Open Access Article
Open Access ArticleA thiol-triggered croconaine–chromene integration to induce ferroptosis and photothermal synergistic efficient tumor ablation†
Xinya
Niu
a,
He
Yang
a,
Xingkang
Wu
c,
Fangjun
Huo
 b,
Kaiqing
Ma
b,
Kaiqing
Ma
 *ad and
Caixia
Yin
*ad and
Caixia
Yin
 *a
*a
aKey Laboratory of Chemical Biology and Molecular Engineering of Ministry of Education, Institute of Molecular Science, Shanxi University, Taiyuan 030006, PR China. E-mail: yincx@sxu.edu.cn
bResearch Institute of Applied Chemistry, Shanxi University, Taiyuan 030006, PR China
cModern Research Center for Traditional Chinese Medicine, Shanxi University, Taiyuan 030006, China
dZhendong Research Institute, Shanxi-Zhendong Pharmaceutical Co., Ltd, Changzhi 047100, China
First published on 16th August 2024
Abstract
Theranostic probes, combining diagnostic and treatment capabilities, have emerged as promising tools in tumor precision medicine. However, existing probes with constant fluorescence and photothermal activity can result in low signal-to-background ratios and phototoxicity. In this study, we introduced CM-Croc, a novel probe comprised of chromene and croconaine, selectively triggered by thiol. CM-Croc exhibited turn-on fluorescence and released croconaine for photothermal therapy. The croconaine moiety possesses high photothermal conversion efficiency up to 55%. Besides, it demonstrated potent activity against various cancer cell lines at low micromolar concentrations, including drug-resistant variants, through enhanced photothermal therapy combined with the ferroptosis effect. What's more, CM-Croc was proved to inhibit the activity of GPX4 to induce ferroptosis. Finally, CM-Croc was demonstrated to be the first croconaine-derived SOP, which targeted tumors and significantly inhibited tumor growth in vivo following intravenous administration with irradiation. This study showed CM-Croc's potential for enhancing tumor precision medicine.
Introduction
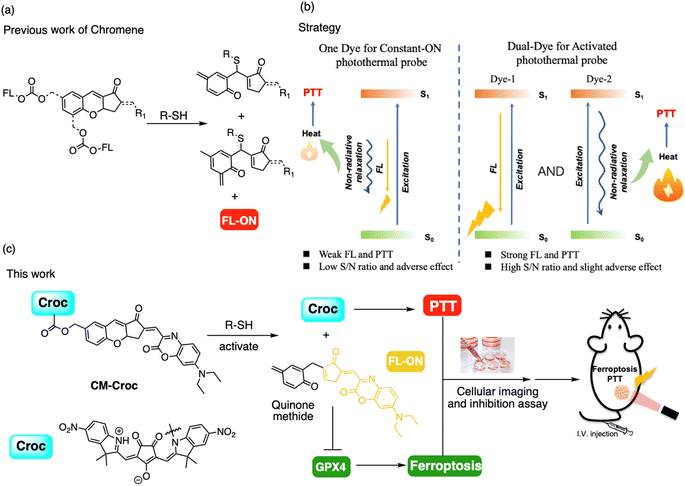
Photothermal therapy (PTT), a method that harnesses the absorption and conversion of light to induce hyperthermia for cancer cell destruction, has gained prominence as a noninvasive strategy in cancer treatment,1,2 especially for drug-resistant tumors.3 The integration of tumor- and organelle-targeted small organic photothermal agents (SOPs) has demonstrated effective tumor inhibition during PTT.4–6 Remarkably, Tang and colleagues have developed novel dual-targeted SOPs as PTT agents by targeting tumor tissue and mitochondria for fluorescence imaging (FI) of the tumor and selectively killing cancer cells.7 However, the “always-on” approach in PTT probes has raised concerns, leading to adverse effects such as thermal damage to normal tissues, inflammation, and patient discomfort (Fig. 1).8,9 Moreover, the energy is limited for the fluorescence and photothermal effect when only one dye absorbs a photon. Although activable (off–on) photosensitizers have been developed for photodynamic therapy (PDT),10–12 activable PTT probes, especially those utilizing SOPs, have received limited attention.13,14 Additionally, the activation for photoacoustic imaging (PAI) has been reported,15 which could be combined with the photothermal effect for diagnosis and therapy.16 Despite this promising synergy, PAI generally exhibits lower sensitivity and specificity compared to fluorescence imaging (FI). Furthermore, the complexity of data interpretation in PAI poses additional challenges. Simultaneously, significant efforts have been directed toward combining PTT with the inhibition of heat shock protein (HSP) expression to enhance the therapeutic efficacy of the photothermal effect.17 Notably, the upregulation of intracellular reactive oxygen species (ROS) during the process of ferroptosis offers a pathway for inhibiting HSP expression. As a result, the combined approach of ferroptosis and PTT is proposed as a promising strategy for effectively targeting cancer cells.18,19Croconaine (Croc) compounds belong to the donor–acceptor–donor (D–A–D) class of zwitterionic molecules with an intense absorption in the near-infrared (NIR) region and high thermal efficiency, especially in acid environments.20,21 Despite their considerable potential for bioimaging applications, the intrinsic instability stemming from self-aggregation and the limited tumor-targeting capability of Croc have constrained their broad biomedical utilization in vivo.22,23 To address these limitations, chemical conjugation techniques have been extensively employed to enhance the physicochemical properties and functionalities of Croc.24 However, it is noteworthy that the introduction of chemical conjugation may compromise the thermostability of Croc when applied to photothermal therapy.
Our recent work has focused on the development of a thiol–chromene “click” nucleophilic reaction, resulting in the creation of a turn-on probe for thiol detection with remarkable selectivity and high signal-to-background ratios.25–28 Additionally, in our previous studies,29,30 we extended the utility of the thiol-triggered cascade reaction to generate quinone methide and release functional components, such as near-infrared (NIR) fluorophores and organelle targeting groups. Building upon this foundation, we envisioned the prospect of integrating the chromene component with the Croc structure to produce a thiol-responsive probe. This hybrid probe not only facilitated high signal-to-background ratio fluorescence imaging but also activated the Croc moiety for PTT. Importantly, quinone methide (QM) generated in this cascade reaction was postulated to inhibit glutathione peroxidase 4 (GPX4) and induce ferroptosis.31 The high level of thiol in the tumor region could facilitate the controlled release of p-quinone methide and Croc,32 enabling a combined approach involving both chemotherapy and thermotherapy.
As a proof-of-concept, we engineered probe CM-Croc by combining chromene and Croc for a novel approach to integrated photothermal therapy and ferroptosis-based cancer theranostics. CM-Croc demonstrated the ability to effectively penetrate cells and selectively target thiol in the mitochondria. Moreover, when administered intravenously, CM-Croc exhibited a remarkable propensity to accumulate in tumor regions, enabling clear differentiation between the tumor and normal tissue based on thiol levels, thus achieving high contrast. Notably, our research showed the release of QM, which was found to inhibit GPX4 activity and induce ferroptosis. The anti-cancer effect was significantly enhanced when combined with irradiation, particularly against HCT-116 and drug-resistant cell lines (HT-29, HCT116 p53−/−), achieving potent inhibition at low micromolar concentrations. Furthermore, CM-Croc demonstrated outstanding in vivo therapeutic efficacy through the combination of PTT and ferroptosis induction. Importantly, to the best of our knowledge, CM-Croc represents the first application of a Croc-derived SOP for in vivo photothermal therapy through intravenous administration. Overall, our work has introduced a robust and reliable tool for advancing theranostic research in the field of cancer treatment.
Results and discussion
Probe design and synthesis
In our previous investigation, we synthesized the chromene structure from (2-hydroxy-5-methyl-1,3-phenylene) dimethanol, employing a two-step synthesis approach (Scheme S1†).26 Subsequently, we chose the coumarin moiety as the candidate for condensation with chromene, resulting in the formation of compound 4 under established reaction conditions.27 Notably, the structural substitution of oxygen with nitrogen within coumarin moiety 4 led us to anticipate an extended emission wavelength compared to our previous findings. Compound 4 was then subjected to a reaction with triphosgene and Croc (5) to provide the final probe CM-Croc (1) with a moderate yield.Spectral properties
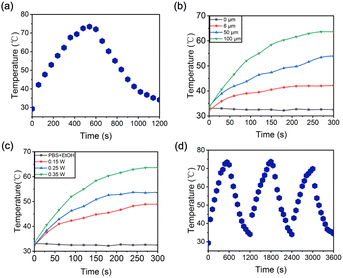
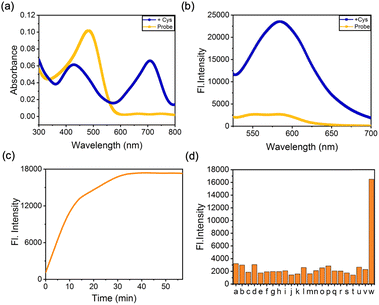
The assessment of CM-Croc's capacity to release the chromene–coumarin moiety was conducted through the examination of UV-vis absorption and fluorescence spectra (Fig. 2a, b and S1†). Notably, the absorption peak at 700 nm exhibited a substantial increase upon the introduction of thiol compounds, suggesting the liberation of the Croc moiety in comparison to the absorption profile of Croc in isolation (Fig. S2†). It is noteworthy that while the absorbance behaviour appeared typical under standard conditions, a noteworthy enhancement was expected under the acidic milieu characteristic of tumor regions, in line with previous findings.33 A conspicuous fluorescence “turn-on” effect at 580 nm from the coumarin part was observed upon interaction with thiols through the mechanism reported in our previous work.30 However, the emission wavelength was notably shorter than that of the substructure devoid of the Croc component (Fig. S3†). Additionally, kinetic investigations revealed the completion of the reaction within a period of 30(34) minutes (Fig. 2c). Intriguingly, in contrast to our previously developed probe,29,30 the reaction rate of CM-Croc was comparatively sluggish. Based on these two sets of results, it was postulated that a direct interaction occurred between the Croc moiety and the chromene–coumarin segment. Subsequently, the selectivity of CM-Croc for thiols was scrutinized (Fig. 2d). The results indicated that only thiol elicited significant alterations in fluorescence emission at 580 nm.Subsequently, we investigated the photophysical attributes of CM-Croc, with a particular focus on its photothermal effect. Employing 660 nm laser irradiation at a power density of 0.35 W cm−2, we observed a rapid elevation in temperature change within the CM-Croc aqueous solution, reaching 70 °C within the initial 10 minutes subsequent to thiol treatment, as illustrated in Fig. 3a and S4.† Conversely, when the photothermal effect was examined in the absence of cysteine (Cys), the results indicated an insignificant temperature change when compared to the Cys-treated group (Fig. S5†). To provide a quantitative assessment of the photothermal conversion efficiency (PCE), we calculated it to be approximately 55% based on the observed photothermal effects and time constants, as depicted in Fig. S6.† It is noteworthy that this PCE value for CM-Croc surpasses the majority of previously reported small molecular photothermal agents, including ICG (∼3.1%) (Table S1†).34 Furthermore, we explored the concentration and power density-dependent photothermal efficiency of CM-Croc, affirming its versatility and potential efficacy (Fig. 3b and c). In addition to these observations, we examined the stability of CM-Croc, a pivotal factor for an effective photothermal probe. Following irradiation with a 660 nm laser for three on–off cycles, CM-Croc (at a concentration of 100 μM) consistently maintained a maximum temperature of around 70 °C, thus underscoring its remarkable photothermal stability (Fig. 3d). These cumulative findings emphasize the promising photothermal properties of CM-Croc, positioning it as a noteworthy candidate for effective photothermal therapy.
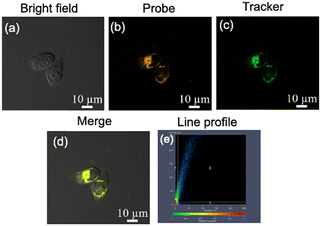
Next, the ability of probe CM-Croc to penetrate the cell membrane was evaluated (Fig. S7†). The results indicated that probe CM-Croc could enter the cell well. Moreover, when the cells were pretreated with N-methylmaleimide or exogenous, the intracellular fluorescence decreased or increased, respectively, which indicated that fluorescence occurred after CM-Croc reacted with the thiols in the living cells. Subsequently, the colocalization experiment was conducted. It is surprising that probe CM-Croc selectively localized in mitochondria (Fig. 4) and the Pearson's coefficient is around 0.90, which was probably due to the nitrogen heterocycles with N–H bonds.35
Imaging in cells and tumors
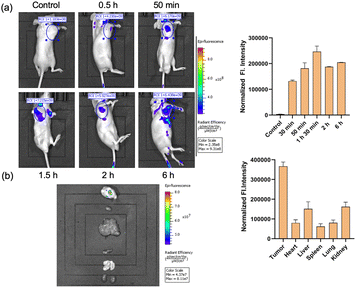
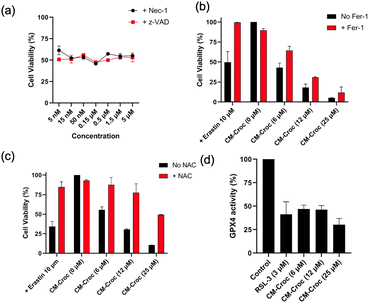
In the subsequent phase of our investigation, we aimed to assess the viability of imaging of tumors with CM-Croc. To accomplish this, we intravenously administered CM-Croc to BALB/c nude mice with subcutaneous HCT-116 tumors. Subsequent to the administration, we observed a discernible fluorescence signal within the tumor tissue as early as 1 hour post-injection. This signal reached its peak intensity at approximately 1.5 hours post-injection (Fig. 5a). Following this, the experimental mouse was euthanized, and the associated fluorescence imagery unambiguously delineated the tumor (Fig. 5b). Notably, our findings revealed that other organs exhibited relatively weaker fluorescence signals, with the liver being one such example. This observation was in alignment with our initial hypothesis, substantiating the notion that CM-Croc possesses the capability to accumulate within the tumor region. Moreover, it shows CM-Croc's potential to effectively discriminate between tumor and normal tissues based on variations in thiol levels.Subsequently, ES-2 cells were subjected to initial treatment with CM-Croc, alongside the use of the apoptosis inhibitor z-VAD-fmk (z-VAD) and the necroptosis inhibitor necrostatin-1 (Nec-1), as depicted in Fig. 6a. Notably, it was observed that the cytotoxicity induced by CM-Croc remained unmitigated by the presence of z-VAD and Nec-1, implying that CM-Croc did not influence apoptosis or necroptosis. Furthermore, when ES-2 cells were exposed to varying concentrations of CM-Croc for a duration of 24 hours, a dose-dependent inhibition of ES-2 cell viability was evident. In contrast, when ES-2 cells were co-incubated with CM-Croc and the ferroptosis inhibitor ferrostatin-1 (Fer-1), a substantial rescue of cell death was observed (Fig. 6b). This protective effect was also mirrored when N-acetyl cysteine (NAC), serving as an antioxidant, was introduced alongside CM-Croc (Fig. 6c). Notably, a parallel set of results were obtained when HCT-116 cells were exposed to CM-Croc (Fig. S8†). CM-Croc continued to exhibit substantial inhibitory effects on HCT-116 cells in a concentration-dependent manner, specifically through ferroptosis. Importantly, the positive control agents, erastin and RSL3, displayed negligible inhibitory effects on the growth of HCT-116 cells after 24 hours of treatment, which was consistent with the previous result.36 Moreover, CM-Croc showed weak toxicity toward the normal colorectal cell NCM-460 even at 25 μM, which was probably due to the low level of thiol in the normal cells (Fig. S9†).
CM-Croc, by virtue of its ability to react with thiols and generate quinone methide, was postulated to exert inhibitory effects on GPX4. This led to the proposition that CM-Croc might induce redox dyshomeostasis and elevate intracellular oxidative stress, ultimately triggering ferroptosis. In order to scrutinize this hypothesis, we investigated the inhibitory effect of CM-Croc on GPX4 at cellular levels. As shown in Fig. 6d, the activity of the intracellular GPX4 enzyme treated with CM-Croc significantly decreased compared with that treated with positive control (RSL-3).
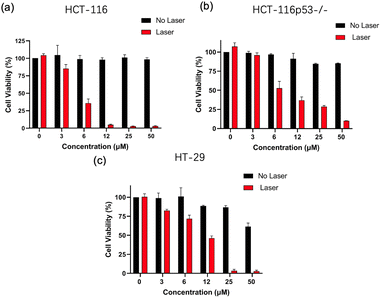
Afterward, we assessed the synergistic chemo- and photothermal toxicity of CM-Croc on HCT-116 cells in vitro. Prior to exposing HCT-116 cells to laser treatment, various laser power settings were tested (Fig. S10†). These preliminary experiments revealed a significant reduction in HCT-116 cell viability when the laser power was adjusted to 0.35 W cm−2. Following this, HCT-116 cells were incubated with CM-Croc for a duration of 4 hours, while simultaneously subjecting them to laser irradiation at a power of 0.35 W cm−2 for 5 minutes. The results demonstrated that cell viability decreased in a concentration-dependent manner under these conditions. In contrast, the control group that did not receive laser treatment exhibited comparatively weaker inhibitory effects over the same 4 hour period (Fig. 7a). Additionally, it is noteworthy that drug-resistant colon cancer cell lines, including HCT-116 p53−/− and HT-29, also exhibited heightened sensitivity to the combined chemo- and thermotherapy, as illustrated in Fig. 7b and c. These findings underscore the enhanced efficacy of CM-Croc in inhibiting the growth of HCT-116 cells and its drug-resistant variants when subjected to combined chemo- and thermotherapy.
Building upon the promising results obtained from our in vitro experiments, we proceeded to investigate the in vivo anticancer potential of CM-Croc. To achieve this, we employed xenograft mouse models bearing HCT-116 cell-derived tumors. Initially, the in vivo photothermal effect of CM-Croc was explored under 660 nm laser excitation (0.2 W cm−2) one hour after CM-Croc injection in an intravenous manner (as demonstrated in Fig. S11†). Notably, the temperature rapidly escalated from 35.6 °C to 64.7 °C within a span of 5 minutes (Fig. S12†). In contrast, the temperature changes in the PBS group and the Croc group were approximately 7.3 °C and 5.8 °C, respectively. These findings substantiate CM-Croc's ability to accumulate at the tumor site and release croconaine for PTT.
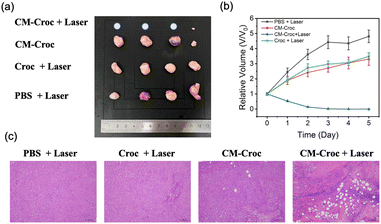
Subsequently, we evaluated the therapeutic impact on tumor growth by monitoring the tumor volume. As illustrated in Fig. 8, the control group (PBS and laser treatment) exhibited rapid tumor growth without significant inhibitory effects. In contrast, CM-Croc and Croc with the laser group displayed modest inhibitory effects, whereas CM-Croc and the laser group demonstrated substantial tumor suppression. These results emphasize the collaborative efficacy of the combined chemotherapy and photothermal therapy in more effectively and specifically inhibiting cancer cells.
In order to assess the biosafety and potential side effects of the photothermal agent, histological examination was conducted on major organs, including the heart, liver, spleen, lung, kidney, and the tumor, after 5 days of treatment (as shown in Fig. S13†). The results revealed no observable necrosis or injury in the major organs. Notably, the tumor tissue displayed clear signs of necrosis in the CM-Croc and laser group. Furthermore, no significant fluctuations in body weight were observed during the five-day treatment, underscoring the excellent biosafety profile of CM-Croc (Fig. S14†). These findings support the potential of CM-Croc as a safe and effective approach for cancer treatment in vivo.
Conclusion
In summary, we have successfully developed a novel probe, CM-Croc, for the purpose of integrated photothermal and ferroptosis-based cancer theranostics. This probe responded to thiol activation, releasing croconaine and enabling the activation of fluorescence with high signal-to-background ratios. Importantly, CM-Croc exhibited exceptional PCE and maintained good photothermal stability. Additionally, CM-Croc possessed outstanding tumor-targeting capabilities, allowing for the clear differentiation of tumor tissue from normal tissue in vivo following intravenous administration. Furthermore, CM-Croc has been demonstrated to induce ferroptosis in a concentration-dependent manner through the inhibition of GPX4. Significantly, our findings reveal that cancer cell lines, including drug-resistant variants, exhibit heightened sensitivity to the combined chemo- and thermotherapy, even at low micromolar concentrations with low laser power. Finally, the synergistic anticancer effects of CM-Croc have been corroborated in a xenograft mouse model of tumors. This approach presents a promising strategy, offering the potential for the design of more adaptable and switchable photothermal probes, aimed at achieving combined chemo- and thermotherapy. These advancements hold substantial promise for the development of innovative and effective cancer treatment strategies.Data availability
The authors confirm that the data supporting the findings of this study are available within the article. The supporting data include experimental procedures and analytical data (NMR and HRMS), which can be found in the ESI.† Copies of NMR spectra are also provided.Author contributions
K. M. and C. Y. conceived the project. X. N. and H. Y. synthesized the dyes and conducted photophysical characterization and analysis. H. Y. and X. W. performed fluorescence imaging experiments. X. N. and F. H. characterized structures. All authors participated in writing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (22077076, 22325703, 22377071, and U23A6009), the Research Project Supported by the Shanxi Scholarship Council of China (2023-012, 2022-002), the Shanxi Province Science Foundation (no. 202203021221009), and the Scientific Instrument Center of Shanxi University (201512).Notes and references
- X. Li, J. F. Lovell, J. Yoon and X. Chen, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef PubMed.
- H. S. Jung, P. Verwilst, A. Sharma, J. Shin, J. L. Sessler and J. S. Kim, Chem. Soc. Rev., 2018, 47, 2280–2297 RSC.
- L. B. Carpin, L. R. Bickford, G. Agollah, T. K. Yu, R. Schiff, Y. Li and R. A. Drezek, Breast Cancer Res. Treat., 2011, 125, 27–34 CrossRef PubMed.
- X. Guo, N. Yang, W. Ji, H. Zhang, X. Dong, Z. Zhou, L. Li, H. Shen, S. Yao and W. Huang, Adv. Mater., 2021, 33, 2007778 CrossRef PubMed.
- H. Jung, J. Lee, K. Kim, S. Koo, P. Verwilst, J. L. Sessler, C. Kang and J. Kim, J. Am. Chem. Soc., 2017, 139, 9972–9978 CrossRef CAS PubMed.
- R. Wang, X. Li and J. Yoon, ACS Appl. Mater. Interfaces, 2021, 13, 19543–19571 CrossRef CAS PubMed.
- H. Wang, J. Chang, M. Shi, W. Pan, N. Li and B. Tang, Angew. Chem., Int. Ed., 2019, 58, 1057–1061 CrossRef CAS PubMed.
- X. Deng, Z. Shao and Y. Zhao, Adv. Sci., 2021, 8, 2002504 CrossRef CAS PubMed.
- M. Salimi, S. Mosca, B. Gardner, F. Palombo, P. Matousek and N. Stone, Nanomaterials, 2022, 12, 922 CrossRef CAS PubMed.
- J. Tian, B. Li, F. Zhang, Z. Yao, W. Song, Y. Tang, Y. Ping and B. Liu, Angew. Chem., Int. Ed., 2023, 135, e202307288 CrossRef.
- M. Zhao, Y. Zhang, J. Miao, H. Zhou, Y. Jiang, Y. Zhang, M. Miao, W. Chen, W. Xing, Q. Li and Q. Miao, Adv. Mater., 2023, 36, 2305243 CrossRef PubMed.
- X. Chen, Z. Zhang, W. Luo, Z. Zhuang, Z. Zhao, L. Wang, D. Wang and B. Tang, Biomaterials, 2022, 287, 121680 CrossRef CAS PubMed.
- X. Meng, J. Zhang, Z. Sun, L. Zhou, G. Deng, S. Li, W. Li, P. Gong and L. Cai, Theranostics, 2018, 8, 6025–6034 CrossRef CAS PubMed.
- H. Han, H. Wang, P. Jangili, M. Li, L. Wu, Y. Zang, A. C. Sedgwick, J. Li, X. He, T. D. James and J. Kim, Chem. Soc. Rev., 2023, 52, 879–920 RSC.
- H. Knox, T. Kim, Z. Zhu and J. Chan, ACS Chem. Biol., 2018, 13, 1838–1843 CrossRef CAS PubMed.
- Y. Liu, P. Bhattarai, Z. Dai and X. Chen, Chem. Soc. Rev., 2019, 48, 2053–2108 RSC.
- X. Zhang, S.-S. Xue, W. Pan, H. Wang, K. Wang, N. Li and B. Tang, Chem. Commun., 2023, 59, 235–238 RSC.
- M. Chang, Z. Hou, M. Wang, C. Yang, R. Wang, F. Li, D. Liu, T. Peng, C. Li and J. Lin, Angew. Chem., Int. Ed., 2021, 60, 12971–12979 CrossRef CAS PubMed.
- S. He, Y. Jiang, J. Li and K. Pu, Angew. Chem., Int. Ed., 2020, 59, 10633–10638 CrossRef CAS PubMed.
- S. Lei, Y. Zhang, N. T. Blum, P. Huang and J. Lin, Bioconjugate Chem., 2020, 31, 2072–2084 CrossRef CAS PubMed.
- Y. Wen, H. H. McGarraugh, C. L. Schreiber and B. D. Smith, Chem. Commun., 2020, 56, 6977–6980 RSC.
- C. R. Ouyang, P. You, F. Lu, H. Mei, J. Yu, Z. Zhu and C. Zhou, Dyes Pigm., 2024, 221, 111780 CrossRef CAS.
- J. S. Al-Otaibi, Y. S. Mary, Y. S. Mary and R. Thomas, Spectrochim. Acta, Part A, 2022, 264, 120233 CrossRef PubMed.
- F. Zeng, L. Tang, Q. Zhang, C. Shi, Z. Huang, S. R. Y. Nijiati, X. Chen and Z. Zhou, Angew. Chem., Int. Ed., 2022, 134, e202112925 CrossRef.
- K. Ma, L. Zhao, Y. Yue, F. Huo, J. Chao and C. Yin, Anal. Chem., 2020, 92, 15936–15942 CrossRef CAS PubMed.
- F. Huo, Y. Sun, J. Su, Y. Yang, J. Chao and C. Yin, Org. Lett., 2009, 11, 4918 CrossRef CAS PubMed.
- K. Ma, L. Zhao, Y. Yue and C. Yin, Chem. Phys. Rev., 2022, 3, 011302 CrossRef CAS.
- Y. Yang, K. Zhou, M. Ma, H. Liu, M. Jin, C. Yin, S. Wang and J. Zhang, Chem. Eng. J., 2023, 452, 139020 CrossRef.
- Y. Yang, T. Zhou, M. Jin, K. Zhou, D. Liu, X. Li, F. Huo, W. Li and C. Yin, J. Am. Chem. Soc., 2020, 142, 1614–1620 CrossRef PubMed.
- K. Ma, H. Yang, T. Shen, Y. Yue, L. Zhao, X. G. Liu, F. Huo and C. Yin, Chem. Sci., 2022, 13, 3706–3712 RSC.
- D. Qi, L. Xing, L. Shen, W. Sun, C. Cai, C. Xue, X. Song, H. Yu, H. Jiang and C. Li, Chin. Chem. Lett., 2022, 33, 4595–4599 CrossRef.
- Y. Wang, P. Pigeon, W. Li, J. Yan, P. M. Dansette, M. Othman, M. J. McGlinchey and G. Jaouen, Eur. J. Med. Chem., 2022, 234, 114202 CrossRef CAS PubMed.
- S. Guha, G. K. Shaw, T. M. Mitcham, R. R. Bouchard and B. D. Smith, Chem. Commun., 2016, 52, 120–123 RSC.
- L. Weng and Y. Liu, Drug Discov. Today, 2021, 26, 2045–2052 CrossRef PubMed.
- W. Ma, B. Xu, R. Sun, Y. J. Xu and J. F. Ge, J. Mater. Chem. B, 2021, 9, 2524–2531 RSC.
- E. Llabani, R. W. Hicklin, H. Y. Lee, S. E. Motika, L. A. Crawford, E. Weerapana and P. J. Hergenrother, Nat. Chem., 2019, 11, 521–532 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc03688c |
| This journal is © The Royal Society of Chemistry 2024 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: