Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Heterogeneously catalyzed thioether metathesis by a supported Au–Pd alloy nanoparticle design based on Pd ensemble control†
Takehiro
Matsuyama
 a,
Takafumi
Yatabe
a,
Takafumi
Yatabe
 *ab,
Tomohiro
Yabe
*ab,
Tomohiro
Yabe
 a and
Kazuya
Yamaguchi
a and
Kazuya
Yamaguchi
 *a
*a
aDepartment of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: kyama@appchem.t.u-tokyo.ac.jp; yatabe@appchem.t.u-tokyo.ac.jp; Fax: +81-3-5841-7220
bPrecursory Research for Embryonic Science and Technology (PRESTO), Japan Science and Technology Agency (JST), 4-1-8 Honcho, Kawaguchi, Saitama 332-0012, Japan
First published on 26th June 2024
Abstract
C–S bond metathesis of thioethers has gained attention as a new approach to the late-stage diversification of already existing useful thioethers with molecular frameworks intact. However, direct or indirect thioether metathesis is scarcely reported, and heterogeneously catalyzed systems have not been explored. Here, we develop heterogeneously catalyzed direct thioether metathesis using a supported Au–Pd alloy nanoparticle catalyst with a high Au/Pd ratio. The Au-diluted Pd ensembles suppress the strong π-adsorption of diaryl thioethers on the nanoparticles and promote transmetalation via thiolate spill-over onto neighboring Au species, enabling an efficient direct thioether metathesis.
Introduction
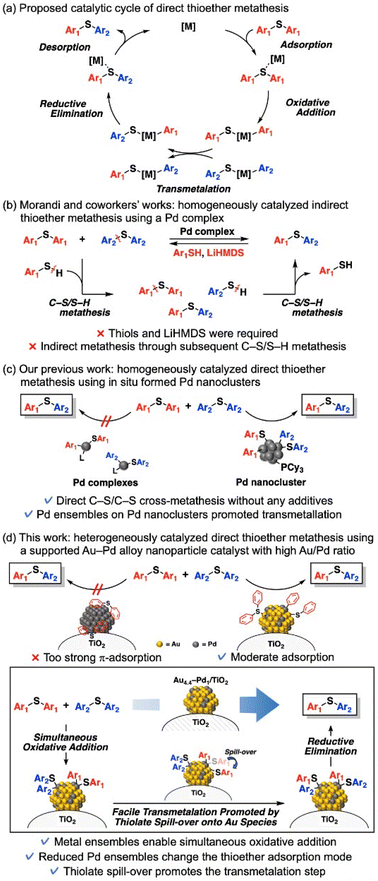
Multimetallic nanocatalysts can exhibit higher catalytic performance and/or selectivity than their monometallic counterparts. This high performance is usually attributed to three alloy effects—ensemble, ligand, and strain effects1—which are difficult to study in isolation. Nevertheless, according to some reports, the dominant effect is metal ensembles on the nanoparticle surfaces.1a The decreased ensembles of active metal species alter the adsorption configuration of molecules2 or exhibit a single atom-like character,3 resulting in unique catalytic properties. Such catalyst designs based on ensemble control are uniquely applicable to nanoparticle catalysts and have the potential to realize novel molecular transformations.Diaryl thioethers are widely used in polymers, natural products, bioactive compounds, and pharmaceuticals.4a–c Late-stage diversification of diaryl thioethers, which enables functionalization and transformation of complex molecules without disrupting their building blocks, has therefore become an important goal in synthetic organic chemistry, medicinal science, and materials science.4 One desirable approach is C–S bond metathesis of diaryl thioethers, which synthesizes novel diaryl thioethers from already existing useful thioethers. The first C–S bond metathesis of diaryl thioethers was reported by Morandi et al.,5a who also constructed porous organic polymers with two- and three-dimensional cores under mild reaction conditions (∼80 °C) using a homogeneous Pd complex catalyst.5b Theoretically, direct C–S bond metathesis of diaryl thioethers can proceed through the following catalytic cycle: adsorption and oxidative addition of thioethers to Pd species, transmetalation between oxidative adducts, and then reductive elimination and desorption of thioethers from Pd species (Scheme 1a). However, the direct metathesis does not occur in their reports5a,b possibly because the transmetalation step is prohibitively difficult on Pd complex catalysts. Thus, thiols and lithium bis(trimethylsilyl)amide are required for their reaction systems: indirect C–S/C–S cross-metathesis via C–S/S–H metathesis between diaryl thioethers and thiols (Scheme 1b).5 Quite recently, Audisio et al. reported Ni-catalyzed direct C–S/C–S cross-metathesis of various thioethers for isotope labelling without using thiols and bases;6 however, C–S bond metathesis of diaryl thioethers was not demonstrated. On the other hand, recently, we achieved the first example of direct C–S bond metathesis of diaryl thioethers using only Pd acetate and tricyclohexylphosphine (PCy3) as catalyst precursors.7 The active species of metathesis was confirmed as in situ-formed Pd nanoclusters, which likely enable direct crossover between two oxidative adducts (Scheme 1c). However, heterogeneously catalyzed C–S bond metathesis of diaryl thioethers, including indirect metathesis, is demanded for practical use and green sustainable chemistry but has not been attained. In fact, supported monometallic Pd nanoparticles hardly catalyze C–S bond metathesis between phenyl sulfide (1a) and p-tolyl sulfide (1b), even with PCy3 (Table S1†).7 Clearly, an additional catalyst design is required for heterogeneously catalyzed thioether metathesis.
This study proposes a heterogeneously catalyzed efficient direct C–S bond metathesis of diaryl thioethers using a TiO2-supported Au–Pd alloy nanoparticle catalyst (Au4.4–Pd1/TiO2) without any additives (Scheme 1d). This catalytic system exhibits a wide substrate scope and functional group tolerance; moreover, the catalyst can be reused a few times. Catalyst characterization and density functional theory (DFT) calculations of cluster models showed that when the Pd ensembles are diluted by Au alloying, the changed adsorption mode of thioethers on the nanoparticles lowers the adsorption/desorption energy and enables C–S bond metathesis. We also suggest that oxidative addition of thioethers produces thiolate species on the Pd species, which migrate to the Au species and promote the transmetalation step, thereby achieving efficient thioether metathesis.
Results and discussion
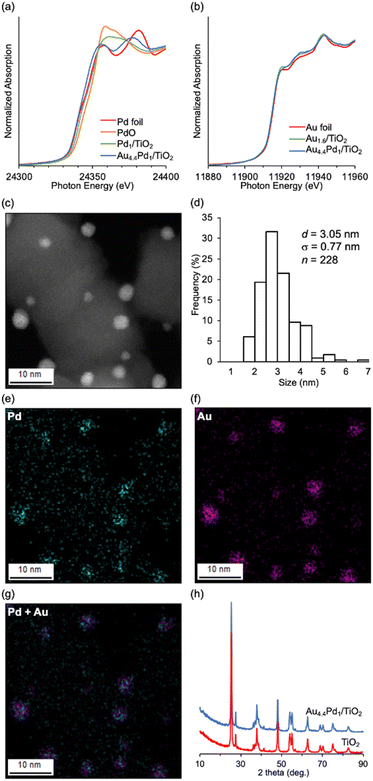
Au4.4–Pd1/TiO2 was prepared via simultaneous deposition–precipitation of Au and Pd hydroxides (Au/Pd molar ratio = 4.4) on TiO2, followed by reduction using NaBH4 in deionized water. The Pd K-edge and Au LIII-edge X-ray absorption near edge structure (XANES) spectra of Au4.4–Pd1/TiO2 were similar to those of Pd foil and Au foil, respectively (Fig. 1a and b), indicating zero valence of Pd and Au, as inferred from the Pd 3d and Au 4f X-ray photoelectron spectra of Au4.4–Pd1/TiO2 (Fig. S1†). From high-angle annular dark-field-scanning transmission electron microscopy (HAADF-STEM) images, the mean diameter of the TiO2-supported metal nanoparticles was determined as 3.05 nm (σ = 0.77 nm; Fig. 1c and d). From the almost coincident locations of Pd and Au species in the STEM-energy-dispersive spectroscopy (EDS) mapping of Au4.4–Pd1/TiO2 (Fig. 1e–g) and the fitting of the Pd K-edge and Au LIII-edge extended X-ray absorption fine structure spectra (indicating that the scatterings originated from Au–Pd bonds; see Fig. S2 and Table S2†), we inferred that Au–Pd alloy nanoparticles were supported on TiO2. Moreover, the X-ray diffraction (XRD) patterns of Au4.4–Pd1/TiO2 and TiO2 were comparable (Fig. 1h), confirming that the TiO2 structure was unchanged during the catalyst preparation.Table 1 lists the reaction conditions for investigating the effect of Pd-based supported nanoparticle catalysts on C–S bond metathesis between 1a and 1b. A quantitative metathesis can obtain two equivalents of phenyl p-tolyl sulfide (1ab) on the basis of 1b. Based on our previous report,7 we first investigated a monometallic TiO2-supported Pd nanoparticle catalyst but 1ab was hardly obtained (Table 1, entry 1).‡ Among several TiO2-supported Pd-based bimetallic nanoparticle catalysts, the Au–Pd alloy nanoparticle catalyst exhibited the highest catalytic performance for C–S bond metathesis (Table S3†). We therefore prepared supported Au–Pd alloy nanoparticle catalysts with various Au/Pd ratios (Aux–Pd1/TiO2, x: Au/Pd molar ratio). The catalytic performance of metathesis improved with increasing Au/Pd ratio in the catalyst, reaching a 1ab yield of 55% for Au4.4–Pd1/TiO2 (Table 1, entries 2–5). Au1.6/TiO2 hardly catalyzed the reaction and a physical mixture of Au1.6/TiO2 and Pd1/TiO2 did not improve the 1ab yield (Table 1, entries 6 and 7), indicating that Au–Pd alloy formation is essential for the present C–S bond metathesis. After optimizing the supports (Table S4†), solvents (Table S5†), reaction temperatures (Table S6†), and 1a/1b ratio (Table S7†), 1ab was efficiently produced in 77% yield (Table 1, entry 8).
| Entry | Catalyst | Conversion (%) | Yield (%) | |
|---|---|---|---|---|
| 1a | 1b | 1ab | ||
| a Conditions: 1a (0.5 mmol), 1b (0.1 mmol), catalyst (Pd: 2.5 mol%), xylene (2 mL), 120 °C, 3 h, Ar (1 atm). Conversions and yields were determined by GC. b Au1.6/TiO2 (Au: 4.0 mol%). c 140 °C, 24 h. | ||||
| 1 | Pd1/TiO2 | 9 | 11 | 1 |
| 2 | Au0.7–Pd1/TiO2 | 9 | 20 | 11 |
| 3 | Au1.4–Pd1/TiO2 | 14 | 36 | 25 |
| 4 | Au3.0–Pd1/TiO2 | 19 | 62 | 52 |
| 5 | Au4.4–Pd1/TiO2 | 18 | 66 | 55 |
| 6b | Au1.6/TiO2 | 10 | 8 | <1 |
| 7b | Pd1/TiO2 + Au1.6/TiO2 | <1 | 3 | 3 |
| 8c | Au4.4–Pd1/TiO2 | 21 | 84 | 77 |
The C–S bond metathesis of 1a and 1b immediately ceased after hot filtration of Au4.4–Pd1/TiO2 (Fig. S3†), and inductively coupled plasma-atomic emission spectroscopy (ICP-AES) detected almost zero Pd and Au species in the filtrate (Pd: below the detection limit, Au: 0.004% of the Au used in the reaction). Therefore, the observed catalysis was truly heterogeneous. Although the catalyst could be regenerated via calcination in an air followed by reduction with NaBH4 and reused twice without significant loss of the final yield (Fig. S4†), the final 1ab yield dropped at the 4th use. The average size of Au–Pd nanoparticles in the Au4.4–Pd1/TiO2 after the 1st use observed by HAADF-STEM (d = 3.70 nm, σ = 1.12 nm) was a little larger than that of the fresh catalyst (d = 3.05 nm, σ = 0.77 nm) (Fig. 1c, d, and S5†), and a new peak assigned to Au(111) appeared in the XRD pattern of the catalyst after the 4th reuse (Fig. S6†), indicating that the aggregation of Au–Pd nanoparticles is one reason for the deactivation of the catalytic activity.
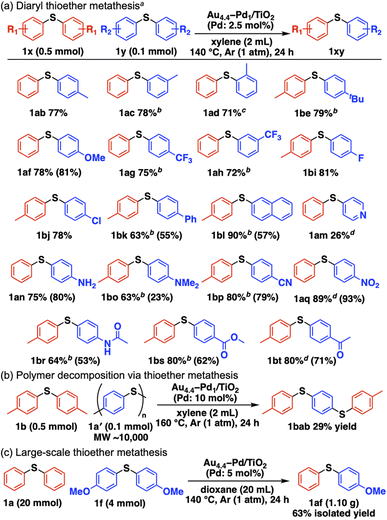
Scheme 2a summarizes the substrate scope of Au4.4–Pd1/TiO2-catalyzed C–S bond metathesis of diaryl thioethers.§ Symmetrical methyl-substituted thioethers at the para, meta, and ortho positions afforded the corresponding unsymmetrical thioethers in good yields (1ab–1ad). Other electron-donating groups, including 4-tert-butyl and 4-methoxy groups, also afforded their metathesis products (1be, 1af). This system is applicable to thioether metathesis with electron-withdrawing trifluoromethyl groups (1ag, 1ah) and halogenated thioethers with fluoro and chloro groups (1bi, 1bj). 4-Biphenyl-, 2-naphthyl-, and 4-pyridyl-substituted thioethers also served as competent metathesis partners (1bk, 1bl, 1am). Thioethers with amino (1an), N,N-dimethylamino (1bo), cyano (1bp), nitro (1aq), acetamide (1br), methyl ester (1bs), and acetyl (1bt) functional groups were converted into the corresponding unsymmetrical thioethers without disrupting the functional groups. Moreover, commercial polyphenylene sulfide (PPS, 1a′) decomposed into 1,4-bis[(4-methylphenyl)thio]benzene (1bab) via metathesis between 1a′ and 1b in the presence of Au4.4–Pd1/TiO2 (Scheme 2b). Gram-scale synthesis from 1a and 1f afforded the metathesis product 1af (1.10 g, 63% isolated yield) (Scheme 2c).
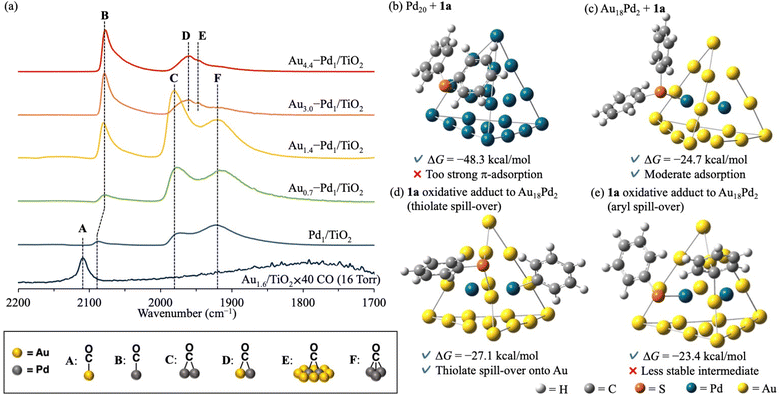
The effect of alloying on the present C–S bond metathesis of thioethers was determined from diffuse reflectance infrared Fourier transform (CO-DRIFT) spectra of adsorbed CO on Au1.6/TiO2, Pd1/TiO2, and Aux–Pd1/TiO2 (Fig. 2a). In the CO-DRIFT spectra of Pd1/TiO2, Au0.7–Pd1/TiO2, and Au1.4–Pd1/TiO2, which exhibited low catalytic activity for the C–S bond metathesis (Table 1, entries 1–3), three peaks around 2070, 1980, and 1920 cm−1 were observed assignable to linear (B), bridged (C), and three-fold (F) CO species on zero-valent Pd species, respectively.9 In the CO-DRIFT spectra of Au3.0–Pd1/TiO2 and Au4.4–Pd1/TiO2 showing high catalytic activity (Table 1, entries 4 and 5), peaks C and F were absent while new peaks at 1950 and 1940 cm−1 were attributable to CO species bridged on Au and Pd species (D) and on Pd species surrounded by Au species (E), respectively.9d As the Pd and Au–Pd alloy nanoparticles were similarly sized (Fig. 1c, d and S7†) and the peak of the CO species on Pd did not shift with increasing Au/Pd ratio in Aux–Pd1/TiO2 (Fig. 2a), we attributed the high catalytic activity of Au4.4–Pd1/TiO2 to the diluted Pd ensembles rather than to the ligand effect.
Next, the ensemble effect on the present C–S bond metathesis was investigated through DFT calculations on Pd20 and Au18Pd2 cluster models. Referring to previous reports, we adopted Gaussian 16 (M06 functional with SDD basis sets for Au and Pd and 6-31G(d,p) basis sets for H, C, and S)10 (see ESI for the calculation methods and model selection) (Fig. S8–S10).† The adsorption Gibbs energy of 1a was calculated at the center site of the Pd20 or Au18Pd2 cluster model.¶ The adsorption Gibbs energy of 1a on Pd20 is very high (ΔG = −48.3 kcal mol−1), indicating that the metathesis product cannot easily desorb and the reaction is strongly inhibited (Fig. 2b). In contrast, 1a adsorbed on Au18Pd2 has a moderate ΔG(−24.7 kcal mol−1) and the reaction can proceed (Fig. 2c). The optimized structure of 1a on Pd20 clarifies strong π-adsorption between 1a and the Pd20 facets, whereas 1a on Au18Pd2 adheres via coordination of its S atom to the Pd center with almost no π-adsorption. Corroborating this finding, the natural-bond orbital charge of the phenyl group is higher in Pd20-adsorbed 1a than in free 1a, probably due to π-back donation from the Pd species to the phenyl rings of 1a,11 but is lower in Au18Pd2–adsorbed 1a than in free 1a, possibly due to σ-donation from the S atom to the Pd atom (Fig. S11†). Facilitated by the weak π-back donation ability of the Au species,12 the Pd ensembles diluted with Au alloy changed the 1a adsorption mode and lowered the adsorption energy to enable the reaction (Scheme 1d).|| Moreover, as indicated in the optimized structure of 1a oxidative adducts to Au18Pd2 (Fig. S12 and S13†), the thiolate species produced by thioether oxidative addition on Pd species can transfer to the Au species without forming very stable structures (Fig. 2d), whereas the 1a oxidative adduct via aryl spill-over to Au species gives a slightly higher ΔG (Fig. 2e).¶ Considering the aforementioned indirect thioether metathesis on Pd complexes,5 thiolate spill-over onto the Au species probably promotes the transmetalation, enabling an efficient direct diaryl thioether metathesis with no additives (Scheme 1d).
Conclusions
In conclusion, we achieved the first heterogeneously catalyzed direct C–S bond metathesis of diaryl thioethers with a wide substrate scope and functional group tolerance using Au4.4–Pd1/TiO2. Catalyst characterization and DFT calculations revealed the likely causes of the high catalytic activity of Au4.4–Pd1/TiO2: moderate adsorption energy of thioethers on the Au–Pd alloy nanoparticles (conferred by diluted Pd ensembles) and thiolate spillover onto Au species (which promotes subsequent transmetalation). These findings are anticipated to guide the development of novel molecular transformations using multimetallic catalysts, especially by harnessing metal ensembles.Data availability
The data (experimental procedures and characterization data) that support this article is available within the article and its ESI.†Author contributions
T. Yatabe and K. Y. conceived and supervised the project. T. M. performed most of the experiments. T. Yabe performed the XAFS measurements. All authors contributed to data analysis and discussed the results. T. M. and T. Yatabe wrote the manuscript with feedback from K. Y. and T. Yabe.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by JSPS KAKENHI Grant No. 22H04971. This work was supported by JST, PRESTO Grant Number JPMJPR227A, Japan. A part of this work was conducted at the Advanced Characterization Nanotechnology Platform of the University of Tokyo, supported by “Nanotechnology Platform” of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. We thank Ms. Mari Morita (The University of Tokyo) for her assistance with the HAADF-STEM and EDS analyses. The computation was performed using Research Center for Computational Science, Okazaki, Japan (Project: 23-IMS-C002). T. M. was supported by the JSPS through the Research Fellowship for Young Scientists (Grant No. 23KJ0669).Notes and references
- (a) F. Gao and D. W. Goodman, Chem. Soc. Rev., 2012, 41, 8009 RSC; (b) H. Li, K. Shin and G. Henkelman, J. Chem. Phys., 2018, 149, 174705 CrossRef PubMed; (c) P. Liu and J. K. Nørskov, Phys. Chem. Chem. Phys., 2001, 3, 3814 RSC.
- H. Ishikawa, S. Yamaguchi, A. Nakata, K. Nakajima, S. Yamazoe, J. Yamasaki, T. Mizugaki and T. Mitsudome, JACS Au, 2022, 2, 419 CrossRef CAS PubMed.
- M. T. Greiner, T. E. Jones, S. Beeg, L. Zwiener, M. Scherzer, F. Girgsdies, S. Piccinin, M. Armbrüster, A. Knop-Gericke and R. Schlögl, Nat. Chem., 2018, 10, 1008 CrossRef CAS PubMed.
- (a) C.-F. Lee, Y.-C. Liu and S. S. Badsara, Chem.–Asian J., 2014, 9, 706 CrossRef CAS PubMed; (b) K. L. Dunbar, D. H. Scharf, A. Litomska and C. Hertweck, Chem. Rev., 2017, 117, 5521 CrossRef CAS PubMed; (c) E. A. Ilardi, E. Vitaku and J. T. Njardarson, J. Med. Chem., 2014, 57, 2832 CrossRef CAS PubMed; (d) J. Lou, Q. Wang, P. Wu, H. Wang, Y.-G. Zhou and Z. Yu, Chem. Soc. Rev., 2020, 49, 4307 RSC . For the reviews of synthesis of thioethers, see; (e) P. Chauhan, S. Mahajan and D. Enders, Chem. Rev., 2014, 114, 8807 CrossRef CAS PubMed; (f) T. Kondo and T. Mitsudo, Chem. Rev., 2000, 100, 3205 CrossRef CAS PubMed; (g) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS PubMed.
- (a) Z. Lian, B. N. Bhawal, P. Yu and B. Morandi, Science, 2017, 356, 1059 CrossRef CAS PubMed; (b) M. A. Rivero-Crespo, G. Toupalas and B. Morandi, J. Am. Chem. Soc., 2021, 143, 21331 CrossRef CAS PubMed; (c) B. N. Bhawal and B. Morandi, Angew. Chem., Int. Ed., 2019, 58, 10074 CrossRef CAS PubMed.
- B. Mouhsine, M. Norlöff, J. Ghouilem, A. Sallustrau, F. Taran and D. Audisio, J. Am. Chem. Soc., 2024, 146, 8343 CrossRef CAS PubMed.
- T. Matsuyama, T. Yatabe, T. Yabe and K. Yamaguchi, Catal. Sci. Technol., 2024, 14, 76 RSC.
- T. Matsuyama, T. Yatabe and K. Yamaguchi, Org. Biomol. Chem., 2024, 22, 579 RSC.
- (a) T. Matsuyama, T. Yatabe, T. Yabe and K. Yamaguchi, ACS Catal., 2022, 12, 13600 CrossRef CAS; (b) C.-W. Yi, K. Luo, T. Wei and D. W. Goodman, J. Phys. Chem. B, 2005, 109, 18535 CrossRef CAS PubMed; (c) X. Xu and D. W. Goodman, J. Phys. Chem., 1993, 97, 7711 CrossRef CAS; (d) B. Zhu, G. Thrimurthulu, L. Delannoy, C. Louis, C. Mottet, J. Creuze, B. Legrand and H. Guesmi, J. Catal., 2013, 308, 272 CrossRef CAS.
- (a) R. N. Dhital, C. Kamonsatikul, E. Somsook, K. Bobuatong, M. Ehara, S. Karanjit and H. Sakurai, J. Am. Chem. Soc., 2012, 134, 20250 CrossRef CAS PubMed; (b) X.-F. Lang, P.-G. Yin, T.-T. You and L. Guo, ChemPhysChem, 2012, 13, 237 CrossRef CAS PubMed; (c) R. Miyazaki, X. Jin, D. Yoshii, T. Yatabe, T. Yabe, N. Mizuno, K. Yamaguchi and J. Hasegawa, Catal. Sci. Technol., 2021, 11, 3333 RSC; (d) D. Takei, T. Yatabe, T. Yabe, R. Miyazaki, J. Hasegawa and K. Yamaguchi, JACS Au, 2022, 2, 394 CrossRef CAS PubMed.
- T. Wang, Y. Xu, J. Yang, X. Ju, W. Ding and Y. Zhu, ChemCatChem, 2019, 11, 3770 CrossRef CAS.
- (a) L. Ouyang, G.-J. Da, P.-F. Tian, T.-Y. Chen, G.-D. Liang, J. Xu and Y.-F. Han, J. Catal., 2014, 311, 129 CrossRef CAS; (b) J. H. Lee, J. Cho, M. Jeon, M. Ridwan, H. S. Park, S. H. Choi, S. W. Nam, J. Han, T.-H. Lim, H. C. Ham and C. W. Yoon, J. Mater. Chem. A, 2016, 4, 14141 RSC; (c) W. Wang, R. J. Lewis, B. Lu, Q. Wang, G. W. Hutchings, J. Xu and F. Deng, ACS Catal., 2024, 14, 2522 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc02732a |
| ‡ Previously, we reported slight thioether metathesis between 1a and 1b in the presence of a hydroxyapatite-supported mono-metallic Pd nanoparticle catalyst8 but under very different reaction conditions, using much more catalyst (Pd: 20 mol%) at a higher reaction temperature (160 °C) for 24 h. |
| § As for the products that are difficult to be isolated by column chromatography, GC charts after the reaction can be found in Fig. S14.† |
| ¶ The other adsorption sites are discussed in detail in the ESI.† |
| || Previously we identified in situ formed Pd nanoclusters as the active species of diaryl thioether metathesis.7 In that case, π-adsorption of diaryl thioethers on Pd nanoclusters was probably suppressed by moderate steric hindrance of the PCy3 ligand, enabling the metathesis to proceed. |
| This journal is © The Royal Society of Chemistry 2024 |