Open Access Article
Open Access ArticleSulfur oxidation states manipulate excited state electronic configurations for constructing highly efficient organic type I photosensitizers†
Jianye
Gong
a,
Xiaopeng
Wang
 b,
Weijing
Zhang
a,
Yifan
Wu
a,
Kai
Li
a,
Renmanduhu
Sha
a,
Lingxiu
Liu
a,
Chunbin
Li
a,
Lina
Feng
a,
Guoyu
Jiang
b,
Weijing
Zhang
a,
Yifan
Wu
a,
Kai
Li
a,
Renmanduhu
Sha
a,
Lingxiu
Liu
a,
Chunbin
Li
a,
Lina
Feng
a,
Guoyu
Jiang
 a,
Jianguo
Wang
a,
Jianguo
Wang
 *a and
Ben Zhong
Tang
*a and
Ben Zhong
Tang
 c
c
aInner Mongolia Key Laboratory of Fine Organic Synthesis Department, College of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot 010021, P. R. China. E-mail: wangjg@iccas.ac.cn
bXi'an Modern Chemistry Research Institute, Xi'an 710069, P. R. China
cSchool of Science and Engineering, Shenzhen Institute of Aggregate Science and Technology, The Chinese University of Hong Kong, Shenzhen, Shenzhen, Guangdong 518172, P. R. China
First published on 16th July 2024
Abstract
The multiple relaxation processes of excited states are a bridge connecting molecular structures and properties, providing enormous application potential for organic luminogens. However, a systematic understanding and manipulation of the relationship between the molecular structure, excited state relaxation processes, and properties of organic luminogens is still lacking. Herein, we report a strategy for manipulating excited state electronic configurations through the regulation of the sulfur oxidation state to construct eminent organic type I PSs. Combined with the experimental results and theoretical calculations, we have successfully revealed the decisive role of high sulfur oxidation states in promoting ROS production capacity. Impressively, a higher sulfur oxidation state can reduce the singlet–triplet energy gap (ΔEST), increase the matching degree of transition configurations, promote the changes of the excited state electronic configurations, and boost the effective ISC proportion by enhancing intramolecular interactions. Therefore, DBTS2O with the highest sulfur oxidation state exhibits the strongest type I ROS generation ability. Additionally, guided by our strategy, a water-soluble PS (2OA) is designed and synthesized, showing selective imaging capacity and photokilling ability against Gram-positive bacteria. This study broadens the horizons for both molecular design and mechanism study of high-performance organic type I PSs.
Introduction
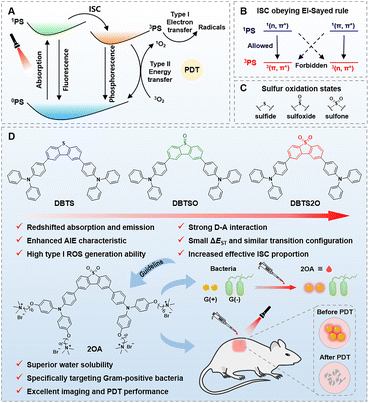
The relaxation processes of excited organic luminogens include several complex procedures such as vibrational relaxation (VR), internal conversion (IC), intersystem crossing (ISC), reverse intersystem crossing (RISC), fluorescence emission, phosphorescence emission, etc.1–7 These procedures determine the different photophysical behaviors and provide the basis for diverse applications. For decades, regulating the relaxation processes of excited states has become a universal strategy to control the functionality of organic luminogens. For example, manipulating the VR process improved the photothermal conversion efficiency to enhance the photothermal performance of organic phototheranostic agents,8,9 while promoting the ISC process facilitated phosphorescence properties to realize multiple information encryption of organic room temperature phosphorescence (RTP) materials.10,11 By adjusting the RISC process, efficient organic light-emitting diodes can be achieved.12,13 Generally, the relaxation processes of excited states are closely related to the molecular structure of organic luminogens.14–18 Unambiguously, it is significant to comprehensively understand the relaxation processes of excited molecules and to elucidate the structure–activity relationship for developing organic functional luminogens.19–21Photodynamic therapy (PDT) is a modern therapy with non-invasiveness, high specificity, controllable spatio-temporal selectivity and low side effects and shows great potential in clinical applications.22–24 In recent years, numerous organic luminogens have been successfully developed as photosensitizers (PSs) and applied in PDT due to their good biosafety, easily modifiable structures and excellent fluorescence properties.25,26 In the PDT process (Scheme 1A), the singlet excited state of PSs (1PS) undergoes the ISC process to form the triplet excited state of PSs (3PS) after light excitation. Subsequently, 3PS interacts with oxygen through electron transfer (type I) or energy transfer (type II) procedures to generate reactive oxygen species (ROS) which can cause irreversible oxidative damage to biological macromolecules (such as proteins, nucleic acids, lipids, etc.). Therefore, an efficient ISC process is the key to high performance PSs.27,28 Notably, type I PSs have attracted extensive attention due to their less oxygen-dependent features. Although several design strategies have been reported to improve the ROS production capacity of organic PSs, it is still a huge challenge to construct superior organic type I PSs by enhancing the ISC efficiency.29 According to the El-Sayed rule (Scheme 1B), the ISC process occurs between excited states with different electronic configurations.30–32 In other words, the transition from the 1(n, π*) singlet excited state to the 3(π, π*) triplet excited state or from the 1(π, π*) singlet excited state to the 3(n, π*) triplet excited state is allowed. Therefore, facilitating the transformation of the excited state electronic configurations into an allowed transition mode is an effective method to improve ISC efficiency. However, there are few reports about manipulating the electron configurations of excited states. Additionally, a small singlet-triplet energy gap (ΔEST) and a similar transition configuration of the excited state are also essential for boosting the ISC process.33,34 Collectively, these factors need to be fulfilled in the construction of excellent organic PSs. Changing the sulfur oxidation states in sulfides (Scheme 1C) could influence the photophysical properties of molecules by adjusting the relaxation processes of excited states.35–37 This has been shown to be effective in increasing photoluminescence quantum yields and promoting RTP.38–43 However, regulating sulfur oxidation states to manipulate excited state electronic configurations for constructing organic type I PSs remains unexplored. Predictably, high sulfur oxidation states can provide the following advantages: (1) strong intramolecular donor–acceptor (D–A) interactions; (2) multiple intermolecular interactions; (3) abundant (n, π*) electronic configurations. Therefore, it is expected to promote the ISC process by regulating sulfur oxidation states for obtaining brilliant organic type I PSs.
Based on the above discussions, a strategy of manipulating excited state electronic configurations by regulating sulfur oxidation states was proposed to enhance ISC efficiency for constructing organic type I PSs (Scheme 1D). As a proof-of-concept, three D–A–D type (triphenylamine group as a donor and dibenzothiophene group as an acceptor) organic luminogens (DBTS, DBTSO, and DBTS2O) with an aggregation-induced emission (AIE) feature were designed and synthesized by subtly regulating the sulfur oxidation state of the dibenzothiophene group. DBTS, DBTSO, and DBTS2O showed different sulfur oxidation states from sulfide to sulfoxide to sulfone, respectively. Excitedly, DBTS2O exhibited the most redshifted absorption and fluorescence emission as well as the highest type I ROS generation efficiency. Single-crystal analysis indicated that DBTS2O with the highest sulfur oxidation state had abundant intermolecular interactions, implying a compact molecular packing. Theoretical calculations showed that the sulfur oxidation state could significantly affect the electronic configurations of excited states. On one hand, higher sulfur oxidation states enhanced intramolecular D–A interactions, resulting in a smaller ΔEST and a more similar transition configuration of the excited state. On the other hand, higher sulfur oxidation states changed the proportion of excited state electronic configurations, thus increasing the effective ISC from 1(n, π*) to 3(π, π*) or/and from 1(π, π*) to 3(n, π*). These two factors jointly promoted the ISC process of DBTS2O, resulting in its superior type I ROS generation ability. Furthermore, guided by this strategy, an AIE PS (2OA) with good water solubility was synthesized. 2OA showed excellent selective imaging and photodynamic killing against Gram-positive bacteria. Collectively, this study elucidates the structure–activity relationship between the sulfur oxidation state and ROS production capacity, providing valuable insights for the development of efficient organic type I PSs.
Results and discussion
Firstly, DBTS, DBTSO, and DBTS2O with different sulfur oxidation states were synthesized via a one-step Suzuki coupling reaction. The detailed synthesis routes and characterization data are provided in the ESI (Schemes S1–S3 and Fig. S1–S12†). The photophysical properties of the three luminogens in DMSO were studied by UV-vis and photoluminescence (PL) spectroscopy. As shown in Fig. 1A, the absorption maxima of DBTS, DBTSO, and DBTS2O peaked at 335, 370, and 375 nm, respectively. And the maximal emission peaks were located at 415, 550, and 564 nm, respectively. DBTS2O showed the most redshifted absorption and emission as well as a 189 nm Stokes shift. The electron cloud distribution of the highest occupied molecular orbitals (HOMOs) and the lowest unoccupied molecular orbitals (LUMOs) for DBTS, DBTSO, and DBTS2O were calculated using density functional theory (DFT). The HOMOs of the three luminogens were mainly localized on the triphenylamine groups, while the LUMOs were mainly localized on the dibenzothiophene group with different sulfur oxidation states (Fig. S13†). The HOMO–LUMO energy gaps were calculated to be 5.90, 5.48, and 5.35 eV, respectively, indicating a gradual enhancement of intramolecular D–A interactions. Additionally, the AIE properties of DBTS, DBTSO, and DBTS2O were studied in DMSO/toluene mixtures. As depicted in Fig. 1B and C and S14,† the PL intensity of the three luminogens slowly increased as the toluene fraction (ft) was raised from 0% to 80%. When the toluene fraction reached 99%, the PL intensity of these compounds was obviously enhanced by 3.6-fold, 4.7-fold, and 11.9-fold that in pure DMSO solution, suggesting the typical AIE feature. Moreover, the emissions of DBTS, DBTSO, and DBTS2O were gradually blue-shifted with the increase in ft due to the twisted intramolecular charge transfer (TICT) effect. Remarkably, DBTS2O showed the strongest AIE properties among the three luminogens. Subsequently, the ROS generation abilities of the three AIE luminogens (AIEgens) were further investigated under white light irradiation. Initially, dichlorofluorescein (DCFH) was used as an indicator to detect total ROS production. As shown in Fig. 1D and S15,† after white light irradiation, the PL intensity of DCFH in the presence of DBTS barely showed any change, while the PL intensity of DBTSO + DCFH and DBTS2O + DCFH increased rapidly with prolonged irradiation time. As we expected, the ROS generation efficiency of DBTS2O was higher than that of DBTSO. To confirm the species of ROS, different commercial indicators were used. The ˙OH and O2˙− generation efficiencies were studied by using aminophenyl fluorescein (APF) and dihydrorhodamine 123 (DHR123) as indicators, respectively. The results were similar to those obtained with DCFH as an indicator. The PL intensity of APF or DHR123 was clearly enhanced in the presence of DBTSO and DBTS2O after white light irradiation, and DBTS2O exhibited superior generation efficiency of ˙OH and O2˙− (Fig. 1E and F, S16 and S17†). In contrast, no distinct change was observed in DBTS + APF or DBTS + DHR123 solution. When 9,10-anthracenediyl-bis(methylene) dimalonic acid (ABDA) was utilized to assess 1O2, no obvious changes were recorded (Fig. 1G and S18†). These results demonstrated that DBTS2O with the highest sulfur oxidation state had a stronger type I ROS production ability compared to DBTS and DBTSO. In addition, electron spin resonance (ESR) spectroscopy using 5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide (BMPO) and 4-amino-2,2,6,6-tetramethylpiperidine (TEMP) as the spin-trap agents was employed to further confirm the generation of free radicals and 1O2. As shown in Fig. 1H, in the presence of BMPO and the three AIEgens, only DBTSO and DBTS2O showed typical ESR signals of free radicals, while DBTS had no ESR signal after white light irradiation. However, no 1O2 ESR signals of the three AIEgens were observed in TEMP-trapping ESR spectra after white light irradiation. These results further verified the type I ROS production ability of DBTSO and DBTS2O.To understand the mechanism of enhanced ROS production efficiency by a high sulfur oxidation state, single crystal analysis of the three AIEgens was carried out. The single crystals of DBTS (CCDC: 2325250), DBTSO (CCDC: 2325253), and DBTS2O (CCDC: 2325255) were obtained by slow evaporation of ethyl acetate solution (Table S1†). As illustrated in Fig. S19–S21,† the three AIEgens showed similar molecular packing due to the small change of the sulfur oxidation state in molecular structures. The same molecular unit was extracted from the three crystals, which were then separated into three trimers for analysis of intermolecular interactions. In detail, only C–H⋯π interactions were observed in the three trimers of DBTS. For DBTSO and DBTS2O, however, S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H interactions were gradually observed with the increase in the sulfur oxidation state. Notably, DBTS2O trimers exhibited more S
O⋯H interactions were gradually observed with the increase in the sulfur oxidation state. Notably, DBTS2O trimers exhibited more S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H interactions compared to DBTSO (Fig. 2A). In order to intuitively reflect the difference in intermolecular interactions of DBTS, DBTSO, and DBTS2O, the visualization of intermolecular interactions was conducted based on independent gradient model (IGM) analysis44,45 using the Multiwfn program46 and visual molecular dynamics (VMD) program.47 As shown in Fig. S22–S25,† plentiful green isosurfaces appeared in the DBTS2O trimers, indicating their rich intermolecular interactions. In addition, Hirshfeld surface analysis48–50 was further performed to quantitatively evaluate the intermolecular interactions of the three AIEgen crystals. Over 90% of total intermolecular interactions were attributed to weak intermolecular C⋯H, H⋯H, and other interactions for DBTS, DBTSO, and DBTS2O. As the sulfur oxidation state increased, the ratio of S⋯H interaction decreased from 5.1% to 2.0% to 0%, while the ratio of O⋯H interaction increased from 0% to 5.7% to 10.9% (Fig. 2B and S26†). These results suggested that changing the sulfur oxidation state from sulfide to sulfoxide to sulfone effectively strengthened the intermolecular interactions, promoting tight packing in the aggregated state. Moreover, the enhanced O⋯H interaction also potentially affected the changes in the excited state electron configurations.
O⋯H interactions compared to DBTSO (Fig. 2A). In order to intuitively reflect the difference in intermolecular interactions of DBTS, DBTSO, and DBTS2O, the visualization of intermolecular interactions was conducted based on independent gradient model (IGM) analysis44,45 using the Multiwfn program46 and visual molecular dynamics (VMD) program.47 As shown in Fig. S22–S25,† plentiful green isosurfaces appeared in the DBTS2O trimers, indicating their rich intermolecular interactions. In addition, Hirshfeld surface analysis48–50 was further performed to quantitatively evaluate the intermolecular interactions of the three AIEgen crystals. Over 90% of total intermolecular interactions were attributed to weak intermolecular C⋯H, H⋯H, and other interactions for DBTS, DBTSO, and DBTS2O. As the sulfur oxidation state increased, the ratio of S⋯H interaction decreased from 5.1% to 2.0% to 0%, while the ratio of O⋯H interaction increased from 0% to 5.7% to 10.9% (Fig. 2B and S26†). These results suggested that changing the sulfur oxidation state from sulfide to sulfoxide to sulfone effectively strengthened the intermolecular interactions, promoting tight packing in the aggregated state. Moreover, the enhanced O⋯H interaction also potentially affected the changes in the excited state electron configurations.
To systematically elucidate the structure–activity relationship between high sulfur oxidation states and outstanding ROS production efficiency, theoretical calculations based on the crystal structure were carried out. The electrostatic potential (ESP) distributions51–54 of DBTS, DBTSO, and DBTS2O are displayed in Fig. 3A. Notably, the acceptor of DBTS had weak electron-withdrawing properties, and there was no significant difference in ESP between the donor and acceptor. When the sulfur atom of the dibenzothiophene group was oxidized, the strong electronegativity of the oxygen atom enhanced the local electron-withdrawing properties, resulting in a more negative ESP for the sulfoxide and sulfone parts of DBTSO and DBTS2O. Meanwhile, the molecular dipole moment gradually increased. DBTS2O with the highest sulfur oxidation state showed the largest molecular dipole moment of 8.34 D, indicating its strong intramolecular D–A interaction. Furthermore, in order to gain a deeper understanding of the ESP differences among the three AIEgens, the surface areas of ESP were also analyzed. DBTS2O had a larger surface area with ESP values of −45, −35, −25, 15, and 25 kcal mol−1, while it had a smaller area at −15 and 5 kcal mol−1 than DBTS and DBTSO (Fig. 3B). The positive average ESP value (![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) +) and the negative average ESP value (
+) and the negative average ESP value (![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) −) of DBTS2O were calculated to be 10.07 and −9.97 kcal mol−1, respectively. These results not only demonstrated that the change in the sulfur oxidation state significantly affected the strength of D–A interaction, but also further illustrated the strongest D–A interaction of DBTS2O. Subsequently, the electronic configurations of excited states for the three AIEgens were studied using time-dependent density functional theory (TD-DFT). As shown in Fig. 3C, the energy levels of the first singlet excited state (S1) and the first triplet excited state (T1) for DBTS, DBTSO, and DBTS2O gradually decreased with the enhancement of intramolecular D–A interactions. The calculated ΔEST of DBTS, DBTSO, and DBTS2O were 0.76, 0.74, and 0.72 eV, respectively. Meanwhile, the transition configurations of S1 and T1 also changed from H → L+1 to H → L, and the matching degree gradually increased (Fig. S27–S29 and Table S2†). The smaller ΔEST and the more similar transition configuration of the excited state suggested that the increase in the sulfur oxidation state could promote the ISC process by enhancing intramolecular D–A interactions. More importantly, according to the El-Sayed rule, the ISC process from 1(n, π*) to 3(π, π*) or from 1(π, π*) to 3(n, π*) is favored. Thus, the electronic configurations of the excited states for DBTS, DBTSO, and DBTS2O were studied to assess transition modes by using natural transition orbital (NTO) analysis. Considering the proportion difference in different electron configurations, the type of electron configuration with a smaller proportion in the allowed transition mode was used to determine the effective transition. The proportion sum of the two effective transition modes was the final effective ISC proportion. The effective ISC proportions of DBTS, DBTSO, and DBTS2O were calculated to be 29.13%, 40.21%, and 41.50%, respectively, indicating that DBTS2O underwent the most efficient ISC process among the three AIEgens (Fig. 3C). The NTOs of excited states and the calculated details of electron configuration proportions for the three AIEgens are displayed in Fig. S30–S32 and Tables S3–S5.† Collectively, by increasing the sulfur oxidation state, the intramolecular D–A interaction could be significantly enhanced, resulting in a reduced ΔEST, an increased matching degree of transition configurations, changes in the excited state electronic configurations and a higher effective ISC proportion, which ultimately improved ROS generation efficiency. These results clearly elucidated the structure–activity relationship between high sulfur oxidation states and superior ROS production efficiency.
−) of DBTS2O were calculated to be 10.07 and −9.97 kcal mol−1, respectively. These results not only demonstrated that the change in the sulfur oxidation state significantly affected the strength of D–A interaction, but also further illustrated the strongest D–A interaction of DBTS2O. Subsequently, the electronic configurations of excited states for the three AIEgens were studied using time-dependent density functional theory (TD-DFT). As shown in Fig. 3C, the energy levels of the first singlet excited state (S1) and the first triplet excited state (T1) for DBTS, DBTSO, and DBTS2O gradually decreased with the enhancement of intramolecular D–A interactions. The calculated ΔEST of DBTS, DBTSO, and DBTS2O were 0.76, 0.74, and 0.72 eV, respectively. Meanwhile, the transition configurations of S1 and T1 also changed from H → L+1 to H → L, and the matching degree gradually increased (Fig. S27–S29 and Table S2†). The smaller ΔEST and the more similar transition configuration of the excited state suggested that the increase in the sulfur oxidation state could promote the ISC process by enhancing intramolecular D–A interactions. More importantly, according to the El-Sayed rule, the ISC process from 1(n, π*) to 3(π, π*) or from 1(π, π*) to 3(n, π*) is favored. Thus, the electronic configurations of the excited states for DBTS, DBTSO, and DBTS2O were studied to assess transition modes by using natural transition orbital (NTO) analysis. Considering the proportion difference in different electron configurations, the type of electron configuration with a smaller proportion in the allowed transition mode was used to determine the effective transition. The proportion sum of the two effective transition modes was the final effective ISC proportion. The effective ISC proportions of DBTS, DBTSO, and DBTS2O were calculated to be 29.13%, 40.21%, and 41.50%, respectively, indicating that DBTS2O underwent the most efficient ISC process among the three AIEgens (Fig. 3C). The NTOs of excited states and the calculated details of electron configuration proportions for the three AIEgens are displayed in Fig. S30–S32 and Tables S3–S5.† Collectively, by increasing the sulfur oxidation state, the intramolecular D–A interaction could be significantly enhanced, resulting in a reduced ΔEST, an increased matching degree of transition configurations, changes in the excited state electronic configurations and a higher effective ISC proportion, which ultimately improved ROS generation efficiency. These results clearly elucidated the structure–activity relationship between high sulfur oxidation states and superior ROS production efficiency.
As mentioned above, the ROS production efficiency of organic photosensitizers could be effectively enhanced by adjusting the sulfur oxidation state. According to our strategy, a cationic derivative of DBTS2O, namely 2OA, was designed and synthesized (Scheme S4 and Fig. S33–S40†). Notably, the lipid-water partition coefficient of 2OA was −2.30, indicating its excellent water solubility (Fig. 4A), which is favorable for biological applications.55–57 Therefore, the optical properties of 2OA were evaluated in aqueous solution. 2OA showed a maximal absorption at 386 nm and an emission maximum at 593 nm (Fig. 4B). The Stokes shift reached 207 nm, which effectively avoids the interference of the excitation light and is suitable for fluorescence imaging applications.58–60 Subsequently, the AIE characteristic of 2OA was investigated in a water/THF mixture with THF as a poor solvent due to the excellent water solubility of 2OA. 2OA had almost no emission in aqueous solution and showed 83-fold emission enhancement in a 99% THF fraction, indicating its AIE characteristic (Fig. 4C and S41†). The ROS generation abilities of 2OA were further tested. Different indicators, DCFH, hydroxyphenyl fluorescein (HPF), and DHR123, were employed to detect ROS production ability. Chlorin e6 (Ce6) was used as a reference. As expected, Fig. 4D and E and S42–S44† show that 2OA exhibited a higher efficiency in ROS production compared to Ce6 under different indicator conditions. However, the 1O2 production efficiency of Ce6 was higher than that of 2OA (Fig. S45†). These results confirmed that 2OA was a highly efficient PS, primarily generating type I ROS.
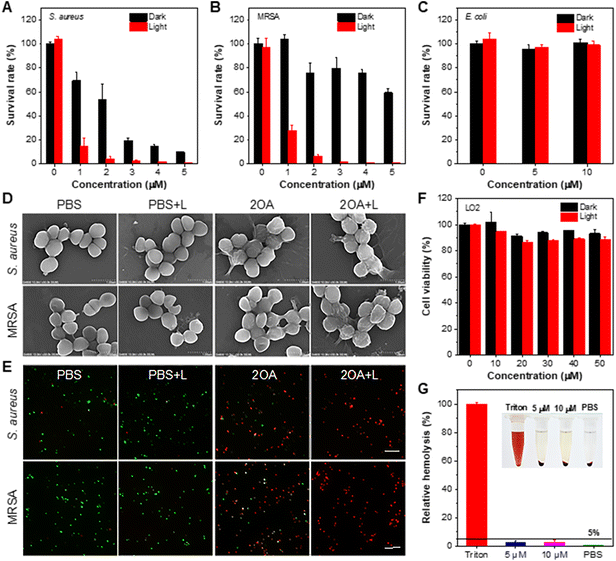
The annual death toll from antimicrobial resistance is on the rise, with projections indicating a staggering 10 million fatalities globally by 2050.61,62 This alarming trend poses a significant risk to both human health and public health security.63–65 PDT has been recognized as an effective method for treating drug-resistant bacterial infections due to its unique killing mechanism and its ability to prevent antimicrobial resistance.66–69 2OA not only had excellent photophysical properties and ROS production ability, but also positive charges that were conducive to binding with the negatively charged surfaces of bacteria. Therefore, 2OA was expected to be applied for imaging and photodynamic killing of drug-resistant bacteria. Gram-positive bacteria S. aureus, methicillin-resistant S. aureus (MRSA) and Gram-negative bacteria E. coli were selected as representative bacteria. In the preliminary bacterial imaging experiment, stark fluorescent signals were clearly observed in S. aureus and MRSA after being incubated with 2OA (Fig. S46†). However, the fluorescence emission in E. coli was barely visible. This could be attributed to the dense outer membrane structure of Gram-negative bacteria, which provides enormous obstacles to drug binding. These results were consistent with the changes in PL spectra before and after incubating 2OA with bacteria (Fig. S47†). Subsequently, S. aureus and MRSA were co-cultured with E. coli respectively under the same conditions and then incubated with 2OA. Interestingly, as shown in Fig. 4G and S48,†S. aureus and MRSA were selectively lit-up, while E. coli showed a low fluorescent signal. And the fluorescence intensity ratios were 5.3-fold and 6.7-fold, respectively (Fig. S49†). These results solidly suggested the brilliant performance of 2OA for accurate discrimination of Gram-positive bacteria. Moreover, 2OA had the ability to perform 3D imaging on S. aureus and MRSA with a high signal-to-noise ratio (Fig. 4H and S50†). To further verify the selective binding capacity of 2OA for Gram-positive bacteria, the changes in zeta potential (ξ) of S. aureus, MRSA, and E. coli before and after incubation with 2OA were studied. As depicted in Table S6,† the ξ values of both S. aureus and MRSA showed a positive shift after incubation with 2OA, while the ξ value of E. coli remained almost unchanged. These data provided further evidence of effective binding between 2OA and Gram-positive bacteria.
Encouraged by its selective imaging capacity and excellent ROS production ability, the photodynamic antibacterial performance of 2OA was investigated through the plate count method. As shown in Fig. 5A–C and S51,† 2OA at 5 μM was able to eliminate more than 99% of S. aureus and MRSA upon white light irradiation. However, although the concentration of 2OA was increased to 10 μM, the survival rate of E. coli remained unchanged under light conditions. These data indicated the specific killing ability of 2OA against Gram-positive bacteria. Subsequently, to evaluate the bacteriostatic process of 2OA in detail, the growth curves of S. aureus, MRSA and E. coli in the presence of 2OA with varying concentrations were monitored for 10 h. As shown in Fig. S52,† the 90% minimum inhibitory concentration (MIC90) values of 2OA against S. aureus and MRSA under light conditions were as low as 6.54 μM and 6.56 μM, respectively, further indicating that 2OA had an outstanding photodynamic killing effect on Gram-positive bacteria, including drug-resistant bacteria. The scanning electron microscopy (SEM) analysis results showed that the smooth membrane surfaces of S. aureus and MRSA were vigorously crumpled and collapsed after treatment with 2OA under white light irradiation, resulting in bacterial death (Fig. 5D). Moreover, we also visualized the antibacterial effect of 2OA by live/dead fluorescent staining. In comparison to the control group in the absence of 2OA, obvious red fluorescence, a marker of bacterial death, was observed in the presence of 2OA for both S. aureus and MRSA, particularly after being irradiated with white light (Fig. 5E, S53 and S54†). These data were in good agreement with the results of the in vitro antibacterial experiments. Good biocompatibility is another key parameter to evaluate the performance of PSs. Therefore, the cytotoxicity of 2OA toward LO2 cells was assessed by MTT assay. As shown in Fig. 5F, 2OA demonstrated negligible toxicity toward LO2 cells with an increasing concentration of 50 μM under both dark and light conditions. Furthermore, the blood compatibility of 2OA was also evaluated through a hemolysis experiment. The hemolysis analysis shown in Fig. 5G suggested that 2OA had excellent blood compatibility even at a high concentration of 10 μM. These results systematically showed the good biocompatibility of 2OA.
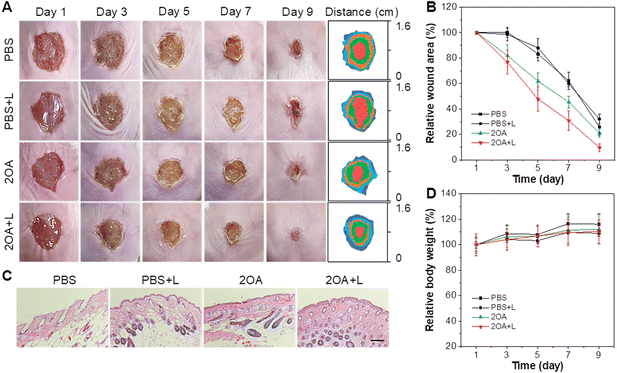
Combining the results of the in vitro antibacterial experiments and the biocompatibility assessments, we further evaluated the antibacterial activity of 2OA in vivo. An animal model with MRSA-infected wounds was established on the dorsal skin of mice. The mice were randomly divided into four groups, including PBS only (PBS group), PBS with white light irradiation (PBS + L group), 2OA only (2OA group), and 2OA with white light irradiation (2OA + L group). The photographs of the wounds during the healing process were recorded. As shown in Fig. 6A and B, the 2OA + L group maintained a faster rate of wound healing from day 3 onwards compared to the other groups. By day 9, the wounds in the 2OA + L group were almost completely healed, while the other groups still showed visible scabs. The hematoxylin and eosin (H&E) staining of wound tissue on day 9 showed that the epidermal and dermal layers of the wound tissue in the 2OA + L group were intact and thick, indicating better wound healing (Fig. 6C). Therefore, the in vivo experiments sufficiently demonstrated that 2OA could effectively treat wound infections caused by MRSA. In addition, the biosafety of 2OA was further assessed in the process of animal experiments. As depicted in Fig. S55 and S56,† the parameters of blood routine and blood biochemistry analysis for liver and kidney functions were within the normal range. Meanwhile, no obvious damage was found in all organs of the mice treated with 2OA (Fig. S57†). Besides, the body weight of the mice in each group showed minimal changes during the treatment process (Fig. 6D). These results powerfully evidenced the excellent biosafety of 2OA.
Conclusions
In summary, we proposed a facile strategy involving the manipulation of excited state electronic configurations using sulfur oxidation states to construct highly efficient organic type I PSs. Three D–A–D type AIEgens, DBTS, DBTSO, and DBTS2O, were designed and synthesized with different sulfur oxidation states. Therein, DBTS2O showed the most redshifted optical properties and the highest type I ROS generation efficiency. Notably, theoretical calculations uncovered deeper origins. A high sulfur oxidation state could significantly affect the electronic configurations of excited states by enhancing intramolecular D–A interactions. Higher sulfur oxidation states not only reduced ΔEST and increased the matching degree of transition configurations, but also increased the proportion of effective ISC, resulting in the excellent ROS generation ability of DBTS2O. According to this strategy, a highly water-soluble AIE PS (2OA) was synthesized. 2OA could selectively bind Gram-positive bacteria and provide high signal-to-noise ratio imaging. Moreover, the outstanding photokilling efficacy against Gram-positive bacteria in vitro and favorable biocompatibility have promoted the effective use of 2OA for treating MRSA-infected wounds. This work not only elucidates the structure–activity relationship of enhanced type I ROS production capacity caused by a high sulfur oxidation state, but also successfully develops a water-soluble AIE PS against Gram-positive bacteria guided by this strategy, which provides new ideas for the development of efficient organic type I PSs.Data availability
All data (experimental procedures and characterization) that support the findings of this study are available within the article and its ESI. Crystallographic data for DBTS, DBTSO, and DBTS2O have been deposited at the Cambridge Structural Database (CSD) of the Cambridge Crystallographic Data Centre (CCDC) with the CCDC numbers 2325250, 2325253, and 2325255 on the CSD website (https://www.ccdc.cam.ac.uk/).†Author contributions
Guoyu Jiang, Jianguo Wang and Ben Zhong Tang contributed to the conceptualization and supervision. Jianye Gong, Xiaopeng Wang, Weijing Zhang, Yifan Wu, Kai Li, Renmanduhu Sha, Lingxiu Liu, Chunbin Li and Lina Feng contributed to the methodology. Jianye Gong and Weijing Zhang contributed to the software. Jianye Gong, Guoyu Jiang and Jianguo Wang contributed to the original draft writing. Jianye Gong, Guoyu Jiang, Jianguo Wang and Ben Zhong Tang contributed to the review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22165020, 22161034, and 22371157), the Grassland Talent Program of Inner Mongolia Autonomous Region of China (12000-12102807), the Science and Technology Leading Talent Team in Inner Mongolia Autonomous Region (2022LJRC0001), the Science and Technology Program of Inner Mongolia (2021GG0154), the Program for Innovative Research Team in Universities of Inner Mongolia Autonomous Region (NMGIRT2324) and the Project of Innovation Research in Postgraduate in Inner Mongolia (B20231022Z). BALB/c mice (6–8 weeks old, average body weight 16–18 g) were purchased from SPF Biotechnology Co., Ltd (Beijing, China) and all animals received care in compliance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals. All procedures were approved by the Institutional Animal Care and Use Committee at the Inner Mongolia University (IMU-2022-mouse-047).Notes and references
- K. Chen, Y. Zhang, Y. Lei, W. Dai, M. Liu, Z. Cai, H. Wu, X. Huang and X. Ma, Nat. Commun., 2024, 15, 1269 CrossRef CAS PubMed.
- W. Dai, Y. Jiang, Y. Lei, X. Huang, P. Sun, J. Shi, B. Tong, D. Yan, Z. Cai and Y. Dong, Chem. Sci., 2024, 15, 4222–4237 RSC.
- H. Deng, G. Li, H. Xie, Z. Yang, Z. Mao, J. Zhao, Z. Yang, Y. Zhang and Z. Chi, Angew. Chem., Int. Ed., 2024, 63, e202317631 CrossRef CAS PubMed.
- Kenry, C. Chen and B. Liu, Nat. Commun., 2019, 10, 2111 CrossRef CAS PubMed.
- V.-N. Nguyen, Z. Zhao, B. Z. Tang and J. Yoon, Chem. Soc. Rev., 2022, 51, 3324–3340 RSC.
- P. Wang, B. Wu, M. Li, Y. Song, C. Chen, G. Feng, D. Mao, F. Hu and B. Liu, ACS Nano, 2023, 17, 10365–10375 CrossRef CAS.
- G. Jiang, J. Yu, J. Wang and B. Z. Tang, Aggregate, 2022, 3, e285 CrossRef CAS.
- C. Li, G. Jiang, J. Yu, W. Ji, L. Liu, P. Zhang, J. Du, C. Zhan, J. Wang and B. Z. Tang, Adv. Mater., 2023, 35, e2208229 CrossRef.
- M. Zheng, Q. Yang, C. Lu, X. Wu, W. Yan and D. Liu, Drug Discovery Today, 2023, 28, 103598 CrossRef CAS PubMed.
- M. Gao, J. Ren, Y. Gong, M. Fang, J. Yang and Z. Li, Aggregate, 2024, 5, e462 CrossRef CAS.
- F. Xiao, H. Gao, Y. Lei, W. Dai, M. Liu, X. Zheng, Z. Cai, X. Huang, H. Wu and D. Ding, Nat. Commun., 2022, 13, 186 CrossRef CAS.
- J. Fang, Z. Liu, J. Li, N. Zhuang, D. Yang, D. Ma, B. Z. Tang and Z. Zhao, Adv. Opt. Mater., 2023, 11, 2300835 CrossRef CAS.
- Y. Xiao, H. Wang, Z. Xie, M. Shen, R. Huang, Y. Miao, G. Liu, T. Yu and W. Huang, Chem. Sci., 2022, 13, 8906–8923 RSC.
- Y. Liu, M. Gu, Q. Ding, Z. Zhang, W. Gong, Y. Yuan, X. Miao, H. Ma, X. Hong, W. Hu and Y. Xiao, Angew. Chem., Int. Ed., 2023, 62, e202214875 CrossRef CAS.
- J. Sun and X. He, Aggregate, 2022, 3, e282 CrossRef CAS.
- S. Yang, J. Zhang, Z. Zhang, R. Zhang, X. Ou, W. Xu, M. Kang, X. Li, D. Yan, R. T. K. Kwok, J. Sun, J. W. Y. Lam, D. Wang and B. Z. Tang, J. Am. Chem. Soc., 2023, 145, 22776–22787 CrossRef CAS.
- Y. Yang, Q. Jiang and F. Zhang, Chem. Rev., 2023, 124, 554–628 CrossRef.
- D. Zhang, C. Li, G. Zhang, J. Tian and Z. Liu, Acc. Chem. Res., 2024, 57, 625–635 CAS.
- J. Gong, L. Liu, C. Li, Y. He, J. Yu, Y. Zhang, L. Feng, G. Jiang, J. Wang and B. Z. Tang, Chem. Sci., 2023, 14, 4863–4871 RSC.
- J. Guo, J. Dai, X. Peng, Q. Wang, S. Wang, X. Lou, F. Xia, Z. Zhao and B. Z. Tang, ACS Nano, 2021, 15, 20042–20055 CrossRef CAS.
- R.-N. Su, Q.-Q. Pan, G.-Y. Ding, J. Sun, L.-L. Wen, K.-Z. Shao, S.-B. Wang, G.-G. Shan and Z.-M. Su, J. Mater. Chem. C, 2023, 11, 9908–9915 RSC.
- C. Li, J. Liu, Y. Hong, R. Lin, Z. Liu, M. Chen, J. W. Y. Lam, G. H. Ning, X. Zheng, A. Qin and B. Z. Tang, Angew. Chem., Int. Ed., 2022, 61, e202202005 CrossRef CAS PubMed.
- K. X. Teng, D. Zhang, B. K. Liu, Z. F. Liu, L. Y. Niu and Q. Z. Yang, Angew. Chem., Int. Ed., 2024, 63, e202318783 CrossRef CAS.
- G. Zhang, X. Chen, X. Chen, K. Du, K. Ding, D. He, D. Ding, R. Hu, A. Qin and B. Z. Tang, ACS Nano, 2023, 17, 14800–14813 CrossRef CAS PubMed.
- Y. Wu, J. Li, Z. Shen, D. Wang, R. Dong, J. Zhang, Y. Pan, Y. Li, D. Wang and B. Z. Tang, Angew. Chem., Int. Ed., 2022, 61, e202212386 CrossRef CAS PubMed.
- G. Yang, J. S. Ni, Y. Li, M. Zha, Y. Tu and K. Li, Angew. Chem., Int. Ed., 2021, 60, 5386–5393 CrossRef CAS PubMed.
- Z. Zhang, M. Kang, H. Tan, N. Song, M. Li, P. Xiao, D. Yan, L. Zhang, D. Wang and B. Z. Tang, Chem. Soc. Rev., 2022, 51, 1983–2030 RSC.
- Q. Wang, C. Li, Y. Song, Q. Shi, H. Li, H. Zhong, J. Wang and F. Hu, Chem. Sci., 2023, 14, 684–690 RSC.
- T. C. Pham, V.-N. Nguyen, Y. Choi, S. Lee and J. Yoon, Chem. Rev., 2021, 121, 13454–13619 CrossRef CAS.
- H. Ma, Q. Peng, Z. An, W. Huang and Z. Shuai, J. Am. Chem. Soc., 2018, 141, 1010–1015 CrossRef.
- Z. Xu, Y. He, H. Shi and Z. An, SmartMat, 2022, 4, e1139 CrossRef.
- W. Zhao, Z. He, J. W. Y. Lam, Q. Peng, H. Ma, Z. Shuai, G. Bai, J. Hao and B. Z. Tang, Chem, 2016, 1, 592–602 CAS.
- Z. An, C. Zheng, Y. Tao, R. Chen, H. Shi, T. Chen, Z. Wang, H. Li, R. Deng, X. Liu and W. Huang, Nat. Mater., 2015, 14, 685–690 CrossRef CAS.
- J. Yu, H. Ma, W. Huang, Z. Liang, K. Zhou, A. Lv, X.-G. Li and Z. He, JACS Au, 2021, 1, 1694–1699 CrossRef CAS PubMed.
- C. D. Cruz, P. R. Christensen, E. L. Chronister, D. Casanova, M. O. Wolf and C. J. Bardeen, J. Am. Chem. Soc., 2015, 137, 12552–12564 CrossRef CAS PubMed.
- E. Varathan and A. Patnaik, J. Phys. Chem. A, 2019, 123, 8755–8765 CrossRef CAS PubMed.
- J. Yuan, Z. Xu and M. O. Wolf, Chem. Sci., 2022, 13, 5447–5464 RSC.
- P. R. Christensen, J. K. Nagle, A. Bhatti and M. O. Wolf, J. Am. Chem. Soc., 2013, 135, 8109–8112 CrossRef CAS.
- K.-M. Tong, J. Toigo, B. O. Patrick and M. O. Wolf, Inorg. Chem., 2023, 62, 13662–13671 CrossRef CAS.
- K. Yamamoto, W. Imai, S. Kanamori, K. Yamamoto and Y. Nakamura, J. Org. Chem., 2023, 88, 4003–4007 CrossRef CAS.
- J. Yuan, L. Jiang, T. Nishimura, E. R. Sauvé, D. Hean, K. Maeda and M. O. Wolf, J. Org. Chem., 2022, 87, 12315–12322 CrossRef CAS PubMed.
- Y. Wen, H. Liu, S.-T. Zhang, G. Pan, Z. Yang, T. Lu, B. Li, J. Cao and B. Yang, CCS Chem., 2021, 3, 1940–1948 CrossRef CAS.
- Z. Xu, C. Climent, C. M. Brown, D. Hean, C. J. Bardeen, D. Casanova and M. O. Wolf, Chem. Sci., 2021, 12, 188–195 RSC.
- C. Lefebvre, G. Rubez, H. Khartabil, J. C. Boisson, J. Contreras-Garcia and E. Henon, Phys. Chem. Chem. Phys., 2017, 19, 17928–17936 RSC.
- T. Lu and Q. Chen, J. Comput. Chem., 2022, 43, 539–555 CrossRef CAS.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS PubMed.
- M. A. Spackman and D. Jayatilaka, CrystEngComm, 2009, 11, 19–32 RSC.
- M. A. Spackman and J. J. McKinnon, CrystEngComm, 2002, 4, 378–392 CAS.
- P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka and M. A. Spackman, J. Appl. Crystallogr., 2021, 54, 1006–1011 CrossRef CAS PubMed.
- H. Yao, Y. Cui, D. Qian, C. S. Ponseca, A. Honarfar, Y. Xu, J. Xin, Z. Chen, L. Hong, B. Gao, R. Yu, Y. Zu, W. Ma, P. Chabera, T. Pullerits, A. Yartsev, F. Gao and J. Hou, J. Am. Chem. Soc., 2019, 141, 7743–7750 CrossRef CAS PubMed.
- T. Lu and Q. Chen, J. Mol. Model., 2020, 26, 315 CrossRef CAS PubMed.
- J. Zhang and T. Lu, Phys. Chem. Chem. Phys., 2021, 23, 20323–20328 RSC.
- Y. Cui, P. Zhu, H. Hu, X. Xia, X. Lu, S. Yu, H. Tempeld, R. A. Eichel, X. Liao and Y. Chen, Angew. Chem., Int. Ed., 2023, 62, e202304931 CrossRef CAS.
- L. Liu, C. Li, J. Gong, Y. Zhang, W. Ji, L. Feng, G. Jiang, J. Wang and B. Z. Tang, Angew. Chem., Int. Ed., 2023, 62, e202307776 CrossRef CAS PubMed.
- A. Ji, H. Lou, C. Qu, W. Lu, Y. Hao, J. Li, Y. Wu, T. Chang, H. Chen and Z. Cheng, Nat. Commun., 2022, 13, 3815 CrossRef CAS PubMed.
- C. Zhou, Z. Li, Z. Zhu, G. W. N. Chia, A. Mikhailovsky, R. J. Vázquez, S. J. W. Chan, K. Li, B. Liu and G. C. Bazan, Adv. Mater., 2022, 34, 2201989 CrossRef CAS.
- L. Feng, C. Li, L. Liu, X. Chen, G. Jiang, J. Wang and B. Z. Tang, Angew. Chem., Int. Ed., 2023, 62, e202212673 Search PubMed.
- C.-J. Wu, X.-Y. Li, T. Zhu, M. Zhao, Z. Song, S. Li, G.-G. Shan and G. Niu, Anal. Chem., 2022, 94, 3881–3887 CrossRef CAS.
- J. Liu, W. Zhang, X. Wang, Q. Ding, C. Wu, W. Zhang, L. Wu, T. D. James, P. Li and B. Tang, J. Am. Chem. Soc., 2023, 145, 19662–19675 CrossRef CAS PubMed.
- J. Li, G. Pan, G. V. Zyryanov, Y. Peng, G. Zhang, L. Ma, S. Li, P. Chen and Z. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 40864–40876 CrossRef CAS PubMed.
- P. Xiao, Z. Shen, D. Wang, Y. Pan, Y. Li, J. Gong, L. Wang, D. Wang and B. Z. Tang, Adv. Sci., 2021, 9, 2104079 CrossRef.
- Z. Pang, N. Ren, Y. Wu, J. Qi, F. Hu, Y. Guo, Y. Xie, D. Zhou and X. Jiang, Adv. Mater., 2023, 35, 2303562 CrossRef CAS PubMed.
- S. Shen, Y. Huang, A. Yuan, F. Lv, L. Liu and S. Wang, CCS Chem., 2021, 3, 129–135 CrossRef CAS.
- T. Li, Y. Wu, W. Cai, D. Wang, C. Ren, T. Shen, D. Yu, S. Qiang, C. Hu, Z. Zhao, J. Yu, C. Peng and B. Z. Tang, Adv. Sci., 2022, 9, 2202485 CrossRef CAS PubMed.
- Q. Guo, S. Xue, J. Feng, C. Peng, C. Zhou and Y. Qiao, Adv. Healthcare Mater., 2023, 12, 2300818 CrossRef CAS.
- Q. Li, Y. Li, T. Min, J. Gong, L. Du, D. L. Phillips, J. Liu, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams, R. T. K. Kwok, C. L. Ho, K. Li, J. Wang and B. Z. Tang, Angew. Chem., Int. Ed., 2019, 59, 9470–9477 CrossRef PubMed.
- X. Wu, M. Yang, J. S. Kim, R. Wang, G. Kim, J. Ha, H. Kim, Y. Cho, K. T. Nam and J. Yoon, Angew. Chem., Int. Ed., 2022, 61, e202200808 CrossRef CAS PubMed.
- E. Y. Yu, J. H. C. Chau, M. M. S. Lee, T. H. Koo, R. Lortz, J. W. Y. Lam, R. T. K. Kwok, Y. Li and B. Z. Tang, ACS Nano, 2024, 18, 1907–1920 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2325250, 2325253 and 2325255. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc03039g |
| This journal is © The Royal Society of Chemistry 2024 |