Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Making sense of chemical equilibrium: productive teacher–student dialogues as a balancing act between sensemaking and managing tension†
Ylva
Hamnell-Pamment

Department of Educational Sciences, Lund University, Box 117, SE-221 00 Lund, Sweden. E-mail: ylva.hamnell-pamment@uvet.lu.se
First published on 17th October 2023
Abstract
Navigating the observational, symbolic, and theoretical knowledge domains of chemistry is crucial for chemistry sensemaking. However, this has been shown to be particularly challenging for students of chemistry. In order to reach government standards for sensemaking in the chemistry subject, it is important to investigate how chemistry teachers can sustain sensemaking practices in their classrooms. In this study, conversation analysis was used to study videotaped teacher–student dialogues at upper secondary school practical lessons in chemical equilibrium. Common patterns in how sensemaking was produced in interaction were found in four experienced chemistry teachers’ sensemaking dialogues with students. The data show how the teachers use coordinated actions in conversations to create a balance between (1) managing sensemaking dialogues in the laboratory classroom on a moment-to-moment basis through connecting theory and experience, and (2) managing the tension between exposing students’ knowledge gaps and presenting the students as competent as part of the interaction. The results of the study indicate that resolving tension in interaction is an important part of teacher–student sensemaking in chemistry, and also identify the chemical equation as a possible tool for sensemaking progression. The detailed examples of teacher–student sensemaking can be used as models for chemistry teachers interested in how sensemaking can be achieved practically.
Introduction
Recent decades have seen increased interest in science education in how students use language to make sense of scientific phenomena (Lemke, 1990; Norris and Phillips, 2003; Lemke, 2004; Osborne, 2010; Fang, 2016; Taber, 2017). According to the sensemaking construct established by Odden and Russ (2019a), sensemaking in science learning has been defined as ‘a dynamic process of building an explanation in order to resolve a gap or inconsistency in knowledge … built in one's own words, through an iterative process of construction and critique’ while at the same time connecting to prior knowledge and lived experience (p. 199). According to Odden and Russ (2019a), sensemaking can be seen as both a stance to science learning (figuring something out), a cognitive practice (integrating knowledge and connecting representations) and a discursive practice (constructing and critiquing an explanation). Recent research has shown that classrooms that are rich in sensemaking dialogues have significantly higher learning gains for students than classrooms that are low in sensemaking dialogues (Cannady et al., 2019). The research interest in sensemaking has been mirrored by changes in government policy, for instance in the United States, where standards have moved further toward using language to make sense of phenomena as an essential part of students’ classroom practices (Hakuta et al., 2013; Lee et al., 2018).However, students often struggle to make sense of science (Osborne, 2010; Taber, 2017). Challenges have been observed with sensemaking in science classrooms, especially with regard to connecting theory to observable phenomena (Gunstone and White, 1981; Kind et al., 2011; Hofstein and Kind, 2012). Hence, it has been suggested that teachers need to give students active support in connecting concepts to laboratory experiences (Abrahams and Millar, 2008). In the case of the chemistry classroom, similar issues with making sense of chemistry phenomena have been noted (Ben-Zvi et al., 1988; Kind, 2004; Barke et al., 2010). In addition, it is well established that students of chemistry often have difficulty differentiating between the observational and the particulate knowledge domains of chemistry, as well as effectively using the symbolic language of chemistry (Gabel et al., 1987; Ben-Zvi et al., 1988; Andersson, 1990; Treagust et al., 2003; Johnstone, 2006; Talanquer, 2008; Haigh et al., 2012; Stieff et al., 2013; Hernández et al., 2014; Becker et al., 2015). Based on these difficulties, it has been suggested that more research is needed regarding how these knowledge domains can be connected successfully in chemistry classroom dialogues between teachers and students as part of classroom sensemaking (Xu, 2022). However, successful sensemaking also depends on whether students feel comfortable sharing their ideas in the classroom, or if the students feel the teacher is intent on “proving them wrong” and therefore stop sharing their ideas (Criswell, 2012, p. 26). The present study explores how chemistry teachers at upper secondary school can support student sensemaking in teacher–student conversations, both through supporting sensemaking about chemistry and through managing the social interaction within the teacher–student relationship.
Review of relevant literature
Supporting sensemaking in the chemistry classroom
Research in science education has explored dialogical teacher–student sensemaking from several angles. It has been proposed that teachers can support students in their sensemaking through helping students articulate vexing questions (Odden and Russ, 2019b) and introducing alternative concepts that can contribute to students being able to bring their reasoning forward (Odden, 2021). It has also been shown that experimental work can support sensemaking through stimulating a dialectic between reasoning about experimental data and reasoning about models (Russ and Odden, 2017), and that student confusion, surprise and curiosity is connected to enhanced sensemaking at upper secondary level (Vilhunen et al., 2022). General teacher practices that support sensemaking noted in the literature are asking questions, making connections, increasing challenges in conversations, establishing conversational norms and differentiating instruction (Fitzgerald and Palincsar, 2019). In the chemistry classroom, it has been shown that teacher-established norms, such as those where students are expected to provide particulate-level explanations, can be effective in scaffolding student sensemaking practices (Becker et al., 2013). Conversations where teachers help students make the connection between observations and particulate-level knowledge through questioning, shifting focus, and elaborating on student answers, have also been shown to be beneficial to student sensemaking (Warfa et al., 2014; Towns et al., 2019; Becker et al., 2015).With regard to how productive teacher–student sensemaking dialogues are best maintained, it has been suggested that there might be a tension between (a) the need for students to express their own reasoning, and (b) the need for teachers to monitor that same reasoning in terms of scientific correctness (Russ and Berland, 2019), which might affect how well teachers can guide productive classroom sensemaking in terms of correctness. This can be related to whether students perceive they have the teacher's support, which has been shown to be an important factor that influences student engagement in sharing their tentative ideas and asking questions (Beghetto, 2009), thereby engaging in sustained sensemaking. Teachers exposing students’ alternative ideas of chemical phenomena and labelling them as incorrect can lead to classroom conversational tension due to students feeling exposed and made to look bad (Criswell, 2012), or can lead to guarded student interaction due to fear of failing (Oliveira, 2010). However, if questions are aimed at getting students to articulate their ideas and reasoning, this can lead to students being presented as ‘complementary experts’ in the interaction, which may lead to longer and more sophisticated contributions from students (Oliveira, 2010, p. 445). Students being presented as competent or less competent as a result of classroom interaction and their perceived achievement then affects their academic self-concept; that is, how they evaluate their own academic ability and their expectations of success (Bong and Skaalvik, 2002). Their academic self-concept in turn predicts their continued achievement (Wu et al., 2021).
Hence, how teachers position and guide students through the sensemaking dialogue is vital for how the sensemaking progresses. Encouraged students who feel a high sense of self-efficacy (an aspect of the academic self-concept; see Bong and Skaalvik, 2002) have better problem-solving capacity, higher active engagement and greater perseverance in the face of difficulty (Bandura, 1989), all of which are important components that contribute to sustained sensemaking (Odden and Russ, 2019b). Conversely, student frustration has been connected with disengagement from the task at hand (D’Mello and Graesser, 2012). In the subject of chemistry, language complexity also poses a challenge for students (Markic and Childs, 2016), whereby the medium through which sensemaking takes place can become a barrier for student participation. Tensions arising in teacher–student conversations in the chemistry classroom may be related to the importance of students feeling competent in the language of chemistry in order to feel academically competent in the subject in general (Rüschenpöhler and Markic, 2020).
Interaction disconnect and threat to students’ self-perception can both be linked to Weick's (1995) view on sensemaking as driven partly by a need for a person to maintain a positive self-image in a situation where a gap appears between what is observed and what is expected. A person's experiences of highly complex phenomena, confusion, surprise, or discrepancies in explanations that are pointed out by another all qualify as triggers to sensemaking (Weick's, 1995). According to Weick (1995), if the gap between the perceived and expected reality is not resolved, this will lead to an emotional response that can be negative if the person sees a perceived threat, for instance to their perceived self-image or to the continuation of an expected turn of events. This can lead to a narrowing of perception for the person and a tendency to use familiar rather than recently introduced models to describe phenomena (Weick's, 1995).
Hence, the way in which teachers manage conversational tension and the display of student competency in interaction is vital for how sensemaking can progress in the chemistry classroom. However, no studies so far have investigated examples of how teachers can work to produce sustained dialogical sensemaking in the chemistry classroom, or in science education in general. Considering the challenges that chemistry educators face regarding chemistry sensemaking, it becomes particularly important to study how teachers enact and sustain chemistry sensemaking dialogues with students, and how tension in conversation is managed in these dialogues. This study explores how four experienced chemistry teachers work to produce sustained sensemaking dialogues with students while managing tension in conversation. The aim is to increase knowledge about how chemistry teachers can facilitate productive, sustained sensemaking conversations in their classrooms.
Chemical equilibrium as a topic of study of sensemaking in chemistry
The topic of chemical equilibrium is regarded as a threshold concept in learning chemistry (Talanquer, 2015), and many alternative conceptions have been noted in student populations (Driel and Gräber, 2002; Kind, 2004). The concept of chemical equilibrium undergoes several changes in terms of how it is presented to students, and its definition is gradually changed and refined throughout the students’ schooling (Driel and Gräber, 2002; see Fig. 1). At upper secondary schools in most Western countries, a qualitative introduction to chemical equilibrium is given with a basis in kinetics, followed by the learning of the Equilibrium Law (Law of Mass Action) (Driel and Gräber, 2002).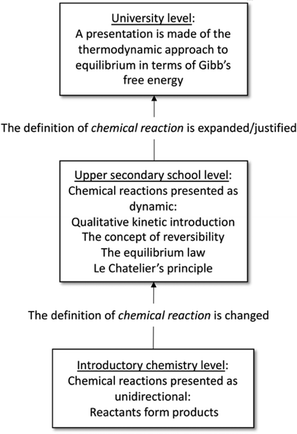 | ||
| Fig. 1 Overview of how the concept of chemical equilibrium is presented in stages throughout education from introductory to university level in many Western countries (Driel and Gräber, 2002). Initially, reactions are presented as proceeding to completion. This definition is then modified at upper secondary school as the chemical equilibrium concept is introduced. The thermodynamic derivation of chemical equilibrium is usually presented at university level (Driel and Gräber, 2002). | ||
One issue with learning chemical equilibrium is that chemical reactions are presented as unidirectional at introductory chemistry level, and then as dynamic at upper secondary school level (Driel and Gräber, 2002). Issues with generating static instead of dynamic meanings appear to persist at university level (Yan and Talanquer, 2015). Hence, the study of sensemaking of the threshold concept of chemical equilibrium at upper secondary school level was regarded as a focus that could be beneficial to chemistry educators.
Theoretical framework
Odden and Russ's (2019a) definition of sensemaking was utilised as a framework for defining sensemaking in the chemistry classroom. This framework has been used previously (Hunter et al., 2021). Odden and Russ (2019a) defined sensemaking as noticing a gap in one's understanding, commonly through an observation, and trying to resolve it, connecting to prior knowledge and lived experience. To capture tension in the teacher–student dialogues, Odden and Russ's construct was expanded using Weick's (1995) definition of sensemaking in social interaction and Goffman's definition of the self (1955).In addition to the sensemaking definition by Odden and Russ (2019a), Weick's (1995) definition of sensemaking included the factors of socialisation, following cues, deciding what is plausible, and sense of self, where he proposes that sensemaking is ‘grounded in identity construction’ (p. 61). According to Weick (1995), if a gap in understanding remains unresolved, this can lead to a threat to a person's sense of self and tension in interaction. The tension arises as the person encounters a discrepancy between the need to confirm the self-concept (perceiving oneself as competent) and the inability to make sense as part of the interaction (Weick, 1995).
Using the theories of Goffman (1955), the sense of self can be defined as an image of the self that arises from social interaction, where the self is defined both as an actor and a construction. Hence, the encounter, where sense is being made, is also a place where a person's self is gradually constructed through self-evaluation (Goffman, 1955). This self-evaluation emerges both from the person's self-judgement and the evaluation made by others based on the actions played out the encounter (Goffman, 1955). During teacher–student sensemaking, the teacher's evaluation of the student in interaction becomes particularly important for the student's self-concept, as the teacher's judgement takes precedence over the students’ judgement in these types of interactions (Hutchby and Wooffitt, 2008).
Aim and research question
The purpose of the study was to explore how teachers promote sensemaking during teacher–student conversations on chemical equilibrium, and how the teachers manage possible instances of tension arising from their monitoring of student reasoning. The study utilised conversation analysis of dialogues between experienced teachers and upper-secondary-school students. To include student observations and various experiences relating to chemical phenomena in the teacher–student conversations, observations were conducted of teacher–student dialogues during practical lessons on the topic of chemical equilibrium at upper secondary schools in Sweden. The study of productive classroom sensemaking teacher–student dialogues through conversation analysis can show how extended sensemaking is managed by teachers on a moment-to-moment basis. Furthermore, possible teacher actions identified in promoting sustained sensemaking can be used as models for teachers who wish to further promote sensemaking in their daily practice.The research question posed was: What actions do teachers use to promote sustained sensemaking in conversations with students while teaching the topic of chemical equilibrium during practical lessons, and how is tension managed in these conversations?
Methods
Research rationale and study design
The data used for this study were taken from a larger research project, which focused on the study of student learning of chemical equilibrium in the chemistry laboratory in four schools in Sweden. The research project utilised purposive sampling to approach schools. This meant schools were chosen to represent a range of grade intake levels, settings (one small town school, one university town school, and two city schools) and two school systems (represented by three Swedish school classes from the Natural Sciences programme‡ and two International Baccalaureate school classes). Video recordings collected for this research project that contained teacher–student dialogues on chemistry theory were chosen as data for the present study. In addition, student achievement levels and teacher interviews about the learning context, also collected through the project, were used to complement the video data.The analytic method chosen for the study was conversation analysis, and the study design was emergent, grounded in data, and inductive, with a focus on teacher facilitation of student learning of chemical equilibrium. This meant the aim and research question emerged as the data were being analysed (Miles et al., 2014). Conversation analysis can provide evidence on intersubjectivity in interaction, or a ‘known-in-common world’ of the conversation participants, at the same time as each participant's contribution is continuously shaped by, and re-shapes, the dialogue as it progresses (Heritage, 1984, p. 216). As a method, conversation analysis is based on detailed analysis of verbal and non-verbal interactions and focuses on what the conversational turns indicate about the overall organisation of the participants’ behaviour (Heritage, 1984). Therefore, conversation analysis was regarded as an appropriate method for the inductive study of teacher facilitation of chemistry learning. Video data is particularly useful for this kind of analytic method as it provides additional nonverbal information, including gestures and direction of eye gaze, which are important for contextualisation of the utterances (Cowan, 2014). Finally, interviews with teachers on the learning context can give information on the students’ previously covered topics, and which concepts the teachers use to teach chemical equilibrium, which provides additional evidence on which concepts students might be expected to use in class. Because the conversations were analysed as belonging to a local teaching context, this meant the coding had an ethnographic approach (Altheide and Schneider, 2013).
In the present study, a qualitative approach was taken to studying the dialogues in the data. Qualitative methodologies carry low generalisability (Cohen et al., 2018), but can also be used to explore context and nuance in data that cannot be covered by statistical analysis (Flyvbjerg, 2001).
Participants
| Name (pseudonym) | Setting of school | School system | Average grades of students being admitted to school |
|---|---|---|---|
| Anna | City | Swedish | High |
| Lars | Small town | Swedish | Medium |
| Cecilia | City | Swedish | Low |
| Erik | University town | International Baccalaureate | High |
Learning context
All students had followed roughly the same teaching sequence prior to practical lesson, which included an introduction to reversibility with a focus on particle collisions and kinetics, followed by a (sometimes brief) introduction to the Equilibrium Law and, in some cases, Le Chatelier's principle.The four practical lessons that focused on exploration of shift in chemical equilibrium all had a similar setup: (1) teacher theoretical/practical introduction and handing out of lab sheets; (2) students setting up their equipment with teacher support; (3) the students executing small experiments on shift in chemical equilibrium (either related to iron thiocyanate or cobalt chloride equilibrium), discussing answers to questions on the lab sheet amongst themselves and with the teacher; and (4) a teacher summary of the theory or referral to a continued conversation during the next lesson. All teachers introduced the relevant theory on the board at the beginning of the practical lesson as part of the introduction, except for Lars, who introduced it on the board gradually in response to student questions. The theory discussed during the lesson was assessed later as part of the chemical equilibrium subtopic. An example lab sheet is found in Appendix A.
Data collection
All classrooms were filmed from the back and at an angle to ensure that the students who did not wish to be filmed could participate in the practical lesson. The teacher carried a lapel microphone and the video camera had a separate microphone that recorded background noise. Additionally, a table camera was set up to film the whiteboard. The researcher sat at the back of the classroom by the camera, taking observational notes, from the beginning of the class (starting before the students entered) and throughout the recording session.The teachers were interviewed twice for validity purposes, firstly in order to collect contextual information about the practical lesson, and secondly for participant verification. Hence, the interviews were transcribed but not coded. The first interview took place before the practical lesson, where the teachers were asked to describe the students, their teaching goals and the learning context. This information was later used to inform the interpretation of the video data when contextual information was needed, such as which concepts had been taught in the previous classes. The second interview took place after the practical session and teachers were asked to reflect on the video clips from the practical lesson and verify that the students, as well as they themselves, had behaved as they normally do during conversations. Only one teacher commented on the students’ behaviour, saying they were a little calmer than usual during the introduction to the lab (perhaps because many were absent during that day). Two teachers appeared to forget that they were being recorded during the lesson: one stated this explicitly after the lesson, and the other walked around with the microphone still turned on outside of the classroom.
Data coding and analysis
In total, 5 hours and 34 minutes of video were analysed. All longer teacher–student conversations regarding the theory of chemical equilibrium (spanning 2–3 minutes or longer) were first transcribed in great detail using conversation analysis (Heritage, 1984), including pauses, hesitations, eye gaze, and movements (including gestures, hands indicating towards the whiteboard or lab sheet, symbols being drawn in the air and movement to and from the conversation). These conversations were then coded descriptively alongside the video material in Transana to inductively explore common themes. Several waves of coding were used that included cycles of coding and discussion of video materials in continuous movements between transcripts and video data. These discussions about the material and codes were held on a regular basis with an external professor specialised in conversation analysis.The teacher–student dialogues were first inductively described using conversation analysis, and the patterns in the data were then identified as sensemaking interactions (recently defined by Odden and Russ, 2019a,b), based on a review of science education literature related to student reasoning. The actions identified in the data as being part of sensemaking involved gaps in student knowledge being displayed, students connecting prior knowledge and experience in their own words to make sense of what they saw, and explanations gradually being built (Odden and Russ, 2019a,b). For an example of the initial analysis, see Appendix B. After the theoretical focus of the article had been identified, the conversations in the transcripts and video materials were analysed in the form of process coding (a type of coding that uses gerunds to describe actions unfolding over time in certain sequences; Miles et al., 2014), and the aim and research question emerged as the data was related to the research literature. When the overarching themes relating to sensemaking were identified, all shorter conversations were transcribed, leading to further adjustments of the codes. This meant all interactions between teachers and students that contained a sensemaking component were changed into film clips (46 in total) and coded in Transana. After an analysis of the conversational structure (see Appendix C), 41 sensemaking conversations were identified in the data. Common themes, categories and examples of codes from the process coding analysis are shown in Table 2. Constant comparison analysis was utilised throughout, which meant that codes were repeatedly checked for internal consistency (Cohen et al., 2018, pp. 719–720). A codebook of code definitions was kept throughout, and coding was accompanied by regular and frequent memoing.
| Themes | Categories | Examples of actions |
|---|---|---|
| a ‘Linking to alternative theoretical concepts’ could be an action belonging to Theme 2 or Theme 3, but it occurred in all conversations. | ||
| Theme 1 – connecting observations or experiences to theory (41) | Connecting an observational experience to theory (37) | Connecting the colour change of the mixture to the chemical equation |
| Connecting an experience of working with a chemical solution to theory (16) | Connecting the addition of a solution with a proposed reaction | |
| Connecting a symbolic representation of an experience to theory (e.g., a symbol spoken of as a chemical solution) (4) | Pointing to the chemical equation and referring to the memory of the experiment, then referring to particle collisions | |
| Theme 2 – linking to alternative concepts (41) | Linking to alternative theoretical conceptsa (38) | Introducing the concept of ‘reaction rate’ to the conversation |
| Linking concepts to chemical equation (2) | Introducing the chemical equation to the conversation when talking about reversibility | |
| Theme 3 – managing tension (40) | Linking to alternative theoretical conceptsa (16) | Asking the student to think in terms of ‘concentration’ when the student cannot give the required answer |
| Providing a hint (11) | Indicating at the answer with a gesture while asking | |
| Reformulating a student statement (8) | Claiming the student meant to say a shift in position of equilibrium occurred | |
| Pointing out the difficulty of the task (4) | Saying that the experiment was quick and hard to see | |
| Giving a direct explanation (27) | Explaining colour change in terms of reaction rates to the student in response to the student struggling with sensemaking | |
| Providing acts of inclusion (using inclusive pronouns) (7) | Using “we” when referring to past experiments performed in class | |
| Confirming a student response as valid (2) | Acknowledging that things may be expressed in different ways | |
| Using humour (4) | Making a friendly joke after the student claims not to know | |
For the purposes of publication, transcripts were translated by the first author – a qualified chemistry teacher, native Swedish speaker and C2 proficiency holder – who studied the transcripts extensively. The translations were checked by a native Swedish lecturer at the university's English department. However, nuances may have been lost in the translation, which according to recommendations for discourse analysis (Belczyk-Kohl, 2016) is shown as separate from the original transcript.
Conversation analysis is useful for the study of sensemaking dialogues, as it can show how the practice of making sense is managed by the conversation participants. In addition, conversation analysis can identify how tension is managed; that is, how agreements and disagreements are managed reflexively in different conversational contexts through the study of, for instance, silences, hesitations, clarifying questions, repair, and mitigations in conversations (Pomerantz, 1984; Pomerantz and Heritage, 2012). Regarding validity, conversation analysis is a careful and detailed way of studying the structured, social organisation of interaction, through analysis of question–answer pairs in dialogue as empirical data that show how actors reflexively organise and make sense of their local social setting (Heritage, 1984). Although conversation analysis can sometimes concern single cases, its focus is to find general patterns of interaction through rigorous description of the participants’ actions, maximising generalisability of accounts through the study of many examples, and paying close attention to deviant cases (Hutchby and Wooffitt, 2008). Hence, it is a method that is particularly suited for inductive analysis. From this perspective, conversation analysis can be viewed as a method that is high in validity through its thick description and focus on overall patterns of actions (Geertz, 1973; Miles et al., 2014). To explore how teachers and students carry out sensemaking in teaching dialogues, the method was regarded as suitable.
Ethical considerations
Results
Common trends in the data
The practical lessons all contained an introduction by the teacher, practical teacher–student dialogues as the experiments were set up, and then sensemaking dialogues between the teacher and the students (either with individual students or with students in pairs) as the students attempted to answer the questions on their lab sheets. Two of the teachers ended the class with a summary on the board, whereas the other two teachers produced the summary on the board gradually as the class progressed.All of the sensemaking dialogues in the data contained two essential parts. In the first part of the dialogue, the students’ pre-knowledge was expressed in their own words. This pre-knowledge was expressed either through a question or an utterance, or through their own attempt to connect chemistry theory with their observation of the novel phenomenon that they were asked to explain. This was followed, in the second part of the dialogue, by a teacher–student sensemaking sequence guided by the teacher, in which an observation or an experience was connected to theory using the student's own words (concepts previously covered in class and known to the student as evidenced by the interaction and teacher interviews), in order to resolve a gap in knowledge that was exposed in the conversation. A full breakdown of the dialogic structure, exemplified in relation to one of the dialogues, is shown in Appendix C.
Three common approaches to supporting extended sensemaking appeared in the teacher–student dialogues defined as sensemaking conversations (see Appendix C) during the chemistry practical lessons. These were: (1) relating observations or experiences to theory; (2) linking to alternative concepts to open up new paths of reasoning; and (3) managing tension. In addition, teachers clarified misunderstandings when needed. Hence, all of the sensemaking dialogues had both theoretical and social aspects. To exemplify both the process of sensemaking and the themes uncovered in the data analysis, one sensemaking sequence is described below in its entirety, after which transcript excerpts are used to further illustrate the themes in the data.
Actions used by teachers to achieve sustained sensemaking about chemical phenomena
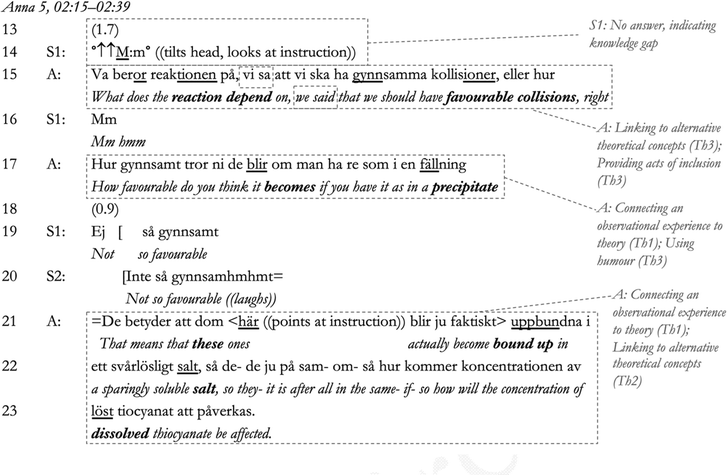
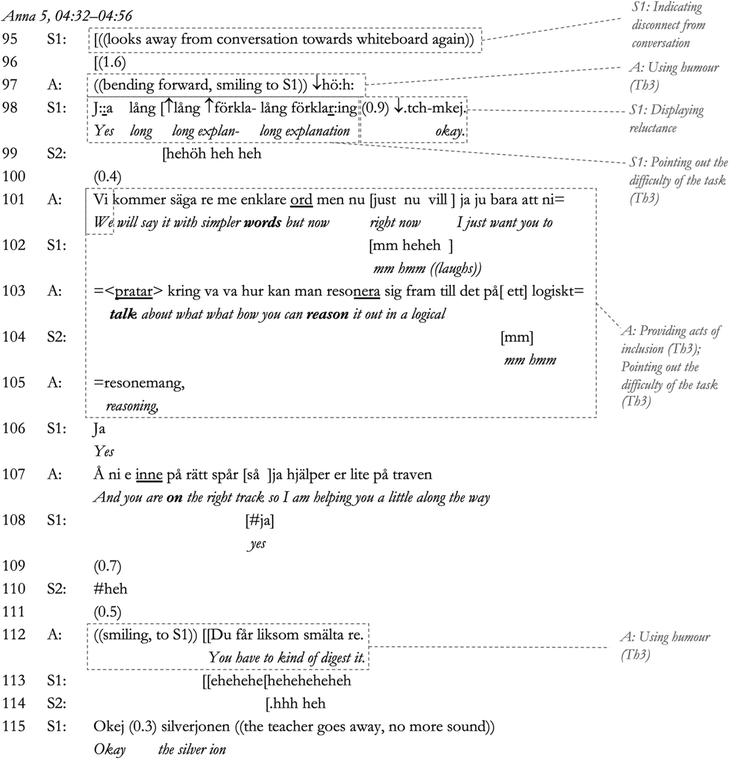
The following conversation, which exemplifies the themes (denoted Th in the transcript and running text, see also Table 2) in the data related to the theoretical and social aspects of sensemaking, comes from a conversation between the teacher Anna and three of her students. Anna taught a class dominated by very high-achieving students in one of the larger cities in Sweden. The conversation was translated from Swedish by the researcher(s)¶ and shows Anna leading the students through sensemaking during a practical lesson on shift in chemical equilibrium. In this particular class, the students are studying iron thiocyanate equilibrium, and are expected to reason about what happens to the equilibrium mixture when they add iron(III) ions, thiocyanate ions, and silver ions.In this first part of the dialogue, Anna initiates the conversation in Line 1 through asking if the students have ‘figured it out’, whereby she contextualises the overall purpose of the lesson; that is, to figure out what happens during the shift in equilibrium. In response to this, student S1 initiates sensemaking in her own words in Lines 2–3 (connecting experience to theory, Th1), which Anna responds to with a questioning yes (‘jaa?’), indicating agreement (Pomerantz, 1984), and an inadequate answer (Gardner, 2012). Due to a lack of final assessment by Anna, S1 is then obliged to elaborate (Gardner, 2012) in Line 5, followed by another withheld assessment by Anna in Line 6. S1 is then again obliged to continue (Lines 7–8). However, in Lines 9–10 there is first a pause and then a ‘tch’-sound and a questioning repeat (‘jaa?’ [yes?]), indicating a reluctance to agree on behalf of Anna (Pomerantz, 1984; Lindström, 1999), to which S1 immediately responds by partially withdrawing her answer (‘kinda’ [typ], line 11). The affirmatory yes, combined with displayed reluctance, can be reasonably deduced to indicate an underlying positive, encouraging position with regard to the student, and it is notable that a direct contradiction of the student's sensemaking is avoided. In response to the students’ withdrawal, Anna's explicitly assesses the response as being valid from a different perspective (‘Så skulle man ju kunna säga’ [That's one way of putting it of course], Line 10). The confirmation of the previous position by Anna minimises the rejection of S1's statement through agreeing with its validity (Pomerantz and Heritage, 2012) and thereby alleviates tension arising from the indication of rejection or disagreement (Anna managing tension, Th3). As S1's statement is confirmed as a valid perspective, S1's competence can be reasonably assumed to be upheld in the interaction.
Anna then initiates teacher–student sensemaking in the second part of the dialogue through a request for a change of focus from the concept of reactivity (silver ions react with thiocyanate ions, Lines 5–7) to the effect that precipitation (connecting experience to theory, Th1) has on the equilibrium reaction (Lines 10–12), opening up for sensemaking using the theory of chemical equilibrium. This teacher–student sensemaking part of the conversation, which contains a narrative made up from teacher cues for sensemaking and student responses, then continues all the way to Line 71 in the transcript.
The teacher–student sensemaking does not progress immediately. S1 pauses in Line 13, looks away, and expresses a high-pitched but quiet ‘Mm’ in Line 14, indicating that she cannot act on Anna's sensemaking cue; that is, a knowledge gap is revealed (Macbeth, 2011). Continuing immediately in Line 15, Anna then introduces to the sensemaking dialogue concepts previously covered by the students (Anna, Interview 1; the concept of particle collisions [Lines 15–17] and concentration [Line 22]) (managing tension, Th3) and connects back to the students’ experience of the solution (‘fällning’ [precipitate], ‘salt’ [salt], ‘löst’ [dissolved] and ‘lösning’ [solution]; Lines 17, 22, 23, 27 and 30) (connecting experience to theory, Th1). In this way, Anna formulates the problem using words and experiences that are familiar to the students. Thereby, she can be reasonably assumed to give prompts for sensemaking (which the students need to perform in their own words; see Odden and Russ, 2019a) as she is managing the tension that arises when the students struggle to participate. Anna also frames the previous teaching experience as a joint experience using the inclusive pronoun ‘vi’ [we] in Line 15. The use of inclusive pronouns is an action previously proposed to signal positive approval and inclusiveness through referring to the common membership of a scientific community (Bills, 2000) (Anna managing tension again, Th3). In response to Anna's action, S1 again takes part in sensemaking (showing understanding through sensemaking participation; see Macbeth, 2011), and Anna continues to guide student sensemaking through a rhetorical question that simultaneously connects the students’ observation to the theory (that is, precipitate and particle collisions; connecting experience to theory, Th1) and introduces humour into the dialogue (managing tension, Th3, through a signalling of companionship; see Kangasharju and Nikko, 2009). Anna immediately continues to cue student sensemaking in Lines 21–23, with her continuation indicating the question has been answered correctly (Pomerantz and Heritage, 2012; Gardner, 2012). In the next part of the sequence, we can see how she includes both S1 and S2 in the teacher–student sensemaking.
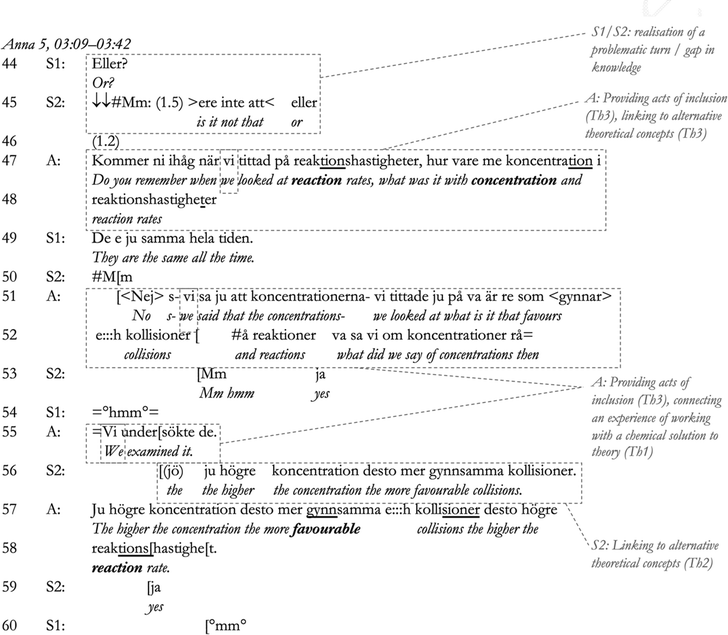
In response to the cue from Anna, S2 elects to take part in sensemaking (Line 24), and the correctness of his response is confirmed by a repeat of S2's answer by Anna, which shows her agreement with the assessment (Pomerantz and Heritage, 2012). This procedure is then repeated for S1 (lines 24–33), whereby S1 elaborates further in Line 34. This statement is followed by an even stronger sign of agreement in Anna's precisely in Line 35 (Pomerantz and Heritage, 2012). In Line 35, Anna then connects this dialogue (which has been linked to the students’ own words) to the equilibrium concept through referring to the equilibrium equation on the whiteboard (linking to alternative concepts, Th2). In response, S1 continues to reason in terms of concentration in Lines 38–40 (connecting observation to theory, in her own words, Th1), and S2 seems to agree (Line 4). Anna reacts to this with another delayed response, indicating dispreferred disagreement (Pomerantz, 1984). In the next excerpt, she again manages tension by guiding the students toward using more contextually appropriate concepts after exposing their reasoning as not sufficient.
In Lines 44–45, S1 and S2 both express what can be described as vexed responses (using the word ‘Eller’ [Or], indicating a realisation of a problematic turn in the conversation; see Lindström, 1999) in response to Anna's hesitation in Lines 42–43. Hence, they acknowledge a gap in their knowledge, which is necessary for the initiation of sensemaking (Odden and Russ, 2019a,b). Anna then explicitly introduces the concept of ‘reaction rate’ in addition to ‘concentration’ (Lines 46–47) to help the students connect the two concepts for sensemaking, combined with continued use of inclusive pronouns in Lines 47–48, a second show of approval and inclusion (Bills, 2000) (managing tension, Th3). S1 then connects the concepts incorrectly (Line 49), which S2 is quick to show he does not agree with a throaty ‘mm’ in Line 50, likely maintaining his display of competence in the interaction. This is followed by a direct correction – ‘Nej’ [No] – by Anna in Line 51, whereby Anna directly continues to refer to a previous joint laboratory experience in a third act of inclusion (the use of the word ‘vi’ [we], Lines 51–55; Bills, 2000) (managing tension, Th3; connecting experience to theory, Th1). Hence, Anna's sensemaking prompt can be said to be combined of both a signalling of her approval (through direct continuation rather than pausing and indicating disagreement; see Pomerantz and Heritage, 2012) and a referral to the students and her being part of the same community (Bills, 2000). S1 indicates she still cannot contribute through a quiet ‘hmm’ (Line 55). Anna then presses for an answer (Line 55), whereby S2 is obliged to continue (Gardner, 2012) and again participates in teacher–student sensemaking (Line 53). S2 continues the sensemaking dialogue with Anna, leading to the production of a correct contribution to the sensemaking dialogue (Line 56, confirmed by Anna in Lines 57–58 through another second assessment (Pomerantz, 1984). S2 then confirms their agreement on Line 59. However, S1's quiet ‘mm’ (Line 60) is likely an attempt to signal to the teacher that she is still not following. In the next excerpt, however, Anna continues to guide teacher–student sensemaking involving S2, displaying their sensemaking for the other students in the group (Macbeth, 2011).
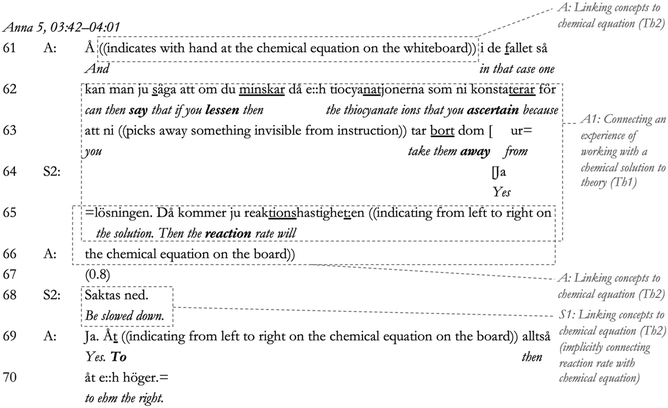
In Lines 61–65, in response to S2's participation, Anna connects the previously established concepts with the new concept of chemical equilibrium (through a second referral to the whiteboard, Line 61) (linking to alternative concepts, Th2), as well as back to the original statement from the students regarding the solution (a connection to their laboratory experience, Lines 62–64; connecting experience to theory, Th1). She then proceeds to connect the reaction rate to chemical equilibrium (represented by the chemical equation) in Lines 65–66 (linking to alternative concepts, Th2). In turn, S2 continues to participate in sensemaking in Line 66 (noting the reaction rate will slow down), whereby Anna confirms and completes S2's sentence, reformulating it in Lines 69–70 (‘Ja … åt e::h höger’ [Yes … to ehm the right]). It is reasonable to say that, in this way, S2's statement is presented as competent. However, we notice a shift in the dialogue in the next part of the sequence.
Having participated successfully in the teacher–student sensemaking narrative, S2 immediately self-elects to elaborate (incorrectly) about equal reaction rates (‘då ere lika’ [then it is the same], Line 71), and Anna possibly realises that she may have guided the students as far as she can in terms of sensemaking (according to her interview, she aimed for the students to “access some of it [the theory] without being given the answers”; Anna, Interview 1, Lines 163–164). Likely managing tension (Th3) through taking over the sensemaking completely, Anna then explains the progression of reaction rates in Lines 72–81, including a check that this makes sense to student S2, which he confirms it does (‘ja nu fattar ja’ [yes now I understand], line 78). Anna confirms having noted this understanding in Line 79 with a yes (‘ja’), before continuing. As Anna finishes the sensemaking in Lines 79–81, S2 displays understanding in Lines 81 and 83 through two more uses of the word yes (‘ja’), showing both his competence and agreement with having made sense. However, at this point S1 has disconnected from the interaction by leaning back and looking away, signalling a third time, and more explicitly, that she is not following (Line 76). In the next excerpt, we find Anna leading another brief section of teacher–student sensemaking, finishing her interaction with S2.
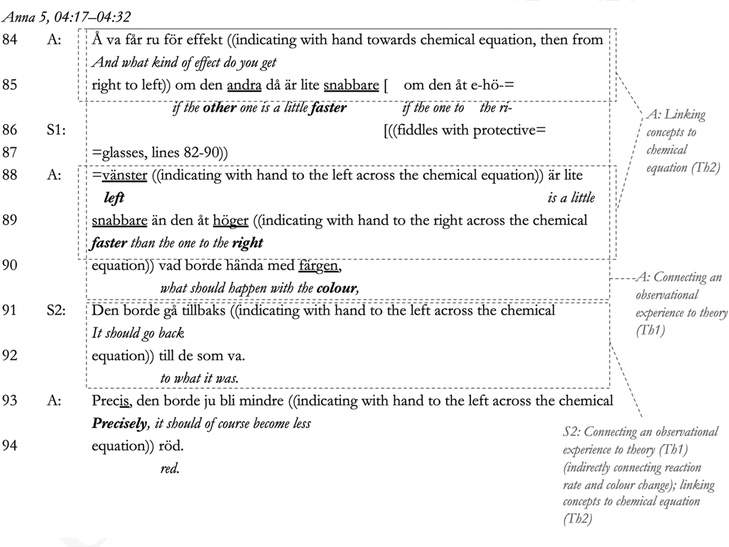
Continuing the sensemaking dialogue in response to S2's display of understanding, Anna connects back to the observation of colour change in Lines 84–90 (connecting observation to theory, Th1). Here, she repeats her initial request for the ‘effekt’ [effect] that initially involved S1, connecting her initial question about the ‘effekt’ [effect] in Line 10 to the concept of reaction rate. She also involves the chemical equation of the equilibrium reaction (indicating to the whiteboard, Lines 88–90) (linking to alternative concepts, Th2). This action connects the original question by Anna, which displayed the students’ initial knowledge gap, with the students’ experience of the colour change and the required theory of chemical equilibrium again, cueing teacher–student sensemaking. In response to this cue, S2 then makes sense of the observed colour change using his own words and gestures towards the chemical equation on the whiteboard (Lines 91–92). Anna confirms this sensemaking, indicating her agreement with the word precisely in Line 93 and a reformulation of S2's statement (Pomerantz, 1984), Line 94. After having finished the sensemaking dialogue with S2, Anna finally responds to student S1, whom after her repeated displays of not following the sensemaking, such as fiddling with her protective glasses in Line 86, has been quiet, looking away.
Anna engages S2 humorously (‘höh’ in Line 97), possibly in order to reduce the tension arising from the challenging situation by confirming the challenge and/or remedying the threat to the student's display of competency (Kangasharju and Nikko, 2009) (managing tension, Th3), which prompts S1 to explain her own lack of competent contribution by referring to the explanation as ‘lång’ [long] (Line 98), and thereby confirming the conversation as challenging (managing tension, Th3). She then expresses a reluctant okay (‘.tch’ indicating reluctance; Pomerantz and Heritage, 2012) with regard to the conversation, which simultaneously produces laughter from S2 (Line 99), possibly a move to indicate companionship (Kangasharju and Nikko, 2009). Anna confirms the definition of the conversation as difficult in Line 101 using an inclusive pronoun (‘Vi kommer säga re me enklare ord’ [We will say it with simpler words]), possibly also indicating approval and belonging (managing tension, Th3) (Bills, 2000). Anna explains that the requirement right now for the students is to reason and talk (Lines 101–105). In this way, the definition of the talk as unusually challenging is confirmed and the perception of S1 as competent in the interaction can be said to be restored (Anna managing tension, Th3). S2 responds by acknowledging Anna's definition of the situation and laughing briefly in Line 102, which is likely an attempt to save face (short laughter in a problematic situation; see Kangasharju and Nikko, 2009). Anna concludes the conversation with humour, jokingly saying that S1 will need to digest it (Line 112), thereby repeating the humorous diffusion of tension and underlining the challenge of the conversation in a final confirmation of S1's competency (managing tension again, Th3).
The above dialogue is an illustrative example of how the teachers in this study worked to connect student experience with the necessary known and new concepts to promote classroom sensemaking, at the same time as they diffused tension arising from threats to students’ display of competency. This tension was produced as the teachers needed to probe for knowledge gaps in order to lead the sensemaking in a productive direction. All the student–teacher dialogues that contained sensemaking in the data showed this pattern. Some details of this pattern are explored in the following sections and an extended analysis of these excerpts can be found in Appendix D.
The dual nature of the chemical equation in teacher–student sensemaking about chemical phenomena
As can be seen in Lines 2–3, Cecilia uses the chemical equation as a reference to student experience when cueing student sensemaking. S4 then uses the chemical equation to mediate the connection between theory and experience as part of making sense of his observations in his own words. Note the long pause (7 seconds, Line 4) as this is achieved.
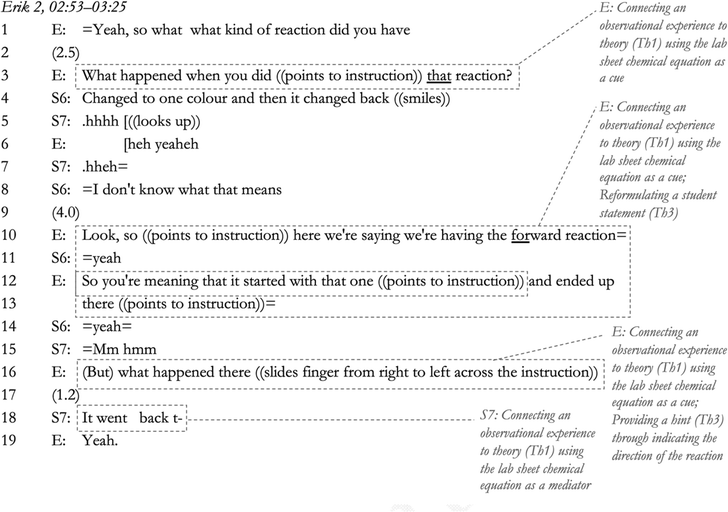
In this excerpt, Erik uses the chemical equation to cue a connection between theory and experience for the students in Lines 3–4, 10–13, and 16–19. As part of this brief interaction, he also reformulates the student statement into a more knowledgeable one (Line 12) and provides a hint (Line 16), which in the end results in S7 taking part in teacher–student sensemaking (Line 18).
As can be observed in these examples, the use of the chemical equation to achieve a connection between experience and theory required some thinking on behalf of the students.
Tension management during teacher–student sensemaking about chemical phenomena
Although some conversations contained more tension management from the teacher than others, all conversations contained at least one theory-laden way of managing tension that arose from student gaps in understanding. This theory-laden way of managing tension meant that moments of tension (pauses or other signs of dispreferred disagreement) that involved a threat to the presented competency of the student were resolved through either linking alternative concepts to the students, hints being given, statements being reformulated, or giving direct explanations. Note that Erik (above) gave an explanation in Lines 10–13 as a response to the student expressing her ignorance in Line 8. This action mirrors the pattern of explanation-giving in the data. Explanations were given either when students asked questions, expressed their ignorance, or could not follow further in the sensemaking dialogue (as shown in the interaction between Anna, S1 and S2, Lines 72–83). It would seem from the data that students professing their incompetence and thereby threatening their own display of competency in the interaction led to the teacher directly assuming the role of sensemaker temporarily, possibly to steer attention away from the threat to the students’ self, and, to model sensemaking for the student (which would be a reasonable move, as less proficient students benefit from teacher modelling; see Kalyuga et al., 2003). This action is clearly illustrated in Erik's conversation above, where he (1) presents the students as competent through a reformulation of their initial sensemaking attempt (reformulating a student statement; Lines 12–13), and (2) helps the students participate in teacher–student sensemaking through providing a hint (Line 16).
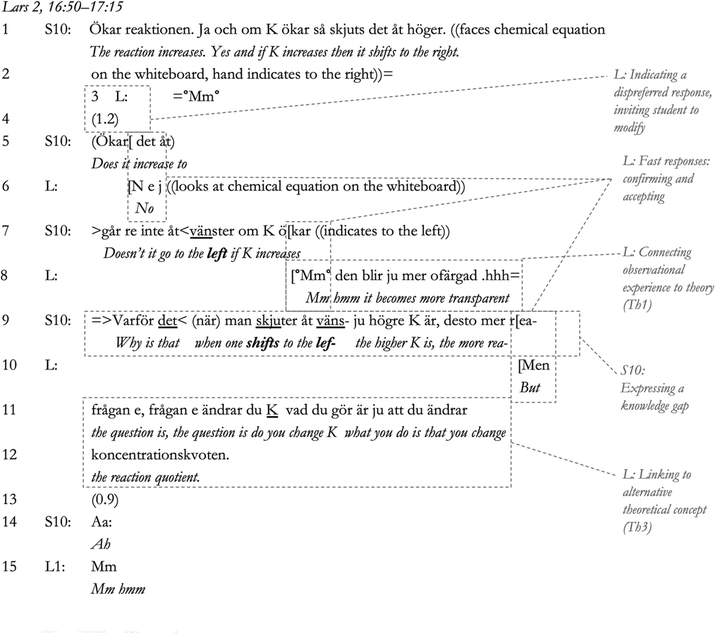
In the above excerpt, Lars can be seen to first invite S11 to modify his statement in Lines 3–4. He then corrects S11 in Line 6, as part of a sequence of fast responses in Lines 6, 8, and 10. Generally, confirming and accepting assessments of the other's statements are shown through turns of talk with no delay (Pomerantz and Heritage, 2012), which can be clearly seen in this sequence. Therefore, it is reasonable to assume that, after being forced to directly correct his student, Lars minimised further negative assessments of the student's knowledge gap in the interaction. We can also see that the interaction led to S11 overtly expressing a knowledge gap in Line 9, which meant that Lars could effectively manage the tension produced by offering an alternative concept for sensemaking in Lines 11–12.
All but one of correction incidents in the data were followed by a tension diffusion act by the teacher that in some way supported the students being displayed as competent in the interaction. An excerpt from this one exception is shown below. Where we enter the conversation below, the student (S11), in response to an initiating question by the teacher, tried for some time to guess why the colour of the solution changed when he added iron(III) ions to the equilibrium mixture of iron thiocyanate. The teacher, Cecilia, hinted at the chemical equation for this reaction written on the board during the conversation but S11 has not yet utilised this resource for reasoning. In the excerpt below, S11 has moved on to attempt to make sense of Experiment 4, which involves the precipitation of silver thiocyanate in order to shift the position of equilibrium.
There is no tension management noted in this conversation, as Cecilia does not act to uphold the student's presentation as competent in response to his attempted sensemaking (however, a minimising of pauses can be seen in the overlapping speech in Lines 3–4 and 12–13, which indicates that, just like Lars, Cecilia acts to minimise the negative impact of her corrections; Pomerantz and Heritage, 2012). Rather, Cecilia asks the student what the instruction says (Lines 15–16), which he admits not having read (Line 17). In response to this, Cecilia confirms having noted that this is the case (Line 18), and then clarifies her expectations for what type of explanation she expects as an enactment of the context of the practical lesson (‘Det är ju de jag vill att ni förklarar’ [This is what I want you to explain], Line 20). This is in line with sensemaking needing to proceed within a predefined context (Weick, 1995), in this case framed by the information given in the instruction. Executing a chemistry practical lesson without reading the instructions could be regarded as breaking the rules of engagement, or the frame within which sensemaking could take place (Persson, 2018; Odden and Russ, 2019a). According to Weick (1995, p. 51), the frame, or the structure of the context within which sensemaking takes place is vital for how sensemaking proceeds as the context provides the cues from which sensemaking can arise. Hence, mildly chastising the student and thereby displaying his actions as less competent could be regarded as a corrective action to re-establish the social order (Persson, 2018, p. 71) and the context for sensemaking (Weick, 1995). With the context under threat, the creation of tension by the teacher would be required to enforce correction and the reestablishment of an environment supportive of sensemaking.
In conclusion, in their sensemaking dialogues all teachers had to manage (a) the tension arising from threats to student's display of competence within the interaction (identified through indicators of dispreferred conversational disagreements with regard to sensemaking; Pomerantz, 1984; Pomerantz and Heritage, 2012; Schegloff, 2007), which was produced by pointing out gaps in student reasoning (or directly contradicting them on some occasions); and (b) the need to create that same tension in order to cue dialogical sensemaking based on the student's current knowledge exposed through the knowledge gap being displayed. At the same time, the teachers used necessary aspects of sensemaking – that is, integrating knowledge (connecting concepts) and connecting theory with experience (Odden and Russ, 2019a) – in combination with face-saving actions to resolve the tension they had created in order for sensemaking to take place. Finally, sensemaking in the data was shown to only occur within a contextual frame of interaction that was pre-defined by the teacher.
Limitations
As this was a qualitative study, the principles underlying sensemaking outlined in the data cannot be proposed to be a general principle for all contexts and occasions. Although the data were collected in authentic and varied classroom contexts, the data sample was comparatively small, which meant that any claims on generalisation were limited. Also, as the data were collected using an emergent, inductive approach, the framework and purpose of the study emerged during the coding process, and the teachers could not verify their sensemaking intentions during data collection.Another limitation of the study was that the video data did not always cover all the gestures from the participants in the conversations. Hence, the importance of gestures, such as in providing cues for sensemaking about dynamic processes (Danielsson, 2016), could not be fully evaluated based on the collected data, although they were transcribed when available.
A third limitation of the study is that although purposive sampling was utilised to maximise variation in the students taking part in the study, all of the teachers were Swedish and the study took place at Swedish schools. The Swedish school system has a long tradition of egalitarianism (Tomasson, 1965). This is shown, for instance, through the schools’ promotion of social responsibility and democratic values, through the teachers’ non-confrontational teaching approaches, by teachers and students wearing informal clothing, and by teachers and students addressing each other by their first names (Gerdin et al., 2019). This egalitarian tradition could create sensemaking opportunities between teachers and students that may not be given at schools in other countries. Therefore, it is possible that teachers of other cultures utilise different actions that are more adapted to their local contexts and student populations.
Discussion and conclusions
Teachers’ actions supporting sensemaking in the chemistry classroom
In this study, sensemaking dialogues between four experienced teachers and their students were studied using conversation analysis. This type of in-depth study of teacher–student sensemaking conversations is novel to the research literature. All of the dialogues, which were inductively coded, contained conversational actions by teachers that promoted sustained sensemaking. These actions involved connecting experience to theory (for instance, cueing a student to connect the colour change of an equilibrium mixture to the concept of concentration), linking to alternative theoretical concepts (for instance, asking the student to reason in terms of reaction rates instead of concentration change) and managing tension (for instance, pointing out the difficulty of a task) in situations where students’ knowledge gaps were exposed. Hence, the data confirm the importance of including connections to theory, lived experience and the student's own words in sensemaking (Odden and Russ, 2019a). In addition, the dialogues showed insights into how the teachers managed the process of sensemaking about chemical phenomena through building on the students’ own expressions, guiding students back and forth between theory and experiences of phenomena or practice, and using both learnt and new concepts to the students as options to sustain sensemaking. As chemistry students often have difficulty navigating the observational and particulate knowledge domains of chemistry (Gabel et al., 1987; Ben-Zvi et al., 1988; Andersson, 1990; Treagust et al., 2003; Johnstone, 2006; Talanquer, 2008; Haigh et al., 2012; Stieff et al., 2013; Hernández et al., 2014; Becker et al., 2015), these examples of experienced teacher practice contribute to the research by showing how the connection between chemistry knowledge domains can be achieved through teacher–student interaction. As students making connections between these domains can be largely dependent on student previous knowledge and classroom context, it has been suggested that more studies are needed to explore how this can be achieved through teacher–student interaction (Xu, 2022). Although previous research has pointed out the importance of questioning, shifting focus and elaborating on student contributions to chemistry classroom dialogues (Warfa et al., 2014; Becker et al., 2015; Towns et al., 2019), the data from this study add to this research by showing how these aspects of sensemaking can be used on a moment-to-moment basis to help students make sense of chemical phenomena.Some dialogues in the data showed how the teacher used the chemical equation as a bridging concept to help students make sense of phenomena. The bridging role of symbols and icons has been proposed to be a versatile tool for building explanations using the language of chemistry (Grosholtz and Hoffmann, 2000; Taber, 2013). In instances where the chemical equation was used in this way, it mediated the connection between student observations and explanations of phenomena, leading to the progression of sensemaking. Thereby, it could be proposed that the chemical equation worked as a mediator for conceptual blending, where two of the students’ separate knowledge domains were merged to promote new ways of thinking about a phenomenon (Fauconnier and Turner, 2002; Odden, 2021). Based on the data, it is possible that the use of the chemical equation in teacher–student conversations could have a special role in mediating the progression of chemistry sensemaking. However, while the symbols of chemistry carry great symbolic utility for chemists, they can be confusing or overwhelming for learners and impede communication (Taber, 2013; Liu and Taber, 2016). Thus, the chemical equation could potentially both be a hindrance and a support in terms of teacher–student sensemaking about chemical phenomena.
The sensemaking dialogues in the data also depended on the contextual framing provided by the teacher. From the perspective of frames and framing (Goffman, 1974; Persson, 2018), conversational resources such as the chemical equation can be seen as a guide for the consensus of what is to be talked about, and how. In terms of sensemaking, the chemical equation can also be viewed as a cue that guides sensemaking down a preferred path (Weick, 1995). Hence, norms established by teachers to support classroom sensemaking can be both based on subject-based criteria on how to reason (Becker et al., 2013), and context, as was shown explicitly in Cecilia's conversation, where a lack of framing lead to the breakdown of sensemaking.
Tension management provided by teachers during teacher–student chemistry sensemaking
The study also described how sensemaking dialogues in chemistry can involve a delicate balance between sensemaking and tension. Tension that arose in situations where teachers appeared reluctant to openly present students as less competent occurred in all dialogues and was a ubiquitous aspect of sensemaking within the contexts that were studied. Anna's conversation with S1 is a clear example of student disengagement from the dialogue and teacher management of tension through restoring the student's presentation as competent. The results underline the importance of considering the emotional aspects of sensemaking within social contexts, as outlined by Weick (1995), who pointed out the role of identity construction in sensemaking. The possible role of classroom tension as an aspect of sensemaking in the science classroom has been previously proposed by Odden and Russ (2019b), who discovered the important role of vexing questions in sustaining sensemaking in student–student conversations. The data confirm previous studies showing that teacher–student dialogues are sensitive to threats to the display of student competence in the interaction (Oliveira, 2010; Criswell, 2012). Based on the ubiquitous presence of tension management in the sensemaking conversations guided by the experienced chemistry teachers in the present study, the results indicate that managing tension in teacher–student sensemaking is important when teaching chemistry. Students who feel competent in communicating using the language of chemistry also feel competent in their academic competence in the subject (Rüschenpöhler and Markic, 2020), which could explain the teachers’ actions. As frustration can lead to disengagement from the task at hand (D’Mello and Graesser, 2012) and lead to feelings of low self-efficacy and poor performance (Bandura, 1989), it would seem natural for experienced chemistry teachers to put value on supporting students’ sense of competency while helping them make sense of phenomena. Positive support for student academic self-concepts has been shown to influence both student engagement (Raufelder et al., 2015) and impact their long-term achievement (Wu et al., 2021). Resolving tension in interaction can also help students manage emotions that unchecked could lead to a narrowing of perception and a reluctance to engage with new material (Weick, 1995). The results from the present study support Weick's (1995) proposition that sensemaking involves an aspect of identity construction, and suggest that the chemistry teachers in the present study not only supported theoretical sensemaking during their lessons, but also the students’ academic self-concept in the subject of chemistry.Implications
Research studies on sensemaking are fairly new to chemistry education (Hunter et al., 2021). Therefore, recommendations for researchers that suggest how to best examine sensemaking in chemistry classrooms are important for the progression of the research field. It has been recommended (Hunter et al., 2021) that sensemaking studies in chemistry should include data collection on references in classroom conversations to real-world contexts, as well as tracking the building of explanations. Extrapolating on the data from the present study, and using the wider definition of sensemaking by Weick (1995), it is suggested that studies on sensemaking in chemistry in addition to this should include the collection of data such as student emotion, student self-perception in terms of their academic and/or language competency in the subject of chemistry, and dialogical tension as added explanations to why classroom sensemaking succeeds or does not succeed. As a person's emotional state can affect their perception during sensemaking (Weick, 1995), studies focusing on students’ emotional experience in relation to teacher–student sensemaking is a possible avenue for further research on sensemaking in the chemistry classroom context.The study presented in this paper has also given a detailed insight into how four experienced teachers used the inherent components of sensemaking interweaved with tension diffusion practices in order to sustain sensemaking dialogues in the chemistry classroom. These examples of successful practice could be useful to both teachers and teacher educators as a model for reflection about how to improve sensemaking in chemistry classrooms. In the present study, this was achieved through managing sustained theoretical sensemaking while displaying students as competent contributors. Probing student knowledge, connecting phenomena and theory, suggesting alternative concepts, careful use of the chemical equation, and supporting the display of students as competent through for instance reformulating student answers and changing the use of pronouns, are all simple conversational actions that could potentially bring great benefit in classroom sensemaking interactions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research study is the product of a collaboration between the Department of Chemistry and the Department of Educational Sciences at Lund University. The article is a product of the joint effort of author writing and seminar discussions. The author wishes to thank Professor Vesa Leppänen for taking the time for regular discussions during the data analysis and for giving feedback on the manuscript.References
- Abrahams I. and Millar R., (2008), Does practical work really work? A study of the effectiveness of practical work as a teaching and learning method in school science, Int. J. Sci. Educ., 30(17), 1945–1969.
- Altheide D. L. and Schneider C. J., (2013), Qualitative media analysis, London: SAGE Publications.
- Andersson B., (1990), Pupil's conceptions of matter and its transformations (age 12–16), Stud. Sci. Educ., 18, 53–85.
- Bandura A., (1989), Regulation of cognitive processes through perceived self-efficacy, Dev. Psychol., 25(5), 729–735.
- Barke H.-D., Hazari A. and Yitbarek S., (2010), Misconceptions in chemistry: addressing perceptions in chemical education, Berlin: Springer.
- Becker N., Rasmussen C., Sweeney G., Wawro M., Towns M. and Cole R., (2013), Reasoning using particulate nature of matter: an example of a sociochemical norm in a university-level physical chemistry class, Chem. Educ. Res. Pract., 14(1), 81–94.
- Becker N., Stanford C., Towns M. H. and Cole R., (2015), Translating across macroscopic, submicroscopic, and symbolic levels: the role of instructor facilitation in an inquiry-oriented physical chemistry class, Chem. Educ. Res. Pract., 16(4), 769–785.
- Beghetto R. A., (2009), Correlates of intellectual risk taking in elementary school science, J. Res. Sci. Teach., 46(2), 210–223.
- Belczyk-Kohl Y., (2016), Some remarks on transcript translation in discourse analysis, Eur. J. Appl. Linguist., 4(1), 139–164.
- Ben-Zvi R., Eylon B. and Silberstein J., (1988), Theories, principles and laws, Educ. Chem., 25, 89–92.
- Bills L., (2000), Politeness in teacher-student dialogue in mathematics: a socio-linguistic analysis, For Learn. Math., 20(2), 40–47.
- Bong M., and Skaalvik E. M., (2002), Academic self-concept and self-efficacy: how different are they really? Educ. Psychol. Rev., 18(1): 1–40.
- Cannady M. A., Vincent-Ruz P., Chung J. M. and Schunn C. D., (2019), Scientific sensemaking supports science content learning across disciplines and instructional contexts, Contemp. Educ. Psychol., 59, 1–15.
- Cohen L., Manion L. and Morrison K., (2018), Research methods in education, London: Routledge.
- Cowan K., (2014), Multimodal transcription of video: examining interaction in early years classrooms, Classr. Discourse, 5(1), 6–21.
- Criswell B. A., (2012), Reducing the degrees of freedom in chemistry classroom conversations, Chem. Educ. Res. Pract., 13(1), 17–29.
- Danielsson K., (2016), Modes and meaning in the classroom – the role of different semiotic resources to convey meaning in science classrooms, Linguist. Educ., 35, 88–99.
- D’Mello S. and Graesser A., (2012), Dynamics of affective states during complex learning, Learn. Instr., 22(2), 145–157.
- Driel J. H. and Gräber W., (2002), The teaching and learning of chemical equilibrium, in: Gilbert J. K., de Jong O., Justi R., Treagust D. F., and van Driel J. H. (ed.), Chemical education: towards research-based practice, Dordrecht: Kluwer Academic Publishers, pp. 271–292.
- Fang Z., (2016), Text complexity in the US common core state standards: a linguistic critique, Aust. J. Lang. Lit., 39(3), 195–206.
- Fauconnier G. and Turner M., (2002), The way we think: conceptual blending and the mind's hidden complexities, New York, NY: Basic books.
- Fitzgerald M. S. and Palincsar A. S., (2019)., Teaching practices that support student sensemaking across grades and disciplines: a conceptual review, Rev. Res. Educ., 43(1), 227–248.
- Flyvbjerg B., (2001), Making social science matter, Cambridge: Cambridge University Press.
- Gabel D. L., Samuel K. V. and Hunn D., (1987), Understanding the particulate nature of matter, J. Chem. Educ., 64(8), 695–697.
- Gardner R., (2012), Conversation Analysis in the Classroom. The Handbook of Conversation Analysis, Chichester: John Wiley and Sons, pp. 593–611.
- Geertz C., (1973), Thick description: toward an interpretive theory of culture, The interpretation of cultures: selected essays, New York, NY: Basic Books, pp. 3–30.
- Gerdin, G., Philpot, R. A., Larsson, L., Schenker, K., Linnér S., Moen K. M., Westlie K., Smith W., and Legge M., (2019), Researching social justice and health (in)equality across different school Health and Physical Education contexts in Sweden, Norway and New Zealand, Eur. Phy. Educ. Rev., 25(1), 273–290.
- Goffman E., (1955), On face-work: an analyisis of ritual elements in social interaction, Psychiatry, 18(3), 213–231.
- Goffman E., (1974), Frame analysis: an essay on the organization of experience, Cambridge, MA: Harvard University Press.
- Grosholtz E. R. and Hoffmann R., (2000), How symbolic and iconic languages bridge the two worlds of the chemist: a case study from contemporary bioorganic chemistry, in Bhushan N. and Rosenfeld S. (ed.), Of minds and molecules: new philosophical perspectives on chemistry, Oxford: Oxford University Press, pp. 230–247.
- Gunstone R. F. and White R. T., (1981), Understanding of gravity, Sci. Educ., 65(3), 291–299.
- Haigh M., France B., and Gounder R., (2012), Compounding confusion? When illustrative practical work falls short of its purpose – a case study, Res. Sci. Educ., 42(5), 967–984.
- Hakuta K., Santos M. and Fang Z., (2013), Challenges and opportunities for language learning in the context of the CCSS and the NGSS, J. Adolesc. Adult Lit., 56(6), 451–454.
- Heritage J., (1984), Garfinkel and ethnomethodology, Cambridge: Polity Press.
- Hernández G. E., Criswell B. A., Kirk N. J., Sauder D. G. and Rushton G. T., (2014), Pushing for particulate level models of adiabatic and isothermal processes in upper-level chemistry courses: a qualitative study, Chem. Educ. Res. Pract., 15(3), 354–365.
- Hofstein A. and Kind P. M., (2012), Learning in and from science laboratories, in: Fraser B. J., Tobin K. G., and McRobbie C. J. (ed.), Second international handbook of science education, Dordrecht: Springer, pp. 189–207.
- Hunter K. H., Rodriguez J. M. G. and Becker N. M., (2021), Making sense of sensemaking: using the sensemaking epistemic game to investigate student discourse during a collaborative gas law activity, Chem. Educ. Res. Pract., 22(2), 328–346.
- Hutchby I. and Wooffitt R., (2008), Conversation analysis, Cambridge: Polity Press.
- International Baccalaureate Organization, (2017), Diploma programme grade descriptors. Available at: https://www.ibo.org/contentassets/0b0b7a097ca2498ea50a9e41d9e1d1cf/dp-grade-descriptors-en.pdf (Accessed: 6 April 2023).
- Johnstone A. H., (2006), Chemical education research in Glasgow in perspective, Chem. Educ. Res. Pract., 7(2), 49–63.
- Kalyuga S., Ayres P., Chandler P., and Sweller J., (2003), The expertise reversal effect, Educ. Psychol., 38(1), 23–31.
- Kangasharju H. and Nikko T., (2009), Emotions in organizations: joint laughter in workplace meetings, J. Bus. Commun., 46(1): 100–119.
- Kind V., (2004), Beyond appearances: students’ misconceptions about basic chemical ideas. A report prepared for the Royal Society of Chemistry, London: Royal Society of Chemistry.
- Kind P. M., Kind V., Hofstein A. and Wilson J., (2011), Peer argumentation in the school science laboratory – exploring effects of task features, Int. J. Sci. Educ., 33(18), 2527–2558.
- Lee O., Grapin S. and Haas A., (2018), Talk in the science classroom, in Bailey A. L., Maher C. A., and Wilkinson L. C. (ed.), Language, literacy and learning in the STEM disciplines: how language counts for english learners, New York, NY: Routledge, pp. 35–52.
- Lemke J. L., (1990), Talking science: language, learning and values, Westport, CT: Ablex Publishing Corporation.
- Lemke J. L., (2004), The literacies of science, in Saul, E. W. (ed.), Crossing borders in literacy and science instruction: perspectives on theory and practice, Arlington, VA: NSTA Press, pp. 33–47.
- Lindström A., (1999), Språk som social handling: grammatik, prosodi och interaktion i svenska samtal, PhD thesis, Uppsala University, Acta Universitatis Upsaliensis.
- Liu Y. and Taber K. S., (2016), Analysing symbolic expressions in secondary school chemistry: their functions and implications for pedagogy, Chem. Educ. Res. Pract., 17(3), 439–451.
- Macbeth D., (2011), Understanding understanding as an instructional matter. J. Pragmat., 43(2), 438–451 DOI:10.1016/j.pragma.2008.12.006.
- Markic S. and Childs P. E., (2016), Language and the teaching and learning of chemistry. Chem. Educ. Rese. Pract., 17(3), 434–438.
- Miles M. B., Huberman A. M. and Saldaña J., (2014), Qualitative analysis: a methods sourcebook, London: SAGE Publications.
- Norris S. P. and Phillips L. M., (2003), How literacy in its fundamental sense is central to scientific literacy, Sci. Educ., 87(2), 224–240.
- Odden T. O. B., (2021), How conceptual blends support sensemaking: a case study from introductory physics, Sci. Educ., 105(5), 989–1012.
- Odden T. O. B. and Russ R. S., (2019a), Defining sensemaking: bringing clarity to a fragmented theoretical construct, Sci. Educ., 103(1), 187–205.
- Odden T. O. B. and Russ R. S., (2019b), Vexing questions that sustain sensemaking, Int. J. Sci. Educ., 41(8), 1052–1070.
- Oliveira A. W., (2010), Improving teacher questioning in science inquiry discussions through professional development, J. Res. Sci. Teach., 47(4), 422–453.
- Osborne J., (2010), Arguing to learn in science: the role of collaborative, critical discourse, Science, 328(5977), 463–466.
- Persson A., (2018), Framing social interaction: continuities and cracks in Goffman's frame analysis, London: Routledge.
- Pomerantz A., (1984), Agreeing and disagreeing with assessments: some features of preferred/dispreferred turn shapes, in Atkinson J. M. and Heritage J. (ed.), Structures of social action, Cambridge: Cambridge University Press, pp. 57–101. Retrieved from https://scholarsarchive.library.albany.edu/cas_communication_scholar.
- Pomerantz A. and Heritage J., (2012), Preference, in Sidner J. and Stivers T. (ed.), The handbook of conversation analysis, Malden, MA: John Wiley and Sons, pp. 210–228.
- Raufelder D., Sahabandu D., Martínez G. S. and Escobar V., (2015), The mediating role of social relationships in the association of adolescents’ individual school self-concept and their school engagement, belonging and helplessness in school, Educ. Psychol., 35(2): 137–157.
- Rüschenpöhler L., and Markic S., (2020). Secondary school students’ chemistry self-concepts: gender and culture, and the impact of chemistry self-concept on learning behaviour, Chem. Educ. Res. Pract., 21(1), 209–219.
- Russ R. S. and Berland L. K., (2019), Invented science: a framework for discussing a persistent problem of practice, J. Learn. Sci., 28(3), 279–301.
- Russ R. S. and Odden T. O. B., (2017), Intertwining evidence- and model-based reasoning in physics sensemaking: an example from electrostatics, Phys. Rev. Phys. Educ. Res., 13(2), 020105.
- Schegloff E. A., (2007), Sequence organization in interaction – a primer in conversation analysis, Cambridge: Cambridge University Press.
- Skolverket, (2022), Kemi. Available at: https://www.skolverket.se/undervisning/gymnasieskolan/laroplan-program-och-amnen-i-gymnasieskolan/gymnasieprogrammen/amne?url=-996270488%2Fsyllabuscw%2Fjsp%2Fsubject.htm%3FsubjectCode%3DKEM%26version%3D2%26tos%3Dgyandsv.url=12.5dfee44715d35a5cdfa92a3 (Accessed: 6 April 2023).
- Stieff M., Ryu M. and Yip J. C. (2013). Speaking across levels – generating and addressing levels confusion in discourse, Chem. Educ. Res. Pract., 14(4), 376–389.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168.
- Taber K. S., (2017), Teaching and learning chemistry, in: Taber K. S. and Akpan B. (ed.), Science education: an international course companion, Rotterdam: Sense Publishers, pp. 325–341.
- Talanquer V., (2008), Students’ predictions about the sensory properties of chemical compounds: additive versus emergent frameworks, Sci. Educ., 92(1), 96–114.
- Talanquer V., (2015), Threshold concepts in chemistry: the critical role of implicit schemas, J. Chem. Educ., 92(1), 3–9.
- The Swedish Research Council, (2011), God forskningssed (Vetenskapsrådets rapportserie 2011:1), Stockholm.
- Tomasson R. F., (1965), From elitism to egalitarianism in Swedish education, Sociol. Educ., 38(3), 203–223.
- Towns M. H., Cole R. S., Moon A. C. and Stanford C., (2019), Argumentation in physical chemistry, in Erduran S. (ed.), Advances in chemistry education, The Royal Society of Chemistry, pp. 247–274.
- Treagust D. F., Chittleborough G. and Mamiala T. L., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25(11), 1353–1368.
- Vilhunen E., Chiu M. H., Salmela-Aro K., Lavonen J. and Juuti K., (2022), Epistemic emotions and observations are intertwined in scientific sensemaking: a study among upper secondary physics students, Int. J. Sci. Math. Educ., 21, 1545–1566.
- Warfa A. R. M., Roehrig G. H., Schneider J. L. and Nyachwaya J., (2014), Role of teacher-initiated discourses in students’ development of representational fluency in chemistry: a case study, J. Chem. Educ., 91(6), 784–792.
- Weick K. E., (1995), Sensemaking in organizations, Thousand Oaks, CA: SAGE Publications.
- Wu H., Guo Y., Yang Y., Zhao L., Guo C., (2021), A meta-analysis of the longitudinal relationship between academic self-concept and academic achievement, Educ. Psychol. Rev., 33(4): 1749–1778.
- Xu L., (2022), Towards a social semiotic interpretation of the chemistry triangle: student exploration of changes of state in an Australian secondary science classroom, Int. J. Sci. Math. Educ., 20(4), 705–726.
- Yan F. and Talanquer V., (2015), Students’ ideas about how and why chemical reactions happen: mapping the conceptual landscape, Int. J. Sci. Educ., 37(18), 3066–3092.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3rp00249g |
| ‡ This is the only upper secondary school programme in the Swedish school system that covers chemical equilibrium. |
| § In the Swedish grading system, the highest grade (A) can be said to correspond to extensive and nuanced reasoning, a C to extensive reasoning, an E to synoptic but satisfactory reasoning and an F to not fulfilling the requirements for E. A B grade fulfils the requirements for Grade C but also, to a large extent, Grades A and D work equivalently as a grade in-between E and C (Skolverket, 2022). The IB achievement levels range from 1 (fragmentary knowledge) to 7 (comprehensive knowledge) (International Baccalaureate Organization, 2017). |
¶ Anna is labelled ‘A’ and the student is labelled ‘S’. ‘[’ denotes overlapping speech, ‘#’ a creaky voice, ‘< >’ slower speech, ‘:’ a prolonged vowel, ‘==’ a continuation of a turn or speakers latching onto each other's utterances, ‘°word°’ a softer voice, ‘↑’ and ‘↓’ a temporary markedly higher or lower pitch, ‘![[w with combining low line]](https://www.rsc.org/images/entities/char_0077_0332.gif) ![[o with combining low line]](https://www.rsc.org/images/entities/char_006f_0332.gif) ![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif) ![[d with combining low line]](https://www.rsc.org/images/entities/char_0064_0332.gif) ’ denotes emphasis, ‘.hh’ indicates an intake of breath, ‘heh’ indicates laughter, and parentheses denote pauses and their length in seconds. The transcript translation (following recommendations by Belczyk-Kohl, 2016) uses standardised language, meaning the word order has been changed where the languages differ, and has interchanged Swedish idioms or interjections for appropriate idioms or interjections in English. It also shows words that are emphasised in the original transcript in bold, and is translated utterance-by-utterance to retain the social meaning of the original (Belczyk-Kohl, 2016).Anna is labelled ‘A’ and the student is labelled ‘S’. ‘[’ denotes overlapping speech, ‘#’ a creaky voice, ‘ < >’ slower speech, ‘:’ a prolonged vowel, ‘ = =’ a continuation of a turn or speakers latching onto each other's utterances, ‘°word°’ a softer voice, ‘↑’ and ‘↓’ a temporary markedly higher or lower pitch, ‘ ’ denotes emphasis, ‘.hh’ indicates an intake of breath, ‘heh’ indicates laughter, and parentheses denote pauses and their length in seconds. The transcript translation (following recommendations by Belczyk-Kohl, 2016) uses standardised language, meaning the word order has been changed where the languages differ, and has interchanged Swedish idioms or interjections for appropriate idioms or interjections in English. It also shows words that are emphasised in the original transcript in bold, and is translated utterance-by-utterance to retain the social meaning of the original (Belczyk-Kohl, 2016).Anna is labelled ‘A’ and the student is labelled ‘S’. ‘[’ denotes overlapping speech, ‘#’ a creaky voice, ‘ < >’ slower speech, ‘:’ a prolonged vowel, ‘ = =’ a continuation of a turn or speakers latching onto each other's utterances, ‘°word°’ a softer voice, ‘↑’ and ‘↓’ a temporary markedly higher or lower pitch, ‘![[w with combining low line]](https://www.rsc.org/images/entities/char_0077_0332.gif) ![[o with combining low line]](https://www.rsc.org/images/entities/char_006f_0332.gif) ![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif) ![[d with combining low line]](https://www.rsc.org/images/entities/char_0064_0332.gif) ’ denotes emphasis, ‘.hh’ indicates an intake of breath, ‘heh’ indicates laughter, and parentheses denote pauses and their length in seconds. The transcript translation (following recommendations by Belczyk-Kohl, 2016) uses standardised language, meaning the word order has been changed where the languages differ, and has interchanged Swedish idioms or interjections for appropriate idioms or interjections in English. It also shows words that are emphasised in the original transcript in bold, and is translated utterance-by-utterance to retain the social meaning of the original (Belczyk-Kohl, 2016). ’ denotes emphasis, ‘.hh’ indicates an intake of breath, ‘heh’ indicates laughter, and parentheses denote pauses and their length in seconds. The transcript translation (following recommendations by Belczyk-Kohl, 2016) uses standardised language, meaning the word order has been changed where the languages differ, and has interchanged Swedish idioms or interjections for appropriate idioms or interjections in English. It also shows words that are emphasised in the original transcript in bold, and is translated utterance-by-utterance to retain the social meaning of the original (Belczyk-Kohl, 2016). |
| This journal is © The Royal Society of Chemistry 2024 |