Open Access Article
Open Access ArticleZinc chloride-catalyzed Grignard addition reaction of aromatic nitriles†
Manabu
Hatano
 *a,
Kisara
Kuwano
a,
Riho
Asukai
a,
Ayako
Nagayoshi
a,
Haruka
Hoshihara
a,
Tsubasa
Hirata
*a,
Kisara
Kuwano
a,
Riho
Asukai
a,
Ayako
Nagayoshi
a,
Haruka
Hoshihara
a,
Tsubasa
Hirata
 a,
Miho
Umezawa
b,
Sahori
Tsubaki
b,
Takeshi
Yoshikawa
a,
Miho
Umezawa
b,
Sahori
Tsubaki
b,
Takeshi
Yoshikawa
 *b and
Ken
Sakata
*b and
Ken
Sakata
 *b
*b
aFaculty of Pharmaceutical Sciences, Kobe Pharmaceutical University, Higashinada, Kobe 658-8558, Japan. E-mail: mhatano@kobepharma-u.ac.jp
bFaculty of Pharmaceutical Sciences, Toho University, Miyama, Funabashi, Chiba 274-8510, Japan. E-mail: takeshi.yoshikawa@phar.toho-u.ac.jp; ken.sakata@phar.toho-u.ac.jp
First published on 13th May 2024
Abstract
In the alkyl addition reaction of aromatic nitriles using Grignard reagents, ketones are formed after hydrolysis. However, this addition reaction is often slow compared to that using reactive organolithium(I) reagents. In this study, we improved the reaction by using zinc(II)ates, which are generated in situ using Grignard reagents and zinc chloride (ZnCl2) as a catalyst. As a result, the corresponding ketones and amines were obtained via hydrolysis and reduction, respectively, in good yields under mild reaction conditions. Scale-up reactions are also demonstrated. Interestingly, using a catalytic amount of ZnCl2 was more effective than using a stoichiometric amount of zinc(II)ates. Possible transition states are proposed on the basis of the active zinc(II)ate species, and DFT calculations were carried out to elucidate a plausible reaction mechanism.
Introduction
Aromatic nitriles are versatile compounds that are widely used in the synthesis of natural products, pharmaceuticals, agrochemicals, and industrial materials.1 Nitriles are not only equivalents of carbonyl compounds, but also a valuable source of nitrogen, and they are easily transformed into amines, amidines, imino esters, triazines, amides, carboxylic acids, ketones, and aldehydes. Due to the low reactivity of aromatic nitriles, traditional organometallic reagents, such as highly reactive organolithium(I) compounds,2 have often been used for the conversion of aromatic nitriles to alkylphenones. Although organomagnesium(II) reagents, i.e., Grignard reagents,3 have also been used for this purpose, reflux conditions are usually required because Grignard reagents are much less reactive than organolithium(I) reagents (Scheme 1a).4 Moreover, α-deprotonation from imide intermediates to produce enamines often results in byproducts during long reactions. In the quest for more reactive alternatives to Grignard reagents, Cu(I) catalysts were found to promote the reaction;5 however, heating conditions are still required. Subsequently, innovative methods involving solvent-free conditions,6 aqueous conditions,7 2-methyltetrahydrofuran,8 and flow-system conditions have been investigated with respect to their potential to replace Cu(I) catalyst systems. In this context, we have previously reported a highly effective zinc chloride (ZnCl2)-catalyzed Grignard addition reaction of ketones under mild reaction conditions (Scheme 1c),9,10 which stands in contrast to the noncatalyzed Grignard addition reactions of ketones that usually result in the formation of byproducts and the recovery of starting materials (Scheme 1b). Remarkably, since zinc(II)ates derived from ZnCl2 and Grignard reagents have stronger nucleophilicity than basicity, alkyl transfer may proceed rather than the α-deprotonation of ketones that usually occurs when using Grignard reagents alone. Accordingly, we envisioned that ZnCl2 could promote the Grignard addition reaction of aromatic nitriles under mild reaction conditions (Scheme 1d). Considering that Grignard reagents are relatively stable and commercially available, using Grignard reagents instead of relatively unstable organolithium(I) reagents for the alkyl addition to aromatic nitriles would be preferable both from a laboratory and industrial perspective. Herein, we report the ZnCl2-promoted Grignard addition reaction of aromatic nitriles, which affords the corresponding ketones and amines.Results and discussion
We started our investigation by examining the addition of n-butyl lithium(I) (n-BuLi) and magnesium(II) reagents (1.1–3.3 equiv.) to benzonitrile 1a (1 equiv.) in the presence or absence of ZnCl2 (0–1.1 equiv.) in tetrahydrofuran (THF) (Table 1). In the absence of ZnCl2, the reaction using n-BuLi was completed within 1 h at −78 °C, and the corresponding ketone (3aa) was obtained in >99% yield after routine hydrolysis with aqueous HCl (entry 1). In contrast, the reaction using n-BuMgCl (2a), which is much less reactive than n-BuLi, did not proceed at −78 °C (entry 2). Unexpectedly, the combined use of 2a (3.3 equiv.) and ZnCl2 (1.1 equiv.), which can be expected to give the corresponding reactive zinc(II)ate (1.1 equiv.) in situ, did not promote the reaction at −78 °C (entry 3). However, the reaction proceeded at 25 °C to give 3aa in 77% yield after 3 h (entry 4). Interestingly, reducing the amount of ZnCl2 (0.2 equiv., 20 mol%) was more effective than using a stoichiometric amount of ZnCl2 (1.1 equiv.), and 3aa was obtained in an improved yield of 88% (entry 5). Furthermore, the amount of 2a could be reduced from 3.3 to 2.3 equiv., affording almost the same yield (87%; entry 6). Finally, by extending the reaction time to 6 h, 3aa was obtained in 95% yield (entry 7). Using n-BuMgBr (2a′) instead of 2a afforded 3aa in 93% yield (entry 8). For practical use, commercially available 0.5 M ZnCl2 in THF and non-hygroscopic dichloro(N,N,N′,N′-tetramethylethylenediamine)zinc(II) (ZnCl2(tmeda))11 can be used in place of ZnCl2, which provided 3aa in 91% and 87% yield, respectively (entries 9 and 10). In contrast to inexpensive ZnCl2, which is hygroscopic and requires melt-drying in vacuo before use, these forms of zinc(II) species are very convenient because they can be used without pre-treatment. We also examined the reactions of 1a with 2a or 2a′ alone as control experiments under the optimized conditions, which furnished 3aa in 18–55% yield (entries 11–14). Overall, the addition of ZnCl2 was found to effectively promote the Grignard addition reaction of aromatic nitrile 1a.| Entry | Reagents | Conditions | Yield (%) of 3aab |
|---|---|---|---|
| a The reaction was carried out using PhCN (1a; 2 mmol), n-BuMX (2.2–6.6 mmol), and ZnCl2 (0–2.2 mmol) in THF at −78 °C or 25 °C for 1–6 h, unless otherwise noted. b Isolated yield. c 0.5 M ZnCl2 in THF was used. d Dichloro(N,N,N′,N′-tetramethylethylenediamine)zinc(II) was used instead of ZnCl2. | |||
| 1 | n-BuLi (1.1 equiv.) | −78 °C, 1 h | >99 |
| 2 | n-BuMgCl (2a) (1.1 equiv.) | −78 °C, 1 h | 0 |
| 3 | 2a (3.3 equiv.) + ZnCl2 (1.1 equiv.) | −78 °C, 1 h | 1 |
| 4 | 2a (3.3 equiv.) + ZnCl2 (1.1 equiv.) | 25 °C, 3 h | 77 |
| 5 | 2a (3.3 equiv.) + ZnCl2 (0.2 equiv.) | 25 °C, 3 h | 88 |
| 6 | 2a (2.3 equiv.) + ZnCl2 (0.2 equiv.) | 25 °C, 3 h | 87 |
| 7 | 2a (2.3 equiv.) + ZnCl2 (0.2 equiv.) | 25 °C, 6 h | 95 |
| 8 | n-BuMgBr (2a′) (3.3 equiv.) + ZnCl2 (0.2 equiv.) | 25 °C, 6 h | 93 |
| 9c | 2a (3.3 equiv.) + ZnCl2 in THF (0.2 equiv.) | 25 °C, 6 h | 91 |
| 10d | 2a (3.3 equiv.) + ZnCl2(tmdea) (0.2 equiv.) | 25 °C, 6 h | 87 |
| 11 | 2a (1.1 equiv.) | 25 °C, 3 h | 18 |
| 12 | 2a (2.3 equiv.) | 25 °C, 3 h | 33 |
| 13 | 2a (2.3 equiv.) | 25 °C, 6 h | 54 |
| 14 | 2a′ (2.3 equiv.) | 25 °C, 6 h | 55 |
With the optimized reaction conditions in hand, various aromatic nitriles (1) and Grignard reagents (2) were investigated in the presence of 20 mol% of ZnCl2 catalyst (Scheme 2). The primary alkyl Grignard reagents 2b (Me), 2c (Et), 2d (i-Bu), 2e (Bn), 2f (BnCH2), and 2g (n-octyl) could be successfully applied to the reaction of 1a, and the corresponding products (3ab–ag) were obtained in improved yield compared with the reactions without ZnCl2 catalyst (for details, see brackets b in Scheme 2). It is noteworthy that in the reactions giving 3af and 3ag, the amount of ZnCl2 could be reduced from 20 to 10 mol% and that of the Grignard reagent could be reduced from 2.3 to 1.3 equiv. In particular, 3ad, 3af, and 3ag were obtained in drastically improved yield, since 2d, 2f, and 2g are less reactive than 2a–c and 2e. A sterically hindered and therefore less reactive secondary alkyl Grignard reagent (2h; c-pentyl), also furnished the corresponding product (3ah) in improved yield (83%) compared to the 19% yield obtained in the absence of ZnCl2. Next, 1a was replaced with 1b–d in order to examine the effect of a simple methyl substituent at the p-, m-, or o-position of the benzonitrile. Even for the sterically demanding 1d, which contains an o-Me substituent, the reactions proceeded smoothly in the presence of ZnCl2 catalyst to produce 3ba–da in 86–90% yield. The electron-donating methyl substituent probably decreases slightly the reactivity, and the yields of the reactions in the absence of the ZnCl2 catalyst were lower (21–44%) than in the case of 1a (54%). In this context, the reactions of reactive 1e with an electron-withdrawing p-CF3-substituent and of less reactive 1f with an electron-donating p-MeO-substituent gave contrasting results; the noncatalyzed reaction with 2a alone gave 3ea in 73% yield and 3fa in 15% yield, whereas 3ea and 3fa were obtained in quantitative yields (>99%) in the presence of ZnCl2. In addition, substrate 1g with a 2-naphthyl moiety, which would be less favored than 1a due to steric and electronic reasons, was suitable for the present catalytic reaction, and the desired product (3ga) was obtained in 90% yield.
A ZnCl2-catalyzed double alkyl addition to isophthalonitrile (1h) with 2a was also carried out (eqn (1)), which afforded the desired double adduct (3ha′) in 92% yield; this stands in sharp contrast to the noncatalyzed version of the reaction (28% yield). Interestingly, the single alkyl adduct 3ha was not obtained when using 2a alone. This implies that a coordinating reagent at the adjacent imido group in pre-3ha would promote the alkyl transfer even without Zn(II) activation; thus, pre-3ha would be easily converted to pre-3ha′. For additional information, aliphatic nitriles were investigated instead of aromatic nitriles. However, even under ZnCl2-catalyzed conditions, α-deprotonation of aliphatic nitriles occurred, resulting in almost complete recovery of substrates and/or α-addition to another nitrile (e.g., aldol-type products). For example, the reaction of isobutyronitrile 1i with 2f afforded the desired product (3if) in merely 20% yield, whereas the noncatalyzed reaction gave 3if in 11% yield (eqn (2)). In addition, using phenyl-magnesium(II) reagent 2i instead of the alkylmagnesium(II) reagents in the reaction with 4-chlorobenzonitrile (1j) (eqn (3)) afforded 3ji in 54% yield irrespective of the presence or absence of ZnCl2. Although the reason is not clear at present, the failure to generate the corresponding triphenyl-zinc(II)ate and/or its low nucleophilicity could explain this result. Overall, the reactions with aliphatic nitriles and arylmagnesium(II) reagents are a limitation of the present ZnCl2 catalysis.
 | (1) |
 | (2) |
 | (3) |
Next, encouraged by the results of the reaction giving 3ah in Scheme 2, the isopropyl addition to aromatic nitriles (1) with i-PrMgCl (2j; 2.3 equiv.) in the presence of ZnCl2 was examined, since reactions of secondary alkyl Grignard reagents are often sluggish due to steric effects (Scheme 3). For aryl or heteroaryl nitriles, the ZnCl2-catalyzed reactions smoothly furnished the desired i-Pr-adducts (3aj–fj and 3jj–mj) in 70–99% yield. These results stand in sharp contrast to those obtained in the absence of ZnCl2 (see brackets b in Scheme 3).12 It is also noteworthy that the differences in yield in the presence or absence of ZnCl2 are larger than the difference in yield when using primary alkyl Grignard reagents (Scheme 2). It should furthermore be noted that halogen moieties are tolerated, and that 2-fluorobenzonitrile (1m) and 4-chlorobenzonitrile (1n) can be used. Unfortunately, the reaction with 3-bromobenzonitrile (1n) did not proceed well because a magnesium–halogen exchange reaction occurred exclusively,13 and the corresponding protonated product PhCN (1a) was recovered in 96% yield.
Next, we examined the synthesis of amines via reduction using sodium borohydride (NaBH4) instead of the routine hydrolysis under acidic conditions with aqueous HCl (Scheme 4). After alkylation of 1a with 2a in the presence of ZnCl2, MeOH was added at 0 °C and the mixture was stirred for 10 min. Since unprotected imine 4aa would be unstable, the residue obtained after rapid extraction with diethyl ether and water followed by concentration of the organic phase was treated with NaBH4 in MeOH at 60 °C for 1 h. As a result, α-n-butylbenzylamine 5aa was obtained in 89% yield, together with α-n-butylbenzylalcohol 6aa in 3% yield as a byproduct. The reduction of hydrolysis product 3aa derived from imine intermediate 4aa afforded 6aa. Reaction products derived from 1a and 2b or 2j also provided the corresponding amine 5ab in 75% yield or 5aj in 83% yield. In addition, when p-, m-, or o-Me substituted benzonitriles 1b–d were used with 2a instead of 1a, the corresponding amines (5ba, 5ca, and 5da) were obtained in 83–94% yield.
To demonstrate the synthetic utility of this catalytic reaction, some scale-up reactions were conducted (Scheme 5). The ZnCl2-catalyzed reaction of 10 mmol (1.768 g) of 4-chlorobenzonitrile (1j) with i-PrMgCl (2j) proceeded smoothly even when using reduced amounts of both 2j (from the usual 2.3 to 1.3 equiv.) and ZnCl2 (from the usual 20 to 10 mol%), and the desired ketone (3jj) was obtained in quantitative yield (1.826 g) (Scheme 5a). In addition, the ZnCl2-catalyzed reaction of 10 mmol (1.031 g) of PhCN (1a) with n-octylMgCl (2g; 1.3 equiv.) proceeded smoothly in the presence of 10 mol% of ZnCl2. After a routine workup procedure, the resulting ketone (3ag) was used without purification for the subsequent ZnCl2 (10 mol%)-catalyzed alkylation reaction of 3ag with 2j (1.3 equiv.) (Scheme 5b). As a result, the corresponding tertiary alcohol (7) was obtained in 98% yield (2.580 g) over two steps based on 1a. ZnCl2 was very effective in both steps shown in Scheme 5b, whereas the product yield was low in the absence of ZnCl2 (see brackets in Scheme 5; 24% and 15% yield, respectively).
 | ||
| Scheme 5 Scalable ZnCl2-catalyzed alkyl addition reactions of aromatic nitriles and sequential alkyl addition to a ketone. | ||
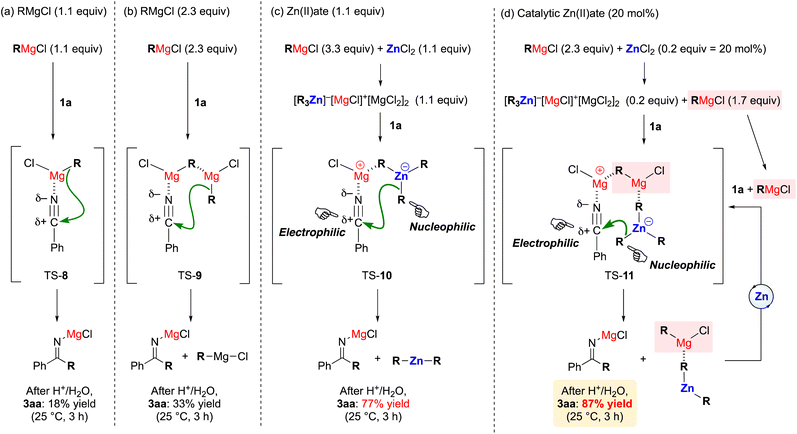
Subsequently, we turned our attention to mechanistic aspects, and a plausible reaction mechanism is proposed in Fig. 1. In particular, Fig. 1a–d show the key results shown in Table 1, entries 11, 12, 4, and 6. In the case of 1.1 equiv. of n-BuMgCl (2a) alone (Fig. 1a), the nitrogen of the nitrile moiety would coordinate to the Mg(II) center, and the alkyl group (R = n-Bu) would attack the carbon atom of the nitrile moiety (TS-8). This would result in a slow reaction (18% yield) due to the relatively large distance between the nucleophilic R carbon atom and the electrophilic nitrile carbon atom. Meanwhile, in the case of 2.3 equiv. of 2a alone (Fig. 1b), two molecules of 2a would disproportionate and dimerize (TS-9).3 As a result, the attack distance would be shortened and the yield slightly improved (33% yield). Furthermore, in the case of 3.3 equiv. of 2a and 1.1 equiv. of ZnCl2, which would produce 1.1 equiv. of the zinc(II)ate in situ (Fig. 1c), the Mg(II) center could be positively ionized and the Zn(II) center negatively ionized (TS-10).14 This would greatly enhance both the nucleophilicity of the R carbon atom and the electrophilicity of the nitrile carbon atom, improving the yield in the case of Fig. 1c (77% yield), albeit the attack distance would not change compared to the case shown in Fig. 1b. Finally, in the case of 2.3 equiv. of 2a and 20 mol% of ZnCl2 (Fig. 1d), there would be an excess of 2a given that the amount of zinc(II)ate depends on the amount of ZnCl2 (20 mol%), which would integrate with the zinc(II)ate to form a trimer complex with two Mg(II) and one Zn(II) centers, to which the nitrile moiety could coordinate (TS-11). This trimeric structure can be also envisaged for the possible structures shown in Fig. 1b and c. Again, it is worth noting that the Mg(II) center would be positively ionized and the Zn(II) center would be negatively ionized in this structure. Moreover, the distance between the nucleophilic R carbon atom and the electrophilic nitrile carbon atom would be shortened. Therefore, despite using a catalytic amount of ZnCl2, the yield was further improved (87% yield). After the alkyl transfer, the trimer complex with two Mg(II) and one Zn(II) centers would be regenerated in the presence of an excess of 2a, thus closing the catalytic cycle.
As shown in Fig. 2, chloro-bridged TS-11′ could also be envisaged instead of TS-11. Moreover, an alternative geminal pathway via TS-12 or a vicinal pathway via TS-11 or TS-11′ cannot be ruled out. Therefore, to gain more insight into the reaction mechanism and to discriminate between the possible transition states, DFT calculations were conducted for the methyl addition to 1a with 2b using a catalytic amount of ZnCl2 in THF. In general, solutions of Grignard reagents contain a variety of chemical molecules, such as MeMgCl, Me2Mg, and MgCl2, under the Schlenk equilibrium (eqn (4)).
| 2MeMgCl ⇌ Me2Mg + MgCl2 | (4) |
Therefore, the formal reactant MeMgCl is a condensed representation of numerous mono-, di-, and polynuclear species that coexist in the Schlenk equilibrium. The crystal structure obtained by adding ZnCl2 also suggests the formation of a more complex structure.14 In addition, two mechanistic possibilities, i.e., nucleophilic polar and radical mechanisms, have long been discussed (Fig. 3).15 Although the mechanism of the Grignard addition reaction of nitriles is difficult to elucidate in detail, some theoretical studies on the mechanism of Grignard addition reactions of aldehydes and ketones have already been reported.16–18 In computational studies on the Schlenk equilibrium, one of the pioneering studies on the Grignard reactions of carbonyl compounds with CH3MgCl suggested that dinuclear species are more reactive than mononuclear species in a nucleophilic polar addition process.17 In this regard, in the nucleophilic polar mechanism, the interaction between the vicinal Mg–CH3 moiety and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moiety, i.e., a four-center interaction, facilitates the addition of the methyl group (Fig. 3a).
O moiety, i.e., a four-center interaction, facilitates the addition of the methyl group (Fig. 3a).
Moreover, in studies concerned with explicit solvent models,18 molecular-dynamics simulations have shown that the Mg(II) centers of mono- and dinuclear species of CH3MgCl can accommodate a variable number of solvent molecules in their first coordination spheres. In particular, for the Grignard addition reactions of aldehydes and ketones in THF, the possible pathways were examined on the basis of the Schlenk equilibrium for the mono- and dinuclear species, which showed that the conformational space including the explicit treatment of THF was important.18 Indeed, THF molecules can effectively and flexibly bind to Mg(II) centers, thus stabilizing the nucleophilic polar pathway by compensating for the lack of chemical bonds on the Mg(II) centers. In addition, it has been noted that the radical reaction cannot occur unless a substrate with a low-lying π* (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) orbital, such as in fluorenone but not benzaldehyde, coordinates to the Mg(II) center.18
O) orbital, such as in fluorenone but not benzaldehyde, coordinates to the Mg(II) center.18
According to these previous studies,16–18 we comprehensively examined in this study the ZnCl2-catalyzed Grignard addition reaction between benzonitrile (1a) and CH3MgCl (2b) explicitly including THF (eqn (5); for computational details, see Section 10 in the ESI†). Since the orbital energy of the π* orbital in benzonitrile (−1.25 eV) is slightly higher than that of benzaldehyde (−1.52 eV), the radical reaction pathway can be considered unlikely; instead, we focused on the nucleophilic polar mechanism.
 | (5) |
Fig. 4 shows the reaction pathways examined for the model Grignard addition reaction of benzonitrile (1a) with mono- and dinuclear species of CH3MgCl (2b). In complex I, the mononuclear Grignard species CH3MgCl(THF)2 is coordinated to 1a. The methyl carbon atom in the Grignard species is 4.81 Å away from the nitrile carbon atom. A nucleophilic attack of the methyl carbon atom on the nitrile carbon atom would give the corresponding imine complex Vvia transition state TS(I–V) (Fig. S4†). The activation energy of TS(I–V) from I is relatively high (21.77 kcal mol−1), although it is lower than that of the transition state without any THF molecules, i.e., TS(I–V)0 (29.26 kcal mol−1; Fig. S2†). This indicates that the coordination of THF to the Grignard reagent plays an important role in this reaction. The pathway viaTS(I–V) corresponds to that via TS-8 shown in Fig. 1a.
Next, we examined the reaction with dinuclear Grignard species in the presence of a large amount of Grignard reagents. Addition of one more CH3MgCl(THF) molecule to I gives a dinuclear reactant species with a bridged structure, i.e., IIg (ΔG = −1.25 kcal mol−1) or IIv (ΔG = −0.93 kcal mol−1). In IIg, two Mg(II) centers are bridged by one chloride ion and one THF molecule. The distance between the carbon atom in the geminal methyl group and the nitrile carbon atom is 3.76 Å, which is shorter than that in I (4.81 Å). This suggests that the geminal methyl group in IIg is more prone to attack the nitrile group than that in I. It should also be noted here that the ΔG of the transition state for the attack of the methyl group on the nitrile carbon to afford VIg, i.e., TS(IIg–VIg), is 16.75 kcal mol−1 (geminal pathway in Fig. 2), which is lower than that of TS(I–V) for the mononuclear Grignard species. Elimination of the unreacted Grignard species CH3MgCl(THF) from VIg gives V. Meanwhile, in IIv, two Mg(II) centers are bridged by one chloride ion and one methyl group. The bridging methyl group in IIv could easily migrate to the other Mg(II) center via transition state  (ΔG = 1.67 kcal mol−1) to give
(ΔG = 1.67 kcal mol−1) to give  (ΔG = 1.65 kcal mol−1); thus, IIv and
(ΔG = 1.65 kcal mol−1); thus, IIv and  are in equilibrium. From IIv, the attack of the vicinal methyl group on the nitrile carbon atom would proceed via transition state TS(IIv–VIv), where the geminal methyl group transfers to the other Mg(II) center in parallel via a four-center interaction, to give VIv (vicinal pathway in Fig. 2). The ΔG of TS(IIv–VIv) is 18.32 kcal mol−1, which is also lower than that of TS(I–V) for the mononuclear Grignard species. TS(IIv–VIv) corresponds to TS-9 in Fig. 1b. The results obtained using these dinuclear Mg(II) calculation models support the experimental results, indicating that a large amount of Grignard reagents facilitates the addition of the methyl group.
are in equilibrium. From IIv, the attack of the vicinal methyl group on the nitrile carbon atom would proceed via transition state TS(IIv–VIv), where the geminal methyl group transfers to the other Mg(II) center in parallel via a four-center interaction, to give VIv (vicinal pathway in Fig. 2). The ΔG of TS(IIv–VIv) is 18.32 kcal mol−1, which is also lower than that of TS(I–V) for the mononuclear Grignard species. TS(IIv–VIv) corresponds to TS-9 in Fig. 1b. The results obtained using these dinuclear Mg(II) calculation models support the experimental results, indicating that a large amount of Grignard reagents facilitates the addition of the methyl group.
Next, we examined the reaction pathways of the Zn(II)-catalyzed system. Coordination of Me2Zn to IIg or  would give trimer complexes IIIg (ΔG = 0.86 kcal mol−1) or IIIv (ΔG = −2.25 kcal mol−1), respectively. In IIIg, the Zn(II) center is coordinated to the geminal methyl group. The distance between the carbon atom in the methyl group of Me2Zn and the nitrile carbon atom (3.52 Å) is even shorter than that of IIg (3.76 Å). The methyl group on the Zn(II) center could then attack the nitrile carbon atom via a transition state in which the Zn(II) center exhibits a trigonal-planar arrangement (TS(IIIg–IVg)), which corresponds to TS-12 in Fig. 2, to give IVg (geminal pathway in Fig. 4). In TS(IIIg–IVg), a four-center interaction between the Zn–Me moiety and the C
would give trimer complexes IIIg (ΔG = 0.86 kcal mol−1) or IIIv (ΔG = −2.25 kcal mol−1), respectively. In IIIg, the Zn(II) center is coordinated to the geminal methyl group. The distance between the carbon atom in the methyl group of Me2Zn and the nitrile carbon atom (3.52 Å) is even shorter than that of IIg (3.76 Å). The methyl group on the Zn(II) center could then attack the nitrile carbon atom via a transition state in which the Zn(II) center exhibits a trigonal-planar arrangement (TS(IIIg–IVg)), which corresponds to TS-12 in Fig. 2, to give IVg (geminal pathway in Fig. 4). In TS(IIIg–IVg), a four-center interaction between the Zn–Me moiety and the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond is observed. The ΔG of TS(IIIg–IVg) (14.10 kcal mol−1) is lower than that of TS(IIg–VIg) (16.75 kcal mol−1). In the pathway viaTS(IIIg–IVg), the methyl group bonded to the Mg(II) center migrates to the Zn(II) center in parallel with the attack of the methyl group. Subsequently, elimination of both Me2Zn and CH3MgCl(THF) from IVg would give V. In the case of IIIv, the Zn(II) center is coordinated to the vicinal methyl group. The attack of the methyl group on the Zn(II) center on the nitrile carbon atom would afford IVvvia a transition state wherein the Zn(II) center adopts a tetrahedral arrangement (TS(IIIv–IVv)), which corresponds to TS-11′ in Fig. 2 (vicinal pathway in Fig. 4). The ΔG of TS(IIIv–IVv) (15.12 kcal mol−1) is lower than that of TS(IIv–VIv). Since the last two pathways viaTS(IIIg–IVg) or TS(IIIv–IVv) would correspond to the Zn(II)-catalyzed reaction using a large amount of Grignard reagents (TS-11 in Fig. 1d and TS-11′ and TS-12 in Fig. 2), the computational results strongly support that the Zn(II)-catalyzed system has higher reactivity than the noncatalytic version.
N bond is observed. The ΔG of TS(IIIg–IVg) (14.10 kcal mol−1) is lower than that of TS(IIg–VIg) (16.75 kcal mol−1). In the pathway viaTS(IIIg–IVg), the methyl group bonded to the Mg(II) center migrates to the Zn(II) center in parallel with the attack of the methyl group. Subsequently, elimination of both Me2Zn and CH3MgCl(THF) from IVg would give V. In the case of IIIv, the Zn(II) center is coordinated to the vicinal methyl group. The attack of the methyl group on the Zn(II) center on the nitrile carbon atom would afford IVvvia a transition state wherein the Zn(II) center adopts a tetrahedral arrangement (TS(IIIv–IVv)), which corresponds to TS-11′ in Fig. 2 (vicinal pathway in Fig. 4). The ΔG of TS(IIIv–IVv) (15.12 kcal mol−1) is lower than that of TS(IIv–VIv). Since the last two pathways viaTS(IIIg–IVg) or TS(IIIv–IVv) would correspond to the Zn(II)-catalyzed reaction using a large amount of Grignard reagents (TS-11 in Fig. 1d and TS-11′ and TS-12 in Fig. 2), the computational results strongly support that the Zn(II)-catalyzed system has higher reactivity than the noncatalytic version.
Conclusions
In summary, we have developed a ZnCl2-catalyzed Grignard addition reaction of aromatic nitriles by virtue of the in situ generation of reactive zinc(II)ate species. The corresponding ketones and amines were successfully obtained via hydrolysis and reduction, respectively, in good yield under mild reaction conditions. A scale-up reaction of a ketone and sequential tertiary alcohol synthesis were also demonstrated by taking advantage of the this ZnCl2 catalysis. Furthermore, computational DFT calculations were performed to propose plausible reaction mechanisms and transition states in order to investigate the origin of the higher efficiency of a catalytic amount of ZnCl2 compared to the use of a stoichiometric amount of zinc(II)ates.Data availability
All data associated with this article are available from ESI.†Author contributions
M. Hatano conceived and directed the project and designed the experiments. M. Hatano, K. Kuwano, R. Asukai, A. Nagayoshi, H. Hoshihara, and T. Hirata performed the experimental studies and analyzed the results. M. Umezawa, S. Tsubaki, T. Yoshikawa, and K. Sakata performed the DFT calculations. M. Hatano, T. Yoshikawa, and K. Sakata prepared the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support was partially provided by JSPS KAKENHI grant JP20H02735 and JP24K08419 (to M. H.). Some of the calculations were performed using resources of the Research Center for Computational Science, Okazaki, Japan (Project: 23-IMS-C047).Notes and references
- (a) Z. Rappoport, The chemistry of the cyano group, John Wiley & Sons, London, 1970 Search PubMed; (b) A. J. Fatiadi, Preparation and Synthetic Applications of Cyano Compounds, ed. S. Patai and Z. Rappoport, Wiley-VCH, New York, 1983 Search PubMed; (c) R. C. Larock, Comprehensive Organic Transformations: A Guide to Functional Group Preparations, Wiley-VCH, Weinheim, 1999, pp. 1621–1927 Search PubMed; (d) P. Pollak, Nitriles. Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000 Search PubMed; (e) G. Yan, Y. Zhang and J. Wang, Adv. Synth. Catal., 2017, 359, 4068–4105 CrossRef CAS.
- For selected textbooks on organolithium(I) reagents, see: (a) B. J. Wakefield, The Chemistry of Organolithium Compounds, Pergamon Press, Oxford, 1974 Search PubMed; (b) J. Clayden, Organolithiums: Selectivity for Synthesis, Pergamon, Oxford, 2002 Search PubMed; (c) Z. Z. Rappoport and I. Marek, The Chemistry of Organolithium Compounds: R-Li (Patai's Chemistry of Functional Groups), Wiley-VCH, Weinheim, 2004 CrossRef; (d) Z. Z. Rappoport and I. Marek, The Chemistry of Organolithium Compounds: R-Li (Patai's Chemistry of Functional Groups), Wiley-VCH, Weinheim, 2006, vol. 2 CrossRef.
- For selected reviews and textbooks on Grignard reagents, see: (a) Y.-H. Lai, Synthesis, 1981, 585–604 CrossRef CAS; (b) B. J. Wakefield, Organomagnesium Methods in Organic Chemistry, Academic Press, San Diego, 1995 Search PubMed; (c) G. S. Silverman and P. E. Rakita, Handbook of Grignard Reagents, Marcel Dekker, New York, 1996 CrossRef; (d) H. G. Richey Jr, Grignard Reagents: New Development, Wiley, Chichester, 2000 Search PubMed; (e) P. Knochel, Handbook of Functionalized Organometallics, Wiley-VCH, Weinheim, 2005 CrossRef; (f) H. Yorimitsu, in Science of Synthesis, Knowledge Updates (2011), George Thieme, Stuttgart, 2010, ch. 7.6.5.6, pp. 1–9 Search PubMed; (g) H. Yorimitsu, in Science of Synthesis, Knowledge Updates (2011), George Thieme, Stuttgart, 2010, ch. 7.6.10.9, pp. 11–19 Search PubMed.
- (a) L. S. Cook and B. J. Wakefield, J. Chem. Soc., Perkin Trans. 1, 1980, 2392–2397 RSC; (b) F. J. Weiberth and S. S. Hall, J. Org. Chem., 1986, 51, 5338–5341 CrossRef CAS.
- (a) F. J. Weiberth and S. S. Hall, J. Org. Chem., 1987, 52, 3901–3904 CrossRef CAS; (b) M. Ortiz-Marciales, L. M. Tirado, R. Colón, M. L. Ufret, R. Figueroa, M. Lebrón, M. DeJesús, J. Martínez and T. Malavé, Synth. Commun., 1998, 28, 4067–4075 CrossRef CAS.
- For the addition of RLi under solvent-free conditions, see: D. Elorriaga, F. Carrillo-Hermosilla, B. Parra-Cadenas, A. Antiñolo and J. García-Álvarez, ChemSusChem, 2022, 15, e202201348 CrossRef CAS PubMed.
- For the addition of RLi in water and glycerol, see: (a) G. Dilauro, M. Dell'Aera, P. Vitale, V. Capriati and F. M. Perna, Angew. Chem., Int. Ed., 2017, 56, 10200–10203 CrossRef CAS PubMed; (b) M. J. Rodríguez-Álvarez, J. García-Álvarez, M. Uzelac, M. Fairley, C. T. O'Hara and E. Hevia, Chem.–Eur. J., 2018, 24, 1720–1725 CrossRef PubMed.
- For the addition of n-BuMgBr in 2-methyltetrahydrofuran, see: W. Zhong, Y. Wu and X. Zhang, J. Chem. Res., 2009, 2009, 370–373 CrossRef.
- We have already reported the catalytic and stoichiometric use of ZnCl2 with Grignard reagents for carbonyl compounds; for details, see: (a) M. Hatano, S. Suzuki and K. Ishihara, J. Am. Chem. Soc., 2006, 128, 9998–9999 CrossRef CAS PubMed; (b) M. Hatano, S. Suzuki and K. Ishihara, Synlett, 2010, 2010, 321–324 CrossRef; (c) M. Hatano, O. Ito, S. Suzuki and K. Ishihara, Chem. Commun., 2010, 46, 2674–2676 RSC; (d) M. Hatano, O. Ito, S. Suzuki and K. Ishihara, J. Org. Chem., 2010, 75, 5008–5016 CrossRef CAS PubMed; (e) M. Hatano, K. Yamashita, M. Mizuno, O. Ito and K. Ishihara, Angew. Chem., Int. Ed., 2015, 54, 2707–2711 CrossRef CAS PubMed; (f) M. Hatano, K. Yamashita and K. Ishihara, Org. Lett., 2015, 17, 2412–2415 CrossRef CAS PubMed; (g) M. Hatano, M. Mizuno and K. Ishihara, Org. Lett., 2016, 18, 4462–4465 CrossRef CAS PubMed.
- Lanthanide halides are known as good additives in the Grignard addition reaction of ketones; for details, see: (a) T. Imamoto, N. Takiyama, K. Nakamura, T. Hatajima and Y. Kamiya, J. Am. Chem. Soc., 1989, 111, 4392–4398 CrossRef CAS; (b) A. Krasovskiy, F. Kopp and P. Knochel, Angew. Chem., Int. Ed., 2006, 45, 497–500 CrossRef CAS PubMed.
- A ZnCl2(tmeda)-catalyzed nucleophilic substitution reaction of chlorosilanes with RMgX has been reported by Oshima and coworkers; for details, see: K. Murakami, H. Yorimitsu and K. Oshima, J. Org. Chem., 2009, 74, 1415–1417 CrossRef CAS PubMed.
- The product yield for the noncatalyzed reactions of heteroaryl nitriles 1k and 1l with 2j was relatively high, probably due to the presence of directing O- or N-moieties at the ortho-position, similar to the phenomena as seen in the reaction of 1h (see eqn (1)).
- (a) P. Knochel, W. Dohle, N. Gommermann, F. F. Kneisel, F. Kopp, T. Korn, I. Sapountzis and V. A. Vu, Angew. Chem., Int. Ed., 2003, 42, 4302–4320 CrossRef CAS PubMed; (b) H. Shinokubo and K. Oshima, Eur. J. Org Chem., 2004, 2004, 2081–2091 CrossRef.
- Since ZnCl2 should be smoothly converted to anionic zinc(II)ates in situ, the corresponding Zn(II)-function as Lewis acid catalysts to activate the nitrile moiety can be reasonably excluded. D. R. Armstrong, W. Clegg, P. García-Alvarez, M. D. McCall, L. Nuttall, A. R. Kennedy, L. Russo and E. Hevia, Chem.–Eur. J., 2011, 17, 4470–4479 CrossRef CAS PubMed.
- (a) E. C. Ashby, Pure Appl. Chem., 1980, 52, 545–569 CrossRef CAS; (b) E. C. Ashby and W. E. Becker, J. Am. Chem. Soc., 1963, 85, 118–119 CrossRef CAS; (c) K. Maruyama and T. Katagiri, J. Am. Chem. Soc., 1986, 108, 6263–6270 CrossRef CAS; (d) T. Holm, Acta Chem. Scand., 1966, 20, 2821–2828 CrossRef CAS; (e) S. G. Smith and G. Su, J. Am. Chem. Soc., 1964, 86, 2750–2751 CrossRef CAS; (f) E. C. Ashby, J. Laemmle and H. M. Newmann, Acc. Chem. Res., 1974, 7, 272–280 CrossRef CAS; (g) H. Yamataka, T. Matsuyama and T. Hanafusa, J. Am. Chem. Soc., 1989, 111, 4912–4918 CrossRef CAS.
- M. Uchiyama, S. Nakamura, T. Ohwada, M. Nakamura and E. Nakamura, J. Am. Chem. Soc., 2004, 126, 10897–10903 CrossRef CAS PubMed.
- (a) S. Yamazaki and S. Yamabe, J. Org. Chem., 2002, 67, 9346–9353 CrossRef CAS PubMed; (b) T. Mori and S. Kato, J. Phys. Chem. A, 2009, 113, 6158–6165 CrossRef CAS PubMed.
- (a) R. M. Peltzer, O. Eisenstein, A. Nova and M. Cascella, J. Phys. Chem. B, 2017, 121, 4226–4237 CrossRef CAS PubMed; (b) R. M. Peltzer, J. Gauss, O. Eisenstein and M. Cascella, J. Am. Chem. Soc., 2020, 142, 2984–2994 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, characterization data, computational details, and copies of 1H NMR and 13C NMR spectra of compounds. See DOI: https://doi.org/10.1039/d4sc01659a |
| This journal is © The Royal Society of Chemistry 2024 |