Identifying the critical features of resonance: instructors’ intentions for the teaching and learning of resonance in General Chemistry I and Organic Chemistry I
Sabrina
Barakat
 a and
MaryKay
Orgill
a and
MaryKay
Orgill
 *b
*b
aDepartment of Chemistry and Biochemistry, University of Nevada, Las Vegas, Las Vegas, NV, USA
bDepartment of Chemistry and Biochemistry, University of Nevada, Las Vegas, Las Vegas, NV, USA. E-mail: marykay.orgill@unlv.edu
First published on 4th January 2024
Abstract
Resonance is a fundamental chemistry concept first introduced to students in General Chemistry I (GCI), reintroduced in Organic Chemistry I (OCI), and then utilized throughout other higher-level chemistry courses. Student difficulties with resonance are well documented. Instruction is one potential source of student difficulties. What instructors choose to expose students to and how they choose to expose students to concepts related to resonance is influenced by instructors’ intentions for learning. As a first step in understanding and addressing the difficulties students encounter when learning about resonance, we qualitatively examined what instructors intend for their students to understand about and do with resonance in the courses in which it is first introduced, GCI and OCI. The instructors we interviewed identified eleven critical features of resonance that they deemed important for students to learn about. We found that GCI and OCI instructors in this study identified many of the same critical features of resonance. However, there were differences in what they expected students to know about and do with those critical features. GCI and OCI instructors also identified critical features unique to their courses. Overall, while discussing the critical features, the instructors tended to emphasize an operational versus conceptual understanding of resonance, which may partially explain students’ focus on using resonance instead of understanding it, as has been reported previously in the literature. The instructor-identified critical features presented herein have important implications for teaching and learning, as instructors’ perceptions determine what they expose their students to during instruction and ultimately influence what students have the possibility to learn about resonance.
Introduction
Resonance is a fundamental chemistry concept that scientists use to not only explain the behaviors of groups of molecules but also make predictions about and understand various chemical phenomena, such as the mechanisms of enzymatic reactions. Resonance is typically introduced to students in General Chemistry I (GCI), is later revisited in Organic Chemistry I (OCI), and then, while not formally taught again, is utilized throughout other chemistry courses. Chemistry instructors agree that understanding resonance is vital to student success in chemistry (Duis, 2011). However, even with repeated exposure to the concept and continued attempts by practitioners to clarify the concept (e.g., Gero, 1954; Richardson, 1986; Delvigne, 1989; Silverstein, 1999; Lin, 2007), educational research has shown that students have many challenges understanding and using resonance (e.g., Taber, 2002; Kim et al., 2019; Petterson et al., 2020; Xue and Stains, 2020; Brandfonbrener et al., 2021).To address the difficulties students have with understanding and using resonance, we need to know something about where those difficulties come from. There are many potential sources of student difficulties, but one is from instruction. What instructors choose to expose students to and how they choose to expose students to concepts related to resonance is influenced by many factors, including the instructors’ intentions for learning (i.e., what they think is important for students to know about a particular concept) (Marton and Booth, 1997; Hora, 2014). Therefore, an important first step in understanding and addressing the challenges students encounter when learning about resonance, and the goal of this study, is to look at what instructors deem critical for their students to understand about and do with resonance in the classes where it is first introduced, GCI and OCI.
Literature review
In the context of science education, instructors often share the common goal of having their students come to a shared, scientifically accepted understanding of a concept. While instructors cannot make students learn, it is their responsibility to provide students with the opportunity to learn by exposing them to the specific features of a concept (later defined as critical features) that will help them come to the desired understanding of a concept (Marton and Booth, 1997). What instructors choose to present to students about a concept (via curricular and instructional design) depends on their own knowledge and beliefs about the concept (Bussey et al., 2013; Atieh et al., 2022). Thus, instructors’ perceptions of a concept can affect students’ opportunity to come to the desired understanding of a concept (Hattie, 2008). We aim to understand GCI and OCI instructors’ perceptions of resonance as these perceptions will ultimately influence what the instructor presents in class and, consequently, what students will have the opportunity to learn about resonance. To further establish the necessity of this research, we will first discuss some of the common difficulties students have while learning about resonance in chemistry and the possible sources of these difficulties. We will then discuss the limited research regarding instructors’ perceptions of what students should learn about resonance.Student difficulties with resonance
Chemistry students have many difficulties understanding and using resonance (e.g., Betancourt-Perez et al., 2010; Kim et al., 2019; Finkenstaedt-Quinn et al., 2020; Petterson et al., 2020; Xue and Stains, 2020; Brandfonbrener et al., 2021; Braun et al., 2022; Atieh et al., 2022; Tetschner and Nedungadi, 2023). For instance, research consistently indicates that students struggle to conceptualize resonance. They misinterpret resonance structures as real entities that exist in nature alternating back and forth when, actually, the most correct structure (the resonance hybrid) is a mental melding of the different resonance structures (Taber, 2002; Kim et al., 2019; Xue and Stains, 2020). Aside from conceptual difficulties, students struggle to identify if a molecule exhibits resonance. They often look for specific structural cues that indicate resonance, such as a lone pair of electrons (Finkenstaedt-Quinn et al., 2020; Petterson et al., 2020; Watts et al., 2021). When these are not explicitly shown, students often cannot correctly identify if a molecule exhibits resonance (Finkenstaedt-Quinn et al., 2020; Petterson et al., 2020; Watts et al., 2021). Even when they do identify that a molecule exhibits resonance, students often struggle to draw valid resonance structures (with or without the use of curved arrows) (Betancourt-Perez et al., 2010; Petterson et al., 2020; Braun et al., 2022; Tetschner and Nedungadi, 2023). For example, similar to and likely tied to students' difficulties drawing Lewis structures (Cooper et al., 2010), students draw resonance structures that violate the octet rule or have incorrect formal charges (Betancourt-Perez et al., 2010).After learning about resonance, students are expected to apply their understanding to different chemical contexts, such as in acid–base chemistry or while solving reaction mechanisms. Unfortunately, research shows that while students identify the importance of resonance in these contexts, they struggle to apply their understanding of it (Cartrette and Mayo, 2011; McClary and Talanquer, 2011a; 2011b; Shah et al., 2018; Petterson et al., 2020; Dood and Watts, 2023). For example, while determining the relative acidity of a set of compounds, students recognized that resonance was an important factor to consider but lacked an underlying understanding of why resonance was important (McClary and Talanquer, 2011b), instead relying on the heuristic “More resonance forms-more acid strength” (McClary and Talanquer, 2011b, p. 1448). In another study, students were unsure how resonance affected reactivity and struggled to use the concept to determine the mechanistic steps of a reaction (Petterson et al., 2020). Overall, the research indicates that students have multiple difficulties in both understanding and applying the concept of resonance.
Possible sources of student difficulties with resonance
There are many possible reasons why resonance is challenging for students. One possible reason students might hold misconceptions about resonance is the term itself. Researchers suggest that the term “resonance” might contribute to students' belief that resonance structures are real entities that alternate or resonate back and forth (Kerber, 2006; Carle and Flynn, 2020). Kerber (2006) and Carle and Flynn (2020) argue that resonance should be called “delocalization” to address this misconception. Furthermore, other researchers have attributed students' conceptual difficulties with resonance to the representations themselves. That is, students lack representational competence, and along with the high cognitive load associated with drawing resonance structures, they struggle to interpret meaningful information from resonance structures (Taber, 2002; Kim et al., 2019; Braun et al., 2022). To address this difficulty, Kim et al. (2019) developed an instructional intervention that required students to create their own representations of resonance and to discuss the strengths and weaknesses of the chemical representations they drew. This intervention helped more students come to a scientifically accepted understanding of resonance (Kim et al., 2019).Another possible source of student difficulties is instruction/instructional materials. Textbooks often influence both what students focus on as they learn about resonance and what instructors choose to present to students about resonance. Carle and Flynn (2020) used content analysis to identify ten essential learning outcomes related to resonance. They analyzed how those essential learning outcomes are taught, practiced, and assessed in seven organic chemistry textbooks. They found that the essential learning outcomes requiring a conceptual understanding of resonance, such as those related to the resonance hybrid, were vastly underrepresented in textbook explanations and practice questions. More operational aspects of resonance, such as drawing resonance structures, were well-represented in the textbooks and assessments. Atieh et al. (2022) argue that this could be a possible reason why students tend to focus on an operational understanding of resonance rather than a conceptual understanding. Specifically, this lack of focus on the conceptual aspects of resonance could negatively influence students’ ability to draw resonance structures and apply resonance to different chemical contexts (Brandfonbrener et al., 2021; Braun et al., 2022). Based on their findings, Carle and Flynn (2020) suggest that instructors teach their students how to draw and interpret the resonance hybrid in order to help them develop a more conceptual and scientifically accepted understanding of resonance.
Instructors’ intentions for student learning related to resonance
Of course, what instructors choose to present to students about a concept depends on their own knowledge and beliefs about the concept (Bussey et al., 2013; Atieh et al., 2022). Thus, following Carle and Flynn's (2020) suggestion described at the end of the previous section would first require instructors to perceive the resonance hybrid as an important feature of resonance for their students to learn about. Unfortunately, there is limited information about what exactly it is that instructors believe students should know about and do with resonance (i.e., conceptually and operationally).To our knowledge, no studies have explored GCI instructors’ perceptions of resonance. Thus, the remainder of this literature review will focus on what OCI instructors want their students to know about and do with resonance. We should note that while, in the current study, we are only interested in Organic Chemistry I instructors’ perceptions of resonance, two studies we will discuss (Betancourt-Perez et al., 2010; Carle and Flynn, 2020) examined the perceptions of both Organic Chemistry I and Organic Chemistry II instructors. In all, we have identified five main studies that have, in some way, examined instructors’ beliefs about what their organic chemistry students should understand about and do with resonance (Betancourt-Perez et al., 2010; Carle and Flynn, 2020; Xue and Stains, 2020; Atieh et al., 2022; Tetschner and Nedungadi, 2023). It should be noted that the following discussion does not address all of the results from the studies; as relevant to this study, we will focus on what instructors have identified as important for students to know about and do with resonance (i.e., not on student results). We will first discuss the studies that address only Organic Chemistry I courses, followed by those that address Organic Chemistry I and Organic Chemistry II courses, in order of publication.
In their respective studies, Xue and Stains (2020) and Atieh et al. (2022) interviewed first-semester organic chemistry instructors about their approaches to teaching resonance. Xue and Stains (2020) interviewed two OCI instructors from the same institution about how they teach resonance in their first-semester organic chemistry classrooms. They found that both instructors (Instructors 1 and 2) who participated in the study believed it was important for students to know how to draw/identify resonance using curved arrows and to identify the major resonance contributor. The instructors also both emphasized that they believed students should be able to draw/identify resonance by recognizing specific patterns in molecular structure that typically indicate resonance (e.g., an allylic lone pair). There were, however, differences in what the instructors emphasized while discussing their teaching practices. Instructor 1 believed students should be able to draw resonance structures and identify possibilities of resonance in structures using an understanding of hybridization. In other words, he believed students should understand that resonance typically involves sp and sp2 hybridized atoms. Instructor 2 emphasized the importance of students understanding (1) the limitations of Lewis structures for expressing the bonding in real molecules, (2) that the resonance hybrid represents the real structure of a molecule, and (3) that resonance structures are not real entities and do not exist in nature (a common misconception about resonance).
Like Xue and Stains (2020), Atieh et al. (2022) found key differences in what instructors believe students should know about and do with resonance in organic chemistry. Atieh et al. (2022) interviewed fifteen first-semester organic chemistry instructors from varying institutions about their enacted pedagogical content knowledge (ePCK) of the resonance hybrid. An instructor's ePCK can be defined as what they intend to teach, how they teach, or what they reflect on about their teaching (Atieh et al., 2022). The researchers found that most of the instructors' ePCK could be categorized into two main groups based on what the instructors said they teach about the resonance hybrid: (1) instructors who perceive value in a conceptual understanding of the resonance hybrid and teach students about the resonance hybrid (including addressing common student misconceptions about resonance) but do not assess students’ understanding of it and (2) instructors who do not initially teach students about the resonance hybrid (although they might do so at a later time) and do not assess students’ understandings of it. The fact that these instructors believe the resonance hybrid is important for students to understand, yet they do not assess students’ understandings of it, is interesting as students often only pay attention to things that they know will be assessed (Van Etten et al., 1997).
Tetschner and Nedungadi (2023) collected information regarding what first-semester organic chemistry instructors perceive as important for students to learn about resonance as part of the process of developing a resonance-focused concept inventory. The inventory included 15 multiple-choice questions designed to probe students’ conceptual understandings of resonance, focusing on five broad categories related to resonance: “(1) resonance theory, (2) resonance structures and the resonance hybrid, (3) using curved arrows to draw resonance structures, (4) identifying the best resonance structure, and (5) resonance and stability (applications of resonance)” (Tetschner and Nedungadi, 2023, p. 3798). To validate the content of their concept inventory, the researchers asked chemistry instructors to comment on the relevance of each question. Based on their responses, it was clear that the instructors agreed that each of the five broad categories represented information that they believed students should understand about resonance. Overall, the questions within each of these categories addressed many of the same learning objectives related to resonance previously identified in the research literature as important for students to understand about resonance (Betancourt-Perez et al., 2010; Carle and Flynn, 2020; Xue and Stains, 2020; Atieh et al., 2022).
Carle and Flynn's (2020) study, introduced briefly in the previous section, provides information regarding what first and second-semester organic chemistry instructors believe students should understand about and do with resonance (please note that the researchers use the term “delocalization” to refer to resonance in their article). Before consulting with organic chemistry instructors, the researchers performed a content analysis using seven popular organic chemistry textbooks to identify any information related to resonance concepts, including information about the application of resonance in different chemical contexts (e.g., using resonance to determine relative acidity/basicity). The content analysis yielded 33 learning outcomes associated with resonance concepts in OCI and II. The researchers then asked five organic chemistry instructors to review and rank each of the 33 learning outcomes. The instructors’ responses were statistically analyzed to determine the importance of each learning outcome using a content validity ratio (Zamanzadeh et al., 2015). This analysis resulted in ten learning outcomes that instructors identified as essential for learning about resonance-related concepts in the organic chemistry series. These essential learning outcomes align with the findings from other studies (Betancourt-Perez et al., 2010; Xue and Stains, 2020; Atieh et al., 2022; Tetschner and Nedungadi, 2023) and also include additional learning objectives related to how students should be able to apply their understanding of resonance. In general, the ten essential learning outcomes are as follows: (1) identify/draw resonance structures, (2) draw resonance structures using curved arrows, (3) assess and justify the major and minor resonance contributors, (4) draw the resonance hybrid, (5) understand how hybridization relates to resonance, (6) determine the aromaticity of a molecule, (7) use resonance to determine relative molecular stability, (8) use resonance to explain relative acidity/basicity, (9) use resonance to determine/justify nucleophilic and electrophilic sites on a molecule, and (10) use resonance to predict and justify reaction mechanisms.
Table 1 lists the features of resonance that the instructors in these studies identified as important for students to understand to be successful in the organic chemistry series.
| Important aspects of resonance based on instructors’ perceptions of resonance | Supporting publication(s) |
|---|---|
| Students should understand the limitations of Lewis structures and the “need” for resonance. | Xue and Stains (2020); Tetschner and Nedungadi (2023) |
| Students should understand how to identify resonance and draw resonance structures for a molecule or ion. | Betancourt-Perez et al., 2010; Carle and Flynn (2020); Xue and Stains (2020); Tetschner and Nedungadi (2023) |
| Students should understand how to use curved arrows to draw resonance structures. | Betancourt-Perez et al., 2010; Carle and Flynn (2020); Xue and Stains (2020); Tetschner and Nedungadi (2023) |
| Students should understand how to identify the major and minor resonance contributors to the resonance hybrid. | Betancourt-Perez et al., 2010; Carle and Flynn (2020); Xue and Stains (2020); Tetschner and Nedungadi (2023) |
| Students should understand the resonance hybrid and/or draw the resonance hybrid. | Betancourt-Perez et al., 2010; Carle and Flynn (2020); Xue and Stains (2020); Atieh et al., (2022); Tetschner and Nedungadi (2023) |
| Students should understand how to identify specific patterns in molecular structures that indicate resonance structures can be drawn. | Xue and Stains (2020) |
| Students should understand how hybridization can be used to determine if resonance structures can be drawn for a molecule or ion. | Carle and Flynn (2020); Xue and Stains (2020) |
| Students should understand how to apply resonance in chemical contexts (e.g., reaction mechanisms). | Carle and Flynn (2020); Tetschner and Nedungadi (2023) |
The current study aims to understand GCI and OCI instructors’ perceptions of what students should know about and be able to do with resonance, as these perceptions will ultimately influence what instructors present in class and, as a consequence, what students will have the opportunity to learn about resonance. To our knowledge, this research provides the first research-based information about what GCI instructors intend for their students to learn about resonance. It also contributes to the research literature by further establishing what OCI instructors want their students to understand about resonance using thick and rich descriptions.
Theoretical framework
This study was informed by variation theory (Bussey et al., 2013). This framework focuses on how and why students can experience the same phenomenon differently and how that information can inform classroom teaching and learning (Tan, 2009; Bussey et al., 2013). Variation theory assumes that the differences in students’ understanding of a concept result from the differences in what students pay attention to or ignore during a particular learning event. A commonplace example might be useful. If two individuals attend the same live concert, their perceptions of the event will differ based on the features they focus on at the event. One individual might be drawn to the lyrics of the songs, interpreting how the words relate to their own life, thus concluding that the concert was an emotional and introspective event. The other individual might concentrate on the instrumentation, focusing less on the lyrics and more on the musical complexity. This individual's opinion of the concert might be that it was technically impressive. Despite both people attending the same live concert, their experience and interpretation of the event were different based on what they focused on at the concert.Object(s) of learning
In a study guided by variation theory, a given phenomenon or concept is studied from three perspectives: (1) what the instructor wants students to learn about the phenomenon or concept; (2) how students experience the phenomenon or concept during a learning event; (3) what students come to understand about the phenomenon or concept. In variation theory, the phenomenon or concept to be experienced is called the object of learning. In other words, it is the something that is to be learned by the student (Marton and Booth, 1997). In this study, the object of learning is resonance. The student's understanding of the object of learning is affected by what the instructor intends for students to understand about a particular concept and what the instructor presents to students in the classroom. Therefore, three different perspectives of the object of learning are examined:1. Intended object of learning: this perspective focuses on what instructors intend students to understand about a concept (Marton and Tsui, 2004).
2. Enacted object of learning: this perspective focuses on what actually happens during the learning event, regardless of what the instructor intended (Marton and Tsui, 2004; Runesson, 2005).
3. Lived object of learning: this perspective focuses on what students actually came to understand about the object of learning (Marton and Tsui, 2004).
The focus of the current study is the intended object of learning and, more specifically, on identifying the features that instructors deem “critical” for students' developing a correct understanding of resonance.
Critical features
Chemistry instructors often desire students to come to a shared, scientifically accepted understanding of a concept. Specific features of the object of learning (e.g., resonance) are critical for developing a correct understanding of the concept (Marton and Tsui, 2004). Variation theory refers to these features as critical features. In this study, we have defined a critical feature as anything the instructors (who participated in this study) deemed critical for developing a correct understanding of resonance, which included both what students should know about resonance and what students should be able to do with resonance. If instructors want their students to come to a shared understanding, they must direct students' attention to the critical features of a given concept (Marton and Booth, 1997).Purpose of study and research question
The current study is part of a larger study focused on investigating the teaching and learning of resonance. Here, we specifically focus on instructors’ perceptions of what students should know about resonance and what students should be able to do with resonance, as those beliefs will affect what instructors present in class and, as a consequence, what students will have the opportunity to learn about. The following research question guided the study: What do GCI and OCI instructors intend for their students to understand about resonance? We decided to focus our study on GCI and OCI, as these are the courses in which resonance is typically introduced and formally taught. We have also decided to focus on what instructors want students to know about and do with resonance, but not its application in different chemical contexts.Methodology
Participants and setting
After obtaining approval from the Institutional Review Board, we recruited GCI and OCI instructors from a community college, a state college, and a large research-focused university in the Southwestern United States. Ultimately, five GCI instructors and five OCI instructors agreed to speak with us about what they intend for students to know about and do with resonance. We found that the instructors’ beliefs were not institutionally specific, meaning their beliefs were not dependent on the type of institution they taught at. Thus, we will not identify the instructors’ institution or institution type to further protect their identities. All of the instructors that participated in this study have a PhD in chemistry but have different numbers of years of teaching experience (e.g., first semester to several years teaching).Overall, we interviewed five GCI instructors and five OCI instructors about what they believe students should learn about resonance in GCI. One instructor (Maryann) was interviewed twice because she taught both GCI and OCI and could offer insight about teaching resonance in both courses.
Interview protocol
The instructor interviews were semi-structured and lasted approximately 45–60 minutes. After discussing the instructors’ professional backgrounds, we asked a series of questions to determine what they believe students should know about and do with resonance. First, to establish a shared understanding of resonance, we asked the instructors to describe the concept in their own words and why/if they believed it was important for students to learn about resonance. Next, we asked instructors questions related to what they expected students to know about resonance before coming into their classes. This information was collected as it might influence what instructors decide to teach and provide insight into what instructors believe and/or have identified in their students' prior knowledge about resonance. We also asked when and how they typically teach resonance in their courses.We then asked instructors what they believe students should understand about resonance in their classes and how they assess those understandings, followed by questions about the challenges of teaching and learning about resonance. For example, we asked instructors what their students need help understanding about resonance and about any specific misunderstandings they have noticed their students have while learning about resonance. This information provides context regarding why instructors teach resonance the way they do and/or why they emphasize or exclude specific aspects of resonance while teaching about it. Finally, we ended the interviews by asking instructors what they believe to be the most important aspects for students to understand about resonance and what is not as important for students to understand about resonance.
Data analysis
Pseudonyms were created for each instructor, and artifacts were re-created to maintain the participants’ confidentiality. Interviews were audio recorded and then transcribed verbatim. We organized and analyzed the transcripts using MAXQDA software (VERBI Software 2021). The interviews were read, reread, and iteratively coded for what the instructors identified as critical features students should understand about and do with resonance using thematic analysis (Creswell, 2012). The codes (which are the descriptions of the critical features) were then grouped based on similarity, which resulted in collapsing some codes with each other. Once the codes were grouped, the critical features were defined using the supporting textual evidence. We defined a critical feature of resonance as anything at least one of the participating instructors believed a student should understand or be able to do in order to develop a correct understanding of resonance. For example, all of the GCI and OCI instructors who participated in this study wanted their students to know that resonance structures only differ in the placement of electrons and to be able to draw valid resonance structures for a given species. Thus, “resonance structures” was coded as a critical feature. The first author completed the initial coding of the transcripts. The critical features, code definitions, and textual evidence were reviewed by the second author and by a colleague with a doctorate in chemistry education. After discussion, the codes were revised until a consensus (complete agreement) was reached.Results
Here, we present our results related to the intended object of learning (i.e., the focus of this study). Instructors in the current study identified a limited number of critical features of resonance. Both GCI and OCI instructors mentioned some of these. Other critical features were mentioned only by GCI instructors or only by OCI instructors. In the current section, we will discuss the critical features mentioned by both GCI and OCI instructors. Next, we will discuss the critical features mentioned by only GCI instructors, followed by the critical features mentioned by only OCI instructors. Before presenting the critical features, we first establish the instructors’ perceptions of the importance of the concept of resonance in chemistry.Instructors’ perceptions of the importance of resonance
While prior educational research has indicated that chemistry instructors believe resonance is an important chemistry topic (Duis, 2011), it was important to establish that the instructors participating in this study shared similar beliefs. All instructors participating in this study believed resonance was an important topic for students to learn. The GCI instructors tended to emphasize that resonance was not a large course focus but knew it was important for students to learn about because they would need to understand the topic if they took other higher-level chemistry courses. For example, Maryann, who taught general and organic chemistry, stated, “They [her general chemistry students] are going to need it in organic chemistry.” OCI instructors, on the other hand, gave more specific examples of why they believed resonance was a “fundamental” topic for students to learn about. They emphasized that students need to master the topic to predict and solve reaction mechanisms, determine relative acidities of molecules, and interpret data from spectroscopic instruments, such as NMR instruments. While the instructors might have shared different reasons why they believe resonance to be an important concept for students to learn, there was a clear consensus on its importance in chemistry curricula. This shared consensus establishes the context for identifying what the instructors participating in this study believe to be the critical features of resonance.Critical features of resonance
The instructors in the current study identified eleven critical features of resonance (Table 2). The critical features were categorized based on which type of instructor identified them—OCI instructors and GCI instructors, GCI instructors, or OCI instructors. Within these three categories, we discuss the critical features in descending order of the total number of instructors who identified them. We also distinguish between what instructors want students to know about a particular critical feature and what instructors want students to do with that critical feature.| Identified by General Chemistry I (GCI) and Organic Chemistry I (OCI) instructors, GCI instructors, or OCI instructors | Critical feature | Description of critical feature |
|---|---|---|
| GCI and OCI instructors | ||
| Lewis structures | Students should understand Lewis's model of chemical bonding (i.e., Lewis structures) and that a single Lewis structure cannot adequately represent the bonding in some molecules. | |
| Resonance structures | Students should understand that resonance structures only differ in the placement of electrons (i.e., electron movement), not atoms. | |
| Resonance hybrid | Students should understand that resonance structures are not real entities and do not exist in nature. They should also understand that the actual, most correct structure, the resonance hybrid, is a mental melding of the different resonance structures. | |
| Octet rule | Students should understand that second-row elements should not exceed an octet while drawing resonance structures. | |
| Formal charge | Students should understand that atoms in resonance structures can have different formal charges, but the overall net charge of the molecule does not change. They should also understand how formal charge can be used to identify the major resonance contributor. | |
| Major and minor resonance contributors | Students should understand that there are certain instances in which one (or more) of the resonance structures is the major contributor to the resonance hybrid. | |
| GCI instructors | ||
| Bond length | Students should understand that there can be differences between the expected and observed bond lengths predicted by Lewis structures of certain molecules and how resonance can explain these discrepancies. | |
| OCI instructors | ||
| Pattern recognition | Students should understand that resonance structures can be identified by recognizing specific patterns in molecular structure. | |
| Curved arrows | Students should understand that curved arrows are used to draw resonance structures. | |
| Delocalized and localized lone pairs | Students should understand the difference between electrons that participate in resonance (i.e., delocalized) and those that do not (i.e., localized). | |
| Hybridization | Students should understand how hybridization affects whether there is resonance or not. | |
Critical features identified by General and Organic Chemistry I instructors
The critical features in this category were identified by at least one GCI instructor and one OCI instructor participating in this study: they are Lewis structures, resonance hybrid, octet rule, formal charge, and major and minor resonance contributors. The fact that both GCI and OCI instructors identified these features as important for their students to learn suggests that some OCI instructors might expect students coming into organic chemistry to have prior knowledge about and experience with these critical features. However, it should be noted that aside from expecting students to be able to draw Lewis structures, some of the OCI instructors in this study shared that, based on personal experience, they expected students to come into their class with very little understanding of resonance.Lewis structures
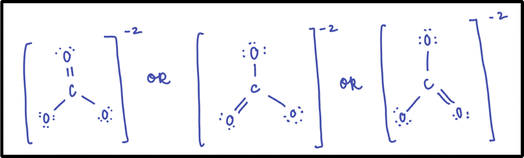
All of the instructors in this study indicated the importance of understanding Lewis's model of chemical bonding (i.e., Lewis structures) and knowing that a single Lewis structure cannot adequately represent the bonding in some molecules. They expected students to be able to draw and interpret Lewis structures in order to develop a correct understanding of resonance. When we asked Annabelle how she introduces resonance in her GCI classroom, she shared that she asks students to draw the Lewis structure for the polyatomic ion carbonate before drawing it on the board herself (Fig. 1). She will then ask students if they drew the double bond in the same place as she did and if they still believed they were correct. After this discussion with students, she said she would draw all of the resonance structures for the polyatomic ion with the word “or” written between them (Fig. 1). Typically, the students agree that each structure is a valid Lewis structure, but they do not know why there are multiple correct structures. Annabelle introduces resonance to her students to explain why. Other GCI instructors in this study took similar approaches to Annabelle.The OCI Instructors also believed that Lewis structures is a critical feature for developing a correct understanding of resonance; however, they described this critical feature as one that students should come into the course having already mastered. For example, one instructor stated, “I don’t have time to cover Lewis structures […], and I just said [to students], you did this in [GCI]”.
Regarding what the GCI and OCI instructors wanted students to do with this critical feature, there was consensus that students should be able to draw a valid Lewis structure, including lone pairs, and determine the formal charge for individual atoms in the molecule. Students should then be able to look at a Lewis structure and determine if resonance is possible. This was limited to relatively simple molecules or ions in GCI (e.g., carbonate) and larger, more complex molecules in OCI (e.g., CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHOCH2CH(CH3)2).
CHOCH2CH(CH3)2).
Resonance structures
Resonance structures was another critical feature mentioned by all of the instructors in this study. Both GCI and OCI instructors described the importance of understanding that resonance structures only differ in the placement of electrons, not atoms. The instructors wanted students to know that resonance structures are two-dimensional representations showing electron “movement” between atoms. For example, Ryan said “…You're writing Lewis structures where you're moving electrons around.” Initially, we assumed that instructors were using phrases like “electron movement” to describe the fact that the electrons are “moved” from one atom to another in the drawings of different resonance structures; and this was, indeed, true for some of the instructors we interviewed. However, as we continued our analysis, we found that a few of the instructors in this study expressed the misconception that resonance structures are real entities that exist in nature and that electrons were literally moving as a molecule transitioned from one resonance structure to another. For example, during her interview, Annabelle stated that “electrons are moving and that there's not always one stable structure for something.” Her phrasing suggests that she believes molecules that exhibit resonance have multiple stable structures instead of a single resonance hybrid in which the molecule's electron density is spread out amongst multiple atoms. Resonance structures do not depict the actual movement of electrons but, rather, “treat electrons as if they [are moving]” (Klein, 2012, p. 70) because this is useful for thinking about chemical reactions. In other words, as a participant in this study stated, “Resonance is a tool to predict reaction mechanisms; it's not a real phenomenon. Resonance structures don’t actually exist.” It will be important for future research to examine potential misconceptions that instructors themselves might hold about resonance, as this will impact what students can learn about the concept.All of the instructors we interviewed believed it was important that students could draw resonance structures for a molecule or ion. The GCI instructors kept their expectations relatively simple; they told students when a molecule or ion exhibited resonance and expected students to be able to draw the resulting resonance structures on homework, quizzes, or exams without the use of curved arrows (described in a later section). There were typically one or two exam questions dedicated to assessing resonance. For example, Kory said that he asks his students to “write one or more Lewis [resonance] structures for the nitrate ion.”
The OCI instructors wanted students to do more with resonance structures, as resonance is more of a course focus for them. They expected students to identify if a species exhibited resonance by using specific molecular structure patterns (described in a later section). Unlike the GCI instructors, this group also expected students to use curved arrows to draw resonance structures. For example, Anthony stated:
So, I guess top priority is being able to spot when there are resonance structures. So, look for multiple bonds, and that has to be adjacent to either another multiple bond or a charge of some sort. So, first step, when is there going to be a resonance structure possible? Sort of identifying that. The next tier above that is, what are those resonance structures? Can you draw them? Can you ideally push the arrows correctly?
Furthermore, while assessing resonance for the first time, the OCI instructors explained that they typically provide students with a molecular structure and require students to identify if resonance is possible and, if so, to draw all possible resonance structures using curved arrows. Interestingly, none of the instructors in this study mentioned using double-headed arrows between resonance structures or brackets to distinguish the resonance structures, as is commonly depicted in textbooks.
Resonance hybrid
All but one of the GCI and OCI instructors participating in this study mentioned the resonance hybrid as a critical feature. For instance, many instructors in this study identified the importance of understanding that resonance structures are not real entities and do not exist in nature and that the actual, most correct structure, the resonance hybrid, is a mental melding of the different resonance structures for a given molecule or ion. The instructors' responses regarding what they believed students should know about this critical feature varied. The GCI instructors reported discussing the resonance hybrid more than the OCI instructors. Kory and Ryan (GCI instructors) took special care to point out student misconceptions about the resonance hybrid that they have noticed in their classrooms. For example, Ryan emphasized while introducing resonance structures to students, “[the molecule] does not flip back and forth between these different resonance structures. Rather, we say it's like a hybrid of all three resonance structures.” This particular misconception that Ryan is addressing (that resonance structures are in equilibrium, flipping back and forth) has been widely reported in the literature (e.g., Taber, 2002; Kim et al., 2019; Xue and Stains, 2020; Petterson et al., 2020). Interestingly, it was also a misconception that some of the other instructors from both groups in this study seemed to reinforce and/or express themselves while describing “electron movement” in resonance structures, as mentioned previously.OCI instructors believed students should understand the resonance hybrid; however, they did not believe it was as important in their course as did the GCI instructors. Nevertheless, some of the OCI instructors shared that they quickly reviewed the resonance hybrid during class to dispel the misconception that resonance structures are real entities that alternate back and forth. For example, Joe explains to students “that the conjugated double bond is not really a double bond, single bond, double bond; it is actually a mixture [of the two bonds].”
A few instructors who participated in this study identified what they wanted students to be able to do with the resonance hybrid (e.g., drawing it), but none of the instructors formally assessed students' understanding of the resonance hybrid on homework assignments or exams. Nevertheless, two GCI instructors and one OCI instructor explained a desire for students to “visualize resonance” via a provided drawing of the resonance hybrid (none of the instructors expected students to draw the resonance hybrid on their own).
Octet rule
Most of the GCI and OCI instructors in this study believed it was important for students to understand the octet rule and, more specifically, to recognize that second-row elements cannot have expanded octets in Lewis structures. This understanding establishes a context for introducing resonance concepts. For example, one GCI instructor, Destiny, shared that her students questioned why there could be multiple correct Lewis structures that keep the octet rule for the same molecule. The students’ questioning gives Destiny a reason to introduce resonance to her class.While both GCI and OCI instructors were relatively vague regarding what they wanted students to know about this critical feature, the instructors were clear regarding what they wanted students to do with it. Instructors from both groups wanted students to use the octet rule to decipher if a resonance structure was valid. For example, Brad (OCI instructor) expected students to construct a resonance structure and then “decide whether that structure is acceptable. Like, does it follow the octet rule?” Unfortunately, some instructors shared that this critical feature was challenging for students. Dallas shared that his organic chemistry students struggle to decipher how many bonds certain elements can typically form, often resulting in their drawing too many bonds to a given atom.
Formal charge
Formal charge is essential to understanding the differences between resonance structures for the same species. Most of the GCI and OCI instructors who participated in this study wanted students to understand that, while a given atom can have a different formal charge in different resonance structures, the overall net charge of the molecule does not change from one resonance structure to another. The instructors believed this was important because it is one method students can use to determine the major resonance contributor out of a group of resonance structures.The instructors were relatively vague when describing what they wanted students to know about this critical feature. Dallas, who teaches OCI, discussed this critical feature the most. While discussing his approach to teaching resonance, he drew the molecule in Fig. 2 as an example. After drawing the resonance structures for the molecule using curved arrows, he said, “It's really easy to get lost in resonance structures. I always try to remind my students to remember formal charges; no reactions are occurring, so there should be the same sum of formal charges across every resonance structure.” He wrote a note under the resonance structures he drew to emphasize his point (Fig. 2).
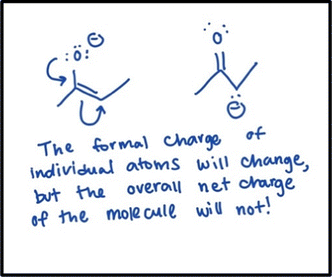 | ||
| Fig. 2 Dallas’ example showing that the net charge of a molecule does not change between resonance structures. | ||
Students’ ability to use formal charge to identify the major resonance contributors or the most stable structure was a concern for both GCI and OCI instructors. The instructors spent more time describing what they wanted students to do with this critical feature than what they wanted students to know about it. When Ryan was asked about the type of practice and homework he assigns his GCI students related to resonance, he stated:
I might draw a couple of molecules and ask them to determine the number [of resonance structures], the formal charge of each atom present and then tell me which [resonance structure] is more likely, you know, the predominant resonance structure and explain why. So that's kind of about the depth.
Similarly, Anthony, an OCI instructor, shared that he spends time explaining how formal charge can be used to identify the most stable resonance structure. He stated, “If there's a particular charge present, for example, if it's a negative charge, I mentioned that you want your negative charges to be on your more electronegative atoms because that's just the more stable position.”
Major and minor resonance contributors
Most of the GCI and OCI instructors in this study wanted students to understand that not all of the resonance structures that can be drawn for a molecule will always equally contribute to the resonance hybrid. Instead, they wanted students to understand that there are certain instances in which one (or more) of the resonance structures for a molecule is the major resonance contributor (some instructors referred to the major resonance contributor as the “dominant” or “prominent” contributor).The GCI and OCI instructors shared similar beliefs regarding what students should be able to do relative to this critical feature: they expected students to identify the major and minor resonance contributors for a given molecule. For example, when we asked Brad, an OCI instructor, what type of practice problems and test questions he asks students to solve after first learning about resonance, he said, “I ask them to draw the resonance [structures] and [figure out] if they are equivalent or not equivalent. Then they need to figure out which is the major form and which is the minor form.” The GCI instructors described similar expectations. Interestingly, the instructors did not connect this critical feature to the resonance hybrid. In other words, they did not specifically discuss wanting students to understand that the major resonance structure contributes more to the overall structure of the resonance hybrid than do the minor resonance structure(s). Instead, they simply mentioned it was important for students to identify major and minor structures without pointing out what the major and minor structures were contributing to.
Critical features identified by General Chemistry I instructors
There was only one critical feature mentioned exclusively by GCI instructors participating in our study. This critical feature was bond length, which is discussed below.Bond length
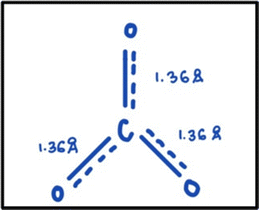
A couple of the GCI instructors believed it was important for students to understand how resonance can explain the differences between bond lengths predicted by Lewis structures and the observed bond lengths for certain molecules. Ryan and Annabelle expected students to know that electrons can be shared over multiple atoms, which results in an intermediate bond length compared to that predicted by individual Lewis or resonance structures. Ryan said:I guess just my main hope is that they'd have a little better understanding of what molecules really are and how these atoms are connected. And that the bonds [i.e., electrons] can be kind of shared over multiple atoms, as opposed to like having discrete double bonds and single bonds, but there's like, kind of a middle ground. […] If they get that feeling and appreciation, I've done my job.
Similarly, when we asked Annabelle how she typically introduces resonance in her class, she referred to a structure of the carbonate molecule (Fig. 1, shown previously) and stated:
What would you expect to measure if we could take a snapshot of this [see Fig. 1] and measure this distance, this distance, and this distance [pointed to each of the bonds on Fig. 1]? So expected, we would measure a carbon–oxygen double bond at 1.23 angstroms. And you would expect me to measure two carbon–oxygen single bonds at 1.43 angstroms. If this is how it was if we took a snapshot, maybe the double bond would be here, [if we took another snapshot, it could be] here or here [pointing to the double bond in each resonance structure in Fig. 1].
What actually happens? So actually, if we took a snapshot of this, we would have something that looks like this [draws Fig. 3] where we have three equal bonds at 1.36 angstroms. So, it's not really that these electrons are confined here. They are actually in kind of fluid motion all around. And the double bond is actually being shared between all of these atoms. So, we wouldn't say it's a single, a single and a double bond. We would say each of these has a bond length of 1 and 1/3 kind of a bond order. They each get their own [bond], and then they're all sharing that second one.
Although the two instructors agreed that it was important for their students to understand this critical feature, they disagreed on what they expected students to do with it. Annabelle wanted students to be able to calculate and predict bond lengths (but not draw the resonance hybrid) on homework and exams, as she does in class. Ryan, however, does not expect students to do so. Instead, he perceived this critical feature only as an important way to introduce resonance to students to gain a deeper appreciation of the underlying concepts of resonance.
Critical features identified by Organic Chemistry I instructors
Only OCI instructors identified the remaining critical features to be discussed: pattern recognition, curved arrows, delocalized and localized lone pairs, and hybridization.Pattern recognition
All OCI instructors in this study agreed it was important for students to understand that they can use specific patterns in molecular structures in order to determine if a molecule will exhibit resonance or not. The instructors broadly referred to these patterns as the “five different patterns of resonance.” The patterns are (1) allylic lone pair, (2) allylic positive charge, (3) lone pair adjacent to positive charge, (4) pi bond between two atoms of differing electronegativity, and (5) conjugated pi bonds enclosed in a ring (Klein, 2012, p. 81).In general, the instructors wanted students to recognize each pattern listed above and, if a pattern was present in a molecular structure, use the corresponding resonance structures to solve a problem. When Maryann was asked to go over how she teaches resonance to her organic chemistry students, she provided a basic introduction to resonance before going over each of the possible patterns of resonance. She expected that her students (1) recognize that the allylic lone pair “could move,” as shown in Fig. 4 and (2) draw the corresponding resonance structure.
Curved arrows
All but one OCI instructor participating in this study identified curved arrows as a critical feature. These instructors wanted students to understand that curved arrows are used to draw resonance structures. None of the instructors discussed what they wanted students to know about curved arrows, such as what the head or tail of the arrow represents or that curved arrows do not represent the actual movement of electrons (i.e., they are merely a tool used to construct resonance structures).The OCI instructors expected students to use curved arrows while drawing resonance structures on homework, quizzes, and exams. Some instructors specifically examined and graded students’ use of curved arrows, ensuring that the resulting structure matched what their curved arrows depicted. The instructors generally agreed that students' success with resonance in organic chemistry largely depends on their ability to use curved arrows.
Delocalized and localized lone pairs
All but one OCI instructor identified delocalized and localized lone pairs as a critical feature of resonance. The instructors wanted students to understand the difference between electrons that participate in resonance (i.e., delocalized electrons) and those that do not (i.e., localized electrons). While most instructors identified this critical feature, they were vague about what they wanted students to know and do with it, simply indicating they wanted students to identify a lone electron pair and decide if it would participate in resonance.Hybridization
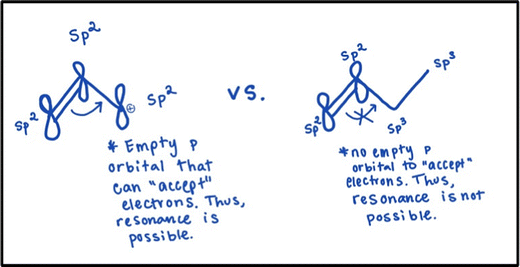
Joe was the only instructor that identified this critical feature. In fact, he introduces his students to hybridization before he discusses the five resonance patterns previously mentioned. He requires students to identify the hybridization of an individual atom in a molecule (e.g., sp, sp2, sp3). After students can do this, Joe introduces the concept of resonance. Combining these two concepts (i.e., resonance and hybridization), he teaches students that, for resonance to occur, there needs to be sp2 or sp hybridized orbitals with empty p orbitals to “accept” electrons. sp3 hybridized atoms do not have an empty p orbital and cannot accept electrons. Joe expects students to apply this information to decide if a species exhibits resonance and believes that students should mainly use their knowledge of hybridization to solve problems involving resonance (as opposed to relying only on pattern recognition). Fig. 5 provides an example of this approach.Discussion
In a study guided by variation theory, the object of learning (in this case, resonance) is studied from the instructor, classroom, and student perspectives. This study focused on the instructor perspective. Specifically, we examined what GCI and OCI instructors perceive to be critical for their students to know about and be able to do in order to develop a correct understanding of resonance. Here, we present a discussion of (1) how our findings relate to the research literature, (2) instructors’ emphasis on the operational versus the conceptual aspects of the critical features of resonance, and (3) differences in what the GCI and OCI instructors deem important to understand about resonance.Overview of how our findings relate to the research literature
The instructors we interviewed identified eleven critical features of resonance (Table 2). In general, these align with previous reports of what students should know about and be able to do with the concept of resonance (see Table 1). We should note that while the instructors in this study indicated the importance of the resonance hybrid, none expected students to be able to draw the resonance hybrid, nor did they assess students’ understanding of the resonance hybrid (aside from Annabelle asking students to predict bond length). These results align with similar findings in the research literature that indicate that instructors believe the resonance hybrid is important—yet not all instructors believe they should assess what students know about it or if they can draw it (Xue and Stains, 2020; Atieh et al., 2022). We also identified additional critical features of resonance not yet reported in the research literature: octet rule, formal charge, and delocalized and localized lone pairs. It will be important for future studies to examine specific relationships between students’ understanding of these concepts and their understanding of resonance.Instructors emphasize operational vs. conceptual aspects of the critical features of resonance
The instructors in the current study identified both what they wanted students to know (conceptual understandings) and what they wanted students to do (operational understandings) with the critical features of resonance. That said, in identifying these critical features, instructors often prioritized what students should do with resonance over what students should know. For example, while many instructors in this study clearly explained that they wanted students to use formal charge to identify the major and minor resonance contributors, they were relatively vague when describing what they wanted students to know about formal charge's relationship to resonance. If this emphasis parallels what happens in class, students would have more opportunities to build their operational understandings of resonance compared with their conceptual understandings (Carle and Flynn, 2020; Xue and Stains, 2020; Atieh et al., 2022).Furthermore, the instructors in the current study were more likely to report assessing students’ operational understandings of resonance than their conceptual understandings of resonance. As students tend to pay more attention to skills and concepts that will be assessed, the instructors’ assessment practices could also reinforce the students’ developing operational understandings of resonance over their conceptual understandings (Van Etten et al., 1997). In fact, Brandfonbrener et al. (2021) argue that an assessment focused on operational aspects of resonance could partially explain why many students continue to hold conceptual misconceptions about resonance. This may also partially explain why students exhibit difficulties with using and applying resonance, as Braun et al. (2022) found that students who had correct conceptual understandings of resonance were more likely to draw valid resonance structures (i.e., to exhibit correct operational understandings of resonance) than students who do not have correct conceptual understandings of resonance.
Finally, the fact that instructors in the current study emphasized operational understandings of resonance might also explain why some of them, specifically the OCI instructors, expressed misconceptions about the resonance hybrid, as their lack of focus on the conceptual aspects of resonance limits situations in which they have to confront the validity of their own ideas (Kruse and Roehrig, 2005).
Differences in what General Chemistry I and Organic Chemistry I instructors deem important to understand about resonance
While many of the GCI and OCI instructors participating in our study identified some of the same critical features of resonance, there were differences in what the instructors expected students to know about and do with those critical features. The instructors also identified critical features unique to the course they taught (i.e., GCI or OCI).Although neither type of instructor focused on conceptual understandings of resonance relative to operational understandings, the GCI instructors in the current study reported discussing critical features that address students' conceptual understandings of resonance more often than did the OCI instructors. They also identified an additional critical feature not mentioned by the OCI instructors: bond length. This critical feature focuses on an underlying understanding of the resonance hybrid. Nevertheless, as previously discussed, only one of the GCI instructors reported assessing students on these more conceptual aspects of resonance (i.e., Annabelle reported assessing students on bond length).
Overall, those teaching organic chemistry expected students to do more with resonance than those teaching general chemistry in this study. The OCI instructors used larger, more complex molecules to teach resonance. The OCI instructors also identified four additional critical features (pattern recognition, curved arrows, delocalized and localized lone pairs, and hybridization) not mentioned by the GCI instructors. Interestingly, although these critical features require a more advanced understanding of chemistry in general, they largely involve operational understandings of resonance specifically. For example, students must use curved arrows to draw resonance structures or use pattern recognition to identify if a molecule exhibits resonance.
Conclusions and implications
This study employed variation theory. Specifically, we investigated what GCI and OCI instructors in our study intended for their students to learn about resonance (i.e., the intended object of learning). Our participants identified eleven critical features of resonance that students should learn about in GCI and OCI courses. Both groups of instructors identified many of the same critical features (Lewis structures, resonance structures, resonance hybrid, octet rule, formal charge, major and minor resonance contributors). However, there were differences in what the instructors expected students to know about and do with those critical features. For example, those teaching OCI used larger, more complex molecules to teach resonance.The instructors also identified critical features unique to their course (i.e., GCI or OCI). The GCI instructors were the only ones who identified the critical feature bond length. While the OCI instructors were the only ones who identified the critical features pattern recognition, curved arrows, and hybridization. Overall, while discussing the critical features, we found that instructors tended to emphasize the operational aspects of resonance versus the conceptual aspects of resonance. For example, while the instructors in this study indicated the importance of the resonance hybrid, none expected students to be able to draw the resonance hybrid, nor did they report directly assessing students’ understanding of the resonance hybrid. If this emphasis parallels what happens in class, we argue that this might partially explain why previous research has found that students focus on using resonance instead of developing a conceptual understanding of it. Based on these findings and their connections to the research literature, in the sections that follow, we provide suggestions for research and practice.
Implications for research
According to variation theory, identifying what instructors want their students to know about a concept (the intended object of learning and the focus of this study) is not sufficient evidence to inform change in the classroom. This is because students’ understanding (the lived object of learning) of the object of learning is not only affected by what the instructor intends for students to understand about a particular concept but also what the instructor presents to students in the classroom (the enacted object of learning). Thus, a study guided by variation must examine the object of learning from all three perspectives (instructor, classroom, and student). Information from each of these perspectives can be compared to draw overarching results and to guide the improvement of instructional materials and teaching practices (Bussey et al., 2013).Thus, it will be important in the future to examine how the instructor-identified critical features (the intended object of learning) align with what is presented in the classroom and with the instructional materials/textbooks available to students (the enacted object of learning). This comparison will allow researchers to identify potential differences in what instructors intend to teach about resonance versus what students actually had the opportunity to learn about resonance. This is of particular concern, considering the instructors in this study discussed critical features of resonance not identified in Carle and Flynn's (2020) study. Thus, these additional features are potentially not being covered in textbooks and exams, limiting where students have the opportunity to learn about them.
It will also be important to determine how the critical features identified by the instructors (the intended object of learning) align with what students are actually coming to understand about resonance in the classroom (the lived object of learning). Any differences between the intended and enacted objects of learning provide an opportunity for critical examination of current classroom practice (the enacted object of learning) and can inform potential changes to that classroom practice. For example, if students are not understanding something the instructor intends them to understand, the instructor might reflect on why that is. They might ask themselves: “Why is that? Am I not teaching it? Am I not emphasizing it enough? Am I teaching it in a way that promotes misconceptions?”
Finally, it is also important to compare what happens in the classroom (the enacted object of learning) to what students ultimately integrate into their understandings of resonance (the lived object of learning), as this comparison highlights what students pay attention to when learning about resonance. This may bring to light concepts that require more emphasis and attention by the instructor, as students might not be paying attention to them during instruction.
We plan to examine these perspectives and how they relate to each other as an extension of the study presented here. This information will help us better understand potential reasons why students struggle to understand resonance in the ways their instructors intend them to and what we can do about it to improve classroom teaching and learning of resonance. In the meantime, in the section that follows, we provide possible suggestions for practitioners based on our current findings and the research literature.
Implications for practice
The current study has focused on what instructors perceive to be critical for their students to know about and do with resonance to be successful in their courses. Our results indicate that while instructors believe a conceptual understanding of resonance is important, they emphasize the operational aspects while teaching and assessing students. This may partially explain why students focus on using resonance rather than understanding it, as was reported by Brandfonbrener et al. (2021). In an effort to align instructors' intentions with what is made available to learn in the classroom, we suggest instructors (1) engage students in activities to discuss conceptual aspects of resonance and (2) assess students’ conceptual understandings of resonance.Engage students in activities to discuss conceptual aspects of resonance
Kim et al. (2019) and Brandfonbrener et al. (2021) provide useful teaching interventions/strategies that address students’ conceptual understandings of resonance. Specifically, according to Kim et al.'s (2019) study, instructors should focus on developing students' metarepresentational competence related to resonance. This involves having students create their own representations of resonance and discussing the representations' strengths and weaknesses. A writing-to-learn assignment (Hayes, 1996) similar to that designed by Brandfonbrener et al. (2021) might also be useful in developing students’ conceptual understanding of resonance. Such an assignment should encourage students to articulate, reflect, and elaborate on their conceptual understandings of resonance. Finally, considering that some of the instructors in our study expressed misconceptions about the resonance hybrid, we encourage instructors to reflect on their own conceptual understandings of resonance, as these misunderstandings could profoundly impact student learning, and should be addressed before leading student discussions on the concept.Assess students’ conceptual understandings of resonance
It seems reasonable that instructors would assess students in their classrooms as they have experienced in the past. If this is true, it is possible that instructors do not have experience with conceptual assessments of resonance. Thus, despite believing a conceptual understanding of resonance to be important, it might not be reflected in their assessments of the concept. We suggest that instructors examine their assessment questions to ensure that they match what they intend for students to learn about resonance. Tetschner and Nedungadi's (2023) resonance concept inventory provides examples of the types of questions that could be used to elicit and assess students’ conceptual understandings of resonance.Limitations
We have identified several possible limitations to our study. First, while this study was not limited to a single institution, it was limited to a specific region of the Southwestern United States with a limited sample of participants. Thus, the transferability of these results should be thoughtfully considered. Second, the findings from this study represent our interpretations of the participating instructors’ perceptions related to what they believe students should understand about and do with resonance. While we believe we captured the most accurate representation of instructors’ perceptions, there were interview questions to which instructors provided short or vague responses, making it difficult to fully capture their beliefs. Furthermore, it is possible that instructors’ responses to the interview questions do not actually reflect how they teach resonance in their classes. Each of these potential limitations should be taken into account when considering the applicability of these findings to another context.Conflicts of interest
We have no conflicts of interest to declare.Acknowledgements
We would like to thank all the instructors who participated in this study. We would also like to thank Nicole Baldwin, who assisted in data analysis, and the reviewers, who provided insightful feedback.References
- Atieh E. L., Mitchell-Jones J. K., Xue D. and Stains M., (2022), Variations in the Teaching of Resonance—An Exploration of Organic Chemistry Instructors’ Enacted Pedagogical Content Knowledge, in Graulich N. and Shultz G. (ed.), Student Reasoning in Organic Chemistry, The Royal Society of Chemistry.
- Betancourt-Pérez R., Olivera L. J. and Rodríguez J. E., (2010), Assessment of organic chemistry students’ knowledge of resonance-related structures, J. Chem. Educ., 87(5), 547–551.
- Brandfonbrener P. B., Watts F. M. and Shultz G. V., (2021). Organic chemistry students’ written descriptions and explanations of resonance and its influence on reactivity, J. Chem. Educ., 98, 3431–3441.
- Braun I., Langner A. and Graulich N., (2022), Let's draw molecules: students’ sequential drawing processes of resonance structures in organic chemistry, Front. Educ., 7, 1–24.
- Bussey T. J., Orgill M. and Crippen K. J., (2013), Variation theory: a theory of learning and a useful theoretical framework for chemical education research, Chem. Educ. Res. Pract., 14(1), 9–22.
- Carle M. S. and Flynn A. B., (2020), Essential learning outcomes for delocalization (resonance) concepts: How are they taught, practiced, and assessed in organic chemistry? Chem. Educ. Res. Pract., 21(2), 622–637.
- Cartrette D. P. and Mayo P. M., (2011), Students’ Understanding of Acids/Bases in Organic Chemistry Contexts, Chem. Educ. Res. Pract., 12(1), 29–39.
- Cooper M. M., Grove N. and Underwood S. M. (2010). Lost in Lewis structures: an investigation of student difficulties in developing representational competence, J. Chem. Educ., 87(8), 869–874.
- Creswell J. W., (2012), Educational Research: Planning, Conducting, and Evaluating Quantitative and Qualitative Research, Pearson.
- Delvigne F., (1989), A visual aid for teaching the resonance concept, J. Chem. Educ., 66(6), 461.
- Dood A. J. and Watts F. M., (2023), Mechanistic reasoning in organic chemistry: a scoping review of how students describe and explain mechanisms in the chemistry education research literature, J. Chem. Educ., 99(8), 2864–2876.
- Duis J. M., (2011), Organic chemistry educators‚ perspectives on fundamental concepts and misconceptions: an exploratory study, J. Chem. Educ., 88(3), 346–350.
- Finkenstaedt-Quinn S. A., Watts F. M., Petterson M. N., Archer S. R., Snyder-White E. P. and Shultz G. V., (2020), Exploring student thinking about addition reactions, J. Chem. Educ., 97(7), 1852–1862.
- Gero A., (1954), Predicting reaction of a resonance hybrid from minor canonical structures, J. Chem. Educ., 31(3), 136.
- Hattie J., (2008), Visible learning: A synthesis of over 800 metaanalyses relating to achievement, Routledge.
- Hayes J. R., (1996), A new framework for understanding cognition and affect in writing, in Levy C. M. and Randsdell S. (ed.), The science of writing: Theories, methods, individual differences, and applications. Mahwah, NJ: Lawrence Erbaum Associates.
- Hora M. T., (2014), Exploring faculty beliefs about student learning and their role in instructional decision-making, Rev. Higher Educ., 38(1), 37–70.
- Kerber R. C., (2006), If It's Resonance, What Is Resonating? J. Chem. Educ., 83(2), 223.
- Kim T. D., Wright L. K. and Miller K., (2019), An examination of students’ perceptions of the Kekulé resonance representation using a perceptual learning theory lens, Chem. Educ. Res. Pract., 20(4), 659–666.
- Klein D., (2012), Organic Chemistry, John Wiley & Sons, Inc.
- Kruse R. A. and Roehrig G. H., (2005), A comparison study: assessing teachers' conceptions with the chemistry concepts inventory, J. Chem. Educ., 82(8), 1246–1251.
- Lin S., (2007), Aromatic bagels: an edible resonance analogy, J. Chem. Educ., 84(5), 779.
- Marton F. and Booth, S., (1997), Learning and awareness. Mahwah, NJ: Lawrence Erlbaum Associates.
- Marton F. and Tsui A. B. M., (2004), Classroom discourse and the space of learning, Mahwah, NJ: Lawrence Erlbaum Associates.
- McClary L. M. and Talanquer V., (2011a), College chemistry students' mental models of acids and acid strength, J. Res. Sci. Teach., 48(4), 396–413.
- McClary L. M. and Talanquer V., (2011b), Heuristic reasoning in chemistry: Making decisions about acid strength, Int. J. Sci. Educ., 33(10), 1433–1454.
- Petterson M. N., Watts F. M., Snyder-White E. P., Archer S. R., Shultz G. V. and Finkenstaedt-Quinn S. A., (2020), Eliciting student thinking about acid–base reactions via app and paper–pencil based problem solving, Chem. Educ. Res. Pract., 21(1), 878–892.
- Richardson W. S., (1986), Teaching the concept of resonance with transparent overlays, J. Chem. Educ., 63(6), 518.
- Runesson U., (2005), Beyond discourse and interaction. Variation: a critical aspect for teaching and learning mathematics, Cambridge J. Educ., 35(1), 69–87.
- Shah L., Rodriguez C. A., Bartoli M. and Rushton G. T., (2018), Analysing the impact of a discussion-oriented curriculum on first-year general chemistry students' conceptions of relative acidity, Chem. Educ. Res. Pract., 19, 543–557.
- Silverstein T. P., (1999), The “big dog-puppy dog” analogy for resonance, J. Chem. Educ., 76(2), 206.
- Taber K. S., (2002), Compounding quanta: probing the frontiers of student understanding of molecular orbitals, Chem. Educ. Res. Pract., 3(2), 159–173.
- Tan K., (2009), Variation theory and the different ways of experiencing educational policy, Educ. Res. Policy Pract., 8(2), 95–109.
- Tetschner G. and Nedungadi S., (2023), Obtaining validity evidence during the design and development of a resonance concept inventory, J. Chem. Educ., 100 (1), 2795–3805.
- Van Etten S., Freebern G. and Pressley M. (1997). College students' beliefs about exam preparation, Contem. Educ. Psychol., 22(2), 192–212.
- softwareVERBI Software, (2021), MAXQDA 2022, computer program, Berlin: VERBI Software.
- Watts F. M., Zaimi I., Kranz, D., Graulich, N. and Shultz G. V., (2021), Investigating students’ reasoning over time for case comparisons of acyl transfer reaction mechanisms, Chem. Educ. Res. Pract., 22(2), 364–381.
- Xue D. and Stains M., (2020), Exploring students’ understanding of resonance and its relationship to instruction, J. Chem. Educ., 97(4), 894–902.
- Zamanzadeh V., Ghahramanian A., Rassouli M., Abbaszadeh A. and H. Alavi-Majd, (2015), Design and implementation content validity study: development of an instrument for measuring patient-centered communication, J. Caring Sci., 4(2), 165–178.
| This journal is © The Royal Society of Chemistry 2024 |