Open Access Article
Open Access ArticleBoron-doped double [6]carbohelicenes: a combination of helicene and boron-doped π-systems†
Yujia
Liu
,
Liuzhong
Yuan
,
Zengming
Fan
,
Jingyuan
Yang
,
Yue
Wang
 and
Chuandong
Dou
and
Chuandong
Dou
 *
*
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: chuandong@jlu.edu.cn
First published on 8th July 2024
Abstract
Helicenes, featuring unique helical structures, have a long history as three-dimensional polycyclic aromatic hydrocarbons (PAHs). Incorporation of heteroatoms into helicenes may alter their electronic structures and achieve unexpected physical properties. Here, we disclose fusion of boron-doped π-systems onto helicenes as an efficient strategy to design boron-doped carbohelicenes. Two boron-doped double [6]carbohelicenes were synthesized, which possess the C58B2 and C86B2 polycyclic π-skeletons containing two [6]helicene subunits, respectively. The C86B2 molecule thus represents the largest-size helicene-based boron-doped PAH. A thorough investigation reveals that the helicene moieties and boron atoms endow the polycyclic π-systems with delocalized electronic structures, and well-tunable ground-state and excited-state photophysical properties. It is notable that the C58B2 molecule displays excited-state stimulated emission behavior and amplified spontaneous emission (ASE) properties in not only the blend films with various doped concentrations but also the pure film. To our knowledge, it is the first example of ASE-active [n]helicene (n ≥ 6), and moreover, such robust ASE performance has rarely been observed in PAHs, demonstrating the promising utility of boron-doped carbohelicenes for laser materials.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) have received much attention because of their intriguing optical, electronic and magnetic properties, as well as potential applications in organic light-emitting diodes (OLEDs), organic field-effect transistors (OFETs), organic solar cells (OSCs), etc.1–8 Owing to the great advance of synthetic chemistry, various π-extended PAHs with well-defined sizes, edge structures and conformations have been successfully developed, in which their electronic structures and physical properties are mainly determined by their topological conjugated structures.9–16 In particular, by controlling the zigzag, armchair and/or cove edge states, stimulated emission (SE) behaviors and amplified spontaneous emission (ASE) properties have been remarkably achieved in PAHs, demonstrating that they will be a promising class of optical-gain materials for organic lasers, photonics and optoelectronics.17–20 However, as large-size topologies and zigzag edge structures usually lead to open-shell electronic states and non-fluorescence nature,21–23 such ASE-active PAHs are still rare and limited to only a few examples, such as dibenzo[hi,st]ovalene, zigzag-edged nanographenes and molecular ribbons.24–29 Therefore, it is needed to develop new ASE-active PAH systems, and moreover, this research may further promote the development of PAH chemistry and materials.Helicenes, helically ortho-fused polyaromatics, have a long history as three-dimensional PAHs.30 Their helical structures can induce unique intrinsic chirality despite the absence of asymmetric carbons and chiral centers, and thereby bestow interesting nonplanarity, optical rotation and dynamic behaviors, as well as other physical-organic properties.31,32 Until now, π-extension of simple helicenes, e.g. [5]helicene and [6]helicene, has led to a variety of helicene systems with amazing topological structures, including elongated helicenes, multiple helicenes and expanded helicenes, such as A,33B (ref. 34) and C (ref. 29) (Fig. 1a).33–41 Moreover, their outstanding optical, electrical and chiroptical properties, as well as synthetic chemistry and material applications have been intensively studied. Nevertheless, aside from these wonderful helicene research studies, the new space of helicene systems still needs to be explored.
Boron atoms have one less electron than carbon atoms, and feature a characteristic vacant p-orbital. Boron-doping of PAHs as a powerful strategy has generated a large family of boron-doped PAHs, which not only displayed some unusual properties, such as Lewis acidity, control over supramolecular polymerization, photo-responsive fluorescence and dynamic modulation of (anti)aromaticity and diradical properties, but also were employed as organic catalysts and optoelectronic materials.42–45 However, owing to the inherent high reactivity of the boron moiety and complicated multi-step syntheses, the design and synthesis of boron-doped PAHs with large-size π-frameworks and/or well-tuned edge structures remains challenging.46–54 For instance, only a few pristine boron-doped helicene derivatives (such as D and E) have been developed.55–57 Moreover, most of them are based on [4]helicene, and until very recently, only one boron-doped [6]helicene F was constructed.57 We recently developed several large-size boron-doped PAHs via implementing controllable cyclization on conjugated organoboranes.58–60 Among them, two boron-doped [4]carbohelicenes exhibit intriguing ASE activity in the blend films.61,62 We thus envisioned that precise control over topological structures of boron-doped PAHs may afford not only more sophisticated π-scaffolds but also organic emitters with desirable ASE performance.
Herein, we disclose fusion of boron-doped π-systems onto helicenes as an efficient strategy to design boron-doped carbohelicenes. Two boron-doped double [6]carbohelicenes 1 and 2 (Fig. 1b) that feature fully fused C58B2 and C86B2 π-conjugated frameworks containing two [6]helicene subunits, respectively, were successfully synthesized. Thus, we achieved a combination of helicene and boron-doped π-systems and created the largest-size record of helicene-based boron-doped PAHs. Their molecular geometries, aromaticity and electronic structures, as well as ground-state and excited-state photophysical properties have been studied in detail. Notably, the C58B2 molecule displays stimulated emission behavior and amplified spontaneous emission properties in the various blend films and even pure film, which have rarely been achieved in PAHs. Therefore, this study provides not only two new examples of boron-doped carbohelicenes, but also opens a new direction for the design of functional helicenes and efficient ASE-active PAHs using organoboron chemistry.
Results and discussion
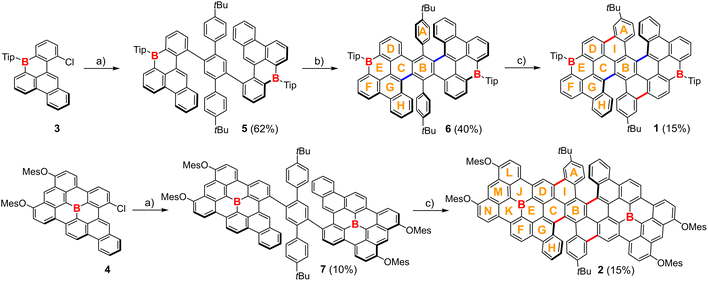
As shown in Scheme 1, the target B-doped carbohelicenes 1 and 2 were synthesized starting from two key B-containing building blocks 3 and 4, which were first reported by Feng's group and our group, respectively.61,63 The Suzuki–Miyaura cross-coupling reactions between 3/4 and 2,5-di-(4-tert-butylphenyl)-1,4-bis-bpin-benzene produced the precursors 5/7. The oxidative cyclization reaction of 5 with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and CH3SO3H in dry CH2Cl2 led to the cyclization of rings B and G, and generated a partially fused product 6. The Scholl reaction with FeCl3 was then performed on 6, yielding compound 1via the cyclization of rings A and D. Based on these synthetic results, we treated compound 7 with FeCl3 and directly prepared the desired product 2 with a larger size. Compounds 1 and 2 were purified using silica gel column chromatography without any special precautions (Fig. S1†). As determined by high-resolution mass spectrometry (HRMS), only one intense peak is observable in their HRMS spectra, and the experimental isotopic distributions agree well with the stimulated data, thus confirming the molecular formula of C96H88B2 for 1 and C130H92B2O4 for 2 (Fig. S2†).The conformations of B-doped carbohelicenes 1 and 2 were studied by density functional theory (DFT) calculations at the B3LYP/6-311G(d) level of theory. For 1, there are three possible stereoisomers, including 1-(P,P), 1-(P,M) and 1-(M,M) (Fig. 2 and S4†). The optimized geometries of 1-(P,P) and 1-(M,M) have the C58B2 π-frameworks, which are composed of two [6]helicene substructures and two B-containing π-systems with the B atoms doped into the zigzag edges (Fig. S5†). The dihedral angles are ca. 58° for the terminal rings of [6]helicene moieties, leading to their highly twisted conformations. As shown in the energy diagram, the enantiomers 1-(P,P) and 1-(M,M) are thermodynamically more stable than 1-(P,M) with the meso form by 2.2 kcal mol−1. Additionally, the clean proton signals in the 1H NMR spectrum of 1 are consistent with the theoretical data of 1-(P,P) (Fig. S3†). These results indicate that 1-(P,P) and 1-(M,M) were obtained as the sole products in the synthetic process. The interconversion from 1-(P,P) to 1-(P,M) proceeds through the transition state (1-TS1) with face-to-face oriented terminal rings of [6]helicene subunits. The activation free energy (45.7 kcal mol−1) for 1-TS1 is much higher than that of [6]helicene, also higher than that of [7]helicene, and comparable with that of double [6]helicene B.34 These B-doped carbohelicenes thus may preserve the rigidity of helicenes and thereby bestow high isomerization barriers. For 2, its optimized (P,P)-geometry has the C86B2 π-framework, comprising two [6]helicene substructures and two larger B-centered π-systems (Fig. S5†). To our knowledge, compound 2 represents the largest-size helicene-edged B-doped PAHs.
 | ||
| Fig. 2 Energy diagram for the isomerization from 1-(P,P) to 1-(P,M), calculated at the B3LYP/6-311G(d) level of theory. | ||
To illustrate the electronic structures of B-doped carbohelicenes, we investigated the aromaticity of 1 and 2 along with the partially fused 6, using nucleus independent chemical shift (NICS) and anisotropy of the induced current density (ACID) calculations at the B3LYP/6-311+G(d) and B3LYP/6-311G(d) levels, respectively.64 The optimized (P,P)-geometries of 1 and 2 were employed as the model structures for the calculations. As shown in Fig. 3, largely negative NICS(1)ZZ values are observed for rings A/B/D/F/H (in red) in 6 and 1, and rings A/B/D/F/H/M (in red) for 2, suggestive of their essence of aromatic sextet rings. The corresponding molecular forms of 6, 1 and 2 shown in Scheme 1 mainly contribute to their electronic structures. In the ACID plots, the π electrons of 6 display obviously localized and clockwise ring current circuits, thus indicating its typical local aromaticity. For 1, its π electrons are mainly delocalized along the periphery with a clockwise current, and also flow along the new C–C bonds between rings A and D. It is notable that the π electrons cross over the central ring B, which acts as a special bridge that effectively connects the two B-containing π-skeletons. Thus, different from 6, B-doped carbohelicene 1 exhibits unusual crossed-circle aromaticity and efficient π-delocalization. For 2, clockwise ring currents along the larger-size periphery and bypassing through ring B are observed, indicating the contribution of π-extension to molecular aromaticity. It can be concluded that the central hexagon in these two B-doped helicenes is a crucial bridge, which links two B-containing moieties and contributes to the formation of two helical substructures, further producing delocalized π-electronic structures.
 | ||
| Fig. 3 Calculated NICS(1)ZZ values and ACID plots (contribution from π electrons only) of (a and d) 6, (b and e) 1 and (c and f) 2. The tBu, Mes and OMes groups are removed for clarity. | ||
Reflecting efficient π-conjugated structures, B-doped carbohelicenes 1 and 2 exhibit red-shifted and broad absorption bands. In toluene, 1 shows an absorption band that covers the range of 300–630 nm and the longest absorption maximum (λabs) at 591 nm (Fig. 4a and S7,† and Table 1). For 6, it shows an absorption band around 300–530 nm with one intense peak at 453 nm and a shoulder band at 490 nm. Compared with that of 6, the absorption spectrum of 1 is red-shifted exceeding 100 nm, despite the formation of only two more C–C bonds, thus highlighting the importance of its efficient π-conjugation. In the absorption spectrum of 2, a broader absorption band around 300–740 nm is observed, along with a λabs of 700 nm. The unchanged time-dependent absorption spectra reveal their high ambient stability (Fig. S9†). Based on the absorption onsets, the optical band gap (Eoptg) is estimated to be 2.41 eV for 6, 1.99 eV for 1 and 1.68 eV for 2, respectively. On the other hand, toluene solutions of 6 and 1 emit bright green and red fluorescence, respectively, with a maximum emission wavelength (λem) and fluorescence quantum yield (ΦF) of 513/547 nm and 0.21 for 6 and 618/650 nm and 0.22 for 1. The fluorescence bands exhibit small solvent effects on the solvent polarity (Fig. S7†). For 2, the fluorescence is not detected in its solution. The photophysical properties of 1 are distinct from those of all-carbon double [6]helicene B (λabs, 506 nm; λem, 525 nm in chloroform) and B-doped double [5]carbohelicene C56B2 (λabs, 477 nm; λem, 504 nm in toluene),34,61 thus demonstrating the significant effects of B-doping and π-electron delocalization.
Time-dependent density functional theory (TD-DFT) calculations were performed at the B3LYP/6-311G(d) level of theory to further reveal their absorption properties and electronic structures. The LUMOs and HOMOs of 6 and 1 are delocalized over their polycyclic frameworks, respectively (Fig. 4b). From 6 to 1, the HOMO level is enhanced by 0.16 eV and the LUMO level is decreased by 0.41 eV, leading to the decreased energy gap, which can be ascribed to the effect of π-extension on 1. The all-carbon analogue 1C was employed for comparison. From 1C to 1, the LUMO and HOMO energies are lowered by 0.71 and 0.36 eV, respectively, and the energy gap is decreased by 0.35 eV. These changes are attributed to the B atoms in 1, whose vacant p-orbitals obviously contribute to the LUMO and HOMO, thus implying the vital role of B-doping in the electronic structure of carbohelicene. Compared with 1, 2 exhibits higher LUMO and HOMO energies by 0.19 and 0.55 eV, respectively, along with a narrower band gap. According to the TD-DFT calculations, the calculated transition energies, wavelengths and oscillator strengths reproduce well with the experimental spectra of 6, 1 and 2. It is notable that all of their low-energy absorption bands involve the HOMO → LUMO transitions, in which the B atom contributes to the LUMOs (Fig. S14–S16†). For 2, its HOMO is mainly localized on the central region of the π-skeleton, whereas the LUMO is obviously distributed on the B-containing moieties. This suggests that the non-emission essence of 2 is ascribed to its intramolecular charge transfer process. Moreover, the calculated low-energy absorption of 1C appears at 518 nm (Fig. S17†). This demonstrates that B-doping and π-extension greatly impact the red-shifted absorption properties of B-doped carbohelicenes.
To gain insight into their excited-state photophysical properties, we performed femtosecond transient absorption (fs-TA) characterization on 6, 1 and 2. In the wide-range fs-TA spectra, 6 and 2 in toluene exhibit ground-state bleach (GSB) signals around 459/492 nm and 640/705 nm, respectively, which agree well with their characteristic absorption signals in the steady-state absorption spectra (Fig. 5). Excited-state absorption (ESA) bands are observed at 500–750 nm for 6 and 475–615 nm for 2. By contrast, 1 exhibits a distinctive fs-TA spectrum. Two peaks at 510/547 nm and one overwhelming band around 584–623 nm are observable, which are tentatively ascribed to the GSB regions. The ESA bands appear at 450–495 and 715–750 nm. It is notable that one unexpected negative bump at 675 nm is observed. As this signal is not present in the steady-state absorption spectrum but seems to be the long-wavelength tail of the fluorescence spectrum, we can assign it to the stimulated emission characteristic of 1.18,19 Thus, compound 1 features unique SE behavior in the excited state. We further conducted fs-TA measurements on the 1/polystyrene (PS) blend film (20 wt%) and the pure film of 1. The two films show not only GSB signals around 600 nm but also weak SE bands around 675 nm. Such preserved SE performance may be owing to less non-radiative decays in the inert PS matrix film, and weak molecular aggregation and an inhibited intermolecular charge-transfer process in the pristine film (Fig. S10,†vide infra).
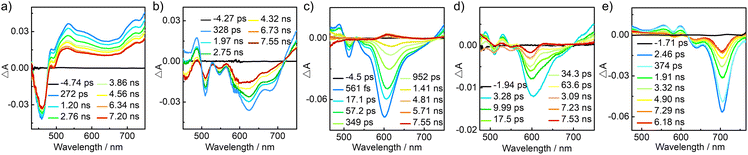 | ||
| Fig. 5 Femtosecond transient absorption spectra of (a) 6 in toluene, (b) 1 in toluene, (c) the 1/PS blend film (20 wt%) and (d) the pristine film of 1, as well as (e) 2 in toluene. | ||
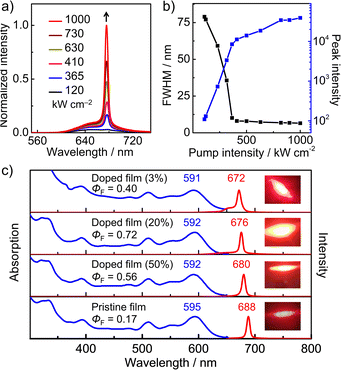
The intriguing excited-state SE behavior of 1 promotes us to explore its amplified spontaneous emission properties, because ASE is not only a photonics process where the spontaneous emission is amplified owing to the optical-gain action but also a basic requirement for lasing materials.65–67 The ASE characteristics of the 1/PS blend film (20 wt%) are shown in Fig. 6a and b. Enhancing the pump laser energy over a certain threshold leads to the appearance of an emission peak at 676 nm, which obviously increases and narrows, finally resulting in a small full width at half maximum (FWHM) of 6.5 nm. This emission wavelength agrees with the SE signal in the fs-TA spectrum, and moreover, the dependence of FWHM versus pump energy and the nonlinear gain of emission peak intensity versus pump energy are obtained. These results undoubtedly verify the ASE activity of 1, and the threshold is determined to be 120 kW cm−2. This threshold value is relatively high, but comparable to that of several large-size PAHs and our reported boron-doped molecular carbon (Table S5†).24–29,61,62 Furthermore, almost no changes are observed for the ASE intensities of this 1/PS blend film upon irradiation for 20 min under air at a laser energy of 500 kW cm−2 (Fig. S12†), indicative of its remarkable photostability. To our knowledge, it is the first example of ASE-active [n]helicene (n ≥ 6). For 6 and 2, they do not display any ASE signals, in consistence with their unobserved excited-state SE behaviors.
Finally, to reveal the remarkable effects of double [6]helicene substructures, we studied the photophysical properties of 1 at various film states. The blend films with different doped concentrations and the pure film exhibit very similar absorption spectra with the long-wavelength absorption peak around 590 nm (Fig. 6c), which is also almost identical to that of 1 in toluene. Moreover, the emission peaks of its films and solution all appear at ca. 619 nm (Fig. S8†). The fluorescence quantum yields of the 3 wt%, 20 wt% and 50 wt% blend films are 0.40, 0.72 and 0.56, respectively, which are higher than that of the pure film (ΦF = 0.17) and the solution (ΦF = 0.22). These results suggest that the molecules are well dispersed and fixed into the PS matrix, leading to the restricted vibration of helicene moieties and the rotation of the tip groups, which can minimize non-radiative decay channels of the excited state. For the pure film, the helicene substructures along with the peripheral substituents effectually inhibit intermolecular π–π stacking and molecular aggregation, further depressing the intermolecular charge-transfer process and preventing fluorescence quenching to a certain extent. We then conducted ASE measurements on the blend and pure films of 1. These films show the typical dependences of FWHM and emission peak intensity versus pump energy. The ASE wavelengths are red-shifted from 672 nm to 688 nm (Fig. S11†), and the fluorescence band around 650 nm is quenched for the 50 wt% blend and pristine films. These fluorescence changes are probably due to the formation of a small portion of excimers at higher concentration.25,68 The threshold value is estimated to be 258 kW cm−2 for the 3 wt% blend and 120 kW cm−2 for the 50 wt% blend. The radiative constants (kr) for the 3 wt%, 20 wt% and 50 wt% blend films were determined to be 6.40 × 107 s−1, 1.70 × 108 s−1 and 1.30 × 108 s−1 (Fig. S13†), respectively. It is thus concluded that a higher radiative rate may lead to a smaller threshold value. Notably, the ASE properties can be achieved for the blend films with distinct doped concentrations and even pristine films of 1. Until now, for the reported ASE-active PAHs, the preparation of the blend films with low concentration is highly required to recover SE behavior and realize ASE properties.18,19,24–29 In this study, B-doped carbohelicene 1 displays ASE properties in the various blend films and even pure film. Such robust ASE performance has never been reported for the family of helicenes and has also been rarely observed in PAHs. This demonstrates that B-doped carbohelicenes are very promising to develop film-insensitive ASE-active materials for organic lasers and photonics.
Conclusions
We succeeded in the synthesis of two boron-doped double [6]carbohelicenes, which were designed by fusion of boron-doped π-systems onto helicenes. They have fully fused C58B2 and C86B2 π-conjugated frameworks containing two [6]helicene subunits, respectively. Thus, they can be regarded as a combination of helicene and boron-containing π-systems, and moreover, the C86B2 molecule represents the largest-size helicene-based B-doped PAH. The helicene substructures and boron atoms endow the polycyclic π-systems with delocalized electronic structures and well-tunable ground-state and excited-state photophysical properties. Notably, the C58B2 molecule displays stimulated emission behavior and amplified spontaneous emission properties in the blend films and even pure film, along with good photostability. Therefore, the results demonstrate the potential of boron-doped carbohelicenes as robust ASE-active materials. This study provides a design strategy for functional helicenes using organoboron chemistry, which can be applied to the construction of more sophisticated photonic materials.Data availability
The data that support the findings of this study are available in the ESI† of this article.Author contributions
Y. L., Y. W., and C. D. conceived the idea. Y. L. synthesized the compounds and fully evaluated their properties. Y. L., L. Y., Z. F., and J. Y. performed the theoretical calculations. Y. L., Y. W., and C. D. wrote the manuscript, and all authors discussed and commented on the manuscript. C. D. directed the project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 52373182 and 22175074) and Jilin Scientific and Technological Development Program (no. 20220101054JC).Notes and references
- A. Narita, X.-Y. Wang, X. Feng and K. Müllen, Chem. Soc. Rev., 2015, 44, 6616–6643 RSC.
- M.-W. Wang, W. Fan, X. Li, Y. Liu, Z. Li, W. Jiang, J. Wu and Z. Wang, ACS Nano, 2023, 17, 20734–20752 CrossRef PubMed.
- M. Grzybowski, B. Sadowski, H. Butenschön and D. T. Gryko, Angew. Chem., Int. Ed., 2020, 59, 2998–3027 CrossRef CAS.
- H. Ito, Y. Segawa, K. Murakami and K. Itami, J. Am. Chem. Soc., 2019, 141, 3–10 CrossRef CAS.
- M. A. Majewski and M. Stępień, Angew. Chem., Int. Ed., 2019, 58, 86–116 CrossRef CAS PubMed.
- Y. Segawa, H. Ito and K. Itami, Nat. Rev. Mater., 2016, 1, 15002 CrossRef CAS.
- T.-H. Shi and M.-X. Wang, CCS Chem., 2021, 3, 916–931 CrossRef CAS.
- S. H. Pun and Q. Miao, Acc. Chem. Res., 2018, 51, 1630–1642 CrossRef CAS PubMed.
- Y. Tanaka, N. Fukui and H. Shinokubo, Nat. Commun., 2020, 11, 3873 CrossRef CAS.
- S. Zank, J. M. Fernández-García, A. J. Stasyuk, A. A. Voityuk, M. Krug, M. Solà, D. M. Guldi and N. Martín, Angew. Chem., Int. Ed., 2022, 61, e202112834 CrossRef CAS PubMed.
- T. Kirschbaum, F. Rominger and M. Mastalerz, Angew. Chem., Int. Ed., 2020, 59, 270–274 CrossRef CAS.
- S. Ma, J. Gu, C. Lin, Z. Luo, Y. Zhu and J. Wang, J. Am. Chem. Soc., 2020, 142, 16887–16893 CrossRef CAS PubMed.
- X.-J. Zhao, H. Hou, X.-T. Fan, Y. Wang, Y.-M. Liu, C. Tang, S.-H. Liu, P.-P. Ding, J. Cheng, D.-H. Lin, C. Wang, Y. Yang and Y.-Z. Tan, Nat. Commun., 2019, 10, 3057 CrossRef PubMed.
- W. Jiang and Z. Wang, J. Am. Chem. Soc., 2022, 144, 14976–14991 CrossRef CAS PubMed.
- N. Ogawa, Y. Yamaoka, H. Takikawa, K.-i. Yamada and K. Takasu, J. Am. Chem. Soc., 2020, 142, 13322–13327 CrossRef CAS PubMed.
- M. Mahl, M. A. Niyas, K. Shoyama and F. Würthner, Nat. Chem., 2022, 14, 457–462 CrossRef CAS.
- I. D. W. Samuel and G. A. Turnbull, Chem. Rev., 2007, 107, 1272–1295 CrossRef CAS PubMed.
- G. M. Paternò, Goudappagouda, Q. Chen, G. Lanzani, F. Scotognella and A. Narita, Adv. Opt. Mater., 2021, 9, 2100508 CrossRef.
- G. M. Paternò, L. Moretti, A. J. Barker, Q. Chen, K. Müllen, A. Narita, G. Cerullo, F. Scotognella and G. Lanzani, Adv. Funct. Mater., 2019, 29, 1805249 CrossRef.
- B. Liu, M. Chen, X. Liu, R. Fu, Y. Zhao, Y. Duan and L. Zhang, J. Am. Chem. Soc., 2023, 145, 28137–28145 CrossRef CAS PubMed.
- W. Zeng and J. Wu, Chem, 2021, 7, 358–386 CAS.
- J. Liu and X. Feng, Angew. Chem., Int. Ed., 2020, 59, 23386–23401 CrossRef CAS PubMed.
- Z. X. Chen, Y. Li and F. Huang, Chem, 2021, 7, 288–332 CAS.
- G. M. Paternò, Q. Chen, X.-Y. Wang, J. Liu, S. G. Motti, A. Petrozza, X. Feng, G. Lanzani, K. Müllen, A. Narita and F. Scotognella, Angew. Chem., Int. Ed., 2017, 56, 6753–6757 CrossRef PubMed.
- Y. Zou, V. Bonal, S. Moles Quintero, P. G. Boj, J. M. Villalvilla, J. A. Quintana, G. Li, S. Wu, Q. Jiang, Y. Ni, J. Casado, M. A. Díaz-García and J. Wu, Angew. Chem., Int. Ed., 2020, 59, 14927–14934 CrossRef CAS PubMed.
- V. Bonal, R. Muñoz-Mármol, F. Gordillo Gámez, M. Morales-Vidal, J. M. Villalvilla, P. G. Boj, J. A. Quintana, Y. Gu, J. Wu, J. Casado and M. A. Díaz-García, Nat. Commun., 2019, 10, 3327 CrossRef.
- R. Muñoz-Mármol, F. Gordillo, V. Bonal, J. M. Villalvilla, P. G. Boj, J. A. Quintana, A. M. Ross, G. M. Paternò, F. Scotognella, G. Lanzani, A. Derradji, J. C. Sancho-García, Y. Gu, J. Wu, J. Casado and M. A. Díaz-García, Adv. Funct. Mater., 2021, 31, 2105073 CrossRef.
- Y. Gu, V. Vega-Mayoral, S. Garcia-Orrit, D. Schollmeyer, A. Narita, J. Cabanillas-González, Z. Qiu and K. Müllen, Angew. Chem., Int. Ed., 2022, 61, e202201088 CrossRef CAS PubMed.
- X. Xu, G. Serra, A. Villa, R. Muñoz-Mármol, S. Vasylevskyi, M. Gadea, A. Lucotti, Z. Lin, P. G. Boj, R. Kabe, M. Tommasini, M. Á. Díaz-García, F. Scotognella, G. M. Paternò and A. Narita, Chem. Sci., 2022, 13, 13040–13045 RSC.
- M. Rickhaus, M. Mayor and M. Juríček, Chem. Soc. Rev., 2016, 45, 1542–1556 RSC.
- Y. Shen and C.-F. Chen, Chem. Rev., 2012, 112, 1463–1535 CrossRef CAS PubMed.
- M. Gingras, Chem. Soc. Rev., 2013, 42, 1051–1095 RSC.
- G.-F. Huo, T. M. Fukunaga, X. Hou, Y. Han, W. Fan, S. Wu, H. Isobe and J. Wu, Angew. Chem., Int. Ed., 2023, 62, e202218090 CrossRef CAS PubMed.
- T. Fujikawa, Y. Segawa and K. Itami, J. Am. Chem. Soc., 2015, 137, 7763–7768 CrossRef CAS.
- S. Jia, S. Li, Y. Liu, W. Qin and H. Yan, Angew. Chem., Int. Ed., 2019, 58, 18496–18501 CrossRef CAS.
- T. Hosokawa, Y. Takahashi, T. Matsushima, S. Watanabe, S. Kikkawa, I. Azumaya, A. Tsurusaki and K. Kamikawa, J. Am. Chem. Soc., 2017, 139, 18512–18521 CrossRef CAS.
- B. Liu, M. Böckmann, W. Jiang, N. L. Doltsinis and Z. Wang, J. Am. Chem. Soc., 2020, 142, 7092–7099 CrossRef CAS.
- W. Yang, N. Li, J. Miao, L. Zhan, S. Gong, Z. Huang and C. Yang, CCS Chem., 2022, 4, 3463–3471 CrossRef CAS.
- J. Wang, C. Shen, G. Zhang, F. Gan, Y. Ding and H. Qiu, Angew. Chem., Int. Ed., 2022, 61, e202115979 CrossRef CAS.
- M. Navakouski, H. Zhylitskaya, P. J. Chmielewski, T. Lis, J. Cybińska and M. Stępień, Angew. Chem., Int. Ed., 2019, 58, 4929–4933 CrossRef CAS.
- W. Fu, V. Pelliccioli, R. Casares-López, J. M. Cuerva, M. Simon, C. Golz and M. Alcarazo, CCS Chem., 2024 DOI:10.31635/ccschem.024.202404088.
- M. Hirai, N. Tanaka, M. Sakai and S. Yamaguchi, Chem. Rev., 2019, 119, 8291–8331 CrossRef CAS PubMed.
- L. Ji, S. Griesbeck and T. B. Marder, Chem. Sci., 2017, 8, 846–863 RSC.
- E. von Grotthuss, A. John, T. Kaese and M. Wagner, Asian J. Org. Chem., 2018, 7, 37–53 CrossRef CAS.
- A. Escande and M. J. Ingleson, Chem. Commun., 2015, 51, 6257–6274 RSC.
- J. M. Farrell, C. Mützel, D. Bialas, M. Rudolf, K. Menekse, A.-M. Krause, M. Stolte and F. Würthner, J. Am. Chem. Soc., 2019, 141, 9096–9104 CrossRef CAS PubMed.
- T. Huang, Z. Ding, H. Liu, P.-A. Chen, L. Zhao, Y. Hu, Y. Yao, K. Yang and Z. Zeng, Chin. Chem. Lett., 2024, 35, 109117 CrossRef CAS.
- N. Ando, T. Yamada, H. Narita, N. N. Oehlmann, M. Wagner and S. Yamaguchi, J. Am. Chem. Soc., 2021, 143, 9944–9951 CrossRef CAS.
- Z. Fan, W. Sun, Y. Yang, J. Guo, C. Dou and Y. Wang, Chin. Chem. Lett., 2023, 34, 107729 CrossRef CAS.
- F. Miyamoto, S. Nakatsuka, K. Yamada, K.-i. Nakayama and T. Hatakeyama, Org. Lett., 2015, 17, 6158–6161 CrossRef CAS.
- C. Dou, S. Saito, K. Matsuo, I. Hisaki and S. Yamaguchi, Angew. Chem., Int. Ed., 2012, 51, 12206–12210 CrossRef CAS.
- V. M. Hertz, M. Bolte, H.-W. Lerner and M. Wagner, Angew. Chem., Int. Ed., 2015, 54, 8800–8804 CrossRef CAS.
- J.-J. Zhang, L. Yang, F. Liu, G. Serra, Y. Fu, A. Lucotti, A. A. Popov, M. Tommasini, J. Ma and X. Feng, Angew. Chem., Int. Ed., 2023, 62, e202312055 CrossRef CAS PubMed.
- R. J. Kahan, D. L. Crossley, J. Cid, J. E. Radcliffe, A. W. Woodward, V. Fasano, S. Endres, G. F. S. Whitehead and M. J. Ingleson, Chem. Commun., 2018, 54, 9490–9493 RSC.
- D. L. Crossley, R. J. Kahan, S. Endres, A. J. Warner, R. A. Smith, J. Cid, J. J. Dunsford, J. E. Jones, I. Vitorica-Yrezabal and M. J. Ingleson, Chem. Sci., 2017, 8, 7969–7977 RSC.
- J. Radtke, K. Schickedanz, M. Bamberg, L. Menduti, D. Schollmeyer, M. Bolte, H.-W. Lerner and M. Wagner, Chem. Sci., 2019, 10, 9017–9027 RSC.
- M. Schnitzlein, K. Shoyama and F. Würthner, Chem. Sci., 2024, 15, 2984–2989 RSC.
- L. Yuan, Y. Wang and C. Dou, Org. Mater., 2023, 5, 191–201 CrossRef CAS.
- L. Yuan, J. Guo, Y. Yang, K. Ye, C. Dou and Y. Wang, CCS Chem., 2023, 5, 876–884 CrossRef CAS.
- W. Sun, J. Guo, Z. Fan, L. Yuan, K. Ye, C. Dou and Y. Wang, Angew. Chem., Int. Ed., 2022, 61, e202209271 CrossRef CAS PubMed.
- Y. Liu, L. Yuan, J. Guo, W. Sun, Y. Wang and C. Dou, Angew. Chem., Int. Ed., 2023, 62, e202306911 CrossRef CAS.
- Z. Fan, Y. Liu, T. Zhang, Y. Wang and C. Dou, CCS Chem., 2024 DOI:10.31635/ccschem.024.202403953.
- J.-J. Zhang, M.-C. Tang, Y. Fu, K.-H. Low, J. Ma, L. Yang, J. J. Weigand, J. Liu, V. W.-W. Yam and X. Feng, Angew. Chem., Int. Ed., 2021, 60, 2833–2838 CrossRef CAS.
- M. Randić, Chem. Rev., 2003, 103, 3449–3605 CrossRef PubMed.
- A. J. C. Kuehne and M. C. Gather, Chem. Rev., 2016, 116, 12823–12864 CrossRef CAS PubMed.
- C. Adachi and A. S. D. Sandanayaka, CCS Chem., 2020, 2, 1203–1216 CrossRef CAS.
- K. Wang and Y. S. Zhao, Chem, 2021, 7, 3221–3231 CAS.
- R. Muñoz-Mármol, N. Zink-Lorre, J. M. Villalvilla, P. G. Boj, J. A. Quintana, C. Vázquez, A. Anderson, M. J. Gordon, A. Sastre-Santos, F. Fernández-Lázaro and M. A. Díaz-García, J. Phys. Chem. C, 2018, 122, 24896–24906 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, synthesis and characterization of 1, 2 and 6. See DOI: https://doi.org/10.1039/d4sc03124e |
| This journal is © The Royal Society of Chemistry 2024 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: