Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mechanochemically-induced glass formation from two-dimensional hybrid organic–inorganic perovskites†
Chumei
Ye
 ab,
Giulio I.
Lampronti
ab,
Giulio I.
Lampronti
 a,
Lauren N.
McHugh
a,
Lauren N.
McHugh
 c,
Celia
Castillo-Blas
c,
Celia
Castillo-Blas
 a,
Ayano
Kono
a,
Celia
Chen
ab,
Georgina P.
Robertson
a,
Liam A. V.
Nagle-Cocco
a,
Ayano
Kono
a,
Celia
Chen
ab,
Georgina P.
Robertson
a,
Liam A. V.
Nagle-Cocco
 b,
Weidong
Xu
d,
Samuel D.
Stranks
b,
Weidong
Xu
d,
Samuel D.
Stranks
 bd,
Valentina
Martinez
bd,
Valentina
Martinez
 e,
Ivana
Brekalo
e,
Ivana
Brekalo
 e,
Bahar
Karadeniz
e,
Krunoslav
Užarević
e,
Bahar
Karadeniz
e,
Krunoslav
Užarević
 e,
Wenlong
Xue
e,
Wenlong
Xue
 f,
Pascal
Kolodzeiski
f,
Chinmoy
Das
f,
Pascal
Kolodzeiski
f,
Chinmoy
Das
 fg,
Philip
Chater
fg,
Philip
Chater
 h,
David A.
Keen
h,
David A.
Keen
 i,
Siân E.
Dutton
i,
Siân E.
Dutton
 b and
Thomas D.
Bennett
b and
Thomas D.
Bennett
 *a
*a
aDepartment of Materials Science and Metallurgy, University of Cambridge, 27 Charles Babbage Road, Cambridge, Cambridgeshire CB3 0FS, UK. E-mail: tdb35@cam.ac.uk
bCavendish Laboratory, University of Cambridge, J. J. Thomson Avenue, Cambridge, Cambridgeshire CB3 0HE, UK
cDepartment of Chemistry, University of Liverpool, Crown Street, Liverpool, L69 7ZD, UK
dDepartment of Chemical Engineering and Biotechnology, University of Cambridge, Philippa Fawcett Drive, Cambridge, Cambridgeshire CB3 0AS, UK
eDivision of Physical Chemistry, Ruđer Bošković Institute, Zagreb, Croatia
fAnorganische Chemie, Fakultät für Chemie und Chemische Biologie, Technische Universität Dortmund, Otto-Hahn-Straße 6, 44227, Dortmund, Germany
gDepartment of Chemistry, SRM University-AP, Andhra Pradesh-522240, India
hDiamond Light Source Ltd., Diamond House, Harwell Campus, Didcot, Oxfordshire OX11 0QX, UK
iISIS Facility, Rutherford Appleton Laboratory, Harwell Campus, Didcot, Oxfordshire OX11 0QX, UK
First published on 19th April 2024
Abstract
Hybrid organic–inorganic perovskites (HOIPs) occupy a prominent position in the field of materials chemistry due to their attractive optoelectronic properties. While extensive work has been done on the crystalline materials over the past decades, the newly reported glasses formed from HOIPs open up a new avenue for perovskite research with their unique structures and functionalities. Melt-quenching is the predominant route to glass formation; however, the absence of a stable liquid state prior to thermal decomposition precludes this method for most HOIPs. In this work, we describe the first mechanochemically-induced crystal-glass transformation of HOIPs as a rapid, green and efficient approach for producing glasses. The amorphous phase was formed from the crystalline phase within 10 minutes of ball-milling, and exhibited glass transition behaviour as evidenced by thermal analysis techniques. Time-resolved in situ ball-milling with synchrotron powder diffraction was employed to study the microstructural evolution of amorphisation, which showed that the crystallite size reaches a comminution limit before the amorphisation process is complete, indicating that energy may be further accumulated as crystal defects. Total scattering experiments revealed the limited short-range order of amorphous HOIPs, and their optical properties were studied by ultraviolet-visible (UV-vis) spectroscopy and photoluminescence (PL) spectroscopy.
Introduction
Recently, hybrid glasses derived from hybrid materials, including coordination polymers (CPs), metal–organic frameworks (MOFs) and hybrid organic–inorganic perovskites (HOIPs), have attracted broad interest across materials science.1–3 These glassy materials not only inherit chemical diversity and compositional tunability from their crystalline counterparts but also show advantages such as optical transparency and high moldability, as demonstrated in some other glasses.4 The intrinsic disorder in hybrid glasses can endow materials with attractive functionalities and show promising applications in areas such as gas adsorption, separation, and ion transport.5–7The most popular approach to forming glasses from hybrid materials is melt-quenching; i.e., heating a crystalline sample above its melting point (Tm), followed by rapid cooling to achieve vitrification. However, this is limited to a few thermally stable hybrid materials that melt prior to decomposition.1 Mechanochemistry has recently emerged as a powerful tool for the green synthesis of a variety of solid materials as it reduces or minimises the consumption of organic solvents and thermal energy.8 In addition to yielding crystalline materials by grinding or ball-milling solid starting reagents,9,10 it also allows for the amorphisation or vitrification of various hybrid materials. Without the need to form stable liquids as in melt-quenching, this direct mechanochemically-induced vitrification method of preparing a glass from a crystal may accordingly be applicable to a wider range of materials, which may form glasses. Thus far, mechanochemically-induced hybrid glasses have been reported in several CPs and MOFs, including phosphate-azole frameworks M2+(1,2,4-trizole)2(H2PO4)2 (M2+ = Cd2+, Cr2+, Mn2+),11,12 Ag-tripodal nitrile frameworks [Ag(mL1)(CF3SO3)]·C6H6 and [Ag(pL2)(CF3SO3)]·C6H6 (mL1 = 1,3,5-tris(3-cyanophenylethynyl)benzene, pL2 = 1,3,5-tris(4-cyanophenylethynyl)benzene),13,14 Prussian blue analogues,15 and zeolitic imidazolate frameworks (ZIFs) ZIF-62 (Zn(Im)2−x(bIm)x, Im = imidazolate, bIm = benzimidazolate, x = 0.35, 0.5, 1.0).16 However, at present, there are no reports involving the vitrification of HOIPs through mechanical milling, though the glasses have been obtained via the melt-quenching of three-dimensional [TPrA][M(dca)3]17 (TPrA+ = tetrapropylammonium, M2+ = Mn2+, Fe2+, Co2+, dca = dicyanamide) and two-dimensional (2D) (S-NEA)2PbBr4 (S-NEA+ = (S)-(−)-1-(1-naphthyl)ethylammonium)18 hybrid perovskites. It should be noted that the HOIPs after amorphisation are not strictly based on the perovskite structure-type.
In this study, we describe the first example of mechanochemically-induced crystal-glass transformation of both a melting and a non-melting HOIP crystal. The effect of milling time on their glassy behaviour was investigated using various thermal techniques. Time-resolved in situ synchrotron X-ray diffraction and X-ray total scattering were employed to probe the microstructural evolution of amorphisation, and to compare the difference in local structure between the crystalline and glassy phases. Finally, their optical properties were compared through UV-vis absorption and PL spectroscopy.
Results and discussion
Crystal structure and X-ray diffraction
Two 2D HOIPs, chiral (S-NEA)2PbBr4 and racemic (1-(1-naphthyl)ethylammonium)2PbBr4 ((rac-NEA)2PbBr4), were synthesised following the reported methodology.19 Both compounds are composed of alternatively stacked organic bilayers and inorganic layers of corner-sharing [PbBr6] octahedra (Fig. 1a and b). (S-NEA)2PbBr4 and (rac-NEA)2PbBr4 both crystallise in the monoclinic system, with a space group of P21 and P21/c, respectively. The chiral spacers S-NEA+ cause symmetry-breaking helical distortions in the inorganic lead bromide framework through asymmetric H-bonding interactions, which are absent in the racemic analogue.19 Powder X-ray diffraction (PXRD) was performed on the as-synthesised (S-NEA)2PbBr4 (Fig. 1c) and (rac-NEA)2PbBr4 (Fig. 1d), and their purity was confirmed through Pawley refinements (see Fig. S1, S2, Tables S1 and S2 in ESI†).20,21 Fully amorphous am(S-NEA)2PbBr4 (am = mechanically amorphised) and am(rac-NEA)2PbBr4 were produced after 30 minutes of ball-milling at 30 Hz using one stainless steel ball and exhibit featureless PXRD patterns typical of amorphous materials (Fig. 1c and d).Thermal analysis, CHN analysis and 1H NMR
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were further conducted on am(S-NEA)2PbBr4 products (Fig. 2a and S3–S5†). No obvious mass loss (<1 wt%) was observed below 200 °C in TGA. The DSC upscan showed typical glass transition behaviour, which is an intrinsic characteristic of a glass and features a reversible transition from a relatively hard and brittle “glassy” phase to a softer and more “liquid-like” phase.4 The glass transition temperature (Tg = 51 °C) of am(S-NEA)2PbBr4 was lower than that of a glass sample produced via melt-quenching (Fig. S5†).18 Similar behaviour has been observed in several CP glasses14 and MOF glasses.16 Following the glass transition, an exothermic event was observed at 94 °C in the DSC profile (Fig. 2a). PXRD confirmed that it arose from recrystallisation, as the annealed am(S-NEA)2PbBr4 is isostructural to the parent crystalline phase (Fig. S6, S7 and Table S3†). It was also observed that am(S-NEA)2PbBr4 recrystallised in an ambient environment within 9 hours (Fig. S8–S10 and Tables S4 and S5†). When stored in the freezer at ca. 0 °C (Fig. S11a†), or stored under vacuum at room temperature (Fig. S11b†), the recrystallisation process of am(S-NEA)2PbBr4 was slowed, though weak Bragg peaks of the crystalline counterpart appeared again after 48 hours. This suggests that storage at low temperature or isolation from moisture (Fig. S12†) is beneficial in hindering the glass-crystal transformation. CHN microanalysis (Table S6†) and 1H NMR spectroscopy (Fig. S13†) confirmed that the organic cations (S-NEA)+ were well preserved in am(S-NEA)2PbBr4 upon glass formation. In addition to the chiral HOIP, the glassy phase of the racemic analogue was also observed after ball-milling, which exhibited a Tg of 49 °C and a recrystallisation temperature (Tx) of 84 °C (Fig. 2b and S14†). To our knowledge this is the first report of a glassy phase of (rac-NEA)2PbBr4 as decomposition of the melt prevents glass formation via conventional melt-quenching (Fig. S3b†).18Effect of milling time on glassy behaviour
To examine the effect of ball-milling time on the thermal behaviour of am(S-NEA)2PbBr4, ex situ ball-milling treatments were performed on crystalline (S-NEA)2PbBr4 powders for 5, 10, 30 and 60 minutes. The products are thus denoted as am(S-NEA)2PbBr4-x, where x is the milling time in minutes. A weak crystalline peak at 4.6° was retained in the PXRD of am(S-NEA)2PbBr4-5 (Fig. 3a), suggesting that the amorphisation was not completed within 5 minutes. However, after 10 minutes of ball-milling, only diffuse scattering was observed, supporting its full transformation to the amorphous phase. Additionally, the product remained amorphous after 60 minutes of ball-milling with no further phase changes. DSC measurements showed that the Tgs of am(S-NEA)2PbBr4-10, am(S-NEA)2PbBr4-30 and am(S-NEA)2PbBr4-60 were 56 °C, 51 °C and 48 °C, respectively (Fig. 3b). This decreasing Tg likely arises from a weaker network connectivity upon longer milling time.22 The recrystallisation temperature and the difference in enthalpy of recrystallisation (ΔHx) are 97 °C and −22 ± 1 J g−1 (am(S-NEA)2PbBr4-10), 94 °C and −20 ± 1 J g−1 (am(S-NEA)2PbBr4-30), 92 °C and −19 ± 1 J g−1 (am(S-NEA)2PbBr4-60), respectively (Fig. 3b and S15†). FTIR spectra of crystalline (S-NEA)2PbBr4 and am(S-NEA)2PbBr4-x with different milling times were collected from 4000–550 cm−1 to confirm the presence of the organic (S-NEA)+ cation in the structure. They showed identical absorbance, including N–H and C–H stretching at around 3000 cm−1, aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching at 1580 cm−1 and C–H bending at 770 cm−1, indicating that no new chemical bonds were formed during the milling process, and that the structure of the organic cations remained intact (Fig. S16†).
C stretching at 1580 cm−1 and C–H bending at 770 cm−1, indicating that no new chemical bonds were formed during the milling process, and that the structure of the organic cations remained intact (Fig. S16†).
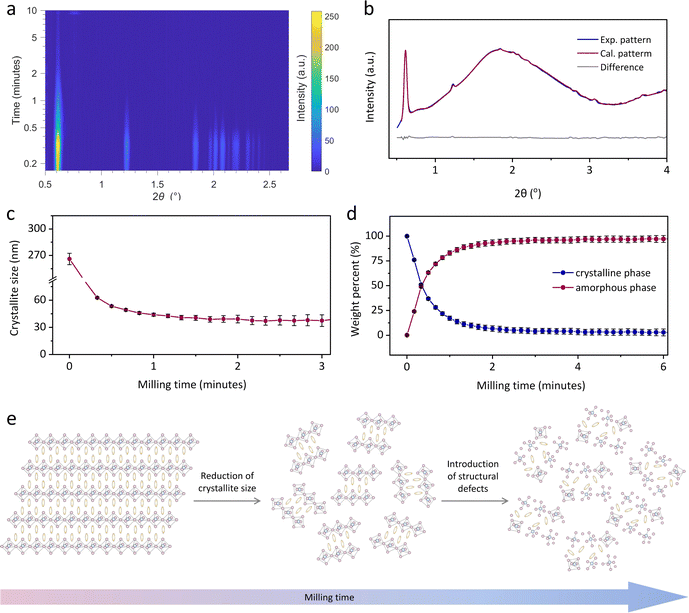
Microstructural evolution upon ball-milling
To further illustrate the mechanochemically-induced amorphisation in 2D HOIPs, time-resolved in situ (TRIS) ball-milling synchrotron PXRD data was collected at the PETRA III beamline P02.1 in DESY (Fig. 4).23 It revealed that crystalline (S-NEA)2PbBr4 took ca. 5 minutes to fully amorphise (Fig. 4a and S17†), which was quicker than amorphisation under laboratory milling (Fig. 3a). This is attributed to the slightly different milling conditions. For example, the in situ measurements were performed in a PMMA milling jar (Fig. S18†) to allow the penetration of X-ray beams, while conventional laboratory ball-milling experiments used stainless steel milling jars. The subsequent Rietveld refinements24 were carried out on the diffraction data using TOPAS Academic V7 (ref. 21) to demonstrate the microstructural evolution (Fig. S19–S22†).25,26 Details of the refinement strategy are reported in the ESI.† The sample used in the in situ experiment was found to contain a minor impurity (<2% in weight) of lead oxide (Pb3O4), probably an impurity from the synthesis. This did not change in abundance during the experiment nor did it affect the amorphisation process. Fig. 4b shows an example of a Rietveld refinement, indicating the quality of the fit. During measurements, the Scherrer crystal size decreased rapidly from ca. 300 nm to reach what looks to be a comminution average crystallite size limit of ca. 40 nm within 20 seconds, when the amorphous fraction is less than 50 wt% (Fig. 4c and d).26,27 Milling causes a decrease in the crystal quality in the powder, initially through crystal comminution with an increase in crystal surface energy. At some comminution limit,‡ further breakage is not possible, we accordingly hypothesise that the excess energy is stored in the nanocrystallites as structural defects (Fig. 4e). The accumulation of defects in the crystallites generally results in microstrain contribution to peak broadening, which is expected to be more significant at higher 2θ angle compared to the contribution of crystal size. Unfortunately, the weak signal of the reflections in the higher angle range did not allow for reliable estimates of the microstrain contribution to peak broadening beyond the first few scans. Following complete amorphisation, further heating towards ca. 80 °C resulted in rapid recrystallisation of am(S-NEA)2PbBr4 within 5 minutes (at ca. 50 °C, see Fig. S23†), highlighting the strong tendency for am(S-NEA)2PbBr4 to recrystallise.Local structure analysis from pair distribution functions
The atomic structures of crystalline (S-NEA)2PbBr4 and the corresponding mechanochemically-induced glass were probed via synchrotron X-ray total scattering conducted at beamline I15-1 at the Diamond Light Source. The absence of crystallinity in the amorphous HOIP is evidenced by the lack of sharp features in the structure factor (Fig. S24†). Pair distribution functions (PDFs) of crystalline (S-NEA)2PbBr4 and am(S-NEA)2PbBr4 were obtained from the Fourier transformation of the corrected total scattering data (Fig. 5). As expected, the crystalline HOIP contains long-range oscillations in the D(r) on length scales above 10 Å, whereas the glassy sample PDF is relatively featureless in this region (Fig. 5a). The lack of distinct correlations over longer distances in am(S-NEA)2PbBr4 confirms its transformation to the glass phase.At low-r, the PDF profiles for crystal and glass are similar below 5 Å (Fig. 5b). Peak assignment was carried out using PDFgui,28 where partial PDFs for atom pairs were calculated based on the crystal structure (Fig. S25†). Note that correlations involving strongly-scattering Pb (and to a lesser extent Br) atoms tend to dominate the PDF signal. The most intense feature at 3 Å (labelled 1 in Fig. 5b) is assigned to the nearest Pb–Br distance, while the second peak at 4.2 Å (labelled 2 in Fig. 5b) corresponds to Br–Br interactions within the same [PbBr6]2− octahedron. Correlations 3 to 4 are dominated by Pb–Pb and Pb–Br interactions between two neighbouring octahedra, respectively. These features reveal that the limited short-range order is preserved after the crystal-glass phase transition of HOIPs.
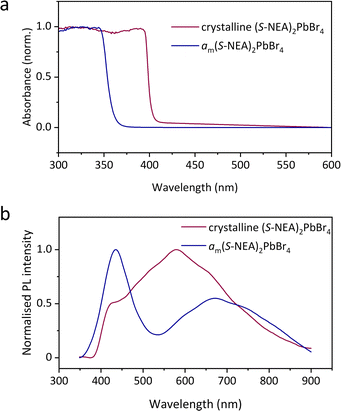
Optical properties
The optical properties of both crystalline HOIPs and amHOIPs were studied by UV-vis absorption and PL spectroscopy at ambient temperature (Fig. 6). The absorption onset is located at approximately 400 nm for crystalline (S-NEA)2PbBr4 powders at ambient temperature (Fig. 6a), which is in accordance with the reported result for the crystalline film on soda lime glass.18 It shifts to approximately 360 nm for am(S-NEA)2PbBr4, suggesting that the mechanochemically-induced crystal-glass transformation results in a significant change of the bandgap structure. The PL spectra of crystalline (S-NEA)2PbBr4 exhibits a broad emission at around 600 nm (Fig. 6b), consistent with the reported literature.19 It may originate from self-trapped excitonic emission19 or halide vacancies,29 which has been commonly observed in two-dimensional hybrid perovskites.30–32 In comparison to the crystalline phase, the PL emission of am(S-NEA)2PbBr4 shows a significant shift in wavelength, agreeing well with the absorption spectra (Fig. 6a), while the intensity decreases drastically upon mechanochemically-induced crystal-glass transformation (Fig. S26†).Conclusions
In conclusion, we demonstrate the formation of amorphous am(S-NEA)2PbBr4 and am(rac-NEA)2PbBr4via ball-milling. These amHOIPs show clear glass transitions and recrystallisation upon heating despite not being melt-quenched. The relatively low Tg-values of amHOIPs point to greater glass processability, which opens exciting new opportunities for their industrial implementation. Time-resolved in situ synchrotron powder X-ray diffraction monitored the microstructural evolution of amorphisation, indicating that during the milling process, the crystallites reach a crystallite size comminution limit well before the amorphisation process reaches completion. Further energy may be accumulated as structural defects. The am(S-NEA)2PbBr4 retains limited short-range order as determined by pair distribution function measurements. UV-vis absorption study and photoluminescence spectroscopy show that the optical properties of the glasses formed from HOIPs are significantly different from those of their crystalline counterparts. This mechanochemically-induced amorphisation approach to achieving the crystal-glass transformation of HOIPs circumvents the requirement to melt-quench a crystalline material to form a glass. It provides a promising case study, illustrating that ball-milling may be applied to a wide variety of glass-forming systems in addition to conventional coordination polymers and metal–organic frameworks.Experimental
Materials
Lead(II) bromide (99.999%) was purchased from Aldrich. (S)-(−)-1-(1-naphthyl)ethylamine (99%) and 1-(1-naphthyl)ethylamine (99%) were purchased from Thermo Fisher and Fluorochem, respectively. Hydrobromic acid (47 wt% HBr in H2O) was purchased from VMR Chemicals. Diethyl ether (99.8+%) was purchased from Sigma-Aldrich. Methanol was purchased from Fisher Scientific. All reagents were used without further purification.Synthesis of chiral (S-NEA)2PbBr4
PbBr2 (0.24 mmol, 90 mg) and (S)-(−)-1-(1-naphthyl)ethylamine (0.48 mmol, 78 μL) were dissolved in a mixed solution of hydrobromic acid (1.0 mL) and deionized water (2.4 mL) at 95 °C in a sealed vial. The resultant solution was gradually cooled down to room temperature over a period of two days to obtain crystals with flake-like morphologies, which were then filtered, washed with diethyl ether and dried in vacuum at 90 °C for 12 hours. This synthetic method was designed based on the reported synthesis of (S-NEA)2PbBr4.18Synthesis of racemic (rac-NEA)2PbBr4
Crystals of (rac-NEA)2PbBr4 were grown in a similar way from a solution of PbBr2 (90 mg, 0.24 mmol) and 1-(1-naphthyl)ethylamine (78 μL, 0.48 mmol) in hydrobromic acid (1.0 mL) and methanol (2.4 mL).Powder X-ray diffraction (PXRD)
Finely ground samples were compacted into 5 mm flat plate discs and data were collected on the Bruker D8 ADVANCE, using CuKα radiation (λ = 1.5418 Å) as the X-ray source. Measurements were carried out at room temperature over the 2θ range of 3° to 70° for all materials, with a step size of 0.02° and measurement time of 0.750 seconds per step. Pawley refinements20 were performed using TOPAS-Academic Version 7.21 The lattice parameters were refined over the 2θ range of 3–70° against the values obtained from the published Crystallographic Information Files CCDC 2015618 for (S-NEA)2PbBr4 and CCDC 2015614 for (rac-NEA)2PbBr4.19Lab ball-milling experiments
Approximately 200 mg of finely ground samples were placed in a 10 mL stainless steel ball-mill jar with one 10 mm stainless steel ball of around 4 g. The powders were ball-milled at 30 Hz for different durations including 5, 10, 30 and 60 minutes at room temperature. After the ball-milling process finished, samples were immediately taken out for further measurements.Time-resolved in situ (TRIS) ball-milling powder X-ray diffraction
In situ synchrotron PXRD experiments were performed at PETRA III beamline P02.1 (λ = 0.207351 Å), DESY Germany. Data was collected on a Varex XRD4343CT detector, and milling was conducted using a modified IST-636 ball mill, controlled remotely from outside the experimental hutch. Beam alignment and calibration were performed using a Si standard in a poly(methyl methacrylate) (PMMA) milling jar. Data was processed by removing the amorphous background contribution from PMMA. To investigate the amorphisation process, around 100 mg finely ground (S-NEA)2PbBr4 powders were placed into a 10 mL PMMA jar with one 10 mm stainless-steel ball of around 4 g and then milled at 30 Hz for around 40 minutes at room temperature. Following this, the milling ball was removed, and a heating jacket was subsequently attached to the jar to observe recrystallisation.TRIS ball-milling PXRD data analysis
To obtain the classic one-dimensional PXRD pattern, the collected two-dimensional diffraction images were integrated with the DAWN Science package. Time-resolved diffractograms were generated by MATLAB R2023a using the MATLAB scripts developed by Dr Stipe Lukin. The structure was first optimized via Rietveld refinement using laboratory powder diffraction data with TOPAS Academic V7 (see ESI† for details). Determination of the instrumental resolution function and sequential Rietveld refinements were then performed according to the strategies previously described.25,26 Details of the sequential refinements are described in the ESI.† Raw data and sequential input files for TOPAS are supplied as ESI.†Thermogravimetric analysis (TGA)
TGA measurements were performed on a METTLER TOLEDO TGA2. Approximately 2–5 mg of evacuated samples were placed on an alumina crucible. Data were collected under argon in the range of 30–800 °C with a ramp rate of 10 °C min−1. Data analysis was performed using the TA Instruments Universal Analysis software package.Differential scanning calorimetry (DSC)
DSC experiments were performed on a NETZSCH DSC 214 Polyma and data were processed by the Proteus Analysis software. Approximately 2–5 mg of samples were placed into an aluminium crucible with a pierced lid, compressed by a hand press kit and situated at the sample position in DSC. An empty aluminium crucible was used as a reference. Samples were heated to 190 °C at 10 °C min−1 unless otherwise stated. Tm was taken as the onset of the melting endotherm, while Tg was taken as the mid-point of the change in gradient of the heat flow.Simultaneous TGA-DSC (SDT)
SDT measurements were performed on a TA Instruments SDT-Q65. Approximately 2–5 mg of evacuated samples were placed on an alumina crucible. Data were collected under argon in the range of 30–800 °C with a ramp rate of 10 °C min−1.CHN microanalysis
CHN combustion analysis experiments were performed using a CE440 Elemental Analyser, EAI Exeter Analytical Inc. It was operated with the tolerances of ±0.2% for the C and ±0.1% for the H and N. Approximately 2–5 mg of sample was used for each run, and two measurements were collected per sample.Fourier-transform infrared spectroscopy (FT-IR)
FT-IR measurements on powder samples were performed on a Bruker Tensor 27 FTIR spectrometer. Data were collected in transmission mode between 600 cm−1 and 4000 cm−1 at room temperature.1H nuclear magnetic resonance spectroscopy (1H NMR)
1H NMR experiments were performed in a Bruker Advance III HD 500 MHz spectrometer at room temperature. Around 1–5 mg samples of am(S-NEA)2PbBr4 were dissolved in DMSO-d6 solution and sonicated for 5 minutes to allow for complete dissolution. Spectra were calibrated using TMS as a standard. Data were processed using MestReNova V14.0.0.X-ray total scattering and pair distribution function (PDF)
X-ray total scattering data were collected at beamline I15-1, Diamond Light Source, UK (EE20038) on crystalline (S-NEA)2PbBr4 and am(S-NEA)2PbBr4 samples. Finely ground samples were loaded into borosilicate glass capillaries (0.78 mm inner diameter) to 3.68 cm for (S-NEA)2PbBr4 and 3.70 cm for am(S-NEA)2PbBr4. The filled capillaries were then sealed with clay before being mounted onto the beamline. Total scattering data were collected at ambient temperature for the background (i.e., empty instrument), empty borosilicate capillary and for both samples in a Q range of 0.4–26 Å−1 (λ = 0.161669 Å). The total scattering data were processed to account for absorption corrections and various scattering corrections (background scattering, multiple scattering, container scattering and Compton scattering) in a Q range of 0.4–20 Å−1. Subsequent Fourier transformation of the processed total scattering data resulted in a real space pair distribution function G(r) for each material. In this work, we use the D(r) form of the pair distribution function to accentuate high r correlations. All total scattering data were processed using GudrunX software following well documented procedures.33–35Ultraviolet-visible (UV-vis) spectroscopy
UV-vis experiments were performed on crystalline (S-NEA)2PbBr4 and am(S-NEA)2PbBr4 at ambient temperature using an Agilent UV-vis spectrophotometer, in the range 200–800 nm. Finely ground samples were sandwiched between two glass slides and then sealed using glue for measurements. Two clean glass slides without samples were also sealed together, and its spectra was taken as background.Steady-state photoluminescence (PL) spectroscopy
PL spectra of crystalline (S-NEA)2PbBr4 and am(S-NEA)2PbBr4 were recorded on the FLS1000 fluorescence spectrometer (Edinburgh Instruments) at room temperature. Excitation was achieved using 300 nm monochromatic light generated from a xenon arc lamp. The resulting PL spectra were acquired using a double-grating Czerny–Turner monochromator and a photomultiplier tube detector. Finely ground samples were sandwiched between two quartz slides and then sealed using clamps for measurements.Data availability
The datasets supporting this article have been uploaded as part of the ESI.†Author contributions
C. Y. conceptualised and designed the project. C. Y. synthesised all the materials. V. M., B. K., C. Y., I. B. and K. U. collected the in situ ball-milling powder X-ray diffraction data and G. I. L. analysed the data. P. K., W. X. (Technische Universität Dortmund), C. D., C. Y. and T. D. B. collected the X-ray total scattering data, while C. Y. analysed the data with inputs from C. C. B., P. C. and D. A. K., C. Y. and W. X. (University of Cambridge) collected the photoluminescence spectra. C. Y. performed and analysed all other experiments. L. N. M., A. K., C. C., G. P. R. and L. N. C. contributed with useful discussions. S. E. D. and T. D. B. supervised the project and acquired funding. C. Y. wrote the manuscript with input from all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank funding and support from St Edmund's College (C. Y.), the Royal Society for a university research fellowship, URF\R\211013 (T. D. B), Leverhulme Trust for a Philip Leverhulme Prize (2019) (T. D. B. and L. N. M.), University of Liverpool (L. N. M.), the Winton Programme for the Physics of Sustainability (S. E. D.), the Cambridge Trusts and EPSRC Cambridge NanoDTC, EP/S022953/1 (C. C.), EPSRC scholarship, EP/R513180/1 (L. A. V. N.-C.), the Leverhulme Trust for a Research Project Grant, RPG-2020-005 (C. C. B. and T. D. B.), the Croatian Science Foundation, IP-2020-02-4702 (V. M., I. B., B. K. and K. U.), the Royal Society and Tata Group, UF150033 (S. D. S.), and the EPSRC, EP/V027131/1. (S. D. S. and W. X.). The authors acknowledge DESY (Hamburg, Germany), a member of the Helmholtz Association HGF, for the provision of experimental facilities (proposal I-20221330 EC). Parts of this research were carried out at PETRA III and we would like to thank Martin Etter and Volodymyr Baran for assistance in using beamline P02.1. We extend our gratitude to Diamond Light Source, Rutherford Appleton Laboratory, United Kingdom, for the provision of synchrotron access to Beamline I15-1. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising from this submission.Notes and references
- C. Ye, L. N. McHugh, C. Chen, S. E. Dutton and T. D. Bennett, Angew. Chem., Int. Ed., 2023, 62, e202302406 CrossRef CAS PubMed.
- N. Ma and S. Horike, Chem. Rev., 2022, 122, 4163–4203 CrossRef CAS PubMed.
- R. Lin, M. Chai, Y. Zhou, V. Chen, T. D. Bennett and J. Hou, Chem. Soc. Rev., 2023, 52, 4149–4172 RSC.
- T. D. Bennett and S. Horike, Nat. Rev. Mater., 2018, 3, 431–440 CrossRef.
- L. N. McHugh and T. D. Bennett, J. Mater. Chem. A, 2022, 10, 19552–19559 RSC.
- Z. Yang, Y. Belmabkhout, L. N. McHugh, D. Ao, Y. Sun, S. Li, Z. Qiao, T. D. Bennett, M. D. Guiver and C. Zhong, Nat. Mater., 2023, 22, 888–894 CrossRef CAS PubMed.
- S. S. Nagarkar, S. Horike, T. Itakura, B. L. Ouay, A. Demessence, M. Tsujimoto and S. Kitagawa, Angew. Chem., Int. Ed., 2017, 56, 4976–4981 CrossRef CAS PubMed.
- V. Martinez, T. Stolar, B. Karadeniz, I. Brekalo and K. Užarević, Nat. Rev. Chem, 2023, 7, 51–65 CrossRef CAS PubMed.
- F. Palazon, Y. E. Ajjouri and H. J. Bolink, Adv. Energy Mater., 2020, 10, 1902499 CrossRef CAS.
- D. Prochowicz, M. Saski, P. Yadav, M. Grätzel and J. Lewiński, Acc. Chem. Res., 2019, 52, 3233–3243 CrossRef CAS PubMed.
- W. Chen, S. Horike, D. Umeyama, N. Ogiwara, T. Itakura, C. Tassel, Y. Goto, H. Kageyama and S. Kitagawa, Angew. Chem., Int. Ed., 2016, 55, 5195–5200 CrossRef CAS PubMed.
- Y. Ohara, A. Hinokimoto, W. Chen, T. Kitao, Y. Nishiyama, Y. L. Hong, S. Kitagawa and S. Horike, Chem. Commun., 2018, 54, 6859–6862 RSC.
- C. Das, T. Ogawa and S. Horike, Chem. Commun., 2020, 56, 8980–8983 RSC.
- C. Das and S. Horike, Faraday Discuss., 2021, 225, 403–413 RSC.
- N. Ma, R. Ohtani, H. M. Le, S. S. Sorensen, R. Ishikawa, S. Kawata, S. Bureekaew, S. Kosasang, Y. Kawazoe, K. Ohara, M. M. Smedskjaer and S. Horike, Nat. Commun., 2022, 13, 4023 CrossRef CAS PubMed.
- M. F. Thorne, A. F. Sapnik, L. N. McHugh, A. M. Bumstead, C. Castillo-Blas, D. S. Keeble, M. Diaz Lopez, P. A. Chater, D. A. Keen and T. D. Bennett, Chem. Commun., 2021, 57, 9272–9275 RSC.
- B. K. Shaw, A. R. Hughes, M. Ducamp, S. Moss, A. Debnath, A. F. Sapnik, M. F. Thorne, L. N. McHugh, A. Pugliese, D. S. Keeble, P. Chater, J. M. Bermudez-Garcia, X. Moya, S. K. Saha, D. A. Keen, F. X. Coudert, F. Blanc and T. D. Bennett, Nat. Chem., 2021, 13, 778–785 CrossRef CAS PubMed.
- A. Singh, M. K. Jana and D. B. Mitzi, Adv. Mater., 2021, 33, e2005868 CrossRef PubMed.
- M. K. Jana, R. Song, H. Liu, D. R. Khanal, S. M. Janke, R. Zhao, C. Liu, Z. V. Vardeny, V. Blum and D. B. Mitzi, Nat. Commun., 2020, 11, 4699 CrossRef CAS PubMed.
- G. S. Pawley, J. Appl. Crystallogr., 1981, 14, 357–361 CrossRef CAS.
- A. A. Coelho, J. Appl. Crystallogr., 2018, 51, 210–218 CrossRef CAS.
- Q. Zheng, Y. Zhang, M. Montazerian, O. Gulbiten, J. C. Mauro, E. D. Zanotto and Y. Yue, Chem. Rev., 2019, 119, 7848–7939 CrossRef CAS PubMed.
- A.-C. Dippel, H.-P. Liermann, J. T. Delitz, P. Walter, H. Schulte-Schrepping, O. H. Seeck and H. Franz, J. Synchrotron Radiat., 2015, 22, 675–687 CrossRef CAS PubMed.
- H. M. Rietveld, J. Appl. Crystallogr., 1969, 2, 65–71 CrossRef CAS.
- P. P. Mazzeo, G. I. Lampronti, A. A. Michalchuk, A. M. Belenguer, A. Bacchi and F. Emmerling, Faraday Discuss., 2023, 241, 289–305 RSC.
- G. I. Lampronti, A. A. L. Michalchuk, P. P. Mazzeo, A. M. Belenguer, J. K. M. Sanders, A. Bacchi and F. Emmerling, Nat. Commun., 2021, 12, 6134 CrossRef CAS PubMed.
- K. Linberg, P. Szymoniak, A. Schönhals, F. Emmerling and A. A. Michalchuk, Chem.–Eur. J., 2023, 29, e202302150 CrossRef CAS PubMed.
- C. Farrow, P. Juhas, J. Liu, D. Bryndin, E. Božin, J. Bloch, T. Proffen and S. Billinge, J. Phys.: Condens. Matter, 2007, 19, 335219 CrossRef CAS PubMed.
- S. Kahmann, D. Meggiolaro, L. Gregori, E. K. Tekelenburg, M. Pitaro, S. D. Stranks, F. De Angelis and M. A. Loi, ACS Energy Lett., 2022, 7, 4232–4241 CrossRef CAS PubMed.
- J. Ma, C. Fang, C. Chen, L. Jin, J. Wang, S. Wang, J. Tang and D. Li, ACS Nano, 2019, 13, 3659–3665 CrossRef CAS PubMed.
- M. D. Smith, A. Jaffe, E. R. Dohner, A. M. Lindenberg and H. I. Karunadasa, Chem. Sci., 2017, 8, 4497–4504 RSC.
- X. Wang, W. Meng, W. Liao, J. Wang, R. G. Xiong and Y. Yan, J. Phys. Chem. Lett., 2019, 10, 501–506 CrossRef CAS PubMed.
- A. K. Soper, GudrunN and GudrunX: programs for correcting raw neutron and X-ray diffraction data to differential scattering cross section, Tech. Rep. RAL-TR-2011-013, Science & Technology Facilities Council, Swindon, UK, 2011 Search PubMed.
- D. A. Keen, J. Appl. Crystallogr., 2001, 34, 172–177 CrossRef CAS.
- D. A. Keen, Crystallogr. Rev., 2020, 26, 143–201 CrossRef.
- J. E. Field, M. Farhat and S. M. Walley, J. Mater. Sci., 2014, 49, 3780–3784 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc00905c |
| ‡ Communition limit: particles of brittle materials are expected to become smaller with longer milling time until a brittle–ductile transition occurs, as there is no longer sufficient stored energy in the particles to initiate and further propagate cracks throughout the particles.36 |
| This journal is © The Royal Society of Chemistry 2024 |