Open Access Article
Open Access ArticleSystemic regulation of binding sites in porous coordination polymers for ethylene purification from ternary C2 hydrocarbons†
Yi
Li
a,
Yanxin
Wu
a,
Jiaxin
Zhao
a,
Jingui
Duan
 *ab and
Wanqin
Jin
*ab and
Wanqin
Jin
 *a
*a
aState Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing Tech University, Nanjing 211816, China. E-mail: duanjingui@njtech.edu.cn; wqjin@njtech.edu.cn
bState Key Laboratory of Chemistry and Utilization of Carbon Based Energy Resources, College of Chemistry, Xinjiang University, Urumqi, 830017, China
First published on 22nd May 2024
Abstract
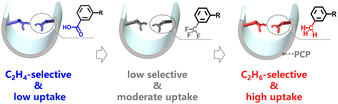
The global demand for poly-grade ethylene (C2H4) is increasing annually. However, the energy-saving purification of this gas remains a major challenge due to the similarity in molecular properties among the ternary C2 hydrocarbons. To address this challenge, we report an approach of systematic tuning of the pore environment with organic sites (from –COOH to –CF3, then to –CH3) in porous coordination polymers (PCPs), of which NTU-73-CH3 shows remarkable capability for the direct production of poly-grade C2H4 from ternary C2 hydrocarbons under ambient conditions. In comparison, the precursor structure of NTU-73-COOH is unable to purify C2H4, while NTU-73-CF3 shows minimal ability to harvest C2H4. This is because the changed binding sites in the NTU-73-series not only eliminate the channel obstruction caused by the formation of gas clusters, but also enhance the interaction with acetylene (C2H2) and ethane (C2H6), as validated by in situ crystallographic and Raman analysis. Our findings, in particular the systematic tuning of the pore environment and the efficient C2H4 purification by NTU-73-CH3, provide a blueprint for the creation of advanced porous families that can handle desired tasks.
Introduction
Ethylene (C2H4) is used as an important building block in industry for the production of value-added organics.1–3 Currently, C2H4 is mainly obtained by separating the downstream of cracking of naphtha, C2 hydrocarbon gas mixtures during the steam cracking process.4–6 Typically, acetylene (C2H2) is removed by solvent extraction or catalytic hydrogenation, which requires large equipment, as well as large amounts of solvents or higher temperature.7,8 Subsequently, ethane (C2H6) is removed through cryogenic distillation, a type of huge energy-consuming process.9The energy-intensive and cost-effective processes have spurred research into the development of energy-efficient approaches.10 Adsorptive separation, with a significant character of energy efficiency, has been considered as a kind of alternative or transition technology.11 Porous coordination polymers (PCPs),12–16 covalent organic frameworks (COFs),17,18 zeolites,19–21 and carbon materials22 have been explored for gas separation. Thanks to the rational pore tuning and straightforward pore functionalization, PCPs have shown good performance as adsorbents for various gas separations, but only a few examples can purify C2H4 from binary mixtures containing C2H2 or C2H6. However, from an energy point of view, it is crucial to remove both by-products in one-step with an adsorbent, but the challenge is enormous.23–25
In general, the preferential adsorption of C2H2 over C2H4 requires the pore to contain highly polar sites (such as inorganic anionic pillars and open metal sites) due to the larger quadrupole moment (7.2 × 10−26vs. 1.5 × 10−26 esu cm2) and higher acidity (pKa: 45 vs. 26) of C2H2, resulting in strong host–C2H2 electrostatic interactions.26 Conversely, the greater polarizability of C2H6 (44.7 × 10−25vs. 42.5 × 10−25 cm3) contributes to the relatively weak van der Waals interactions with the structural framework modified low polarity sites.27–30 To address this conflict, very few studies have been reported, primarily by exploiting the supramolecular interactions. The early framework of TJT-100 demonstrates the simultaneous capture of C2H2 and C2H6 through a hierarchy of interactions between the electronegative carboxylate O atoms and the gases.11 A similar interaction has also been observed in NPU-1.31 By incorporating the –NH2 group or exposing the N site, UiO-67-NH2 and Al-PyDC also show preferential adsorption of C2H2 and C2H6.32,33 Additionally, the cyclopentadiene-cobalt functional group in the UPC-series allows for efficient separation of C2 hydrocarbon mixtures. More promisingly, the synergistic sorbent separation technology (also called Lego-brick strategy) enables the production of high-purity C2H4 from a quaternary mixture of C2H6/C2H4/C2H2/CO2 by utilizing tandem packing of carefully selected PCPs, with each PCP acting as a separator for binary mixtures.25,34 Although these individual materials exhibit the desired functions, further systematic exploration is urgently needed due to the abundance and facile tunability of the supramolecular sites.
We are interested in the separation of light hydrocarbons using finely designed PCPs.35–39 Recently, we have reported a new family of PCPs with crab-like carboxylic pincers, which allows for high C2H2/C2H4 selectivity and an unprecedented ability to obtain high-purity forms of both gases.40 However, the strong binding interaction allows the absorbed C2H2 to form a tetrameric gas cluster at the channel neck, which strongly blocks the accessibility of the adjacent large cavity. Inspired by the unique porous nature of NTU-73 and also by the function of supramolecular sites, we report here a systematic tuning of functional sites in this porous platform, where the carboxylic pincers on the pore wall (corresponding L1: 3,5-di(1H-imidazol-1-yl)benzoic acid) were replaced with –CF3 (corresponding L2: 1,1′-(5-(trifluoromethyl)-1,3-phenylene)bis(1H-imidazole)) or –CH3 (corresponding L3: 1,1′-(5-methyl-1,3-phenylene)bis(1H-imidazole)) (Scheme 1). The highly stable NTU-73-CH3 exhibits a remarkable ability to directly produce poly-grade C2H4 from ternary C2 hydrocarbons, while, NTU-73-CF3 and NTU-73-COOH can only exhibit minimal capacity or are unable to achieve C2H4 purification at all. These results reflect the positive effect of changing functional sites on strengthening the host–guest interactions and the stability of the framework.
Experimental section
General procedures of the experiments and simulation are available in the ESI.†Synthesis of NTU-73-series
Results and discussion
Crystal structures and characterization
Solvent-thermal reactions of copper(II) hexafluorozirconium with corresponding ligands yielded polyhedron-shaped crystals (NTU-73-series). These crystals all crystallized in a tetragonal I4122 space group with the formula of [Cu(L)2ZrF6]·xGuest (Table S1†). Two of our newly synthesized crystals (NTU-73-CF3 and NTU-73-CH3) have the same coordination mode as that of NTU-73-COOH. The asymmetric unit of all three PCPs consists of one ligand, half a Cu2+ ion and half a ZrF62− anion. Each Cu node is coordinated by two F atoms from the two ZrF62− anions and four imidazole N atoms from four ligands (Fig. S3–S5†). Additionally, two helical chains linked by ZrF62− anions are present in the frameworks (Fig. S6†). Differently, the carboxylic-modified channel wall observed from two different directions in NTU-73-COOH has been replaced by a couple of –CF3 or –CH3 groups in NTU-73-CF3 and NTU-73-CH3 (Fig. S7–S12†), respectively. Furthermore, there is an overall trend of increasing pore size (NTU-73-COOH: 4.5 × 5.2 Å2 and 3.9 × 5.6 Å2; NTU-73-CF3: 3.7 × 5.2 Å2 and 4.2 × 7.8 Å2; NTU-73-CH3: 4.4 × 5.4 Å2 and 6.2 × 9.2 Å2) as the functional group size gradually decreases (Fig. 1). Considering the unique ability of NTU-73-COOH to separate C2H2/C2H4 and the systematic changes in the pore environment, including binding sites and pore size, we expected this platform to be highly efficient for the C2 ternary mixture separation. The phase purity of the crystals was confirmed by powder X-ray diffraction (PXRD) as the diffraction peaks of the synthesized and activated NTU-73-series were in good agreement with the simulated patterns (Fig. S13–S15†). | ||
| Fig. 1 Structure of the NTU-73-series: local views of the nanospace of NTU-73-COOH (a and d), NTU-73-CF3 (b and e) and NTU-73-CH3 (c and f) in different directions. | ||
Pore evaluation
The permanent porosity of the NTU-73-series was investigated through N2 (77 K) and CO2 (195 K) adsorption measurements (Fig. 2a and S22†). Although NTU-73-COOH exhibits negligible N2 uptake, the two newly prepared PCPs show improved N2 adsorption, with maximum uptakes of 193 and 295 cm3 g−1, respectively. Interestingly, all three PCPs show type-I and significant CO2 adsorption isotherms. The maximum uptake increases from 63 (NTU-73-COOH) to 250 cm3 g−1 (NTU-73-CH3). For consistency, the Brunauer–Emmett–Teller surface areas of the NTU-73-series were calculated based on CO2 isotherms. The BET surface areas of the NTU-73-series were calculated to be 228, 889 and 1078 m2 g−1, respectively, with corresponding pore volumes of 0.039, 0.153 and 0.185 m3 g−1 (Table S2†). Additionally, the pore size distribution is consistent with the crystal structures, verifying the impact of the functional groups on pore size tuning (Fig. S23†).Single-component adsorption and selectivity
Single-component adsorption isotherms of C2H2, C2H4 and C2H6 were collected for the three PCPs (Fig. 2b–d and S24–S35†), respectively. With the carboxylate pincers, exposed in the nanochannel, NTU-73-COOH shows the lowest gas uptake (C2H2: 32.3 cm3 g−1, C2H4: 12.9 cm3 g−1 and C2H6: 11.8 cm3 g−1) among the three PCPs. Not only that, the adsorption capacity of C2H4 is slightly higher than that of C2H6, indicating an impossible harvesting of pure C2H4 in one-step from the ternary C2 mixtures, although it shows promising ability in binary C2H2/C2H4 separation. After replacing the –COOH groups with –CF3 groups, NTU-73-CF3 shows a significantly increased gas uptake at 100 kPa (C2H2: 77.7 cm3 g−1, C2H4: 62.0 cm3 g−1, and C2H6: 62.7 cm3 g−1), in which the C2H6 uptake is slightly higher than that of C2H4. Comparing the structural nature of the above two, the decrease in the polarity of the pore surface could alter the sequence of host–C2H4 and –C2H6 interactions. As expected, the adsorption capacities of C2 gases show a further increase in NTU-73-CH3 (C2H2: 98.0 cm3 g−1; C2H4: 77.8 cm3 g−1; C2H6: 89.9 cm3 g−1), particularly, the C2H6 uptake is higher than that of C2H4 (12.1 cm3 g−1, 100 kPa, 298 K). Notably, this uptake difference is much higher than that of the benchmark materials of MOF-303 (−0.5 cm3 g−1),41 NPU-1 (6.7 cm3 g−1),31 TJT-100 (5.8 cm3 g−1),11 and is approaching that of Al-PyDC (17.0 cm3 g−1, 296 K)33 and UPC-612 (17.5 cm3 g−1)6 (Table S3†). In addition, despite the gradual decrease in pore channel polarity, C2H2 uptake is always higher than that of C2H4, about 20.0 cm3 g−1, in the NTU-73-series (Fig. 2e and S36†). In other words, systematically tuned organic sites endow significant functional changes, where NTU-73-CH3 is expected to achieve efficient C2H4 purification from C2 ternary mixtures.Static adsorption selectivity and isosteric heats
To further evaluate the gas separation performance, the adsorption selectivities of C2H6/C2H4 and C2H2/C2H4 at 298 K were calculated via the ideal adsorbed solution theory (IAST) after fitting the single-component adsorption isotherms to the Langmuir–Freundlich equation (Fig. S37–S49†). For the equimolar C2H6/C2H4 mixtures, the adsorption selectivity values were found to be 0.66 for NTU-73-COOH, 1.18 for NTU-73-CF3 and 1.33 for NTU-73-CH3. Importantly, the selectivity of NTU-73-CH3 is higher than that of TJT-100 (1.2) and NPU-1 (1.32), and close to that of UPC-612 (1.4), MOF-303 (1.7), Al-PyDC (1.9, 296 K) under almost the same conditions (Table S3†). Additionally, among the top-tier (C2H6–C2H4 uptake difference larger than 10 cm3 g−1) C2H2–C2H6-selective adsorbents, the C2H2/C2H4 (1/99, v/v) selectivity (3.2 at 298 K, 100 kPa) of NTU-73-CH3 is only lower than that of Al-PyDC (4.3 at 296 K, 100 kPa) (Table S4†). Therefore, the high C2H6–C2H4 uptake difference and the good selectivities make NTU-73-CH3 a promising candidate for C2H4 purification from C2 ternary mixtures.To evaluate the interaction strength of C2H2, C2H4 and C2H6 in the NTU-73-series, the isosteric heat (Qst) was calculated after fitting the adsorption isotherms by the virial equation at 273, 283 and 298 K, respectively (Fig. 2f and S50–S59†). The Qst value of C2H2 (NTU-73-COOH: 41.00 kJ mol−1, NTU-73-CF3: 39.83 kJ mol−1, NTU-73-CH3: 37.06 kJ mol−1) is higher than that of C2H4 (NTU-73-COOH: 35.78 kJ mol−1, NTU-73-CF3: 32.12 kJ mol−1, NTU-73-CH3: 29.90 kJ mol−1) and C2H6 (NTU-73-COOH: 26.10 kJ mol−1, NTU-73-CF3: 32.39 kJ mol−1, NTU-73-CH3: 35.43 kJ mol−1) in the NTU-73-series at low coverage, while an inversion of the host–C2H4 and host–C2H6 interactions was observed: a higher Qst of C2H4 over C2H6 in NTU-73-COOH changes to a higher Qst of C2H6 over C2H4 in NTU-73-CH3. These observations are consistent with the adsorption isotherms. In addition, these relatively low adsorption enthalpy values indicate a relatively low energy consumption for regeneration of the PCPs.
Gas-loaded crystallographic and Raman analysis
The isosteric heat is a kind of statistical result, making it difficult to accurately describe the role of functional sites. Therefore, to better explore the reversal phenomenon of C2H4 and C2H6 adsorption and Qst values, gas-loaded crystallographic analysis was performed at 298 K (Fig. 3 and Table S5†).42 Despite the disordered nature, the trapped gases can be clearly found in these three PCPs. For NTU-73-COOH⊃C2H4, two C2H4 molecules form three hydrogen bonds with the OCOOH of the free carboxylic pincers, of which, the shortest distance is 1.8973 Å. Meanwhile, intermolecular interactions (dCC2H4–H⋯CC2H4: 2.3748 to 2.8025 Å) have been found between these two molecules (Fig. 3a). For NTU-73-COOH⊃C2H6, two groups of hydrogen bonds (dCC2H6–H⋯OCOOH: 2.3338 to 2.9779 Å and g CC2H6–H⋯Nimidazole) with relatively longer distances were observed. In addition, the slightly larger distance leads to the disappearance of the intermolecular interactions (Fig. 3b). This clear change can therefore explain the fact that NTU-73-COOH binds C2H4 more strongly than C2H6. However, in contrast to our expectation, the distance between the gases and the –CF3 group is relatively far. So as that, both molecules are located around the coordinated ZrF62− ions. This is due to the relatively weak degree of electronegativity of FCF3 compared to that of FZrF6. However, the presence of the –CF3 group influences the configuration of the adsorbed gas molecules. As can be seen, the two F atoms of ZrF62− form two hydrogen bonds with C2H4 and C2H6, respectively. The shortest distances of the hydrogen bonds (dCC2H6–H⋯FZrF6: 2.6094 Å and dCC2H4–H⋯FZrF6: 2.6647 Å) are very close to each other, indicating similar host–guest interactions (Fig. 3c and d). In NTU-73-CH3, both gases are also observed around the coordinated ZrF62− ions. Without the withdrawal effect of the organic F sites from –CF3, the configuration of the adsorbed C2H4 shows a slight change, wherein the distance of the two hydrogen bonds (CC2H4–H⋯FZrF6) becomes close to each other (2.9352 Å and 2.9775 Å) (Fig. 3e). Additionally, the end-on mode of CC2H6–H⋯FZrF6 in NTU-73-CF3 changes to a chelate connection in NTU-73-CH3; particularly, the distance of the two CC2H6–H⋯FZrF6 (2.1757 Å and 2.3150 Å) is shorter than that of CC2H4–H⋯FZrF6 in NTU-73-CF3 (Fig. 3f). Therefore, these results not only explain the phenomenon of the adsorption trend of C2H4 and C2H6 in NTU-73-series, but also can strengthen the molecular insights of fine-tuning the pore environment by functional organic groups. | ||
| Fig. 3 C2H4- and C2H6-loaded crystallographic analysis of NTU-73-COOH (a and b), NTU-73-CF3 (c and d) and NTU-73-CH3 (e and f). | ||
Given the promising nature of NTU-73-CH3, the stabilizing effect of the pore environment towards C2H4 and C2H6 was further evaluated by Raman spectra. After dosing a highly pure gas (He, C2H4 or C2H6, 1 bar, 298 K) into the glass tube with fully activated NTU-73-CH3, respectively, the corresponding spectra were recorded in the range of 1000 to 4000 cm−1 (Fig. 4 and S60†). As can be seen, the peaks at 2887 cm−1 and 2942 cm−1 belonging to the stretching vibration of C2H6 have been identified,43 but no additional peaks other than those belonging to NTU-73-CH3 were observed under the C2H4 atmosphere (theoretical position: 1689 cm−1). Therefore, it is clear that NTU-73-CH3 not only has a relatively stronger host–C2H6 interaction, but also can stabilize a certain number of C2H6 molecules in a periodic arrangement, which is consistent with the results of the crystallographic analysis.
Breakthrough experiments
To validate the separation performance under dynamic conditions, breakthrough experiments were conducted on the NTU-73-series (Fig. 5 and S61–S69†). All initially activated samples were loaded into the column and further activation was achieved by He sweeping until no signals were detected. Breakthrough experiments were then carried out using a binary mixture of C2H2/C2H4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v) at first, a typical industrial composition. Compared to NTU-73-COOH, both NTU-73-CF3 and NTU-73-CH3 showed a certain loss of breakthrough time interval, but still demonstrated the separation ability towards C2H2/C2H4. Further experiments were conducted on C2H6/C2H4 mixtures. At a volume ratio of 1
99, v/v) at first, a typical industrial composition. Compared to NTU-73-COOH, both NTU-73-CF3 and NTU-73-CH3 showed a certain loss of breakthrough time interval, but still demonstrated the separation ability towards C2H2/C2H4. Further experiments were conducted on C2H6/C2H4 mixtures. At a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, C2H6 breaks through slightly earlier than C2H4 for the NTU-73-COOH sample bed. Interestingly, high purity C2H4 can be obtained directly at the outlet of the NTU-73-CF3 and NTU-73-CH3 columns, of which, NTU-73-CH3 has a longer C2H6–C2H4 breakthrough interval than NTU-73-CF3 (4.8 min g−1vs. 4.0 min g−1). To further investigate the influence of ratio on this separation, a feed gas with a ratio of 1
15, C2H6 breaks through slightly earlier than C2H4 for the NTU-73-COOH sample bed. Interestingly, high purity C2H4 can be obtained directly at the outlet of the NTU-73-CF3 and NTU-73-CH3 columns, of which, NTU-73-CH3 has a longer C2H6–C2H4 breakthrough interval than NTU-73-CF3 (4.8 min g−1vs. 4.0 min g−1). To further investigate the influence of ratio on this separation, a feed gas with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, the most common ratio found in the cracking process, was introduced into the column. NTU-73-COOH was unable to separate this mixture, while NTU-73-CF3 and NTU-73-CH3 retained their separation abilities. Among them, NTU-73-CH3 showed the longest breakthrough time interval (4.6 min g−1vs. 2.7 min g−1vs. 0 min g−1), owing to its higher selectivity for C2H6/C2H4 and its greater uptake capacity for C2H6 (Fig. 5a–c). This trend was also observed in experiments using equimolar C2H6/C2H4 mixtures.
9, the most common ratio found in the cracking process, was introduced into the column. NTU-73-COOH was unable to separate this mixture, while NTU-73-CF3 and NTU-73-CH3 retained their separation abilities. Among them, NTU-73-CH3 showed the longest breakthrough time interval (4.6 min g−1vs. 2.7 min g−1vs. 0 min g−1), owing to its higher selectivity for C2H6/C2H4 and its greater uptake capacity for C2H6 (Fig. 5a–c). This trend was also observed in experiments using equimolar C2H6/C2H4 mixtures.
Encouraged by the successful separation of the binary mixtures, C2H2/C2H4 and C2H6/C2H4, the separation ability of the NTU-73-series towards ternary mixtures C2H2/C2H6/C2H4 (1/9/90, v/v/v) was further investigated, respectively. As expected, both C2H6 and C2H4 elute out simultaneously from the NTU-73-COOH bed (Fig. 5d). Despite the separation potential, high-yield C2H4 cannot be produced by NTU-73-CF3 (Fig. 5e). Only NTU-73-CH3 allows significant production of high purity (>99.9%) C2H4 (0.52 mmol g−1 (STP)) (Fig. 5f and S70†). This value is higher than that of CAU-23 (0.18 mmol g−1), TJT-100 (0.41 mmol g−1), and UPC-612 (0.47 mmol g−1), but lower than that of MOF-303 (1.35 mmol g−1) and Azole-Th-1 (1.34 mmol g−1) (Tables S3 and S4†).
Stability and recyclability
Stability is another crucial factor in measuring the performance of adsorbents.44 The crystals of NTU-73-COOH, NTU-73-CF3 and NTU-73-CH3 were immersed in chemical solutions (pH = 2, pH = 7 and pH = 12) for 3 days or exposed to air for 7 days, respectively. The PXRD patterns of the samples were then collected and compared with the simulated patterns (Fig. S71–S73†). The results reveal that some of the characteristic peaks of NTU-73-COOH and NTU-73-CF3 were weakened or disappeared after immersion in certain solutions, in particular, the diffraction peaks almost completely disappeared in the alkaline solution (pH = 12), suggesting the structural instability. However, the crystals of NTU-73-CH3 retained almost the same diffraction peaks after treatment under these harsh conditions. Additionally, the C2H6 uptakes of these treated samples are nearly the same (Fig. S74†), indicating that the introduction of the hydrophobic –CH3 group plays a crucial role in protecting the coordination bonds and then stabilizing the framework. Thanks to this good stability, NTU-73-CH3 demonstrates no loss of performance during the cycling breakthrough experiments (Fig. S75†).Conclusions
In response to the demand for C2H4 purification, we herein report an approach of systematic tuning of the porous environment with organic sites (from –COOH to –CF3 and then to –CH3) in PCPs, wherein the highly stable NTU-73-CH3 exhibits remarkable capability for direct production of poly-grade C2H4 from ternary C2 hydrocarbons under ambient conditions. In addition, the molecular insights derived from in situ crystallographic and Raman analysis confirm the positive effect of the –CH3 group in tuning the configuration and strength of the adsorbed C2H4 and C2H6. This study not only highlights the importance of systemic regulation, but also illustrates how changes in functional groups within PCPs can profoundly affect the host–guest interaction and separation performance. Moreover, the strategy of systematic organic functionalization can be extended to other porous families, offering a powerful tool for tailoring high-performance PCPs for desired applications.Data availability
All data can be found in the main text and ESI.†Author contributions
J. D. and W. J. conceived the idea of this work. Y. L. carried out the experiments and analyzed the results. J. D. and Y. L. wrote the paper. All authors gave valuable suggestions for the final draft.Conflicts of interest
The authors declare no competing interests.Acknowledgements
We are thankful for the financial support of the National Natural Science Foundation of China (22171135), the National Natural Science Foundation of Jiangsu Province (BK20231269) and the State Key Laboratory of Materials-Oriented Chemical Engineering (SKL-MCE-23A18).References
- S. Gong, C. Shao and L. Zhu, Energy, 2021, 217, 119401 CrossRef CAS.
- S. M. Sadrameli, Fuel, 2016, 173, 285–297 CrossRef CAS.
- G. D. Wang, Y. Z. Li, W. J. Shi, L. Hou, Y. Y. Wang and Z. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202205427 CrossRef CAS PubMed.
- Z. B. Bao, D. Y. Xie, G. G. Chang, H. Wu, L. Y. Li, W. Zhou, H. L. Wang, Z. G. Zhang, H. B. Xing, Q. W. Yang, M. J. Zaworotko, Q. L. Ren and B. L. Chen, J. Am. Chem. Soc., 2018, 140, 4596–4603 CrossRef CAS PubMed.
- Q. Ding, Z. Zhang, Y. Liu, K. Chai, R. Krishna and S. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202208134 CrossRef CAS PubMed.
- Y. T. Wang, C. L. Hao, W. D. Fan, M. Y. Fu, X. K. Wang, Z. K. Wang, L. Zhu, Y. Li, X. Q. Lu, F. N. Dai, Z. X. Kang, R. M. Wang, W. Y. Guo, S. Q. Hu and D. F. Sun, Angew. Chem., Int. Ed., 2021, 60, 11350–11358 CAS.
- F. Studt, F. Abild-Pedersen, T. Bligaard, R. Z. Sorensen, C. H. Christensen and J. K. Norskov, Science, 2008, 320, 1320–1322 CrossRef CAS PubMed.
- Z. Xu, X. Xiong, J. Xiong, R. Krishna, L. Li, Y. Fan, F. Luo and B. Chen, Nat. Commun., 2020, 11, 3163–3172 CrossRef CAS.
- K. Z. Su, W. J. Wang, S. F. Du, C. Q. Ji and D. Q. Yuan, Nat. Commun., 2021, 12, 3703–3710 CrossRef CAS PubMed.
- J.-W. Cao, S. Mukherjee, T. Pham, Y. Wang, T. Wang, T. Zhang, X. Jiang, H.-J. Tang, K. A. Forrest, B. Space, M. J. Zaworotko and K.-J. Chen, Nat. Commun., 2021, 12, 6507–6515 CrossRef CAS PubMed.
- H. G. Hao, Y. F. Zhao, D. M. Chen, J. M. Yu, K. Tan, S. Q. Ma, Y. Chabal, Z. M. Zhang, J. M. Dou, Z. H. Xiao, G. Day, H. C. Zhou and T. B. Lu, Angew. Chem., Int. Ed., 2018, 57, 16067–16071 CrossRef CAS PubMed.
- Z. Q. Zhang, S. B. Peh, Y. X. Wang, C. J. Kang, W. D. Fan and D. Zhao, Angew. Chem., Int. Ed., 2020, 59, 18927–18932 CrossRef CAS PubMed.
- S. H. Yang, A. J. Ramirez-Cuesta, R. Newby, V. Garcia-Sakai, P. Manuel, S. K. Callear, S. I. Campbell, C. C. Tang and M. Schroder, Nat. Chem., 2015, 7, 121–129 CrossRef CAS PubMed.
- P. Q. Liao, W. X. Zhang, J. P. Zhang and X. M. Chen, Nat. Commun., 2015, 6, 8697–8706 CrossRef PubMed.
- C. Gu, N. Hosono, J. J. Zheng, Y. Sato, S. Kusaka, S. Sakaki and S. Kitagawa, Science, 2019, 363, 387–391 CrossRef CAS PubMed.
- Q. Ding, Z. Q. Zhang, C. Yu, P. X. Zhang, J. Wang, X. L. Cui, C. H. He, S. G. Deng and H. B. Xing, Sci. Adv., 2020, 6, 122617 Search PubMed.
- C. H. He, Y. Wang, Y. Chen, X. Q. Wang, J. F. Yang, L. B. Li and J. P. Li, ACS Appl. Mater. Interfaces, 2020, 12, 52819–52825 CrossRef CAS PubMed.
- L. C. Jiang, Y. Y. Tian, T. Sun, Y. L. Zhu, H. Ren, X. Q. Zou, Y. H. Ma, K. R. Meihaus, J. R. Long and G. S. Zhu, J. Am. Chem. Soc., 2018, 140, 15724–15730 CrossRef CAS PubMed.
- Y. Z. Liu, Y. Wu, W. W. Liang, J. J. Peng, Z. Li, H. H. Wang, M. J. Janik and J. Xiao, Chem. Eng. Sci., 2020, 220, 115636 CrossRef CAS.
- P. J. Bereciartua, A. Cantin, A. Corma, J. L. Jorda, M. Palomino, F. Rey, S. Valencia, E. W. Corcoran, P. Kortunov, P. I. Ravikovitch, A. Burton, C. Yoon, Y. Wang, C. Paur, J. Guzman, A. R. Bishop and G. L. Casty, Science, 2017, 358, 1068–1071 CrossRef CAS PubMed.
- Y. C. Chai, X. Han, W. Y. Li, S. S. Liu, S. K. Yao, C. Wang, W. Shi, I. Da-Silva, P. Manuel, Y. Q. Cheng, L. D. Daemen, A. J. Ramirez-Cuesta, C. C. Tang, L. Jiang, S. H. Yang, N. J. Guan and L. D. Li, Science, 2020, 368, 1002–1006 CrossRef CAS PubMed.
- W. W. Liang, Y. Wu, H. Y. Xiao, J. Xiao, Y. W. Li and Z. Li, Chem.–Eur. J., 2018, 64, 3390–3399 CAS.
- C. Xiao, J. D. Tian, Q. H. Chen and M. C. Hong, Chem. Sci., 2024, 15, 1570–1610 RSC.
- P. Zhang, Y. Zhong, Y. Zhang, Z. Zhu, Y. Liu, Y. Su, J. Chen, S. Chen, Z. Zeng, H. Xing, S. Deng and J. Wang, Sci. Adv., 2022, 8, eabn9231 CrossRef CAS PubMed.
- K. J. Chen, D. G. Madden, S. Mukherjee, T. Pham, K. A. Forrest, A. Kumar, B. Space, J. Kong, Q. Y. Zhang and M. J. Zaworotko, Science, 2019, 366, 241–246 CrossRef CAS PubMed.
- Q. Ding, Z. Q. Zhang, Y. L. Liu, K. G. Chai, R. Krishna and S. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202208134 CrossRef CAS PubMed.
- M. Kang, S. Yoon, S. Ga, D. W. Kang, S. Han, J. H. Choe, H. Kim, D. W. Kim, Y. G. Chung and C. S. Hong, Adv. Sci., 2021, 8, 2004940 CrossRef CAS.
- M. J. Kang, D. W. Kang, J. H. Choe, H. Kim, D. W. Kim, H. Park and C. S. Hong, Chem. Mater., 2021, 33, 6193–6199 CrossRef CAS.
- J. Y. Pei, J. X. Wang, K. Shao, Y. Yang, Y. J. Cui, H. Wu, W. Zhou, B. Li and G. D. Qian, J. Mater. Chem. A, 2020, 8, 3613–3620 RSC.
- R. B. Lin, H. Wu, L. B. Li, X. L. Tang, Z. Q. Li, J. K. Gao, H. Cui, W. Zhou and B. L. Chen, J. Am. Chem. Soc., 2018, 140, 12940–12946 CrossRef CAS PubMed.
- B. Y. Zhu, J. W. Cao, S. Mukherjee, T. Pham, T. Zhang, T. Wang, X. Jiang, K. A. Forrest, M. J. Zaworotko and K. J. Chen, J. Am. Chem. Soc., 2021, 143, 1485–1492 CrossRef CAS PubMed.
- X. W. Gu, J. X. Wang, E. Y. Wu, H. Wu, W. Zhou, G. D. Qian, B. L. Chen and B. Li, J. Am. Chem. Soc., 2022, 144, 2614–2623 CrossRef CAS PubMed.
- E. Wu, X.-W. Gu, D. Liu, X. Zhang, H. Wu, W. Zhou and G. Qian, Nat. Commun., 2023, 14, 6146–6157 CrossRef CAS PubMed.
- H. R. Sun, F. Q. Chen, R. D. Chen, J. Q. Li, L. D. Guo, Y. Liu, F. X. Shen, Q. W. Yang, Z. G. Zhang, Q. L. Ren and Z. B. Bao, Small, 2023, 19, 2208182 CrossRef CAS PubMed.
- Q. Dong, X. Zhang, S. Liu, R. B. Lin, Y. Guo, Y. Ma, A. Yonezu, R. Krishna, G. Liu, J. Duan, R. Matsuda, W. Jin and B. Chen, Angew. Chem., Int. Ed., 2020, 59, 22756–22762 CrossRef CAS PubMed.
- Q. Dong, Y. Huang, K. Hyeon-Deuk, I. Y. Chang, J. Wan, C. Chen, J. Duan, W. Jin and S. Kitagawa, Adv. Funct. Mater., 2022, 32, 2203745 CrossRef CAS.
- Y. Huang, J. Wan, T. Pan, K. Ge, Y. Guo, J. Duan, J. Bai, W. Jin and S. Kitagawa, J. Am. Chem. Soc., 2023, 145, 24425–24432 CrossRef CAS PubMed.
- J. Wan, H. L. Zhou, K. Hyeon-Deuk, I. Y. Chang, Y. Huang, R. Krishna and J. Duan, Angew. Chem., Int. Ed., 2023, 62, e202316792 CrossRef CAS PubMed.
- H. Wang, Y. Duan, Y. Wang, Y. Huang, K. Ge, S. Wang, B. Zheng, Z. Wang, J. Bai and J. Duan, ACS Appl. Mater. Interfaces, 2022, 14, 13550–13559 CrossRef CAS PubMed.
- Y. F. Duan, Y. H. Huang, C. Q. Wang, Q. Wang, K. Ge, Z. Y. Lu, H. J. Wang, J. G. Duan, J. F. Bai and W. Q. Jin, Chem. Sci., 2023, 14, 4605–4611 RSC.
- H.-M. Wen, C. Yu, M. Liu, C. Lin, B. Zhao, H. Wu, W. Zhou, B. Chen and J. Hu, Angew. Chem., Int. Ed., 2023, 62, e202309108 CrossRef CAS PubMed.
- Q. Dong, Y. Huang, J. Wan, Z. Lu, Z. Wang, C. Gu, J. Duan and J. Bai, J. Am. Chem. Soc., 2023, 145, 8043–8051 CrossRef CAS PubMed.
- R. Fantoni, K. Vanhelvoort, W. Knippers and J. Reuss, Chem. Phys., 1986, 110, 1–16 CrossRef CAS.
- Q. B. Dong, J. M. Wan, H. H. Chen, Y. H. Huang and J. G. Duan, ACS Appl. Mater. Interfaces, 2023, 15, 39606–39613 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of the three crystals, PXRD, TGA, IR, sorption isotherms, IAST, breakthrough experiments and fitting parameters. CCDC 2337168–2337175. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc02659d |
| This journal is © The Royal Society of Chemistry 2024 |