Open Access Article
Open Access ArticlePillar[5]arenes decorated with six-membered-ring aromatics at all the substitution positions†
Tomoya
Kaneda
a,
Kenichi
Kato
 *a,
Shunsuke
Ohtani
*a,
Shunsuke
Ohtani
 a and
Tomoki
Ogoshi
a and
Tomoki
Ogoshi
 *ab
*ab
aDepartment of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto, 615-8510, Japan. E-mail: katok@sbchem.kyoto-u.ac.jp; ogoshi@sbchem.kyoto-u.ac.jp
bWPI Nano Life Science Institute, Kanazawa University, Kakuma-machi, Kanazawa, 920-1192, Japan
First published on 6th June 2024
Abstract
Macrocyclic molecules have characteristic properties different from linear ones, such as high symmetry and guest-inclusion ability. To bring drastic changes to these properties, direct introduction of many substituents is a challenging but effective tool. Herein, we attain direct installation of ten six-membered-ring aromatic π-units into both rims of a pillar[5]arene. In contrast to previous pillar[n]arenes with less hindered five-membered-ring units, which showed conformational complexity and crushed crystal structures, the per-phenyl-substituted pillar[5]arene has a cylinder-shaped crystal structure with a dichloromethane inside the cavity and is obtained as a single pair of D5-symmetric enantiomers. The average dihedral angles between the core and peripheral benzene rings sharply increase from 38° to 66°. These differences indicate the importance of local steric repulsion on both rims for determining the structures and properties of macrocycles.
Introduction
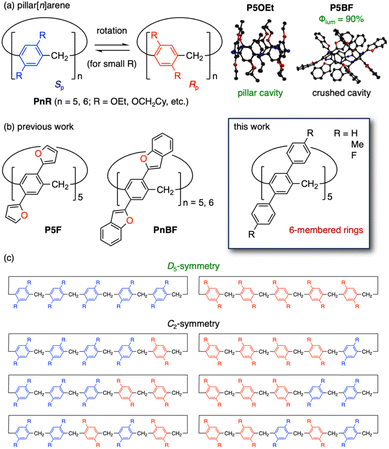
Macrocyclic compounds, such as cyclodextrins,1 crown ethers,2 calix[n]arenes,3 and cycloparaphenylenes,4 are expected to be applied in switchable systems,5a drug delivery,5b and chiral recognition5c because of their unique ability to incorporate guest compounds into the cavities. Such properties of macrocycles are determined by the chemical structures of component units and linkers. Therefore, alteration of the components is a powerful strategy to gain novel macrocycles. For example, simple changes of calix[n]arene π-units produced different classes of macrocycles, including calix[n]pyrroles,6 pillar[n]arenes,7 prism[n]arenes,8a pagoda[n]arenes,8b,c and saucer[n]arenes.8d However, the synthesis of oligomeric macrocycles often competes against the formation of linear polymers to some extent and even small alteration of the monomeric units or reaction conditions may lead to severe drops in the product yields or changes of preferred ring sizes.6–10 By contrast, post-functionalization of macrocycles is a reliable method to alter the properties. However, small effect by one substituent often requires amplification by multi-fold reactions in a highly efficient and selective manner, which is rarely attained in most macrocycle platforms.11Pillar[n]arene is a highly symmetric cylinder-shaped macrocyclic oligomer of 1,4-dialkoxybenzene with methylene linkers (Fig. 1a).7,12 The pre-installed alkoxy groups are deprotected and resulting phenolic ones are widely used for re-alkylation,13b acylation,13c sulfonation,13d,14a and so on.13a,e,f These modifications have provided a variety of combinations with other functional segments whereas they have hardly perturbed the electronic properties of pillar[n]arene cores.
The alkoxy groups have also enabled ortho-functionalization15 and direct replacement with amino,16a,b cyano,16c,d and aryl substituents17 at a few positions.14 In addition, we recently found that per-arylation of triflates14via Suzuki–Miyaura cross-coupling is possible at one and both rims of pillar[n]arenes, overcoming swing suppression and accompanying low reactivity caused by bulky groups on the other rim.17f,18 The first per-aryl-substituted pillar[n]arenes, PnBF (n = 5, 6) and P5F (Fig. 1b),18 were obtained by choosing benzofuran- and furan-2-boronic acids respectively, which have less hindered five-membered rings and lack hydrogen atoms on one of two adjoining positions.
Similar to most pillar[n]arene derivatives, P5F and P6BF gave dynamic mixtures of eight or more possible isomers (Fig. 1c) due to the π-unit rotation roughly on the 1H NMR time scale at room temperature. On the other hand, P5BF was resolved into three pairs of enantiomers by chiral high-performance liquid chromatography (HPLC) because the benzofuran rings were large enough to suppress the rotation. This result made a sharp contrast to conventional pillar[n]arenes with bulky alkoxy substituents, which yield a single pair of chirality-aligned enantiomers regardless of synthetic routes.12,13e,19 Furthermore, the single crystals of P5BF and P5F showed crushed structures with larger average tilt angles of 29–30° (Table S5-2 in the ESI†) than the angle for a P5OEt crystal without guest inclusion (20°).20 These results indicated that stable conformations of pillar[n]arenes are determined not only by steric repulsion between substituents on the benzene units but also by multipoint CH/O, CH/π and other weak interactions between them.
In this study, we further optimize per-arylation reactions of pillar[5]arene to extend the scope to benzene analogues with versatile functionalities. Using a carbene-coordinating Pd-PEPPSI-iPr catalyst,21 several benzene analogues can be fully attached to all the substitution positions owing to the excellent stability of the catalyst at elevated temperature. Different from P5BF, per-phenyl-substituted product P5Ph provides a single D5-symmetric pair with slow conformational interconversion at room temperature and takes a symmetric pillar shape including a dichloromethane in the cavity.
Results and discussion
Full substitution with six-membered-ring aromatics
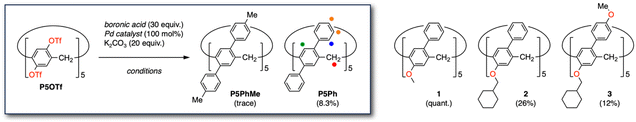
Initially, per-arylation of pillar[5]arene triflate P5OTf (ref. 14a) was attempted with 4-methylphenylboronic acid under the conditions used for the synthesis of per-benzofuranyl pillar[5]arene P5BF (ref. 18) (Table 1, entries 1 and 2). However, the reaction did not proceed completely even at higher temperature (entry 3). Mass spectrometry (MS) results included clear peaks for pillar[5]arenes with up to nine-fold substitution but showed only slight peaks for target P5PhMe. The 1H NMR spectrum did not provide a D5-symmetric set of sharp peaks expected for the P5PhMe. These results suggested that steric hindrance around the reaction sites was a highly important factor for the direct installation of π-units into pillar[5]arene rims. The installation of benzene derivatives would suffer from more severe steric repulsion due to the six-membered rings with hydrogen atoms at both 2,6-positions than that of furan ones due to the five-membered rings missing a hydrogen on each oxygen atom.| Entry | Boronic acid | Pd catalyst | Conditions | Product | Yield |
|---|---|---|---|---|---|
| a Solvent (4.0 mL, 1,4-dioxane/H2O = 4/1). b Solvent (8.2 mL, 1,4-dioxane/mesitylene/H2O = 4/4/1). c 1,4-Dioxane (2.0 mL). d Boronic acid (60 equiv.), Pd catalyst (200 mol%), K2CO3 (40 equiv.), and 1,4-dioxane (2.0 mL). | |||||
| 1 (ref. 18) | Benzofuran-2-boronic acid | XPhos Pd G3 | 1,4-Dioxane/H2O, 100 °C, 21 h | P5BF | 50% |
| 2 | 4-Methylphenylboronic acid | XPhos Pd G3 | 1,4-Dioxane/H2O, 100 °C, 21 h | P5PhMe | Not detected |
| 3b | 4-Methylphenylboronic acid | XPhos Pd G3 | 1,4-Dioxane/mesitylene/H2O, 120 °C, 20 h | P5PhMe | Trace |
| 4c | 4-Methylphenylboronic acid | Pd-PEPPSI-iPr | 1,4-Dioxane, 60 °C, 24 h | P5PhMe | Trace |
| 5c | Phenylboronic acid | Pd-PEPPSI-iPr | 1,4-Dioxane, 100 °C, 24 h | P5Ph | 8.3% |
| 6,d | Phenylboronic acid | Pd-PEPPSI-iPr | 1,4-Dioxane, 100 °C, 48 h | P5Ph | 4.4% |
Then, we changed the XPhos Pd G3 catalyst to Pd-PEPPSI-iPr,21 which is extremely air- and water-stable, in hopes of remaining active for sufficient duration without forming de-complexed palladium. As expected, the reaction proceeded sufficiently at 60 °C for 24 h (entry 4), giving a MS peak of P5PhMe at m/z = 1351.7093 (calcd for [C105H91]+: 1351.7115 [M + H]+). However, the yield was too low to isolate the target. Introduction of sterically demanding substituents into pillar[n]arenes was reported to be largely affected by bulkiness not only near the reaction sites but also on the other rim because of limited unit-swing motions. For example, the yield for introduction of five phenyl rings into a methoxy pillar[5]arene was quantitative (1),17e whereas that into a cyclohexylmethoxy one was 26% (2).17f Further drop to 12% was observed when five 4-methoxyphenyl rings were attached (3). Accordingly, we replaced 4-methylphenylboronic acid with phenylboronic acid to minimize the steric repulsion and raised the reaction temperature to 100 °C (entry 5). This entry afforded a D5-symmetric set of 1H NMR signals, enabling isolation of the target P5Ph in 8.3% yield after column chromatography on silica gel and recrystallization from CH2Cl2/n-hexane (Fig. S3-1 and S4-1†). Finally, the boronic acid, catalyst, and base were doubled in anticipation of increasing yield, which did not lead to further improvement (entry 6).
Characterization of pristine per-phenyl-substituted pillar[5]arene
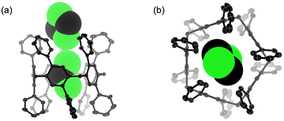
Typical per-alkoxy pillar[5]arenes show excellent guest-inclusion abilities with linear alkyl segments bearing electron-withdrawing or cationic groups in solution.7a–c,23 In contrast, previous per-aryl pillar[5]arene P5BF did not show such behaviour because its cavity was crushed in a major conformation and the core benzene units were no longer electron-rich without alkoxy substituents. The host ability of P5Ph was also investigated by 1H NMR spectroscopy. Upon addition of neutral and cationic guests (1,2-dicyanoethane, 1,4-dicyanobutane, and n-octyltrimethylammonium hexafluorophosphate), no peak shift or peak appearance/disappearance was detected in CDCl3 solution (Fig. S3-6 to S3-8†). These results revealed that P5Ph did not form complexes with these guest molecules in solution, despite the cavity in the crystal structure like those for per-alkoxy pillar[5]arenes. The poor host ability of P5Ph was probably because the cavity was less electron-rich, and the rims were more hindered than those of per-alkoxy compounds (Fig. S5-3†).
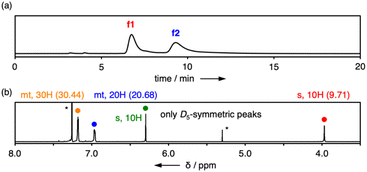
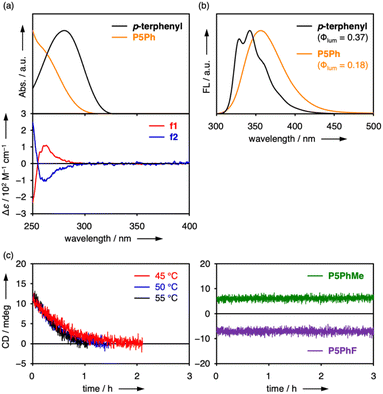
The separated isomers, f1 and f2, were then evaluated by ultraviolet/visible (UV/vis) absorption, fluorescence (FL), and circular dichroism (CD) spectroscopy in CHCl3 (Fig. 4a and b). The P5Ph isomers showed broad absorption at around 270 nm and fluorescence at around 360 nm with a quantum yield (Φlum) of 0.18. In comparison with p-terphenyl, which corresponds to the π-unit structure, P5Ph showed blue-shifted absorption because the p-terphenyl backbones of P5Ph had more twisted structures with smaller conjugative effect than the p-terphenyl structure. In fact, the average dihedral angle of P5Ph was 66° in the single crystal while that of p-terphenyl had been reported to be 14–26°.24 By contrast, the fluorescence band showed the opposite trend to the absorption despite weak conjugation at the ground state. The red-shifted fluorescence implies that the p-terphenyl units in P5Ph had small dihedral angles enabling conjugation within the units and through–space interactions between the units became operative at the excited states. In the CD spectra, positive and negative peaks appeared around 260 nm as a symmetrical pair for f1 and f2, which were assigned to Rp and Sp isomers, respectively, by using a theoretically calculated spectrum of the (Rp)-structure (Fig. S8-5†). The dissymmetry factor was ca. 2 × 10−3, being comparable to that of P5BF (1 × 10−3).
Substituent-dependent panel-rotation barriers
The obtained products, P5PhMe and P5PhF, were separated into two fractions by chiral HPLC (Fig. S7-2 and S7-3†). These fractions were revealed to be pairs of D5-symmetric enantiomers by 1H NMR spectra (Fig. S3-2 and S3-3†). The UV/vis absorption, FL, and CD spectra of P5PhMe and P5PhF were almost the same with only slight peak shifts as those of P5Ph (Fig. S6-1 and S6-2†). On the other hand, CD peaks of these fractions did not diminish even after heating at 80 °C (Fig. 4c). These compounds have slightly larger atoms or groups at para-positions than P5Ph, and these small differences made the unit rotation suppressed efficiently. These phenomena were consistent with the fact that the efficiency of cross-coupling reactions was highly sensitive to the aryl groups of boronic acids. In per-alkoxy pillar[5]arenes, even per-dodecyloxy9b and per-(2-cyclohexylethoxy)19a derivatives showed conformational interconversion due to the flexibility of alkyl chains. Therefore, the sharp increase of the rotational barrier in the present series was ascribed to the unique rigidity of directly per-arylated pillar[5]arenes.
Conclusions
In this work, we accomplished the direct installation of six-membered-ring aromatic π-units at all the substitution positions of a pillar[5]arene. In the Suzuki–Miyaura cross coupling, use of a carbene-coordinating Pd complex was a key to success due to the high stability at elevated temperature. Although six-membered-ring reagents were expected to offer a large number of options and expand the possibilities of pillar[5]arenes, the scope of reaction was rather limited because small drops in reaction efficiency led to a large decrease in overall yields and increasing steric hindrance during the reaction disturbed the progress of further cross coupling.Each of the obtained compounds provided a pair of D5-symmetric enantiomers by the locally hindered substituents suppressing the unit rotation. Variable-temperature CD intensity monitoring of P5Ph revealed that the activation energy at 25 °C (ΔG‡25°C) was 95.3 kJ mol−1. After optical resolution by chiral HPLC, heating an enantiopure fraction caused racemization but did not produce any C2-symmetric isomers. These results indicated that the D5-symmetric isomer was selectively obtained, which was different from P5BF obtained as a mixture of six isomers. On the other hand, heating at 80 °C could not cause any unit rotation for P5PhMe and P5PhF because of slight differences in the steric bulkiness at substituent para-positions. Although P5Ph did not serve as a host for neutral and cationic linear molecules in solution, a single-crystal structure revealed that pillar-shaped P5Ph could incorporate a dichloromethane in its cavity. Overall, the structures and properties of macrocycles were largely changed by the increase of steric hindrance in P5Ph from P5F and P5BF, which had less hindered five-membered-ring segments and resulting crushed crystal structures. Non-alkoxy pillar[n]arenes are an emerging class of functional macrocycles and are pursued by all the synthetic means in our group.
Data availability
The data that support the findings of this study are available in the ESI† of this article.Author contributions
T. O. supervised and administrated this project. T. K. and K. K. performed conceptualization. T. K. conducted the experiments. T. K. and K. K. prepared the draft and initial version of ESI. All authors reviewed and edited the draft and ESI.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers JP21K14611 (Early-Career Scientists, K. K.), JP23H04027 (Transformative Research Areas (A), K. K.), and JP22H00334 (Scientific Research (A), T. O.), JST CREST Grant Number JPMJCR18R3 (T. O.), and MEXT World Premier International Research Center Initiative (WPI), Japan. K. K. thanks Masuyakinen basic research foundation and The Kyoto University Foundation for financial support.Notes and references
- G. Crini, Review: A History of Cyclodextrins, Chem. Rev., 2014, 114, 10940–10975 CrossRef CAS PubMed.
- G. W. Gokel, W. M. Leevy and M. E. Weber, Crown Ethers: Sensors for Ions and Molecular Scaffolds for Materials and Biological Models, Chem. Rev., 2004, 104, 2723–2750 CrossRef CAS PubMed.
- (a) R. Kumar, A. Sharma, H. Singh, P. Suating, H. S. Kim, K. Sunwoo, I. Shim, B. C. Gibb and J. S. Kim, Revisiting Fluorescent Calixarenes: From Molecular Sensors to Smart Materials, Chem. Rev., 2019, 119, 9657–9721 CrossRef CAS PubMed; (b) F. Sansone, L. Baldini, A. Casnati and R. Ungaro, Calixarenes: from biomimetic receptors to multivalent ligands for biomolecular recognition, New J. Chem., 2010, 34, 2715–2728 RSC.
- (a) S. Yamago, E. Kayahara and T. Iwamoto, Organoplatinum-Mediated Synthesis of Cyclic π-Conjugated Molecules: Towards a New Era of Three-Dimensional Aromatic Compounds, Chem. Rec., 2014, 14, 84–100 CrossRef CAS PubMed; (b) M. R. Golder and R. Jasti, Syntheses of the Smallest Carbon Nanohoops and the Emergence of Unique Physical Phenomena, Acc. Chem. Res., 2015, 48, 557–566 CrossRef CAS PubMed; (c) S. E. Lewis, Cycloparaphenylenes and related nanohoops, Chem. Soc. Rev., 2015, 44, 2221–2234 RSC; (d) Y. Segawa, A. Yagi, K. Matsui and K. Itami, Design and Synthesis of Carbon Nanotube Segments, Angew. Chem., Int. Ed., 2016, 55, 5136–5158 CrossRef CAS PubMed; (e) Q.-H. Guo, Y. Qiu, M.-X. Wang and J. F. Stoddart, Aromatic hydrocarbon belts, Nat. Chem., 2021, 13, 402–419 CrossRef CAS PubMed; (f) D. Imoto, A. Yagi and K. Itami, Carbon Nanobelts: Brief History and Perspective, Precis. Chem., 2023, 1, 516–523 CrossRef CAS.
- (a) A. Blanco-Gómez, P. Cortón, L. Barravecchia, I. Neira, E. Pazos, C. Peinador and M. D. García, Controlled binding of organic guests by stimuli-responsive macrocycles, Chem. Soc. Rev., 2020, 49, 3834–3862 RSC; (b) M. J. Webber and R. Langer, Drug delivery by supramolecular design, Chem. Soc. Rev., 2017, 46, 6600–6620 RSC; (c) W. Xu, M. Cheng, S. Zhang, Q. Wu, Z. Liu, M. K. Dhinakaran, F. Liang, E. G. Kovaleva and H. Li, Recent advances in chiral discrimination on host–guest functionalized interfaces, Chem. Commun., 2021, 57, 7480–7492 RSC.
- (a) D. S. Kim and J. L. Sessler, Calix[4]pyrroles: versatile molecular containers with ion transport, recognition, and molecular switching functions, Chem. Soc. Rev., 2015, 44, 532–546 RSC; (b) G. Cafeo, F. H. Kohnke, M. F. Parisi, R. P. Nascone, G. L. La Torre and D. J. Williams, The Elusive β-Unsubstituted Calix[5]pyrrole Finally Captured, Org. Lett., 2002, 4, 2695–2697 CrossRef CAS PubMed; (c) Y. Inaba, Y. Nomata, Y. Ide, J. Pirillo, Y. Hijikata, T. Yoneda, A. Osuka, J. L. Sessler and Y. Inokuma, Calix[3]pyrrole: A Missing Link in Porphyrin-Related Chemistry, J. Am. Chem. Soc., 2021, 143, 12355–12360 CrossRef CAS PubMed.
- (a) P. J. Cragg and K. Sharma, Pillar[5]arenes: fascinating cyclophanes with a bright future, Chem. Soc. Rev., 2012, 41, 597–607 RSC; (b) M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Pillararenes, A New Class of Macrocycles for Supramolecular Chemistry, Acc. Chem. Res., 2012, 45, 1294–1308 CrossRef CAS PubMed; (c) T. Ogoshi, T.-a. Yamagishi and Y. Nakamoto, Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS PubMed; (d) T. Ogoshi, S. Kanai, S. Fujinami, T.-a. Yamagishi and Y. Nakamoto, para-Bridged Symmetrical Pillar[5]arenes: Their Lewis Acid Catalyzed Synthesis and Host–Guest Property, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed; (e) D. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang and H. Meier, A Facile and Efficient Preparation of Pillararenes and a Pillarquinone, Angew. Chem., Int. Ed., 2009, 48, 9721–9723 CrossRef CAS PubMed; (f) Y. Ma, X. Chi, X. Yan, J. Liu, Y. Yao, W. Chen, F. Huang and J.-L. Hou, per-Hydroxylated Pillar[6]arene: Synthesis, X-ray Crystal Structure, and Host–Guest Complexation, Org. Lett., 2012, 14, 1532–1535 CrossRef CAS PubMed.
- (a) P. Della Sala, R. Del Regno, C. Talotta, A. Capobianco, N. Hickey, S. Geremia, M. De Rosa, A. Spinella, A. Soriente, P. Neri and C. Gaeta, Prismarenes: A New Class of Macrocyclic Hosts Obtained by Templation in a Thermodynamically Controlled Synthesis, J. Am. Chem. Soc., 2020, 142, 1752–1756 CrossRef CAS PubMed; (b) X.-N. Han, Y. Han and C.-F. Chen, Pagoda[4]arene and i-Pagoda[4]arene, J. Am. Chem. Soc., 2020, 142, 8262–8269 CrossRef CAS PubMed; (c) X.-N. Han, Q.-S. Zong, Y. Han and C.-F. Chen, Pagoda[5]arene with Large and Rigid Cavity for the Formation of 1:2 Host–Guest Complexes and Acid/Base-Responsive Crystalline Vapochromic Properties, CCS Chem., 2022, 4, 318–330 CrossRef CAS; (d) J. Li, H.-Y. Zhou, Y. Han and C.-F. Chen, Saucer[n]arenes: Synthesis, Structure, Complexation, and Guest-Induced Circularly Polarized Luminescence Property, Angew. Chem., Int. Ed., 2021, 60, 21927–21933 CrossRef CAS PubMed.
- (a) T. Ogoshi, K. Kitajima, K. Umeda, S. Hiramitsu, S. Kanai, S. Fujinami, T.-a. Yamagishi and Y. Nakamoto, Lewis acid-catalyzed synthesis of dodecamethoxycalix[4]arene from 1,3,5-trimethoxybenzene and its conformational behavior and host–guest property, Tetrahedron, 2009, 65, 10644–10649 CrossRef CAS; (b) T. Ogoshi, K. Kitajima, T. Aoki, S. Fujinami, T.-a. Yamagishi and Y. Nakamoto, Synthesis and Conformational Characteristics of Alkyl-Substituted Pillar[5]arenes, J. Org. Chem., 2010, 75, 3268–3273 CrossRef CAS PubMed; (c) S. T. Schneebeli, C. Cheng, K. J. Hartlieb, N. L. Strutt, A. A. Sarjeant, C. L. Stern and J. F. Stoddart, Asararenes—A Family of Large Aromatic Macrocycles, Chem.–Eur. J., 2013, 19, 3860–3868 CrossRef CAS PubMed; (d) F. Chen, T. Tanaka, Y. Hong, W. Kim, D. Kim and A. Osuka, ortho-Phenylene-Bridged Cyclic Oligopyrroles: Conformational Flexibilities and Optical Properties, Chem.–Eur. J., 2016, 22, 10597–10606 CrossRef CAS PubMed; (e) K. Kise, F. Chen, K. Kato, T. Tanaka and A. Osuka, Cyclic Hybrids of Alternately Linked 2,5-Pyrrolylenes and 3,4-Thienylenes, Chem. Lett., 2017, 46, 1319–1322 CrossRef CAS; (f) X.-N. Han, Y. Han and C.-F. Chen, Chem. Soc. Rev., 2023, 52, 3265–3298 RSC.
- (a) J.-Y. Shin, H. Furuta, K. Yoza, S. Igarashi and A. Osuka, meso-Aryl-Substituted Expanded Porphyrins, J. Am. Chem. Soc., 2001, 123, 7190–7191 CrossRef CAS PubMed; (b) Y. Tanaka, J.-Y. Shin and A. Osuka, Facile Synthesis of Large meso-Pentafluorophenyl-Substituted Expanded Porphyrins, Eur. J. Org. Chem., 2008, 1341–1349 CrossRef CAS; (c) X.-B. Hu, Z. Chen, L. Chen, L. Zhang, J.-L. Hou and Z.-T. Li, Pillar[n]arenes (n = 8–10) with two cavities: synthesis, structures and complexing properties, Chem. Commun., 2012, 48, 10999–11001 RSC; (d) T. Ogoshi, N. Ueshima, T. Akutsu, D. Yamafuji, T. Furuta, F. Sakakibara and T.-a. Yamagishi, The template effect of solvents on high yield synthesis, co-cyclization of pillar[6]arenes and interconversion between pillar[5]- and pillar[6]arenes, Chem. Commun., 2014, 50, 5774–5777 RSC; (e) Y. Chao, T. U. Thikekar, W. Fang, R. Chang, J. Xu, N. Ouyang, J. Xu, Y. Gao, M. Guo, H. Zuilhof and A. C.-H. Sue, “Rim-Differentiated” Pillar[6]arenes, Angew. Chem., Int. Ed., 2022, 61, e202204589 CrossRef CAS PubMed.
- (a) N. Kubota, Y. Segawa and K. Itami, η6-Cycloparaphenylene Transition Metal Complexes: Synthesis, Structure, Photophysical Properties, and Application to the Selective Monofunctionalization of Cycloparaphenylenes, J. Am. Chem. Soc., 2015, 137, 1356–1361 CrossRef CAS PubMed; (b) E. Kayahara, R. Qu and S. Yamago, Bromination of Cycloparaphenylenes: Strain-Induced Site-Selective Bis-Addition and Its Application for Late-Stage Functionalization, Angew. Chem., Int. Ed., 2017, 56, 10428–10432 CrossRef CAS PubMed; (c) N. Narita, Y. Kurita, K. Osakada, T. Ide, H. Kawai and Y. Tsuchido, A dodecamethoxy[6]cycloparaphenylene consisting entirely of hydroquinone ethers: unveiling in-plane aromaticity through a rotaxane structure, Nat. Commun., 2023, 14, 8091 CrossRef CAS PubMed; (d) H. Shudo, M. Kuwayama, Y. Segawa, A. Yagi and K. Itami, Half-substituted fluorocycloparaphenylenes with high symmetry: synthesis, properties and derivatization to densely substituted carbon nanorings, Chem. Commun., 2023, 59, 13494–13497 RSC.
- (a) S. Fa, T. Kakuta, T.-a. Yamagishi and T. Ogoshi, Conformation and Planar Chirality of Pillar[n]arenes, Chem. Lett., 2019, 48, 1278–1287 CrossRef CAS; (b) J.-F. Chen, J.-D. Ding and T.-B. Wei, Pillararenes: fascinating planar chiral macrocyclic arenes, Chem. Commun., 2021, 57, 9029–9039 RSC; (c) C. Shi, H. Lia, X. Shic, L. Zhao and H. Qiu, Chiral pillar[n]arenes: Conformation inversion, material preparation and applications, Chin. Chem. Lett., 2022, 33, 3613–3622 CrossRef CAS; (d) K. Kato, S. Fa and T. Ogoshi, Alignment and Dynamic Inversion of Planar Chirality in Pillar[n]arenes, Angew. Chem., Int. Ed., 2023, 62, e202308316 CrossRef CAS PubMed.
- (a) N. L. Strutt, H. Zhang, S. T. Schneebeli and J. F. Stoddart, Functionalizing Pillar[n]arenes, Acc. Chem. Res., 2014, 47, 2631–2642 CrossRef CAS PubMed; (b) T. Ogoshi, R. Shiga, M. Hashizume and T.-a. Yamagishi, “Clickable” pillar[5]arenes, Chem. Commun., 2011, 47, 6927–6929 RSC; (c) L.-Z. Liu, X. Qin, W.-G. Duan, H.-F. Huang, W.-X. Zhang, Q.-Q. Zhou and Y. Huang, Aggregation-induced near-infrared absorption of a pillar[5]arene trimer by charge transfer interaction, Dyes Pigm., 2018, 158, 390–395 CrossRef CAS; (d) W. Xue, P. Y. Zavalij and L. Isaacs, Pillar[n]MaxQ: A New High Affinity Host Family for Sequestration in Water, Angew. Chem., Int. Ed., 2020, 59, 13313–13319 CrossRef CAS PubMed; (e) T. Ogoshi, K. Demachi, K. Masaki and T.-a. Yamagishi, Diastereoselective synthesis of meso-pillar[6]arenes by bridging between hydroquinone units in an alternating up-and-down manner, Chem. Commun., 2013, 49, 3952–3954 RSC; (f) Y. Chen, M. He, B. Li, L. Wang, H. Meier and D. Cao, A monophosphoryl copillar[5]arene: synthesis and host–guest complexation with alkanols, RSC Adv., 2013, 3, 21405–21408 RSC.
- Sterically less bulky alkyne and alkene units were introduced at all the substitution positions: (a) T. Ogoshi, K. Umeda, T.-a. Yamagishi and Y. Nakamoto, Through-space π-delocalized Pillar[5]arene, Chem. Commun., 2009, 4874–4876 RSC; (b) Y. Li, Y. Segawa, A. Yagi and K. Itami, A Nonalternant Aromatic Belt: Methylene-Bridged [6]Cycloparaphenylene Synthesized from Pillar[6]arene, J. Am. Chem. Soc., 2020, 142, 12850–12856 CrossRef CAS PubMed.
- (a) N. L. Strutt, H. Zhang, S. T. Schneebeli and J. F. Stoddart, Amino-Functionalized Pillar[5]arene, Chem.–Eur. J., 2014, 20, 10996–11004 CrossRef CAS PubMed; (b) M. r. Bojtár, A. s. Simon, P. Bombicz and I. n. Bitter, Expanding the Pillararene Chemistry: Synthesis and Application of a 10 + 1 Functionalized Pillar[5]arene, Org. Lett., 2017, 19, 4528–4531 CrossRef PubMed; (c) E. Li, K. Jie, Y. Fang, P. Cai and F. Huang, Transformation of Nonporous Adaptive Pillar[4]arene[1]quinone Crystals into Fluorescent Crystals via Multi-Step Solid–Vapor Postsynthetic Modification for Fluorescence Turn-on Sensing of Ethylenediamine, J. Am. Chem. Soc., 2020, 142, 15560–15568 CrossRef CAS PubMed; (d) Z. Wang, Y. A. Liu, H. Yang, W.-B. Hu and K. Wen, ortho-Functionalization of Pillar[5]arene: An Approach to Mono-ortho-Alkyl/Aryl-Substituted A1/A2-Dihydroxypillar[5]arene, Org. Lett., 2022, 24, 1822–1826 CrossRef CAS PubMed.
- (a) W.-B. Hu, W.-J. Hu, X.-L. Zhao, Y. A. Liu, J.-S. Li, B. Jiang and K. Wen, A1/A2-Diamino-Substituted Pillar[5]arene-Based Acid–Base-Responsive Host–Guest System, J. Org. Chem., 2016, 81, 3877–3881 CrossRef CAS PubMed; (b) C. Han, D. Zhao, Z. Lü, F. Zhan, L. Zhang, S. Dong and L. Jin, Synthesis of a Difunctionalized Pillar[5]arene with Hydroxyl and Amino Groups at A1/A2 Positions, Eur. J. Org. Chem., 2019, 2019, 2508–2512 CrossRef CAS; (c) G. Wang, H. Qiang, Y.-Z. Guo, J. Yang, K. Wen and W.-B. Hu, Systematic rim cyano-functionalization of pillar[5]arene and corresponding host–guest property varieties, Org. Biomol. Chem., 2019, 17, 4600–4604 RSC; (d) C.-L. Song, Z. Li, J.-R. Wu, T. Lu and Y.-W. Yang, Intramolecular Through-Space Interactions Induced Emission of Pillar[4]arene[1]dicyanobenzene, Chem. Mater., 2022, 34, 10181–10189 CrossRef CAS.
- (a) N. L. Strutt, D. Fairen-Jimenez, J. Iehl, M. B. Lalonde, R. Q. Snurr, O. K. Farha, J. T. Hupp and J. F. Stoddart, Incorporation of an A1/A2-Difunctionalized Pillar[5]arene into a Metal–Organic Framework, J. Am. Chem. Soc., 2012, 134, 17436–17439 CrossRef CAS PubMed; (b) T. Ogoshi, D. Yamafuji, T. Akutsu, M. Naito and T.-a. Yamagishi, Achiral guest-induced chiroptical changes of a planar-chiral pillar[5]arene containing one π-conjugated unit, Chem. Commun., 2013, 49, 8782–8784 RSC; (c) N. L. Strutt, H. Zhanga and J. F. Stoddart, Enantiopure pillar[5]arene active domains within a homochiral metal–organic framework, Chem. Commun., 2014, 50, 7455–7458 RSC; (d) J.-F. Chen, X. Yin, B. Wang, K. Zhang, G. Meng, S. Zhang, Y. Shi, N. Wang, S. Wang and P. Chen, Planar Chiral Organoboranes with Thermoresponsive Emission and Circularly Polarized Luminescence: Integration of Pillar[5]arenes with Boron Chemistry, Angew. Chem., Int. Ed., 2020, 59, 11267–11272 CrossRef CAS PubMed; (e) One rim of pillar[5]arene was fully replaced with aryl groups: P. Demay-Drouhard, K. Du, K. Samanta, X. Wan, W. Yang, R. Srinivasan, A. C. H. Sue and H. Zuilhof, Functionalization at Will of Rim-Differentiated Pillar[5]arenes, Org. Lett., 2019, 21, 3976–3980 CrossRef CAS PubMed; (f) K. Kato, Y. Kurakake, S. Ohtani, S. Fa, M. Gon, K. Tanaka and T. Ogoshi, Discrete Macrocycles with Fixed Chirality and Two Distinct Sides: Dipole-Dependent Chiroptical Response, Angew. Chem., Int. Ed., 2022, 61, e202209222 CrossRef CAS PubMed.
- K. Kato, T. Kaneda, S. Ohtani and T. Ogoshi, Per-Arylation of Pillar[n]arenes: An Effective Tool to Modify the Properties of Macrocycles, J. Am. Chem. Soc., 2023, 145, 6905–6913 CrossRef CAS PubMed.
- (a) T. Ogoshi, K. Masaki, R. Shiga, K. Kitajima and T.-a. Yamagishi, Planar-Chiral Macrocyclic Host Pillar[5]arene: No Rotation of Units and Isolation of Enantiomers by Introducing Bulky Substituents, Org. Lett., 2011, 13, 1264–1266 CrossRef CAS PubMed; (b) T. Ogoshi, N. Ueshima, T. Akutsu, D. Yamafuji, T. Furuta, F. Sakakibara and T.-a. Yamagishi, The template effect of solvents on high yield synthesis, co-cyclization of pillar[6]arenes and interconversion between pillar[5]- and pillar[6]arenes, Chem. Commun., 2014, 50, 5774–5777 RSC; (c) S. Mirzaei, D. Wang, S. V. Lindeman, C. M. Sem and R. Rathore, Highly Selective Synthesis of Pillar[n]arene (n = 5, 6), Org. Lett., 2018, 20, 6583–6586 CrossRef CAS PubMed.
- K. Jie, M. Liu, Y. Zhou, M. A. Little, A. Pulido, S. Y. Chong, A. Stephenson, A. R. Hughes, F. Sakakibara, T. Ogoshi, F. d. r. Blanc, G. M. Day, F. Huang and A. I. Cooper, Near-Ideal Xylene Selectivity in Adaptive Molecular Pillar[n]arene Crystals, J. Am. Chem. Soc., 2018, 140, 6921–6930 CrossRef CAS PubMed.
- C. J. O'Brien, E. A. B. Kantchev, C. Valente, N. Hadei, G. A. Chass, A. Lough, A. C. Hopkinson and M. G. Organ, Easily Prepared Air- and Moisture-Stable Pd–NHC (NHC=N-Heterocyclic Carbene) Complexes: A Reliable, User-Friendly, Highly Active Palladium Precatalyst for the Suzuki–Miyaura Reaction, Chem.–Eur. J., 2006, 12, 4743–4748 CrossRef PubMed.
- (a) K. Kato, S. Ohtani, S. Fa and T. Ogoshi, Discrete and Continuous One-Dimensional Channels Based on Pillar[n]arenes, Bull. Chem. Soc. Jpn., 2021, 94, 2319–2328 CrossRef CAS; (b) S. Ohtani, K. Kato, S. Fa and T. Ogoshi, Host–Guest chemistry based on solid-state pillar[n]arenes, Coord. Chem. Rev., 2022, 462, 214503 CrossRef CAS; (c) L.-L. Tan, H. Li, Y. Tao, S. X.-A. Zhang, B. Wang and Y.-W. Yang, Pillar[5]arene-Based Supramolecular Organic Frameworks for Highly Selective CO2-Capture at Ambient Conditions, Adv. Mater., 2014, 26, 7027–7031 CrossRef CAS PubMed; (d) T. Ogoshi, R. Sueto, M. Yagyu, R. Kojima, T. Kakuta, T.-a. Yamagishi, K. Doitomi, A. K. Tummanapelli, H. Hirao, Y. Sakata, S. Akine and M. Mizuno, Molecular weight fractionation by confinement of polymer in one-dimensional pillar[5]arene channels, Nat. Commun., 2019, 10, 479 CrossRef PubMed; (e) K. Kato, K. Maeda, M. Mizuno, Y. Nishina, S. Fa, S. Ohtani and T. Ogoshi, Room-Temperature Ring-Opening Polymerization of δ-Valerolactone and ε-Caprolactone Caused by Uptake into Porous Pillar[5]arene Crystals, Angew. Chem., Int. Ed., 2022, 61, e202212874 CrossRef CAS PubMed.
- (a) Z. Zhang, B. Xia, C. Han, Y. Yu and F. Huang, Syntheses of Copillar[5]arenes by Co-oligomerization of Different Monomers, Org. Lett., 2010, 12, 3285–3287 CrossRef CAS PubMed; (b) T. Ogoshi, R. Shiga, T.-a. Yamagishi and Y. Nakamoto, Planar-Chiral Pillar[5]arene: Chiral Switches Induced by Multiexternal Stimulus of Temperature, Solvents, and Addition of Achiral Guest Molecule, J. Org. Chem., 2011, 76, 618–622 CrossRef CAS PubMed; (c) X. Shu, S. Chen, J. Li, Z. Chen, L. Weng, X. Jia and C. Li, Highly effective binding of neutral dinitriles by simple pillar[5]arenes, Chem. Commun., 2012, 48, 2967–2969 RSC.
- A. P. Rice, F. S. Tham and E. L. Chronister, A Temperature Dependent X-ray Study of the Order–Disorder Enantiotropic Phase Transition of p-Terphenyl, J. Chem. Crystallogr., 2013, 43, 14–25 CrossRef CAS.
- Representative studies on rotational behaviours: (a) K. Du, P. Demay-Drouhard, K. Samanta, S. Li, T. U. Thikekar, H. Wang, M. Guo, B. v. Lagen, H. Zuilhof and A. C.-H. Sue, Stereochemical Inversion of Rim-Differentiated Pillar[5]arene Molecular Swings, J. Org. Chem., 2020, 85, 11368–11374 CrossRef CAS PubMed; (b) Y. Nagata, M. Suzuki, Y. Shimada, H. Sengoku, S. Nishida, T. Kakuta, T.-a. Yamagishi, M. Suginome and T. Ogoshi, Holding of planar chirality of pillar[5]arene by kinetic trapping using host–guest interactions with achiral guest solvents, Chem. Commun., 2020, 56, 8424–8427 RSC; (c) J. Ji, X. Wei, W. Wu, C. Fan, D. Zhou, K. Kanagaraj, G. Cheng, K. Luo, X.-G. Meng and C. Yang, The More the Slower: Self-Inhibition in Supramolecular Chirality Induction, Memory, Erasure, and Reversion, J. Am. Chem. Soc., 2022, 144, 1455–1463 CrossRef CAS PubMed; (d) K. Wada, S. Ohtani, K. Kato and T. Ogoshi, Stable planar chirality of arylated pillar[6]arene and its thermal response, Tetrahedron Lett., 2024, 135, 154891 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, NMR and MS spectra, X-ray crystallographic data, optical spectra, HPLC charts, and results of theoretical calculations. CCDC 2332554. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc01042f |
| This journal is © The Royal Society of Chemistry 2024 |