of strong binding affinity and molecular sieving should have great potential in preventing competitive adsorption and achieving the most effective purification and separation. Its high efficiency and application potential have been demonstrated in multi-component separation.36,46–48 The construction of coexistent C2H6- and C3H8-selective adsorption sites in one MOF system is difficult because C2H6 and C3H8 tend to occupy the same sites, with C3H8 being preferred. However, utilizing the difference in kinetic diameter between C3H8 (5.1 Å) and C2H6 (4.4 Å) to discriminate between them could be an effective method to eliminate competitive adsorption (Table S1†), leading to enhanced MOF performance in the key C2H6/CH4 step. Overall, the combination of nanotraps with the molecular sieving effect is expected to facilitate multiple and strong interactions and widen the thermodynamic difference between C3H8, C2H6 and CH4. It will also help install sites targeting selective adsorption for C3H8 and C2H6 to reduce their competitive adsorption and, therefore, maximize the separation performance (Scheme 1).
 | ||
| Scheme 1 A proposed strategy for paraffin separation with the synergistic effect of C–H⋯π interactions and nanotraps. | ||
Herein, a promising example of bi-nanotraps is demonstrated. In two newly constructed MOFs (SNNU-185/186), the smaller type of nanotraps with appropriate size and shape is ideally suited for accommodating C2H6 based on the thermodynamic-molecular sieving mechanism and the larger nanotraps are more advantageous for trapping C3H8 thanks to the thermodynamic interaction difference. In the meantime, the large channels serve as mass transfer pathways, promoting gas molecules to enter the adsorption sites from pore walls. As a result, multiple C–H⋯π interactions and highly discriminating interaction sites are achieved in one unprecedented MOF system, contributing to benchmark −Qst for C2H6 and the exceptionally large −Qst difference between C2H6 and CH4. The overall effect is greatly increased thermodynamic difference and weakened competitive adsorption. Together with excellent adsorption capability and high stability, SNNU-185 and SNNU-186 can produce ultra-high purity CH4 (>99.9999%) at flow rates of 4/6 mL min−1 with top-level productivities for CH4 (6.85 and 6.10 mmol g−1), and top-notch capture capacities for C2H6 (1.23 and 0.90 mmol g−1) and C3H8 (2.33 and 2.15 mmol g−1) in breakthrough experiments. GCMC simulation provides a molecular level insight and mechanistic explanation of the role of bi-nanotraps. This work not only provides promising materials for natural gas upgrade, but also reveals an effective design philosophy toward the development of porous coordination polymers for challenging multi-component separation processes.
Results and discussion
Hydrothermal reactions of 1,3,5-tris(4-pyridyl)-benzene (TPB) or 2,4,6-tri(4-pyridinyl)-1-pyridine (TPP), 2,5-pyridinedicarboxylic acid (2,5-PDC) and cobalt acetate hydrate were used to synthesize SNNU-185 (with TPB) and SNNU-186 (with TPP) (Fig. S1 and S2†). From the single crystal analysis, they are found to be isostructural and crystallize in the hexagonal space group P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) c2 with the formula of {[Co3(μ3-OH)][Co(2,5-PDC)2]3(TPB/TPP)3}n (Fig. S3 and Table S2†), which is isostructural with our reported SNNU-54 (ref. 49) synthesized under different conditions (Fig. S4 and S5†). SNNU-185 and SNNU-186 were selected to demonstrate the feasibility of the aromatic ring-confined bi-nanotrap strategy for efficiently and separately trapping C2H6 and C3H8 based on a thermodynamic-molecular sieving coupling mechanism. As shown in Fig. 1, both SNNU-185 and SNNU-186 contain two distinct Co atoms that form two types of secondary building units (SBU I and SBU II). The Co1 center is six-coordinated by four O-donors from four different 2,5-PDC ligands, one N-donor from TPB/TPP and one central μ3-OH. Three Co1 atoms form a [Co3(μ3-OH)(COO)6] trimer (Fig. 1a and b), acting as a 9-connected node. The Co2 atom (Fig. 1c and d) is hexacoordinated in a distorted octahedral configuration formed by two carboxylate O and two pyridine N atoms from two 2,5-PDC ligands, and two N atoms from TPB/TPP ligands. Chelate rings are on the same side, forming [Co(2,5-PDC)2(4-pyridine)2] MOLs (metal–organic linkers) in a cis-configuration, which is considered a 4-connected node. Two Co1 trinuclear clusters and three Co2 MOLs connect with each other to build a trigonal bipyramid-type cage along the c-axis, which is further extended into 1D {[Co3(μ3-OH)][Co(PDC)2]3}n chains (Fig. 1g).
c2 with the formula of {[Co3(μ3-OH)][Co(2,5-PDC)2]3(TPB/TPP)3}n (Fig. S3 and Table S2†), which is isostructural with our reported SNNU-54 (ref. 49) synthesized under different conditions (Fig. S4 and S5†). SNNU-185 and SNNU-186 were selected to demonstrate the feasibility of the aromatic ring-confined bi-nanotrap strategy for efficiently and separately trapping C2H6 and C3H8 based on a thermodynamic-molecular sieving coupling mechanism. As shown in Fig. 1, both SNNU-185 and SNNU-186 contain two distinct Co atoms that form two types of secondary building units (SBU I and SBU II). The Co1 center is six-coordinated by four O-donors from four different 2,5-PDC ligands, one N-donor from TPB/TPP and one central μ3-OH. Three Co1 atoms form a [Co3(μ3-OH)(COO)6] trimer (Fig. 1a and b), acting as a 9-connected node. The Co2 atom (Fig. 1c and d) is hexacoordinated in a distorted octahedral configuration formed by two carboxylate O and two pyridine N atoms from two 2,5-PDC ligands, and two N atoms from TPB/TPP ligands. Chelate rings are on the same side, forming [Co(2,5-PDC)2(4-pyridine)2] MOLs (metal–organic linkers) in a cis-configuration, which is considered a 4-connected node. Two Co1 trinuclear clusters and three Co2 MOLs connect with each other to build a trigonal bipyramid-type cage along the c-axis, which is further extended into 1D {[Co3(μ3-OH)][Co(PDC)2]3}n chains (Fig. 1g).
Significantly, each cage is decorated with six aromatic rings (from 2,5-PDC) and each aromatic ring layer has three aromatic rings which are distributed in a staggered pattern from top to bottom (Fig. 1h). The available inner cavity is ∼7.2 Å × 7.2 Å and the window size is ∼5.3 Å × 4.8 Å (Fig. 1h and S3c†). This cage size and environment match well with the size and shape of C2H6 and C3H8, acting as “aromatic ring-confined nanotrap 1” which is expected to promote the formation of strong host–guest interactions. Moreover, because the inner cavity of nanotrap 1 is more compatible with C3H8, this type of large nanotrap can act as C3H8-selective interaction sites. The 3D framework of SNNU-185/186 is formed when each cage chain connects six neighboring chains via six TPB/TPP ligands. The resulting small-sized channels (Fig. 1i) are also modified by six aromatic rings (from peripheral pyridine rings of TPB/TPP) in a staggered pattern from top to bottom, which are referred to as “aromatic ring-confined nanotrap 2” (Fig. 1j). This small nanotrap 2 has a pore size of about 4.8 Å × 4.8 Å and a window size of about 4.3 Å × 4.8 Å (Fig. S3d†). By summarizing and analyzing MOF materials with high C2H6/CH4 separation performance such as Ni(TMBDC)(DABCO)0.5 (ref. 2) (5.0 Å), ZUL-C2 (ref. 4) (5.3 Å), Ni-MOF 1 (ref. 50) (5.7 Å), and SNNU-Bai69 (ref. 3) (6.4 Å), it can be concluded that such pore sizes favor the formation of strong interactions with C2H6 through C–H⋯π bonds and can amplify the thermodynamic gap between C2H6 and CH4 to the maximum extent. Furthermore, considering the size-exclusion potential of C3H8 as shown in KAUST-7,51,52 Y-abtc,53 Co-gallate,54 JNU-3a,55 and NTU-85-WNT56 which have aperture sizes of about 4.7 Å, 4.7 Å, 5.2 Å, 5.3 Å and 4.6 Å respectively, this small-size channel is expected to limit C3H8 entry to some extent, thus creating C2H6-selective interaction sites based on a molecular sieving mechanism. Finally, the large channel decorated with oxygen atoms from uncoordinated carboxylic acids can also interact with gas molecules. However, considering its large pore size, the main role of the large channel might be to facilitate gas diffusion, allowing gas molecules to enter size-selective adsorption sites from pore walls. It can be concluded that the construction of C2H6-selective nanotraps, C3H8-selective nanotraps and mass transfer channels is achieved in SNNU-185 and SNNU-186 (Fig. 1k–m). Such a structural arrangement lays the foundation for efficient separation and recovery of C2H6 and C3H8 from natural gas.
PXRD patterns of the as-synthesized SNNU-185 and SNNU-186 samples align well with the calculated patterns obtained from the single crystals, indicating their successful synthesis with high purity (Fig. S6†). Also, a decagram scale synthesis of SNNU-186 was carried out under reflux conditions for 3 days. As shown in Fig. S7 and S8,† impurity-free SNNU-186 (∼12.7 g) could be easily obtained without loss of crystallinity, demonstrating its scalability. The TG analysis data showed that the as-synthesized and solvent-exchanged SNNU-185 and SNNU-186 are stable up to around 573 K, indicating their high thermal stability (Fig. S9†). Overall, the architecture of shape/size-matched bi-nanotraps, combined with size selectivity based on molecular sieving mechanisms and high stability inspired us to further investigate their C3H8/C2H6/CH4 separation performance.
The permanent porosity of activated SNNU-185 and SNNU-186 was confirmed using N2 adsorption–desorption isotherms at 77 K. As shown in Fig. S10,† both MOFs exhibit microporous type I sorption isotherms with calculated Brunauer–Emmett–Teller (BET) surface areas of 886 m2 g−1 and 875 m2 g−1 for SNNU-185 and SNNU-186, respectively. Single component adsorption isotherms for CH4, C2H6, and C3H8 on SNNU-185 and SNNU-186 were measured at different temperatures (273, 283 and 298 K) and at pressures up to 1 bar (Fig. 2a, b and S11†). Taking advantage of the bi-nanotrap structure, SNNU-185/186 adsorbed much more C3H8 and C2H6 than CH4 under the same conditions, indicating their potential for C3H8/C2H6/CH4 separation. At 298 K and 1.0 bar, the C2H6 storage capacity of SNNU-185 and SNNU-186 can reach 69.8 cm3 g−1 (3.12 mmol g−1) and 74.3 cm3 g−1 (3.32 mmol g−1), respectively. These values exceed those of many well-known reported MOF adsorbents, such as Zn-BPZ-SA5 (2.97 mmol g−1), ZUL-C1 (ref. 4) (2.95 mmol g−1), ZUL-C2 (ref. 4) (2.82 mmol g−1), BSF-3 (ref. 38) (2.35 mmol g−1), SNNU-Bai69 (ref. 3) (2.0 mmol g−1), ECUT-Th-10a11 (1.72 mmol g−1) and UiO-66-Naph34 (1.24 mmol g−1). The C3H8 isotherms of SNNU-185 and SNNU-186 at 273/298 K exhibited saturated uptakes of 98.6/94.0 cm3 g−1 (4.40/4.20 mmol g−1) and 108.0/97.1 cm3 g−1 (4.82/4.33 mmol g−1), respectively, surpassing those of most MOF materials such as ZUL-C1 (ref. 4) (2.72 mmol g−1), ZUL-C2 (ref. 4) (2.52 mmol g−1), BSF-3 (ref. 38) (2.98 mmol g−1), Ni-MOF 1 (ref. 50) (3.56 mmol g−1) and LIFM-ZZ-1 (ref. 9) (4.06 mmol g−1). Thanks to strong interactions from thermodynamic C3H8-selective nanotraps, the C3H8 uptake shows steep adsorption at low pressure, which is beneficial for capturing C3H8. For C2H6, steep adsorption at low pressure especially at 0–50 mmHg can also be observed, which might be attributed to strong interaction with C2H6-selective nanotraps. In addition, considering the presence of water and acidic gases such as H2S and SO2 in raw natural gas, detailed stability tests were further performed. After being treated under different conditions including soaking in water, exposure to aqueous solutions with different pH values or exposed to air for an extended period, satisfactory water stability and pH stability of these two MOFs were verified by adsorption/desorption tests (Fig. 2c).
To measure the binding affinities between the host surface and guest gas molecules, the adsorption enthalpy (−Qst) of C3H8, C2H6 and CH4 in SNNU-185/186 was calculated (Fig. 2d, S12 and Table S3†). Significantly, SNNU-186 shows the highest −Qst value of 48.8 kJ mol−1 for C2H6 compared to all reported MOF materials used for C3H8/C2H6/CH4 separation such as ZUL-C2 (ref. 4) (45 kJ mol−1), Ni(TMBDC)(DABCO)0.5 (ref. 2) (36 kJ mol−1), ZUL-C1 (ref. 4) (33 kJ mol−1) and SNNU-Bai69 (ref. 3) (30.6 kJ mol−1) (Table S4†). Importantly, SNNU-185 and SNNU-186 exhibit the largest −Qst difference between C2H6 and CH4 among MOF materials used for natural gas upgrading (Fig. 2e). This benchmark −Qst for C2H6 and the largest thermodynamic interaction difference between C2H6 and CH4 could be attributed to the bi-nanotrap structure which fully takes advantage of the synergistic effects of C–H⋯π interactions and nanotraps. As a result, multiple and strong C–H⋯π interactions and an increased thermodynamic interaction difference between gas molecules were achieved, which are beneficial for improving the performance in the key C2H6/CH4 step. Due to strong C–H⋯π interactions in C3H8-nanotraps and the rejection of C3H8 by C2H6-nanotraps, the −Qst values for C3H8 in SNNU-185/186 are moderate (48.1/47.2 kJ mol−1).
Ideal adsorbed solution theory (IAST) was used to further evaluate the separation potential of SNNU-185/186 for 50/50 C2H6/CH4 mixtures and 50/50 C3H8/CH4 mixtures at 298 K (Fig. 2f, S13–15 and Table S5†). At 1 kPa, for C2H6/CH4, the IAST selectivities of SNNU-185 and SNNU-186 are 43.4 and 52.1, respectively. For 50/50 C3H8/CH4, the selectivity values of SNNU-185 and SNNU-186 at 298 K and 100 kPa are 132.5 and 126.0, respectively. These values are not top-level but still higher than those of many well-known MOF materials, such as MIL-101-Cr30 (84.3), ZUL-C1 (ref. 4) (73), UiO-66 (ref. 34) (65) and ECUT-Th-10a11 (54.5) under the same conditions.
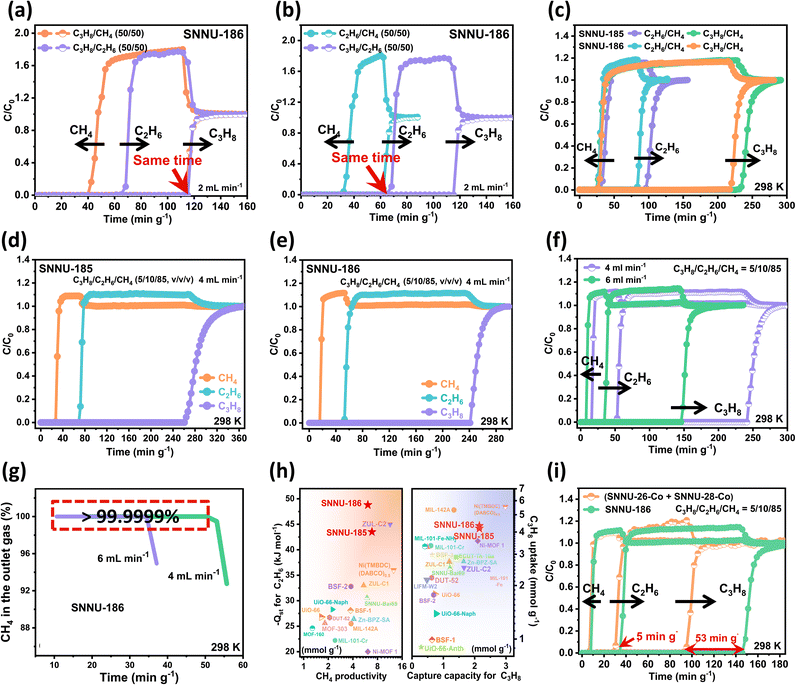
Considering that the relatively small window size of the nanotraps might influence the gas diffusion behaviour, kinetic mass transfer factors were investigated. The adsorption kinetics of C2H6 and C3H8 were evaluated using the time-dependent uptake profile. As shown in Fig. S16,† both C2H6 and C3H8 with similar slopes could achieve complete desorption within similar timeframes, indicating their similar diffusion behaviour, thus excluding their diffusion rate differences as a key factor in their sorption properties. Demonstrating the extent of exclusiveness of bi-nanotraps is crucial and the key is to prove that C2H6 and C3H8 do not affect each other during the separation process. Since the selectivity of “bi-nanotraps” results from both “thermodynamics” and the “molecular sieving” mechanism rather than thermodynamics alone, and the effectiveness of “bi-nanotraps” in weakening competitive adsorption can be demonstrated when C2H6 and C3H8 coexist, two-component breakthrough tests were performed to provide evidence for the “bi-nanotrap” effect (Fig. 3a, b and S17–19†). As shown in Fig. 3a, whether mixed with CH4 or C2H6, the breakthrough time of C3H8 was not affected (∼115 min g−1), implying that C2H6 does not affect the adsorption of C3H8. Moreover, whether mixed with CH4 or C3H8, the breakthrough time of C2H6 was not affected (∼62 min g−1, Fig. 3b), implying that C3H8 does not affect the adsorption of C2H6 as well. Therefore, once gases enter the “bi-nanotrap” structure, it is expected that C3H8 will be adsorbed in C3H8-selective nanotrap 1 and C2H6 will be adsorbed in C2H6-selective nanotrap 2. Clearly, “bi-nanotraps” play a crucial role in removing the competitive adsorption between C2H6 and C3H8, thus improving C3H8/C2H6/CH4 separation performance.
Inspired by the increased thermodynamic interaction difference and exclusive interaction sites, and encouraged by the satisfactory gas uptake and potential separation ability of activated SNNU-185/186, further experimental dynamic breakthrough experiments were performed to evaluate their C3H8/C2H6/CH4 separation performance. As shown in Fig. 3c and S20,† CH4 eluted out first due to its lowest adsorption capacity and weakest affinity with the frameworks, while C2H6 and C3H8 were trapped until their saturation sorption. For 20/80 C2H6/CH4 and 20/80 C3H8/CH4 mixtures with a total flow rate of 2 mL min−1 at 298 K, C2H6/C3H8 was retained for additional 66.0/204.0 min g−1 on SNNU-185, and 58.0/193.6 min g−1 on SNNU-186. Considering the practical composition of natural gas, experimental breakthrough tests with a feed gas of ternary C3H8/C2H6/CH4 (5/10/85, v/v/v) mixtures at flow rates of 4/6 mL min−1 were carried out at 298 K. As shown in Fig. 3d–g and S21,† SNNU-185 and SNNU-186 can produce ultra-high purity CH4 (>99.9999%) with exceptional productivities for CH4. The CH4 productivity of SNNU-185/186 was calculated to be 6.85/6.10 mmol g−1, surpassing those of most top-performing MOFs such as SNNU-Bai69 (ref. 3) (5.93 mmol g−1), ZUL-C1 (ref. 4) (5.42 mmol g−1), BSF-1/2/3 (ref. 1, 14 and 38) (3.75/3.79/4.60 mmol g−1), UiO-66-NaPh34 (2.25 mmol g−1), MIL-101-Cr30 (2.66 mmol g−1), and Zn-BPZ-SA5 (1.56 mmol g−1); it is comparable to that of MOF-303 (ref. 57) (7.97 mmol g−1), and is only lower than those of ZUL-C2 (ref. 4) (1 mL min−1, 11.4 mmol g−1) and Ni(TMBDC)(DABCO)0.5 (ref. 2) (4 mL min−1, 12.6 mmol g−1) (Table S6†). When the experimental breakthrough tests were performed at a high flow rate of 6 mL min−1, the CH4 purity still reached 99.9999%, which can be attributed to the multiple interactions between C2H6 and MOF frameworks, as well as the increased interaction difference between C2H6 and CH4.
Furthermore, considering the importance of C2H6 and C3H8 recovery, the breakthrough capture capacities of SNNU-185 and SNNU-186 for C2H6 and C3H8 were calculated accordingly. SNNU-185/186 possess outstanding C2H6 and C3H8 capture capacities of 1.23/0.90 mmol g−1 and 2.33/2.15 mmol g−1, respectively, which are superior to those of most MOF materials and are comparable to those of top-level MOF materials such as ZUL-C2 (ref. 4) (2.13/1.66 mmol g−1), ZUL-C1 (ref. 4) (0.98/1.19 mmol g−1) and Ni-MOF 1 (ref. 50) (0.78/2.10 mmol g−1) (Table S6†). As shown in Fig. 3h, when considering CH4 productivity, breakthrough capture capacities for C3H8, and C3H8 uptake, SNNU-185 and SNNU-186 exhibit the best performance for CH4 purification as well as for C2H6 and C3H8 recovery. Notably, the excellent separation performance of SNNU-185/186 is based on both “thermodynamics” and the “bi-nanotrap effect” in contrast to other MOFs that rely only on thermodynamics. As a result, although the thermodynamics-based IAST selectivities of SNNU-185/186 for C2H6/CH4 are moderate, thanks to the guest-specific interactions, competitive adsorption between C2H6 and C3H8 is weakened and the practical separation performance is improved (Table S6†). Furthermore, considering the presence of CO2 in raw natural gas and the challenges associated with its removal,27 breakthrough experiments were conducted to provide an assessment of the impact of CO2 contaminants. As shown in Fig. S22 and S23,† SNNU-186 could effectively separate C2H6/CO2/CH4 = 15/4/81 (v/v/v) and C3H8/CO2/CH4 = 4/4/92 (v/v/v) containing 4% CO2. Overall, SNNU-185 and SNNU-186 are highly competitive candidates for natural gas upgrade.
Given that there are two types of pores in SNNU-185/186, comparative experiments were conducted to demonstrate the advantage of using a single material with two types of pores (∼5 Å and ∼7 Å) over using a mixture of two MOFs with one type of pore each. First, since many factors such as metal centers, open metal sites (OMSs), functional groups can strongly influence the adsorption behaviour of MOFs, it is necessary to ensure the same metal center (Co center) and a similar chemical environment (decorated with aromatic rings, N sites, no OMSs). Bearing the above factors in mind, two reported MOFs, SNNU-26-Co58 (Co-BDC-TPP, with a pore size of ∼5 Å) and SNNU-28-Co58 (Co-2,6-NDC-TPP, with a pore size of ∼7 Å) were selected (Table S7†). As shown in Fig. 3i and S24,† under the same conditions, SNNU-186 exhibited better practical separation performance, confirming that using one MOF with two types of pores is more favourable for the C3H8/C2H6/CH4 separation process.
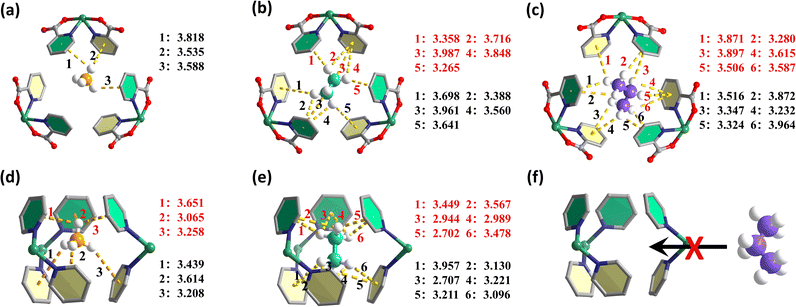
To give a mechanistic explanation of the role and effectiveness of the bi-nanotrap structure, and to gain a molecular-level insight into the host–guest interactions and adsorption behaviors of C3H8, C2H6 and CH4, Grand Canonical Monte Carlo (GCMC) simulations were performed (Fig. 4 and S25†). As shown in Fig. 4a–c, the large-type nanotrap with a pore size of 7.2 Å can trap CH4, C2H6 and C3H8via multiple C–H⋯π bonds with distances between 3.535 Å and 3.818 Å (3 bonds) for CH4, 3.265 Å and 3.987 Å (10 bonds) for C2H6, and 3.232 Å and 3.964 Å (12 bonds) for C3H8. Thanks to the higher number of H atoms in C3H8 and better size matching, these large nanotraps are more favorable for C3H8, forming more and stronger C–H⋯π bonds, and are thus considered thermodynamic C3H8-selective nanotraps. As for the small-type nanotraps with a pore size of 4.8 Å, they do not allow C3H8 molecules to enter due to the pore size limitation (Fig. 4d–f and S25†). However, C2H6 molecules can enter and bind to aromatic rings of TPP ligands on the surface of the nanotraps via a large number of strong and shape-matching C–H⋯π interactions with short distances (2.702–3.957 Å, 12 bonds), implying the exceptionally strong interactions between C2H6 and frameworks as well as preferential adsorption selectivity for C2H6 (Fig. 4e). As a result, C2H6-selective nanotraps are successfully constructed based on the dual integrated thermodynamic-molecular sieving mechanism. CH4 molecules interact with both kinds of nanotraps via fewer and weaker interactions (Fig. 4a and d). Clearly, the construction of thermodynamic C3H8-selective nanotraps and coupled thermodynamic-molecular sieving C2H6-selective nanotraps in the bi-nanotrap structure provides a reasonable explanation for the benchmark performance of SNNU-185 and SNNU-186 for C3H8/C2H6/CH4 separation. When C2H6 and C3H8 molecules coexist, they tend to preferentially occupy different and size-matching sites to form multiple and strong interactions, thus leading to a performance breakthrough.
Conclusions
In summary, a promising aromatic ring-confined bi-nanotrap strategy for excellent natural gas upgrading has been demonstrated here. The perfectly size/shape-matched C2H6-selective nanotraps and C3H8-selective nanotraps enable C2H6 and C3H8 to be preferentially trapped via abundant and extra-strong C–H⋯π bonds. Such a combination of thermodynamic-based nanotraps with molecular sieving-based size exclusion enables multiple, powerful and shape-matched interactions, and selective interaction sites, which is unprecedented. As a result, the goal of increasing the thermodynamic difference and reducing competitive adsorption was achieved. With excellent thermal/chemical stability and satisfactory gas sorption properties, the two MOFs reported here can produce high purity CH4 at high flow rates along with achieving first-class productivities for CH4, C2H6 and C3H8. This work not only creates highly ideal adsorbents with benchmark practical performance for natural gas upgrading, but also introduces a design concept of installing selective bi-nanotraps and fully exploiting the integrated thermodynamic-molecular sieving mechanism for the development of high-performance absorbents for more challenging multi-component gas systems.Data availability
All the associated data are available in the ESI.†Author contributions
Q.-G. Z. and S.-Y. L. conceived the idea of this research. S.-Y. L. carried out the experiments, analyzed the results and wrote the manuscript. Q.-G. Z. led the project and edited the manuscript. X. B. edited the manuscript. All authors participated in and contributed to the preparation of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22071140), the Natural Science Foundation of Shaanxi Province (2021JLM-20), the Youth Innovation Team of Shaanxi Universities (2023), and the Fundamental Research Funds for the Central Universities (GK202307009).Notes and references
- Y. Zhang, L. Yang, L. Wang, S. Duttwyler and H. Xing, Angew. Chem., Int. Ed., 2019, 58, 8145–8150 CrossRef CAS PubMed
.
- Y. Wu, Z. Liu, J. Peng, X. Wang, X. Zhou and Z. Li, ACS Appl. Mater. Interfaces, 2020, 12, 51499–51505 CrossRef CAS PubMed
.
- M. Ding, Q. Wang, H. Cheng and J. Bai, CrystEngComm, 2022, 24, 2388–2392 RSC
.
- J. Zhou, T. Ke, F. Steinke, N. Stock, Z. Zhang, Z. Bao, X. He, Q. Ren and Q. Yang, J. Am. Chem. Soc., 2022, 144, 14322–14329 CrossRef CAS PubMed
.
- G.-D. Wang, R. Krishna, Y.-Z. Li, Y.-Y. Ma, L. Hou, Y.-Y. Wang and Z. Zhu, ACS Mater. Lett., 2023, 5, 1091–1099 CrossRef CAS
.
- Y. He, W. Zhou, G. Qian and B. Chen, Chem. Soc. Rev., 2014, 43, 5657–5678 RSC
.
- J. Shen, A. Dailly and M. Beckner, Microporous Mesoporous Mater., 2016, 235, 170–177 CrossRef CAS
.
- J. Li, X. Luo, N. Zhao, L. Zhang, Q. Huo and Y. Liu, Inorg. Chem., 2017, 56, 4141–4147 CrossRef CAS PubMed
.
- Z. Zeng, W. Wang, X. Xiong, N. Zhu, Y. Xiong, Z. Wei and J.-J. Jiang, Inorg. Chem., 2021, 60, 8456–8460 CrossRef CAS PubMed
.
- Z. Ke, H. Xiao, Y. Wen, S. Du, X. Zhou, J. Xiao and Z. Li, Ind. Eng. Chem. Res., 2021, 60, 4668–4676 CrossRef CAS
.
- L. Wang, W. Zhang, J. Ding, L. Gong, R. Krishna, Y. Ran, L. Chen and F. Luo, Nano Res., 2023, 16, 3287–3293 CrossRef CAS
.
- J.-R. Li, R. J. Kupplera and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC
.
- K. Adil, Y. Belmabkhout, R. S. Pillai, A. Cadiau, P. M. Bhatt, A. H. Assen, G. Maurinb and M. Eddaoudi, Chem. Soc. Rev., 2017, 46, 3402–3430 RSC
.
- Y. Zhang, L. Yang, L. Wang, X. Cui and H. Xing, J. Mater. Chem. A, 2019, 7, 27560–27566 RSC
.
- B. Li, M. Chrzanowski, Y. Zhang and S. Ma, Coord. Chem. Rev., 2016, 307, 106–129 CrossRef CAS
.
- X. Zhao, Y. Wang, D.-S. Li, X. Bu and P. Feng, Adv. Mater., 2018, 30, 1705189 CrossRef PubMed
.
- E. D. Bloch, W. L. Queen, R. Krishna, J. M. Zadrozny, C. M. Brown and J. R. Long, Science, 2012, 335, 1606–1610 CrossRef CAS PubMed
.
- S. Yang, A. J. Ramirez-Cuesta, R. Newby, V. Garcia-Sakai, P. Manuel, S. K. Callear, S. I. Campbell, C. C. Tang and M. Schröder, Nat. Chem., 2015, 7, 121–129 CrossRef CAS PubMed
.
- Q. Liu, S. G. Cho, J. Hilliard, T.-Y. Wang, S.-C. Chien, L.-C. Lin, A. C. Co and C. R. Wade, Angew. Chem., Int. Ed., 2023, e202218854 CAS
.
- L. Wang, H. Huang, X. Zhang, H. Zhao, F. Li and Y. Gu, Coord. Chem. Rev., 2023, 484, 215111 CrossRef CAS
.
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed
.
- H.-C. Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415–5418 RSC
.
- B. Li, H.-M. Wen, W. Zhou, J. Q. Xu and B. Chen, Chem, 2016, 1, 557–580 CAS
.
- S. Rupam and C. D. Madhab, Coord. Chem. Rev., 2021, 442, 213998 CrossRef
.
- X. Han and S. Yang, Angew. Chem., Int. Ed., 2023, e202218274 CAS
.
- H. Wang, D. Luo, E. Velasco, L. Yu and J. Li, J. Mater. Chem. A, 2021, 9, 20874–20896 RSC
.
- P.-Q. Liao, W.-X. Zhang, J.-P. Zhang and X.-M. Chen, Nat. Commun., 2015, 6, 8697 CrossRef PubMed
.
- L. Li, R.-B. Lin, R. Krishna, H. Li, S. Xiang, H. Wu, J. Li, W. Zhou and B. Chen, Science, 2018, 362, 443–446 CrossRef CAS PubMed
.
- H. Zeng, X.-J. Xie, M. Xie, Y.-L. Huang, D. Luo, T. Wang, Y. Zhao, W. Lu and D. Li, J. Am. Chem. Soc., 2019, 141, 20390–20396 CrossRef CAS PubMed
.
- L.-Z. Qin, X.-H. Xiong, S.-H. Wang, L. Zhang, L.-L. Meng, L. Yan, Y.-N. Fan, T.-A. Yan, D.-H. Liu, Z.-W. Wei and C.-Y. Su, ACS Appl. Mater. Interfaces, 2022, 14, 45444–45450 CrossRef CAS PubMed
.
- R.-B. Lin, H. Wu, L. Li, X.-L. Tang, Z. Li, J. Gao, H. Cui, W. Zhou and B. Chen, J. Am. Chem. Soc., 2018, 140, 12940–12946 CrossRef CAS PubMed
.
- J. Pei, J.-X. Wang, K. Shao, Y. Yang, Y. Cui, H. Wu, W. Zhou, B. Li and G. Qian, J. Mater. Chem. A, 2020, 8, 3613–3620 RSC
.
- Y. Ye, Y. Xie, Y. Shi, L. Gong, J. Phipps, A. M. Al-Enizi, A. Nafady, B. Chen and S. Ma, Angew. Chem., Int. Ed., 2023, e202302564 CAS
.
- L. Zhang, X.-H. Xiong, L.-L. Meng, L.-Z. Qin, C.-X. Chen, Z.-W. Wei and C.-Y. Su, J. Mater. Chem. A, 2023, 11, 12902–12909 RSC
.
- J. Peng, J. Zhong, Z. Liu, H. Xi, J. Yan, F. Xu, X. Chen, X. Wang, D. Lv and Z. Li, ACS Appl. Mater. Interfaces, 2023, 15, 41466–41475 CrossRef CAS PubMed
.
- Q. Dong, Y. Huang, K. Hyeon-Deuk, I.-Y. Chang, J. Wan, C. Chen, J. Duan, W. Jin and S. Kitagawa, Adv. Funct. Mater., 2022, 32, 2203745 CrossRef CAS
.
- M. Wriedt, J. P. Sculley, A. A. Yakovenko, Y. Ma, G. J. Halder, P. B. Balbuena and H.-C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 9804–9808 CrossRef CAS PubMed
.
- L. Wang, W. Sun, S. Duttwyler and Y. Zhang, J. Solid State Chem., 2021, 299, 122167 CrossRef CAS
.
- L. Yang, X. Cui, Q. Yang, S. Qian, H. Wu, Z. Bao, Z. Zhang, Q. Ren, W. Zhou, B. Chen and H. Xing, Adv. Mater., 2018, 30, 1705374 CrossRef PubMed
.
- O. T. Qazvini, R. Babarao, Z.-L. Shi, Y.-B. Zhang and S. G. Telfer, J. Am. Chem. Soc., 2019, 141, 5014–5020 CrossRef CAS PubMed
.
- Z. Niu, X. Cui, T. Pham, P. C. Lan, H. Xing, K. A. Forrest, L. Wojtas, B. Space and S. Ma, Angew. Chem., Int. Ed., 2019, 58, 10138–10141 CrossRef CAS PubMed
.
- Y.-Y. Xue, S.-N. Li, Y.-C. Jiang, M.-C. Hu and Q.-G. Zhai, J. Mater. Chem. A, 2019, 7, 4640 RSC
.
- Z. Niu, X. Cui, T. Pham, G. Verma, P. C. Lan, C. Shan, H. Xing, K. A. Forrest, S. Suepaul, B. Space, A. Nafady, A. M. Al-Enizi and S. Ma, Angew. Chem., Int. Ed., 2021, 60, 5283–5288 CrossRef CAS PubMed
.
- Y. Ye, S. Xian, H. Cui, K. Tan, L. Gong, B. Liang, T. Pham, H. Pandey, R. Krishna, P. C. Lan, K. A. Forrest, B. Space, T. Thonhauser, J. Li and S. Ma, J. Am. Chem. Soc., 2022, 144, 1681–1689 CrossRef CAS PubMed
.
- H. Zhu, Y. Wang, X. Wang, Z.-W. Fan, H.-F. Wang, Z. Niu and J.-P. Lang, Chem. Commun., 2023, 59, 5757 RSC
.
- Y. Wang, N.-Y. Huang, X.-W. Zhang, H. He, R.-K. Huang, Z.-M. Ye, Y. Li, D.-D. Zhou, P.-Q. Liao, X.-M. Chen and J.-P. Zhang, Angew. Chem., Int. Ed., 2019, 58, 7692–7696 CrossRef CAS PubMed
.
- L. Yang, S. Qian, X. Wang, X. Cui, B. Chen and H. Xing, Chem. Soc. Rev., 2020, 49, 5359–5406 RSC
.
- F. Zheng, R. Chen, Z. Zhang, Q. Yang, Y. Yang, Q. Ren and Z. Bao, Cell Rep. Phys. Sci., 2022, 3, 100903 CrossRef CAS
.
- Y.-Y. Xue, S.-N. Li, Y.-C. Jiang, M.-C. Hu and Q.-G. Zhai, J. Mater. Chem. A, 2019, 7, 4640 RSC
.
- X.-X. Zhang, X.-Z. Guo, S.-S. Chen, H.-W. Kang, Y. Zhao, J.-X. Gao, G.-Z. Xiong and L. Hou, Chem. Eng. J., 2023, 466, 143170 CrossRef CAS
.
- A. Cadiau, K. Adil, P. M. Bhatt, Y. Belmabkhout and M. Eddaoudi, Science, 2016, 353, 137–140 CrossRef CAS PubMed
.
- Y. Cheng, B. Joarder, S. J. Datta, N. Alsadun, D. Poloneeva, D. Fan, R. Khairova, A. Bavykina, J. Jia, O. Shekhah, A. Shkurenko, G. Maurin, J. Gascon and M. Eddaoudi, Adv. Mater., 2023, 2300296 CrossRef CAS PubMed
.
- H. Wang, X. Dong, V. Colombo, Q. Wang, Y. Liu, W. Liu, X.-L. Wang, X.-Y. Huang, D. M. Proserpio, A. Sironi, Y. Han and J. Li, Adv. Mater., 2018, 30, 1805088 CrossRef PubMed
.
- B. Liang, X. Zhang, Y. Xie, R.-B. Lin, R. Krishna, H. Cui, Z. Li, Y. Shi, H. Wu, W. Zhou and B. Chen, J. Am. Chem. Soc., 2020, 142, 17795–17801 CrossRef CAS PubMed
.
- H. Zeng, M. Xie, T. Wang, R.-J. Wei, X.-J. Xie, Y. Zhao, W. Lu and D. Li, Nature, 2021, 595, 542–548 CrossRef CAS PubMed
.
- Q. Dong, Y. Huang, J. Wan, Z. Lu, Z. Wang, C. Gu, J. Duan and J. Bai, J. Am. Chem. Soc., 2023, 145, 8043–8051 CrossRef CAS PubMed
.
- S. Xian, J. Peng, H. Pandey, T. Thonhauser, H. Wang and J. Li, Engineering, 2023, 23, 56–63 CrossRef CAS
.
- Y.-Y. Xue, X.-Y. Bai, J. Zhang, Y. Wang, S.-N. Li, Y.-C. Jiang, M.-C. Hu and Q.-G. Zhai, Angew. Chem., Int. Ed., 2021, 60, 10122–10128 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2268660 and 2268661. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc04387a |
| This journal is © The Royal Society of Chemistry 2024 |