Open Access Article
Open Access ArticleDual ligand-enabled iron and halogen-containing carboxylate-based photocatalysis for chloro/fluoro-polyhaloalkylation of alkenes†
Wanru
Han‡
a,
Zhenyan
Zhao‡
a,
Kui
Jiang
a,
Yu
Lan
 *abc,
Xuehan
Yu
a,
Xiaoyu
Jiang
a,
Wei
Yang
a,
Donghui
Wei
*abc,
Xuehan
Yu
a,
Xiaoyu
Jiang
a,
Wei
Yang
a,
Donghui
Wei
 *a,
Shi-Jun
Li
*a,
Shi-Jun
Li
 *ab and
Linbin
Niu
*ab
*ab and
Linbin
Niu
*ab
aCollege of Chemistry, Pingyuan Laboratory, Zhengzhou University, 100 Science Avenue, Zhengzhou 450001, Henan, China. E-mail: lanyu@cqu.edu.cn; donghuiwei@zzu.edu.cn; lishijunzong@zzu.edu.cn; nlb@zzu.edu.cn
bState Key Laboratory of Antiviral Drugs, Pingyuan Laboratory, Henan Normal University, Xinxiang 453007, Henan, China
cSchool of Chemistry and Chemical Engineering, Chongqing Key Laboratory of Chemical Theory and Mechanism, Chongqing University, Chongqing 401331, China
First published on 4th November 2024
Abstract
Herein, we demonstrate a practical dual ligand-enabled iron photocatalysis paradigm—converting all kinds of halogen-containing carboxylates (CnXmCOO−, X: F, Cl, Br) into CnXm radicals for the valuable chloro/fluoro-polyhaloalkylation of non-activated alkenes with easily available trichloroacetonitrile/Selectfluor as the electrophilic halogenation reagent. The modular in situ assembly of the effective iron and CnXmCOO−-based light-harvesting species using the two ligands—OMe/CF3-substituted bipyridine and acetonitrile/trichloroacetonitrile is evidenced by detailed mechanistic studies. The late-stage modification, low loading amount of iron (TON: 257) and feasible gram-scale synthesis show the utility of this protocol. We thus anticipate that the dual ligand-enabled iron photocatalysis paradigm may facilitate activation and transformation of inert bulk chemicals.
Introduction
Using abundant bulk chemicals to synthesize fine chemicals through a sustainable catalytic strategy is significant in the field of synthetic chemistry.1 Regarding the efficient activation of inert bulk chemicals, general considerations or solutions are to increase the energy input such as resorting to high temperatures,2 noble metal catalysts,3 strong or special oxidants/reductants/acids/bases,4 UV light,5etc.6 Due to the urgent demand for green and sustainable chemistry,7 balancing the requirement of reducing the energy input/consumption and the growing interest of chemists in the activation of inert chemicals is what chemists should imminently address (Fig. 1a).The nontoxicity and abundance of iron endow iron-catalyzed synthetic chemistry with strong sustainability and potential application prospects in bio-medicine and industrial manufacturing fields.8 The low cost and easily available iron(III) salts have been playing versatile roles in the synthetic chemistry community owing to their remarkable Lewis acidity9 and redox reactivity. With respect to the redox character of iron(III), it has exhibited the ability to oxidise some active radical species10 or electron rich compounds such as anilines11 and phosphines.12 To achieve iron-mediated oxidative activation of inert compounds (alkane as an example), strong stoichiometric oxidants are usually required to yield high valent iron(IV or V)oxo species that can dominate the activation and transformation of inert alkanes for the generation of value-added alcohols/ketones, which is a representative mode for iron-catalyzed functionalization of inert compounds (Fig. 1b).13 By contrast, the oxidative ability of iron(III) is relatively moderate, hence direct iron(III)-dominated oxidative activation of inert compounds is still rare.
The input of light energy into iron(III) enhances the redox activity to a certain degree.14 Photo-induced iron ligand-to-metal charge transfer (LMCT) possesses excellent ability to produce radical species from abundant chemical feedstocks like alkyl carboxylates (tBuCOO− as a representative, Eox1/2 = +1.26 V versus SCE),15–18 in which the necessary prerequisite is the suitable coordination of the substrate to the iron center. It means that activating those inert compounds with exceedingly weak coordination capacity in this fashion is helpless and doubtful. Very recently, through cooperating iron photocatalysis and redox-active thiol catalysis, West and Xia elegantly reported direct decarboxylation of F-containing carboxylic acids for hydrofluoroalkylation of alkenes.19 The extremely high oxidative potential of TFA (CF3COO−, Eox1/2 > +2.4 V versus SCE) and the strong acid system make the corresponding activation mechanism of this special method complicated and confusing (Fig. 1c).
To develop a general strategy for the activation and transformation of inert and weakly coordinating compounds via metal-based photocatalysis, we hope to choose abundant and alkalescent halogen-containing carboxylates (CnXmCOO−, X: F, Cl, Br) as the investigational object to circumvent the strong acid conditions,20 which is conducive to bringing the effect of the external ligand into full play.21 Given the fact that the role of the external ligand in 3d metal-based LMCT chemistry is usually intricate but significant, herein, we adopted the dual ligand-enabled strategy to efficiently assemble iron and CnXmCOO−-based light-harvesting species for CnXm radical production under visible light conditions,22 thus successfully achieving chloro/fluoro-polyhaloalkylation of non-activated alkenes with easily available trichloroacetonitrile/Selectfluor as the electrophilic halogenation reagent (Fig. 1d). OMe/CF3-substituted bipyridine (bpy) and acetonitrile/trichloroacetonitrile indeed serve as the crucial dual ligands to achieve the desired LMCT between CnXmCOO− and the iron(III) center and furnish the decarboxylation of CnXmCOO− under visible light rather than UV light.
Results and discussion
To quickly obtain photochemical insights into iron(III) and CnXmCOO−-based light-harvesting species, stoichiometric photolysis experiments of Fe(CF3COO)3 were first carried out. As we expected, the high energy light (λmax = 365 nm) irradiation could induce the decomposition of Fe(CF3COO)3 to the CF3 radical, which was captured by a non-activated alkene 1, and the generated radical adduct was further quenched through the halogen atom transfer (XAT) with Cl3CCN, delivering the chloro-trifluoromethylation product of alkene (Fig. 2a, entry 1).23 This process failed under visible light (λmax = 450 nm) or darkness conditions (entries 2 and 3). To reduce the energy consumption in the activation of CF3COO−, the external ligands were investigated to achieve the release of the CF3 radical under visible light conditions. Bpy L1 was incapable of controlling the effective decarboxylation of CF3COO− to access the chloro-trifluoromethylation of alkene, whereas its derivatives functionalized with electron-donating/withdrawing functional groups (L2 and L3) both showed the potential of being a competent ligand (entries 4–6).Based on the preliminary results of the above stoichiometric experiments, a hypothetical catalytic cycle is illustrated in Fig. 2b. We proposed that a suitable electrophilic halogenation reagent (VIII or IX) should not only participate in the halogen-atom transfer (XAT) with the radical adduct (VII) that was generated from the CnXm radical (V) and alkene (VI) for the designed chloro/fluoro-polyhaloalkylation (XIV or XV), but also its concomitant electrophilic carbon/nitrogen radical species (X or XI) generated from the XAT would be responsible for the oxidation of [FeII/L] species (III) to regenerate the [FeIII/L] complex (I). Notably, the additional effect of the ligand also possibly affected the process of [FeII/L] to [FeIII/L], indicating that the choosing of the competent ligand is first and foremost for investigating conditions. After our detailed investigations (Fig. S14 and S15†), it was found that the chloro-trifluoromethylation and fluoro-chlorodifluoromethylation of alkenes via visible light-induced iron catalysis could be accomplished in good yields and regioselectivity under the regulation of L2 and L4, respectively (Fig. 2c). Regarding the choosing of the halogenation reagent, it was necessary to mention the result that commonly used chlorine sources such as trichloromethane, ethyl chloroacetate analogue, NCS (N-chlorosuccinimide), etc. could not yield the desired chloro-trifluoromethylation product (Fig. S17†), and bromo-trifluoromethylation of alkene by this method was infeasible (Fig. S18†).
With the optimized conditions in hand, the scope of this protocol was explored in terms of various non-activated alkenes' chloro/fluoro-polyhaloalkylation (Fig. 3). We first examined the reactivity of benzoate-containing terminal olefins. The different alkyl carbon chain structures of olefins did not disturb the reactivity and both provided good yields (4 and 5). Electron-donating or electron-withdrawing substituents in benzoate groups were compatible (6–8), and the substituents installed at ortho and meta positions also provided satisfactory yields (9–11). The alkene assembled with valuable benzotriazole successfully introduced the Cl and CF3 groups in 74% yield under our standard conditions (12). Moreover, sulfonate/phenoxy-modified alkenes, simple hexadecane and α-methyl olefin were all well tolerated (13–17). Besides the above-mentioned terminal olefins, the successful chloro-trifluoromethylation of the internal olefin enriched the diversity of the product (18). Significantly, the synthetic utility of this method in achieving fluoro-polyhaloalkylation24 of non-activated alkenes was elaborated for the divergent synthesis of complex fluorine-containing molecules (19–25). Unfortunately, conjugated alkenes were not suitable for this reaction (Fig. S12†).
 | ||
| Fig. 3 Chloro/fluoro-polyhaloalkylation of alkenes via iron photocatalysis. a 24 h reaction time. b 0.5 mmol Selectfluor was used. Isolated yields. | ||
Furthermore, a wide variety of alkenes derived from pharmaceutical molecules were examined for the late-stage functionalization to extend this practical synthetic platform. The medicine oxaprozin used for rheumatoid arthritis and postoperative analgesia could be modified with halogen-containing alkyl in moderate yield (26). It is noteworthy that the performance of the derivative of piperonylic acid was amazing with 83% yield, whose sensitive ternary ring structure was well preserved (27). Likewise, ciprofibrate as an oral medicine for treating endogenous hypercholesterolemia and hypertriglyceridemia in adults, its derivative could also be achieved chloro-trifluoromethylation smoothly (28). The modification based on amino acid N-Boc-D-proline (29) increased the opportunities to discover late-model biomolecules. Of special note, eugenol possesses antioxidant, anticancer, anti-inflammatory, and antimicrobial activities, and its analogue gave moderate yield (30), despite the existence of the sensitive phenolic hydroxyl group. Besides, the late-stage chloro-trifluoromethylation, chloro-chlorodifluoromethylation, chloro-bromodifluoromethylation and chloro-pentafluoroethylation of loxoprofen/febuxostat/flurbiprofen derivatives demonstrated intriguing synthesis potential for new drug research and development (31–40). Importantly, the utility and sustainability of this method were further highlighted by the gram-scale synthesis and low loading amount of the iron catalyst (TON = 257).
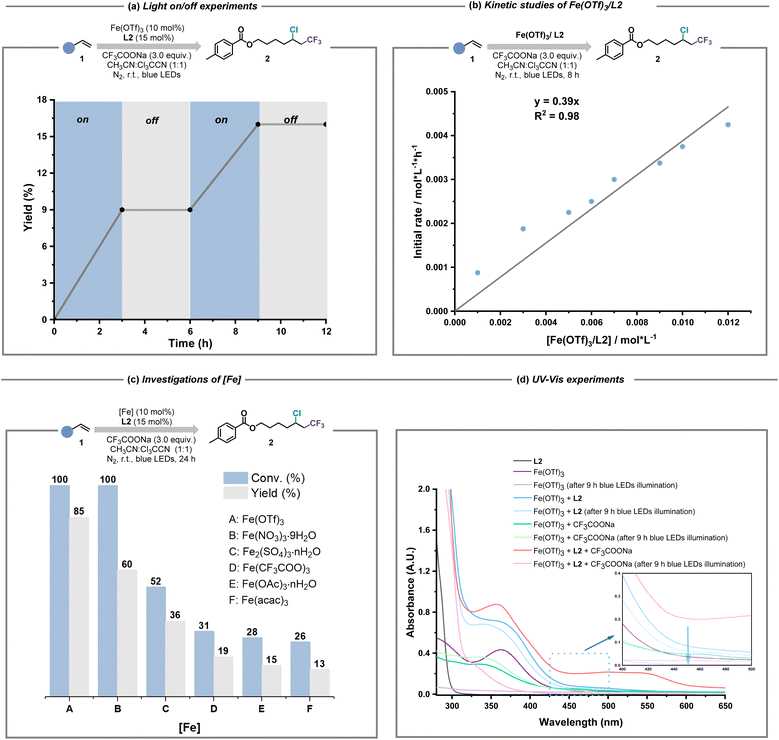
Based on the encouraging results of this ligand-enabled iron photocatalysis under visible light conditions, we turned our attention to the mechanistic studies. When stoichiometric radical scavenger (TEMPO/BHT) was subjected to the standard conditions (Fig. S30†), the desired chloro-trifluoromethylation process was severely inhibited, in which the radical adduct of the CF3 radical and BHT was detected by electrospray ionization high-resolution mass spectrometry (ESI-HRMS, Fig. S31†). The existence of CF3 radical species was also evidenced by the designed radical probe experiment (Fig. S32†). Theoretically, the production of the CnXm radical required the excitation of iron and CnXmCOO−-based light-harvesting species, meaning that continuous blue light irradiation should be necessary. The light on/off experiments verified this viewpoint (Fig. 4a). According to the fact that the combination of Fe(OTf)3 and L2 showed first-order dependence during the kinetic studies (Fig. 4b), we realized that how to assemble iron salt/L2, CnXmCOO− and other potential ligands in this system into iron-based light-harvesting species for CnXm radical release is the most crucial factor for the whole catalytic cycle. Because of the weaker coordination ability of OTf− and NO3− than that of CnXmCOO−, easily dissociated iron salts like Fe(OTf)3 and Fe(NO3)3·9H2O whose coordination anions prefer to be exchanged by excess CnXmCOO−, showed good catalytic performance. In contrast, the efficiency of Fe(acac)3 and Fe(OAc)3·nH2O was unsatisfactory, probably resulting from the stagnant ligand exchange between CnXmCOO− and acac−/OAc− (Fig. 4c). Moreover, the fact that only when Fe(OTf)3, L2 and CnXmCOO− are all present under the irradiation of blue light, can the iron(II) intermediate be observed, was revealed by UV-Vis experiments (Fig. 4d and S29†).
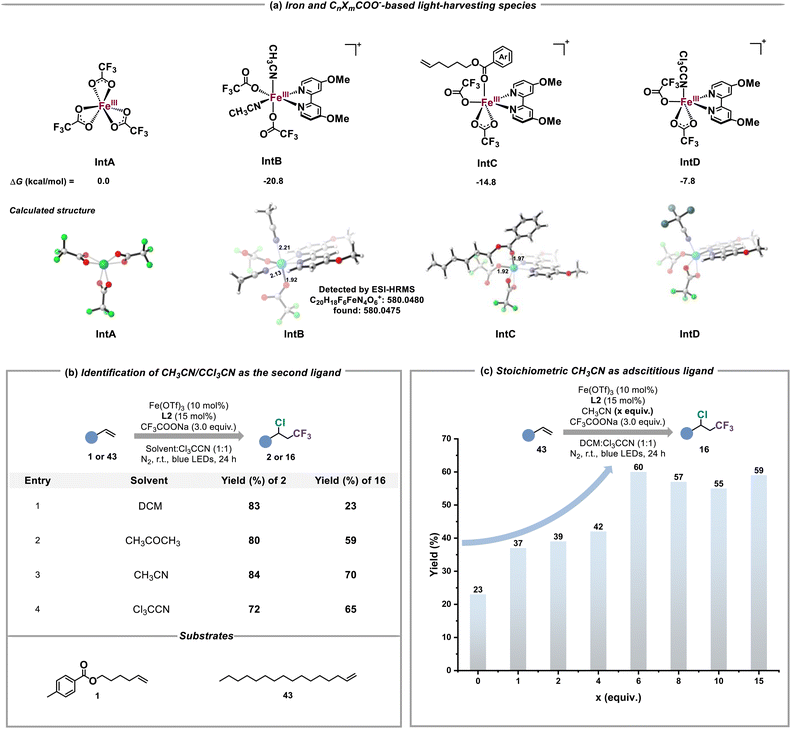
To quickly determine the possible structure of iron(III) and CnXmCOO−-based light-harvesting species, DFT calculations were carried out (Tables S1 and S2†). As shown in Fig. 5a, the suitable coordination of L2 and CH3CN to Fe(CF3COO)3 (IntA) to form IntB is thermodynamically favorable (ΔG = −20.8 kcal mol−1), which also shows an obvious absorption band at 454 nm and an effective LMCT between CF3COO− and the iron(III) center in its excited state (Fig. S21–S24†). This result revealed that CH3CN served as the second ligand to construct IntB. The ESI-HRMS experiment also identified the real existence of IntB under the standard conditions (Fig. S34†). Given that some alkenes were equipped with the ester scaffold whose coordination capability in comparison to CnXmCOO− and nitriles should not be ignored, IntC as a possible LMCT-light-harvesting species could not be excluded. In contrast, the electron withdrawing inductive effect of the Cl group weakens the structural stability of IntD, potentially causing the low efficiency of producing CnXm radicals. Furthermore, we compared the performance of alkene 1 and 43 in different solvents such as CH3CN, dichloromethane (DCM), acetone and CCl3CN to prove the necessity of the second ligand for the activation of inert CnXmCOO− (Fig. 5b). Due to the fact that there is no coordination effect between the iron(III) catalyst and DCM/43, the chloro-trifluoromethylation of 43 under the condition of DCM as solvent was inefficient (entry 1). By contrast, replacing DCM with acetone obviously enhanced the yield of 16 (entry 2). The similar reactivities of 43 in CH3CN and CCl3CN solvent explained the possibility that IntD truly served as alternative iron and CnXmCOO−-based light-harvesting species in the absence of acetonitrile (entries 3 and 4). We also studied the relationship between the final yields of 16 and the amounts of adscititious CH3CN (Fig. 5c). With the increase of the equivalent of CH3CN, the desired reactivity for chloro-trifluoromethylation gradually improved, confirming that CH3CN is the more efficient second ligand than CCl3CN (Fig. S33†).
Conclusion
In conclusion, we have developed a general and practical method in which dual ligand-enabled iron photocatalysis was leveraged for the activation of halogen-containing carboxylates under blue light conditions, thus achieving the chloro/fluoro-polyhaloalkylation of non-activated alkenes with broad functional group compatibility. Mechanistic studies revealed that the most likely real iron and CnXmCOO−-based light-harvesting species is assembled by the external dual ligand—OMe/CF3-substituted bipyridine and acetonitrile/trichloroacetonitrile. Extending this strategy to the activation of more inert bulk chemicals is underway in our laboratory.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Conceptualization: W. H., Z. Z., K. J., Y. L., D. W., S. L., and L. N.; methodology: W. H., Z. Z., K. J., Y. L., X. Y., D. W., S. L., and L. N.; investigation: W. H., Z. Z., K. J., Y. L., X. Y., D. W., S. L., and L. N.; supervision: Y. L., D. W., S. L., and L. N.; writing—original draft: W. H., Z. Z., K. J., Y. L., D. W., S. L., and L. N.; writing—review & editing: W. H., Z. Z., K. J., Y. L., X. Y., X. J., W. Y., Y. L., D. W., S. L., and L. N.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This project was supported by the National Natural Science Foundation of China (Grant No. 22101265, 21903071 and 21822303); Promotion Projects for Key Research & Development in Henan Province, China (Grant No. 222102310042); China Postdoctoral Science Foundation (Grant No. 2022M712866 and 2023M733212); Joint Fund of Key Technologies Research & Development Program of Henan Province (Grant No. 222301420006); The Ministry of Science and Technology of the People’s Republic of China. The authors are also grateful for the support from Center of Advanced Analysis & Gene Sequencing of Zhengzhou University.Notes and references
- (a) C. K. Prier, D. A. Rankic and D. W. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (b) P. Bellotti, H.-M. Huang, T. Faber and F. Glorius, Chem. Rev., 2023, 123, 4237–4352 CrossRef CAS PubMed; (c) J. F. Hartwig and M. A. Larsen, ACS Cent. Sci., 2016, 2, 281–292 CrossRef CAS PubMed; (d) H. Jiang and A. Studer, Chem. Soc. Rev., 2020, 49, 1790–1811 RSC; (e) J. M. Narayanam and C. R. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC; (f) D. Ravelli, S. Protti and M. Fagnoni, Chem. Rev., 2016, 116, 9850–9913 CrossRef CAS PubMed; (g) N. Holmberg-Douglas and D. A. Nicewicz, Chem. Rev., 2022, 122, 1925–2016 CrossRef CAS PubMed.
- (a) M. Chen and S. L. Buchwald, Angew. Chem., Int. Ed., 2013, 52, 11628–11631 CrossRef CAS PubMed; (b) K. Matsui, E. Tobita, M. Ando and K. Kondo, Chem. Lett., 1981, 10, 1719–1720 CrossRef.
- (a) E. J. Cho, T. D. Senecal, T. Kinzel, Y. Zhang, D. A. Watson and S. L. Buchwald, Science, 2010, 328, 1679–1681 CrossRef CAS PubMed; (b) N. Rodríguez and L. J. Goossen, Chem. Soc. Rev., 2011, 40, 5030–5048 RSC; (c) J. D. Weaver, A. Recio, A. J. Grenning and J. A. Tunge, Chem. Rev., 2011, 111, 1846–1913 CrossRef CAS PubMed; (d) Y. Wei, P. Hu, M. Zhang and W. Su, Chem. Rev., 2017, 117, 8864–8907 CrossRef CAS PubMed; (e) X. Li, T. Yang, J. Li, X. Li, P. Chen, Z. Lin and G. Liu, Nat. Chem., 2023, 15, 862–871 CrossRef CAS PubMed.
- (a) Y. Fujiwara, J. A. Dixon, F. O'Hara, E. D. Funder, D. D. Dixon, R. A. Rodriguez, R. D. Baxter, B. Herlé, N. Sach, M. R. Collins, Y. Ishihara and P. S. Baran, Nature, 2012, 492, 95–99 CrossRef CAS PubMed; (b) D. Yin, D. Su and J. Jin, Cell Rep. Phys. Sci., 2020, 1, 100141 CrossRef CAS; (c) M. Tang, F. Draper, L. N. Pham, C. C. Ho, H. Huang, J. Sun, S. C. Thickett, M. L. Coote, T. U. Connell and A. C. Bissember, J. Org. Chem., 2024, 89, 2683–2690 CrossRef CAS PubMed.
- (a) C. Lai and T. E. Mallouk, J. Chem. Soc., Chem. Commun., 1993, 1359–1361 RSC; (b) W. Liu, X. Yang, Z.-Z. Zhou and C.-J. Li, Chem, 2017, 2, 688–702 CrossRef CAS; (c) T.-T. Yuan, J. Chen, L. N. Pham, S. Paul, L. V. White, J. Li, P. Lan, M. L. Coote, M. G. Banwell and Y.-T. He, Org. Chem. Front., 2023, 10, 4649–4657 RSC.
- (a) C. Depecker, H. Marzouk, S. Trevin and J. Devynck, New J. Chem., 1999, 23, 739–742 RSC; (b) H. Morimoto, T. Tsubogo, N. D. Litvinas and J. F. Hartwig, Angew. Chem., Int. Ed., 2011, 50, 3793–3798 CrossRef CAS PubMed.
- (a) C.-J. Li, Green Chem., 2016, 18, 1836–1838 RSC; (b) P.-Z. Wang, W.-J. Xiao and J.-R. Chen, Nat. Rev. Chem., 2023, 7, 35–50 CrossRef PubMed; (c) J. Xuan, Z.-G. Zhang and W.-J. Xiao, Angew. Chem., Int. Ed., 2015, 54, 15632–15641 CrossRef CAS PubMed; (d) S. Das, C. Zhu, D. Demirbas, E. Bill, C. De and B. List, Science, 2023, 379, 494–499 CrossRef CAS PubMed.
- (a) A. Guérinot and J. Cossy, Top. Curr. Chem., 2016, 374, 49 CrossRef PubMed; (b) A. Fürstner, ACS Cent. Sci., 2016, 2, 778–789 CrossRef PubMed.
- (a) Z. Huang, L. Jin, Y. Feng, P. Peng, H. Yi and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 7151–7155 CrossRef CAS PubMed; (b) M.-Q. Tian, Z.-Y. Shen, X. Zhao, P. J. Walsh and X.-H. Hu, Angew. Chem., Int. Ed., 2021, 60, 9706–9711 CrossRef CAS PubMed.
- (a) W. Jian, L. Ge, Y. Jiao, B. Qian and H. Bao, Angew. Chem., Int. Ed., 2017, 56, 3650–3654 CrossRef CAS PubMed; (b) F. Du, S.-J. Li, K. Jiang, R. Zeng, X.-C. Pan, Y. Lan, Y.-C. Chen and Y. Wei, Angew. Chem., Int. Ed., 2020, 59, 23755–23762 CrossRef CAS PubMed.
- (a) T. Taniguchi, Y. Sugiura, H. Zaimoku and H. Ishibashi, Angew. Chem., Int. Ed., 2010, 49, 10154–10157 CrossRef CAS PubMed; (b) J. Y. Hwang, A. Y. Ji, S. H. Lee and E. J. Kang, Org. Lett., 2020, 22, 16–21 CrossRef CAS PubMed.
- (a) B. Liu, F. Jin, T. Wang, X. Yuan and W. Han, Angew. Chem., Int. Ed., 2017, 56, 12712–12717 CrossRef CAS PubMed; (b) T. Tanaka, R. Yazaki and T. Ohshima, J. Am. Chem. Soc., 2020, 142, 4517–4524 CrossRef CAS PubMed.
- (a) T. J. Collins and A. D. Ryabov, Chem. Rev., 2017, 117, 9140–9162 CrossRef CAS PubMed; (b) X. Huang and J. T. Groves, Chem. Rev., 2018, 118, 2491–2553 CrossRef CAS PubMed; (c) P. R. Montellano, Chem. Rev., 2010, 110, 932–948 CrossRef PubMed; (d) P. Xu, W. Fan, P. Chen and G. Liu, J. Am. Chem. Soc., 2022, 144, 13468–13474 CrossRef CAS PubMed.
- L. H. Groot, A. Ilic, J. Schwarz and K. Wärnmark, J. Am. Chem. Soc., 2023, 145, 9369–9388 CrossRef PubMed.
- (a) Z. Li, X. Wang, S. Xia and J. Jin, Org. Lett., 2019, 21, 4259–4265 CrossRef CAS PubMed; (b) G. Feng, X. Wang and J. Jin, Eur. J. Org. Chem., 2019, 2019, 6728–6732 CrossRef CAS; (c) S.-C. Kao, K.-J. Bian, X.-W. Chen, Y. Chen, A. A. Martí and J. G. West, Chem Catal., 2023, 3, 100603 CrossRef CAS PubMed; (d) L. M. Denkler, M. A. Shekar, T. S. Ngan, L. Wylie, D. Abdullin, T. Hett, F. H. Pilz, B. Kirchner, O. Schiemann, P. Kielb, A. Bunescu, M. Engeser and G. Schnakenburg, Angew. Chem., Int. Ed., 2024, e202403292 CAS.
- (a) F. Juliá, ChemCatChem, 2022, 14, e202200916 CrossRef; (b) Q. Zhang, S. Liu, J. Lei, Y. Zhang, C. Meng, C. Duan and Y. Jin, Org. Lett., 2022, 24, 1901–1906 CrossRef CAS PubMed; (c) B. Niu, K. Sachidanandan, M. V. Cooke, T. E. Casey and S. Laulhé, Org. Lett., 2022, 24, 4524–4529 CrossRef CAS PubMed; (d) S. Oh and E. E. Stache, J. Am. Chem. Soc., 2022, 144, 5745–5749 CrossRef CAS PubMed; (e) D. Gygi, M. I. Gonzalez, S. J. Hwang, K. T. Xia, Y. Qin, E. J. Johnson, F. Gygi, Y.-S. Chen and D. G. Nocera, J. Am. Chem. Soc., 2021, 143, 6060–6064 CrossRef CAS PubMed; (f) M. I. Gonzalez, D. Gygi, Y. Qin, Q. Zhu, E. J. Johnson, Y. S. Chen and D. G. Nocera, J. Am. Chem. Soc., 2022, 144, 1464–1472 CrossRef CAS PubMed; (g) Y. Jin, Q. Zhang, L. Wang, X. Wang, C. Meng and C. Duan, Green Chem., 2021, 23, 6984–6989 RSC; (h) Z.-Y. Dai, S.-Q. Zhang, X. Hong, P.-S. Wang and L.-Z. Gong, Chem Catal., 2022, 2, 1211–1222 CrossRef CAS; (i) Y. Jin, L. Wang, Q. Zhang, Y. Zhang, Q. Liao and C. Duan, Green Chem., 2021, 23, 9406–9411 RSC; (j) G. Zhang, Z. Zhang and R. Zeng, Chin. J. Chem., 2021, 39, 3225–3230 CrossRef CAS; (k) Y. C. Kang, S. M. Treacy and T. Rovis, ACS Catal., 2021, 11, 7442–7449 CrossRef CAS PubMed.
- (a) W. Liu, Q. Wu, M. Wang, Y. Huang and P. Hu, Org. Lett., 2021, 23, 8413–8418 CrossRef CAS PubMed; (b) N. Xiong, Y. Li and R. Zeng, Org. Lett., 2021, 23, 8968–8972 CrossRef CAS PubMed; (c) T. Xue, Z. Zhang and R. Zeng, Org. Lett., 2022, 24, 977–982 CrossRef CAS PubMed.
- (a) M. Zhang, J. Zhang, Q. Li and Y. Shi, Nat. Commun., 2022, 13, 7880 CrossRef CAS PubMed; (b) K.-J. Bian, S.-C. Kao, D. Nemoto Jr, X.-W. Chen and J. G. West, Nat. Commun., 2022, 13, 7881 CrossRef CAS PubMed; (c) H. Lindner, W. M. Amberg and E. M. Carreira, J. Am. Chem. Soc., 2023, 145, 22347–22353 CrossRef CAS PubMed; (d) W. Zhang, T. Liu, H. T. Ang, P. Luo, Z. Lei, X. Luo, M. J. Koh and J. Wu, Angew. Chem., Int. Ed., 2023, 62, e202310978 CrossRef CAS PubMed.
- (a) K.-J. Bian, Y.-C. Lu, D. Nemoto Jr, S.-C. Kao, X. Chen and J. G. West, Nat. Chem., 2023, 15, 1683–1692 CrossRef CAS PubMed; (b) J. Qi, J. Xu, H. T. Ang, B. Wang, N. K. Gupta, S. R. Dubbaka, P. O'Neill, X. Mao, Y. Lum and J. Wu, J. Am. Chem. Soc., 2023, 145, 24965–24971 CAS; (c) X.-K. Qi, L.-J. Yao, M.-J. Zheng, L. Zhao, C. Yang, L. Guo and W. Xia, ACS Catal., 2024, 14, 1300–1310 CrossRef CAS.
- X.-Y. Jiang, Y. Lan, Y. Hao, K. Jiang, J. He, J. Zhu, S. Jia, J. Song, S.-J. Li and L. Niu, Nat. Commun., 2024, 15, 6115 CrossRef CAS PubMed.
- During the preparation of this manuscript, Juliá-Hernández et al. published the photo-induced iron catalyzed decarboxylation of trifluoroacetates: S. Fernández-García, V. O. Chantzakou and F. Juliá-Hernández, Angew. Chem., Int. Ed., 2023, 63, e202311984 CrossRef PubMed.
- A. J. Fernandes, R. Giri, K. N. Houk and D. Katayev, Angew. Chem., Int. Ed., 2024, e202318377 CAS.
- (a) S. Kamijo, S. Yokosaka and M. Inoue, Tetrahedron, 2012, 68, 5290–5296 CrossRef CAS; (b) X.-J. Tang and W. R. Dolbier Jr, Angew. Chem., Int. Ed., 2015, 54, 4246–4249 CrossRef CAS PubMed; (c) D. B. Bagal, G. Kachkovskyi, M. Knorn, T. Rawner, B. M. Bhanage and O. Reiser, Angew. Chem., Int. Ed., 2015, 54, 6999–7002 CrossRef CAS PubMed; (d) J. Fang, Z.-K. Wang, S.-W. Wu, W.-G. Shen, G.-Z. Ao and F. Liu, Chem. Commun., 2017, 53, 7638–7641 RSC; (e) K.-Y. Ye, G. Pombar, N. Fu, G. Sauer, I. Keresztes and S. Lin, J. Am. Chem. Soc., 2018, 140, 2438–2441 CrossRef CAS PubMed; (f) M. Alkan-Zambada and X. Hu, Organometallics, 2018, 37, 3928–3935 CrossRef CAS; (g) X. Sun, H.-X. Ma, T.-S. Mei, P. Fang and Y. Hu, Org. Lett., 2019, 21, 3167–3171 CrossRef CAS PubMed; (h) H. Sun, G. Cui, H. Shang and B. Cui, J. Org. Chem., 2020, 85, 15241–15255 CrossRef CAS PubMed; (i) P. Lian, W. Long, J. Li, Y. Zheng and X. Wan, Angew. Chem., Int. Ed., 2020, 59, 23603–23608 CrossRef CAS PubMed; (j) V. S. Kostromitin, A. A. Zemtsov, V. V. Levin and A. D. Dilman, Adv. Synth. Catal., 2021, 363, 5336–5340 CrossRef CAS; (k) P. Basnet, G. Hong, C. E. Hendrick and M. C. Kozlowski, Org. Lett., 2021, 23, 433–437 CrossRef CAS PubMed; (l) X.-H. Li, G.-C. Yang, J.-F. Gong and M.-P. Song, Org. Biomol. Chem., 2022, 20, 4815–4825 RSC; (m) D. Wu, W. Fan, L. Wu, P. Chen and G. Liu, ACS Catal., 2022, 12, 5284–5291 CrossRef CAS; (n) C. Wu, X. Hui, D. Zhang, M. Zhang, Y. Zhu and S. Wang, Green Chem., 2022, 24, 1103–1108 RSC; (o) M. Zhang, J. Zhang and M. Oestreich, Nat. Synth., 2023, 2, 439–447 CrossRef; (p) X.-J. Ren, P.-W. Liao, H. Sheng, Z.-X. Wang and X.-Y. Chen, Org. Lett., 2023, 25, 6189–6194 CrossRef CAS PubMed; (q) Y. Xie, Z. Zhang, B. Zhang, N. He, M. Peng, S. Song, B. Wang and F. Yu, J. Org. Chem., 2024, 89, 8521–8530 CrossRef CAS PubMed; (r) J. Elfert, N.-L. Frye, I. Rempel, C. G. Daniliuc and A. Studer, Nat. Commun., 2024, 15, 7230 CrossRef CAS PubMed; (s) K.-J. Bian, D. Nemoto Jr, X.-W. Chen, S.-C. Kao, J. Hoosona and J. G. West, Chem. Sci., 2024, 15, 124–133 RSC.
- (a) S. Kindt and M. R. Heninrich, Chem.–Eur. J., 2014, 20, 15344–15348 CrossRef CAS PubMed; (b) W. Yu, X.-H. Xu and F.-L. Qing, Adv. Synth. Catal., 2015, 357, 2039–2044 CrossRef CAS; (c) Z. Liu, H. Chen, Y. Lv, X. Tan, H. Shen, H.-Z. Yu and C. Li, J. Am. Chem. Soc., 2018, 140, 6169–6175 CrossRef CAS PubMed; (d) A. S. Porzer, E.-M. Alvarez, H. Friedrich and M. R. Heinrich, Chem.–Eur. J., 2019, 25, 2786–2792 CrossRef PubMed; (e) Q. Wang, Y. Qu, H. Tian, Y. Liu, H. Song and Q. Wang, Chem.–Eur. J., 2019, 25, 8686–8690 CrossRef CAS PubMed; (f) J.-L. Li, Y.-Q. Liu, W.-L. Zou, R. Zeng, X. Zhang, Y. Liu, B. Han, Y. He, H.-J. Leng and Q.-Z. Li, Angew. Chem., Int. Ed., 2020, 59, 1863–1870 CrossRef CAS PubMed; (g) S. Ma, F. Li, G. Zhang, L. Shi and X. Wang, ACS Catal., 2021, 11, 14848–14853 CrossRef CAS; (h) A. Granados, R. K. Dhungana, M. Sharique, J. Majhi and G. A. Molander, Org. Lett., 2022, 24, 4750–4755 CrossRef CAS PubMed; (i) V. S. Kostromitin, A. O. Sorokin, V. V. Levin and A. D. Dilman, Org. Lett., 2023, 25, 6598–6602 CrossRef CAS PubMed; (j) Y. Li, Y. Dong, X. Wang, G. Li, H. Xue, W. Xin, Q. Zhang, W. Guan and J. Fu, ACS Catal., 2023, 13, 2410–2421 CrossRef CAS; (k) V. S. Kostromitin, V. V. Levin and A. D. Dilman, J. Org. Chem., 2023, 88, 6252–6262 CrossRef CAS PubMed; (l) R.-X. Liu, W. Wang and L. Yang, Adv. Synth. Catal., 2024, 366, 1643–1648 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc04038d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |