Open Access Article
Open Access ArticleIn situ Nafion-nanofilm oriented (002) Zn electrodeposition for long-term zinc-ion batteries†
Da
Zhang
a,
Ziyang
Song
a,
Ling
Miao
a,
Yaokang
Lv
 b,
Lihua
Gan
b,
Lihua
Gan
 a and
Mingxian
Liu
a and
Mingxian
Liu
 *a
*a
aShanghai Key Lab of Chemical Assessment and Sustainability, School of Chemical Science and Engineering, Tongji University, Shanghai, 200092, P. R. China. E-mail: liumx@tongji.edu.cn
bCollege of Chemical Engineering, Zhejiang University of Technology, Hangzhou 310014, P. R. China
First published on 22nd February 2024
Abstract
Dendrite growth and parasitic reactions of a Zn metal anode in aqueous media hinder the development of up-and-coming Zn-ion batteries. Optimizing the crystal growth after Zn nucleation is promising to enable stable cyclic performance of the anode, but directly regulating specific crystal plane growth for homogenized Zn electrodeposition remains highly challenging. Herein, a perfluoropolymer (Nafion) is introduced into an aqueous electrolyte to activate a thermodynamically ultrastable Zn/electrolyte interface for long-term Zn-ion batteries. The low adsorption energy (−2.09 eV) of Nafion molecules on Zn metal ensures the in situ formation of a Nafion-nanofilm during the first charge process. This ultrathin artificial solid electrolyte interface with zincophilic –SO3− groups guides the directional Zn2+ electrodeposition along the (002) crystal surface even at high current density, yielding a dendrite-free Zn anode. The synergic Zn/electrolyte interphase electrochemistry contributes an average coulombic efficiency of 99.71% after 4500 cycles for Zn‖Cu cells, and Zn‖Zn cells achieve an ultralong lifespan of over 7000 h at 5 mA cm−2. Besides, Zn‖MnO2 cells operate well over 3000 cycles. Even at −40 °C, Zn‖Zn cells achieve stable Zn2+ plating/stripping for 1200 h.
Introduction
Aqueous zinc-ion batteries (ZIBs) have emerged as one of the advanced electrochemical energy storage systems, characterized by their excellent safety, low cost, and high energy and power densities (820 mA h g−1 and 5855 mA h cm−3).1–6 However, the Zn metal anode in ZIBs encounters significant challenges, including the formation of disordered dendrites and grievous interface polarization reactions (Zn anode corrosion and hydrogen evolution), which restrict cycling performance.7–12 Conspicuously, the low plating/stripping efficiency of the anode at high current densities (≥5 mA cm−2) and in low-concentration Zn salt (1 M and below) significantly hinders the practical application of ZIBs.13–16 Typically, protecting the Zn anode by organic–inorganic composite coating is an effective strategy, but the slow ion transport caused by the thicker organic hydrophobic layer limits the diffusion of Zn2+ at the solid phase interface (SEI), resulting in an increase in the nucleation potential of Zn2+ ions. Besides, organic co-solvents disrupt the hydrogen bonding network of the electrolyte and reduce the ionic conductivity of the original electrolyte. Accordingly, it is necessary to achieve deposition induction and rapid diffusion of Zn2+ ions.Profiting from the stable structure and the abundant zincophilic groups, polymers are widely used as electrode protective layers and electrolyte additives to deliver a widened electrochemical window and accelerate the dissociation of Zn salts.17–22 As a common cation transfer polymer separator, the protons of Nafion are gradually replaced by Zn2+ as the cycling proceeds, causing uniform Zn2+ transport at the electrode/electrolyte interface. Specifically, Nafion is combined with zeolite through a solution pouring method to construct an organic–inorganic layer, where the zincophilic Nafion benefits the transport of zinc ions and inorganic zeolites with 0.74 nm micropores inhibit the side reactions at the anode/electrolyte interface to prevent dendrite growth.23 However, such pouring strategy generally yields a micrometer-scale protective layer which is not conducive to the rapid plating/stripping of zinc ions.24–29 Beyond that, the present Nafion-based layer at the Zn anode often results in uncontrollable Zn nucleation with a (101) and (110)-dominated texture due to high Zn2+ concentration polarization, causing deteriorated hydrogen evolution reaction (HER) and loose Zn plating.30–32 In contrast, the (002) crystal plane is the most stable texture due to its lowest surface nucleation energy, which can homogenize the Zn electrodeposition to suppress the growth of dendrites and HER reactions, achieving long-term ZIBs.33–35 Therefore, reducing the thickness of the Nafion-based protective layer from the micrometer to nanometer for rapid plating/stripping of zinc ions, accompanied by decreasing the surface nucleation energy for achieving stable Zn electrodeposition to inhibit the HER is expected to boost the anode reversibility and long-term ZIBs, yet not reported.
In this work, we introduce perfluoropolymer (Nafion) molecules to construct an anode micro-electric field and activate a thermodynamically ultrastable zinc anode/electrolyte interface for propelling high-performance ZMBs. The spontaneous adsorption of Nafion (NAF) molecules on the Zn anode ensures the in situ generation of zincophilic NAF-film, which regulates the directional and dendrite-free deposition of solvated Zn2+ ions, significantly improving the plating/stripping efficiency of the Zn anode. Therefore, Zn‖Zn cells using a Zn(OTF)2-NAF (OTF− = CF3SO3−) electrolyte can cycle over 5000 h, and Zn‖Cu cells achieve an average CE of 99.71% after 4500 cycles at 5 mA cm−2. Significantly, Zn‖MnO2 cells exhibit remarkable cyclic stability over 3000 cycles. Even at −40 °C, the reversible plating/stripping of Zn‖Zn cells can work over 1200 h. This work offers a design avenue of dendrite-free Zn deposition for ultrastable ZMBs.
Results and discussion
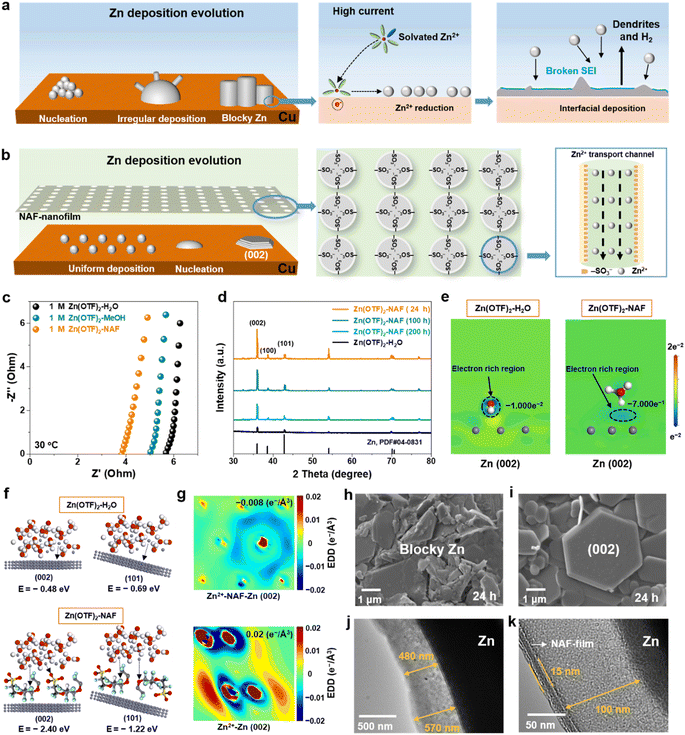
First-principles calculations and systemic experiments were performed to study the Zn2+ transport mechanisms in Zn(OTF)2-H2O and Zn(OTF)2-NAF electrolytes.36–39 For the Zn(OTF)2-H2O electrolyte interface (Fig. 1a), when the solvated Zn2+ ions migrate to the anode/electrolyte interface, the inhomogeneous Zn2+ plating and Zn(OTF)2 decomposition result in potential differentiation at the Zn anode, further leading to the fracturing of the SEI and the generation of H2 (Fig. S1†). In comparison, NAF molecules show a high binding energy with Zn metal (Fig. S2–S4 and Table S1†), which indicates their great predilection to form a NAF-nanofilm on the Zn anode.40–45 The unique –SO3− functional groups in NAF molecules accept Zn2+ ion transportation to the anode interface, avoiding excessive plating on the Cu collector. When a smooth NAF-nanofilm is formed at the anode/electrolyte interface (Fig. 1b), the Zn anode exhibits dense Zn2+ plating even at high current densities.The ionic conductivity of Zn2+ ions in various electrolytes was analyzed by electrochemical impedance spectroscopy (EIS) using the formula σ = L/RS (where L, R, and S are the separator thickness, bulk resistance, and area, respectively). The Zn(OTF)2-NAF electrolyte shows the smallest resistance of 3.7 Ω (Fig. 1c), corresponding to the fastest Zn2+ conductivity of 1.22 × 10−2 S cm−1 (Table S2†). The cyclic voltammetry (CV) curves of Zn‖Cu cells show the enhancement of Zn ion transport kinetics by the Zn(OTF)2-NAF electrolyte. With the increase of scan rate from 1 to 4 mV s−1, the thicker SEI affects the transfer rate of Zn2+ ions, resulting in lower plating/stripping efficiency in the Zn(OTF)2-H2O electrolyte (Fig. S5†). Compared to the Zn(OTF)2-H2O electrolyte, the higher amount of Zn2+ ions transported in the Zn(OTF)2-NAF electrolyte (0.85 vs. 0.33) also demonstrates the rapid transfer and storage of Zn2+ ions in the solid/liquid phase interface (Fig. S6†). Of note, there is no significant polarization reaction at the Zn anode demonstrating stable Zn2+ plating/stripping. The X-ray diffraction (XRD) patterns of the Cu substrate of Zn‖Cu cells after cycling at 1 mA cm−2 in various electrolytes show obviously different Zn2+ plating/stripping (Fig. 1d). Fig. 1e demonstrates the geometric models of charge distributions at the Zn/electrolyte interface. Among different Zn/electrolyte interfaces, Zn–NAF interaction and significant charge transfer induce a larger binding energy of Zn(OTF)2, which accelerates the interfacial Zn2+ transfer. The Zn deposited through the NAF-nanofilm, unlike the previous Nafion–zeolite protective layer,46–51 preferentially grows along the (002) crystal surface to form a dense anode after 200 h, due to a lower binding energy of the NAF-nanofilm and zinc metal anode (Fig. 1f).52 Notably, the charge density of Zn2+ ions deposited in the Zn(OTF)2-NAF electrolyte is more uniform compared to that in the Zn(OTF)2-H2O electrolyte, which is crucial for dense Zn nucleation and a dendrite-free anode (Fig. 1g). Even when the plating/stripping continues for 2000 h, Zn2+ ions still preferentially nucleate along the (002) crystal plane (Fig. S7†).
The Zn2+ deposition evolution in electrolytes was analyzed by field emission scanning electron microscopy (SEM) and transmission electron microscopy (TEM). For the Zn(OTF)2-H2O electrolyte (Fig. 1h), a large amount of blocky Zn appeared on the Cu substrate after a plating/stripping of 24 h. As the operating time increases to 200 h, the irregular growth of dendrites on the Zn anode inevitably leads to SEI damage and Zn(OTF)2 consumption (Fig. S8†). In contrast, the deposition morphology in the Zn(OTF)2-NAF electrolyte is smoother (predominantly hexagonal deposition), maintaining a stable (002) crystal growth (Fig. 1i). The interface of directional crystal growth ((002) plane) reduces the formation of dendrites and delays the corrosion rates of Zn anodes (Fig. S9 and S10†). TEM images clearly show the plating of Zn2+ in different electrolytes. With a lower current density (1 mA cm−2 and 0.5 mA h cm−2), an inhomogeneous SEI was obtained on the Zn anode, about 480 and 570 nm (Fig. 1j). Besides, obvious fluctuations occur at the Zn anode interface at 5 mA cm−2 (Fig. S11†), resulting in uneven SEI thickness and disordered Zn deposition. Notably, the NAF-nanofilm (15 nm in thickness) and SEI (100 nm in thickness) can be observed at the interface between the anode and electrolyte (Fig. 1k), which promises high-kinetics Zn2+ transfer. Even at 10 mA cm−2, the SEI in the Zn(OTF)2-NAF electrolyte maintains higher crystallinity compared to that in the Zn(OTF)2-H2O electrolyte (Fig. S12†).
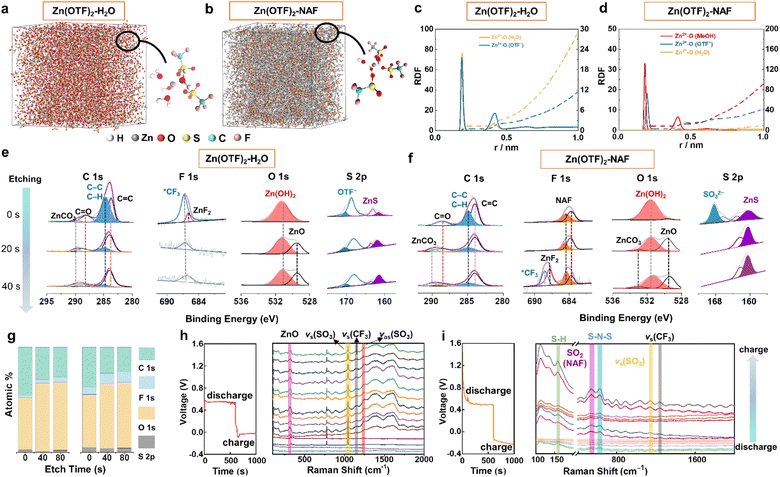
Ab initio molecular dynamics (AIMD) simulations and density functional theory (DFT) calculations were used to explore the solvation structure and coordination potential during the (dis)charging of ZIBs. For 2 M Zn(OTF)2-H2O electrolyte (Fig. 2a and S13†), the Zn2+ solvation structure includes five H2O molecules and one OTF− ion. The more solvated the H2O molecules, the more severe the hydrogen evolution reactions (Fig. S14–16†). Consequently, NAF co-migrated with Zn2+ ions to form a NAF-nanofilm on the Zn anode, effectively guiding the stable deposition of Zn2+ ions (Fig. 2b). Compared with the Zn(OTF)2-H2O electrolyte, the solvated structure of the Zn(OTF)2-NAF electrolyte indicates the decreased proportion of H2O molecules around Zn2+ ions in the solvated shell (Fig. 2c and d). According to molecular dynamics simulations,53 the coordination probability of OTF− with Zn2+ in the Zn(OTF)2-NAF electrolyte is significantly higher than that in the Zn(OTF)2-H2O electrolyte, triggering favorable solvated environments to limit the hydrogen evolution reaction of H2O molecules, and boosting the Zn2+ plating/stripping efficiency at high current densities. The chemical components on the surface of the Zn anode were detected by Ar+ sputtering X-ray photoelectron spectroscopy (XPS). To maintain the uniformity and integrity of the SEI, all cells were subjected to two constant-current (dis)charging cycles. The elemental contents of the SEI show significant differences for different electrolytes (Fig. S17†). For the Zn(OTF)2-H2O electrolyte, the inorganic components in the SEI are mainly ZnCO3, ZnO, ZnF2, and ZnS (Fig. 2e). Notably, ZnF2 almost disappears with the increase of sputtering time (20 and 40 s), indicating the scarcity of high Zn2+ conductivity components in the SEI, and the uneven SEI impedes the rapid transfer and plating/stripping of Zn2+. The detected characteristic peak of OTF− confirms the continuous decomposition of Zn(OTF)2 during the (dis)charging of ZIBs.54
For Zn(OTF)2-MeOH (MeOH = methanol) electrolyte (Fig. S18†), ZnF2 (684.8 eV) and *CF3 (689.4 eV)55 can be captured after the etching time reaches 20 s (Table S3†). The results indicate the formation of a ZnF2 and ZnCO3 polycrystalline phase SEI. However, the solvation structure of Zn2+ ions in the Zn(OTF)2-MeOH electrolyte damages the formed SEI and causes sustained consumption of Zn(OTF)2, restricting the long-term cycling performance of ZIBs. Therefore, specific protection needs to be implemented for the Zn anode. Even when the etching time reaches 40 s, the detected ZnF2 confirms that NAF-SEI has a more complete polycrystalline phase structure (Fig. 2f). As the sputtering time increases, the SO3− fraction of NAF is captured on the Zn surface, which verifies the self-adsorption of NAF with Zn.56 Besides, the protection of the NAF-nanofilm on the Zn anode is confirmed by the disappearance of OTF− ions. Fig. 2g shows the variation in elemental content of the formed SEI in different electrolytes. The introduction of NAF solution increases the ratio of ZnF2 and ZnCO3 content in the SEI.
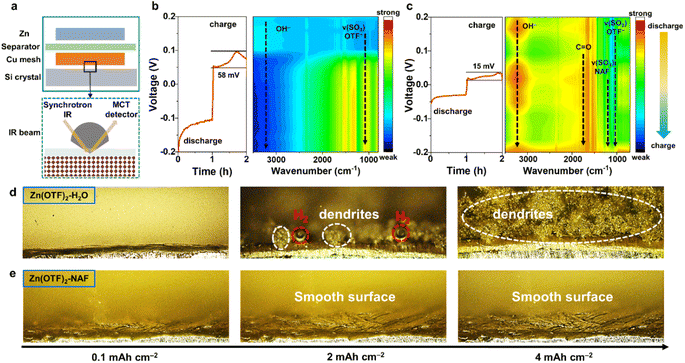
In situ Raman spectroscopy was applied to monitor the Raman peak variations of the Zn anode in the Zn(OTF)2-H2O electrolyte (Fig. S19†). During discharging (Zn2+ migrating to the Cu collector), a significant Zn(OTF)2 component is detected, which is reflected by the peaks at 1036 and 1256 cm−1 (Fig. 2h).57 The characteristic peak of Zn(OTF)2 (1224 cm−1)58 is accurately observed, further confirming the severe hysteresis in the desolvation process of Zn(OTF)2. For the Zn(OTF)2-NAF electrolyte, a noticeable voltage drop at the beginning of the voltage–time curve is attributed to the binding between NAF and Zn (Fig. 2i). Besides, the S–H binding sites of NAF at 156 cm−1 are captured.59 The characteristic peaks of vs(SO3) and vas(SO3) derived from Zn(OTF)2 can be detected in the Zn(OTF)2-H2O electrolyte, confirming the precipitation of a large amount of Zn(OTF)2. In contrast, the characteristic peak of Zn(OTF)2 in the Zn(OTF)2-NAF electrolyte is very weak, indicating little Zn(OTF)2 precipitates at the Zn/electrolyte interface and ensuring the long-term stability of ZIBs. The in situ attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy was utilized to analyse the evolution pattern of the solvation conformation in electrolytes (Fig. 3a). The process of Zn2+ plating/stripping was studied under constant-current density (1 mA cm−2) (dis)charging. An obvious OTF− peak (1032 cm−1) is captured during discharging (Fig. 3b and S20†), indicating that OTF− molecules remain at the anode/electrolyte interface during Zn2+ stripping.
In the voltage–time curves of Zn2+ plating/stripping, the cell using the Zn(OTF)2-H2O electrolyte has a higher overpotential than that using Zn(OTF)2-NAF. A sharp increase in voltage (0.0958 V) is even observed at the end of the charging process (voltage–time curve constant coordinate for 1.7 h, Fig. 3b), which is attributed to the precipitation and decomposition of Zn(OTF)2, leading to an increase in the proportion of H2O molecules around solvated Zn2+ ions, producing more hydrogen gas. Besides, the reversible change of MeOH molecules during (dis)charging indicates the rapid migration of Zn2+ ions (Fig. 3c). More importantly, the appearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak suggests a possible bimolecular Cannizzaro disproportionation of MeOH molecules to form methoxy, followed by dehydrogenation to CH2O−. The generated CH2O− promotes a charge-rich state in the solvation sheath of Zn2+ ions and shields it from H2O molecules, which is crucial for rapid Zn2+ transport.60–62 The dendrite growth and hydrogen production on the Zn anode were directly observed through in situ depth of field (DOF) microscopy. For the Zn(OTF)2-H2O electrolyte, the generation of bubbles can be observed when the Zn2+ plating capacity reaches 0.1 mA h cm−2 (Fig. 3d). As the discharging process continues, obvious gray-black dendrites appear on the Zn anode, accompanied by severe corrosion after deposition (Fig. S21†). Due to the direct contact between the Zn anode and electrolyte, similar phenomena can also be examined in the Zn(OTF)2-MeOH electrolyte (Fig. S22†). Without the regulation by the NAF-nanofilm, the Zn2+ transport kinetics is slower, and the growth of dendrites remains uncontrolled. Compared with the Zn(OTF)2-H2O electrolyte, the Zn corrosion can be delayed in the Zn(OTF)2-MeOH electrolyte because MeOH significantly reduces the proportion of active H2O molecules in the solvation sheath of Zn2+ ions. No bubbles are observed when the plating capacity reaches 4 mA h cm−2, and the color of the deposited Zn is almost the same as that of the original Zn (Fig. 3e). The in situ DOF plating results further indicate the substantially suppressed dendrite growth and H2 generation in the Zn(OTF)2-NAF electrolyte.
O peak suggests a possible bimolecular Cannizzaro disproportionation of MeOH molecules to form methoxy, followed by dehydrogenation to CH2O−. The generated CH2O− promotes a charge-rich state in the solvation sheath of Zn2+ ions and shields it from H2O molecules, which is crucial for rapid Zn2+ transport.60–62 The dendrite growth and hydrogen production on the Zn anode were directly observed through in situ depth of field (DOF) microscopy. For the Zn(OTF)2-H2O electrolyte, the generation of bubbles can be observed when the Zn2+ plating capacity reaches 0.1 mA h cm−2 (Fig. 3d). As the discharging process continues, obvious gray-black dendrites appear on the Zn anode, accompanied by severe corrosion after deposition (Fig. S21†). Due to the direct contact between the Zn anode and electrolyte, similar phenomena can also be examined in the Zn(OTF)2-MeOH electrolyte (Fig. S22†). Without the regulation by the NAF-nanofilm, the Zn2+ transport kinetics is slower, and the growth of dendrites remains uncontrolled. Compared with the Zn(OTF)2-H2O electrolyte, the Zn corrosion can be delayed in the Zn(OTF)2-MeOH electrolyte because MeOH significantly reduces the proportion of active H2O molecules in the solvation sheath of Zn2+ ions. No bubbles are observed when the plating capacity reaches 4 mA h cm−2, and the color of the deposited Zn is almost the same as that of the original Zn (Fig. 3e). The in situ DOF plating results further indicate the substantially suppressed dendrite growth and H2 generation in the Zn(OTF)2-NAF electrolyte.
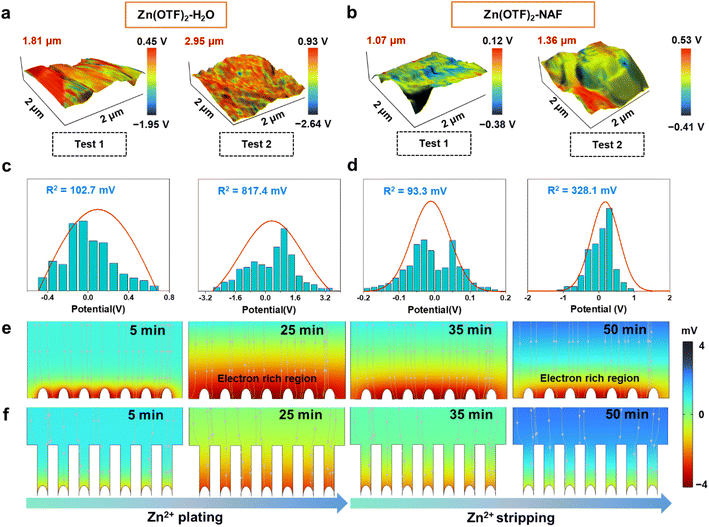
Scanning probe microscopy (SPM) was applied to investigate the plating morphology and interface potential of the Zn anode.63 Based on the working principle and test results of SPM, the calculation rules of the Zn anode interface potential are obtained:
| Test 1: Vsample − Vtip = (Φtip − Φsample)/e | (1) |
| Test 2: VHOPG − Vtip = (Φtip − ΦHOPG)/e | (2) |
| e(Test 1) = Φtip − Φsample | (3) |
| e(Test 2) = Φtip − ΦHOPG | (4) |
Based on formulae (1) and (2), the conversion formulae (3) and (4) between the probe potential and the sample surface potential, and further formula (5) for calculating the surface potential of the sample can be obtained. Φsample and Φtip denote the test sample surface potential and probe tip potential, respectively. ΦHOPG is a fixed applied potential of 4.5 eV.
| Φsample = ΦHOPG + e(Test 1 − Test 2) | (5) |
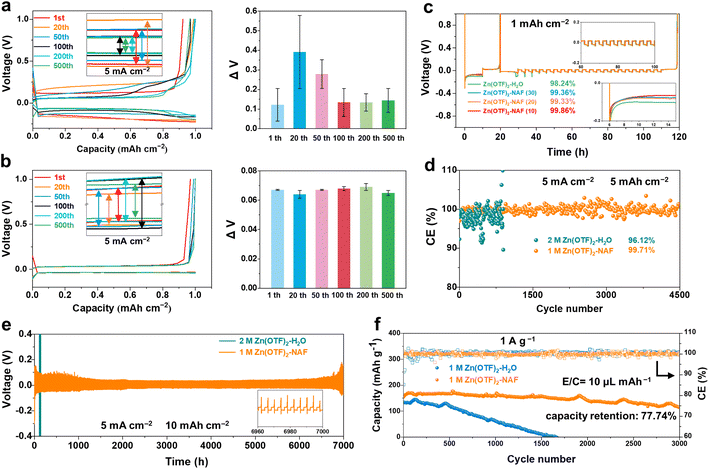
The height difference of the Zn anode after plating reaches 1.81 and 2.95 μm (plating capacities are 1 and 2 mA h cm−2) in the Zn(OTF)2-H2O electrolyte (Fig. 4a), together with a minimum local potential value of −2.64 V and a maximum value of 0.93 V through the interfacial potential distribution maps. In contrast, more uniform deposition and potential are achieved in the Zn(OTF)2-NAF electrolyte, as reflected by the height difference of 1.36 μm and the minimum potential value of −0.41 V (Fig. 4b). As a result, the average surface potentials in Zn(OTF)2-H2O and Zn(OTF)2-NAF electrolytes are determined to be 5.67 and 4.94 eV. The larger average potential on the sample surface indicates the more disordered interfacial charge distribution and irregular Zn2+ deposition. The fitted interfacial average potentials in the Zn(OTF)2-H2O electrolyte are 102.7 and 817.4 mV, which are inferior to those of the Zn(OTF)2-NAF electrolyte (93.3 and 328.1 mV, Fig. 4c and d). The more homogeneous interfacial potential implies a lower activation energy for Zn2+ ion migration, thus enabling the dissociation of Zn(OTF)2 at the Zn/NAF interface and accelerating the transport efficiency of Zn2+ ions.64,65 Besides, the Zn2+ plating test in the Zn(OTF)2-MeOH electrolyte (Fig. S23†) confirms the attenuated desolvation of Zn2+ ions by the NAF-nanofilm. Without the protection of the NAF-nanofilm, the solvation effect remains the main reason for the slow Zn2+ plating/stripping. Based on the deposition potential analysis, the potential simulation of the Zn2+ plating/stripping process was performed through the COMSOL potential field.66 During Zn2+ plating (from 5 to 25 min) in Zn(OTF)2-H2O electrolyte, a local micro-electric field is formed due to the polarization of the electrolyte concentration near the Zn anode (Fig. 4e), which exacerbates the hydrogen evolution reaction (HER) of solvation H2O molecules and dendrite growth. The potential gradient near the Zn anode remains less than 0 mV during the stripping (35 to 50 min). In the Zn(OTF)2-NAF electrolyte, a microporous film (NAF-nanofilm) is formed on the Zn anode, and the migration of Zn2+ is induced by –SO3F groups on the NAF-nanofilm. The solvation structure fails to reach the Zn anode (Fig. 4f), resulting in a smaller potential gradient compared with the Zn(OTF)2-H2O electrolyte after 25 min. During Zn2+ stripping from the anode interface (35 to 50 min), the potential gradient response of the Zn/NAF/electrolyte interface is faster than that of Zn/electrolyte, which is attributed to the rapid co-migration of dissociated Zn(OTF)2 with the solvated structure. For the ZnSO4-NAF-H2O electrolyte (Fig. S24–S27†), the micro-electric field constructed by the NAF-nanofilm also enhances the electrochemical performance of ZIBs. The electrochemical properties of Zn‖Cu, Zn‖Zn, and Zn‖MnO2 cells were systematically studied to verify the facilitation of solvation reconfiguration and Zn2+ ion transportation channels in modified electrolytes on Zn2+ plating/stripping. According to the voltage–capacity curves of Zn‖Cu cells in Zn(OTF)2-H2O and Zn(OTF)2-NAF electrolytes (Fig. 5a and b), there is no significant difference in the plating/stripping voltage even after 500 cycles. The maximum voltage drop in the Zn(OTF)2-H2O electrolyte is 0.39 V (20th cycle), which is attributed to the concentration polarization of the interfacial electrolyte and the disordered deposition of Zn2+. Drastic voltage fluctuations lead to the decomposition of Zn(OTF)2 and dendrite growth. The maximum voltage drop in the Zn(OTF)2-NAF electrolyte is only 0.069 V (200th of the cycles), thus ensuring the long-term cycling of ZIBs. The modified Aurbach method was used to analyze the Zn2+ desolvation kinetics.67,68 The NAF-nanofilm significantly improves the Zn2+ plating/stripping coulombic efficiency (CE) (Fig. 5c), with the highest value of 99.86%.
Fig. 5d shows the Zn2+ plating/stripping CE of Zn‖Cu cells using different electrolytes at 5 mA cm−2. An impressive CE of 99.71% is achieved for Zn‖Cu cells using the Zn(OTF)2-NAF electrolyte after 4500 cycles. The corresponding charge–discharge curves show more stable voltage fluctuations and gradually decreasing polarization reactions (from the 1st to 4000th cycles), proving the improvement of coulombic efficiency in Zn2+ ion plating/deposition (Fig. S28†). For comparison, Zn‖Cu cells using the Zn(OTF)2-H2O electrolyte show a CE of 96.12%. Despite the Zn2+ plating/stripping in the Zn(OTF)2-MeOH electrolyte reaching 2700 h (while 280 h for the Zn(OTF)2-H2O electrolyte), the deposition overpotential of Zn2+ is continuously increased from 0.3 to 0.65 V. Benefiting from the regulation of Zn2+ transportation channels at the Zn anode, the NAF-nanofilm accelerates Zn2+ transport in the anode/electrolyte interface. Consequently, Zn‖Zn cells using the Zn(OTF)2-NAF electrolyte exhibit remarkable cycling stability with Zn2+ plating/stripping for over 7000 h at 5 mA cm−2 (Fig. 5e).
Besides, the cell capacity starts to decrease after 100 cycles, corresponding to the gradual depletion of Zn(OTF)2 and the continuous breakage/generation of the SEI (Fig. S29†). In contrast, the Zn‖MnO2 cell using the Zn(OTF)2-NAF electrolyte at 30 °C demonstrates stable 3000 (dis)charging cycles at 1 A g−1 with a capacity retention of 77.74% (Fig. 5f). Similar to previous studies,69 the binding of MeOH to Zn2+ ions improves the ionic conductivity of the Zn(OTF)2-NAF electrolyte at low temperatures. Therefore, Zn‖Zn cells achieve stable plating/stripping at 1 mA cm−2 for 1200 h even at −40 °C (Fig. S30†). Even at −40 °C, Zn‖MnO2 cells using the Zn(OTF)2-NAF electrolyte demonstrate stable 2000 (dis)charging cycles at 0.5 A g−1 with a capacity retention of 66.41% (Fig. S31 and S32†). Zn(OTF)2-NAF with excellent electrochemical performance highlights its promising application prospects in advanced ZIBs (Tables S4 and S5†), especially in harsh environments.
Conclusion
In conclusion, Nafion as an additive is introduced into an aqueous electrolyte to construct Zn2+ ion directional transmission channels for establishing an ultrastable Zn/electrolyte interface for advanced ZIBs. NAF molecules allow the in situ generation of a zincophilic nanofilm which serves as directional Zn2+ transport channels with a more negative binding energy to induce Zn2+ nucleation along the (002) crystal surface. Benefiting from the artificial ion transportation channels, the dendrite growth and anode corrosion are substantially controlled in Zn(OTF)2-NAF electrolyte. Consequently, Zn‖Cu cells achieve an impressive coulombic efficiency of 99.71% after 4500 cycles, and Zn‖Zn cells demonstrate stable plating/stripping for 7000 h at 5 mA cm−2. Even at −40 °C, the reversible plating/stripping of Zn‖Zn cells can work over 1200 h. This work offers appealing insights into the microscopic Zn/electrolyte interfacial modulation with obviated Zn-dendrites and H2O-originated parasitic reactions for advanced ZIBs.Data availability
The data that support the findings of this study are available within the article and its ESI,† or from the corresponding author on reasonable request.Author contributions
The authors contributed to this work in the following ways: conceptualization (M. Liu, D. Zhang, Z. Song); data curation (D. Zhang); formal analysis (M. Liu, D. Zhang); funding acquisition (M. Liu, L. Gan, Y. Lv); investigation (M. Liu, D. Zhang, L. Miao); methodology (M. Liu, D. Zhang, Z. Song, L. Gan, L. Miao, Y. Lv); project administration (M. Liu); supervision (M. Liu); validation (M. Liu, L. Gan, Y. Lv); visualization (M. Liu, D. Zhang, L. Miao); writing – original draft (D. Zhang); writing – review & editing (M. Liu, D. Zhang, Z. Song).Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 22272118, 22172111, 21905207 and 22309134), the Science and Technology Commission of Shanghai Municipality, China (No. 22ZR1464100, 20ZR1460300 and 19DZ2271500), China Postdoctoral Science Foundation (2022M712402), Shanghai Rising-Star Program (23YF1449200), Zhejiang Provincial Science and Technology Project (2022C01182), and the Fundamental Research Funds for the Central Universities (22120210529 and 2023-3-YB-07).Notes and references
- L. Cao, D. Li, T. Pollard, T. Deng, B. Zhang, C. Yang, L. Chen, J. Vatamanu, E. Hu, M. J. Hourwitz, L. Ma, M. Ding, Q. Li, S. Hou, K. Gaskell, J. T. Fourkas, X. Q. Yang, K. Xu, O. Borodin and C. Wang, Nat. Nanotechnol., 2021, 16, 902–910 CrossRef CAS PubMed.
- Z. Song, L. Miao, H. Duan, L. Ruhlmann, Y. Lv, D. Zhu, L. Li, L. Gan and M. Liu, Angew. Chem., Int. Ed., 2022, 61, e202208821 CrossRef CAS PubMed.
- N. Yao, X. Chen, Z. H. Fu and Q. Zhang, Chem. Rev., 2022, 122, 10970–11021 CrossRef CAS PubMed.
- Z. Song, L. Miao, L. Ruhlmann, Y. Lv, L. Li, L. Gan and M. Liu, Angew. Chem., Int. Ed., 2023, 62, e202219136 CrossRef CAS PubMed.
- Z. Wang, J. Huang, Z. Guo, X. Dong, Y. Liu, Y. Wang and Y. Xia, Joule, 2019, 3, 1289–1300 CrossRef CAS.
- Z. Song, L. Miao, Y. Lv, L. Gan and M. Liu, Angew. Chem., Int. Ed., 2023, 62, e202309446 CrossRef CAS PubMed.
- C. Hu, Y. Qin, Z. Song, P. Liu, L. Miao, H. Duan, Y. Lv, L. Xie, M. Liu and L. Gan, J. Colloid Interface Sci., 2024, 658, 856–864 CrossRef CAS PubMed.
- D. Zhang, R. Gu, Y. Yang, J. Ge, J. Xu, Q. Xu, P. Shi, M. Liu, Z. Guo and Y. Min, Angew. Chem., Int. Ed., 2024, e202315122 CAS.
- Z. Cao, P. Zhuang, X. Zhang, M. Ye, J. Shen and P. M. Ajayan, Adv. Energy Mater., 2020, 10, 2001599 CrossRef CAS.
- Y. Pan, S. Liu, K. Sun, X. Chen, B. Wang, K. Wu, X. Cao, W. C. Cheong, R. Shen, A. Han, Z. Chen, L. Zheng, J. Luo, Y. Lin, Y. Liu, D. Wang, Q. Peng, Q. Zhang, C. Chen and Y. Li, Angew. Chem., Int. Ed., 2018, 57, 8614–8618 CrossRef CAS PubMed.
- C. C. Hou, Y. Wang, L. Zou, M. Wang, H. Liu, Z. Liu, H. F. Wang, C. Li and Q. Xu, Adv. Mater., 2021, 33, 2101698 CrossRef CAS.
- L. Miao, Z. Song, W. Du, X. Zheng, Y. Lv, L. Gan and M. Liu, Mater. Chem. Front., 2023, 7, 2731–2749 RSC.
- P. Xiong, Y. Zhang, J. Zhang, S. H. Baek, L. Zeng, Y. Yao and H. S. Park, EnergyChem, 2022, 4, 100076 CrossRef CAS.
- H. Dou, M. Xu, B. Wang, Z. Zhang, G. Wen, Y. Zheng, D. Luo, L. Zhao, A. Yu, L. Zhang, Z. Jiang and Z. Chen, Chem. Soc. Rev., 2021, 50, 986–1029 RSC.
- P. Xiong, C. Lin, Y. Wei, J.-H. Kim, G. Jang, K. Dai, L. Zeng, S. Huang, S. J. Kwon, S. Y. Lee and H. S. Park, ACS Energy Lett., 2023, 8, 2718–2727 CrossRef CAS.
- Y. Zhang, Z. Song, L. Miao, Y. Lv, L. Gan and M. Liu, Angew. Chem., Int. Ed., 2024, 63, e202316835 CrossRef CAS PubMed.
- M. Peng, X. Tang, K. Xiao, T. Hu, K. Yuan and Y. Chen, Angew. Chem., Int. Ed., 2023, 62, e202302701 CrossRef CAS PubMed.
- B. Niu, Z. Li, D. Luo, X. Ma, Q. Yang, Y. E. Liu, X. Yu, X. He, Y. Qiao and X. Wang, Energy Environ. Sci., 2023, 16, 1662–1675 RSC.
- Y. Wu, T. Zhang, L. Chen, Z. Zhu, L. Cheng, S. Gu, Z. Li, Z. Tong, H. Li, Y. Li, Z. Lu, W. Zhang and C. S. Lee, Adv. Energy Mater., 2023, 13, 2300719 CrossRef CAS.
- Q. Zhang, J. Luan, L. Fu, S. Wu, Y. Tang, X. Ji and H. Wang, Angew. Chem., Int. Ed., 2019, 58, 15841–15847 CrossRef CAS PubMed.
- P. Xiong, Y. Kang, N. Yao, X. Chen, H. Mao, W.-S. Jang, D. M. Halat, Z. H. Fu, M. H. Jung, H. Y. Jeong, Y. M. Kim, J. A. Reimer, Q. Zhang and H. S. Park, ACS Energy Lett., 2023, 8, 1613–1625 CrossRef CAS.
- Y. Wang, Q. Li, H. Hong, S. Yang, R. Zhang, X. Wang, X. Jin, B. Xiong, S. Bai and C. Zhi, Nat. Commun., 2023, 14, 3890 CrossRef CAS.
- Y. Cui, Q. Zhao, X. Wu, X. Chen, J. Yang, Y. Wang, R. Qin, S. Ding, Y. Song, J. Wu, K. Yang, Z. Wang, Z. Mei, Z. Song, H. Wu, Z. Jiang, G. Qian, L. Yang and F. Pan, Angew. Chem., Int. Ed., 2020, 59, 16594–16601 CrossRef CAS PubMed.
- H. Qiu, X. Du, J. Zhao, Y. Wang, J. Ju, Z. Chen, Z. Hu, D. Yan, X. Zhou and G. Cui, Nat. Commun., 2019, 10, 5374 CrossRef PubMed.
- Q. Zhang, Y. Ma, Y. Lu, Y. Ni, L. Lin, Z. Hao, Z. Yan, Q. Zhao and J. Chen, J. Am. Chem. Soc., 2022, 144, 18435–18443 CrossRef CAS.
- Y. Xie, J. Huang, T. Kong, X. Zhou, K. Wu, X. Liu, J. Yi, L. Xing and Y. Xia, Energy Storage Mater., 2023, 56, 218–226 CrossRef.
- J. F. Parker, J. S. Ko, D. R. Rolison and J. W. Long, Joule, 2018, 2, 2519–2527 CrossRef CAS.
- L. Zhang, I. A. Rodríguez Pérez, H. Jiang, C. Zhang, D. P. Leonard, Q. Guo, W. Wang, S. Han, L. Wang and X. Ji, Adv. Funct. Mater., 2019, 29, 1902653 CrossRef.
- Q. Yang, Q. Li, Z. Liu, D. Wang, Y. Guo, X. Li, Y. Tang, H. Li, B. Dong and C. Zhi, Adv. Mater., 2020, 32, 2001854 CrossRef CAS.
- W. Yuan, X. Nie, G. Ma, M. Liu, Y. Wang, S. Shen and N. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202218386 CrossRef CAS.
- S. D. Pu, C. Gong, Y. T. Tang, Z. Ning, J. Liu, S. Zhang, Y. Yuan, D. Melvin, S. Yang, L. Pi, J. J. Marie, B. Hu, M. Jenkins, Z. Li, B. Liu, S. C. E. Tsang, T. J. Marrow, R. C. Reed, X. Gao, P. G. Bruce and A. W. Robertson, Adv. Mater., 2022, 34, 2202552 CrossRef CAS PubMed.
- J. Wang, B. Zhang, Z. Cai, R. Zhan, W. Wang, L. Fu, M. Wan, R. Xiao, Y. Ou, L. Wang, J. Jiang, Z. W. Seh, H. Li and Y. Sun, Sci. Bull., 2022, 67, 716–724 CrossRef CAS.
- D. Yuan, J. Zhao, H. Ren, Y. Chen, R. Chua, E. T. J. Jie, Y. Cai, E. Edison, W. Manalastas Jr, M. W. Wong and M. Srinivasan, Angew. Chem., Int. Ed., 2021, 60, 7213–7219 CrossRef CAS PubMed.
- M. Zhou, S. Guo, J. Li, X. Luo, Z. Liu, T. Zhang, X. Cao, M. Long, B. Lu, A. Pan, G. Fang, J. Zhou and S. Liang, Adv. Mater., 2021, 33, 2100187 CrossRef CAS PubMed.
- Z. Huang, Z. Li, Y. Wang, J. Cong, X. Wu, X. Song, Y. Ma, H. Xiang and Y. Huang, ACS Energy Lett., 2023, 8, 372–380 CrossRef CAS.
- H. Zhang, X. Gan, Z. Song and J. Zhou, Angew. Chem., Int. Ed., 2023, 62, e202217833 CrossRef CAS PubMed.
- L. Zhang, J. Huang, H. Guo, L. Ge, Z. Tian, M. Zhang, J. Wang, G. He, T. Liu, J. Hofkens, D. J. L. Brett and F. Lai, Adv. Energy Mater., 2023, 13, 2203790 CrossRef CAS.
- C. Li, R. Kingsbury, L. Zhou, A. Shyamsunder, K. A. Persson and L. F. Nazar, ACS Energy Lett., 2022, 7, 533–540 CrossRef CAS.
- D. Li, L. Cao, T. Deng, S. Liu and C. Wang, Angew. Chem., Int. Ed., 2021, 60, 13035–13041 CrossRef CAS PubMed.
- Q. Li, Y. Wang, F. Mo, D. Wang, G. Liang, Y. Zhao, Q. Yang, Z. Huang and C. Zhi, Adv. Energy Mater., 2021, 11, 2003931 CrossRef CAS.
- H. Tian, G. Feng, Q. Wang, Z. Li, W. Zhang, M. Lucero, Z. Feng, Z.-L. Wang, Y. Zhang, C. Zhen, M. Gu, X. Shan and Y. Yang, Nat. Commun., 2022, 13, 7922 CrossRef CAS PubMed.
- X. Huo, L. Xu, K. Xie, K. Zhang, J. Li, D. Wang and K. Shu, Adv. Energy Mater., 2023, 13, 2203066 CrossRef CAS.
- Z. Hu, F. Zhang, Y. Zhao, H. Wang, Y. Huang, F. Wu, R. Chen and L. Li, Adv. Mater., 2022, 34, 2203104 CrossRef CAS PubMed.
- P. X. Sun, Z. Cao, Y. X. Zeng, W. W. Xie, N. W. Li, D. Luan, S. Yang, L. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2022, 61, e202115649 CrossRef CAS PubMed.
- J. Hao, B. Li, X. Li, X. Zeng, S. Zhang, F. Yang, S. Liu, D. Li, C. Wu and Z. Guo, Adv. Mater., 2020, 32, 2003021 CrossRef CAS PubMed.
- L. Yan, Y. Zhang, Z. Ni, Y. Zhang, J. Xu, T. Kong, J. Huang, W. Li, J. Ma and Y. Wang, J. Am. Chem. Soc., 2021, 143, 15369–15377 CrossRef CAS PubMed.
- K. Zhu, C. Guo, W. Gong, Q. Xiao, Y. Yao, K. Davey, Q. Wang, J. Mao, P. Xue and Z. Guo, Energy Environ. Sci., 2023, 16, 3612–3622 RSC.
- X. Liu, F. Yang, W. Xu, Y. Zeng, J. He and X. Lu, Adv. Sci., 2020, 7, 2002173 CrossRef CAS.
- Y. Zeng, Z. Lai, Y. Han, H. Zhang, S. Xie and X. Lu, Adv. Mater., 2018, 30, 1802396 CrossRef PubMed.
- R. Chen, Q. Liu, L. Xu, X. Zuo, F. Liu, J. Zhang, X. Zhou and L. Mai, ACS Energy Lett., 2022, 7, 1719–1727 CrossRef CAS.
- R. Kim, S. Yuk, J.-H. Lee, C. Choi, S. Kim, J. Heo and H.-T. Kim, J. Membr. Sci., 2018, 564, 852–858 CrossRef CAS.
- Y. Lin, Z. Mai, H. Liang, Y. Li, G. Yang and C. Wang, Energy Environ. Sci., 2023, 16, 687–697 RSC.
- R. Chen, C. Zhang, J. Li, Z. Du, F. Guo, W. Zhang, Y. Dai, W. Zong, X. Gao, J. Zhu, Y. Zhao, X. Wang and G. He, Energy Environ. Sci., 2023, 16, 2540–2549 RSC.
- C. Li, R. Kingsbury, A. S. Thind, A. Shyamsunder, T. T. Fister, R. F. Klie, K. A. Persson and L. F. Nazar, Nat. Commun., 2023, 14, 3067 CrossRef CAS PubMed.
- Y. Wang, B. Liang, J. Zhu, G. Li, Q. Li, R. Ye, J. Fan and C. Zhi, Angew. Chem., Int. Ed., 2023, 62, e202302583 CrossRef CAS.
- S. Carli, M. Bianchi, E. Zucchini, M. Di Lauro, M. Prato, M. Murgia, L. Fadiga and F. Biscarini, Adv. Healthc. Mater., 2019, 8, 1900765 CrossRef PubMed.
- H. Ao, W. Zhu, M. Liu, W. Zhang, Z. Hou, X. Wu, Y. Zhu and Y. Qian, Small Methods, 2021, 5, 2100418 CrossRef CAS PubMed.
- W. Guo, W. Zhang, Y. Si, D. Wang, Y. Fu and A. Manthiram, Nat. Commun., 2021, 12, 3031 CrossRef CAS PubMed.
- S. Liu, J. Vongsvivut, Y. Wang, R. Zhang, F. Yang, S. Zhang, K. Davey, J. Mao and Z. Guo, Angew. Chem., Int. Ed., 2023, 62, e202215600 CrossRef CAS PubMed.
- J. G. Highfield, M. H. Chen, P. T. Nguyen and Z. Chen, Energy Environ. Sci., 2009, 2, 991–1002 RSC.
- G. N. Nomikos, P. Panagiotopoulou, D. I. Kondarides and X. E. Verykios, Appl. Catal. B: Environ., 2014, 146, 249–257 CrossRef CAS.
- C. Li, K. Domen, K. i. Maruya and T. Onishi, J. Catal., 1990, 125, 445–455 CrossRef CAS.
- J. Hao, L. Yuan, Y. Zhu, M. Jaroniec and S. Z. Qiao, Adv. Mater., 2022, 34, 2206963 CrossRef CAS.
- F. Hui and M. Lanza, Nat. Electron., 2019, 2, 221–229 CrossRef.
- Y. Wang, S. A. Skaanvik, X. Xiong, S. Wang and M. Dong, Matter, 2021, 4, 3483–3514 CrossRef CAS.
- P. Shi, J. Ma, M. Liu, S. Guo, Y. Huang, S. Wang, L. Zhang, L. Chen, K. Yang, X. Liu, Y. Li, X. An, D. Zhang, X. Cheng, Q. Li, W. Lv, G. Zhong, Y.-B. He and F. Kang, Nat. Nanotechnol., 2023, 18, 602–610 CrossRef CAS.
- D. Wang, D. Lv, H. Liu, S. Zhang, C. Wang, C. Wang, J. Yang and Y. Qian, Angew. Chem., Int. Ed., 2022, 61, e202212839 CrossRef CAS PubMed.
- M. H. Chung, Electrochim. Acta, 2000, 45, 3959–3972 CrossRef CAS.
- B. D. Adams, J. Zheng, X. Ren, W. Xu and J. G. Zhang, Adv. Energy Mater., 2018, 8, 1702097 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc06935d |
| This journal is © The Royal Society of Chemistry 2024 |