Open Access Article
Open Access ArticleElectronic configuration regulation of single-atomic Mn sites mediated by Mo/Mn clusters for an efficient hydrogen evolution reaction†
Chengyu
Zhang
abc,
Xiangyang
Wang
abc,
Renyuan
Zhao
abc,
Fabrice
Ndayisenga
 abc and
Zhisheng
Yu
abc and
Zhisheng
Yu
 *abc
*abc
aCollege of Resources and Environment, University of Chinese Academy of Sciences, 19A Yuquan Road, Beijing 100049, P. R. China. E-mail: yuzs@ucas.ac.cn; Fax: +86-10-88256057; Tel: +86-10-88256057
bBinzhou Institute of Technology, Weiqiao-UCAS Science and Technology Park, Binzhou City 256606, Shandong Province, P. R. China
cRCEES-IMCAS-UCAS Joint-Lab of Microbial Technology for Environmental Science, Beijing 100085, China
First published on 8th January 2024
Abstract
Tuning the electron distribution of metal single-atom active sites via bimetallic clusters is an effective way to enhance their hydrogen evolution reaction (HER) activity, but remains a great challenge. A biochar-based electrocatalyst (BCMoMn800-2) with both MnN4 active sites and Mo2C/Mn7C3 clusters was synthesized using in situ enriched Mo/Mn biomass as a precursor to trigger the HER. Various characterization and density functional theory (DFT) calculation results indicated that the presence of Mo2C/Mn7C3 clusters in BCMoMn800-2 effectively induced the redistribution of charges at MnN4 sites, reducing the energy of H* activation during the HER. In 0.5 M H2SO4, the overpotential was 27.4 mV at a current density of 10 mA cm−2 and the Tafel slope was 31 mV dec−1, and its electrocatalytic performance was close to that of Pt/C. The electrocatalyst also exhibited excellent electrocatalytic stability and durability. This work might provide a new strategy for solid waste recycling and constructing efficient HER electrocatalysts.
Introduction
Excessive use of fossil fuels has caused climate change and energy crisis, which have aroused widespread concern.1,2 To solve these problems, new renewable energy sources that are carbon-neutral are continuously being explored.3 Hydrogen (H2), as one of the perfect substitutes for fossil fuels, has gradually become an indispensable part of the future sustainable and clean energy system due to its excellent properties in terms of energy density, sustainability and cleanliness.4,5 Currently, the traditional hydrogen production processes are pyrolysis of fossil fuels and reforming of natural gas.6,7 In contrast, the production of green H2 by water electrolysis possesses various advantages and is of great significance for global carbon neutrality.8,9 Moreover, compared with alkaline electrolytes, water exhibits higher current densities and energy conversion efficiencies in acidic electrolytes, which could enhance the efficiency of H2 production in large-scale industries.10,11 However, in such an environment of acidic corrosion or strong oxidation potential, most catalysts are prone to dissolve and fall off from the electrode, resulting in a significant decrease in their catalytic activity and stability, thus limiting their practical applications.12 Therefore, the synthesis of high-efficiency catalysts that could achieve high current density and long-term stability is of great significance for the large-scale application of water electrolysis hydrogen production technology.Traditional noble metal catalysts, such as Pt, exhibit excellent electrocatalytic performance for the hydrogen evolution reaction (HER), but inherent defects (high cost and limited reserves) severely hinder their availability in practical industrial applications.13,14 Therefore, there is an urgent need to develop a non-precious metal-based HER electrocatalyst with high efficiency, acid resistance, and long-term stability. In particular, metal cluster/single-atom catalysts have received widespread attention due to the ability to use metal clusters to tune the electronic structure of single atoms.15–17 Generally, the increase of the metal content could effectively improve the HER electrocatalytic performance of the catalyst, while the excess metal is prone to agglomeration during the pyrolysis process, thereby seriously reducing the HER activity of the catalyst.18,19 Therefore, improving the intrinsic activity of a single active site is an effective way to enhance the electrocatalytic activity for the HER. It has been reported that carbides, such as molybdenum carbide20 and manganese carbide,21 are superior HER electrocatalysts with excellent electrical conductivity, resistance to acid, and alkali environments, and exhibit excellent long-term HER stability. Specifically, carbide clusters could induce changes in the local electronic structure, and reduce the adsorption energy of H* and the energy barrier of H* activation by regulating the electron density of d orbitals and TDOS.22,23 Besides, the introduction of metal single-atom sites could not only regulate the surrounding electronic structure but also serve as the adsorption sites of H*, which facilitate the adsorption/desorption of H* and reduce the energy barrier of the HER process.24,25 Based on the above analysis, it was speculated that the coupling of metal clusters and single atoms might exhibit unexpected HER electrocatalytic properties. Most of the current studies mainly focus on the optimization of the active sites of single atoms and metal clusters, and there are a large number of reports on the synergistic effect between single atoms and metal clusters.26–29 Non-noble metals including Mo and Mn have been proven to exhibit good performance in electrocatalytic hydrogen evolution. The pyrolysis of Mo and C in a reducing atmosphere could generate MoxC. MoxC has a d-band electronic structure similar to Pt, and its flexible controllability and high conductivity ensure its high activity and stability in a wide pH range, thus showing promise as an HER catalyst.11,30 Mn single atoms are an efficient candidate in the research of manganese-based catalyst materials and could be used in many challenging catalytic reactions. The three-dimensional unoccupied orbitals in Mn single atoms could accommodate foreign electrons, thereby effectively reducing the bonding strength with H* intermediates during the HER process. In addition, the introduction of Mn single atoms in carbon-based catalysts could promote the formation of Mn–N–C and enhance HER catalytic activity and stability.15 Previous studies reported that the coupling of MoxC with metals or non-metals is an effective method to improve the kinetics and activity of the HER.31 There are relatively few studies on electrocatalysts with the synergistic effects of Mo and Mn doped together in biochar. How the coordination conditions or structures between MoxC and Mn single atoms affect the HER performance is still a question that needs to be explored. Therefore, it is necessary to further evaluate the HER performance of the catalyst formed by the two and explore its HER mechanism.
Herein, a novel biochar-based HER electrocatalyst (BCMoMn800-2) with Mo2C/Mn7C3 clusters and atomically dispersed MnN4 was prepared from in situ enriched Mo/Mn biomass as a precursor. HAADF-STEM and X-ray absorption near-edge spectroscopy results demonstrate the formation of atomically dispersed MnN4 single atoms and Mo2C/Mn7C3 clusters. Density functional theory (DFT) calculations further elucidated the strong electronic interaction between Mo2C/Mn7C3 clusters and single-atom Mn sites, as well as the electrocatalytic mechanism of the HER. According to the above structural features, BCMoMn800-2 exhibited excellent electrocatalytic activity for the HER. In 0.5 M sulfuric acid, the overpotential was 27.4 mV at a current density of 10 mA cm−2 and the Tafel slope was 31 mV dec−1, thus the electrocatalytic performance was close to that of Pt/C. Moreover, excellent long-term stability and durability were exhibited. Overall, experiments and theoretical calculations confirmed that BCMoMn800-2 with metal clusters and single atoms is a low-cost and promising electrocatalyst for the HER.
Results and discussion
Characterization of electrocatalysts
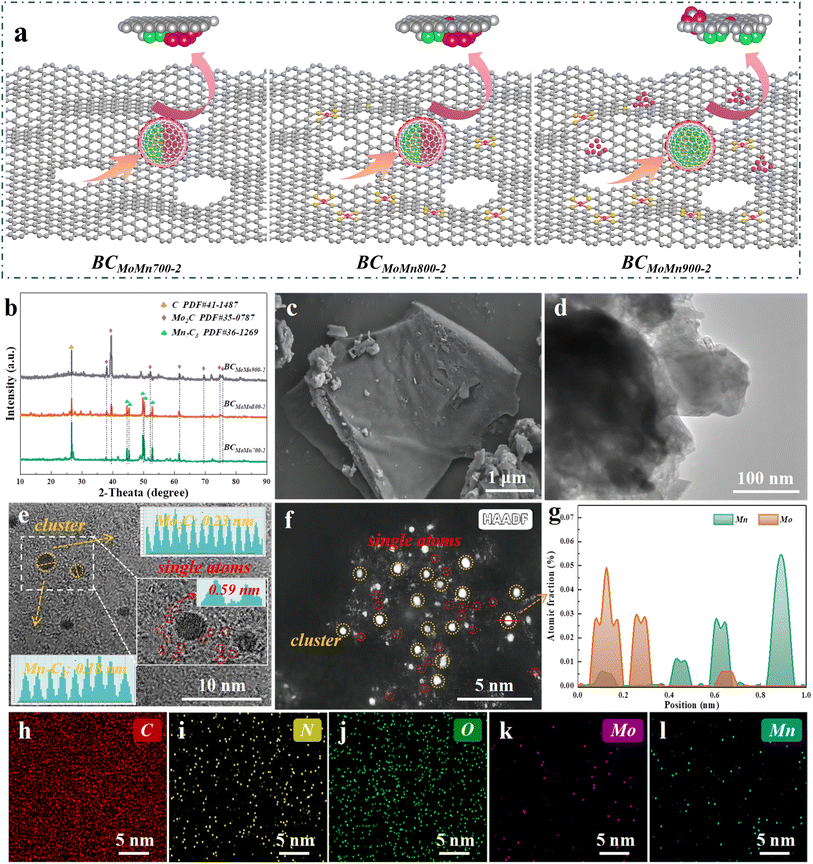
The evolution of the catalyst structure at different pyrolysis temperatures is shown in Fig. 1a. In this study, biomass precursors containing Mo and Mn were prepared by plant absorption and further pyrolyzed at 700, 800, and 900 °C to obtain BCMoMn with a graphene-like structure and abundant defects. Notably, the preparation method of BCMoMn precursors using plant-absorbed metal ions displayed the following advantages. (1) Unlike the conventional impregnation pyrolysis method, this preparation method was beneficial for the uniform distribution of metal elements in biochar. (2) Evenly distributed metal elements were beneficial to the regulation of the local microenvironment of the catalyst. (3) N absorbed during plant growth enhanced the formation of metal single atoms during pyrolysis.The crystalline phases of the synthesized catalysts were detected by X-ray diffraction (XRD). As illustrated in Fig. 1b and S1,† an obvious characteristic diffraction peak was observed at 26.4° in all samples, which matched with graphitic carbon (C, PDF#41-1487).24 With the increase of pyrolysis temperature, the intensity of the diffraction peak gradually increased, indicating that high temperature could transform amorphous carbon into graphitic carbon under the action of Mn/Mo. More importantly, XRD results demonstrated the formation of Mo2C and Mn7C3 in the synthesized samples. Characteristic diffraction peaks of Mo2C (PDF#35-0787) at 2θ = 38.0, 39.4, 52.1, 61.5, 69.6, 74.6, and 75.5° are ascribed to the (002), (101), (102), (110), (103), (112), and (201) facets, respectively.32 Additionally, characteristic diffraction peaks of Mn7C3 (PDF#36-1269) at 2θ = 44.6, 45.3, 49.8, 50.3, and 52.8° are ascribed to the (211), (300), (301), (202), and (220) facets, respectively.33 Notably, after increasing the temperature to 900 °C, the diffraction peak of Mn7C3 almost disappeared, which may be due to the fact that Mn7C3 clusters participated in the growth of the carbon layer during the high-temperature graphitization of biochar.34 Combining XRD patterns analyzed from samples pyrolyzed at 600 °C (Fig. S1b†) and the literature,35,36 it could be speculated that the Mo0/Mn0 formed by reduction at 600 °C acted as a catalyst to start promoting the formation of graphene-like structure. By increasing the temperature to 700–800 °C, the Mo0/Mn0 gradually transformed into Mo2C/Mn7C3 and were gradually wrapped by graphene-like structure. By further raising the temperature to 900 °C, Mn7C3 clusters were gradually consumed and combined in the stable form of graphene-like structure and Mn7C3.
The microstructure of the synthesized samples was characterized by field-emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM) and high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM). FESEM results showed that the BCMoMn800-2 sample possessed a smooth surface structure (Fig. 1c). Compared with the control biochar, the introduction of Mn/Mo did not induce obvious changes in the morphology of biochar. Additionally, TEM images further confirmed the smooth surface structure of BCMoMn800-2 (Fig. 1d). These results indicated that the preparation method achieved a uniform distribution of Mn/Mo in biochar, thereby suppressing the agglomeration of metal nanoparticles during pyrolysis. The HRTEM image clearly exhibited the metal cluster size (only 0.5–1.5 nm) and lattice fringe properties on the biochar substrate (yellow circles and split lines highlighted, Fig. 1e). Notably, two kinds of lattice fringes could be observed with distinct interfaces (dashed yellow lines highlighted). According to the Fast Fourier Transform (FFT) of the cluster lattice fringes (Fig. 1e inset), lattice spacings of 0.23 and 0.18 nm were calculated, corresponding to the (003) crystal plane of Mo2C and (004) crystal plane of Mn7C3, respectively. As reported, the preparation method of plant enrichment could achieve a uniform distribution of metal species in biochar rather than loading them on the surface.37 Of note, obvious carbon stripes were observed at the edge of the lattice (Fig. 1e inset), suggesting that the Mo2C/Mn7C3 clusters were wrapped by the carbon layer. The distance between close bright spots was 0.59 nm (Fig. 1e inset), which was significantly larger than the atomic diameter of Mo/Mn atoms (0.278/0.254 nm), suggesting that the Mo or Mn element was distributed as a single atom in BCMoMn800-2.38 The HAADF-STEM images clearly revealed metal single atoms (highlighted in red circles) and clusters (highlighted in yellow circles) on the biochar matrix (Fig. 1f), indicating the coexistence of single-atom dispersed M–N–C sites and metal clusters.39 The positional relationship of Mo/Mn in metal clusters was further proved by EDS line-scan elemental of BCMoMn800-2 (Fig. 1g), which was consistent with FFT analysis. Additionally, the energy-dispersive X-ray spectroscopy (EDS) images of BCMoMn800-2 confirmed the uniform distribution of N, Mo, and Mn elements on the biochar. More details on electron microscopy characterization of other catalysts are provided in Fig. S2.†
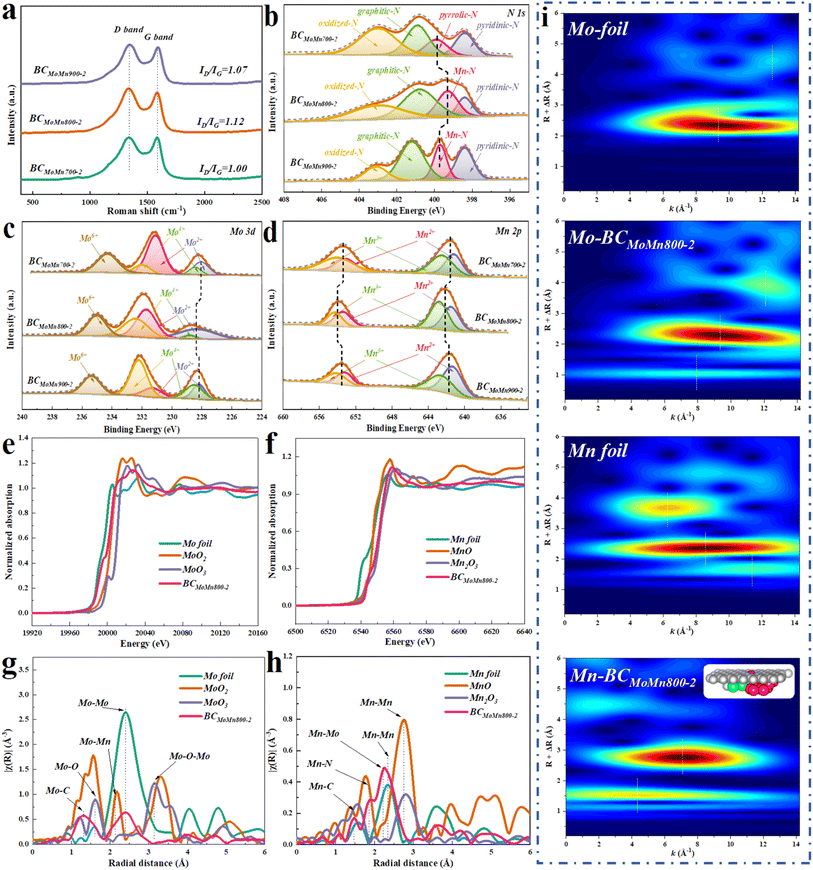
The carbon structural defects of the synthesized catalysts were characterized by Raman spectroscopy. The peaks at 1350 (D band) and 1580 (G band) cm−1 were ascribed to defective/disordered carbon and graphitic structures, respectively, while the ratio of D and G bands (ID/IG) could reflect the defect degree of carbon-based materials.40 As reported, different metal crystalline phases could lead to differences in the structural defects of carbon layers.41 By calculation, the ID/IG values of BCMoMn700-2, BCMoMn800-2, and BCMoMn900-2 were 1.00, 1.12, and 1.07 (Fig. 2a), respectively, indicating that more carbon defect structures were formed in BCMoMn800-2, which were conducive to the adjustment of the surface electronic structure, thereby enhancing the interfacial electron exchange.42 Additionally, the loading amount of Mo/Mn also affected the structure of the carbon (Fig. S3 and Table S1†). The ID/IG values of BCMoMn800-1 and BCMoMn800-3 were 1.05 and 1.18, suggesting that carbon structural defects gradually increased as the content of Mo and Mn increased.
The surface elemental composition and chemical speciation of the synthesized catalysts were detected by X-ray photoelectron spectroscopy (XPS). XPS further confirmed the existence of C, O, N, Mo, and Mn elements on the catalyst's surface (Fig. S4a†). Specifically, all synthesized catalysts exhibited similar C 1s and O 1s XPS spectra (Fig. S4b and c†). As illustrated in Fig. 2b, the high-resolution N 1s spectra of BCMoMn700-2 were fitted with four peaks at 403.1, 400.8, 400.0, and 398.4 eV, corresponding to oxidized-N, graphitic-N, pyrrolic-N, and pyridinic-N.43,44 Notably, the high-resolution N 1s spectra of BCMoMn800-2 and BCMoMn900-2 were fitted with a Mn–N peak at 399.3 eV and 399.8 eV instead of the pyrrolic-N peak,45 and the negative electron shift of 0.5 eV indicated that BCMoMn800-2 possessed a higher N electron density than BCMoMn900-2. Additionally, BCMoMn800-2 exhibited a higher relative content of graphite-N (42.6%) and Mn–N (21.4%) than the others (in Table S2†), which was beneficial for the accelerated electron transfer during the HER, thereby increasing the current density.46,47 The high-resolution Mo 3d spectra of BCMoMn700-2 could be fitted with three groups of peaks at 228.0/231.0, 228.5/232.1, and 232.2/235.3 eV (Fig. 2c), corresponding to Mo2C, MoO2, and MoO3,31,48 where MoO2 and MoO3 may be caused by surface oxidation.49,50 In particular, the Mo2C peaks in BCMoMn800-2 and BCMoMn900-2 were positively shifted by 0.4 and 0.2 eV, indicating that Mo2C on BCMoMn800-2 exhibited a significantly electron-deficient feature than the other two. The high-resolution Mn 2p spectra of BCMoMn700-2 could be fitted with two groups of peaks at 641.2/653.1 and 642.5/654.0 eV (Fig. 2d), which were ascribed to Mn2+ and Mn3+, respectively.45 Similarly, the Mn 2p spectra of BCMoMn800-2 and BCMoMn900-2 exhibited a positive shift, especially BCMoMn800-2 (0.3 eV), indicating the electron loss tendency on Mn sites.51,52 According to the above results, it could be speculated that the Mn single atoms on the graphene-like structure could promote the electron transfer from the Mo2C/Mn7C3 clusters to the graphene-like structure, thereby resulting in the strengthening of electronic metal–support interaction between the Mo2C/Mn7C3 clusters and the carbon layer. This speculation was further confirmed by DFT calculations (Fig. S5–S7†).
The electronic structures and coordination environments of Mo and Mn species in BCMoMn800-2 were detected by X-ray absorption fine structure spectroscopy (XAFS). As illustrated in Fig. 2e, the Mo k-edge X-ray absorption near-edge structure (XANES) spectrum showed that the absorption edge position of Mo in BCMoMn800-2 was between Mo foil and MoO2, indicating that the average valence state of Mo was between 0 and +4. Moreover, Fourier-transformed X-ray absorption fine structure (FT EXAFS) of Mo k-edge revealed obvious Mo–C and Mo–Mo coordination at 1.3 and 2.4 Å (Fig. 2g), further confirming the generation of Mo2C in BCMoMn800-2.53 Additionally, the weak Mo–O (1.7 Å) coordination may be due to the surface oxidation of the sample in air. As illustrated in Fig. 2f, the Mn k-edge XANES spectrum of BCMoMn800-2 was in the vicinity of Mn2O3, indicating that the valence state of Mn was around +3. In the analysis of Mn k-edge FT EXAFS (Fig. 2h), the peaks at 1.4 and 2.3 Å could be ascribed to Mn–C and Mn–Mn coordination, which were the key to prove the existence of Mn7C3.33,54 More importantly, the Mn–N coordination at 1.7 Å was detected in FT EXAFS of BCMoMn800-2.55 As reported,16 due to the complexity of metal–nitrogen–carbon materials, the average coordination number between the metal and nitrogen could not directly prove the specific structure of Metal–Nx. The calculated average coordination number of Mn–N was about 2.0, which may be due to the reduction of the coordination number of N by the Mn atoms adjacent to the cluster heterojunction. Notably, Mo–Mn coordination was also detected at 2.1 Å in the FT EXAFS of both Mo and Mn k-edges, which could be due to the interfacial coupling of clusters formed by Mo2C and Mn7C3. Combined with HAADF-STEM and XRD analysis, it was further confirmed that Mo2C and Mn7C3 in BCMoMn800-2 existed in the form of clusters instead of nanoparticles. The wavelet transform (WT) of the k3-weighted EXAFS spectrum could further reconfirm the above coordination structure (Fig. 2i). According to the above results, it was inferred that the possible structure of BCMoMn800-2 was MnN4 fixed on the graphene layer plane and Mo2C/Mn7C3 clusters below the graphene layer. This structure contained 4 Mn–N bonds and 2 Mn atoms (Fig. 2i inset), so the average coordination number of Mn–N was about 2.0, which was consistent with the detection and calculation results. Mn–N also existed in BCMoMn900-2, but not in BCMoMn700-2 (Fig. S8†). It was speculated that Mn single atoms were generated when the pyrolysis temperature was greater than 700 °C.56 Moreover, Mn single atoms could facilitate the electron transfer from the Mo2C/Mn7C3 cluster to the graphene-like structure by DFT calculation, thereby enhancing the electronic metal–support interaction (Fig. S6†).
HER catalytic performances
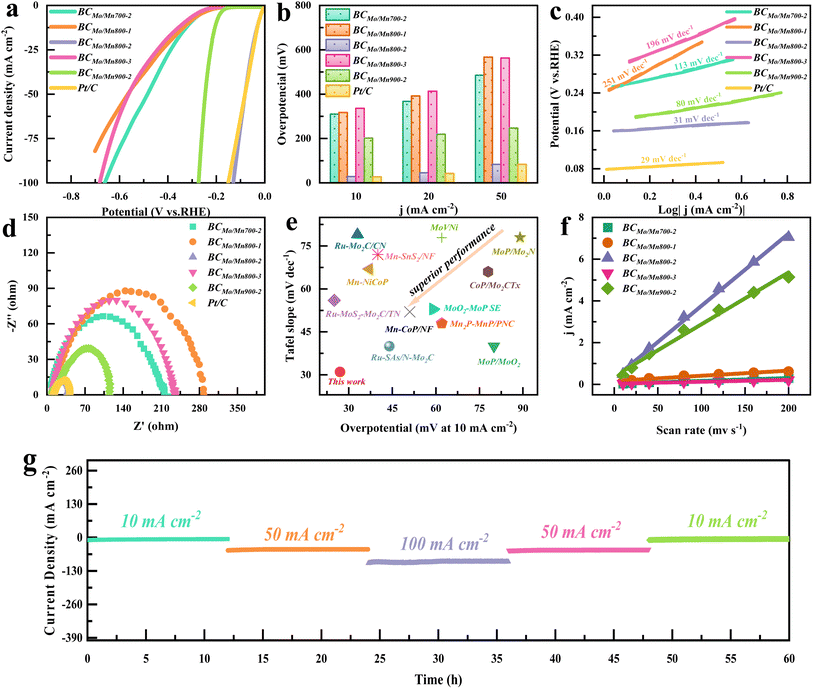
To evaluate the HER electrocatalytic performance of the synthesized catalysts, electrochemical tests were performed in 0.5 M H2SO4 electrolyte. As illustrated in Fig. 3a, the linear sweep voltammetry (LSV) image revealed that BCMoMn800-2 exhibited a higher current density than others, indicating its superior HER catalytic performance. Notably, at a current density of 10 mA cm−2 (Fig. 3b), the overpotential of BCMoMn800-2 was only 27.4 mV, which was close to that of Pt/C (26.4 mV), but much lower than that of BCMoMn700-2 (309.4 mV), BCMoMn800-1 (316.4 mV), BCMoMn800-3 (335.4 mV), and BCMoMn900-2 (201.4 mV). Additionally, even at current densities of 20, 50, and 100 mA cm−2, BCMoMn800-2 still exhibited smaller overpotentials than other catalysts. Subsequently, the HER kinetics of the catalysts were evaluated by calculating the Tafel slope (Fig. 3c). The Tafel slope of BCMoMn800-2 was the smallest, only 31 mV dec−1, which was much smaller than that of BCMoMn700-2 (113 mV dec−1), BCMoMn800-1 (251 mV dec−1), BCMoMn800-3 (196 mV dec−1), and BCMoMn900-2 (80 mV dec−1), and close to that of Pt/C (29 mV dec−1). These results indicated that the HER process of the catalysts followed the Volmer–Heyrovsky mechanism, and the Tafel step was rate-limiting.57 Electrochemical impedance spectroscopy (EIS) further revealed the interfacial electron transfer kinetics of the catalysts in the HER process (Fig. 3d), and the resistance of BCMoMn800-2 was the smallest (only 27.38 Ω), indicating the fastest charge transfer at the interface with the electrolyte. Compared with some of the recently reported advanced Mo/Mn catalysts, BCMoMn800-2 exhibited excellent overpotential and Tafel slope in the HER process compared with the recently reported advanced Mo/Mn-containing catalysts (Fig. 3e).To evaluate the abundance of active sites, the effective electrochemical surface area (ECSA) of the catalyst was detected by the electrochemical double-layer capacitance method (Fig. 3f and S9†). Among the synthesized catalysts, BCMoMn800-2 possessed the largest double-layer capacitance of 34.6 mF cm−2, indicating its larger ECSA and more abundant active sites than others. Overall, from the overpotential, Tafel slope, resistance, and ECSA, it could be obviously shown that BCMoMn800-2 exhibited superior catalytic activity in the HER process. The HER performance of electrodes is not only related to ECSA, but also affected by electrode composition, active surface area, and hydrophilicity.58 Among them, the hydrophilicity of the electrode surface is a key factor affecting the movement behavior of H2 bubbles, thus determining the kinetic performance of the HER reaction. In principle, the formation of small uniform H2 bubbles facilitates its desorption from the electrode without causing polarization of the electrode. The contact angles of BCMoMn800-2 were 32.6, 27.1, 24.0, 19.8, and 14.3° at 0, 100, 200, 500, and 1000 ms, respectively, which exhibited excellent hydrophilicity (Fig. S10†). The optimal hydrophilicity of the BCMoMn800-2 electrode might be due to its abundant mesopores, channels, and defect structures, which accelerated the separation of the generated H2 bubbles and enabled the acidic electrolyte to reach the active catalytic sites.59 From Fig. S11,† the electrode surface of BCMoMn700-2 and BCMoMn900-2 adsorbed a large number of large bubbles generated, resulting in the coverage of active sites on the catalyst surface, thereby reducing the mass transfer rate at the electrode/electrolyte interface. In contrast, the electrode surface of BCMoMn800-2 generated uniform small bubbles and desorbed rapidly, exhibiting superior H2 production efficiency. Therefore, electrodes with smaller contact angles were beneficial for the generation of small/uniform bubbles and their rapid desorption from the electrode surface, thereby maintaining the mass transfer rate at the electrode/electrolyte interface, the effectiveness and stability of electrode activity.60
Cyclic voltammetry (CV) tests were performed to evaluate the electrochemical stability of BCMoMn800-2 during the acidic HER process. After 1000 cycles, HER polarization curves changed slightly, indicating its superior electrochemical stability (Fig. S12†). Additionally, further characterization analysis of the tested materials (Fig. S13 and S14†), including SEM, TEM, XRD, etc., showed that the surface of BCMoMn800-2 maintained good microstructure and crystal phase characteristics. These results indicated that BCMoMn800-2 exhibited superior electrochemical stability during the HER process. Long-term electrolytic stability tests at different current densities were performed to further evaluate the application prospect in the actual process (Fig. 3g). During the continuous experiment of 60 h, even if different current densities were applied, the current density of the reaction system remained stable. Notably, at a current density of 10 mA cm−2, the current density of the BCMoMn800-2 system decreased by less than 10%, indicating its excellent electrochemical stability for acidic HER, showing broad application prospects.
To evaluate the HER electrocatalytic active sites of the synthesized catalysts, the turnover frequency (TOF) was calculated. As illustrated in Fig. S15,† at an overpotential of 250 mV, the BCMoMn800-2 electrode exhibited a superior TOF value of 74.2 s−1 compared to other electrodes, indicating that the BCMoMn800-2 electrode possessed an excellent intrinsic HER electrocatalytic activity. Combined with characterization analysis, this may be due to the synergy between Mo2C–Mn7C3 clusters and MnN4. In detail, Mo2C–Mn7C3 clusters on BCMoMn800-2 provided electrons for H* activation, while MnN4 on graphene was beneficial for electron aggregation and H* adsorption/desorption. It is known that the HER activity of catalysts is not only dependent on the type of active sites but also closely related to the number and size of active sites. Compared with BCMoMn800-2, BCMoMn800-1 exhibited a lower electrocatalytic activity for the HER, which may be attributed to the lower number of active sites due to lower metal loading.61 In contrast, BCMoMn800-3 possessed a higher metal content (Table S1†), but its HER electrocatalytic activity was significantly lower than that of BCMoMn800-2, which might be due to the agglomeration of Mo2C–Mn7C3 clusters caused by the excessive metal loading, reducing the HER electrocatalytic activity of the catalyst. Moreover, from the SEM and HRTEM images (Fig. S2†), the particle size of BCMoMn800-3 was significantly increased compared to that of BCMoMn800-2, demonstrating that the increase of particle size suppressed the electrocatalytic activity of the HER, thus further proving the speculation.
Mechanism and theoretical calculation
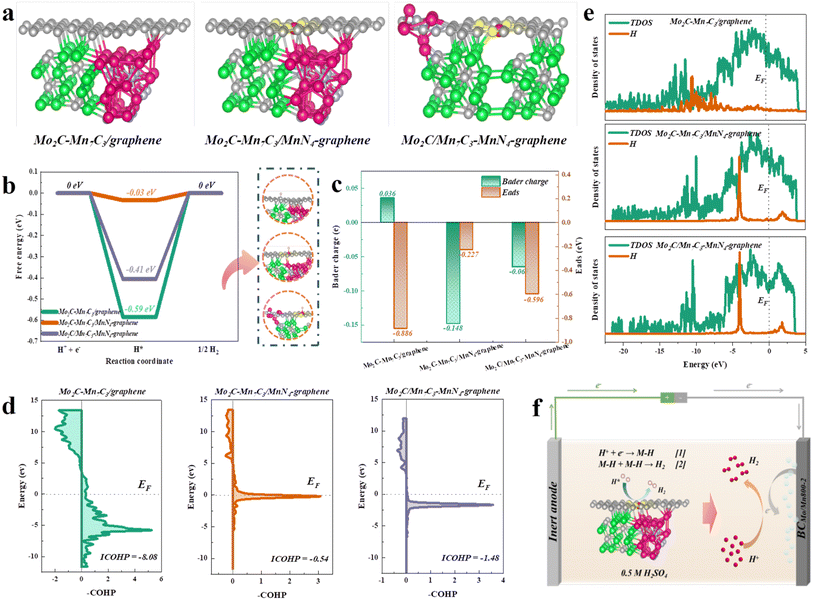
To elucidate the mechanism of the superior HER activity of the synthesized catalysts from the atomic scale, DFT calculations were performed. Based on HAADF-STEM and XAFS characterization analysis, the atomic models of Mo2C–Mn7C3/graphene, Mo2C–Mn7C3/MnN4-graphene, and Mo2C/Mn7C3–MnN4-graphene were constructed and optimized (Fig. 4a and S5†), corresponding to BCMoMn700-2, BCMoMn800-1, and BCMoMn900-2, respectively. As reported,62 a positive value of the Gibbs free energy of H* adsorption indicated that the HER catalyst exhibited poor adsorption of H*, while a negative value might result in difficulty in the desorption of the generated H2 molecules. In acidic systems, among Mo2C–Mn7C3/graphene, Mo2C–Mn7C3/MnN4-graphene, and Mo2C/Mn7C3–MnN4-graphene, Mo2C–Mn7C3/MnN4-graphene exhibited the best Gibbs free energy of H* adsorption (ΔGH* = −0.03 eV, Fig. 4b), which was almost close to the ideal value of 0, indicating its excellent H* adsorption/desorption kinetics.63 Notably, from the charge density difference diagram, the Mo2C–Mn7C3 heterostructure formed in Mo2C–Mn7C3/MnN4-graphene exhibited a large local lattice distortion, resulting in changes in the local electronic structure, thereby increasing the activity of electrons. The Bader charge analysis further demonstrated the superior electron donating ability of the Mo2C–Mn7C3 heterojunction (−0.293 e) compared to Mo2C (−0.197 e, Fig. S6†), and electrons were transferred from Mo2C–Mn7C3 to graphene, which was consistent with XPS analysis. Moreover, the density of states (DOS) at the Fermi level of the metal d orbitals in the Mo2C–Mn7C3 heterostructure was significantly weakened (Fig. S7†), thereby resulting in a weakened adsorption capacity. Besides, the MnN4 on graphene enhanced the charge transfer from Mo2C–Mn7C3 to the graphene layer.Moreover, the electron transfer of different models during the H* adsorption process was investigated. H atoms adsorbed on Mo2C–Mn7C3/MnN4-graphene exhibited a smaller charge transfer of −0.148 e than Mo2C–Mn7C3/graphene and Mo2C/Mn7C3–MnN4-graphene, corresponding to the smaller adsorption energy (Eads) of −0.227 eV (Fig. 4c). The charge transfer of H atoms exhibited a significant linear relationship with Eads (R2 = 0.98, Fig. S16†), indicating that the low charge transfer on hydrogen atoms resulted in the weaker adsorption of H*, thereby accelerating the desorption of H*.
To evaluate the contribution of the bonding and antibonding states of H uptake at the Mo2C–Mn7C3/MnN4-graphene interface, the projected crystal orbital Hamilton population (pCOHP) analysis was performed.64 The integral COHP (ICOHP) of Mo2C–Mn7C3/MnN4-graphene was maximum (−0.54 vs. −8.08 of Mo2C–Mn7C3/graphene, −1.48 of Mo2C/Mn7C3–MnN4-graphene) in all models (Fig. 4d), corresponding to a moderate bonding strength, which was more conducive to the dissociation of H*. Moreover, the overlapping peaks of the H partial density of states on Mo2C–Mn7C3/MnN4-graphene were more pronounced than Mo2C–Mn7C3/graphene and Mo2C/Mn7C3–MnN4-graphene in the PDOS calculations (Fig. 4e), indicating that it was closer to the Fermi level. So, it was more favorable for the activation of H atoms. Overall, these theoretical results indicated the synergistic effect between Mo2C–Mn7C3 clusters and MnN4 to accelerate the adsorption/dissociation of H*, resulting in its excellent HER performance in acidic solution. Correspondingly, a schematic diagram of the HER mechanism is proposed, as shown in Fig. 4f.
Conclusion
In summary, Mo/Mn biochars (BCMoMn) were prepared from in situ enriched Mo/Mn biomass as precursors at different pyrolysis temperatures, especially at 800 °C. The synthesized BCMoMn800-2 exhibited excellent HER electrocatalytic activity. In an acidic electrolyte, the overpotential of BCMoMn800-2 was 27.4 mV at a current density of 10 mA cm−2, and the Tafel slope was 31 mV dec−1, which was close to that of Pt/C. This was ascribed to the unique properties arising from the interaction between MnN4 and Mo2C/Mn7C3 clusters in BCMoMn800-2. DFT calculations revealed that the heterojunction of Mo2C/Mn7C3 clusters in BCMoMn800-2 induced changes in the local electronic structure and charge redistribution of the clusters. It was an indirect electron transfer, resulting in a decrease in charge at the MnN4 site and an increase in electron density around C–N. Moreover, the presence of Mo2C/Mn7C3 clusters reduced the energy required for H* activation and indirectly facilitated the desorption of H*. Crucially, the optimized electronic structure of MnN4 sites facilitated the adsorption/desorption of H*, thereby promoting the kinetic process of the HER. These results indicated that BCMoMn800-2 exhibited superior electrocatalytic performance for the HER to that of BCMoMn700-2 and BCMoMn900-2, which might be mainly derived from the synergistic effect of MnN4 and Mo2C/Mn7C3 clusters rather than the pure increase of metal content. In this study, we successfully synthesized an efficient and stable HER electrocatalyst, clarified the synergy and catalytic mechanism between clusters and single atoms, and provided a new idea for solid waste recycling and the synthesis of electrocatalysts.Experimental section
Materials
Anhydrous ethanol (AR, 99.7%) and Nafion117 solution (5 wt%) were purchased from Aladdin Holdings Group Co., Ltd. Hoagland nutrient solution was purchased from Fuzhou Feijing Biological Technology Co., Ltd. Sodium molybdenum oxide (Na2MoO4, 99%), manganese chloride (MnCl2, 99%) and platinum on carbon (Pt/C, 20%wt) were obtained from Meryer (Shanghai) Chemical Technology Co., Ltd. Sulfuric acid (H2SO4, AR) was purchased from Beijing Chemical Co., Ltd. All chemicals were of analytical grade and used as received without further purification.Synthesis of biochar electrocatalysts in situ doped with Mo2C and single-atomic Mn
The source of electrocatalysts: Reed seedlings were bought with the same biological quality, the roots of reed seedlings were rinsed with running water, and then fixed with white stones into buckets. Subsequently, they were cultured with Hoagland nutrient solution, Na2MoO4 and MnCl2 (300, 500, and 800 mg L−1), and changed every week to ensure the concentration of Mo and Mn ions, which would last for three months. The reed roots were washed with deionized water several times after the completion of growth, and the biomass containing Mo and Mn ions was obtained by drying for 24 h. The reeds were crushed with a grinder for 5 min before use.Synthesis of Mo2C, Mn3C7, and single-atomic Mn nanostructures: The required electrocatalysts were obtained by the pyrolysis of biomass in a vacuum tube furnace at 700, 800, and 900 °C for 2 h, the pyrolysis rate was 3 °C min−1. The obtained biochar was stirred in 1 M H2SO4 for 24 h and then pumped and filtered to obtain a black solid, which was washed with deionized water several times until it approached the pH of deionized water. After that, the extracted biochar was dried for 48 h at a temperature of 80 °C in an oven and marked as BCMo/Mn700-2, BCMo/Mn800-1, BCMo/Mn800-2, BCMo/Mn800-3, and BCMo/Mn900-2. The numbers 1, 2, and 3 represent the increase of metal content in catalysts. Among them, the contents of Mo and Mn in BCMoMn800-2 were detected by inductively coupled plasma optical emission spectrometry (ICP-OES) to be 6.53 and 8.61 wt%, respectively (Table S1†).
Characterization
Scanning electron microscopy (SEM, ZEISS GeminiSEM 300, Germany) and transmission electron microscopy (TEM, FEI Talos F200X, America) were used to record the microscopic morphology of the catalysts. X-ray Diffraction (XRD, Bruker D8 Advance, Germany) was used to analyze the crystal structure of the catalysts. A contact angle/surface tension meter (Lauda Scientific LSA100, Germany) was used to detect the hydrophilicity and hydrophobicity of the catalysts. The surface elemental composition and valence state of the catalysts were determined by X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha, America). Raman spectroscopy (Raman, Horiba LabRAM HR Evolution, Japan) was used to analyze the degree of graphitization of catalysts. Metal elements including Mo and Mn were detected by inductively coupled plasma optical emission spectrometry (ICP-OES, Agilent 5110, America). The X-ray absorption fine structure spectra (XAFS) at the Mo/Mn K-edge of electrocatalysts were recorded at beamlines BL14W1 and BL11B in the Shanghai Synchrotron Radiation Facility (SSRF). Electrochemical measurements are provided in Text S1 of the ESI.†Theoretical calculations
Spin-polarized DFT calculations were performed using the Vienna ab initio simulation package (VASP). The generalized gradient approximation proposed by Perdew, Burke, and Ernzerhof (GGA-PBE) was selected for the exchange–correlation potential. The pseudo-potential was described by the projector-augmented-wave (PAW) method. The geometry optimization was performed until the Hellmann–Feynman force on each atom was smaller than 0.02 eV Å−1. The energy criterion was set to 10−6 eV in the iterative solution of the Kohn–Sham equation.Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.Author contributions
Chengyu Zhang: conceptualization, validation, methodology, investigation, writing-original draft. Xiangyang Wang, Renyuan Zhao: methodology, validation, investigation, writing-review & editing. Fabrice Ndayisenga: supervision, writing-review & editing. Zhisheng Yu: conceptualization, supervision, writing-review & editing, funding acquisition.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This investigation was funded by the Binzhou Institute of Technology, (GYY-DTFZ-2022-003), the Fundamental Research Funds for the Central Universities (E2E40503X2) and the Science and Technology Service Network Initiative Project of the Chinese Academy of Sciences (KFJ-STS-QYZX-112).References
- S. Chu and A. Majumdar, Opportunities and challenges for a sustainable energy future, Nature, 2012, 488, 294–303 CrossRef CAS.
- Y. Sun, Z. Xue, Q. Liu, Y. Jia and G. Li, Modulating Electronic Structure of Metal-Organic Frameworks by Introducing Atomically Dispersed Ru for Efficient Hydrogen Evolution, Nat. Commun., 2018, 8, 1801564–1801570 Search PubMed.
- F. Podjaski, D. Weber, S. Zhang, L. Diehl and B. V. Lotsch, Rational Strain Engineering in Delafossite Oxides for Highly Efficient Hydrogen Evolution Catalysis in Acidic Media, Nat. Catal., 2019, 3, 55–63 CrossRef.
- A. Odenweller, F. Ueckerdt, G. F. Nemet, M. Jensterle and G. Luderer, Probabilistic feasibility space of scaling up green hydrogen supply, Nat. Energy, 2022, 7, 854–865 CrossRef.
- J. Yu, Y. Guo, S. She, S. Miao, M. Ni, W. Zhou, M. Liu and Z. Shao, Bigger is surprisingly better: agglomerates of larger RuP nanoparticles outperform benchmark Pt nanocatalysts for the hydrogen evolution reaction, Adv. Mater., 2018, 30, 1800047–1800056 CrossRef PubMed.
- Y. Bang, S. Park, S. J. Han, J. Yoo, J. H. Song, J. H. Choi, K. H. Kang and I. K. Song, Hydrogen production by steam reforming of liquefied natural gas (LNG) over mesoporous Ni/Al2O3 catalyst prepared by an EDTA-assisted impregnation method, Appl. Catal., B, 2016, 180, 179–188 CrossRef CAS.
- F. Valentini, A. Marrocchi and L. Vaccaro, Liquid Organic Hydrogen Carriers (LOHCs) as H-Source for Bio-Derived Fuels and Additives Production, Adv. Energy Mater., 2022, 12, 2103362–2103390 CrossRef CAS.
- I. Roger, M. A. Shipman and M. D. Symes, Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting, Nat. Rev. Chem, 2017, 1, 0003–0016 CrossRef CAS.
- H. Sun, X. Xu, Y. Song, W. Zhou and Z. Shao, Designing high-valence metal sites for electrochemical water splitting, Adv. Funct. Mater., 2021, 31, 2009779–2009822 CrossRef CAS.
- B. Zhou, R. Gao, J. J. Zou and H. Yang, Surface design strategy of catalysts for water electrolysis, Small, 2022, 18, 2202336–2202361 CrossRef CAS.
- W. Liu, X. Wang, F. Wang, K. Du, Z. Zhang, Y. Guo, H. Yin and D. Wang, A durable and pH-universal self-standing MoC–Mo2C heterojunction electrode for efficient hydrogen evolution reaction, Nat. Commun., 2021, 12, 6776–6785 CrossRef CAS.
- K. Wang, S. Wang, K. S. Hui, H. Gao, D. A. Dinh, C. Yuan, C. Zha, Z. Shao, Z. Tang and K. N. Hui, Synergistically boosting the elementary reactions over multiheterogeneous ordered macroporous Mo2C/NC-Ru for highly efficient alkaline hydrogen evolution, Carbon Energy, 2022, 4, 856–866 CrossRef CAS.
- S. Nong, W. Dong, J. Yin, B. Dong, Y. Lu, X. Yuan, X. Wang, K. Bu, M. Chen, S. Jiang, L. M. Liu, M. Sui and F. Huang, Well-Dispersed Ruthenium in Mesoporous Crystal TiO2 as an Advanced Electrocatalyst for Hydrogen Evolution Reaction, J. Am. Chem. Soc., 2018, 140(17), 5719–5727 CrossRef CAS PubMed.
- J. Chen, C. Chen, M. Qin, B. Li, B. Lin, Q. Mao, H. Yang, B. Liu and Y. Wang, Reversible hydrogen spillover in Ru-WO3-x enhances hydrogen evolution activity in neutral pH water splitting, Nat. Commun., 2022, 13, 5382–5391 CrossRef CAS PubMed.
- L. Gong, J. Zhu, F. Xia, Y. Zhang, W. Shi, L. Chen, J. Yu, J. Wu and S. Mu, Marriage of Ultralow Platinum and Single-Atom MnN4 Moiety for Augmented ORR and HER Catalysis, ACS Catal., 2023, 13, 4012–4020 CrossRef CAS.
- H. Liu, L. Jiang, J. Khan, X. Wang, J. Xiao, H. Zhang, H. Xie, L. Li, S. Wang and L. Han, Decorating Single-Atomic Mn Sites with FeMn Clusters to Boost Oxygen Reduction Reaction, Angew. Chem., Int. Ed., 2023, 135, 1–8 Search PubMed.
- F. Mo, C. Song, Q. Zhou, W. Xue, S. Ouyang, Q. Wang, Z. Hou, S. Wang and J. Wang, The optimized Fenton-like activity of Fe single-atom sites by Fe atomic clusters–mediated electronic configuration modulation, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, 1–12 CrossRef PubMed.
- Q. Wang, M. Ming, S. Niu, Y. Zhang, G. Fan and J. S. Hu, Scalable solid-state synthesis of highly dispersed uncapped metal (Rh, Ru, Ir) nanoparticles for efficient hydrogen evolution, Adv. Energy Mater., 2018, 8, 1801698–1801704 CrossRef.
- D. H. Kweon, M. S. Okyay, S.-J. Kim, J.-P. Jeon, H.-J. Noh, N. Park, J. Mahmood and J.-B. Baek, Ruthenium anchored on carbon nanotube electrocatalyst for hydrogen production with enhanced Faradaic efficiency, Nat. Commun., 2020, 11, 1278–1287 CrossRef CAS PubMed.
- J. Wu, J. Su, T. Wu, L. Huang, Q. Li, Y. Luo, H. Jin, J. Zhou, T. Zhai and D. Wang, Scalable Synthesis of 2D Mo2C and Thickness-Dependent Hydrogen Evolution on Its Basal Plane and Edges, Adv. Mater., 2023, 2209954–2209961 CrossRef CAS PubMed.
- P. Li, G. Zhao, N. Cheng, L. Xia, X. Li, Y. Chen, M. Lao, Z. Cheng, Y. Zhao, X. Xu, Y. Jiang, H. Pan, S. X. Dou and W. Sun, Toward enhanced alkaline hydrogen electrocatalysis with transition metal-functionalized nitrogen-doped carbon supports, Chin. J. Catal., 2022, 43, 1351–1359 CrossRef CAS.
- J. Yang, B. Chen, X. Liu, W. Liu, Z. Li, J. Dong, W. Chen, W. Yan, T. Yao, X. Duan, Y. Wu and Y. Li, Efficient and Robust Hydrogen Evolution: Phosphorus Nitride Imide Nanotubes as Supports for Anchoring Single Ruthenium Sites, Angew. Chem., Int. Ed., 2018, 57, 9495–9500 CrossRef CAS PubMed.
- Q. Hu, K. Gao, X. Wang, H. Zheng, J. Cao, L. Mi, Q. Huo, H. Yang, J. Liu and C. He, Subnanometric Ru clusters with upshifted D band center improve performance for alkaline hydrogen evolution reaction, Nat. Commun., 2022, 13, 3958–3967 CrossRef CAS PubMed.
- Y. Gong, D. Li, C. Luo, Q. Fu and C. Pan, Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors, Green Chem., 2017, 19, 4132–4140 RSC.
- P. Aggarwal, D. Sarkar, K. Awasthi and P. W. Menezes, Functional role of single-atom catalysts in electrocatalytic hydrogen evolution: current developments and future challenges, Coord. Chem. Rev., 2022, 452, 214289–214323 CrossRef CAS.
- J. Ji, Y. Zhang, L. Tang, C. Liu, X. Gao, M. Sun, J. Zheng, M. Ling, C. Liang and Z. Lin, Platinum single-atom and cluster anchored on functionalized MWCNTs with ultrahigh mass efficiency for electrocatalytic hydrogen evolution, Nano Energy, 2019, 63, 103849–103856 CrossRef CAS.
- N. Cheng, S. Stambula, D. Wang, M. N. Banis, J. Liu, A. Riese, B. Xiao, R. Li, T.-K. Sham and L.-M. Liu, Platinum single-atom and cluster catalysis of the hydrogen evolution reaction, Nat. Commun., 2016, 7, 13638–13647 CrossRef CAS PubMed.
- S. Sultan, J. N. Tiwari, A. N. Singh, S. Zhumagali, M. Ha, C. W. Myung, P. Thangavel and K. S. Kim, Single atoms and clusters based nanomaterials for hydrogen evolution, oxygen evolution reactions, and full water splitting, Adv. Energy Mater., 2019, 9, 1900624–1900671 CrossRef.
- L. Zhang, J. Zhu, X. Li, S. Mu, F. Verpoort, J. Xue, Z. Kou and J. Wang, Nurturing the marriages of single atoms with atomic clusters and nanoparticles for better heterogeneous electrocatalysis, Interdiscipl. Mater., 2022, 1, 51–87 CrossRef.
- G. Humagain, K. MacDougal, J. MacInnis, J. M. Lowe, R. H. Coridan, S. MacQuarrie and M. Dasog, Highly Efficient, Biochar-Derived Molybdenum Carbide Hydrogen Evolution Electrocatalyst, Adv. Energy Mater., 2018, 8, 1801461–1801465 CrossRef.
- J.-S. Li, Y. Wang, C.-H. Liu, S.-L. Li, Y.-G. Wang, L.-Z. Dong, Z.-H. Dai, Y.-F. Li and Y.-Q. Lan, Coupled molybdenum carbide and reduced graphene oxide electrocatalysts for efficient hydrogen evolution, Nat. Commun., 2016, 7, 11204–11211 CrossRef CAS PubMed.
- X. Zhou, Z. Yu, Y. Yao, Y. Jiang, X. Rui, J. Liu and Y. Yu, A High-Efficiency Mo2C Electrocatalyst Promoting the Polysulfide Redox Kinetics for Na–S Batteries, Adv. Mater., 2022, 34, 2200479–2200487 CrossRef CAS PubMed.
- D. J. Sprouster, W. Streit Cunningham, G. P. Halada, H. Yan, A. Pattammattel, X. Huang, D. Olds, M. Tilton, Y. S. Chu, E. Dooryhee, G. P. Manogharan and J. R. Trelewicz, Dislocation microstructure and its influence on corrosion behavior in laser additively manufactured 316L stainless steel, Addit. Manuf., 2021, 47, 102263–102275 CAS.
- J. Kang, L. Zhou, X. Duan, H. Sun, Z. Ao and S. Wang, Degradation of Cosmetic Microplastics via Functionalized Carbon Nanosprings, Matter, 2019, 1, 745–758 CrossRef CAS.
- S. Amelinckx, X. B. Zhang, D. Bernaerts, X. F. Zhang, V. Ivanov and J. B. Nagy, A formation mechanism for catalytically grown helix-shaped graphite nanotubes, Science, 1994, 265, 635–639 CrossRef CAS PubMed.
- M. Liu and J. M. Cowley, Encapsulation of manganese carbides within carbon nanotubes and nanoparticles, Carbon, 1995, 33, 749–756 CrossRef CAS.
- Y. Wang, Y. Wang, X. Wang, M. Chang, G. Zhang, X. Mao, Y. Li, J. Wang and L. Wang, Efficient activation of peroxodisulfate by novel bionic iron-encapsulated biochar: the key roles of electron transfer pathway and reactive oxygen species evolution, J. Hazard. Mater., 2023, 443, 130204–130214 CrossRef CAS PubMed.
- Z. Yang, X. Yang, G. An and D. Wang, Regulating spin state of Fe active sites by the P-doping strategy for enhancing peroxymonosulfate activation, Appl. Catal. B Environ., 2023, 330, 122618–122627 CrossRef CAS.
- G. Yang, J. Zhu, P. Yuan, Y. Hu, G. Qu, B.-A. Lu, X. Xue, H. Yin, W. Cheng, J. Cheng, W. Xu, J. Li, J. Hu, S. Mu and J.-N. Zhang, Regulating Fe-spin state by atomically dispersed Mn-N in Fe-N-C catalysts with high oxygen reduction activity, Nat. Commun., 2021, 12, 1734–1743 CrossRef CAS PubMed.
- X. Cui, L. Gao, S. Lei, S. Liang, J. Zhang, C. D. Sewell, W. Xue, Q. Liu, Z. Lin and Y. Yang, Simultaneously crafting single-atomic Fe sites and graphitic layer-wrapped Fe3C nanoparticles encapsulated within mesoporous carbon tubes for oxygen reduction, Adv. Funct. Mater., 2021, 31, 2009197–2009205 CrossRef CAS.
- Y. Wang, P. Lv, J. Pan, J. Chen, X. Liu, M. Hu, L. Wan, K. Cao, B. Liu and Z. Ku, Grain Boundary Elimination via Recrystallization-Assisted Vapor Deposition for Efficient and Stable Perovskite Solar Cells and Modules, Adv. Mater., 2023, 35, 2304625–2304632 CrossRef CAS PubMed.
- X. Wu, Z. Wang, D. Zhang, Y. Qin, M. Wang, Y. Han, T. Zhan, B. Yang, S. Li and J. Lai, Solvent-free microwave synthesis of ultra-small Ru-Mo2C@CNT with strong metal-support interaction for industrial hydrogen evolution, Nat. Commun., 2021, 12, 4018–4027 CrossRef CAS PubMed.
- Z. Yang, X. Wang, M. Zhu, X. Leng, W. Chen, W. Wang, Q. Xu, L.-M. Yang and Y. Wu, Structural revolution of atomically dispersed Mn sites dictates oxygen reduction performance, Nano Res., 2021, 14, 4512–4519 CrossRef CAS.
- X. Xie, L. Peng, H. Yang, G. I. N. Waterhouse, L. Shang and T. Zhang, MIL-101-derived mesoporous carbon supporting highly exposed Fe single-atom sites as efficient oxygen reduction reaction catalysts, Adv. Mater., 2021, 33, 2101038–2101045 CrossRef CAS PubMed.
- J. Feng, H. Gao, L. Zheng, Z. Chen, S. Zeng, C. Jiang, H. Dong, L. Liu, S. Zhang and X. Zhang, A Mn-N3 single-atom catalyst embedded in graphitic carbon nitride for efficient CO2 electroreduction, Nat. Commun., 2020, 11, 4341–4348 CrossRef PubMed.
- D. Guo, R. Shibuya, C. Akiba, S. Saji, T. Kondo and J. Nakamura, Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts, Science, 2016, 351, 361–365 CrossRef CAS PubMed.
- Z. Chen, X. Liao, C. Sun, K. Zhao, D. Ye, J. Li, G. Wu, J. Fang, H. Zhao and J. Zhang, Enhanced performance of atomically dispersed dual-site Fe-Mn electrocatalysts through cascade reaction mechanism, Appl. Catal. B Environ., 2021, 288, 120021–120028 CrossRef CAS.
- L. He, W. Zhang, Q. Mo, W. Huang, L. Yang and Q. Gao, Molybdenum Carbide-Oxide Heterostructures: In Situ Surface Reconfiguration toward Efficient Electrocatalytic Hydrogen Evolution, Angew. Chem., Int. Ed., 2020, 59, 3544–3548 CrossRef CAS PubMed.
- J. Staszak-Jirkovský, C. D. Malliakas, P. P. Lopes, N. Danilovic, S. S. Kota, K.-C. Chang, B. Genorio, D. Strmcnik, V. R. Stamenkovic, M. G. Kanatzidis and N. M. Markovic, Design of active and stable Co–Mo–Sx chalcogels as pH-universal catalysts for the hydrogen evolution reaction, Nat. Mater., 2016, 15, 197–203 CrossRef PubMed.
- J. Zhang, T. Wang, P. Liu, Z. Liao, S. Liu, X. Zhuang, M. Chen, E. Zschech and X. Feng, Efficient hydrogen production on MoNi4 electrocatalysts with fast water dissociation kinetics, Nat. Commun., 2017, 8, 15437–15444 CrossRef CAS PubMed.
- H. Shang, Z. Jiang, D. Zhou, J. Pei, Y. Wang, J. Dong, X. Zheng, J. Zhang and W. Chen, Engineering a metal-organic framework derived Mn-N4-CxSy atomic interface for highly efficient oxygen reduction reaction, Chem. Sci., 2020, 11, 5994–5999 RSC.
- C. Zhu, A. L. Wang, W. Xiao, D. Chao, X. Zhang, N. H. Tiep, S. Chen, J. Kang, X. Wang and J. Ding, In situ grown epitaxial heterojunction exhibits high-performance electrocatalytic water splitting, Adv. Mater., 2018, 30, 1705516–1705523 CrossRef PubMed.
- X. Ye, J. Ma, W. Yu, X. Pan, C. Yang, C. Wang, Q. Liu and Y. Huang, Construction of bifunctional single-atom catalysts on the optimized β-Mo2C surface for highly selective hydrogenation of CO2 into ethanol, J. Energy Chem., 2021, 67, 184–192 CrossRef.
- D. V. Lam, U. N. T. Nguyen, E. Roh, W. Choi, J. H. Kim, H. Kim and S. M. Lee, Graphitic Carbon with MnO/Mn7C3 Prepared by Laser-Scribing of MOF for Versatile Supercapacitor Electrodes, Small, 2021, 17, 2100670–2100678 CrossRef CAS PubMed.
- X. Han, T. Zhang, W. Chen, B. Dong, G. Meng, L. Zheng, C. Yang, X. Sun, Z. Zhuang and D. Wang, Mn-N4 Oxygen Reduction Electrocatalyst: Operando Investigation of Active Sites and High Performance in Zinc–Air Battery, Adv. Energy Mater., 2021, 11, 2002753–2002761 CrossRef CAS.
- P. Cui, C. Liu, X. Su, Q. Yang, L. Ge, M. Huang, F. Dang, T. Wu and Y. Wang, Atomically Dispersed Manganese on Biochar Derived from a Hyperaccumulator for Photocatalysis in Organic Pollution Remediation, Environ. Sci. Technol., 2022, 56, 8034–8042 CrossRef CAS PubMed.
- Y. Li, J. Li, J. Chen, P. Cai, G. Wang, Y. Hou, L. Lei and Z. Wen, RuS2-x quantum dots/rGO as bifunctional hydrogen electrocatalysts for harvesting electrochemical neutralization energy, J. Power Sources, 2020, 472, 228625–228631 CrossRef CAS.
- L. Li, P. Wang, Q. Shao and X. Huang, Metallic nanostructures with low dimensionality for electrochemical water splitting, Chem. Soc. Rev., 2020, 49, 3072–3106 RSC.
- M. Wang, X. Yu, Z. Wang, X. Gong, Z. Guo and L. Dai, Hierarchically 3D porous films electrochemically constructed on gas–liquid–solid three-phase interface for energy application, J. Mater. Chem., 2017, 5, 9488–9513 RSC.
- A. K. Kota, Y. Li, J. M. Mabry and A. Tuteja, Hierarchically Structured Superoleophobic Surfaces with Ultralow Contact Angle Hysteresis, Adv. Mater., 2012, 24, 5838–5843 CrossRef CAS PubMed.
- X. Yu, M. Wang, Z. Wang, X. Gong and Z. Guo, 3D multi-structural porous NiAg films with nanoarchitecture walls: high catalytic activity and stability for hydrogen evolution reaction, Electrochim. Acta, 2016, 211, 900–910 CrossRef CAS.
- R. Li and D. Wang, Superiority of dual-atom catalysts in electrocatalysis: one step further than single-atom catalysts, Adv. Energy Mater., 2022, 12, 2103564–2103596 CrossRef CAS.
- J. Mahmood, F. Li, S.-M. Jung, M. S. Okyay, I. Ahmad, S.-J. Kim, N. Park, H. Y. Jeong and J.-B. Baek, An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction, Nat. Nanotechnol., 2017, 12, 441–446 CrossRef CAS PubMed.
- P. Zhou, L. Tao, S. Tao, Y. Li, D. Wang, X. Dong, T. Frauenheim, X. Fu, X. Lv and S. Wang, Construction of nickel-based dual heterointerfaces towards accelerated alkaline hydrogen evolution via boosting multi-step elementary reaction, Adv. Funct. Mater., 2021, 31, 2104827–2104836 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc06053e |
| This journal is © The Royal Society of Chemistry 2024 |