 Open Access Article
Open Access ArticleComment on “What resources do high school students activate to link energetic and structural changes in chemical reactions? – A qualitative study” by B. Pölloth, D. Diekemper and S. Schwarzer, Chem. Educ. Res. Pract., 2023, 24, 1153
Keith S.
Taber

Faculty of Education, University of Cambridge, UK. E-mail: kst24@cam.ac.uk
First published on 9th December 2023
Abstract
A recent study in Chemistry Education Research and Practice highlights the common pattern of student thinking known as ‘the octet framework’, and notes how it seems to relate to, but be inconsistent with, the octet rule: an idea commonly taught in introductory chemistry classes. The study noted the common feature of learners extending the octet rule into ‘a driving force’ for chemical change, but analysis also noted two other features of the alternative conceptual framework. It is argued here that these research findings reflect a key problem in chemistry education: one that the research community should prioritise for further investigation.
Introduction
Chemistry Education Research and Practice invites comments that offer “an alternative analysis of and/or new insight into… previously published” articles. In this comment I discuss one of the themes highlighted in the recent paper “What resources do high school students activate to link energetic and structural changes in chemical reactions? – A qualitative study” (Pölloth et al., 2023). This study raises several interesting and important themes, but my focus will be on one of these that I believe reflects a critical problem in teaching and learning chemistry. My aim in this comment is not to criticise the work as published, but to (i) develop this particular theme in a little more detail and (ii) argue for stronger implications from the reported results for directing further research.Octets and student thinking
I counted the word ‘octet’ being used 23 times in Pölloth and colleagues’ (2023) paper. The authors report that the notion of the octet figured heavily in student thinking as reflected in their data, in particular, in terms of being considered as ‘a driving force’ for chemical reactions (p. 1163). They relate this to the ‘octet rule’ which they report is taught “in the first years of chemistry” (p. 1163) in the context where they carried out their research, German high schools. They also refer to the octet ‘framework’ having been reported in previous research (p. 1164).Pölloth and colleagues’ argue that the octet rule is a canonical part of chemistry, which is therefore understandably included in elementary chemistry teaching, but that students’ use of the octet rule as found in their study, as a ‘driving force’ for chemical reaction, is invalid. The authors do not specify what they mean by the octet rule (that is, exactly what is being taught in introductory chemistry lessons in German high schools). As they are writing for a professional readership of chemistry educators, they can reasonably expect their readers to know what the ‘octet rule’ is, but their findings reflect previous research that the octet rule seems to be inappropriately applied to explain why chemical reactions occur, even when the reactants fit the rule as much as the products. Moreover, this is not an idiosyncratic notion offered by an outlier in research: in Pölloth and colleagues’ study “eleven [of 16] groups gave the same explanation for the driving force”. This seems to present a phenomenon worthy of further investigation: why do so many students seem to misunderstand/misapply this heuristic? It also raises the question of whether the teaching around this rule needs to be revisited. These are the points I will develop in this comment.
Alternative conceptions and frameworks
The octet framework, as a common pattern of thinking found among chemistry learners, has several aspects, and, although Pölloth and colleagues did not adopt it as a analytical tool for data analysis, the data reported in the paper link to several of these features. In their paper, Pölloth and colleagues (p. 1154) refer to how expert chemical knowledge is highly connected, but how learners commonly acquire knowledge in a more fragmentary manner.Terms such as alternative conception and alternative framework are often used (along with other terms such as misconception, intuitive theory, preconception, etc.) synonymously in the literature (Abimbola, 1988). However, it can be useful to distinguish between (i) discrete alternative conceptions (that can be represented in a single proposition) and (ii) more elaborated frameworks of ideas. So, the idea that ‘the noble gases do not form compounds’ can be considered a conception, although it generalises to a number of cases (xenon does not form compounds, argon does not form compounds, etc.). For a long time, when the term ‘inert gases’ was widely used, this particular conception was considered canonical, but now this would be considered an alternative conception. Other such alternative conceptions might be ‘[all] acids are dangerous’ and ‘[all] metals are magnetic’, or that ‘elements are monatomic’.
An alternative conceptual framework, when used in the sense suggested for the octet framework (Taber, 1998), is of something more elaborated – a set of related conceptions (at least some of which are at odds with canonical science) that form a consistent and mutually reinforcing structure for thinking about some aspects of the natural world (e.g., see Fig. 1). In terms of the account of science offered by the physicist and philosopher of science Mario Bunge (2017/1998), an alternative conceptual framework might be seen as standing in relation to an alternative conception in much the same way that a scientific theory relates to a single hypothesis.
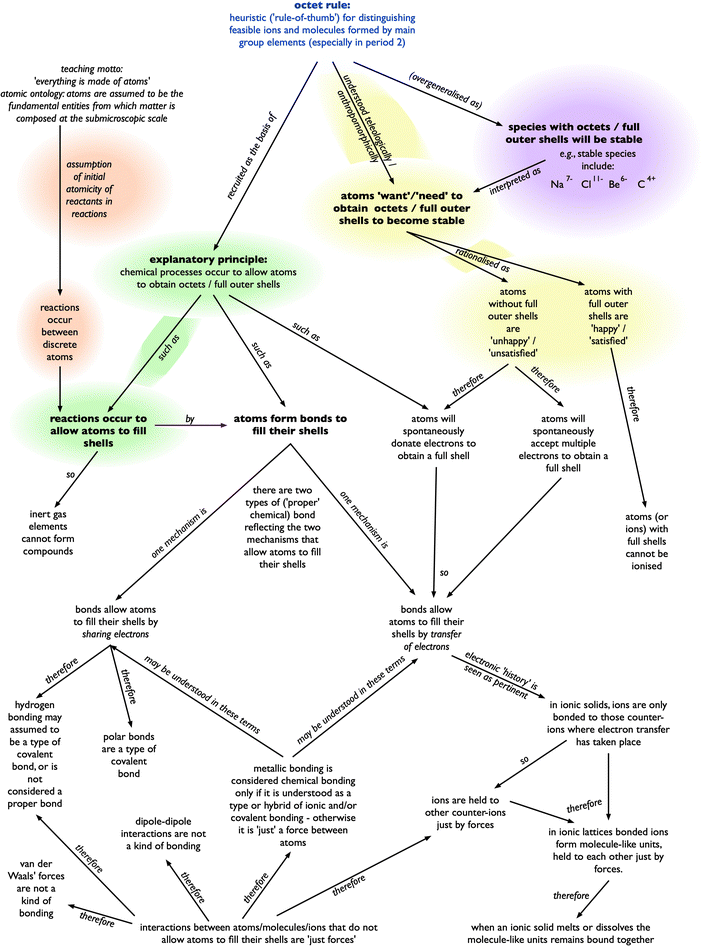 | ||
| Fig. 1 A representation of the octet framework, an alternative conceptual framework encompassing related common alternative conceptions, that seems to derive from interpretation of teaching. (After Fig. 5 in Taber, 2013.) The shading refers to themes discussed in the text. | ||
A once active debate about the nature of learner thinking related to whether students’ alternative ideas at odds with scientific accounts could be considered ‘theory-like’ (Driver, 1989) rather than just being isolated notions (Claxton, 1993). This was seen as an important question as conceptual frameworks that make up an extended set of related and inter-linked ideas are likely to be more resistant to correction by teaching than discrete notions that are not embedded in a supporting network of associations. Given the extensive research-base now available it seems reasonable to state that learners’ alternative thinking about natural phenomena varies along this dimension such that it can sometimes be, but is by no means always, theory-like. Indeed, this characterisation applies to people's thinking more generally.
Pölloth and colleagues draw upon the ‘resources’ perspective on learning and cognition (diSessa, 1988) which sees much thinking as drawing from a repertoire of resources to construct transient conceptual ‘assemblages’ (Brock et al., 2023) for immediate purposes, in contrast to more permanent extensive conceptual frameworks. The development of stable and extensive conceptual structures is often associated with the acquisition of expertise in an area, and, so, with canonical thinking. The octet framework was however proposed as an example of a theory-like conceptual structure commonly acquired in introductory chemistry that is internally coherent, and yet inconsistent with several key areas of the higher grade chemistry curriculum (i.e., non-canonical). This ‘alternative framework’ is represented in Fig. 1.† The core conception in this framework explains chemical processes as occurring to bring about octets or full outer shells; that is, in Pölloth and colleagues’ terms, seeing this as ‘a driving force’ for chemical reactions. This feature is highlighted with green shading in Fig. 1.
The octet framework‡ was proposed following an interview-based study undertaken in an English college (where students, c.16–19 years of age, progressed from a wide range of schools), and where
“students were found to commonly use the octet rule – a heuristic for identifying stable chemical species – as the basis of a principle to explain chemical reactions and chemical bonding: the full shells explanatory principle. From this perspective bonding “is done in order to try to achieve a stable structure, i.e., eight electrons in the outer shell of the atom”. All of the [study participants] used the octet rule as an explanatory principle at some point during the interviews, although the phrasing varied…”
(Taber, 1998, pp. 600–601)
This clearly matches somewhat what was reported in Pölloth and colleagues’ German study (which of course is not only a different educational system, but one where students study in a different language of instruction). The octet framework included other aspects (common across the sample, although those interviewed did not necessarily demonstrate all of these features) as reflected in Fig. 1. Two of these features, beyond the octet rule as a driving force, that are reflected in the data Pölloth and colleagues report to illustrate their analysis were:
• The assumption of initial atomicity
• Use of anthropomorphic language
Students make an assumption that reactants are always atomic
The assumption of initial atomicity is where “learners may assume that any chemical system they are asked to consider has evolved from discrete atoms” (Taber, 1998, p. 601). This feature is highlighted with orange shading in Fig. 1. Pölloth and colleagues report that students commonly explained the reaction between hydrogen and chlorine in terms of the products having octets, despite “already the two reactants Cl2 and H2 have a full valence shell. Thus, the octet rule cannot explain the driving force of this reaction” (p. 1163). It seems that students are making a flawed assumption of initial atomicity.Fig. 2 shows a question from a diagnostic assessment tool included in a Royal Society of Chemistry resource (Taber, 2002). This question asked about a parallel reaction to that used in the German study. Students given this question commonly explained the reaction in terms of the atoms obtaining full shells/fluorine acquiring eight electrons. Yet, as Fig. 2 shows, the question included the information that the reactants were initially molecular (H2, F2). Despite this, the assumption of initial atomically was so strong that even when given this information as part of a question, students commonly gave an explanation that would only be valid for atomic reactants. This seems to be a widespread phenomenon, despite none of the reactions commonly studied in school chemistry (or beyond) being between atomised samples. This conception seems to be widely applied across chemical contexts, and seems to be tenacious. For example, students may explain the formation of sodium chloride in terms of an energetically non-feasible electron transfer from a discrete sodium atom to a discrete chlorine atom, even if they have prepared sodium chloride in the laboratory by neutralisation, starting with reagent solutions (of HCl, NaOH) that already contain the ions found in the product.
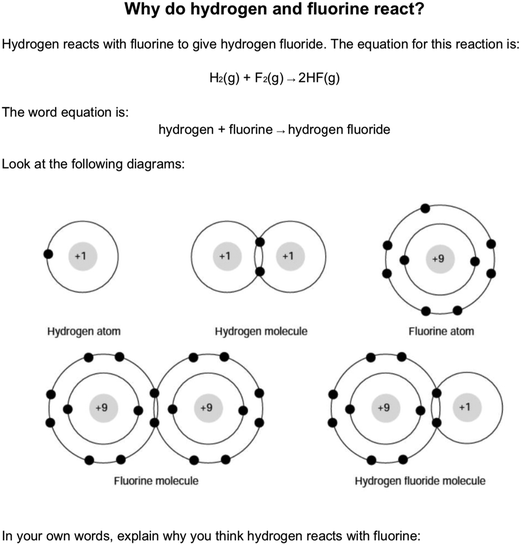 | ||
| Fig. 2 A question about why reactions occur (from Taber, 2002 – reproduced with the permission of the Royal Society of Chemistry). | ||
Anthropomorphic language
Another feature of the octet framework that is reflected in Pölloth and colleagues’ data is the use of anthropomorphic language. This feature is highlighted with yellow shading in Fig. 1. In the study which led to the proposal of the octet framework as an alternative conceptual framework,“…it was very common for students to speak of atoms as if they were sentient actors in the molecular soap opera of chemistry…there were many suggestions that atoms wanted or needed to gain or lose electrons. Indeed this usage was ubiquitous in student comments, although alternatives included atoms preferring, liking, being eager, or having no wish for certain outcomes. When hydrogen bonding in hydrogen fluoride was considered one [participant] accused the fluorine of being greedy for ‘trying to grab two electrons’ from different hydrogen atoms”.
(Taber, 1998, p. 603)
Pölloth and colleagues report students referring to atoms “aiming for the eight electrons” and how a chlorine atom would not “want to react with something that also has seven valence electrons [but rather] want to react with something that has one” (p. 1163). They found students equating stability with not ‘wanting’ to react, and inertness with being ‘very lazy’.
Anthropomorphic explanations are not valid in chemistry, so this raises the question of why they are so common among students. In their paper, Pölloth and colleagues consider the target models presented in the curriculum, and discuss the core and integrating role of energy as a key concept, yet they report than in their study they found that “students seldom refer to energy minimisation to reason on chemical reactivity” (p. 1166).
The suggestion of atoms ‘wanting’ or ‘needing’ or ‘trying to get’ octets or full shells as the basis of chemical explanations has been elicited from secondary students in various other national contexts such as Finland (Joki and Aksela, 2018), Israel (Zohar and Levy, 2019), Sweden (Adbo, 2012) and Turkey (Ünal et al., 2010) and also at undergraduate level in the United States (Nicoll, 2001; Wang and Barrow, 2013) and Australia (Coll and Treagust, 2001) and even among postgraduate students in New Zealand (Coll and Taylor, 2002; Coll and Treagust, 2003). This kind of language can offer the sense of an explanation, but does not amount to a scientific explanation as it relies upon the supposed desires of atoms rather than any natural cause. This makes such explanations teleological as they focus on an assumed desirable end-state without offering a mechanism for its attainment, and such pseudo-explanations are not only found in students’ explanations (Taber and Watts, 2000), but also in textbooks assigned to students (Talanquer, 2007).
Another learner is quoted (in translation) in Pölloth and colleagues’ paper as suggesting “if there aren’t eight electrons, a substance is very reactive because it strives for a stable state” (p. 1164). Students seem to readily come to equate stability with octet or full shell configurations.
The stability of the octet
Clearly there is a sense whereby valence shell octet and/or full outer shell electronic configurations (which is only strictly the same thing in period 2) are associated with stable chemical entities. This is the basis of the octet rule, a heuristic or rule-of-thumb that suggests that main group elements in stable chemical entities (molecules, ions) are likely to have such structures. This is an incredibly useful teaching tool in introductory classes when basic atomic structure is still new, and perhaps strange, to learners. It helps students predict that, for example, the common compound of nitrogen and hydrogen will be NH3 rather than, say, NH2 or NH4; and that salts of calcium will likely include the Ca2+ ion rather than, say, the Ca3+ or Ca2− ion.The rule is limited. It does not help as much with the d-block elements. Copper forms compounds with the Cu+ ion as well as with the Cu2+ ion. It has many exceptions beyond the second period: compounds such as sulphates, PCl5, SF6, inter-halogen compounds, and so forth, with ‘expanded octets’, do not fit the rule. It would rule out any compounds of the inert gases. It is not an absolute guide even in period 2, as CO is stable enough to be a substance of significance, and there are relatively stable ‘electron deficient’ compounds such as BF3.
A major logical problem is that commonly students seem to reverse the idea of the rule: rather than just rule out unlikely species (Cl2+, CH5), it may be used to rule in any species that fits the rule as being inherently stable. This feature is highlighted with mauve shading in Fig. 1. So, it is common for students to think that chemically unlikely species such as Na7−, Be6−, and C4+, as well as Cl11− (i.e., a species with a full outer shell, but not an octet), and even an excited atom such as Cl with electronic configuration 1.8.8, will be stable because they fit the criterion of an octet or full outer shell (Taber, 2009). Students apply this without consideration of context. Na+ is an ion commonly found stabilised in chemical systems such as lattices and hydrated in solution, but Na+ is commonly assumed by learners to be inherently more stable than the neutral atom, such that a sodium atom would spontaneously emit an electron to obtain an octet configuration. Indeed, considering ionisation, neon and argon are commonly assumed to have the highest ionisation enthalpies in their periods because they have stable octet structures (when both fit the general patterns of increasing first ionisation enthalpy across the respective periods).
The belief that an Na+ ion is intrinsically more stable than the sodium atom has also been found in secondary students in Finland (Joki and Aksela, 2018) and among undergraduates in the U.S.A. who also thought the (chemically implausible) Na7− anion would be more stable than the atom (Wang and Barrow, 2013). Israeli secondary students have also been found to assume species with an outer shell octet will be the most stable (Zohar and Levy, 2019).
The significance of the octet framework
The octet framework seems to be a very common alternative conceptual framework that is widely adopted by students in diverse educational contexts, and which seems to satisfy them as offering sound explanations. It concerns core teaching topics in school and college chemistry (stability, bonding, reactions, ionisation). Once acquired, it seems tenacious – explaining chemistry in terms of octets may be retained when other more technically correct ideas have faded. As suggested in Fig. 1, it can lead to learners entertaining a wide range of alternative conceptions.One critical issue seems to be that students have typically already come to think that reactions occur in order to allow atoms to obtain octets or full shells (sometimes, but not always, the same thing) before they are provided with canonical conceptual tools (e.g., bond enthalpies) to learn the scientific accounts. It seems that ‘octet thinking’ comes to replace ‘the explanatory vacuum’ in introductory chemistry classes where no scientific rationale for why chemical reactions occur tends to be offered to students. The octet rule seems to be adopted/adapted as an explanatory principle in the absence of any taught alternative, and typically has several years to be consolidated in student thinking before students move on to more advanced courses where ideas such as bond enthalpies and energy minimisation are usually introduced. However, this can surely only be considered a partial explanation given that – as Pölloth and colleagues remind us – the octet rule should not have any bite as an explanation of reactions where the reactants fit the rule just as well as the products. That is, in just about all reactions met in introductory chemistry.
The source of the octet framework
Some alternative conceptions developed by students may derive from common folk-beliefs that have currency in everyday discourse (Solomon, 1992). Yet it seems unlikely that properties of atomic level structures are commonly part of mealtime discussions in most homes.Another potential source is spontaneously developed notions based on intuitions about the world – common conceptions about force and motion seem to be of this type (diSessa, 1993). DiSessa (1993) has described how people develop what he termed ‘phenomenological primitives’ – features of cognition which reflect the preconscious recognition of common patterns in experience, and which come to be applied as intuitive elements of cognition across domains. These ‘p-prims’ are considered implicit knowledge elements that act as elementary resources to inform expectations and make sense of experience, and which may be automatically recruited in assembling more explicit conceptions (Karmiloff-Smith, 1996), as for example in developing explanations of natural phenomena. This perspective on building up conceptual structures from spontaneously developed cognitive resources has been especially influential in physics education (Hammer, 2000), the domain where diSessa (1993) has described a good many candidate p-prims; but is just as applicable in principle to other domains such as chemistry learning (Taber and García Franco, 2010). P-prims are not domain specific, as they represent intuitions of generalisable patterns, so at least some of diSessa's candidate p-prims are likely to be of wide relevance. However, as diSessa's study was limited to asking students about college physics contexts, it also seems plausible that it would not have revealed any p-prims that are seldom applied in those particular contexts.
Joki and Aksela (2018) have mooted the suggestion that “diSessa's (1993) p-prim of vacuums impel” may be related to the development of the octet framework. It is also possible that the degree to which the octet rule becomes widely adopted as an explanatory principle reflects the recruitment of a common p-prim not identified by diSessa from physics contexts, but which relates to “something about the ‘fullness’, or perhaps the symmetry, of the [full shell] pattern and marks it out as especially significant” (Taber, 2008, p. 1042).
It is not likely that learners commonly, spontaneously, intuit features of the molecular realm; but rather that certain p-prims are (without any conscious awareness) activated in sense-making where they seem to fit with what students are being taught about at this level. Vygostky's (1978) influential theory of learning distinguishes between (i) ideas that are developed spontaneously (e.g., in effect, intuitively) by the learner, and (ii) ideas that are acquired through inter-personal interactions (such as teaching) using symbolic language systems (text, diagram, gesture, etc.): but also argues that extant conceptual ideas learned from others can only be made sense of, and internalised, in terms of existing conceptual resources.
That is, it is certainly possible to learn pre-packaged complex and abstract conceptual material, such as established scientific theories, but only when these can be understood through existing thinking that, directly or indirectly, has its ultimate origin in spontaneous conceptions. Although Vygotsky died (in 1934) long before the debate, referenced above, about whether alternative (non-canonical) patterns of thinking exhibited by science learners could be considered theory-like, he had already proposed a model which suggested that a person's ideas covered a spectrum of levels of abstraction and complexity, but were always, at base, ground in spontaneous notions. An expert's highly abstract and conceptual understanding of some domain differs from the novice's in the extent to which it has iteratively built, stage upon stage, away from the intuitive cognitive resources on which all human conceptualisation ultimately depends.
The ‘resources’ perspective informing Pölloth and colleagues’ work offers an explanation for why learners might interpret chemistry teaching in terms of existing general intuitions/expectations (“productive resources”, p. 1168) about the nature of the world, and this offers a plausible account for why teaching about the octet rule might be over-interpreted by learners. This could certainly explain the origin of the perception of the octet or full shell as ‘télos’, but seems insufficient to explain the widespread and tenacious nature of ‘octet framework thinking’. It might also be feasible that some students, on being taught the octet rule, spontaneously adopt it as the basis of a much more far-reaching explanatory principle, but it would seem incautious to assume this could explain why so many learners seem to acquire the more extensive alternative framework. Something which is not supposed to be taught (reactions occur so atoms can get octets/full outer shells) seems to be learnt much more widely, and retained and applied much more reliably, than many of the notions that are set out in the curriculum as target knowledge, and that are taught and regularly reinforced in classes.
The role of teaching in reinforcing ‘octet thinking’
This raises the question of the possible role of teaching in supporting the development and consolidation of these ideas. Pölloth and colleagues rightly point out that the octet rule is commonly taught in school chemistry, but that “the octet rule cannot explain the driving force of” reactions (p. 1163). It might be wondered if the octet framework is so commonly acquired because teachers in elementary chemistry classes often go beyond teaching the octet rule per se, to implying it offers “the basis of a principle to explain chemical reactions and chemical bonding”, and/or do not correct students when they use this principle in this way. There certainly are reports in the literature suggesting this may be so in some contexts (Dhindsa and Treagust, 2014; Joki and Aksela, 2018).Adbo (2012) reported that a course book used by Swedish upper secondary students was quite explicit in suggesting reactions took place because atoms strived for a noble gas configuration. Presentations suggesting that chemical reactions take place between atomic species (e.g., a carbon atom interacts with four discrete hydrogen atoms; a sodium atom isolated from the metallic lattice interacts with a lone chlorine atom) have been identified in school textbooks (Taber, 2002; Bergqvist, 2017). The use of anthropomorphic language to discuss chemical processes has been reported in textbooks used by English (Taber, 2002), Greek (Tsaparlis et al., 2018) and Swedish (Bergqvist, 2017) secondary students, as well as by Swedish chemistry teachers themselves (Bergqvist, 2017).
In her Swedish study, Bergqvist reported that
“All the textbooks and teachers focused on individual atoms when presenting ionic, covalent, and polar covalent bonding…the octet rule was used explicitly or implicitly as a reason for bonding…Anthropomorphic descriptions were also common and used in many ways, both in textbooks and by teachers”
(Bergqvist, 2017, pp. 92–93)
Whether it transpires that the seemingly ubiquitous nature of ‘octet thinking’ is primarily the creative work of learners facing an explanatory vacuum, or largely derives from teachers and textbook authors doing their best to offer learners some kind of basis for explaining why chemistry occurs, research is indicated to find a solution to the core problem: i.e., the lack of a teaching model of why chemical change occurs, which is both (a) accessible at the introductory level, and yet (b) suitable for developing into the accounts taught in more advanced classes. Without that, it seems likely that learners will continue to adopt ‘the octet rule as a driving force’ in the way Pölloth and colleagues found in their study.
Informing curriculum development
Pölloth and colleagues draw upon the ‘resources’ perspective and this can explain why the use of a notion by a student on a specific occasion cannot be assumed to reflect established thinking and may rather just be the momentary activation and recruitment of a seemingly relevant resource from a diverse repertoire of such resources. Just because a student moots a particular explanation in a specific context on one occasion, it cannot be assumed the same explanation would be offered on another occasion or for a slightly different, but canonically substitutable, example (Palmer, 1997). Yet people do develop ways of thinking which they find productive and commit to, and come to apply widely, over time. Some alternative conceptual frameworks do become well-established in student thinking and are widely used despite contrary science instruction (Watts and Zylbersztajn, 1981). As Pölloth and colleagues suggest “the coherence of the knowledge network is a crucial factor” (p. 1154), and for many learners the idea that a full outer shell or octet of electrons is a driving force for chemical systems offers the core conception around which a coherent understanding of chemistry at the molecular level can be constructed (see Fig. 1).Pölloth and colleagues reiterate the well-rehearsed argument (Jensen, 1995; Taber, 2001; Levy Nahum et al., 2007; Tsaparlis et al., 2018) that in teaching about bonding and structure and reactivity in introductory chemistry, it is important to focus on physical arguments – on charges, forces, energy – as the basis for explaining phenomena. Research suggests that it is not sufficient for such ideas to be adopted in isolation by particular teachers or for specific grades. Research undertaken in Finland suggests that even when introductory chemistry was taught according to a carefully designed scheme to avoid learners treating the octet rule as the basis for explaining chemical change (Joki et al., 2015), students can subsequently take up such thinking whilst studying subsequent chemistry courses (Joki and Aksela, 2018). A detailed longitudinal case study of a student in England suggested that although ‘octet thinking’ found to be initially dominant on transition from secondary school became much less frequent during a two-year college course; octet thinking was again strongly represented in the individual's explanations of chemical phenomena several years after completing the course (Taber, 2003). That students seem so wedded to this alternative way of thinking about chemistry, even after being taught more canonical principles, as Pölloth and colleagues report, is both a key problem in teaching chemistry and – I would suggest – a core phenomenon that it would be valuable for the chemistry education research community to understand better.
The importance of designing ongoing learning experiences around a limited number of core scientific ideas (Key Stage 3 National Strategy, 2002) and of continuity across curriculum experiences is increasingly being recognised, as seen in studies to develop ‘learning progressions’ (Alonzo and Gotwals, 2012). One example of such a curriculum initiative which has been extended from the undergraduate level (Cooper et al., 2012) to include the high school level (Stowe et al., 2019) is ‘CLUE’ (‘Chemistry, Life, the Universe, and Everything’), and such carefully designed curriculum contexts offer useful cases to explore the development of student thinking. If teaching in this area is explored in terms of learning progressions, one possible approach would be to consider whether the octet rule might be productive as a kind of intermediate notion (Driver, 1989) that can be employed to build more advanced thinking. But learning progressions designed to structure curriculum need to be research-informed, and this would first require a better understanding of just how and why this particular idea becomes so readily and firmly recruited into the developing thinking of so many students.
In conclusion
Pölloth, Diekemper and Schwarzer raise a number of interesting issues in their analysis of student data. Here I have focussed on just one of their themes, because it reflects something found widely in other studies – that students commonly come to adopt a taught heuristic as the basis of an invalid explanatory principle that then later becomes widely used in preference to principles taught in upper secondary and higher levels. This does not concern a peripheral aspect of the curriculum, but the central notion of chemical change (and related notions about bonding, chemical stability, ionisation, etc.) It is widely accepted that the octet rule is valuable in introducing elementary ideas about molecules and ions, but it seems that teaching of this heuristic acts as a pedagogic learning impediment – something that is taught in class, but then later comes to interfere with intended learning of canonical chemistry.Pölloth and colleagues’ study is a timely reminder that students commonly adopting (and so adapting) the octet rule as a driving force of chemistry is a well-recognised issue that the chemistry education research community has not yet been able to address. This new study should perhaps be seen as a ‘driving force’ for further research, firstly to better understand precisely how and why this phenomenon develops, and then to explore changes to curriculum and/or teaching practice to address this.
Conflicts of interest
There are no conflicts of interest to declare.References
- Abimbola I. O., (1988), The problem of terminology in the study of student conceptions in science, Sci. Educ., 72(2), 175–184.
- Adbo K., (2012), Relationships between models used for teaching chemistry and those expressed by students, Gothenburg, Sweden: Linnaeus University.
- Alonzo, A. C. and Gotwals, A. W. (ed.), (2012), Learning Progressions in Science: Current Challenges and Future Directions, Sense Publishers.
- Bergqvist A., (2017), Teaching and learning of chemical bonding models: Aspects of textbooks, students’ understanding and teachers’ professional knowledge, Karlstad, Sweden: Karlstad University.
- Brock R., Taber K. S. and Watts D. M., (2023), Assembly required: a microgenetic multiple case study of four students’ assemblages when learning about force, Int. J. Sci. Educ., 1–21 DOI:10.1080/09500693.2023.2269616.
- Bunge M., (2017/1998), Philosophy of Science, Revised edn, Routledge, 1967.
- Claxton G., (1993), Minitheories: a preliminary model for learning science, in Black P. J. and Lucas A. M. (ed.), Children's Informal Ideas in Science, Routledge, pp. 45–61.
- Coll R. K. and Taylor N., (2002), Mental models in chemistry: senior chemistry students’ mental models of chemical bonding, Chem. Educ.: Res. Pract. Eur., 3(2), 175–184.
- Coll R. K. and Treagust D. F., (2001), Learners' mental models of chemical bonding, Res. Sci. Educ., 31(3), 357–382.
- Coll R. K. and Treagust D. F., (2003), Investigation of Secondary School, Undergraduate, and Graduate Learners’ Mental Models of Ionic Bonding, J. Res. Sci. Teach., 40(5), 464–486.
- Cooper M. M., Underwood S. M., Hilley C. Z. and Klymkowsky M. W., (2012), Development and Assessment of a Molecular Structure and Properties Learning Progression, J. Chem. Educ., 89(11), 1351–1357 DOI:10.1021/ed300083a.
- Dhindsa H. S. and Treagust D. F., (2014), Prospective pedagogy for teaching chemical bonding for smart and sustainable learning, Chem. Educ. Res. Pract., 15(4), 435–446 10.1039/C4RP00059E.
- diSessa A. A., (1988), Knowledge in pieces, in Forman G. and Pufall P. (ed.), Constructivism in the Computer Age, Lawrence Erlbaum Publishers.
- diSessa A. A., (1993), Towards an epistemology of physics, Cogn. Instr., 10(2&3), 105–225.
- Driver R., (1989), Students’ conceptions and the learning of science, Int. J. Sci. Educ., 11(special issue), 481–490.
- Hammer D., (2000), Student resources for learning introductory physics, Am. J. Phys., 68(7-Physics Education Research Supplement), S52–S59.
- Jensen W. B., (1995), Logic, History and the Teaching of Chemistry, text of the Keynote Lectures, given at the 57th Annual Summer Conference of the New England Association of Chemistry Teachers, Sacred Heart University, Fairfield, Connecticut.
- Joki J. and Aksela M., (2018), The challenges of learning and teaching chemical bonding at different school levels using electrostatic interactions instead of the octet rule as a teaching model, Chem. Educ. Res. Pract., 19(3), 932–953 10.1039/C8RP00110C.
- Joki J., Lavonen J., Juuti K. and Aksela M., (2015), Coulombic interaction in Finnish middle school chemistry: a systemic perspective on students' conceptual structure of chemical bonding, Chem. Educ. Res. Pract., 16(4), 901–917 10.1039/C5RP00107B.
- Karmiloff-Smith A., (1996), Beyond Modularity: A developmental perspective on cognitive science, MIT Press.
- Key Stage 3 National Strategy, (2002), Framework for teaching science: years 7, 8 and 9, London: Department for Education and Skills.
- Levy Nahum T., Mamlok-Naaman R., Hofstein A. and Krajcik J., (2007), Developing a new teaching approach for the chemical bonding concept aligned with current scientific and pedagogical knowledge, Sci. Educ., 91(4), 579–603 DOI:10.1002/sce.20201.
- Nicoll G., (2001), A report of undergraduates’ bonding misconceptions, Int. J. Sci. Educ., 23(7), 707–730.
- Palmer D., (1997), The effect of context on students’ reasoning about forces, Int. J. Sci. Educ., 19(16), 681–696 DOI:10.1080/0950069970190605.
- Pölloth B., Diekemper D. and Schwarzer S., (2023), What resources do high school students activate to link energetic and structural changes in chemical reactions? – A qualitative study, Chem. Educ. Res. Pract., 24(4), 1153–1173 10.1039/D3RP00068K.
- Solomon J., (1992), Getting to Know about Energy - in School and Society, Falmer Press.
- Stowe R. L., Herrington D. G., McKay R. L. and Cooper M. M., (2019), Adapting a core-idea centered undergraduate general chemistry curriculum for use in high school, J. Chem. Educ., 96(7), 1318–1326.
- Taber K. S., (1998), An alternative conceptual framework from chemistry education, Int. J. Sci. Educ., 20(5), 597–608.
- Taber K. S., (2001), Building the structural concepts of chemistry: some considerations from educational research, Chem. Educ.: Res. Pract. Eur., 2(2), 123–158 10.1039/B1RP90014E.
- Taber K. S., (2002), Chemical Misconceptions - Prevention, Diagnosis and Cure, Royal Society of Chemistry.
- Taber K. S., (2003), Lost without trace or not brought to mind? - a case study of remembering and forgetting of college science, Chemistry Education: Research and Practice, 4(3), 249–277 10.1039/B3RP90016A.
- Taber K. S., (2008), Conceptual resources for learning science: Issues of transience and grain-size in cognition and cognitive structure, Int. J. Sci. Educ., 30(8), 1027–1053 DOI:10.1080/09500690701485082.
- Taber K. S., (2009), College students' conceptions of chemical stability: The widespread adoption of a heuristic rule out of context and beyond its range of application, Int. J. Sci. Educ., 31(10), 1333–1358 DOI:10.1080/09500690801975594.
- Taber K. S., (2013), A common core to chemical conceptions: learners' conceptions of chemical stability, change and bonding, in Tsaparlis G. and Sevian H. (ed.), Concepts of Matter in Science Education, Springer, pp. 391–418.
- Taber K. S. and García Franco A., (2010), Learning processes in chemistry: Drawing upon cognitive resources to learn about the particulate structure of matter, J. Learn. Sci., 19(1), 99–142.
- Taber K. S. and Watts M., (2000), Learners’ explanations for chemical phenomena, Chem. Educ.: Res. Pract. Eur., 1(3), 329–353.
- Talanquer V., (2007), Explanations and Teleology in Chemistry Education, Int. J. Sci. Educ., 29(7), 853–870 DOI:10.1080/09500690601087632.
- Tsaparlis G., Pappa E. T. and Byers B., (2018), Teaching and learning chemical bonding: research-based evidence for misconceptions and conceptual difficulties experienced by students in upper secondary schools and the effect of an enriched text, Chem. Educ. Res. Pract., 19(4), 1253–1269 10.1039/C8RP00035B.
- Ünal S., Coştu B. and Ayas A., (2010), Secondary school students’ misconceptions of covalent bonding, Journal of Turkish Sci. Educ., 7(2), 3–29.
- Vygotsky L. S., (1978), Mind in Society: The development of higher psychological processes, Harvard University Press.
- Wang C. Y. and Barrow L. H., (2013), Exploring conceptual frameworks of models of atomic structures and periodic variations, chemical bonding, and molecular shape and polarity: a comparison of undergraduate general chemistry students with high and low levels of content knowledge, Chem. Educ. Res. Pract., 14, 130–146.
- Watts M. and Zylbersztajn A., (1981), A survey of some children's ideas about force, Phys. Educ., 16(6), 360–365.
- Zohar A. R. and Levy S. T., (2019), Students' reasoning about chemical bonding: The lacuna of repulsion, J. Res. Sci. Teach., 56(7), 881–904 DOI:10.1002/tea.21532.
Footnotes |
| † The suggestion that such a framework is stable should not be equated with it always being applied. Indeed, conceptual development in such circumstances may be considered as a relative shift in the extent to which different (‘competing’) frameworks are activated and applied by a learner in relevant contexts. |
| ‡ The framework, as represented in Fig. 1, was “a composite derived from interpretations of the comments of a range of learners” (Taber, 1998, p. 600), and offered a generalised account of the ideas elicited such that although all those interviewed shared certain core conceptions, not all aspects of the wider framework were expressed by every participant. Some features of the framework had previously been reported as discrete conceptions in prior research. Since the publication of the framework, a range of subsequent research has reported findings that reflect various aspects of the framework. In this comment, only work reporting on the specific aspects of the framework discussed by Pölloth, Diekemper and Schwarzer is discussed. |
| This journal is © The Royal Society of Chemistry 2024 |
