Open Access Article
Open Access ArticleSignificant hydrogen generation via photo-mechanical coupling in flexible methylammonium lead iodide nanowires†
Yucheng
Zhang
a,
Jiawei
Huang
a,
Mengya
Zhu
b,
Zhouyang
Zhang
 b,
Kaiqi
Nie
c,
Zhiguo
Wang
a,
Xiaxia
Liao
a,
Longlong
Shu
b,
Kaiqi
Nie
c,
Zhiguo
Wang
a,
Xiaxia
Liao
a,
Longlong
Shu
 a,
Tingfang
Tian
*a,
Zhao
Wang
a,
Tingfang
Tian
*a,
Zhao
Wang
 *d,
Yang
Lu
*d,
Yang
Lu
 e and
Linfeng
Fei
e and
Linfeng
Fei
 *a
*a
aSchool of Physics and Materials Science, Nanchang University, Nanchang 330031, China. E-mail: feilinfeng@gmail.com; tftian@ncu.edu.cn
bDepartment of Mechanical Engineering, City University of Hong Kong, Kowloon, Hong Kong SAR, China
cInstitute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China
dHubei Key Laboratory of Micro- & Nano electronic Materials and Devices, School of Microelectronics, Hubei University, Wuhan 430062, China. E-mail: wangzhao@hubu.edu.cn
eDepartment of Mechanical Engineering, The University of Hong Kong, Hong Kong SAR, China
First published on 3rd January 2024
Abstract
The flexoelectric effect, which refers to the mechanical-electric coupling between strain gradient and charge polarization, should be considered for use in charge production for catalytically driving chemical reactions. We have previously revealed that halide perovskites can generate orders of higher magnitude flexoelectricity under the illumination of light than in the dark. In this study, we report the catalytic hydrogen production by photo-mechanical coupling involving the photoflexoelectric effect of flexible methylammonium lead iodide (MAPbI3) nanowires (NWs) in hydrogen iodide solution. Upon concurrent light illumination and mechanical vibration, large strain gradients were introduced in flexible MAPbI3 NWs, which subsequently induced significant hydrogen generation (at a rate of 756.5 μmol g−1 h−1, surpassing those values from either photo- or piezocatalysis of MAPbI3 nanoparticles). This photo-mechanical coupling strategy of mechanocatalysis, which enables the simultaneous utilization of multiple energy sources, provides a potentially new mechanism in mechanochemistry for highly efficient hydrogen production.
Introduction
Mechanocatalysis, which triggers chemical reactions by mechanical deformation-induced charge energetics and separation, is an emerging field of advanced catalysis due to its unique potential to achieve the direct conversion of mechanical energy into chemical energy.1 For instance, piezocatalysis, a typical mechanocatalysis, promotes redox reactions directly by mechanical stimuli-induced polarization charges (piezoelectric effect, i.e., the coupling between strain and polarization charges) and the subsequent physical or chemical electron transfer.2 Principally, piezocatalysts with high piezoelectric coefficients and substantial stresses are advantageous for achieving effective piezoelectric charge polarization. Therefore, the low-dimensional piezocatalysts3–5 integrated with the piezoelectric effect and semiconductor properties (where the small size or dimensionality-reduced shape of nanomaterials allows large lattice strain and hence enhanced piezoelectricity)6–8 are highly appealing. These materials are not only attractive for exploring new physical phenomena at the nanoscale, but also potentially capable of driving redox reactions with the assistance of environmental vibrations (waves, wind, etc.).9,10 In this context, there has been a remarkable increase in the studies of piezocatalysis for nanoscale semiconductors in a range of applications, such as dye degradation,11 hydrogen production, CO2 reduction,12 tooth whitening,13 tumor therapy,14 and organic synthesis.15Piezoelectric-induced charge separation occurs when non-centrosymmetric materials are deformed by external stress.16,17 However, upon exposure to random external stresses, internal strain gradients are inevitably introduced in materials, besides the existence of strain.18,19 This has drawn attention to another ubiquitous electromechanical coupling effect, which is the flexoelectric effect.20 Flexoelectricity refers to the electromechanical coupling between strain gradient and polarization,21 which could induce piezoelectric-like polarization charges; this phenomenon widely exists in insulators, liquid crystals, biological samples, and semiconductors22 (whereas piezoelectric materials must be in non-centrosymmetric crystals). In bulk materials, the flexoelectric effect is sometimes negligible due to the small strain gradient within the fracture limitation, but it becomes more significant when the crystal size is shrunk down to the nanoscale.23–25 It is noted that a few studies on flexocatalysis of nanomaterials have been reported, mainly focusing on the applications of dye degradation. Zhonglin Wang et al. suggested that under ultrasonic excitation, reactive species were created due to strain-gradient-induced flexoelectric polarization in MnO2 nanosheets composed of nanoflowers, which could be used to degrade organic pollutants, such as methylene blue.26 Yaojin Wang et al. also demonstrated the flexocatalysis effect in centrosymmetric SrTiO3 nanoparticles for degrading organic dyes (rhodamine B).27
Inspiringly, in our recent work, we have demonstrated that the flexoelectricity in halide perovskites can be significantly enhanced upon the illumination of light, where the materials deliver orders of higher magnitude flexoelectric responses than normal bending-induced flexoelectricity.28 This giant photoflexoelectric effect provides a whole new paradigm for harvesting multiple forms of energy at the same time. Although these halide perovskite materials are well known to suffer severe decomposition in aqueous solutions,29,30 their dynamic equilibrium of dissolution and precipitation in solution can still enable photocatalytic hydrogen iodide (HI) decomposition together with hydrogen (H2) generation.31 For example, Yang Chai et al. have recently evidenced that methylammonium lead iodide (MAPbI3) powder exhibited excellent piezoelectric photocatalytic hydrogen production efficiency in HI aqueous solution.32
Herein, we report the first case of catalytic hydrogen production by photo-mechanical coupling involving the photoflexoelectric effect from flexible MAPbI3 nanowires (NWs) in HI solution. It is worth noting that NWs with a large aspect ratio were used as catalysts in this study because they can generate significant flexoelectricity by experiencing a large strain gradient (see discussions below). The photo-enhanced electromechanical coupling in MAPbI3 NWs achieved a significant hydrogen production rate of 756.5 μmol g−1 h−1 by catalytic decomposition of HI, which is much higher than the reported values from similar systems via photo- or photopiezocatalysis. The development of this photo-mechanical coupling strategy in mechanocatalysis provides a potential “shortcut” in simultaneous utilization of multiple energy sources, which may shed light on new mechanisms for efficient mechanocatalysis.
Results and discussion
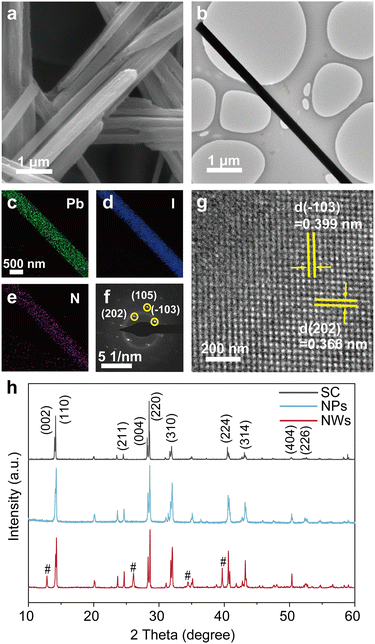
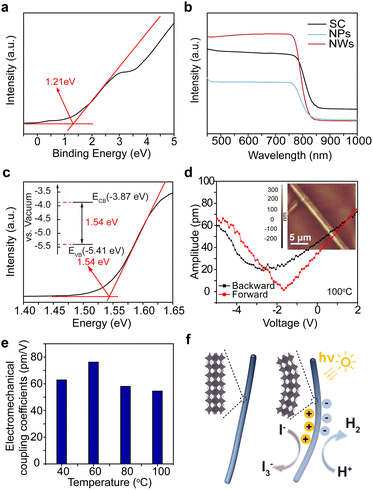
We synthesized MAPbI3 NWs according to the widely reported wet-chemical method.33 MAPbI3 nanoparticles (NPs) and single crystals (SCs) were also synthesized for comparison purposes (see Methods for details). Systematical characterizations were first performed to check the structural information of the as-synthesized samples. Fig. 1a shows the scanning electron microscopy (SEM) image of a crowd of NWs, revealing their typical one-dimensional structures as well as the smooth surfaces (with average diameters of 360 nm and lengths up to 37 μm; see statistical results of their diameters and lengths in Fig. S1†). The transmission electron microscopy (TEM) image in Fig. 1b further demonstrates the smooth surface of a NW. Fig. 1c–e present the energy dispersive spectroscopy (EDS) elemental mappings for Pb, I, and N within a selected NW; the synchronous distributions of these constituent elements imply its uniform composition (one more set of data can be found in Fig. S2†). The selected area electron diffraction (SAED) pattern from an isolated NW in Fig. 1f confirms its single crystalline nature, and the pattern was assigned to a tetragonal structure as reported in previous syntheses.34,35 Furthermore, the high-resolution TEM (HRTEM) image (Fig. 1g) exhibits distinct lattice fringes with a pair of interplanar spacings of 0.399 and 0.366 nm, corresponding to the (−103) and (202) planes of tetragonal MAPbI3, respectively. In addition, the X-ray diffraction (XRD) patterns of MAPbI3 SC, NPs, and NWs (Fig. 1h) show that the diffraction peaks at 14.29° and 28.62° correspond well to the (110) and (220) planes of tetragonal MAPbI3, indicating their preferential orientation along [110] directions.36 The minor existence of PbI2 (refer to the low-intensity peaks at 2θ = 12.84°, 26.09°, 34.46°, and 39.69°) is common in these nanomaterials.37The generation and separation of charge carriers, as well as their subsequent migration into the reaction systems, play a crucial role in the catalytic performance of catalysts. Ultraviolet photoelectron spectroscopy (UPS) and ultraviolet-visible (UV-Vis) absorption spectroscopy were undertaken to construct the band diagram of MAPbI3 NWs. As shown in Fig. 2a, the as-synthesized MAPbI3 NWs exhibit a maximum binding energy of 1.21 eV. The respective valence band positions of the NPs and SC samples were also assessed (Fig. S3†) and are consistent with the recent reports.38,39 The UV-Vis spectra (Fig. 2b) reveal pronounced light absorption within the three samples. The samples present their sharp absorption edges in the infrared region, which signifies that electrons within the samples undergo efficient transitions from the valence band to the conduction band under the irradiation of visible light. The superior light absorption of NWs as compared to NPs and SC, which was possibly caused by the large surface area and the low stacking density of NWs,40,41 implies the higher efficiency of the generation of the energetic electron–hole pairs. Fig. 2c presents the Tauc plot for the optical bandgap calculation of MAPbI3 NWs, which is 1.54 eV (the corresponding values for the SC and NP samples are 1.51 and 1.54 eV, respectively; see Fig. S4,† as well as the comparison of the structures for optical bandgaps of MAPbI3 SC, NWs, and NPs in Fig. S5†). The band diagram of the MAPbl3 NWs revealed that its conduction band minimum (CBM) and valence band maximum (VBM) corresponded to −0.63 and 0.91 V versus the normal hydrogen electrode (NHE), respectively. These energy positions are well-suited for hydrogen reduction (0.046 V versus NHE) and iodine oxidation (0.376 V versus NHE) within our saturated HI aqueous system, as depicted in the inset of Fig. 2c. Therefore, during the catalytic process, the holes from the VBM exhibit stronger oxidation capability compared to I− ions, leading to the oxidation of I− ions to I3− ions, while the electrons from the CBM are more reducible than H+, resulting in the reduction of H+ to form H2 (i.e., 3I− + 2h+ = I3−, 2H+ + 2e− = H2).
We then utilized the piezoelectric response force microscopy (PFM) mode of a scanning probe microscope (SPM) to assess the electromechanical coupling behavior of a single MAPbI3 NW.42 As shown in Fig. 2d, the applied voltage through the PFM tip indeed induced the mechanical response of the underlying NW, which confirms the electromechanical coupling in the MAPbI3 NW. Furthermore, as the Curie temperature (i.e., the piezo-response should become zero above this temperature) of MAPbI3 material is around 60 °C,43 we also tested in situ the PFM response of NWs at a temperature range across its Curie temperature to verify the origin of the electromechanical coupling (Fig. S6 and S7†). The disappearance of hysteresis in the phase-voltage curves above the Curie temperature confirms the ferroelectric to non-ferroelectric transition for the NW (Fig. S7†). However, as it can be seen in Fig. 2e, the electromechanical coupling coefficient was maintained within 76 to 54 pm V−1 (the system has been calibrated by a standard LiNbO3 sample with its d33 of 17.3 pm V−1; see Fig. S8†), which indicated that the NW retained more than 70% of the electromechanical coupling response despite the absence of piezoelectric effect at the temperature beyond Curie point; in brief, it is confirmed that the as-measured electromechanical coupling of MAPbI3 NWs was mainly attributed to the flexoelectric effect.
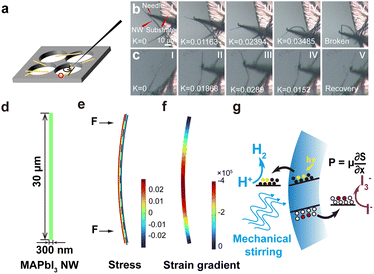
Furthermore, perovskite bulk materials are well known to be extremely fragile, which would be a significant issue for their applications in mechanocatalysis (in which large deformations of materials are essential for charge generation). However, when the dimensions of a sample are reduced toward the nanoscale, they may manifest exceptional flexibility, enabling them to withstand intense mechanical forces such as ultrasonication and stirring without being damaged. In the SEM/TEM observations, we observed a few bending NWs (see Fig. S9† for a representative example), which inspired the high flexibility of these MAPbI3 NWs. Consequently, in situ experiments were conducted to observe the bending behaviors of MAPbI3 NWs (the setup is schematically shown in Fig. 3a; see Methods for details). Upon forced bending by a rigid needle at one end, a single NW endured a large curvature (defined as how much the curve deviates from a straight line) before it was broken; yet under controlled deflection, the NW can be cycled between bending and straight states (as shown in Fig. 3b and c; the K values denoted on panels show the corresponding curvatures). During multiple bending processes, it was observed that the NW exhibited excellent flexibility (Fig. S10; also refer to the ESI Movie†), which is suitable for repeated bending-recovery cycles in mechanocatalysis.
Moreover, finite element method (FEM) simulations were employed to survey the bending-induced strain gradient within a single NW. For a model of MAPbI3 NW, the length is set to 30 μm, and the diameter is set to 300 nm (Fig. 3d). The stress distribution (ε11) within the MAPbI3 NW upon experiencing bending moments at both ends is shown in Fig. 3e, in which one side (the red area) of the NW undergoes tensile stress, while the opposite side (the blue area) experiences compressive stress (the dashed line indicates the neutral plane, where ε = 0). Correspondingly, the strain progressively increases from the center towards the surface, while it decreases from the center to both ends of the NW along the axial direction. The strain gradient, however, manifests distinct variations across the NW due to the structural characteristics of this simply supported NW (Fig. 3f). One can see that the strain gradients at both ends of the NW are relatively low, whereas in the central region, there is significant augmentation in strain gradient (up to 105). This observation demonstrates that the morphology of NW facilitates the formation of strain gradients, subsequently giving rise to significant mechanoelectrical coupling for NWs. The polarization induced by this strain gradient would generate an electric field (E) perpendicular to the radial direction within the MAPbI3 NW, together with the net charges at both surfaces. In our previous work, we obtained a flexoelectric coefficient of ∼2000 μC m−1 for halide perovskites;28 as a result, a polarization strength of about 200 C m−2 in the MAPbI3 NW can be calculated. This significant internal polarization could lead to a completely tilted band structure for MAPbI3 material and drive the efficient dissociation of charge carriers, facilitating electrons and holes to be separated in opposite directions. When MAPbI3 NWs are subjected to a varying field of external mechanical stimulations (i.e., magnetic stirring), the repeated bending and recovery cycles of NWs would drive the random changes in their internal electrical fields (both strengths and directions), which induce the inclinations of the energy levels of NWS and continuous accumulation of charges (which was initiated by photo-induced separation) for catalysis (i.e., photomechanocatalysis).44 In this case, H+ readily combines with the accumulated charges to produce H2 (Fig. 3g).
It is therefore expected that the proposed photo-mechanical coupling in MAPbI3 NWs should deliver significant catalytic performance. Consequently, we evaluated the catalytic performance of MAPbI3 NWs in a MAPbI3-saturated HI solution (though not in water because the material is soluble in water, with a solubility of 0.645 mol L−1 at 20 °C (ref. 31)) under simultaneous light illumination and mechanical stirring (see Methods). The as-synthesized MAPbI3 NWs were added into a homemade quartz vessel filled with MAPbI3-saturated HI solution, which was hermetically sealed and equipped with a magnetic agitator to induce mechanical stimulation along with a xenon lamp for illumination. Gas chromatography (GC) was employed to monitor the H2 production from the reaction system. As anticipated, a substantial quantity of H2 was produced by the photomechanocatalytic HI splitting induced by the flexible MAPbI3 NWs. The results are illustrated in Fig. 4a, which was juxtaposed with the results obtained solely through photocatalysis and mechanocatalysis of the NWs (which were driven by either light or stirring, respectively). The hydrogen generation rates for photomechanocatalysis, mechanocatalysis, and photocatalysis were 756.5, 192.2, and 164.2 μmol g−1 h−1, respectively; in other words, the amounts of H2 generated by the photomechanocatalysis, mechanocatalysis, and photocatalysis in our experimental conditions were recorded as 75.65, 19.22, and 16.42 μmol in 2 hours, respectively (Fig. 4b; the original GC data can be referred to in Fig. S11 and S12†). It is thus evident that the hydrogen production via the photomechanocatalytic process is significantly higher than the combined hydrogen production from the mechanocatalytic and photocatalytic processes, which suggests a remarkable synergistic effect of photo-mechanical coupling on this system. Next, to verify the effect of MAPbI3 morphology on the photomechanocatalytic performance, another group of control experiments was conducted by comparing the hydrogen generation rates of MAPbI3 NWs and NPs, and the time-dependent hydrogen evolution profiles for MAPbI3 NWs and NPs are shown in Fig. 4c. It is clear that the NWs exhibited a significantly higher hydrogen generation rate compared to the NPs (Fig. 4d), demonstrating the geometrical advantages of NWs in flexoelectric charge production,45 as well as in photomechanocatalytic hydrogen production.
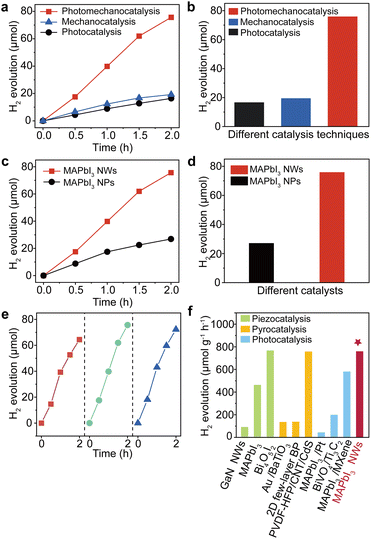 | ||
| Fig. 4 Hydrogen production performances for MAPbI3 NWs via photomechanocatalysis. (a) Time-dependent hydrogen evolution profiles for photomechanocatalysis, photocatalysis, and mechanocatalysis for MAPbI3 NWs. (b) The comparative amounts of hydrogen generated from MAPbI3 NWs after 2 h using different catalysis techniques. (c) Time-dependent hydrogen evolution profiles for MAPbI3 NWs and NPs via photomechanocatalysis. (d) The comparative amounts of hydrogen generated from photomechanocatalysis after 2 h using MAPbI3 NWs and NPs. (e) The test of stability for photomechanocatalytic hydrogen production using MAPbI3 NWs over three cycles. (f) Comparison of hydrogen production rates among different catalytic modes and catalysts in recent reports. The listed examples include piezocatalysis (GaN NWs,3 MAPbI3,32 Bi4O5I2 (ref. 46)), pyrocatalysis (Au/BaTiO3,47 2D few-layer black phosphorene,48 PVDF-HFP/CNT/CdS49), photocatalysis (MAPbI3/Pt,50 BiVO4/Ti3C2 nanosheets,51 MAPbI3/MXene52), and our work. | ||
To mitigate the interference of hydrogen generation from the self-decomposition of HI under illumination and mechanical agitation, we replicated the previously mentioned experiment in the absence of MAPbI3 NWs. No noticeable hydrogen evolution was detected in the scenarios involving sole illumination, sole agitation, or the combination of illumination and agitation (Fig. S13†). In this context, the hydrogen generation in previous experiments can be attributed to the photomechanocatalytic cleavage of HI induced by the MAPbI3 NWs. To the best of our knowledge, the hydrogen production rate of MAPbI3 NWs as reported in this work is one of the highest values for the single-catalyst systems in HI (Table S1†). Moreover, the solar HI splitting efficiency of our experiment can reach up to 2.67% (the calculation can be found in Methods), which represents a significant improvement compared to the existing reports. Moreover, the cycling stability of MAPbI3 NWs as catalysts in photomechanocatalytic hydrogen production was also assessed; as shown in Fig. 4e, the hydrogen production rates have been constantly maintained across three catalytic cycles, demonstrating the good repeatability of MAPbI3 NWs in this catalytic reaction. In addition, a comprehensive analysis was conducted to investigate the microstructural evolution of MAPbI3 NWs during the reaction process. The XRD spectrum of MAPbI3 NWs after catalytic reaction is presented in Fig. S14.† Compared to the XRD pattern of NWs before the reaction (Fig. 1h), the peaks of MAPbI3 remained essentially unchanged, indicating the NWs were basically stable in the catalytic system, while the peaks of PbI2 disappeared as it dissolved in HI. Moreover, the NWs after different reaction times (0, 1, and 2 h) were isolated from the reaction system and further observed under SEM (Fig. S15†). In the prolonged reaction, the NWs exhibited slight surface damage due to mechanical stirring and possible photodegradation, yet the structure of the NWs was largely preserved. We further compared the hydrogen production rate in our experiment with the existing catalytic hydrogen generation approaches, including piezoelectric, pyroelectric, and photocatalytic pathways (as shown in Fig. 4f). The catalytic activity of MAPbI3 NWs with photo-mechanical coupling exhibits a superior rate than, or at least, is comparable to a multitude of extensively investigated piezoelectric, pyroelectric, and photocatalytic systems.
Conclusions
In summary, an effective strategy for hydrogen production using flexible MAPbI3 NWs upon concurrent light illumination and mechanical stirring was demonstrated with a hydrogen yield rate as high as 756.5 μmol g−1 h−1 (the corresponding catalytic efficiency is 2.67%), which surpasses the rates achieved by sole photocatalysis and flexocatalysis by nearly five-fold and four-fold, respectively. The significant photomechanocatalysis from MAPbI3 NWs is attributed to the photo-mechanical coupling, which tilts the band structure of MAPbI3 under large strain gradients and thus accelerates the charge production at the surfaces of NWs upon light illumination. Our work demonstrates the potential of MAPbI3 NWs for hydrogen production via simultaneously harvesting mechanical and solar energy from the environment and may shed light on the paradigm shift in mechanocatalysis.Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.Author contributions
L.-F. F. conceived and supervised the project. L.-F. F. and Y.-C. Z. designed the experiments. J.-W. H. conducted TEM observations and data analysis. M.-Y. Z. and Z.-Y. Z. conducted the in situ bending test. K.-Q. N. and X.-X. L. conducted UPS measurements. Z.-G. W. performed FEM calculations. Y.-C. Z., T.-F. T., Z. W., and L.-F. F. wrote the manuscript with the help and input of all authors. All authors have given their approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was jointly supported by the National Natural Science Foundation of China (51972159 and U21A20500) and Jiangxi Provincial Natural Science Foundation (20212ACB204016, 20192ACB21018, and 20171ACB20006). Y. L. acknowledges the support from the Hong Kong RGC General Research Fund (GRF) project (11200623). Z. W. acknowledges the support from the Hubei Provincial Natural Science Foundation (2023AFA074). L.-F. F. acknowledges the support from Nanchang University. The authors also acknowledge the use of the 4B9B beamline at the Beijing Synchrotron Radiation Facility.References
- L. Chen, Y. Yang, S. Jiang, B. Yang and W. Rao, Multifunctional ferroelectric catalysis for water splitting: classification, synergism, strategies and challenges, Mater. Today Chem., 2023, 30, 101486 CrossRef CAS.
- W. Qian, W. Yang, Y. Zhang, C. R. Bowen and Y. Yang, Piezoelectric materials for controlling electro-chemical processes, Nano-Micro Lett., 2020, 12, 149 CrossRef CAS.
- M. Zhang, S. Zhao, Z. Zhao, S. Li and F. Wang, Piezocatalytic effect induced hydrogen production from water over non-noble metal Ni deposited ultralong GaN nanowires, ACS Appl. Mater. Interfaces, 2021, 13, 10916–10924 CrossRef CAS PubMed.
- Q. Nie, Y. Xie, J. Ma, J. Wang and G. Zhang, High piezo-catalytic activity of ZnO/Al2O3 nanosheets utilizing ultrasonic energy for wastewater treatment, J. Cleaner Prod., 2020, 242, 118532 CrossRef CAS.
- H. Lei, H. Zhang, Y. Zou, X. Dong, Y. Jia and F. Wang, Synergetic photocatalysis/piezocatalysis of bismuth oxybromide for degradation of organic pollutants, J. Alloys Compd., 2019, 809, 151840 CrossRef CAS.
- J. Yu, H. Guo, W. Feng, X. Guo, Y. Zhu, T. Thomas, C. Jiang, S. Liu and M. Yang, Co4N–WNx composite for efficient piezocatalytic hydrogen evolution, Dalton Trans., 2022, 51, 7127–7134 RSC.
- Q. Tang, J. Wu, D. Kim, C. Franco, A. Terzopoulou, A. Veciana, J. Puigmartí-Luis, X.-Z. Chen, B. J. Nelson and S. Pané, Enhanced piezocatalytic performance of BaTiO3 nanosheets with highly exposed {001} facets, Adv. Funct. Mater., 2022, 32, 2202180 CrossRef CAS.
- S. Lin, S. Li, H. Huang, H. Yu and Y. Zhang, Synergetic piezo-photocatalytic hydrogen evolution on CdxZn1-xS solid-solution 1D nanorods, Small, 2022, 18, 2106420 CrossRef CAS.
- C. Wang, C. Hu, F. Chen, T. Ma, Y. Zhang and H. Huang, Design strategies and effect comparisons toward efficient piezocatalytic system, Nano Energy, 2023, 107, 108093 CrossRef CAS.
- S. Li, Z. Zhao, J. Zhao, Z. Zhang, X. Li and J. Zhang, Recent advances of ferro-, piezo-, and pyroelectric nanomaterials for catalytic applications, ACS Appl. Nano Mater., 2020, 3, 1063–1079 CrossRef CAS.
- H. You, X. Ma, Z. Wu, L. Fei, X. Chen, J. Yang, Y. Liu, Y. Jia, H. Li, F. Wang and H. Huang, Piezoelectrically/pyroelectrically-driven vibration/cold-hot energy harvesting for mechano-/pyro- bi-catalytic dye decomposition of NaNbO3 nanofibers, Nano Energy, 2018, 52, 351–359 CrossRef CAS.
- J. Ma, X. Xiong, D. Wu, Y. Wang, C. Ban, Y. Feng, J. Meng, X. Gao, J.-Y. Dai, G. Han, L.-Y. Gan and X. Zhou, Band position-independent piezo-electrocatalysis for ultrahigh CO2 conversion, Adv. Mater., 2023, 35, 2300027 CrossRef CAS.
- Y. Wang, X. Wen, Y. Jia, M. Huang, F. Wang, X. Zhang, Y. Bai, G. Yuan and Y. Wang, Piezo-catalysis for nondestructive tooth whitening, Nat. Commun., 2020, 11, 1328 CrossRef CAS.
- P. Zhu, Y. Chen and J. Shi, Piezocatalytic tumor therapy by ultrasound-triggered and BaTiO3-mediated piezoelectricity, Adv. Mater., 2020, 32, 2001976 CrossRef CAS PubMed.
- K. Kubota, Y. Pang, A. Miura and H. Ito, Redox reactions of small organic molecules using ball milling and piezoelectric materials, Science, 2019, 366, 1500–1504 CrossRef CAS.
- S. Tu, Y. Guo, Y. Zhang, C. Hu, T. Zhang, T. Ma and H. Huang, Piezocatalysis and piezo-photocatalysis: Catalysts classification and modification strategy, reaction mechanism, and practical application, Adv. Funct. Mater., 2020, 30, 2005158 CrossRef CAS.
- J. Liu, W. Qi, M. Xu, T. Thomas, S. Liu and M. Yang, Piezocatalytic techniques in environmental remediation, Angew. Chem., Int. Ed., 2023, 62, 202213927 CrossRef PubMed.
- P. Zubko, G. Catalan and A. K. Tagantsev, Flexoelectric effect in solids, Annu. Rev. Mater. Res., 2013, 43, 387–421 CrossRef CAS.
- T. D. Nguyen, S. Mao, Y.-W. Yeh, P. K. Purohit and M. C. McAlpine, Nanoscale flexoelectricity, Adv. Mater., 2013, 25, 946–974 CrossRef CAS.
- B. Wang, Y. Gu, S. Zhang and L.-Q. Chen, Flexoelectricity in solids: Progress, challenges, and perspectives, Prog. Mater. Sci., 2019, 106, 100570 CrossRef CAS.
- P. V. Yudin and A. K. Tagantsev, Fundamentals of flexoelectricity in solids, Nanotechnology, 2013, 24, 432001 CrossRef CAS PubMed.
- J. Narvaez, F. Vasquez-Sancho and G. Catalan, Enhanced flexoelectric-like response in oxide semiconductors, Nature, 2016, 538, 219–221 CrossRef CAS.
- L. Shu, R. Liang, Z. Rao, L. Fei, S. Ke and Y. Wang, Flexoelectric materials and their related applications: A focused review, J. Adv. Ceram., 2019, 8, 153–173 CrossRef CAS.
- Q. Deng, M. Kammoun, A. Erturk and P. Sharma, Nanoscale flexoelectric energy harvesting, Int. J. Solids Struct., 2014, 51, 3218–3225 CrossRef.
- Z. Wang, C. Li, H. Xie, Z. Zhang, W. Huang, S. Ke and L. Shu, Effect of grain size on flexoelectricity, Phys. Rev. Appl., 2022, 18, 064017 CrossRef CAS.
- T. Wu, K. Liu, S. Liu, X. Feng, X. Wang, L. Wang, Y. Qin and Z. L. Wang, Highly efficient flexocatalysis of two-dimensional semiconductors, Adv. Mater., 2023, 35, 2208121 CrossRef CAS PubMed.
- Z. Liu, X. Wen, Y. Wang, Y. Jia, F. Wang, G. Yuan and Y. Wang, Robust flexo-catalysis in centrosymmetric nanoparticles, Adv. Mater. Technol., 2022, 7, 2101484 CrossRef CAS.
- L. Shu, S. Ke, L. Fei, W. Huang, Z. Wang, J. Gong, X. Jiang, L. Wang, F. Li, S. Lei, Z. Rao, Y. Zhou, R.-K. Zheng, X. Yao, Y. Wang, M. Stengel and G. Catalan, Photoflexoelectric effect in halide perovskites, Nat. Mater., 2020, 19, 605–609 CrossRef CAS PubMed.
- J. Zhao, B. Cai, Z. Luo, Y. Dong, Y. Zhang, H. Xu, B. Hong, Y. Yang, L. Li, W. Zhang and C. Gao, Investigation of the hydrolysis of perovskite organometallic halide CH3NH3PbI3 in humidity environment, Sci. Rep., 2016, 6, 21976 CrossRef CAS PubMed.
- S. Chen, H. Yin, P. Liu, Y. Wang and H. Zhao, Stabilization and performance enhancement strategies for halide perovskite photocatalysts, Adv. Mater., 2023, 35, 2203836 CrossRef CAS.
- S. Park, W. J. Chang, C. W. Lee, S. Park, H.-Y. Ahn and K. T. Nam, Photocatalytic hydrogen generation from hydriodic acid using methylammonium lead iodide in dynamic equilibrium with aqueous solution, Nat. Energy, 2016, 2, 16185 CrossRef.
- M. Wang, Y. Zuo, J. Wang, Y. Wang, X. Shen, B. Qiu, L. Cai, F. Zhou, S. P. Lau and Y. Chai, Remarkably enhanced hydrogen generation of organolead halide perovskites via piezocatalysis and photocatalysis, Adv. Energy Mater., 2019, 9, 1901801 CrossRef CAS.
- Q. Ye, J. Zhang, P. Guo, H. Fan, D. G. Shchukin, B. Wei and H. Wang, Wet-chemical synthesis of surface-passivated halide perovskite microwires for improved optoelectronic performance and stability, ACS Appl. Mater. Interfaces, 2018, 10, 43850–43856 CrossRef CAS PubMed.
- J. Xing, X. F. Liu, Q. Zhang, S. T. Ha, Y. W. Yuan, C. Shen, T. C. Sum and Q. Xiong, Vapor phase synthesis of organometal halide perovskite nanowires for tunable room-temperature nanolasers, Nano Lett., 2015, 15, 4571–4577 CrossRef CAS PubMed.
- P. Bansal and P. Kar, Ultralong micro-belts of luminescent lead halide-based perovskites, Chem. Commun., 2019, 55, 6543–6546 RSC.
- X. Hu, X. Zhang, L. Liang, J. Bao, S. Li, W. Yang and Y. Xie, High-performance flexible broadband photodetector based on organolead halide perovskite, Adv. Funct. Mater., 2014, 24, 7373–7380 CrossRef CAS.
- N. Leupold, K. Schötz, S. Cacovich, I. Bauer, M. Schultz, M. Daubinger, L. Kaiser, A. Rebai, J. Rousset, A. Köhler, P. Schulz, R. Moos and F. Panzer, High versatility and stability of mechanochemically synthesized halide perovskite powders for optoelectronic devices, ACS Appl. Mater. Interfaces, 2019, 11, 30259–30268 CrossRef CAS.
- M. Caputo, N. Cefarin, A. Radivo, N. Demitri, L. Gigli, J. R. Plaisier, M. Panighel, G. Di Santo, S. Moretti, A. Giglia, M. Polentarutti, F. De Angelis, E. Mosconi, P. Umari, M. Tormen and A. Goldoni, Electronic structure of MAPbI3 and MAPbCl3: importance of band alignment, Sci. Rep., 2019, 9, 15159 CrossRef PubMed.
- P.-H. Chang, S.-Y. Liu, Y.-B. Lan, Y.-C. Tsai, X.-Q. You, C.-S. Li, K.-Y. Huang, A.-S. Chou, T.-C. Cheng, J.-K. Wang and C.-I. Wu, Ultrahigh responsivity and detectivity graphene–perovskite hybrid phototransistors by sequential vapor deposition, Sci. Rep., 2017, 7, 46281 CrossRef CAS PubMed.
- G. Zhou, L. Xu, G. Hu, L. Mai and Y. Cui, Nanowires for electrochemical energy storage, Chem. Rev., 2019, 119, 11042–11109 CrossRef CAS.
- S. Sofiane and M. Bilel, Effect of specific surface area on photoelectrochemical properties of TiO2 nanotubes, nanosheets and nanowires coated with TiC thin films, J. Photochem. Photobiol., A, 2016, 324, 126–133 CrossRef CAS.
- L. Fei, Y. Hu, X. Li, R. Song, L. Sun, H. Huang, H. Gu, H. L. W. Chan and Y. Wang, Electrospun bismuth ferrite nanofibers for potential applications in ferroelectric photovoltaic devices, ACS Appl. Mater. Interfaces, 2015, 7, 3665–3670 CrossRef CAS.
- Y.-J. Kim, T.-V. Dang, H.-J. Choi, B.-J. Park, J.-H. Eom, H.-A. Song, D. Seol, Y. Kim, S.-H. Shin, J. Nah and S.-G. Yoon, Piezoelectric properties of CH3NH3PbI3 perovskite thin films and their applications in piezoelectric generators, J. Mater. Chem. A, 2016, 4, 756–763 RSC.
- R. Su, J. Zhang, V. Wong, D. Zhang, Y. Yang, Z.-D. Luo, X. Wang, H. Wen, Y. Liu, J. Seidel, X. Yang, Y. Pan and F.-t. Li, Engineering sub-nanometer hafnia-based ferroelectrics to break the scaling relation for high-efficiency piezocatalytic water splitting, Adv. Mater., 2023, 35, 2303018 CrossRef CAS.
- X. Liang, S. Hu and S. Shen, Effects of surface and flexoelectricity on a piezoelectric nanobeam, Smart Mater. Struct., 2014, 23, 035020 CrossRef CAS.
- C. Wang, C. Hu, F. Chen, H. Li, Y. Zhang, T. Ma and H. Huang, Polar layered bismuth-rich oxyhalide piezoelectrics Bi4O5X2 (X=Br, I): Efficient piezocatalytic pure water splitting and interlayer anion-dependent activity, Adv. Funct. Mater., 2023, 33, 2301144 CrossRef CAS.
- H. You, S. Li, Y. Fan, X. Guo, Z. Lin, R. Ding, X. Cheng, H. Zhang, T. W. B. Lo, J. Hao, Y. Zhu, H.-Y. Tam, D. Lei, C.-H. Lam and H. Huang, Accelerated pyro-catalytic hydrogen production enabled by plasmonic local heating of Au on pyroelectric BaTiO3 nanoparticles, Nat. Commun., 2022, 13, 6144 CrossRef CAS.
- H. You, Y. Jia, Z. Wu, F. Wang, H. Huang and Y. Wang, Room-temperature pyro-catalytic hydrogen generation of 2D few-layer black phosphorene under cold-hot alternation, Nat. Commun., 2018, 9, 2889 CrossRef.
- B. Dai, J. Fang, Y. Yu, M. Sun, H. Huang, C. Lu, J. Kou, Y. Zhao and Z. Xu, Construction of infrared-light-responsive photoinduced carriers driver for enhanced photocatalytic hydrogen evolution, Adv. Mater., 2020, 32, 1906361 CrossRef CAS.
- Y. Wu, P. Wang, X. Zhu, Q. Zhang, Z. Wang, Y. Liu, G. Zou, Y. Dai, M.-H. Whangbo and B. Huang, Composite of CH3NH3PbI3 with reduced graphene oxide as a highly efficient and stable visible-light Photocatalyst for hydrogen evolution in aqueous HI solution, Adv. Mater., 2018, 30, 1704342 CrossRef PubMed.
- Y. Li, Y. Liu, D. Xing, J. Wang, L. Zheng, Z. Wang, P. Wang, Z. Zheng, H. Cheng, Y. Dai and B. Huang, 2D/2D heterostructure of ultrathin BiVO4/Ti3C2 nanosheets for photocatalytic overall water splitting, Appl. Catal., B, 2021, 285, 119855 CrossRef CAS.
- W. Li, F. Wang, Z. Zhang and S. Min, MAPbI3 microcrystals integrated with Ti3C2Tx MXene nanosheets for efficient visible-light photocatalytic H2 evolution, Chem. Commun., 2021, 57, 7774–7777 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc05434a |
| This journal is © The Royal Society of Chemistry 2024 |