Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Molecular imine cages with π-basic Au3(pyrazolate) faces†
Noga
Eren
,
Farzaneh
Fadaei-Tirani
 ,
Rosario
Scopelliti
,
Rosario
Scopelliti
 and
Kay
Severin
and
Kay
Severin
 *
*
Institut des Sciences et Ingénierie Chimiques, École Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland. E-mail: kay.severin@epfl.ch
First published on 1st February 2024
Abstract
One tetrahedral and two trigonal prismatic cages with π-basic Au3(pyrazolate)3 faces were obtained by connection of pre-formed gold complexes via dynamic covalent imine chemistry. The parallel arrangement of the Au3(pyrazolate)3 complexes in the prismatic cages augments the interaction with π-acids, as demonstrated by the encapsulation of polyhalogenated aromatic compounds. The tetrahedral cage was found to act as a potent receptor for fullerenes. The structures of the three cages, as well as the structures of adducts with C60 and C70, could be established by X-ray crystallography.
Introduction
Trinuclear gold complexes of the general formula Au3(pyrazolate)3 (Fig. 1a) were first described by Bonati and co-workers in 1974.1 Following this initial report, Au3(pyrazolate)3 complexes were studied by numerous other groups.2 These investigations have shown that Au3(pyrazolate)3 complexes display high chemical and thermal stability.2,3 Similar to other Au(I) complexes, Au3(pyrazolate)3 trimers are prone to form Au⋯Au contacts in the solid state, and the presence of these aurophilic interactions is often associated with solid-state luminescence.2,4 Luminescence can also be induced by the confinement of multiple Au3(pyrazolate)3 complexes in supramolecular hosts.5 An important feature of Au3(pyrazolate)3 complexes is their variable π-acidity/basicity.2c,6 Most Au3(pyrazolate)3 complexes behave as π-bases.2c,7 However, the trimer Au3[(3,5-CF3)2pz]3 was found to be π-acidic due to the presence of electron-withdrawing CF3 groups.8 | ||
| Fig. 1 The general structure of trinuclear gold pyrazolate complexes (a), and previously reported hexanuclear gold pyrazolate complexes with binaphthyl or ferrocenyl linkers (b). | ||
Au3(pyrazolate)3 complexes have found different applications.2 For example, they were used to form mesogens,9 stimuli-responsive organogels,10 or conductive thin films.11 Furthermore, a Au3(pyrazolate)3 complex was employed as a chemosensor for the selective detection of Ag+ ions,12 and materials based on 2-dimensional nanosheets containing Au3(pyrazolate)3 complexes were used for photocatalytic hydrogen evolution.13
So far, there are few reports about cage-like structures with multiple Au3(pyrazolate)3 units. Thiel and co-workers have synthesized a hexanuclear Au complex, in which two Au3(pyrazolate)3 complexes are connected by three binaphthyl spacers (Fig. 1b).14 The complex was found to display poor solubility, preventing a solution-based characterization. Structurally related complexes with ferrocenyl linkers were described by Meyer and co-workers.15 Again, poor solubility was encountered, hampering a more comprehensive characterization. The limited success in preparing defined complexes with multiple Au3(pyrazolate)3 units is in contrast to what was found for analogous Cu3(pyrazolate)3 and Ag3(pyrazolate)3 complexes. Cu3(pyrazolate)3 and Ag3(pyrazolate)3 complexes have been incorporated into prismatic and antiprismatic cages,16 and some of these cages were found to encapsulate small molecules.16a–c Furthermore, there is a report about an octahedral cage containing four Cu3(pyrazolate)3 complexes,17 and studies about bridged18 or interlocked systems19 with two Cu3(pyrazolate)3-based prisms.
The difficulty in preparing more complex molecular structures with multiple Au3(pyrazolate)3 units is likely related to two features of Au3(pyrazolate)3 complexes. First, metallophilic interactions are stronger for Au3(pyrazolate)3 complexes than for analog Cu3(pyrazolate)3 and Ag3(pyrazolate)3 complexes.2 Stronger intermolecular interaction can lead to reduced solubility. Second, Au3(pyrazolate)3 complexes are rather inert.20 As a result, error correction processes are less efficient during metallosupramolecular syntheses. Notwithstanding these difficulties, we think that molecularly defined nanostructures with multiple Au3(pyrazolate)3 units are worthwhile synthetic targets, because the pronounced π-basicity of Au3(pyrazolate)3 complexes is expected to lead to interesting host properties.
Below, we describe examples of well-soluble molecular cages containing two or four Au3(pyrazolate)3 faces. The cages were obtained by connection of pre-formed gold complexes via dynamic covalent imine chemistry. The presence of the Au complexes enables the molecular recognition of different guest molecules. Notably, a tetrahedral cage was found to be a potent receptor for C60 and C70.
Results and discussion
The substituted pyrazoles 1 and 2 (Scheme 1) were obtained by Suzuki cross-coupling reactions of 4-bromo-3,5-diisopropyl-1-tosyl-1H-pyrazole with the corresponding formylphenylboronic acids, followed by base-induced deprotection (for details, see the ESI†). Subsequent reactions with AuCl(SMe2) in the presence of triethylamine in THF gave the Au3(pyrazolate)3 complexes 3 and 4 (Scheme 1). Both complexes are soluble in chloroform, but they display very poor solubility in acetonitrile and diethyl ether.The trinuclear complexes 3 and 4 were characterized by NMR spectroscopy and single-crystal X-ray diffraction (XRD). The XRD analyses (Fig. 2) confirm that trinuclear complexes have formed.21 In the solid state, 3 and 4 display a co-planar arrangement of the pyrazolate heterocycles, and the Au–N bond distances are within the expected range (1.98 to 2.02 Å). Close intermolecular Au⋯Au contacts are not observed.
 | ||
| Fig. 2 Graphic representation of the molecular structures of 3 and 4 as determined by single-crystal XRD. Hydrogen atoms are not shown. | ||
Organic cages with imine linkages can be obtained in condensation reaction of di/poly-amines with di/poly-aldehydes.22 The trinuclear complexes 3 and 4 appeared to be potentially well-suited for such condensation reactions.23 However, the clean formation of imine cages is often not straightforward, even if the building blocks seem to have an appropriate geometry. Frequently encountered problems include incomplete condensation reactions, the formation of side products (insoluble polymers or mixtures of cages), and structural rearrangements during isolation.24 In the following, we focus on reactions that resulted in the clean formation of a structurally defined cage. A brief discussion of reaction with other amines can be found in the ESI.†
The reaction of complex 3 (2 equiv.) with 1,3-diaminopropane (3 equiv.) in a mixture of dichloromethane and methanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) gave the [2 + 3] condensation product 5 in high yield (Scheme 2). In solution, cage 5 displays high apparent symmetry, with only one set of NMR signals for the six bridging pyrazolate groups.
2) gave the [2 + 3] condensation product 5 in high yield (Scheme 2). In solution, cage 5 displays high apparent symmetry, with only one set of NMR signals for the six bridging pyrazolate groups.
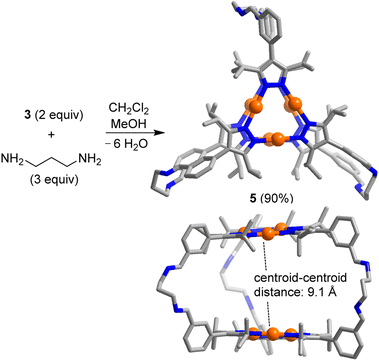 | ||
| Scheme 2 Synthesis of the prismatic cage 5. The graphic representation of the product is based on a crystallographic analysis (view from the top and from the side; hydrogen atoms are not shown). | ||
Single crystals of 5, suitable for an XRD analysis, were obtained by layering of acetonitrile onto a solution of 5 in dichloromethane. The two Au3(pyrazolate)3 complexes in the trigonal prismatic cage 5 are arranged in a parallel fashion, with a distance between the planes of ∼9 Å and a distance between the centroids of 9.1 Å. This spacing suggests that 5 is potentially well-suited to bind ‘flat’ aromatic π-systems.2b The two Au trimers are roughly eclipsed with an angle of ∼6° between them.
The trigonal prismatic cage 6 was obtained in high yield by combining the Au trimer 4 with m-xylylenediamine in a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in C2H2Cl4 (Scheme 3). An XRD analysis of 6 revealed that the height of the prismatic cage, as defined by the distance between the planes of the Au trimers, is ∼7 Å. The overall size of prismatic 6 is significantly larger than that of 5, with a maximum C⋯C distance of 26.8 Å (5: 20.4 Å). In contrast to what was found for 5, one can observe short intermolecular Au⋯Au contacts in crystalline 6 (for details, see the ESI†). The deviation of the Au trimers from a staggered arrangement is ∼25°.
3 in C2H2Cl4 (Scheme 3). An XRD analysis of 6 revealed that the height of the prismatic cage, as defined by the distance between the planes of the Au trimers, is ∼7 Å. The overall size of prismatic 6 is significantly larger than that of 5, with a maximum C⋯C distance of 26.8 Å (5: 20.4 Å). In contrast to what was found for 5, one can observe short intermolecular Au⋯Au contacts in crystalline 6 (for details, see the ESI†). The deviation of the Au trimers from a staggered arrangement is ∼25°.
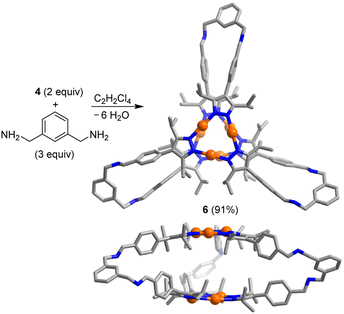 | ||
| Scheme 3 Synthesis of the prismatic cage 6. The graphic representation of the product is based on a crystallographic analysis (view from the top and from the side; hydrogen atoms are not shown). | ||
Tris(2-aminoethyl)amine (TREN) is frequently employed as a building block for the synthesis of imine-based organic cages.22a A mixture of TREN and the Au trimer 4 (ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in CDCl3 gave the [4 + 4] cage 7 (Scheme 4) in nearly quantitative yield as revealed by in situ NMR spectroscopy and ESI mass spectrometry analysis of the reaction mixture. Isolation of 7 was possible by precipitation with acetonitrile (yield: 91%).
1) in CDCl3 gave the [4 + 4] cage 7 (Scheme 4) in nearly quantitative yield as revealed by in situ NMR spectroscopy and ESI mass spectrometry analysis of the reaction mixture. Isolation of 7 was possible by precipitation with acetonitrile (yield: 91%).
 | ||
| Scheme 4 Synthesis of the tetrahedral cage 7. The graphic representation of the product is based on a crystallographic analysis. Hydrogen atoms are not shown. | ||
At room temperature, the 1H NMR spectrum of 7 in CDCl3 showed very broad peaks for the CH signals of the phenylene groups. When cooling the solution to 273 K, four defined signals for the aromatic CH protons were observed. The underlying dynamic phenomenon is likely a hindered rotation of the tightly packed phenylene groups.25 Another noteworthy spectroscopic feature is the presence of two sets of NMR signals for iso-propyl substituents at the pyrazolate ligands. The appearance of two sets of signals is a consequence of the chirality of the TREN-based vertices,26 rending the iso-propyl groups diastereotopic.
A crystallographic analysis of 7 confirmed the tetrahedral shape of the cage (Scheme 4). The edge length of 7 is 24.8 Å (maximum C⋯C distance), making it one of the largest TREN-based imine cages described so far.22a The Au3(pyrazolate)3 complexes panel the four faces of the tetrahedron. As deduced by NMR spectroscopy, the TREN-based vertices show a propeller-like conformation, with the same helical orientation for all four vertices. Residual electron density pointed to the presence of disordered solvent molecules. A solvent mask was calculated, and 2010 electrons were found in a volume of 7652 Å3 in two voids per unit cell.27 The solvent molecules, too disordered to be located in the electron density map, were taken into account using the Olex2 solvent-mask procedure.28
In crystalline 7, close Au⋯Au contacts between the cages are observed (Fig. 3a). The corresponding Au⋯Au distances range from 3.268 to 3.393 Å. These aurophilic interactions are present for three out of the four Au3(pyrazolate)3 complexes in cage 7. To accommodate the Au⋯Au contacts, the Au3(pyrazolate)3 complexes adopt a bent geometry (Fig. 3b), resulting in tetrahedral cages with slightly convex faces. A similar bending of the Au3(pyrazolate)3 complexes was observed for the prismatic cage 6 (Scheme 3, graphic on the bottom). As discussed, this cage shows likewise intermolecular Au⋯Au contacts in the solid state.
 | ||
| Fig. 3 Aurophilic interactions between cages in crystalline 7 (a), and zoom-in on two Au3(pyrazolate)3 complexes in 7, highlighting the bent geometry of the trimers (b). Hydrogen atoms are not shown. | ||
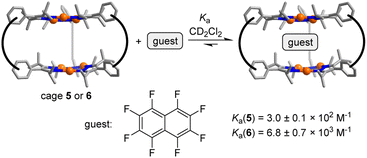
The arrangement of the π-basic Au3(pyrazolate)3 complexes in 5 and 6 suggested that the prismatic cages might be able to act as hosts for π-acidic aromatic compounds. This proposition could be corroborated by NMR studies. The addition of increasing amounts of octafluoronaphthalene to a solution of cage 5 in CD2Cl2 resulted in complexation-induced shifts (CIS) of the 1H NMR signals (for details, see the ESI†). The CIS vs. concentration data could be fitted to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model resulting in an apparent association constant of Ka = 3.0 ± 0.1 × 102 M−1 (Fig. S54†) (Scheme 5).
1 binding model resulting in an apparent association constant of Ka = 3.0 ± 0.1 × 102 M−1 (Fig. S54†) (Scheme 5).
For cage 6, the complexation of octafluoronaphthalene was found to be slow on the 19F NMR time scale, and separate signals for the ‘free’ and the ‘bound’ guest were observed. By integration of the 19F NMR signals, we were able to derive a binding constant of Ka = 6.8 ± 0.7 × 103 M−1 (Fig. S50†). A similar value was derived from 1H NMR data (for details, see the ESI, Fig. S48 and S49†). The tighter binding of octafluoronaphthalene by cage 6 is possibly related to the reduced flexibility of the m-xylylene linkers when compared to the propylene linkers in cage 5. Hexabromobenzene and hexachlorobenzene are likewise bound by 5 and 6, as evidenced by NMR experiments (for details, see the ESI†). The parallel arrangement of the two Au3(pyrazolate)3 complexes in 5 and 6 is of key importance for the complexation of polyhalogenated compounds. In control experiments with the simple Au3(pyrazolate)3 complexes 3 and 4, we were not able to detect an interaction with polyhalogenated compounds.29 Likewise, we were not able to observe a complexation of octafluoronaphthalene by cage 7.
Coinage metal pyrazolate complexes of the general formula [M{3,5-(CF3)2pz}]3 (M = Cu, Ag, Au) were reported to form co-crystals with C60, with four [M{3,5-(CF3)2pz}]3 complexes surrounding C60 in a tetrahedral fashion.30 This finding inspired us to examine if cage 7 would be able to bind fullerenes.31
When C60 (2 equiv.) was added to a solution of cage 7 (1.7 mM) in C2D2Cl4, the formation of the host–guest complex C60⊂7 was detected by mass spectrometry. The complexation of C60 was found to be slow on the NMR time scale, and it could be monitored by 1H NMR spectroscopy. Quantitative formation of C60⊂7 was observed within 12 h. Similar results were obtained with C70. Upon mixing C70 and cage 7 in C2D2Cl4, the adduct C70⊂7 formed in quantitative yield as evidenced by 1H NMR spectroscopy and mass spectrometry. In competition experiments with equal amounts of C60 and C70, we observed the formation of the adducts C60⊂7 and C70⊂7 in nearly equal amounts (∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8).
8).
The structures of C60⊂7 and C70⊂7 in the solid state were analyzed by single-crystal XRD. As expected, the fullerenes are bound in the central cavity of cage 7 (Fig. 4). The distance between the fullerenes and the Au3(pyrazolate)3 complexes, as defined by the closest Au⋯C contacts, is ∼3.4 Å. The average distance between the centroids of the four Au trimers in C60⊂7 is 10.7 Å. For C70⊂7, the corresponding value is 11.3 Å and for 7 it is 11.8 Å. Intermolecular Au⋯Au contacts, as observed for the ‘empty’ cage 7, are observed for C70⊂7 but not for C60⊂7 (for details, see the ESI†).
 | ||
| Fig. 4 Molecular structure of C60⊂7 and C60⊂7 as determined by single-crystal XRD. Hydrogen atoms are not shown. | ||
Conclusions
Molecular cages containing Au3(pyrazolate)3 complexes were obtained by connection of pre-formed gold complexes via dynamic covalent imine chemistry. In contrast to previously described cages with Au3(pyrazolate)3 faces,14,15 the imine cages are soluble in chlorinated organic solvents. It was therefore possible to perform solution-based analyses and to grow single crystals for XRD measurements. The parallel arrangement of the two Au3(pyrazolate)3 complexes in the trigonal prismatic cages 5 and 6 enabled the complexation of polyhalogenated aromatic compounds. Notably, we observed tight encapsulation of octafluoronaphthalene by cage 6 with an apparent binding constant of Ka = 6.8 ± 0.7 × 103 M−1. The tetrahedral cage 7 is able to form adducts with C60 and C70 in a competitive solvent such as tetrachloroethane. Overall, our results provide evidence that the incorporation of Au3(pyrazolate)3 complexes in molecularly defined nanostructures can give compounds with interesting host–guest chemistry.Data availability
The data that support the findings of this study are available in the ESI† of this article.Author contributions
N. E. and K. S. initiated the study, N. E. performed the experiments and analyzed the data, F. F.-T. and R. S. collected and processed the X-ray data, and N. E. and K. S. co-wrote the manuscript. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the École Polytechnique Fédérale de Lausanne (EPFL).References
- F. Bonati, G. Minghetti and G. Banditelli, J. Chem. Soc., Chem. Commun., 1974, 88–89 RSC.
- For review articles, see: (a) J. Zheng, Z. Lu, K. Wu, G.-H. Ning and D. Li, Chem. Rev., 2020, 120, 9675–9742 CrossRef CAS; (b) R. Galassi, M. A. Rawashdeh-Omary, H. V. R. Dias and M. A. Omary, Comments Inorg. Chem., 2019, 39, 287–348 CrossRef CAS; (c) J. Zheng, H. Yang, M. Xie and D. Li, Chem. Commun., 2019, 55, 7134–7146 RSC; (d) M. A. Omray, A. A. Mohamed, M. A. Rawashdeh-Omary and J. P. Fackler Jr, Coord. Chem. Rev., 2005, 249, 1372–1381 CrossRef; (e) A. Burini, A. A. Mohamed and J. P. Fackler, Comments Inorg. Chem., 2003, 24, 253–280 CrossRef CAS.
- G. Minghetti, G. Banditelli and F. Bonati, Inorg. Chem., 1979, 18, 658–663 CrossRef CAS.
- (a) Y. Kuroda, M. Tamaru, H. Nakasato, K. Nakamura, M. Nakata, K. Hisano, K. Fujisawa and O. Tsutsumi, Commun. Chem., 2020, 3, 139 CrossRef CAS PubMed; (b) G. Yang and R. G. Raptis, Inorg. Chem., 2003, 42, 261–263 CrossRef CAS PubMed.
- M. Hanafusa, Y. Tsuchida, K. Matsumoto, K. Kondo and M. Yoshizawa, Nat. Commun., 2020, 11, 6061 CrossRef CAS PubMed.
- S. M. Tekarli, T. R. Cundari and M. A. Omary, J. Am. Chem. Soc., 2008, 130, 1669–1675 CrossRef CAS PubMed.
- Z. Lu, B. Chilukuri, C. Yang, A.-M. M. Rawashdeh, R. K. Arvapally, S. M. Tekarli, X. Wang, C. T. Cardenas, T. R. Cundari and M. A. Omary, Chem. Sci., 2020, 11, 11179–11188 RSC.
- (a) R. Hahn, F. Bohle, S. Kotte, T. J. Keller, S.-S. Jester, A. Hansen, S. Grimme and B. Esser, Chem. Sci., 2017, 9, 3477–3483 RSC; (b) M. A. Omary, M. A. Rawashdeh-Omary, M. W. A. Gonser, O. Elbjeirami, T. Grimes and T. R. Cundari, Inorg. Chem., 2005, 44, 8200–8210 CrossRef PubMed.
- (a) E. Beltrán, J. Barberá, J. L. Serrano, A. Elduque and R. Giménez, Eur. J. Inorg. Chem., 2014, 1165–1173 CrossRef; (b) S. J. Kim, S. H. Kang, K.-M. Park, H. Kim, W.-C. Zin, M.-G. Choi and K. Kim, Chem. Mater., 1998, 10, 1889–1893 CrossRef CAS; (c) J. Barberá, A. Elduque, R. Giménez, F. J. Lahoz, J. A. López, L. A. Oro and J. L. Serrano, Inorg. Chem., 1998, 37, 2960–2967 CrossRef; (d) J. Barberá, A. Elduque, R. Gimenez, L. A. Oro and J. L. Serrano, Angew. Chem., Int. Ed. Engl., 1996, 35, 2832–2835 CrossRef.
- A. Kishimura, T. Yamashita and T. Aida, J. Am. Chem. Soc., 2005, 127, 179–183 CrossRef CAS PubMed.
- L. D. Earl, J. K. Nagle and M. O. Wolf, Inorg. Chem., 2014, 53, 7106–7117 CrossRef CAS PubMed.
- P. K. Upadhyay, S. B. Marpu, E. N. Benton, C. L. Williams, A. Telang and M. A. Omary, Anal. Chem., 2018, 90, 4999–5006 CrossRef CAS PubMed.
- X. Zhu, H. Miao, Y. Shan, G. Gao, Q. Gu, Q. Xiao and X. He, Inorg. Chem., 2022, 61, 13591–13599 CrossRef CAS.
- T. Jozak, Y. Sun, Y. Schmitt, S. Lebedkin, M. Kappes, M. Gerhards and W. R. Thiel, Chem.–Eur. J., 2011, 17, 3384–3389 CrossRef CAS PubMed.
- (a) M. Veronelli, S. Dechert, A. Schober, S. Demeshko and F. Meyer, Eur. J. Inorg. Chem., 2017, 446–453 CrossRef CAS; (b) M. Veronelli, S. Dechert, S. Demeshko and F. Meyer, Inorg. Chem., 2015, 54, 6917–6927 CrossRef CAS PubMed.
- (a) Z.-C. Shi, D.-X. Zhang, S.-Z. Zhan, M. Li, J. Zheng, H. Yang, X.-P. Zhou and D. Li, Isr. J. Chem., 2019, 59, 317–322 CrossRef CAS; (b) L.-H. Li, J.-X. Zhang, S.-K. Jia and G. Yang, Transition Met. Chem., 2016, 41, 107–113 CrossRef CAS; (c) P.-C. Duan, Z.-Y. Wang, J.-H. Chen, G. Yang and R. G. Raptis, Dalton Trans., 2013, 42, 14951–14954 RSC; (d) G.-F. Gao, M. Li, S.-Z. Zhan, Z. Lv, G.-h. Chen and D. Li, Chem.–Eur. J., 2011, 17, 4113–4117 CrossRef CAS.
- M. Grzywa, B. Bredenkötter, D. Denysenko, S. Spirkl, W. Nitek and D. Volkmer, Z. Anorg. Allg. Chem., 2013, 639, 1461–1471 CrossRef CAS.
- J.-H. Wang, M. Li, J. Zheng, X.-C. Huang and D. Li, Chem. Commun., 2014, 50, 9115–9118 RSC.
- S.-Z. Zhan, J.-H. Li, G.-H. Zhang, X.-W. Liu, M. Li, J. Zheng, S. W. Ng and D. Li, Chem. Commun., 2019, 55, 11992–11995 RSC.
- Ligand exchange processes for Au3(pyrazolate)3 complexes were found to be slow at room temperature.
- For examples of tetranuclear Au4(pyrazolate)4 complexes, see: (a) R. A. Smith, R. Kulmaczewski and M. A. Halcrow, Inorg. Chem., 2023, 62, 9300–9305 CrossRef CAS PubMed; (b) K. Fujisawa, Y. Ishikawa, Y. Miyashita and K.-i. Okamoto, Inorg. Chim. Acta, 2010, 363, 2977–2989 CrossRef CAS; (c) G. Yang and R. G. Raptis, Inorg. Chim. Acta, 2003, 352, 98–104 CrossRef CAS.
- For reviews, see: (a) K. S. Gayen, T. Das and N. Chatterjee, Eur. J. Org Chem., 2021, 861–876 CrossRef CAS; (b) K. Acharyya and P. S. Mukherjee, Angew. Chem., Int. Ed., 2019, 58, 8640–8653 CrossRef CAS PubMed; (c) K. Ono and N. Iwasawa, Chem.–Eur. J., 2018, 24, 17856–17868 CrossRef CAS; (d) M. Mastalerz, Acc. Chem. Res., 2018, 51, 2411–2422 CrossRef CAS; (e) G. Zhang and M. Mastalerz, Chem. Soc. Rev., 2014, 43, 1934–1947 RSC; (f) N. M. Rue, J. Sun and R. Warmuth, Isr. J. Chem., 2011, 51, 743–768 CrossRef CAS.
- For the use of metal complexes with pendant aldehyde groups in imine cage synthesis, see: (a) L. S. Lisboa, D. Preston, C. J. McAdam, L. J. Wright, C. C. Hartinger and J. D. Crowley, Angew. Chem., Int. Ed., 2022, 61, e202201700 CrossRef CAS PubMed; (b) L. S. Lisboa, J. A. Findlay, L. J. Wright, C. G. Hartinger and J. D. Crowley, Angew. Chem., Int. Ed., 2020, 59, 11101–11107 CrossRef CAS PubMed; (c) C. Bravin, E. Badetti, F. A. Scaramuzzo, G. Licini and C. Zonta, J. Am. Chem. Soc., 2017, 139, 6456–6460 CrossRef CAS PubMed; (d) S. Bandi and D. K. Chand, Chem.–Eur. J., 2016, 22, 10330–10335 CrossRef CAS PubMed; (e) H. Ding, X. Meng, X. Cui, Y. Yang, T. Zhou, C. Wang, M. Zeller and C. Wang, Chem. Commun., 2014, 50, 11162–11164 RSC; (f) A. Granzhan, C. Schouwey, T. Riis-Johannessen, R. Scopelliti and K. Severin, J. Am. Chem. Soc., 2011, 133, 7106–7115 CrossRef CAS PubMed; (g) A. Granzhan, T. Riis-Johannessen, R. Scopelliti and K. Severin, Angew. Chem., Int. Ed., 2010, 49, 5115–5118 CrossRef PubMed.
- A discussion of potential problems can be found in: T. Hasell and A. I. Cooper, Nat. Rev. Mater., 2016, 1, 16053 CrossRef CAS.
- T. Jiao, L. Chen, D. Yang, X. Li, G. Wu, P. Zeng, A. Zhou, Q. Yin, Y. Pan, B. Wu, X. Hong, X. Kong, V. M. Lynch, J. L. Sessler and H. Li, Angew. Chem., Int. Ed., 2017, 56, 14545–14550 CrossRef CAS PubMed.
- (a) T. Jiao, H. Qu, L. Tong, X. Cao and H. Li, Angew. Chem., Int. Ed., 2021, 60, 9852–9858 CrossRef CAS PubMed; (b) W.-B. Gao, Z. Li, T. Tong, X. Dong, H. Qu, L. Yang, A. C.-H. Sue, Z.-Q. Tian and X.-Y. Cao, J. Am. Chem. Soc., 2023, 145, 17795–17804 CrossRef CAS PubMed.
- Porosity measurements were not performed because crystals of 7 were found to be very fragile.
- O. V. Dolomanov, J. L. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- For the highly π-basic Au3(bzim)3 (bzim = 1-benzylimidazolate), an interaction with octafluoronaphthalene could be detected in solution. See: O. Elbjeirami, M. D. Rashdan, V. Nesterov and M. A. Rawashdeh-Omary, Dalton Trans., 2010, 39, 9465–9468 RSC.
- N. B. Jayaratna, M. M. Olmstead, B. I. Kharisov and H. V. R. Dias, Inorg. Chem., 2016, 55, 8277–8280 CrossRef CAS PubMed.
- (a) X. Chang, Y. Xu and M. Von Delius, Chem. Soc. Rev., 2024, 53, 47–83 RSC; (b) C. Fuertes-Espinosa, M. Pujals and X. Ribas, Chem, 2020, 6, 3219–3262 CrossRef CAS; (c) C. García-Simón, M. Costas and X. Ribas, Chem. Soc. Rev., 2016, 45, 40–62 RSC; (d) D. Canevet, E. M. Pérez and N. Martín, Angew. Chem., Int. Ed., 2011, 50, 9248–9259 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures and experimental details. CCDC 2284420–2284425 and 2309725. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc06280e |
| This journal is © The Royal Society of Chemistry 2024 |