Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unravelling highly oxidized nickel centers in the anodic black film formed during the Simons process by in situ X-ray absorption near edge structure spectroscopy†
Gene
Senges
 a,
Ana Guilherme
Buzanich
a,
Ana Guilherme
Buzanich
 b,
Tilen
Lindič
c,
Tyler A.
Gully
b,
Tilen
Lindič
c,
Tyler A.
Gully
 a,
Marlon
Winter
a,
Marlon
Winter
 a,
Martin
Radtke
b,
Bettina
Röder
b,
Simon
Steinhauer
a,
Beate
Paulus
c,
Franziska
Emmerling
a,
Martin
Radtke
b,
Bettina
Röder
b,
Simon
Steinhauer
a,
Beate
Paulus
c,
Franziska
Emmerling
 b and
Sebastian
Riedel
b and
Sebastian
Riedel
 *a
*a
aFreie Universität Berlin, Fachbereich Biologie, Chemie, Pharmazie, Institut für Chemie und Biochemie – Anorganische Chemie, Fabeckstrasse 34/36, 14195 Berlin, Germany. E-mail: s.riedel@fu-berlin.de
bBAM Federal Institute for Materials Research and Testing, Richard-Willstätter-Str. 11, 12489 Berlin, Germany
cFreie Universität Berlin, Fachbereich Biologie, Chemie, Pharmazie, Institut für Chemie und Biochemie – Theoretische Chemie, Arnimallee 22, 14195 Berlin, Germany
First published on 14th February 2024
Abstract
The Simons process is an electrochemical fluorination method to prepare organofluorine compounds. Despite the wide application, the underlying mechanism is still unclear. We report the investigation of the black film formed on the surface of the anodes in aHF by an in situ Ni K-edge X-ray absorption near edge structure (XANES) investigation. An electrochemical cell for in situ X-ray absorption spectroscopy (XAS) is presented.
The Simons process is an electrochemical method with an exceptionally high functional group tolerance for the generation of industrially important compounds such as triflic acid or perfluorobutane sulfonic acid (PFBS) from their non-fluorinated derivatives.1–6 PFBS, the follow-up and potentially less toxic homologue6,7 of perfluorooctane sulfonic acid (PFOS; annual production of up to 4500 t),8 was similarly used to protect fabrics from water, soil and stain.1,3,6 A row of functionalized perfluorocarbons including perfluorooctyl bromide (PFOB) are of medical interest as oxygen carriers or “blood substitutes”.1,9 Within the broad range of applications for triflic acid,10,11 it depicts the starting material for the production of lithium bis(trifluoromethanesulfonyl)imide (LiTFSI), which is commonly used in electrolytes of Li-ion batteries.11,12 Given the value of the fluorinated compounds produced,1,3,5,6,13 the Simons process is performed with abundant resources, using anhydrous hydrogen fluoride (aHF) as fluoride source, electricity, nickel electrodes, and simple organic starting materials.1,3,14–16 However, in this process also partially fluorinated species are obtained, while simultaneously concurring fragmentation of the products leading to, e.g., CF4, is observed.2,17
The reason for the formation of these species and the mechanism of the Simons process are still under debate.1–3,17,23,31 In principle, two mechanisms have been proposed: (I) either the fluorination proceeds via the electrochemical oxidation of the substrate and consecutive reaction with HF following the ECbECN mechanism (explanation in ref. 32; Fig. 1, left).3,18–23 (II) Alternatively, high potential oxidizers have been proposed (NiF3, NiF4, NiF2⋅F2, or fluorine radicals) to be generated electrochemically, which are reacting consecutively with the organic substrates to the corresponding fluorinated products (Fig. 1, right).1,2,4,17,24–31 Both mechanisms have been investigated in the last decades, while indications were found for both of them.1,3,23,31,33,34 The presence of cationic intermediates as proposed for the ECbECN mechanism is supported by the observation of products that are typical of cationic isomerization processes.2,3,22,23,34 However, as even ammonium salts such as [NMe4]+ are fluorinated a reaction via the ECbECN mechanism of those species would require a dicationic intermediate, which is unlikely to exist.17 The mechanism of electrochemical fluorination (ECF) mediated by nickel-based high potential oxidizers is supported by the fact that nickel electrodes previously anodized under the conditions of the Simons process are able to convert organic molecules to the corresponding fluorinated species even when no electric potential is applied (open circuit conditions).1,2,26,31 This indicates that in the electrochemical process oxidizing nickel agents are involved, mediating the fluorination.1,2,26,31 Further evidence for this hypothesis is provided by Bartlett and coworkers demonstrating that both under Simons conditions and by using the highly pure R-NiF3 acetonitrile is fluorinated yielding similar products.1,2,35,36 Ignat'ev and coworkers observed a black film on the anodes that decomposed rapidly to a yellow-green substance. They proposed that this black film consists of black NiF3 which was reduced to yellow-green NiF2.1,2,36–38 When Ni surfaces exposed to gaseous or liquid HF or after Simons electrochemical fluorination were examined by ex situ X-ray photoelectron spectroscopy (XPS), only NiF2 was detected.39,40 The behavior of nickel anodes was studied in a broad range of hydrogen fluoride based media, but the black film was not investigated in situ and the presence of higher oxidized nickel species during the Simons process remains still unclear.1,29,39,41 Nevertheless several proposed mechanisms use the interaction of an NiF3 surface with the substrate to rationalize the observed products.1 The elucidation of the anodic surface may help to further optimize the Simons process in the future.
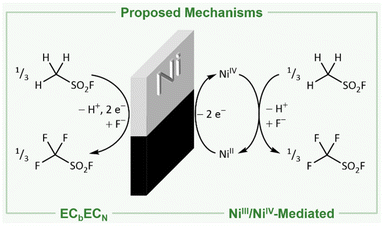 | ||
| Fig. 1 Net reactions for the production of trifluoromethyl sulfonic acid fluoride following the direct electrochemical oxidation via the ECbECN mechanism (left)3,10,18–23 and the fluorination mediated by electrochemically generated NiIII/NiIV (right).1,2,4,10,17,24–31 | ||
Aided by solid state quantum-chemical calculations and reference spectra obtained from the powders of K2NiF6, NiF3 and – for the first time – NiF4, we report an unprecedented in situ X-ray absorption near edge structure (XANES) investigation to reveal the existence of high-valent Ni centers on the surface of the anode in the Simons process.
The Simons process was investigated under conditions closely matching the industrial ones (aHF, Ni electrodes, cell potential of +4.5 to +7.0 V, current density of 0.5 to 3.0 A dm−2).1,3,15,16 We speculated that the black film could be degraded by organic substrates, which could complicate its spectroscopic identification. However, it has been demonstrated by Ignat'ev and coworkers that the organic substrates are fluorinated even when the electrochemical cell is disconnected from the power supply. This means that an oxidizer was produced on the anode before the organic substrate is added, which renders the fluorination independent of the anodic process.26 This justifies the investigation of the anodic process in the absence of an organic substrate.
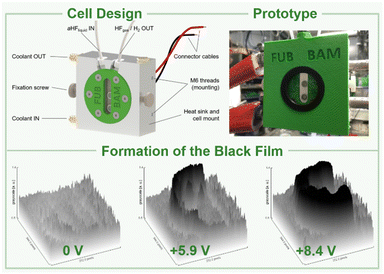
In a preliminary experiment, we studied the formation of the black film in dependence on the applied cell voltage. As already shown by our group, there is only one oxidation feature of the nickel anode at +3.57 V vs. Pt-QRE (platinum quasi-reference electrode), which was attributed to the formation of a higher oxidized nickel species, while at higher potentials no other discriminable oxidation peaks were found in the cyclic voltammogram.42 In several independent experiments we could show that this oxidation process leads to the formation of the black film, as already emphasized by Ignat'ev and coworkers.1,2 After preconditioning a nickel anode at +6.0 V cell voltage, we decreased the potential in 0.1 V steps and observed the stepwise degradation of the black film by chronoamperometric measurements, implying that its layer thickness depends on the voltage applied (Fig. S24 and S25†). As our aim is the in situ characterization of the black film by XANES, it is important to have a layer thickness of at least 50 μm to achieve a sufficient signal-to-noise ratio. Therefore, we polarized a nickel anode at +6.0 V cell voltage for 120 min, removed it from the electrochemical cell and analyzed it by scanning electron microscopy (Fig. S28†). On the anode's surface, particles with an average size of 50 to 100 μm were found, indicating this procedure sufficient to form a black film thick enough for XANES spectroscopy (see the ESI†).
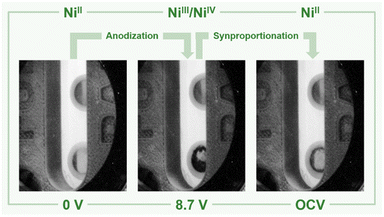
Based on these preliminary analyses, we designed and constructed a setup enabling in situ XANES measurements of the anode's surface in aHF. This setup had to meet certain requirements: (I) resistance against gaseous and liquid aHF, (II) a cell temperature maintained at 0 °C to limit the HF vapor pressure, (III) a window as close as possible to the anode's surface to reduce X-ray absorption by the electrolyte. Therefore, we designed an electrochemical cell, consisting of a polychlorotrifluoroethylene (PCTFE) body with closed circuit cooling, a fluorinated ethylene propylene copolymer (FEP) window (50 μm-foil), planar nickel electrodes embodied in polytetrafluoroethylene (PTFE), and fluorine kautschuk material (FKM) O-ring sealed perfluoroalkoxy alkane (PFA) tubings (Fig. 2, top, and S44 to S51†). The cell was characterized by cyclic voltammetry and showed only one discriminable oxidation feature at +5.25 V cell voltage (Fig. S41†). This deviation from the literature accounts for the overpotential caused by the electrode arrangement required for the planned in situ XANES measurements.42 In a first experiment, the cell was filled with pre-cooled aHF and the surface of the anode was examined by in situ XANES without applying an external potential, revealing that the surface consists of Ni0 and small quantities of NiF2 (Fig. 3 and 4), readily formed by the contact of the nickel electrode with aHF, as previously observed by Scherson and co-workers.40 Then, we applied a cell potential stepwise rising from +5.5 V to +8.9 V and observed the formation of the black film (Fig. 2, bottom, Fig. 3). To further increase the film thickness, an additional conditioning phase at a cell voltage of +8.7 V was maintained for 127 min (Fig. S5 and S7†). After the XANES characterization of the black film its decomposition was further monitored under open-circuit conditions (Fig. S8 and S9†).
Even though we polished the electrodes carefully to a mirror finish, the center of the anode was slightly elevated, resulting in a shorter distance to the FEP foil, and consequently, at this spot no black film could be observed. Therefore, we focused the beam on the ‘blackest’ region of the anode (Fig. S10†).
In the XANES spectrum of the black film we observe a shoulder at 8337.0 eV, and the broader shape right after the edge suggests the contribution of features at 8352.5 and 8354.0 eV. The absorption edge at 8337.0 eV can be attributed to Ni0 of the underlying bulk material of the electrode and the peak at 8352.5 eV is assigned to NiII centers, as confirmed by comparison to a sample of NiF2. More importantly, the absorption maximum at 8354.0 eV is characteristic for highly oxidized nickel centers,38,43 as the XANES spectra of the reference substances K2NiF6, NiF3 and NiF4 feature coinciding white lines at 8354.6 eV (Fig. S35†). The spectra of NiF3 and K2NiF6 measured in transmission resemble the features observed in the spectra in fluorescence (see Fig. S38†). Notably, the XANES spectrum of NiF4, which is the first spectral data obtained from this thermally unstable compound,37 features another peak at 8369.1 eV, while for NiF3 and the black film no maximum was found in this area. These reference spectra were recorded with a low temperature XAS cell (Fig. S52 and S53†).
In order to support these experimental findings, we optimized the structures of binary nickel fluorides and K2NiF6 (Table 1) within the periodic density functional theory (DFT) framework (see the ESI†). Two structures were evaluated for the stoichiometric composition NiF3, one with equal (NiF3, space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c) and one with different Ni–F distances (NiII[NiIVF6], space group R
c) and one with different Ni–F distances (NiII[NiIVF6], space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ). With respect to the different Ni–F distances, a strong distortion of the first F-coordination shell was found for NiII[NiIVF6], in contrast to NiF3. The necessary use of different U values for NiII, NiIII and NiIV centers renders a direct comparison of the energies difficult. However, the energies of NiF3 and NiII[NiIVF6] are comparably large, thus the different synthetic methods for NiF3 – chemically or electrochemically – might force the formation of the monovalent or the mixed-valent species. The comparison of the averaged Ni–F distances of 1.88 and 1.83 Å for NiIII and NiIV, respectively, with 2.02 Å in NiF2, supports our assignment of the features in the XANES spectra. In agreement with these theoretical results and the literature,38,44–46 we determined Ni–F distances of 1.75 (NiIV) and 1.88 Å (NiII) for NiF3 and 1.80 Å for K2NiF6 by their EXAFS (Fig. S40 and Table S2†). These computational results indicate that the black film contains a high-valent nickel fluoride with nickel centers in an oxidation state larger than +II. To further elucidate these observations, we stopped applying a cell potential, thereby initiating the decomposition of the black film while measuring XANES spectra for additional 120 min to monitor the chemical processes behind (Fig. 4). Within 120 min the intensity of the peak at 8352.0 eV was found to significantly increase, while also a peak at 8370.0 eV appears (Fig. 3). As both peaks are indicative for NiF2, it can be assumed that the decomposition of the black film leads to an increase of the NiF2 content. Additionally, we observed a substantial decrease of the absorption at 8337.0 eV (Ni0) comparing the black film and the decomposed film. Thus, simultaneously to the formation of NiF2 the amount of Ni0 decreases, revealing a synproportionation of the high-valent nickel fluoride and the electrode material Ni0 to the decomposition product NiF2.2 This development of the anodic black film is confirmed by linear combination fitting analysis of the spectra (Fig. S18 to S23†). Upon its decomposition at open circuit conditions, the black film decolorized (Fig. 4, right) and the in situ open circuit voltage (OCV) scan showed a residual cell potential of about +2.0 V decreasing over 90 min to approximately 0 V (Fig. S6†). This can be explained by the synproportionation of highly oxidized nickel centers and Ni0 to NiII leading to an anode with an NiF2 surface layer of increased thickness. As the cathode is covered with NiF2 due to passivation by aHF as well,40 after the synproportionation two similar electrodes are obtained, having an electrochemical potential of approximately 0 V.
). With respect to the different Ni–F distances, a strong distortion of the first F-coordination shell was found for NiII[NiIVF6], in contrast to NiF3. The necessary use of different U values for NiII, NiIII and NiIV centers renders a direct comparison of the energies difficult. However, the energies of NiF3 and NiII[NiIVF6] are comparably large, thus the different synthetic methods for NiF3 – chemically or electrochemically – might force the formation of the monovalent or the mixed-valent species. The comparison of the averaged Ni–F distances of 1.88 and 1.83 Å for NiIII and NiIV, respectively, with 2.02 Å in NiF2, supports our assignment of the features in the XANES spectra. In agreement with these theoretical results and the literature,38,44–46 we determined Ni–F distances of 1.75 (NiIV) and 1.88 Å (NiII) for NiF3 and 1.80 Å for K2NiF6 by their EXAFS (Fig. S40 and Table S2†). These computational results indicate that the black film contains a high-valent nickel fluoride with nickel centers in an oxidation state larger than +II. To further elucidate these observations, we stopped applying a cell potential, thereby initiating the decomposition of the black film while measuring XANES spectra for additional 120 min to monitor the chemical processes behind (Fig. 4). Within 120 min the intensity of the peak at 8352.0 eV was found to significantly increase, while also a peak at 8370.0 eV appears (Fig. 3). As both peaks are indicative for NiF2, it can be assumed that the decomposition of the black film leads to an increase of the NiF2 content. Additionally, we observed a substantial decrease of the absorption at 8337.0 eV (Ni0) comparing the black film and the decomposed film. Thus, simultaneously to the formation of NiF2 the amount of Ni0 decreases, revealing a synproportionation of the high-valent nickel fluoride and the electrode material Ni0 to the decomposition product NiF2.2 This development of the anodic black film is confirmed by linear combination fitting analysis of the spectra (Fig. S18 to S23†). Upon its decomposition at open circuit conditions, the black film decolorized (Fig. 4, right) and the in situ open circuit voltage (OCV) scan showed a residual cell potential of about +2.0 V decreasing over 90 min to approximately 0 V (Fig. S6†). This can be explained by the synproportionation of highly oxidized nickel centers and Ni0 to NiII leading to an anode with an NiF2 surface layer of increased thickness. As the cathode is covered with NiF2 due to passivation by aHF as well,40 after the synproportionation two similar electrodes are obtained, having an electrochemical potential of approximately 0 V.
| NiF2a | Ni2F5a | NiF3a | NiII[NiIVF6] | NiF4 | K2NiF6 | |||
|---|---|---|---|---|---|---|---|---|
| a The data were taken from the ref. 42, 43 and 44 respectively. | ||||||||
| SG | P42/mnm | C2/c |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c c |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
P21/c |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
||
| MP | AF | AF | AF | AF | AF | D | ||
| OS (Ni) | +2 | +2 | +3 | +3 | +2 | +4 | +4 | +4 |
| d(Ni–F) | 2.024 | 1.930 | 1.847 | 1.883 | 2.009 | 1.829 | 1.860 | 1.777 |
| 2.024 | 2.154 | 1.834 | 1.883 | 1.987 | 1.828 | 1.860 | 1.777 | |
| 2.024 | 2.171 | 1.941 | 1.883 | 1.950 | 1.860 | 1.933 | 1.777 | |
| 2.024 | 2.154 | 1.834 | 1.883 | 1.986 | 1.817 | 1.836 | 1.777 | |
| 2.016 | 2.171 | 1.941 | 1.883 | 1.970 | 1.817 | 1.933 | 1.777 | |
| 2.016 | 1.930 | 1.847 | 1.883 | 2.003 | 1.824 | 1.836 | 1.777 | |
| d av(Ni–F) | 2.021 | 2.085 | 1.874 | 1.883 | 1.984 | 1.829 | 1.876 | 1.777 |
| E norm | 0 | +0.3297 | +0.2302 | +0.5674 | +1.0091 | — | ||
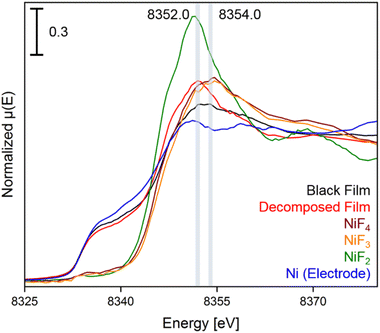 | ||
| Fig. 4 Ni K-edge spectra of the black film (black), the decomposed film (red), and the reference compounds NiF4 (brown), NiF3 (orange), NiF2 (green), and Ni (bulk anode exposed to HF, blue) with an acquisition step size of 0.5 eV. The positions of the maxima of the white lines are highlighted for the black and the decomposed film. The first derivatives of the spectra and time dependent spectra of the black film are shown in Fig. S11 to S17.† | ||
Conclusions
In conclusion, we investigated the black film formed on the surface of the nickel anodes in the Simons process by means of XAS. As it has been speculated for decades that the active species of the Simons process has to be found in the black film, we analyzed the anode's surface employing a tailor-made XAS electrochemical cell. This cell consists of perfluorinated materials and electrodes closely adjustable to the FEP window, allowing for the use of aHF as an electrolyte and enabling in situ Ni–K edge XANES measurements of the anode's surface with a good signal-to-noise ratio. This cell design is not limited to the use of HF and nickel electrodes, but might be adapted for the investigation of numerous other electrochemical processes by XAS or even other methods. Only Ni0 and NiII centers were apparent on the anode prior to the application of a cell potential, while at high potentials XANES spectroscopy revealed the existence of high-valent Ni centers in the black film. Based on our results, the mechanistic understanding and thus the efficiency of the Simons process can be improved.Data availability
Most of the informations is already in the ESI.† However more data is available by the corresponding author.Author contributions
G. S. and S. R. formulated and coordinated the project. G. S. conceptualized the experiments including designs of the XAS cells, with contributions from A. G. B., M. R., B. R., S. S., F. E. The experiments have been performed by G. S. with contributions from M. W., T. A. G. T. L. has performed and analyzed the DFT calculations. B. P. has critically evaluated the computational data and discussed with all coauthors. G. S. visualized experimental data and the graphical representation of the XAS cells with contributions from A. G. B. and B. R. G. S. wrote the first draft of the manuscript. All authors discussed the result of the different disciplines and proofread the publication.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We wish to dedicate this work to Dr Uwe Reinholz (1956–2022), who contributed with all his expertise and full commitment. We want to thank Dr Günther Thiele for the PXRD measurements, Dr Michaël Kulka for the SEM images, Horst Binkowski for manufacturing parts for the preliminary tests, as well as Dr Patrick Voβnacker and Dr Merlin Kleoff (all FU Berlin) for helpful discussions. Experiments were performed at the BAMline at the BESSY-II storage ring (Helmholtz-Zentrum Berlin für Materialien und Energie, HZB). We thank the HZB for the allocation of synchrotron radiation beamtime. We would like to acknowledge the assistance of the Core Facility BioSupraMol supported by the DFG. We thank the CRC 1349 “Fluorine-Specific Interactions” for the financial support of this work and the North German Supercomputing Alliance (Norddeutscher Verbund zur Förderung des Hoch-und Höchstleistungsrechnens HLRN) for computational resources.Notes and references
- N. V. Ignat’ev, in Modern Synthesis Processes and Reactivity of Fluorinated Compounds: Progress in Fluorine Science, ed. H. Groult, F. Leroux and A. Tressaud, Elsevier Science, San Diego, California, 2016, pp. 71–123 Search PubMed.
- N. V. Ignat’ev, U. Welz-Biermann, U. Heider, A. Kucheryna, S. von Ahsen, W. Habel, P. Sartori and H. Willner, J. Fluorine Chem., 2003, 124, 21 CrossRef.
- Y. W. Alsmeyer, W. V. Childs, R. M. Flynn, G. G. I. Moore and J. C. Smeltzer, in Topics in Applied Chemistry, ed. R. E. Banks, B. E. Smart and J. C. Tatlow, Springer US, Boston, MA, 1994, pp. 121–143 Search PubMed.
- P. Sartori and N. Ignat’ev, J. Fluorine Chem., 1998, 87, 157 CrossRef CAS.
- N. S. Rao and B. E. Baker, in Topics in Applied Chemistry, ed. R. E. Banks, B. E. Smart and J. C. Tatlow, Springer US, Boston, MA, 1994, pp. 321–338 Search PubMed.
- R. Renner, Environ. Sci. Technol., 2006, 40, 12 CrossRef PubMed.
- OECD, Results of the 2006 Survey on Production and Use of PFOS, PFAS, PFOA, PFCA, their Related Substances and Products/Mixtures Containing these Substances, 2006 Search PubMed.
- OECD, OECD Hazard Assessment of Perflurooctane Sulphonate (PFOS) and its Salts, 2002 Search PubMed.
- (a) C. Wakselman and A. Lantz, in Topics in Applied Chemistry, ed. R. E. Banks, B. E. Smart and J. C. Tatlow, Springer US, Boston, MA, 1994, pp. 177–194 Search PubMed; (b) K. C. Lowe, in Topics in Applied Chemistry, ed. R. E. Banks, B. E. Smart and J. C. Tatlow, Springer US, Boston, MA, 1994, pp. 555–577 Search PubMed.
- R. D. Howells and J. D. Mc Cown, Chem. Rev., 1977, 77, 69 CrossRef CAS.
- B. A. Shainyan and L. L. Tolstikova, Chem. Rev., 2013, 113, 699 CrossRef CAS PubMed.
- (a) H. Sakaguchi, K. Fujii, S. Sakai, Y. Kobayashi and Y. Kita, DE19533711A1, 1996 Search PubMed; (b) L. Suo, O. Borodin, T. Gao, M. Olguin, J. Ho, X. Fan, C. Luo, C. Wang and K. Xu, Science, 2015, 350, 938 CrossRef CAS PubMed.
- (a) W. H. Pearlson, J. Fluorine Chem., 1986, 32, 29 CrossRef; (b) W. H. Pearlson, US Pat., US3274081A, 1962 Search PubMed; (c) M. Schmeisser and P. Sartori, Chem. Ing. Tech., 1964, 36, 9 CrossRef CAS.
- (a) J. H. Simons and R. D. Dresdner, J. Electrochem. Soc., 1949, 95, 64 CrossRef; (b) J. H. Simons, W. H. Pearlson, T. J. Brice, W. A. Wilson and R. D. Dresdner, J. Electrochem. Soc., 1949, 95, 59 CrossRef; (c) J. H. Simons and W. J. Harland, J. Electrochem. Soc., 1949, 95, 55 CrossRef; (d) J. H. Simons, H. T. Francis and J. A. Hogg, J. Electrochem. Soc., 1949, 95, 53 CrossRef.
- J. H. Simons, J. Electrochem. Soc., 1949, 95, 47 CrossRef CAS.
- J. H. Simons, US Pat., US2519983A, 1950 Search PubMed.
- A. Dimitrov, D. Pfeifer, U. Jonethal, St. Rüdiger and K. Seppelt, J. Fluorine Chem., 1997, 82, 143 CrossRef CAS.
- H. Meinert, J. Mader, W. Röhlke, U. Thewalt and T. Debaerdemaeker, J. Fluorine Chem., 1994, 67, 235 CrossRef CAS.
- I. N. Rozhkov, Russ. Chem. Rev., 1976, 45, 615 CrossRef.
- H. Schmidt and H. Meinert, Angew. Chem., 1960, 72, 109 CrossRef CAS.
- J. Burdon, I. W. Parsons and J. C. Tatlow, Tetrahedron, 1972, 28, 43 CrossRef CAS.
- G. P. Gambaretto, M. Napoli, L. Conte, A. Scipioni and R. Armelli, J. Fluorine Chem., 1985, 27, 149 CrossRef CAS.
- L. Conte and G. Gambaretto, J. Fluorine Chem., 2004, 125, 139 CrossRef CAS.
- R. N. Haszeldine and F. Nyman, J. Chem. Soc., 1956, 2684 RSC.
- S. Rüdiger, A. Dimitrov and K. Hottmann, J. Fluorine Chem., 1996, 76, 155 CrossRef.
- P. Sartori, N. Ignat’ev and S. Datsenko, J. Fluorine Chem., 1995, 75, 157 CrossRef CAS.
- N. Ignat’ev and P. Sartori, J. Fluorine Chem., 2000, 101, 203 CrossRef.
- N. Ignat’ev and P. Sartori, J. Fluorine Chem., 2000, 103, 57 CrossRef.
- A. Dimitrov, S. Rüdiger, N. V. Ignat’ev and S. Datsenko, J. Fluorine Chem., 1990, 50, 197 CrossRef CAS.
- U. Groß, S. Rüdiger and A. Dimitrov, J. Fluorine Chem., 1996, 76, 139 CrossRef.
- T. M. Rangarajan, S. Sathyamoorthi, D. Velayutham, M. Noel, R. P. Singh and R. Brahma, J. Fluorine Chem., 2011, 132, 107 CrossRef CAS.
- The ECbECN mechanism is a four-step mechanism. In the first step (E) the organic molecule is electrochemically oxidized to a radical cation via SET, the second step (Cb) is the elimination of a proton, the formed radical is oxidized electrochemically in the third step (E) to a cation, which reacts in the fourth step (CN) with a fluoride anion. “According to this mechanism the oxidation of the fluoride anions to radicals and a non-ionic origin of the C–F bond are excluded.” (Gambaretto 1985, see also (ref. 18)).
- T. Knuplez, L. N. Schneider, T. Preitschopf, Y. K. J. Bejaoui, L. Zapf, N. Schopper, K. A. M. Maibom, J. A. P. Sprenger, F. Gehrke, S. Lorenzen, R. Graf, R. Bertermann, I. Fischer, N. V. Ignat'ev and M. Finze, Chem.–Eur. J., 2023, 29, e202302701 CrossRef CAS PubMed.
- H. Meinert, R. Fackler, J. Mader, P. Reuter and W. Röhlke, J. Fluorine Chem., 1992, 59, 351 CrossRef CAS.
- J. M. Whalen, L. C. Chacon and N. Bartlett, in Proceedings of the Symposium on Electrochemistry in the Preparation of Fluorine and Its Compounds, ed. W. V. Childs and T. Fuchigami, Electrochemical Society, Pennington, NJ, 1997, vol. 97-15, pp. 1–12 Search PubMed.
- N. Bartlett, R. D. Chambers, A. J. Roche, R. C. H. Spink, L. Chacón and J. M. Whalen, Chem. Commun., 1996, 1049 RSC.
- B. Žemva, K. Lutar, L. Chacón, M. Fele-Beuermann, J. Allman, C. Shen and N. Bartlett, J. Am. Chem. Soc., 1995, 117, 10025 CrossRef.
- C. Shen, L. C. Chacón, N. Rosov, S. H. Elder, J. C. Allman and N. Bartlett, C. R. Acad. Sci. Ser. II, 1999, 2, 557 CAS.
- G. G. Totir, G. S. Chottiner, C. L. Gross and D. A. Scherson, J. Electrochem. Soc., 2001, 148, E262–E266 CrossRef CAS.
- G. G. Totir, G. S. Chottiner, C. L. Gross, W. Ves Childs and D. A. Scherson, J. Electrochem. Soc., 2000, 147, 4212 CrossRef CAS.
- (a) M. Noel, Bull. Electrochem., 1999, 15, 466 CAS; (b) L. Stein, J. M. Neil and G. R. Alms, Inorg. Chem., 1969, 8, 2472 CrossRef CAS; (c) D. J. Wasser, P. S. Johnson, F. W. Klink, F. Kucera and C.-C. Liu, J. Fluorine Chem., 1987, 35, 557 CrossRef CAS; (d) F. G. Drakesmith and D. A. Hughes, J. Fluorine Chem., 1986, 32, 103 CrossRef CAS; (e) N. Watanabe, J. Fluorine Chem., 1983, 22, 205 CrossRef CAS; (f) D. Lines and H. Sutcliffe, J. Fluorine Chem., 1981, 17, 423 CrossRef CAS; (g) N. Watanabe and M. Haruta, Electrochim. Acta, 1980, 25, 461 CrossRef CAS; (h) M. Haruta and N. Watanabe, J. Fluorine Chem., 1976, 7, 159 CrossRef CAS; (i) B. Chang, N. Watanabe and K. Nakanishi, Electrochim. Acta, 1972, 17, 1317 CrossRef CAS; (j) B. Chang, H. Yanase, K. Nakanishi and N. Watanabe, Electrochim. Acta, 1971, 16, 1179 CrossRef CAS; (k) J. A. Donohue, A. Zletz and R. J. Flannery, J. Electrochem. Soc., 1968, 115, 1042 CrossRef CAS; (l) N. Hackerman, E. S. Snavely and L. D. Fiel, Electrochim. Acta, 1967, 12, 535 CrossRef CAS; (m) A. Tasaka, M. Yamanaka, T. Osada and T. Kawagoe, in Proceedings of the Symposium on Electrochemistry in the Preparation of Fluorine and Its Compounds, ed. W. V. Childs and T. Fuchigami, Electrochemical Society, Pennington, NJ, 1997, pp. 144–157 Search PubMed; (n) A. Tasaka, T. Kawagoe, A. Takuwa, M. Yamanaka, T. Tojo and M. Aritsuka, J. Electrochem. Soc., 1998, 145, 1160 CrossRef CAS.
- S. Mattsson, G. Senges, S. Riedel and B. Paulus, Chem.–Eur. J., 2020, 26, 10781 CrossRef CAS PubMed.
- A. L. Hector, E. G. Hope, W. Levason and M. T. Weller, Z. Anorg. Allg. Chem., 1998, 624, 1982 CrossRef CAS.
- T. Lindič, S. Sinha, S. Mattsson and B. Paulus, Z. Naturforsch., B, 2022, 77, 469 CrossRef.
- S. Mattsson and B. Paulus, J. Comput. Chem., 2019, 40, 1190 CrossRef CAS PubMed.
- J. C. Taylor and P. W. Wilson, J. Inorg. Nucl. Chem, 1974, 36, 1561 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc06081k |
| This journal is © The Royal Society of Chemistry 2024 |