A meta-analysis of effectiveness of chemical bonding-based intervention studies in improving academic performance†
Muammer
Çalik
 *a,
Neslihan
Ültay
*a,
Neslihan
Ültay
 b,
Hasan
Bağ
b,
Hasan
Bağ
 c and
Alipaşa
Ayas
c and
Alipaşa
Ayas
 d
d
aDepartment of Elementary Teacher Education, Fatih Faculty of Education, Trabzon University, Trabzon, Türkiye. E-mail: muammer38@hotmail.com
bDepartment of Elementary Teacher Education, Faculty of Education, Giresun University, 28200 Giresun, Türkiye. E-mail: neslihanultay@gmail.com
cDepartment of Elementary Teacher Education, Recep Tayyip Erdoğan University, Rize, Türkiye. E-mail: hsnbag@gmail.com
dFaculty of Education, Bilkent University, Ankara, Türkiye. E-mail: apayas@bilkent.edu.tr
First published on 5th January 2024
Abstract
The purpose of this study is to meta-analytically evaluate research that used chemical bonding-based interventions to improve academic performance. Through meta-analysis, the present study used several keyword patterns (e.g., chemical bonding, experimental, chemistry education, science education) via relevant databases (e.g., ERIC, Springer Link, Taylor & Francis, Wiley Online Library Full Collection, and Scopus) to find chemical bonding-intervention studies. Thus, it included 50 chemical bonding-based intervention papers (15 dissertations, 32 articles, and 3 proceedings). The current meta-analysis found that the overall effect-size of chemical bonding-based intervention studies was 1.007, which shows a large effect. Findings regarding moderator analysis displayed non-significant differences between educational levels and a statistically significant difference between the intervention types. This meta-analysis reveals that the chemical bonding-based intervention studies are effective at improving the participants’ academic performance in terms of chemical bonding. Further, it denotes that when the abstract nature of chemical bonding is overlapped with the features of the intervention type, the interventions (e.g., cooperative learning and enriched learning environment with different methods) result in better academic performance. Since this study, like all meta-analyses, points out consistent and inconsistent findings among published research, further meta-analysis studies should be undertaken to resolve any contradictory findings.
Introduction
Chemical bonding is a concept that differentiates chemistry from other science disciplines (e.g., physics) (Constable and Housecroft, 2020). Thus, it plays a significant role across chemistry curricula at K-12 and university levels (Bergqvist et al., 2016; Hunter et al., 2022; van Dulmen et al., 2023). Students need to understand the concept to draw inferences about structure, function, relationships and properties of matter. In other words, chemical bonding comprehension gives students an opportunity to predict and explain physical and chemical properties of matter (Nahum et al., 2010). For this reason, it is viewed as one of the disciplinary core ideas or anchoring concepts in chemistry (Fensham, 1975; Nahum et al., 2010; Hunter et al., 2022). This means that students’ mastery of chemistry topics such as reactivity, spectroscopy, organic chemistry, and thermodynamics will be compromised unless theories of chemical bonding are first introduced and instructed (Taber and Coll, 2002; Tsaparlis et al., 2021).However, previous studies have reported that students have difficulties understanding the concept of chemical bonding or hold superficial understanding of this topic because of its abstract nature (Özmen, 2004; Ünal et al., 2006; Nahum et al., 2010; Hunter et al., 2022; van Dulmen et al., 2023). Moreover, the concept also includes other complex concepts such as covalent bonds, molecules, ions, giant lattices, and hydrogen bonds that necessitate using the interplay amongst macroscopic, sub-microscopic and symbolic aspects of chemistry/science (Ünal et al., 2006). Further, fully understanding these concepts requires students to be familiar with mathematical and physical concepts and laws that are associated with such key bonding concepts as orbital, electronegativity, electron repulsions, polarity, and Coulomb's law (Nahum et al., 2010). Given its importance in chemistry learning and teaching, the Chemical Bond Approach (CBA), which was released in the USA in the 1950s, has handled chemical bonds as a central topic in teaching chemistry.
The adverse effect(s) of singular and simplistic approaches on students’ conceptual understanding and alternative conceptions of chemical bonding makes teaching chemical bonding difficult (Nahum et al., 2007; van Dulmen et al., 2023). Thus, teachers need to challenge these adverse effects, difficulties and alternative conceptions to enrich their science teaching of chemical bonding and enhance student understanding of chemical bonding (Ünal et al., 2006). Therefore, students’ difficulties or alternative conceptions of chemical bonding have drawn science/chemistry educators’ and teachers’ attention to how to improve chemistry learning and overcome them.
Studies report that the traditional approach to teaching bonding is problematic and often result in misconceptions (Özmen, 2004; Ünal et al., 2006; Nahum et al., 2010; Hunter et al., 2022; van Dulmen et al., 2023). What is more, it directs students to memorize key phrases and facts instead of developing a deeper conceptual understanding of the topic (Nahum et al., 2010; Hunter et al., 2022). Further, it does not help students visualize the abstract nature of chemical bonding, link sub-microscopic, macroscopic, and symbolic aspects of chemistry with each other and meaningfully construct the interplay and interconnections between core concepts (e.g., the particulate nature of matter, atomic structure, and chemical bonding) (e.g., Coll and Treagust, 2002; Ünal et al., 2006; Hunter et al., 2022). Also, when teachers’ content knowledge and pedagogical content knowledge of chemical bonding are limited or superficial, they generally prefer the traditional approach instead of alternative pedagogical ones taking students’ alternative conceptions and difficulties into account and may cause the development of normative ideas about bonding and bonding models (Hunter et al., 2022). For example, when teachers oversimplify octet rule by using improper language that atoms “want” or “need” an octet, their students use similar statements to explain chemical bonding (Hunter et al., 2022). Therefore, chemistry educators have attempted to develop more effective, pedagogically and scientifically sound strategies to teach the concepts of chemical bonding (Teichert and Stacy, 2002; Nahum et al., 2010). Notably, intervention studies have also tested varied methods, strategies, models, and approaches to enable students to grasp a deep conceptual understanding of chemical bonding (Özmen, 2004; Ünal et al., 2006; Nahum et al., 2010; Hunter et al., 2022; van Dulmen et al., 2023). Examples of these interventions include computer-assisted instruction, concept maps, conceptual change, constructivist learning environments, cooperative learning, enriched learning environments with different methods, inquiry-based learning, multiple representations, context-based learning, and problem-based learning. These interventions point how pedagogical tools or preferences of chemical bonding depend on different contexts (e.g., philosophy or theoretical framework of national chemistry curriculum, students’ preparedness, and familiarity with the preferred intervention) (van Dulmen et al., 2023). To gain insights into these variations and implications for practice, a few researchers have systematically reviewed past studies to look for patterns and procedures. The next section provides information about these prior systematic reviews of chemical bonding studies.
Previous systematic reviews of chemical bonding
The related literature includes five systematic review studies that have synthesised and evaluated chemical bonding studies: Özmen (2004), Ünal et al. (2006), Nahum et al. (2010), Hunter et al. (2022) and van Dulmen et al. (2023). Of these review studies, Hunter et al. (2022) thematically reviewed 48 studies (which were published between 2006 and 2020) drawn from four major science education journals (Chemistry Education Research and Practice, International Journal of Science Education, Journal of Chemical Education, and Journal of Research in Science Teaching) indexed by the Education Resources Information Center (ERIC) database. They found that the studies under investigation generally tended to catalogue the students’ alternative conceptions of chemical bonding. Thus, they suggest that future studies should unveil students’ reasoning regarding advanced models of chemical bonding offered by chemistry curricula. Similarly, Nahum et al. (2010) reviewed the external and internal variables that might lead to misconceptions and the problematic issue of using limited teaching/learning models. After defining the nature of the ‘chemical bonding’ concept, they outlined research and developments in teaching about chemical bonding and classified relevant difficulties and alternative conceptions. They showed that there is a huge gap between what is known about learning the ‘chemical bonding’ concept and what has been implemented in educational systems. Apart from the traditional approach, they suggested new approaches to teaching chemical bonding, such as a bottom-up framework, visual models, and instructional techniques. Likewise, Özmen (2004), who handled chemical bonding studies ranging from 1987 to 2001, classified students’ alternative conceptions of chemical bonding via the categories (bond polarity, molecular shape, polarity of molecules, intermolecular forces, the octet rule, and lattices). He suggested that teachers should be informed about how to use conceptual change techniques to remedy students' alternative conceptions of chemical bonding. Also, he offered that textbooks and guide instructional materials should be well-devised to achieve better conceptual understanding of chemical bonding. In a similar vein, Ünal et al. (2006), who thematically analysed 15 chemical bonding studies from 1988 to 2003 in regard to several criteria (e.g., needs, aims, methods of exploring students' conceptions, general knowledge claims, students' alternative conceptions, and implications), found that previous research has mostly used interview-based methods and paper–pencil surveys focusing on students' statements of chemical bonding concepts. They also synthesised that students at different grades have alternative conceptions of chemical bonding that are resistant to change with traditional methods. They suggested that future research should focus on alternative teaching strategies to overcome students’ alternative conceptions rather than categorising or determining them. Finally, van Dulmen et al. (2023) systematically analysed 69 pedagogical content knowledge-related articles drawn from four databases (Web of Science, Scopus, ERIC, and PsycINFO). They reported that cooperative learning and group work positively affect the learning of chemical bonding vis-à-vis other teaching strategies. Further, they recommended a new framework to help teacher educators shape and prioritise chemistry teacher education coursework on how to teach bonding.The aforementioned systematic reviews provided a rich corpus of chemical bonding education that not only incorporates invaluable insights, arguments, and frameworks on how to teach chemical bonding but also illuminates future and further research. However, none of them has used a standard measurement value (e.g., effect-size or Hedges’ g or Cohen d) to explore the effectiveness of chemical bonding-based intervention studies and compare the studies with each other. For example, van Dulmen et al. (2023)'s emphasis on the positive effects of cooperative learning and group work on students’ conceptual understanding/achievement of chemical bonding calls for a meta-analysis study that examines the extent to which these intervention types improve students’ academic performance. Therefore, the current study aims to address this shortcoming with a supplemental and enriched approach to conducting a meta-analysis.
Rationale and significance of the study
Chemistry/science educators have mainly inquired about two principal questions of chemical bonding: what makes it difficult? What pedagogical approaches, methods, and strategies facilitate student learning? (Özmen, 2004; Ünal et al., 2006; Nahum et al., 2010; Hunter et al., 2022; van Dulmen et al., 2023). To respond the second question, they have also implemented different pedagogical approaches, methods, and strategies (e.g., cooperative learning, group work, conceptual change, computer-assisted instruction) (Özmen, 2004; Ünal et al., 2006; Nahum et al., 2010; Hunter et al., 2022; van Dulmen et al., 2023). Of course, a variety of intervention types yields the question, “Which one has the best learning outcomes or results?” Indeed, such a question calls for the current meta-analysis study, which employed a standard measurement criterion (e.g., Hedges’ g) to evaluate and compare them with one another.As chemical bonding is a milestone for learning advanced and related science/chemistry concepts, it is generally taught at the high school and university levels (Fensham, 1975; Nahum et al., 2010; Hunter et al., 2022). Meanwhile, some science curricula (e.g., the Turkish science curriculum) introduce fundamental concepts of chemical bonding at middle school (Ministry of National Education, 2018). As a matter of fact, previous studies have studied with different samples from middle school to university. This also appears the question “Which educational level is better for teaching it?”, which directs the current meta-analysis to handle educational level as a moderator variable.
Luckily, the related literature has incorporated five systematic reviews of chemical bonding (Özmen, 2004; Ünal et al., 2006; Nahum et al., 2010; Hunter et al., 2022; van Dulmen et al., 2023). However, none of them has recruited a standard measurement value (e.g., Hedges’ g) along with a meta-analysis. For this reason, the current study fills in an important gap in the related literature by focusing on meta-analysis, different databases, updated year coverage, and various moderator variables. Thus, it provides invaluable results for chemistry educators, chemistry teachers, curriculum developers, and decision makers on the practical significance of chemical bonding-based intervention studies via evidence with a standard measurement value (e.g., Hedges’ g). For example, it would illuminate the extreme values in chemical bonding-based interventions that can be deeply investigated by future research. Also, given the consistency and efficiency of the chemical bonding-based interventions, it would inform all stakeholders about how to effectively use time, effort, and budget for chemistry education and learning chemistry. For instance, researchers and teachers may seek alternative pedagogical approaches to better teach ‘chemical bonding’ in place of the tested ones. Similarly, chemistry/science educators, teachers, and curriculum developers may have an opportunity to easily access a pedagogical repertoire of chemical bonding and decide which of the intervention types matches better with its nature. Handling the ‘educational level’ as a moderator variable may also give some insights about relative efficacy of interventions in different educational levels (middle school, high school and university). To sum up, this study may shed more light on future decisions and discussions about the effectiveness of chemical bonding-based interventions and the development of guidelines and frameworks for teaching chemical bonding.
The purpose and research questions
The purpose of this study is to meta-analytically evaluate research that used chemical bonding-based interventions to improve academic performance. The following questions guide the present study.● How effective are chemical bonding-based intervention studies on academic performance?
● How do moderator variables (namely, educational level and type of intervention) affect the participants’ academic performance?
Methodology
To address the purpose of the study, the researchers identified and combined research relevant to chemical bonding-based interventions; the findings were then statistically compared via a meta-analysis design (e.g., Üstün and Eryılmaz, 2014; Karadag, 2020). Through a systematic and organized process, the researchers analysed the effects or relationships between dependent (e.g., academic performance) and independent (e.g., chemical bonding-based interventions) variables (Lipsey and Wilson, 2001; Üstün and Eryılmaz, 2014; Sezen-Vekli and Çalik, 2023). Specifically, the current study calculated the effect-sizes of the experimental papers and assessed whether chemical bonding-based interventions are practically significant (e.g., Borenstein et al., 2009; Ellis, 2010; Üstün and Eryılmaz, 2014).Selection criteria
To gain access to chemical bonding-based intervention studies, the present research used many well-known databases and search engines: Academic Search Complete, ERIC, Open Dissertations, Education Source, ULAKBIM, Springer Link, Taylor & Francis, Wiley Online Library Full Collection, Science Direct, ProQuest Dissertations and Theses Global, Google Scholar, Scopus, and the Council of Higher Education Thesis Center in Türkiye. The authors used the following keyword patterns within each of the aforementioned databases and search engines:● Pattern 1: chemical bonding, experimental and chemistry education or science education
● Pattern 2: chemical bonding, treatment and chemistry education or science education
● Pattern 3: chemical bonding, intervention and chemistry education or science education
● Pattern 4: intra-molecular forces, experimental and chemistry education or science education
● Pattern 5: intra-molecular forces, treatment and chemistry education or science education
● Pattern 6: intra-molecular forces, intervention and chemistry education or science education
● Pattern 7: intermolecular forces, experimental and chemistry education or science education
● Pattern 8: intermolecular forces, treatment and chemistry education or science education
● Pattern 9: intermolecular forces, intervention and chemistry education or science education
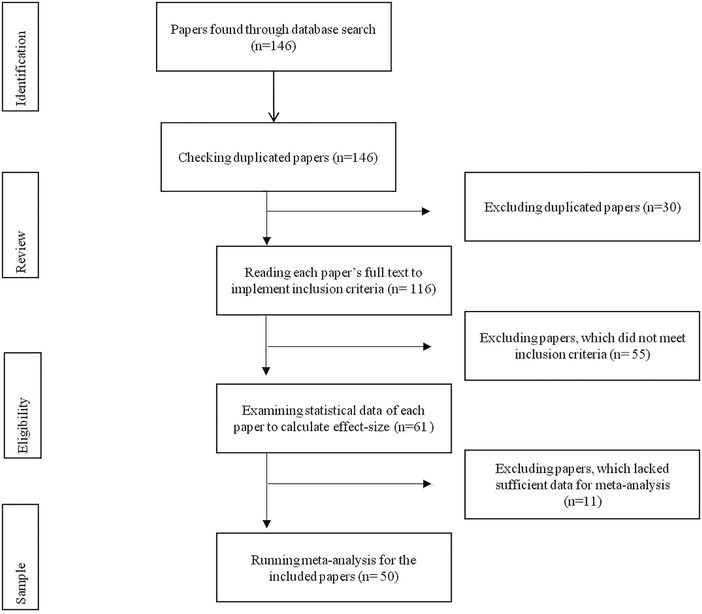
Also, they manually searched the related journals and dissertations by examining the references of recently published papers. As a result of these searches, the authors found 146 potential studies for the meta-analysis. The next step was to critique the papers to ensure they complied with the purpose of the study (see Fig. 1 for a flow chart of this selection process).
The critique of the original set of the papers began with a review to organize the selection process and avoid duplicates. For this review, the authors labelled each study as an in-text citation format. This labelling also helped to determine some papers that were indexed in more than one database or some dissertations that were published in journals as research papers. Therefore, the authors removed 30 papers because of duplications in databases, and research papers from the dissertations.
After the duplicates were removed, the authors assessed eligibility of the papers by carefully reading the full-texts of the papers and applying the inclusion criteria (chemical bonding-based intervention, learning outcomes—conceptual understanding and achievement, quasi- and true-experimental designs, and publication language—Turkish and English). Thereby, the authors excluded 55 papers with pre-experimental design (e.g., Wang, 2007; Zohar; 2020; Lutfi et al., 2021), different dependent variables (e.g., scientific attitude and meanings/definitions of modelling and models) (for example, Barnea and Dori, 1999; Astuti et al., 2019) and different languages (e.g., Heprew) (for example, Frailich, 2007; Fridman, 2020).
Eligibility was also assessed by examining the statistical data of each paper to calculate any effect-size(s). This examination eliminated 11 papers, that lacked sufficient data (e.g., mean, standard deviation, sample size, paired p-value, paired t-value) for meta-analysis. That is, some of the papers only provided either the findings of non-parametric analysis (e.g., mean rank, sum of ranks, and U-value) or qualitative data from pre- and post-interview or limited descriptive statistical results (e.g., frequency and percentage) from open-ended questions. For example, given unclear sample sizes of the experimental and control groups, the authors eliminated Salyani et al. (2020)'s research from the corpus studies.
Study sample
The outcome of the selection process resulted in 50 papers that concentrated on the effectiveness of chemical bonding-based intervention studies in improving academic performance. These papers included 15 dissertations, 32 articles, and three conference proceedings (see Table S1 for characteristics of the studies included in the meta-analysis, ESI†).Nine of the papers contained:
● multiple experimental groups with different cohorts (Ekinci, 2010; Singh and Moono, 2015; Sunyono and Meristin, 2018);
● different modules (subtopics) for home and jigsaw groups (Doymus, 2008);
● different instructional designs (Ulusoy, 2011; Karacop and Doymus, 2013; Korkman, 2018);
● different instructional stages (beginning and end of the course) (Sevim, 2007);
● different parts of the assessment instrument (Tsaparlis et al., 2018).
Since six studies (Sevim, 2007; Doymus, 2008; Ekinci, 2010; Singh and Moono, 2015; Sunyono and Meristin, 2018; Tsaparlis et al., 2018) carried out the same teaching design for their experimental groups, the authors calculated combined effect-sizes for them by using ‘the study as the unit of analysis’ and ‘subgroups within the study’ options via Comprehensive Meta-Analysis (CMA) statistics software. However, because three of them (Ulusoy, 2011; Karacop and Doymus, 2013; Korkman, 2018) carried out different intervention types for their experimental groups, they were viewed as individual studies. For example, the authors handled Karacop and Doymus (2013)'s study as two individual studies in that it included cooperative learning and computer-assisted instruction for their experimental groups as types of intervention. Overall, the current meta-analysis attempted to minimally decrease the number of individual studies to deal with the inflating impacts of multiple experimental studies on the overall effect-size.
Coding procedure
To extract data from the papers, the authors applied a coding form with the following parameters: reference of the paper, sample size, grade, dependent and independent variables, quantitative values (mean scores, standard deviation, t, and p), educational level and type of intervention (Karadag, 2020). While coding educational level and type of intervention for the papers, the criteria were adopted from Çalik et al. (2023). The authors separately coded the papers and their inter-rater consistency value was high: 93%. Any remaining discrepancies were negotiated and resolved to come to consensus.Calculating effect-sizes
To elicit the strength, direction and degree of the relationship between two variables (Borenstein et al. 2009), the authors chose Hedges’ g. Compared Cohen's d, Hedges’ g is more accurate and less biased especially when dealing with relatively small sample sizes (Borenstein et al., 2009; Kansızoğlu, 2017; Güler et al., 2022). When calculating Hedges’ g for the present meta-analysis, the authors imported all statistical data from the Excel sheet into Comprehensive Meta-Analysis (CMA) statistics software. Moreover, the authors copied all calculated effect-sizes to SPSS 21TM to arrange and represent them. This process generated stem and leaf plot to simply show how frequently various effect-size values occur. Given the decision to report combined effect-sizes and two individual effect-sizes from the aforementioned nine papers, the current meta-analysis consisted of 53 studies from 50 unique sources. The corpus of papers provided different datasets to calculate Hedges’ g value:● 43 individual studies (from 41 papers, some of which included several experimental groups and were imported as individual studies) contained mean scores, standard deviations, and sample sizes for the experimental and control groups.
● Four studies (four papers) included the mean scores and sample sizes of the experimental and control groups and independent groups p-values.
● Three studies (three papers) supplied mean scores and sample sizes for the experimental and control groups and independent groups t-values.
● Three studies (two papers, one of which incorporated several experimental groups and was imported as individual study) reported differences in means, sample sizes for the experimental and control groups, and independent group p-values.
Meta-analysis model
Because the present meta-analysis retrieved chemical bonding-based intervention studies from the related literature and intended to generalize its findings, it met the assumptions and nature of the random-effects model (Borenstein, 2019). Further, the values of heterogeneity through the Q-value and I2 test supported this preference to calculate the effect-sizes via a Comprehensive Meta-Analysis (CMA V2) software (e.g., Hedges and Vevea, 1998; Borenstein et al. 2009; Üstün and Eryılmaz, 2014; Karadag, 2020). As seen from Table 1, the Q-value was found to be 606.536, which is higher than the critical value of 69.832 (with 52 degree of freedom for 95% confidence interval). Further, the p-value was statistically less than 0.05. Further, the I2 value was calculated to be 91.427, which means high heterogeneity (Higgins et al., 2003; Borenstein et al., 2009).| Model | Number of studies | Effect-size and 95% confidence interval | Test of null (2-tail) | Heterogeneity | Tau-squared | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Point estimate | Standard error | Variance | Lower limit | Upper limit | Z-Value | P-Value | Q-Value | df (Q) | p-Value | I-Squared | Tau-squared | Standard error | Variance | Tau | ||
| Fixed | 53 | 0.727 | 0.026 | 0.001 | 0.677 | 0.777 | 28.466 | 0.000 | 606.536 | 52 | 0.000 | 91.427 | 0.409 | 0.167 | 0.028 | 0.639 |
| Random | 53 | 1.007 | 0.094 | 0.009 | 0.822 | 1.192 | 10.660 | 0.000 | ||||||||
The second research question called for two moderator variables: educational level and type of intervention. The former includes the following grades: K-8 (kindergarten to grade 8), high school (grades 9–12) and university (undergraduate or bachelor). The studies mentioned ‘types of intervention’ such as computer-assisted instruction, concept maps, conceptual change, constructivist learning environments, cooperative learning, and enriched learning environments with different methods, inquiry-based learning, and multiple representation (see Appendix).
Publication bias: analysis and results
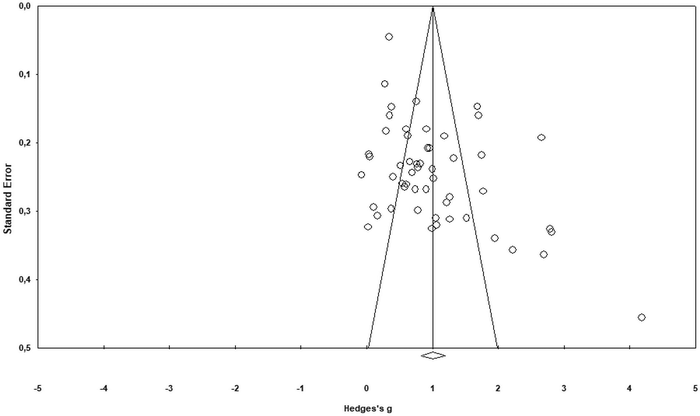
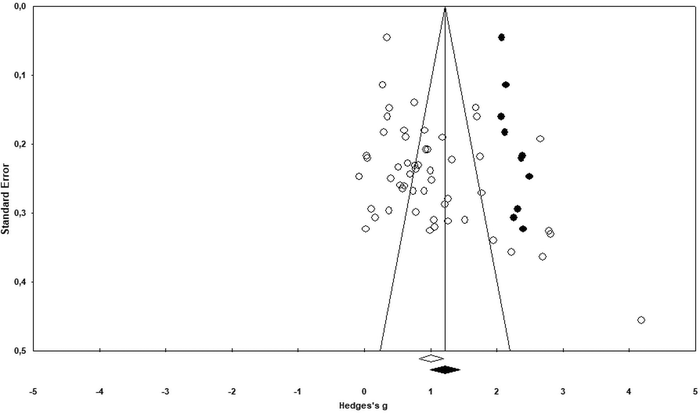
This section describes steps analysing publication bias for the corpus of the collected data. First, a visual inspection of asymmetry was conducted using a funnel plot to make sure small studies with small sample-sizes were not missed (Vevea et al., 2019; Arztmann et al., 2023). Next, the findings were checked using the trim-and-fill method, the Classic fail-safe N and Orwin's fail-safe N (e.g., Üstün and Eryılmaz, 2014). These steps reduced any idiosyncratic contribution to the selection process and helped identify the number of missing studies needed to bring Hedges’ g to below 0.1 or p value to >alpha. The next section provides specific results of the publication bias analysis.The results of the funnel plot (see Fig. 2) display an asymmetrical structure between standard error and effect-size (e.g., Karadag, 2020). This means that the papers with a larger standard error (smaller sample size) might have had larger effects (Rahman and Lewis, 2020). This could be considered as evidence of publication bias. Likewise, the findings of the Duval and Tweedie's trim and fill test (see Table 2) pointed to a difference between the observed (1.007 at 95% confidence interval of 0.822–1.192) and adjusted values (1.219 at 95% confidence interval of 0.997–1.441) that supports the evidence of publication bias in the funnel plot. As seen from Fig. 3 (black dots in the funnel plot), ten studies (as a counterpart data) are needed to offset this asymmetry or cope with the effect of observed asymmetry on the overall effect-size.
| Studies trimmed | Point estimate | Confidence interval (CI) | Q value | ||
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| Observed values | 1.007 | 0.822 | 1.192 | 606.536 | |
| Adjusted values | 10 | 1.219 | 0.997 | 1.441 | 1575.476 |
Fortunately, the difference between the observed and adjusted estimates was 17.39%, which falls into the negligible range (20%) reported by Kepes et al. (2012), Vevea et al. (2019) and Chang et al. (2022). This means that the publication bias could be tolerable for the corpus of data even though some evidence of publication bias was found in the relevant data. However, prior to making a decision about it, the Classic fail-safe N (N = 3013 for p-value of zero and alpha of 0.05) (see Table 3) and Orwin's fail-safe N (N = 333 for the trivial effect size of 0.10 and the mean effect size in missing studies of zero) values were also calculated. The Classic fail-safe N value means that 2960 additional papers with non-significant findings would be necessary to nullify the effect of chemical bonding-based intervention studies on academic performance. Similarly, Orwin's fail-safe N value (see Table 4) indicates that 280 additional papers with an effect-size of zero would be necessary to make the mean effect of this meta-analysis as trivial (Üstün and Eryılmaz, 2014). Further, the failsafe formula [N/(5k + 10)] (k means the total number of the studies in the meta-analysis) appeared to have a high ratio (10.956) for the current meta-analysis, which is higher than the cut-off point (1.00) offered by Mullen et al. (2001). This ratio also complies with the other results of the publication bias analysis. The foregoing values affirmed that the current meta-analysis has the power to sufficiently tolerate future null results. Therefore, its data selection process was robust and not influenced by publication bias.
| Z-Value for observed studies | 30.773 |
| P-Value for observed studies | 0.000 |
| Alpha | 0.050 |
| Tails | 2.000 |
| Z for alpha | 1.960 |
| Number of observed studies | 53 |
| Number of missing studies that would bring p value to >alpha | 3013 |
| Hedges’ g in observed studies | 0.727 |
| Criterion for a ‘trivial’ Hedges’ g | 0.100 |
| Mean Hedges’ g in missing studies | 0.000 |
| Number missing studies needed to bring Hedges’ g under 0.1 | 333 |
Findings
This section presents the findings according to the research questions and interprets the effect-size values with regard to the criteria suggested by Güler et al. (2022): 0.14 and below (negligible); 0.15–0.39 (low); 0.40–0.74 (medium); 0.75–1.09 (large); 1.10–1.44 (very large); and 1.45 and above (perfectly huge).The overall impact of chemical bonding-based intervention studies on academic performance
The findings of stem and leaf plot (Table 5) showed that 21 of the effect-size values were higher than 1.00, while 19 of them were between 0.5 and 1.00. Also, 13 of them were lower than 0.5. In a similar vein, the findings of the essential values (Hedges g, standard error, and p) (see Table 6 and Fig. S1 at ESI,† for the forest plot) indicated that 44 of the effect-sizes were significant (p < 0.05), whereas nine of them were non-significant (p > 0.05). According to the effect-size classification offered by Güler et al. (2022), the negligible effect included five effect-sizes, whilst the low one incorporated seven ones. 10 of them were categorised beneath the medium effect, 14 of them were labelled under the large one. Lastly, the frequencies of the effect-sizes classified under the very large and perfectly huge effects were 5 and 12 respectively. Besides, overall effect-size for the random-effects model was 1.007, which shows a large effect for the effectiveness of chemical bonding-based intervention studies in improving academic performance. Because Munawarah et al. (2020)'s effect-size is considerably larger than any of the others, sensitivity analysis was also conducted to examine its impact on overall effect-size as a potential outlier. When Munawarah et al. (2020)'s study was excluded from the meta-analysis, overall effect-size was calculated to be 0.959. That is, it decreased from 1.007 to 0.959. This difference (0.048) between the overall effect-sizes with and without Munawarah et al. (2020) means that it has minimal impact on the publication bias analysis and would not alter the interpretation of the results.| Frequency | Stem | Leaf |
|---|---|---|
| 1 | −0 | 0 |
| 12 | 0 | 000112233334 |
| 19 | 0 | 5556666677777899999 |
| 9 | 1 | 000012223 |
| 6 | 1 | 567779 |
| 1 | 2 | 2 |
| 4 | 2 | 6678 |
| 1 | 4 | 1 |
| Studies | Hedges' g | Standard error | P-Value | |
|---|---|---|---|---|
| Acar and Tarhan (2008) | 2.698 | 0.364 | 0.000 | |
| Adane (2020) | 1.324 | 0.223 | 0.000 | |
| Adherr et al. (2019) | 1.051 | 0.310 | 0.001 | |
| Anekwe and Opara (2021) | 2.663 | 0.193 | 0.000 | |
| Atasoy et al. (2003) | 0.049 | 0.221 | 0.826 | |
| Bayrak (2005) | 0.606 | 0.261 | 0.020 | |
| Chan (2016) | 0.021 | 0.323 | 0.948 | |
| da Silva et al. (2020) | 0.031 | 0.216 | 0.885 | |
| Doymus (2008) | 0.910 | 0.180 | 0.000 | |
| Ekinci (2010) | 1.180 | 0.190 | 0.000 | |
| Eymur and Geban (2017) | 2.817 | 0.331 | 0.000 | |
| Frailich et al. (2009) | 0.758 | 0.139 | 0.000 | |
| Genel (2008) | 1.686 | 0.147 | 0.000 | |
| Ginting and Juniar (2022) | 0.544 | 0.260 | 0.036 | |
| Gongden et al. (2020) | 2.787 | 0.326 | 0.000 | |
| Ikenna (2014) | 0.658 | 0.227 | 0.004 | |
| İnal (2013) | 0.626 | 0.190 | 0.001 | |
| Iryani et al. (2021) | 0.693 | 0.244 | 0.004 | |
| Karacop and Doymus (2013) | Subgroup 1 | 0.762 | 0.231 | 0.001 |
| Subgroup 2 | 0.779 | 0.236 | 0.001 | |
| Kılıç (2007) | 1.266 | 0.312 | 0.000 | |
| Kırılmazkaya et al. (2014) | 0.740 | 0.268 | 0.006 | |
| Korkman (2018) | Subgroup 1 | −0.077 | 0.247 | 0.756 |
| Subgroup 2 | 0.600 | 0.180 | 0.001 | |
| Kuit and Osman (2021) | 0.515 | 0.233 | 0.027 | |
| Mercy et al. (2019) | 0.296 | 0.182 | 0.105 | |
| Mondal (2012) | 0.817 | 0.231 | 0.000 | |
| Munawarah et al. (2020) | 4.189 | 0.456 | 0.000 | |
| Okorie (2015) | 0.277 | 0.114 | 0.015 | |
| Özmen (2008) | 2.225 | 0.356 | 0.000 | |
| Özmen et al. (2009) | 0.578 | 0.265 | 0.029 | |
| Pabuçcu and Geban (2006) | 0.990 | 0.325 | 0.002 | |
| Pabuçcu and Geban (2012) | 0.162 | 0.307 | 0.597 | |
| Pamuk (2018) | 1.261 | 0.280 | 0.000 | |
| Sarı (2013) | 1.774 | 0.271 | 0.000 | |
| Sentongo et al. (2013) | 1.751 | 0.218 | 0.000 | |
| Sevim (2007) | 1.700 | 0.160 | 0.000 | |
| Sharma (2015) | 0.379 | 0.147 | 0.010 | |
| Side et al. (2020) | 0.400 | 0.249 | 0.108 | |
| Singh and Moono (2015) | 1.520 | 0.310 | 0.000 | |
| Sunyono and Meristin (2018) | 0.350 | 0.160 | 0.029 | |
| Suyanti and Sormin (2016) | 0.905 | 0.268 | 0.001 | |
| Şeker (2012) | 1.947 | 0.340 | 0.000 | |
| Tarhan et al. (2008) | 1.000 | 0.238 | 0.000 | |
| Teichert and Stacy (2002) | 0.103 | 0.295 | 0.726 | |
| Tsaparlis et al. (2018) | 0.346 | 0.045 | 0.000 | |
| Ulusoy (2011) | Subgroup 1 | 0.929 | 0.208 | 0.000 |
| Subgroup 2 | 0.960 | 0.208 | 0.000 | |
| Uzuntiryaki (2003) | 1.061 | 0.321 | 0.001 | |
| Ültay (2015) | 0.374 | 0.296 | 0.207 | |
| Widarti et al. (2019) | 1.220 | 0.288 | 0.000 | |
| Yıldırım et al. (2018) | 0.779 | 0.298 | 0.009 | |
| Zorluoğlu and Sözbilir (2016) | 1.020 | 0.252 | 0.000 | |
| Random | 1.007 | 0.094 | 0.000 |
Moderator variable: educational level
The findings of moderator analysis (Table 7) displayed that the effect-size difference between the educational levels was non-significant (Q-value = 1.996; df = 2; p < 0.05). This means that educational level did not positively influence the participants’ academic performance through chemical bonding-based intervention studies. The mean effect-sizes of the educational levels ranged from 0.783 to 1.084 and showed the n-shaped developmental curve regarding academic performance. Based on the classification criteria offered by Güler et al. (2022), all of them fell into the range of the large effect.| Educational level | k | Point estimate | Standard error | Confidence interval (95%) | Z-Value | p-Value | Q-Value | df (Q) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||||||
| K-8 | 9 | 0.783 | 0.175 | 0.440 | 1.126 | 4.475 | 0.000 | |||
| High school | 34 | 1.084 | 0.125 | 0.839 | 1.329 | 8.677 | 0.000 | |||
| University | 10 | 0.945 | 0.195 | 0.562 | 1.328 | 4.833 | 0.000 | |||
| Total between | 1.996 | 2 | 0.369 | |||||||
Moderator variable: type of intervention
Table 8 points out a statistically significant difference between the intervention types (Q-value = 23.141; df = 7; p < 0.05). This means that the type of intervention positively affected the participants’ academic performance in accordance with chemical bonding-based intervention studies. The mean effect-size for cooperative learning had the highest value (Hedges’ g = 1.766) among the intervention types, while that for constructivist learning environments possessed the lowest value (Hedges’ g = 0.415). In regard to the classification criteria suggested by Güler et al. (2022), the perfectly huge effect contained cooperative learning and an enriched learning environment with different methods, while the very large one involved computer-assisted instruction, concept maps, and conceptual change. The mean effect-size value for multiple representation fell into the range of the large effect; those for constructivist learning environments and inquiry-based learning were classified under the medium one.| Type of intervention | k | Point estimate | Standard error | Confidence interval (95%) | Z-Value | p-Value | Q-Value | df (Q) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||||||
| Computer-assisted instruction | 14 | 1.165 | 0.222 | 0.730 | 1.600 | 5.249 | 0.000 | |||
| Concept map | 2 | 1.391 | 0.181 | 1.036 | 1.746 | 7.689 | 0.000 | |||
| Conceptual change | 7 | 1.175 | 0.188 | 0.808 | 1.543 | 6.265 | 0.000 | |||
| Constructivist learning environment | 3 | 0.415 | 0.251 | −0.077 | 0.908 | 1.652 | 0.099 | |||
| Cooperative learning | 4 | 1.766 | 0.510 | 0.766 | 2.767 | 3.461 | 0.001 | |||
| Enriched learning environment with different methods | 6 | 1.472 | 0.417 | 0.653 | 2.290 | 3.525 | 0.000 | |||
| Inquiry-based learning | 5 | 0.541 | 0.164 | 0.219 | 0.863 | 3.292 | 0.001 | |||
| Multiple representation | 2 | 0.752 | 0.434 | −0.098 | 1.603 | 1.734 | 0.083 | |||
| Total between | 23.141 | 7 | 0.002 | |||||||
Discussion
The overall effect-size value of the chemical bonding-based intervention studies fell into the large effect category (see Table 6 and Fig. S1, ESI†). This confirms that the interventions are effective in improving chemical bonding-based academic performance. In fact, this expected improvement indicates that, as experimental studies, they have accomplished their targeted aims, which strive to help students visualise, conceptualise, and explain chemical bonding.It is important to point out that some of the intervention studies outperformed others. For example, Munawarah et al. (2020), who implemented the enriched learning environment with different methods (e.g., problem-based learning and worksheets) for teaching chemical bonding, produced the highest effect-size (perfectly huge). Their study was crucial for illustrating how the scope, content, and nature of the teaching intervention are important in addressing the purpose of one's research (Pacaci et al., 2023). Moreover, cultural contexts can affect how the interventions fit with the learning goals of the national science/chemistry curriculum or educational priorities. To emphasize this finding, the studies conducted in the following countries, which have high-stakes testing or performance exams in science/chemistry education, had effect-sizes that were within the perfectly huge range: Türkiye (Sevim, 2007; Acar and Tarhan, 2008; Genel, 2008; Özmen, 2008; Şeker, 2012; Sarı, 2013; Eymur and Geban, 2017), Nigeria (Anekwe and Opara, 2021; Gongden et al., 2020), Indonesia (Munawarah et al., 2020), Uganda (Sentongo et al., 2013), and Zambia (Singh and Moono, 2015).
The effect of educational level on academic performance
Table 7 shows that educational level was not a significant moderating variable affecting chemical bonding-based intervention studies on academic performance. The lack of statistical significance between the groups may come from the number of studies. Otherwise, this means that all educational levels can have similar effects on improving chemical bonding-based academic performance. As a matter of fact, all of their mean effect-sizes were classified under the large effect category. These findings suggest that the interventions would be productive at all educational levels.Nonetheless, it is important to note that high school (k = 34) had the highest mean effect-size while K-8 (k = 9) was the lowest. These values align with the effect-sizes of conceptual change strategies reported by Pacaci et al. (2023). Also, the mean effect-size of the studies with high school is very close to the value (Cohen d = 1.06) found by Ahmad et al. (2023). This may result from the nature of the course involving chemical bonding topic. For instance, high school and university include separate chemistry courses, whereas K-8 contains an integrated science course (e.g., biology, chemistry, physics, earth science, and astronomy). Another consideration is that as chemistry concepts become more advanced, more time is needed to study them. Consequently, the duration of the time spent studying chemistry is longer for students in higher grades. It follows that the intervention studies conducted within K-8 took less time than those with older students. For example, the implementation duration of the studies with K-8 (k = 9) generally took 2–3 weeks (e.g., Bayrak, 2005; Ültay, 2015; Pamuk, 2018; Yıldırım et al., 2018), while that for high school (k = 34) and university (k = 10) mostly lasted 4–8 weeks (e.g., Pabuçcu and Geban, 2006; Sevim, 2007; Karacop and Doymus, 2013; Okorie, 2015; Chan, 2016; Adane, 2020).
Although the findings indicate that differences in mean effect-size among educational levels were non-significant, it is worthwhile explaining the n-shaped developmental curve regarding academic performance that was found in the meta-analysis. This curve means that there were notable effects of the interventions at the educational levels, but the effects were more muted in K-8 (k = 9). One reason for this pattern may be that chemistry, as part of an integrated science course, is new for younger learners, so its logical changes would be dramatic. For older students, the pattern may be explained by the fact that their chemistry studies and academic performance are more advanced as a result of epistemological and ontological growth. In other words, the student performance outcomes of the studies’ findings may come from the type of chemical bonding-based assessment implemented at each level (Langbeheim et al., 2023; Pacaci et al., 2023). For example, although multiple choice questions were very common at all educational levels (e.g., Bayrak, 2005; Karacop and Doymus, 2013; Korkman, 2018; Adherr et al., 2019; Ginting and Juniar, 2022), the studies conducted at the university level had the students respond to open-ended questions or two-tier diagnostic questions where they expressed their responses in writing and diagrammed chemical formulas (e.g., Teichert and Stacy, 2002; Karacop and Doymus, 2013; Sari, 2013). Therefore, the academic performance of university students (k = 10) may have been affected by their ability to state, draw, and describe their conceptual understanding of chemical bonding.
The effect of intervention type on academic performance
The meta-analysis revealed that the type of intervention has a statistically significant, positive moderating effect on participants’ academic performance. The following discussion identifies the types of interventions and describes key aspects of the analysed studies. It is important to note that the outcomes of the studies may be affected by which chemical bonding concept students were learning. In other words, some interventions may be more suitable for some chemical bonding topics than others. Nonetheless, in general, the analysis indicated that there are interventions that show strong effects across the studies.The mean effect-size for cooperative learning (k = 4) had the highest value (Hedges’ g = 1.766) amongst the intervention types. This may result from matching the nature of the chemical bonding with the common features of cooperative learning, which requires students to work in groups on a common task. Therefore, aspects of cooperative learning (e.g., positive interdependence, accountability, promotive interactions, teaching interpersonal skills, and group processing) not only encourage students to have intellectual conversations about problem-solving skills and creative thinking, but also facilitate their own learning (e.g., achievement and conceptual understanding) through group interactions (Johnson and Johnson, 1987; Johnson et al., 1998; Rahman and Lewis, 2020; Akkuş and Doymuş, 2022). Furthermore, a socially situated learning environment may have contributed to participants’ meaningful learning of the ‘chemical bonding’ topic (McManus and Gettinger, 1996). Phrased differently, a well-designed cooperative learning, seems to have improved participants’ academic performance (Acar and Tarhan, 2008; Eymur and Geban, 2017) and resulted in a perfectly huge effect.
Another intervention that had a strong effect-size (classified under the perfectly huge effect), was an enriched learning environment with different methods (k = 6). This outcome may come from the power of different learning methods or strategies (e.g., computer-assisted presentation, three-dimensional materials, concept maps, concept networks, evidence-based learning, problem-based learning, worksheets, conceptual change texts, animations, and analogies) that meet various learning styles and pose students’ capacities for learning to increase the efficiency of any intervention (Genel, 2008; Munawarah et al., 2020). Moreover, the studies that merged methods within an enriched learning environment (k = 6) resulted in better learning outcomes than when methods were used in isolation (e.g., Çalik et al., 2023). Therefore, using a variety of teaching methods provides students with a broader sense of learning chemical bonding and increases the probability of them achieving the course objectives (e.g., Zhang, 2014; Çalik et al., 2015).
The mean effect-sizes of intervention studies that used computer-assisted instruction (k = 14), concept map (k = 2), and conceptual change (k = 7) had a very large effect (see Table 8). This effect may result from the nature of these intervention types. For example, computer-assisted instruction models scientific phenomena at the sub-microscopic level and includes enriched technological tools/components (e.g., audio, sound, visualization, interaction, feedback, and hypertext) (Bayraktar, 2001). This modelling may have enhanced students’ capacities for learning and made unfamiliar and abstract concepts (such as chemical bonding) more concrete and familiar (Özmen, 2008; Sentongo et al., 2013; Pamuk, 2018; Gongden et al., 2020; Anekwe and Opara, 2021). Further, computer-assisted instruction may have afforded students the opportunity to bridge their own macroscopic observations to the sub-microscopic level (Barak and Dori, 2005; Frailich et al., 2009).
Concept mapping (k = 2) may provide students with a different way to envision key chemical bonding concepts, using words or short phrases to link and organize the concepts (Novak, 1990, 1998; Singh and Moono, 2015; Adane, 2020). The pedagogical/meta-cognitive engagement germane to concept mapping seems to solidify and facilitate students’ conceptual understanding of the ‘chemical bonding’ topic (Chevron, 2014; Adane, 2020).
Teaching strategies that promote conceptual change (k = 7) may challenge students to consider alternative conceptions and encourage them to replace insufficient concepts with more fruitful and scientifically accepted ones (Hewson and Hewson, 1983; Çalik et al., 2009; Hafizhah Putri et al., 2022). Thus, the use of cognitive and knowledge-based interpretations/processes of conceptual change seems to have supported students’ meaningful learning and sound understanding of chemical bonding concepts (Guzzetti et al., 1993; Çalik et al., 2023). Also, this may result from matching the nature of students’ alternative conceptions of chemical bonding with possible ways and/or strategies of conceptual change (e.g., cognitive conflict, cognitive bridging, and ontological category shift) (Pacaci et al., 2023). Moreover, the mean effect-size (Hedges’ g = 1.175) of conceptual change studies on chemical bonding advocates the findings of Pacaci et al. (2023), who meta-analytically evaluated 218 conceptual change studies in science education (Hedges’ g = 1.10).
The intervention studies that used multiple representations (k = 2) had a mean effect-size value of large (Hedges’ g = 0.752). This means that this type of intervention has some success at assisting students to learn abstract or complicated concepts/knowledge of chemical bonding (Özmen, 2004; Ünal et al., 2006; Nahum et al., 2010; Hunter et al., 2022; van Dulmen et al., 2023). Multiple representation gives an opportunity for students to construct, re-consider and synthesise the same knowledge from multiple perspectives via the use of models, pictures, and a combination of them, or various visual tools (Tsui and Treagust, 2004; Adadan et al., 2010; Namdar and Shen, 2018). It was surprising that this intervention was somewhat effective given that it entails students using abstract thinking to understand abstract concepts of chemical bonding. This means that a theoretical match between multiple representation and chemical bonding seems to have somewhat reflected in practicum. Indeed, there were a couple of intervention studies using multiple representation that had different effect-sizes (Sunyono and Meristin, 2018 [Hedges’ g = 0.350]; and Widarti et al., 2019 [Hedges’ g = 1.220]). It is possible that the overall large effect found in the current meta-analysis resulted from the limited number of intervention studies included in the sample.
Finally, the types of intervention with a medium effect on improving students’ academic performance were constructivist learning environments (k = 3) and inquiry-based learning (k = 5) (see Table 8). These interventions are typically known for being effective because they are student-centred, similar to cooperative learning. The findings of the current study indicate that the topic of chemical bonding may not be suitable for these types of interventions; perhaps the concept is too abstract for constructivist learning and inquiry. Another consideration is the extent to which these types of interventions are incorporated into different cultural contexts. For example, the Turkish science curriculum released in 2000 initially included some elements of constructivist learning theory (Çalık and Ayas, 2008). This means that this type of intervention may be very new for students and authors (Atasoy et al., 2003; Uzuntiryaki, 2003; Mercy et al., 2019). They may have had difficulties involving essential features of constructivism (e.g., eliciting prior knowledge, creating cognitive dissonance, applying new knowledge with feedback, and reflecting on learning) in the learning process or developing instructional materials or lesson plans (Baviskar et al., 2009). Overall, there were inconsistent effect-sizes for constructivist learning environments that may have affected the outcome of the analysis (Atasoy et al., 2003 [Hedges’ g = 0.049]; Uzuntiryaki, 2003 [Hedges’ g = 1.061]; Mercy et al., 2019 [Hedges’ g = 0.296]).
Cultural contexts may have affected the outcomes of intervention studies using inquiry-based learning as well. Inquiry-based learning incorporates a process of enquiry that requests students to answer a question or solve a problem by conducting experiments, collecting and analysing data, and drawing conclusions (Orosz et al., 2022; Ma, 2023). Another important component of inquiry involves asking questions and criticising answers. These actions may differ from earlier learning habits and cultural behaviours or characteristics (Halim et al., 2023). For example, in some cultures students may prefer not to question the knowledge of their teachers and parents. Cultural norms endorse being respectful and polite to people in positions of authority; therefore, it is important to avoid making challenges or posing arguments (e.g., Nusirjan and Fensham, 1987; Halim et al., 2023; Wiyarsi et al., 2023). Therefore, these habits and cultural norms may have caused some adaptation and orientation issues for intervention studies that used inquiry-based learning in different context(s) or countries (Indonesia, Nigeria, and Türkiye) since inquiry-based learning was initially released for Western countries.
Conclusion and implications
The findings of the overall effect-size of this meta-analysis showed that the intervention studies are effective at improving the participants’ chemical bonding-based academic performance. Also, the findings of moderators indicated that educational level is a non-significant moderator of the participants’ chemical bonding-based academic performance while the type of intervention is a significant one. Moreover, some intervention types (cooperative learning and an enriched learning environment with different methods) were better at improving the students’ academic performance than others. In general, the type of intervention that was more suitable for the abstract nature of chemical bonding learning was found to be more effective.Given the significant role of scope, content, and nature of the teaching intervention in improving academic performance, future research should carefully handle these issues within their chemical bonding-based interventions. Since some extrinsic factors (e.g., cultural context) can influence the effectiveness of interventions, future research should consider them while selecting the type of intervention and carefully portray how they fit with the learning goals of the national science/chemistry curriculum. Also, because educational level indicates the n-shaped developmental curve in terms of chemical bonding-based academic performance, further research should explore the reasons and factors affecting this issue.
A key finding of this study is that the effect sizes of some interventions varied greatly (e.g., Acar and Tarhan, 2008; Özmen, 2008; Eymur and Geban, 2017; Gongden et al., 2020; Munawarah et al., 2020; Anekwe and Opara, 2021). Therefore, future researchers should explore the effectiveness of these intervention types on academic performance in different contexts, cultures, and grades. Furthermore, there were some interventions that were used by limited studies and therefore it was impossible to analyse overall effect size across multiple studies. When more researchers consider interventions such as context-based learning, modelling, multiple intelligences, problem-based learning, and enriched texts, more information about the effectiveness of these interventions can be investigated. Finally, this study, like all meta-analyses, points out consistent and inconsistent findings among published research. Therefore, further meta-analysis studies should be undertaken to resolve any contradictory findings.
Limitation of the study
The current study only included papers written in Turkish and English. This may be seen as the first limitation of the study. Although the abstracts of the papers from countries that speak other languages were written in English, the authors were unable to discern whether to include them in the meta-analysis. Furthermore, it was important to avoid the risk of missing or incorrect information by using a translation tool to understand the articles. Therefore, it is recommended that future researchers include sufficient data and results in their abstracts to ensure their possible inclusion in meta-analysis studies. The moderator variables in the current study were limited to education level and intervention type. Unfortunately, very few studies provided sufficient information to analyse moderators such as duration and assessment, which the present study initially planned to handle. This may be considered as the second limitation of the study. Some intervention types included relatively small number of studies. This may be viewed as the third limitation of the study because such factors as assessment type, class size, or role of the researcher in the instructional environment could influence the participants’ academic performance. Moreover, this study excluded the papers published in 2023 in that this year has not been completed. This may be seen as the fourth limitation of the study.Ethical statement
All procedures performed in this study followed the ethical standards of the Department of Health Standards on Human Research (DOH/QD/SD/HSR/0.9) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.Conflicts of interest
There are no conflicts to declare.Appendices
Appendix. Descriptions of the ‘type of intervention’ moderator (Adapted from Çalik et al., 2023, p. 949–950)| Type of intervention | Descriptions |
|---|---|
| Computer-assisted instruction | Refers to the use of computers and computer-based applications (e.g., animation, simulation, educational games, web-based learning environment) in the learning-teaching process to facilitate student learning (Bayraktar, 2001; Tang and Abraham, 2016). Thus, it serves the goals of science education by modelling any scientific phenomenon at sub-microscopic level and enhancing students’ capacities of learning via enriched technological tools/components (e.g., audio, sound, visualization, interaction, feedback and hypertext) (Özmen, 2008; Kırılmazkaya et al., 2014; Adherr et al., 2019; da Silva et al., 2020; Anekwe and Opara, 2021). |
| Concept map | Provides a visual road map showing pathways and relationships between concepts, propositions and phrases (Novak and Gowin, 1984; Novak, 1990). Thus, it not only helps students meaningfully learn concepts, relationships and hierarchies but also clarify or refine their cognitive structures (Singh and Moono, 2015; Adane, 2020; Huynh and Yang, 2023). Therefore, it promotes students’ conceptual understanding and facilitates their abilities of problem-solving and synthesizing concepts (Singh and Moono, 2015). |
| Conceptual change | Intends to replace alternative conceptions or misconceptions with scientifically accepted ones and integrate new conceptions with pre-existing ones (Hewson and Hewson, 1983; Çalık et al., 2009). Thus, it supports meaningful learning and a sound understanding of science concepts that play a generative or organizing role in thought (Guzzetti et al., 1993; Pabuçcu and Geban, 2006; Ültay, 2015). |
| Constructivist learning environment | Claims that learning is an interaction between pre-existing knowledge and new knowledge. Further, students, who actively participate in learning process and take their own responsibility for learning, construct new knowledge in light of their prior conceptions (Guzzetti et al., 1993). To involve essential features of constructivism (e.g., eliciting prior knowledge, creating cognitive dissonance, application of new knowledge with feedback, and reflection on learning) in learning process, such instructional models as learning cycle, generative learning model, 5E learning model are offered (Baviskar et al., 2009). |
| Cooperative learning | Requires students to work in small cooperative groups on a common task, which is in harmony with the goals of science curriculum. Thus, an effective cooperative learning includes such essential features as positive interdependence, accountability, promotive interactions, teaching interpersonal skills and group processing (Johnson et al., 1998; Rahman and Lewis, 2019). Phrased differently, it not only fosters students to have intellectual conversations about problem-solving skills and creative thinking, but also increases group interaction to enhance their own learning (e.g., achievement and conceptual understanding) (Johnson and Johnson, 1990). |
| Enriched learning environment with different methods | Refers to the use of some combinations of different methods (e.g., cooperative learning, animation, worksheet, problem-based learning, animation, analogies and conceptual change text) to increase the effectiveness of any intervention (Özmen et al., 2009; Pabuçcu and Geban, 2012; Karacop and Doymus, 2013; Munawarah et al., 2020). Hence, it merges the advantages of different methods into an enriched teaching intervention to meet various learning styles and pose students’ capacities of learning. For this reason, it purposes to result in better learning outcomes, e.g., achievement and conceptual understanding (Bağ and Çalık, 2022; Er Nas et al., 2022; Türkoguz and Ercan, 2022). |
| Inquiry-based learning | Refers to a process of enquiry that asks students to answer a question or solve a problem by conducting experiments, collecting and analysing data, and drawing conclusions (Orosz et al., 2022; Ma, 2023). Thus, they are able to acquire new knowledge, inquiry skills, and develop attitudes towards science and understand the nature of science as the outcomes of a student-centred method (Ikenna, 2014; Suyanti and Sormin, 2016; Iryani et al., 2021; Ginting and Juniar, 2022). |
| Multiple representation | Involves the use of models, pictures, and a combination of them or various visual tools that enable people to communicate ideas or concepts (Tsui and Treagust, 2004; Adadan et al., 2010). By capturing students’ attention to the concepts to be taught, it improves students’ conceptual understanding (Ainsworth, 1999; Adadan et al., 2010). Since it provides diverse opportunities to students in constructing the same knowledge from multiple perspectives, any weaknesses dealt with one particular representation might be replaced by another one (Adadan et al., 2010). Thus, it facilitates the interpretation of abstract or complicated concepts/knowledge and helps students achieve meaningful learning (Adadan et al., 2010; Sunyono and Meristin, 2018; Widarti et al., 2019; Adadan and Ataman, 2021). |
| Other | Covers different intervention types (e.g., context-based learning, discovery learning model equipped re-lyric songs, drama-assisted instruction, enriched text, explain and integration ideas, learning styles, modelling, multiple intelligence, problem-based learning and remedial learning system) reported by only one paper. |
Acknowledgements
We would like to thank Associate Professor Jennie Farber Lane from Bilkent University, Türkiye for her kind help in language polishing.References
- Acar B. and Tarhan L., (2008), Effects of cooperative learning on students’ understanding of metallic bonding, Res. Sci. Educ., 38, 401–420 DOI:10.1007/s11165-007-9054-9.
- Adadan E. and Ataman M. M., (2021), Promoting senior primary school students’ understanding of particulate nature of matter through inquiry instruction with multiple representations, Education, 49(3), 317–329 DOI:10.1080/03004279.2020.1854960.
- Adadan E., Trundle K. C. and Irving K. E., (2010), Exploring grade 11 students' conceptual pathways of the particulate nature of matter in the context of multirepresentational instruction, J. Res. Sci. Teach., 47(8), 1004–1035 DOI:10.1002/tea.20366.
- Adane F. M., (2020), The effect of using concept map on students’ achievement in chemical bonding: The case of Limat Ber preparatory and secondary school, MPhil thesis, Haramaya University, Haramaya.
- Adherr N. S. K., Ayimah M. W. and Anyetei B., (2019), The effect of computer assisted instruction (CAI) on students’ cognitive achievement in chemical bonding. A case study of schools in the Kwahu east district of Ghana, J. Educ. Practice, 10, 8–13 DOI:10.7176/JEP.
- Ahmad Z., Ammar M., Sellami A. and Al-Thani A., (2023), Effective pedagogical approaches used in high school chemistry education: a systematic review and meta-analysis, J. Chem. Educ., 100(5), 1796–1810 DOI:10.1021/acs.jchemed.2c00739.
- Ainsworth S. E., (1999), The functions of multiple representations, Comput. Educ., 33, 131–152.
- Akkuş A. and Doymuş K., (2022), Effect of subject jigsaw and reading writing presentation techniques on academic achievement of 6th grade, J. Turkish Sci. Educ., 19(2), 496–510.
- Anekwe C. E. and Opara M. F., (2021), Effect of animation as instructional strategy on students' achievement and retention in chemical bonding, Educ. Sustainable Dev., 1(8), 41–50.
- Arztmann M., Hornstra L., Jeuring J. and Kester L., (2023), Effects of games in STEM education: a meta-analysis on the moderating role of student background characteristics, Studies Sci. Educ., 59(1), 109–145 DOI:10.1080/03057267.2022.2057732.
- Astuti T. N., Sugiyarto K. H. and Ikhsan J., (2019), Using virtual reality towards students’ scientific attitude in chemical bonding, Eur. J. Educ. Studies, 6(2), 224–238 DOI:10.5281/zenodo.2958411.
- Atasoy B., Kadayıfçı H. and Akkuş H., (2003), The effect of constructivist approach on 3rd year high school students' misconceptions about chemical bonds and their elimination, J. Turkish Educ. Sci., 1(1), 1–21.
- Bağ H. and Çalık M., (2022), Argumentation-driven educational digital game: a case of matter subject, J. Mater. Educ., 44, 67–76.
- Barak M. and Dori Y. J., (2005), Enhancing undergraduate students’ chemistry understanding through project-based learning in an IT environment, Sci. Educ., 89, 117–139 DOI:10.1002/sce.20027.
- Barnea N. and Dori Y. J., (1999), High-school chemistry students' performance and gender differences in a computerized molecular modeling learning environment, J. Sci. Educ. Technol., 8(4), 257–271.
- Baviskar S. N., Hartle R. T. and Whitney T., (2009), Essential criteria to characterize constructivist teaching: derived from a review of the literature and applied to five constructivist-teaching method articles, Int. J. Sci. Educ., 31(4), 541–550 DOI:10.1080/09500690701731121.
- Bayrak H., (2005), The successes of the eighth grade students of a primary school the retention of knowledge which they learnt, attitudes and perceive of “chemical connection” according on the theory of multiple intelligences the teaching affection, MPhil thesis, Ankara: Gazi University.
- Bayraktar S., (2001), A meta-analysis of the effectiveness of computer-assisted instruction in science education, J. Res. Technol. Educ., 34(2), 173–188 DOI:10.1080/15391523.2001.10782344.
- Bergqvist A., Drechsler M. and Rundgren S.-N. C., (2016), Upper secondary teachers’ knowledge for teaching chemical bonding models, Int. J. Sci. Educ., 38(2), 298–318 DOI:10.1080/09500693.2015.1125034.
- Borenstein M., (2019), Common mistakes in meta-analysis and how to avoid them, Biostat, Inc.
- Borenstein M., Hedges L. V., Higgins J. P. T. and Rothstein H. R., (2009), Introduction to meta-analysis, John Wiley & Sons.
- Çalık M. and Ayas A., (2008), A critical review of the development of the Turkish science curriculum, in R. K. Coll and N. Taylor (ed.), Science Education in Context: An International Examination of the Influence of Context on Science Curricula Development and Implementation, AW Rotterdam, The Netherlands: Sense Publishers B.V., pp. 161–174 DOI:10.1163/9789087902490_014.
- Çalık M., Ayas A. and Coll R. K., (2009), Investigating the effectiveness of an analogy activity in improving students’ conceptual change for solution chemistry concepts, Int. J. Sci. Math. Educ., 7, 651–676 DOI:10.1007/s10763-008-9136-9.
- Çalik M., Ebenezer J., Özsevgeç T., Küçük Z. and Artun H., (2015), Improving science student teachers’ self-perceptions of fluency with innovative technologies and scientific inquiry abilities, J. Sci. Educ. Technol., 24, 448–460 DOI:10.1007/s10956-014-9529-1.
- Çalik M., Ültay N., Bağ H. and Ayas A., (2023), Effectiveness of particulate nature of matter (PNM)-based intervention studies in improving academic performance: a meta-analysis study, Chem. Educ. Res. Pract., 24, 938–955 10.1039/D3RP00027C.
- Chan C. K., (2016), Learning atomic-molecular theory in secondary school: The role of meta-conceptual awareness and modelling skills, PhD thesis, The Chinese University of Hong Kong.
- Chang H. Y., Binali T., Liang J. C., Chiou G. L., Cheng K. H., Lee S. W. Y. and Tsai C. C., (2022), Ten years of augmented reality in education: a meta-analysis of (quasi-) experimental studies to investigate the impact, Comput. Educ., 191, 104641 DOI:10.1016/j.compedu.2022.104641.
- Chevron M. P., (2014), A metacognitive tool: theoretical and operational analysis of skills exercised in structured concept maps, Perspective Sci., 2(1), 46–54 DOI:10.1016/j.pisc.2014.07.001.
- Coll R. K. and Treagust D. F., (2002), Learners’ use of analogy and alternative conceptions for chemical bonding, Australian Sci. Teach. J., 48(1), 24–32. https://search.informit.org/doi/10.3316/aeipt.117423.
- Constable E. C. and Housecroft C. E., (2020), Chemical bonding: the journey from miniature hooks to density functional theory, Molecules, 25(11), 2623. https://search.informit.org/doi:10.3390/molecules25112623.
- da Silva Júnior, J. N., de Sousa Oliveira, J. M., Winum, J. Y., Melo Leite Junior, A. J., Alexandre, F. S. O., do Nascimento, D. M., de Sousa, D. M. Pimenta, A. T. A. and Monteiro, A. J., (2020), Interactions 500: design, implementation, and evaluation of a hybrid board game for aiding students in the review of intermolecular forces during the COVID-19 pandemic, J. Chem. Educ., 97(11), 4049–4054 DOI:10.1021/acs.jchemed.0c01025.
- Doymus, K., (2008) Teaching chemical bonding through jigsaw cooperative learning, Res. Sci. Technol. Educ., 26(1), 47–57 DOI:10.1080/02635140701847470.
- Ekinci M., (2010), The effect of context based teaching method on teaching chemical bonds to 1st grade high school students, MPhil thesis, Gazi University.
- Ellis P. D., (2010), The essential guide to effect sizes: Statistical power, meta-analysis, and the interpretation of research results, Cambridge University Press.
- Er Nas S., İpek Akbulut H., Çalik M. and Emir M. İ., (2022), Facilitating conceptual growth of the mainstreamed students with learning disabilities via a science experimental guidebook: a case of physical events, Int. J. Sci. Math. Educ., 20, 45–67 DOI:10.1007/s10763-020-10140-3.
- Eymur G. and Geban Ö., (2017), The collaboration of cooperative learning and conceptual change: enhancing the students’ understanding of chemical bonding concepts. Int. J. Sci. Math. Educ., 15, 853–871 DOI:10.1007/s10763-016-9716-z.
- Fensham P., (1975), Concept formation, in D. J. Daniels (ed.) New movements in the study and teaching of chemistry, London: Temple Smith.
- Frailich M., (2007), Implementation and evaluation of web-based learning activities on bonding and the structure of matter for 10-th grade chemistry, PhD thesis, Weizmann Institute of Science.
- Frailich, M., Kesner, M. and Hofstein, A., (2009), Enhancing students' understanding of the concept of chemical bonding by using activities provided on an interactive website. J. Res. Sci. Teach., 46(3), 289–310 DOI:10.1002/tea.20278.
- Fridman C., (2020), The development of intuitive and conceptual knowledge of chemical bonding in an embodied learning environment, PhD thesis, University of Haifa.
- Genel İ., (2008), The determination of concept errors concerning “chemical relations” and correction of these errors, MPhil thesis, Yüzüncü Yıl University.
- Ginting S. M. and Juniar A., (2022), The effect of guided inquiry learning model on improving students' learning outcomes and science process skills on chemical bonding materials, Jurnal Pendidikan dan Pembelajaran Kimia, 11(1), 87–96.
- Gongden E. J., Yame P. T. and Ephraim E., (2020), Effects of computer animation instructional strategy on students’ interest and achievement in chemical bonding in Shendam, Am. J. Humanities Soc. Sci. Res., 4, 304–331.
- Güler M., Bütüner S. Ö., Danişman Ş. and Gürsoy K., (2022), A meta-analysis of the impact of mobile learning on mathematics achievement, Educ. Inform. Technol., 27, 1725–1745 DOI:10.1007/s10639-021-10640-x.
- Guzzetti B. J., Snyder T. E., Glass G. V. and Gamas W. S., (1993), Promoting conceptual change in science: a comparative meta-analysis of instructional interventions from reading education and science education, Reading Res. Quarterly, 28(2), 116–159 DOI:10.2307/747886.
- Hafizhah Putri A., Samsudin A. and Suhandi A., (2022), Exhaustive studies before covid-19 pandemic attack of students’ conceptual change in science education: a literature review, J. Turkish Sci. Educ., 19(3), 808–829.
- Halim L., Ramli M. and Nasri N. M., (2023), The cultural dimensions of inquiry-based practices: towards a comprehensive understanding, Asia Pacific J. Educ., 43(4), 1328–1342 DOI:10.1080/02188791.2022.2106941.
- Hedges L. V. and Vevea J. L., (1998), Fixed-and random-effects models in meta-analysis, Psychol. Methods, 3(4), 486–504.
- Hewson P. W. and Hewson M. G., (1983), Effect of instruction using students’ prior knowledge and conceptual change strategies on science learning, J. Res. Sci. Teach., 20, 731–743 DOI:10.1002/tea.3660200804.
- Higgins J. P., Thompson S. G., Deeks J. J. and Altman D. G., (2003), Measuring inconsistency in meta-analyses, Br. Med. J., 327(7414), 557–560 DOI:10.1136/bmj.327.7414.557.
- Hunter K. H., Rodriguez J. M. G. and Becker N. M., (2022), A review of research on the teaching and learning of chemical bonding, J. Chem. Educ., 99(7), 2451–2464 DOI:10.1021/acs.jchemed.2c00034.
- Huynh Q. T. and Yang Y. C., (2023), Impact of fill-in-the-nodes concept maps on low prior-knowledge students learning chemistry: a study on the learning achievements and attitude toward concept maps, Chem. Educ. Res. Pract. 10.1039/D3RP00238A.
- Ikenna I. A., (2014), Remedying students’ misconceptions in learning of chemical bonding and spontaneity through intervention discussion learning model (IDLM), Int. J. Educ. Pedagogical Sci., 8(10), 3259–3262.
- İnal A., (2013), Determining the effects of teaching chemical bonds subject of 9th class with learning styles and traditional learning approach to students’ academic achievement, PhD thesis, Ankara: Gazi University.
- Iryani B., Iswendi and Putra R. F., (2021), Effect of using guided inquiry-based chemical bonding modules on student learning outcomes, J. Phys.: Conf. Ser., 1788, 1–6 DOI:10.1088/1742-6596/1788/1/012022.
- Johnson R. T. and Johnson D. W., (1987), How can we put cooperative learning into practice? Sci. Teach., 54, 46–50.
- Johnson D. and Johnson R., (1990), Cooperative learning and achievement, in S. Sharan (ed.) Cooperative learning: Theory and research, New York: Praeger, pp. 23–37.
- Johnson D. W., Johnson R. T. and Smith K. A., (1998), Cooperative learning returns to college. What evidence is there that it works? Change: The Magazine of Higher Learning, 30, 26–35 DOI:10.1080/00091389809602629.
- Kansızoğlu H. B., (2017), The effect of graphic organizers on language teaching and learning areas: a meta-analysis study, Educ. Sci., 42(191), 139–164 DOI:10.15390/EB.2017.677.
- Karacop A. and Doymus K., (2013), Effects of jigsaw cooperative learning and animation techniques on students’ understanding of chemical bonding and their conceptions of the particulate nature of matter, J. Sci. Educ. Technol., 22, 186–203 DOI:10.1007/s10956-012-9385-9.
- Karadag E., (2020), The effect of educational leadership on students’ achievement: a cross-cultural meta-analysis research on studies between 2008 and 2018, Asia Pacific Educ. Rev., 21, 49–64 DOI:10.1007/s12564-019-09612-1.
- Kepes, S., Banks, G. C., McDaniel, M. and Whetzel, D. L., (2012), Publication bias in the organizational sciences. Organizational Research Methods, 15(4), 624–662 DOI:10.1177/1094428112452760.
- Kılıç, D., (2007), The effect of the teaching with analogies model on elimination of misconceptions of 9th grade students about chemical bonding, MPhil thesis, Ankara: Gazi University.
- Kırılmazkaya G., Keçeci G. and Zengin F., (2014), The effect of computer assisted instruction in science and technology course to teachers and students’ attitudes and achievements, Int. J. Soc. Sci., 30, 453–466 DOI:10.9761/JASSS2622.
- Korkman N., (2018), Comparison of inquiry based different teaching methods in terms of affecting students’ academic success: “The case of chemical bonds”, MPhil thesis, Bozok University.
- Kuit V. K. and Osman K., (2021), CHEMBOND3D e-module effectiveness in enhancing students’ knowledge of chemical bonding concept and visual-spatial skills, Eur. J. Sci. Math. Educ., 9(4), 252–264 DOI:10.30935/scimath/11263.
- Langbeheim E., Akaygun S., Adadan E., Hlatshwayo M. and Ramnarain U., (2023), Relating pictorial and verbal forms of assessments of the particle model of matter in two communities of students, Int. J. Sci. Math. Educ., 21, 2185–2201 DOI:10.1007/s10763-022-10345-8.
- Lipsey M. W. and Wilson D. B., (2001), Practical meta-analysis, Sage Publications.
- Lutfi A., Hidayah R., Sukarmin S. and Dwiningsih K., (2021), Chemical bonding successful learning using the “Chebo collect game”: a case study. JOTSE, 11(2), 474–485 DOI:10.3926/jotse.1265.
- Ma Y., (2023), The effect of inquiry-based practices on scientific literacy: the mediating role of science attitudes, Int. J. Sci. Math. Educ., 21, 2045–2066 DOI:10.1007/s10763-022-10336-9.
- McManus S. and Gettinger M., (1996), Teacher and student evaluations of cooperative learning and observed interactive behaviors, J. Educ. Res., 90, 13–22.
- Mercy O., Grace K. and Michael Y. S., (2019), Effectiveness of a constructivist instructional approach on student academic achievement in chemical bonding in Ahoada East Local Government area of Rivers State, Int. J. Educ. Eval., 5(3), 42–55.
- Ministry of National Education (2018), Turkish science curriculum for grades 3–8. https://mufredat.meb.gov.tr/ProgramDetay.aspx?PID=325.
- Mondal B. C., (2012), The effect of computer animation in the teaching of chemistry at higher secondary level: an experimental study. Indian J. Appl. Res., 2(1), 84–86.
- Mullen B., Muellerleile P. and Bryant B., (2001), Cumulative meta-analysis: a consideration of indicators of sufficiency and stability. Personality Soc. Psychol. Bull., 27(11), 1450–1462 DOI:10.1177/01461672012711006.
- Munawarah M., Haji A. G. and Maulana I., (2020), Developing problem-based worksheet to improve students’ critical thinking skills and learning outcomes in the concept of chemical bonding, J. Phys.: Conf. Ser., 1460, 1–6 DOI:10.1088/1742-6596/1460/1/012099.
- Nahum T. L., Mamlok-Naaman R., Hofstein A. and Krajcik J., (2007), Developing a new teaching approach for the chemical bonding concept aligned with current scientific and pedagogical knowledge, Sci. Educ., 91(4), 579–603 DOI:10.1002/sce.20201.
- Nahum T. L., Mamlok-Naaman R., Hofstein A. and Taber K. S., (2010) Teaching and learning the concept of chemical bonding, Stud. Sci. Educ., 46(2), 179–207 DOI:10.1080/03057267.2010.504548.
- Namdar B. and Shen J., (2018), Knowledge organization through multiple representations in a computer-supported collaborative learning environment, Int. Learn. Environ., 26(5), 638–653 DOI:10.1080/10494820.2017.1376337.
- Novak J. D., (1990), Concept maps and vee diagrams: two meta cognitive tools for science and mathematics education, Instruct. Sci., 19, 29–52 DOI:10.1007/BF00377984.
- Novak J., (1998), Learning, creating, and using knowledge: concept maps as facilitative tools in schools and corporations, J. E-Learn. Knowledge Soc., 6(3), 21–30.
- Novak J. D. and Gowin D. B., (1984), Learning how to learn, Cambridge University Press.
- Nusirjan and Fensham P., (1987), Descriptions and frameworks of solutions and reactions in solutions. Res. Sci. Educ., 17, 139–148. https://search.informit.org/doi/10.3316/aeipt.38772.
- Okorie E. U., (2015), Effects of instructional software package method of teaching (ISPMT) on students’ interest and achievement in chemical bonding, Education, 5(6), 158–165 DOI:10.5923/j.edu.20150506.02.
- Orosz G., Németh V., Kovács L., Somogyi Z. and Korom E., (2022), Guided inquiry-based learning in secondary-school chemistry classes: a case study, Chem. Educ. Res. Pract., 24, 50–70 10.1039/D2RP00110A.
- Özmen H., (2004) Some student misconceptions in chemistry: a literature review of chemical bonding, J. Sci. Educ. Technol., 13(2), 147–159 DOI:10.1023/B:JOST.0000031255.92943.6d.
- Özmen H., (2008), The influence of computer-assisted instruction on students’ conceptual understanding of chemical bonding and attitude toward chemistry: a case for Türkiye, Comput. Educ., 51(1), 423–438.
- Özmen H., Demircioğlu H. and Demircioğlu G., (2009), The effects of conceptual change texts accompanied with animations on overcoming 11th grade students’ alternative conceptions of chemical bonding, Comput. Educ., 52, 681–695.
- Pabuçcu A. and Geban O., (2006), Remediating misconceptions concerning chemical bonding through conceptual change text, Hacettepe Univ. J. Educ., 30, 184–192.
- Pabuçcu A. and Geban Ö., (2012), Students’ conceptual level of understanding on chemical bonding, Int. Online J. Educ. Sci., 4(3), 563–580.
- Pacaci C., Ustun U. and Ozdemir O. F., (2023), Effectiveness of conceptual change strategies in science education: a meta-analysis, J. Res. Sci. Teach., 1–63 DOI:10.1002/tea.21887.
- Pamuk T., (2018), Investigation of the effects on 8th grade students’ academic success and attitude using computer supported teaching in “periodical system” and “chemical bonds” subjects, MPhil thesis, Ordu University.
- Rahman T. and Lewis S. E., (2020), Evaluating the evidence base for evidence-based instructional practices in chemistry through meta-analysis, J. Res. Sci. Teach., 57(5), 765–793 DOI:10.1002/tea.21610.
- Salyani R., Nurmaliah C. and Mahidin M., (2020), Application of the 5E learning cycle model to overcome misconception and increase student learning activities in learning chemical bonding, J. Phys.: Conf. Ser., 1460, 1–7 DOI:10.1088/1742-6596/1460/1/012102.
- Sarı G., (2013), Determination of pre-service elementary science teachers’ misconceptions about chemical bonds and effects of the conceptual change text on the elimination of related misconceptions, MPhil thesis, Ondokuz Mayıs University.
- Şeker A., (2012), Conceptual change oriented instruction and students’ misconceptions in chemical bonding concepts, PhD thesis, Middle East Technical University.
- Sentongo J., Kyakulaga R. and Kibirige I., (2013) The effect of using computer simulations in teaching chemical bonding: experiences with Ugandan learners, Int. J. Educ. Sci., 5(4), 433–441, DOI:10.1080/09751122.2013.11890105.
- Sevim S., (2007), Preparation and application of conceptual change texts on solution and chemical bonding concepts, PhD thesis, Karadeniz Technical University.
- Sezen-Vekli G. and Çalik M., (2023), The effect of web-based biology learning environment on academic performance: a meta-analysis study, J. Sci. Educ. Technol., 32(3), 365–378 DOI:10.1007/s10956-023-10033-4.
- Sharma N., (2015), Developing and studying the effectiveness of computerized two-tier diagnostic test and remedial learning system in science in terms of achievement in science of class IX students, PhD thesis, Devi Ahilya Vishwavidyalaya, Indore.
- Side S., Djangi M. J. and Safitri F., (2020), Influence of discovery learning model equipped re-lyric songs toward learning outcome on chemical bonding subject of tenth-grade natural science student, Adv. Soc. Sci., Educ. Humanities Res., 432, 137–139.
- Singh I. S. and Moono K., (2015), The effect of using concept maps on student achievement in selected topics in chemistry at tertiary level, J. Educ. Practice, 6(15), 106–116.
- Sunyono S. and Meristin A., (2018), The effect of multiple representation-based learning (MRL) to increase students’ understanding of chemical bonding concepts, Jurnal Pendidikan IPA Indonesia, 7(4), 399–406.
- Suyanti R. D. and Sormin E., (2016), Inquiry learning based multimedia towards the student's achievement and creativity on topic chemical bonding, US-China Education Review A, 6(12), 701–707 DOI:10.17265/2161-623X/2016.12.004.
- Taber K. S. and Coll R. K., (2002), Bonding, in Chemical education: Towards research-based practice, Netherlands: Springer.
- Tang H. and Abraham M. R., (2016), Effect of computer simulations at the particulate and macroscopic levels on students’ understanding of the particulate nature of matter, J. Chem. Educ., 93(1), 31–38 DOI:10.1021/acs.jchemed.5b00599.
- Tarhan L., Ayar-Kayali H., Urek R. O. and Acar B., (2008), Problem-based learning in 9th grade chemistry class: ‘Intermolecular forces’, Res. Sci. Educ., 38, 285–300 DOI:10.1007/s11165-007-9050-0.
- Teichert M. and Stacy A., (2002), Promoting understanding of chemical bonding and spontaneity through student explanation and integration of ideas, J. Res. Sci. Teach., 39, 464–496 DOI:10.1002/tea.10033.
- Tsaparlis G., Pappa E. T. and Byers B., (2018), Teaching and learning chemical bonding: research-based evidence for misconceptions and conceptual difficulties experienced by students in upper secondary schools and the effect of an enriched text, Chem. Educ. Res. Pract., 19, 1253–1269 10.1039/C8RP00035B.
- Tsaparlis G., Pantazi G., Pappa E. T. and Byers B., (2021), Using electrostatic potential maps as visual representations to promote better understanding of chemical bonding, Chem. Teacher Int., 3(4), 391–411 DOI:10.1515/cti-2021-0012.
- Tsui C. Y. and Treagust D. F., (2004), Learning genetics with multiple representations: cross-case analyses of student' conceptual status. Paper presented at the National Association for Research in Science Teaching, Vancouver.
- Türkoguz S. and Ercan İ., (2022), Effect of visual anthropomorphic stories on students' understanding of the particulate nature of matter and anthropomorphic discourse, Chem. Educ. Res. Pract., 23(1), 206–225 10.1039/D1RP00057H.
- Ültay N., (2015), The effect of concept cartoons embedded within context-based chemistry: chemical bonding, J. Baltic Sci. Educ., 14(1), 96–108.
- Ulusoy F., (2011), The effect of model applications and computer assisted instruction on learning products in chemistry education: A sample of 12th grade chemical bonds, PhD thesis, Marmara University.
- Ünal S., Çalık M., Ayas A. and Coll R. K., (2006), A review of chemical bonding studies: needs, aims, methods of exploring students’ conceptions, general knowledge claims and students’ alternative conceptions, Res. Sci. Technol. Educ., 24(2), 141–172 DOI:10.1080/02635140600811536.
- Üstün U. and Eryılmaz A., (2014), A research methodology to conduct effective research syntheses: meta-analysis. Educ. Sci., 39(174), 1–32 DOI:10.15390/EB.2014.3379.
- Uzuntiryaki E., (2003), Effectiveness of constructivist approach on students' understanding of chemical bonding concepts, PhD thesis, The Middle East Technical University.
- van Dulmen T. H., Visser T. C., Coenders F. G., Pepin B. and McKenney S., (2023), Learning to teach chemical bonding: a framework for preservice teacher educators, Chem. Educ. Res. Pract., 24, 896–913 10.1039/D2RP00049K.
- Vevea J. L., Coburn K. and Sutton A., (2019), Publication bias, in H. Cooper, L. V. Hedges and J. C. Valentine (ed.), The handbook of research synthesis and meta-analysis, Russell Sage Foundation, 3rd edn, pp. 383–430.
- Wang C. Y., (2007), The role of mental-modeling ability, content knowledge, and mental models in general chemistry students’ understanding about molecular polarity, PhD thesis, University of Missouri.
- Widarti H. R., Marfu’ah S. and Parlan (2019), The effects of using multiple representations on prospective teachers’ conceptual understanding of intermolecular forces, J. Phys.: Conf. Ser., 1227, 1–11 DOI:10.1088/1742-6596/1227/1/012006.
- Wiyarsi A., Çalik M., Priyambodo E. and Dina D., (2023), Indonesian prospective teachers’ scientific habits of mind: a cross-grade study in the context of local and global socio-scientific issues, Sci. Educ. DOI:10.1007/s11191-023-00429-4.
- Yıldırım A., Şekercioğlu A. G. and Yıldırır H. E., (2018), The effect of drama-assisted instruction on 8th-grade science students’ achievement on “chemical bonds” and their attitudes towards the course, J. Balıkesir Univ. Institute Sci. Technol., 20(2), 255–272.
- Zhang L., (2014), A meta-analysis method to advance design of technology-based learning tool: combining qualitative and quantitative research to understand learning in relation to different technology features, J. Sci. Educ. Technol., 23(1), 145–159 DOI:10.1007/s10956-013-9460-x.
- Zohar A. R., (2020), The development of sensory-motor and conceptual knowledge about chemical bonding through activity in an embodied learning environment, PhD thesis, University of Haifa.
- Zorluoğlu S. L. and Sözbilir M., (2016), The effect of analogy method on student's achievement in teaching ionic and covalent bonds, J. Bayburt Educ. Faculty, 11(1), 84–99.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3rp00258f |
| This journal is © The Royal Society of Chemistry 2024 |