Student perceptions of partial charges and nucleophilicity/electrophilicity when provided with either a bond-line, ball-and-stick, or electrostatic potential map for molecular representation
Ayesha
Farheen
 ,
Nia
Martin
and
Scott E.
Lewis
,
Nia
Martin
and
Scott E.
Lewis
 *
*
University of South Florida, USA. E-mail: slewis@usf.edu
First published on 10th November 2023
Abstract
Education in organic chemistry is highly reliant on molecular representations. Students abstract information from representations to make sense of submicroscopic interactions. This study investigates relationships between differing representations: bond-line structures, ball-and-stick, or electrostatic potential maps (EPMs), and predicting partial charges, nucleophiles, and electrophiles. The study makes use of students’ answers in hot-spot question format, where they select partially charged atoms on the image of a molecule and explanations. Analysis showed no significant difference among students when predicting a partially positive atom with each representation; however, more students with EPMs were able to correctly predict the partially negative atom. No difference was observed across representations in students predicting electrophilic character; while representations did influence students identifying nucleophilic character. The affordance of EPMs was that they cued more students to cite relative electronegativity indicating that such students were able to recognize the cause for electron rich/poor areas. This recognition is central to rationalizing mechanisms in organic chemistry. This study offers implications on incorporating EPMs during instruction and provides evidence-based support in how EPMs could be useful in promoting learning on topics that relate to an uneven charge distribution.
Introduction
Organic chemistry is a visual science where a variety of molecular representations are used to communicate chemical concepts (Davidowitz and Rollnick, 2011; Zhou et al., 2023). Representations in organic chemistry can be of many types from skeletal structures to NMR spectra. For the purposes of this study, representations are operationalized as drawings or images that depict the molecule at the submicroscopic level, for example, the molecule of chloromethane can be depicted using a bond-line, ball-and-stick, or electrostatic potential map. An organic chemistry instructor may choose to depict 3D molecules using bond-line structures where students can denote the hybridizations of atoms, dashed-wedge diagrams where students learn the meaning behind a solid versus a dashed line, or a ball-and-stick image that students can rotate on the screen to understand dimensionality. Organic chemistry instructors therefore have a choice in how much they want their students to abstract information from a given representation (Davidowitz and Rollnick, 2011; Jones et al., 2022; Popova and Jones, 2021; Smith, 2023). It therefore becomes essential that students in organic chemistry develop skills to work with the given representations, which leads to the importance of representational competency skills (Kozma and Russell, 2005; Prain and Tytler, 2012; Offerdahl et al., 2017; Talanquer, 2022; Watts et al., 2022; Dood and Watts, 2023).A historical review of ACS exams showed that since 1982, 90% of exam items contain at least one representation (Raker and Holme, 2013) directing to the importance of improving representational competency skills among organic chemistry students. Kozma and Russell (1997) presented representational competency skills and this study investigates one of the skills wherein students are able to identify and analyze features of a representation and use them to carry out the task-at-hand, for example, explanation of chemical concepts. Several studies have investigated the role of representations in students’ understanding of a variety of chemical concepts. Past studies conducted semi-structured interviews with undergraduate organic chemistry students using chemical formulae, Lewis dot diagrams, or bond-lines to investigate topics including applications of hydrogen bonding (Henderleiter et al., 2001), acid–base mental models (McClary and Talanquer, 2011; Cooper et al., 2016), completing reaction mechanisms (Grove et al., 2012; Galloway et al., 2019; Crandell et al., 2020), nucleophiles/electrophiles (Anzovino and Bretz, 2015; Eckhard et al., 2022), and explaining electron pushing formalism (Bhattacharyya and Harris, 2018; Webber and Flynn, 2018; Watts et al., 2020). These studies showed that students either rely on rote memorization for concepts or are unable to explain the why behind mechanisms. Beyond bond-line structures, studies have explored other visual representations such as dashed-wedge diagrams to explain enantiomers (Domin et al., 2008), translation between dashed-wedge, Newman, and Fisher (Olimpo et al., 2015; Ward et al., 2022, p. 39), chair conformations (Head et al., 2005; Decocq and Bhattacharyya, 2019), and reaction coordinate diagrams (Popova and Bretz, 2018a, 2018b; Watts et al., 2022). These studies that went beyond the popular bond-line structures showed that students might be focused on the surface features more than the molecule's functionality, and that they need more support understanding in-depth cues in representations. Two studies looked into organic chemistry students’ use with visual representations including color shown in ball-and-stick (Ealy and Hermanson, 2006; Stull et al., 2012); the latter study made use of the tactile ball-and-stick model of molecules to investigate mental rotation. They concluded that organic chemistry students need more practice in working with such representations and this could be demonstrated by instructors during instruction. The role of representations was also investigated with chemistry graduate students (Bhattacharyya and Bodner, 2005; Kraft et al., 2010; Strickland et al., 2010). These studies made use of bond-line structures with curved arrows showing the electron pushing formalism. Similar to the undergraduate students, graduate students struggled in explaining the why behind curved arrow notations relying on memorization rather than process-oriented thinking. Combined, these studies call for helping students develop the skill of using the given representation to argue for how chemical species interact by promoting focus beyond the surface-level or structural features of the representations (Hand and Choi, 2010; Watts et al., 2021, 2022; Eckhard et al., 2022). Since movement of electrons or attraction between areas of high and low electron densities are necessary to rationalize why chemical species interact, this study therefore investigates how students explain using features of a representation that makes electronic distribution explicit.
Studies investigating students’ understanding of nucleophiles and electrophiles
For this study, the chemical concepts chosen to investigate the role of representations are partial charges and nucleophiles/electrophiles. The reason behind choosing these concepts is owing to their importance in organic chemistry and their reliance on electronic distribution. A national survey of organic chemistry instructors rated determining high/low electron density and recognition of nucleophiles/electrophiles higher than having knowledge of reaction mechanisms in organic chemistry (Bhattacharyya, 2013). Owing to this importance, this study investigated how students might use representations to aid in the process of assigning partial charges on atoms in molecules that are interacting and identifying nucleophiles and electrophiles. In addition, students struggle to connect uneven charge distribution (partial charges) to its effect on reactions, which is central to organic chemistry education. Studies show that partial charges are typically drawn on representations, but in the absence of these drawings, students struggle in connecting implicit charge distribution to its effect on reaction mechanisms (Smith, 2023), activation energy (Caspari et al., 2018) or nucleophilicity/electrophilicity (Frost et al., 2023). Thus, continuous incorporation of representations that make electron distributions explicit may help students understand the effects of implicit charge distribution (Taagepera and Noori, 2000). This study therefore investigates the impact of a representation that makes charge distribution explicit to investigate how well it supports students identifying nucleophilicity/electrophilicity, which is impacted by the charge distribution.Past studies have investigated how students define and consider involvement of nucleophiles/electrophiles in reactions. Interviews with second-semester organic chemistry students showed that students used electronic features such as charges to define nucleophiles and electrophiles but were unable to use these definitions as explanatory for why reactions, such as acid/base, occur (Anzovino and Bretz, 2015; Cartrette and Mayo, 2011). Organic chemistry students’ ideas about nucleophiles/electrophiles seem to be fragmented (Anzovino and Bretz, 2016). Interviews with pairs of organic chemistry students where they had to explain and draw electron-pushing formalism found that students were able to identify nucleophile and electrophile correctly but there was confusion with where electrons (nucleophile to electrophile) or protons (electrophile to nucleophile) transfer (Bhattacharyya and Harris, 2018). Thus, connecting implicit partial charges to their effect on reaction mechanisms cannot be presumed. Crandell and colleagues (2019) also make the argument that students have difficulty in understanding the “source-to-sink” in electron pushing formalism, that is, where electrons move from that might promote understanding of why nucleophiles and electrophiles interact. Studies that have characterized students’ explanations of mechanisms involving nucleophiles and electrophiles also show students’ understanding as surface-level (Dood et al., 2020a; Frost et al., 2023; Yik et al., 2023). These studies pointed to the notion that even though students might know the definition of nucleophiles/electrophiles, they struggle to make sense of their role in reaction mechanisms, that is, relating the effect of uneven charge distribution within chemical species to determining which species will interact. A similar struggle was also observed with graduate chemistry students in explaining the why behind the role of nucleophiles and electrophiles (Strickland et al., 2010). However, in recent studies, we do see students trying to make those connections. Two studies that investigated organic chemistry lab students’ explanations on comparing two mechanisms involving nucleophiles and electrophiles coded for features, wherein charge, induction, electronegativity, and resonance were prominent (Watts et al., 2020; Watts et al., 2021). These studies pointed out that there is value in students bringing back concepts about electron distribution they learned in general chemistry and applying them to reaction mechanisms in organic chemistry. To promote this application of connecting implicit properties of electron distribution to nucleophiles/electrophiles, representations can assist (Ealy and Hermanson, 2006; Graulich, 2015; Crandell et al., 2019; Dood and Watts, 2023), which this study will investigate.
Rationale
It is well established in organic chemistry education literature that students need to explain the why behind reaction mechanisms (Anzovino and Bretz, 2015; Graulich, 2015; Stowe and Cooper, 2017; Caspari et al., 2018; Crandell et al., 2019; Talanquer, 2018, p. 48; Dood and Watts, 2022). For this reason, in this study we ask students to explain how they identified partially charged atoms and then explain the first step of a nucleophilic aryl substitution reaction. The foundation of this study is the relationship between partially charged atoms within a molecule with predicting and explaining nucleophilic substitution. The focus is on electron density because excess or deficient electron density in molecular regions is essential to understand the functionality of nucleophiles and electrophiles (Cartrette and Mayo, 2011; Anzovino and Bretz, 2015).Because representations are used in organic chemistry that depict the implicit properties of electron distributions’ effect on nucleophilicity/electrophilicity, this study investigates the role of three representations in students’ predicting and explaining partially charged atoms (electron density), recognizing nucleophiles and electrophiles, and explaining the first step in the mechanism of a nucleophilic aryl substitution reaction. By explaining the first step in this mechanism, there is an assumption that students are relating the presence of partially charged atoms to recognizing the functionality of nucleophiles and electrophiles in this reaction. Past studies, that have used representations and investigated what students mention when they explain reaction mechanisms have predominantly used bond-line structures or dashed-wedge diagrams (Webber and Flynn, 2018; Watts et al., 2020; Rodemer et al., 2021; Eckhard et al., 2022), while a study also used electrostatic potential maps (Dood et al., 2020b). To date, no studies have compared representations to determine the impact of representations on explanations of reaction mechanisms. Conducting a study comparing representations on explanations adds to the literature to promote students in explaining the why using representations. Thus, the novelty of this study comes in two aspects: (1) investigating two chemical concepts that are needed to explain why chemical species interact, that is, partial charges, and nucleophilicity/electrophilicity and (2) comparing common representations that make charge implicit (bond-line structures and ball-and-stick) and explicit (electrostatic potential maps). Those three representations were chosen owing to how they depict uneven charge distribution. Bond-line structures are ubiquitous in instruction and assessments in organic chemistry owing to their easy construction. Electrostatic potential maps (EPM) show electron density as color variation. In contrast, ball-and-stick images also use varying color to demonstrate atomic identity but not relative electron density. These three representations fit the inquiry. With bond-line structures, students will need to abstract relative electronegativity from atom identity and infer molecular geometry. With ball-and-stick, students are presented molecular geometry but still need to abstract relative electronegativity from atom identity. With EPM, students are provided all the information of ball-and-stick (which is embedded within the representation) and a color map modeling electronic distribution. Thus, comparisons of EPM with bond-line demonstrate the impact of providing students with molecular geometry and electronic distribution versus students implicitly determining molecular geometry and electronic distribution. Comparisons of EPM with ball-and-stick demonstrate the impact of providing only the electronic distribution color map since all other features are identical. Comparisons of ball-and-stick with bond-line structures demonstrate the impact of providing molecular geometry.
Theoretical framework
The study design was informed by the C–R–M model of the visual literacy framework by Schönborn and Anderson (Schönborn and Anderson, 2009, 2010; Anderson et al., 2013). The C in the model refers to students’ prior conceptual knowledge, R is the cognitive abilities or students’ reasoning, while M is the external features of the mode of the representation. R–M is the ability to reason using external features of a representation and in this study, the evaluation of students’ responses across different representations was indicative of how students R–M is cued by differing representations. The application of this framework is also seen in other studies that use a variety of representations to decode students’ understanding (Sunyono et al., 2015; Wright et al., 2017; Ward et al., 2022, p. 38; Coleman et al., 2023). This assumption is also supported by cognitive theories on learning with representations summarized by Rau (2017). Several theories were mentioned in the summary including cognitive theory of multimedia learning (Mayer, 2005), integrated model by text and comprehension (Schnotz, 2005), and structure mapping theory (Gentner, 1983). These describe how students gain representational competency skills, that is, how students map features of a given visual representation to a referent to build internal processes, which they then use to problem-solve. Referent is operationalized as what comes to mind when someone is given a visual representation, for example, when given a bond-line structure of alcohol, the referent could be the molecule of alcohol (a concrete object) or the strength of the nucleophilicity of the molecule (an abstract concept). Rau (2017) summarizes that these theories describe that students struggle in determining features of a visual representation that are relevant and irrelevant to the referent. Features in this context are similar to the external features of the representation (M) (Schönborn and Anderson, 2009). Once students focus on the feature, they access their prior knowledge from the long-term memory and insert it into the internal process they develop. Students then use this internal process for the task at hand using the representation which is the intersection of C–R–M (Schönborn and Anderson, 2009).In organic chemistry, there are a variety of visual representations that show symbols of atoms connected with lines or circles (bond-line, Newman projections, Fischer projections, chair etc.) or colored entities (ball-and-stick, space filling, electrostatic potential maps, isosurface structures etc.). Across representations, a feature might differ in the way it is represented even if it means the same thing. For example, the feature of connectivity or two atoms bonded to each other is represented with a straight line in bond-line structures but a straight line and overlapping colorful spheres in EPM. Therefore, each way the feature is represented (M) requires a different level of abstraction to understand that it means two atoms are bonded to each other (R–M). Since features across visual representations are presented differently but indicate the same concept – mapping a feature to the referent is likely to differ from one visual representation to the other. This leads to retrieval of different prior knowledge from the student's long-term memory and a different process for the task-at-hand using that representation (C–R–M). For example, while seeing a bond-line structure to understand that a straight line represents a bond, students would need to extract from their prior knowledge that the straight line depicts two electrons being shared between two atoms, whereas to understand that overlapping of spheres resulting in different colors students would extract knowledge of an electron cloud of atoms being shared with each other. Thus, the C–R–M model pairs with cognitive theories in learning with representations to offer an explanation that different visual representations can bring forth different processes in students since they have features that map onto similar concepts. Past work has evidenced this possibility by exploring the effect of representations in determining the polarity of a single molecule where polarity was implicit in each molecule (Rau, 2017; Farheen and Lewis, 2021). This study focused on the C–R–M which is what conceptual knowledge (C) do students bring forth while they are explaining (R) the given task at hand using the representation (M).
Research questions
This study is guided by the following research questions to investigate the C–R–M of students, that is, what concepts students bring forth by relating features of the representation to justify charges and nucleophilicity/electrophilicity.1. What is the relationship between the representation and correct prediction of the location for the partial positive or negative regions of a molecule?
2. What concepts do students cite while predicting the location of partial charges and how do representations relate to such concepts?
3. What is the relationship between representation and students’ classifying carbon as electrophilic and nitrogen as nucleophilic in nature?
Methods
Participants, research setting and ethical considerations
The participants for this study were second-semester organic chemistry course students at a research-intensive university in the southeast of the United States. Students from a second-semester organic chemistry course were chosen for two reasons: (1) this cohort has gone through the first-semester organic chemistry training where they learn nucleophilic reaction mechanisms, and (2) this study makes use of students creating mechanisms using the software Marvin JS and this cohort has had experience working with this software in the first-semester organic chemistry course. Three classes of second-semester organic chemistry course were coordinated with the same instructor, syllabus, common class material, and exams. Students were recruited from all three courses and consented to participate on a voluntary basis. Out of 428 students, 81.1% (390) consented to participate in the research study. The institution's IRB approved this study as Study002446.Study design
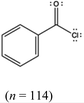
Three surveys were created and students from the second-semester organic chemistry were randomly assigned to one survey. Random assignment was conducted to mitigate the potential for inherent differences among the groups. Students were given five days after their third in-term exam to complete their assigned survey. Upon completion students received a small portion as an extra credit opportunity. Responses from students who consented were analyzed.Each survey differed with the accompanying representation between bond-line, ball-and-stick, and electrostatic potential maps as shown in Fig. 1 and 2. It is essential to point out that this student population has more experience working with bond-line structures as this is the most common representation used in assessments at the research setting. Thus, the results will describe students’ interpretations of ball-and-stick and EPMs without formal training on either. Furthermore, the ball-and-stick and EPM were implemented without a legend or direct instruction on how to interpret the representations, so the results herein may be most applicable to describe students’ initial encounter with these representations. The bond-line structures were created using ChemDraw and the ball-and-stick and electrostatic potential maps using Jmol.
For each survey, the same representation type was used throughout the series of prompts. That is, if a student was assigned to bond-line survey, they were provided only bond-line structures throughout the survey. Instructions that included the electronegativity values and difference in values to indicate a polar bond were given at the top of the survey. Students could use these instructions anytime during the survey. For prompts 1 and 2, students selected the atom with partial positive charge in a benzoyl chloride molecule in the hot-spot and explained their prediction in an essay. Hot-spot is when students are given an image and they are asked to click on the part of the image that they think is the correct answer. For example, a student clicked on the image of a ball-and-stick molecule of benzoyl chloride to indicate the partially positive atom in the molecule. For prompts 3 and 4, students selected the atom with the partial negative charge in ethyl amine and explained their prediction. Prompts 1 and 3 were designed to contain quantitative data for the selection of the atoms with partial charges and prompts 2 and 4 for qualitative data to act as support for their selection. Prompts 1 and 3 were designed to answer the first research question about the impact of representations on students’ prediction of charges, and prompts 2 and 4 the second research question on concepts cited. Hot-spot style was used instead of the traditional multiple-choice as it offered evidence of students clicking on the location in the image as their selection compared to them selecting a textual option from the multiple choices. For prompt 5, students were asked to use MarvinJS to construct a mechanism for the reaction between benzoyl chloride and ethyl amine and upload the mechanism; prompt 6 asked students to explain the first step in the mechanism. Note that students were not informed that the interaction between the two molecules was a nucleophile interacting with an electrophile. Finally, prompt 7 asked students to predict the product. Table 8 in the appendix shows the instructions and survey prompts students received.
Data analysis
To answer the first research question about the relationship between representations and students’ correct prediction of partially charged atoms, the proportion of correct responses were compared between the representations. Chi square analyses were run to test whether the type of representation had a significant influence on students’ correct prediction; effect sizes are reported using Cohen's w where 0.1 is a small effect and 0.3 is a medium effect size (Cohen, 2013).To understand the relationship between representations and students using certain features while predicting partially charged atoms or describing the first step in the mechanism, students’ responses to open-ended prompts were open-coded (Given, 2008, p. 5 of 9). Codebooks for predicting partial charges (Table 9) and explanations of nucleophilic attack (Table 10) are presented in the appendix. Each codebook development took place in the following steps. Two researchers took a subset of responses different from each other and inductively coded to generate two separate codebooks. They came together to merge these codebooks to create a single codebook. This codebook was deductively applied to another subset of responses independently and the researchers came together to discuss disagreements and modified the codebook. This deductive application of the codebook occurred until no changes to the codebook seemed necessary. Once that was achieved, two researchers independently applied the codebook to the entire sample and came together to conduct consensus coding until all disagreements were resolved (O’Connor and Joffe, 2020). One coder was a graduate student and another an undergraduate student who did academically well in organic chemistry. Due to their familiarity with partial charges and nucleophilic aryl substitution reactions, there is trustworthiness in their interpretation of these data (Shenton, 2004). Consensus coding was carried out between the two researchers to further establish trustworthiness that these data were being interpreted appropriately to answer the research question by more than one researcher. By conducting coding with another researcher, this helps to mitigate any biases.
Results
RQ1: What is the relationship between the representation and correct prediction of the location for the partial positive or negative regions of a molecule?Table 1 shows the percentage of students among a representation that correctly determined the carbonyl carbon as the partially positive atom in benzoyl chloride. The percentage of students who made correct predictions across the three representations are similar and range from 83.6% to 87.7%, with no statistically significant difference observed. There was thus no evidence to show a relationship between one representation over the other on students correctly predicting the partially positive atom in benzoyl chloride. Students had a high success rate in predicting the partially positive atom in benzoyl chloride, independent of the representation used.
Table 2 shows the percentage of students among a given representation who correctly predicted that the nitrogen atom is the partially negative atom in ethyl amine. Differences among the representation groups were more pronounced when predicting the partial negative charge, with the percent correct ranging from 79.8% to 97.3%. Pair-wise chi-square analysis found no significant difference between students with bond-line and students with ball-and-stick. There was a statistically significant difference between bond-line and EPM (X2(1, N = 226) = 16.988, p < 0.001, Cohen's w = 0.274, medium effect) and between ball-and-stick and EPM (X2(1, N = 228) = 8.237, p = 0.004, Cohen's w = 0.190, small-medium effect). Students with EPM were more likely to correctly predict that nitrogen is the location of the partial negative charge in ethyl amine compared to the other student groups.
RQ2: What concepts do students cite while predicting partial charges and how do representations relate to such concepts?
As the rationale described, explaining how students predicted partial charges is important in organic chemistry. How students use the representations to map features of representation to predicting partial charges can help instructors learn more on the role of representations in predicting partial charges. Students’ explanations when predicting partial charges were categorized as invoking one or two of the following concepts: relative electronegativity, absolute electronegativity, uneven charge distribution, resonance, color, connectivity, and electronic entities.
Students who used relative electronegativity made explicit comparisons of electronegativity values between bonded atoms. For example, in the case of benzoyl chloride, “I believe that carbon will have a partial positive charge because it is bonded to two atomschlorine and oxygen that are both more electronegative than the carbon, causing the shared electrons to be drawn closer to the chlorine and oxygen resulting in a partial positive carbon. [ball-and-stick]” The same student with ethyl amine indicated “Thenitrogen is more electronegative than hydrogen and carbon, therefore, pulling the shared electrons closer resulting in a partial negative charge. [ball-and-stick]” In both responses, the student describes the relative electronegativity between atoms that share a chemical bond.
In contrast, students who used absolute electronegativity did not make explicit comparisons. For example, “The carbon has boththe electronegative oxygen and chlorideforcing the atom to be partial positive. [bond-line]” Here a student does not mention whether carbon, oxygen, or chlorine, is more electronegative and does not enact comparisons based on bonded atoms. A similar example with the ethyl amine prompt was “the nitrogen will be the electronegative atomin this situation and pulls the electrons in the dipole moments with the neighboring carbon and hydrogens towards itself becoming partially negative. [bond-line]” There are two potential interpretations for why comparisons were not invoked. It is possible that students perceive the concept of electronegativity as an inherit characteristic of the atom. That is, certain atoms have high electronegativity and possess partial negative charges and atoms connected to them possess partial positive charges, without attending to the electronegativity value of the connected atoms. Alternatively, students may be omitting the electronegativity comparison as part of a colloquial phrase. In this interpretation, students may recognize that comparisons are needed but omit this detail in their explanation. Ultimately, absolute electronegativity is seen as ambiguous. Responses that invoked absolute electronegativity were demarcated from relative electronegativity to note the potential ambiguity in their processes.
Students also implicitly used the property of electronegativity, that is, they described the uneven distribution of charges by mentioning pull on the electrons or presence of electron withdrawing/donating groups. For example, “Both the chlorine and theoxygen pull electronsmore than the carbon so the carbon will experience a partial positive charge. [ball-and-stick]” or “The amino group is anelectron withdrawing groupin the molecule. Thenitrogen will draw electrons toward itself. [EPM]” Infrequently, an uneven distribution of charges was cited with relative electronegativity (3 students) or with absolute electronegativity (2 students). Overlaps of codes were infrequent and were not analyzed separately.
Some students also used the concept of resonance to justify the location of the partial charge. For example, a student wrote “The carbonyl carbon has a partial positive charge due to resonance (the oxygen can take the pi bond as a lone pair). [bond-line]”. This reasoning strategy was also observed with students with ethyl amine even though the molecule does not exhibit resonance properties. For example, “Due to resonance, the Nitrogen will carry the negative charge. [ball-and-stick]” Here a student describing ethyl amine used resonance to explain why a nitrogen atom will be the partially negative atom in ethyl amine even though the lone pairs on the nitrogen are not delocalized.
There were also infrequent occurrences where students used the colors in the ball-and-stick or EPM to justify their selection. For example, “You can see in the picture where thered is negativethere will likely be a partial positive charge below it in thedark blue area. [EPM]” or “the atom with the partial positive charge is theblue one. [ball-and-stick, in ethyl amine]” Using this concept showed that some students focused on the surface feature of color to determine the location of partial charges.
Some students cited only connectivity to justify the location of partial charges, which were atoms connected to or bonded to each other. Example statements include “becauseit is a chlorine that's connected to a carbonthat is alsoconnected to an oxygenwith a double bond., [ball-and-stick]” and “theNitrogen is connected to 2 hydrogen atoms, a carbon atom… making it have a partial negative charge. [bond-line]”
In contrast, other students cited electronic entities such as charges, lone pairs, or number of valence electrons to justify their decision. For example, “Thechloride and oxygen both have negative charges, which means that thecarbon is the positivethat pulls down. [bond-line]” and “the nitrogen in NH2 would have the partial negative charge, particularly due to theunshared electrons on the nitrogen. [EPM]” Responses coded for connectivity or electronic entities did not make explicit mention of electronegativity or uneven electron distributions in their justifications.
Table 3 shows the proportion of concepts cued in predicting the partial positive charge within benzoyl chloride, demarcated by the representation provided. The percentages represent the proportion of those receiving a particular representation. For example, among students with bond-line, 28.9% of responses cited relative electronegativity. Electronegativity is the foundational concept that rationalizes the presence of partial charges. Comparing relative electronegativity values between bonded atoms represents a required step in the process for determining partial charges. Absolute electronegativity carries ambiguity over whether the comparison of electronegativity values is conducted but may represent a similar process for some of the respondents. Even when students use uneven charge distribution in their response, it still shows that they are thinking about the position of electrons without mentioning the term electronegativity. Combining the frequency of relative electronegativity, absolute electronegativity, and uneven charge distribution (for the occasional case of overlap, where a student's response received more than one code, those were counted only once when discussing trends among the combination of codes) and comparing across the representations showed a small effect that was not statistically significant (X2(2, N = 342) = 12.514, p > 0.05, Cohen's w = 0.10, small effect). The uniform rate of invoking electronegativity or position of electrons across representations may explain why student success in predicting the location of the partially positive charge was independent of representation.
Concepts that showed a larger difference among the three representations were resonance (X2(2, N = 342) = 12.514, p < 0.05, Cohen's w = 0.19, small-medium effect) and color (X2(2, N = 342) = 7.388, p < 0.05, Cohen's w = 0.15, small effect). The percentage of students with bond-line structures citing resonance was higher than the percentage of students with ball-and-stick or EPM. The presence of lone pairs being explicit within the bond-line structure may cue students to think about resonance since lone pairs are not explicitly visible in either ball-and-stick or EPM. Only two of the representations ball-and-stick and EPM explicitly show color. In ball-and-stick representations the color of the balls was intended to identify atomic identity whereas in EPM color variation in the spheres was intended to signify electron rich/poor areas. Students with EPM infrequently relied exclusively on color as their sole reasoning, representing about one in twenty responses. The use of color in ball-and-stick was less frequent still and appears to note the atomic identity.
Table 4 shows the percentage of students given a specific representation who cited a concept while explaining their prediction of the partial negative charge within ethyl amine. With partial negative, we see a difference among the three representations in citing electronegativity or the position of electrons (relative, absolute, and uneven charge distribution) (X2(2, N = 342) = 12.133, p < 0.05, Cohen's w = 0.19, small-medium effect). The percentage of students with EPM citing relative electronegativity, absolute electronegativity, and uneven charge distribution (80.3%) was higher than the percentage of students with bond-line (59.7%) or ball-and-stick (65.5%) (see Table 4). This could explain why more students with EPM were successful at making the correct prediction about the partial negative charge than students with the other representations.
As with the partial positive charge, in the partial negative charge students use of resonance (X2(2, N = 342) = 7.067, p < 0.05, Cohen's w = 0.15, small effect) and color (X2(2, N = 342) = 7.388, p < 0.05, Cohen's w = 0.15, small effect) differed by representation. Students with a bond-line were more likely to cite resonance, particularly compared to EPM where no students cited resonance. This trend matches the trend observed with partial positive. The reliance on color as an explanation remained constant from the partial positive prompt such that students with EPM cited this more than the other two representations.
Table 5 shows the proportion of students who accurately predicted the partial positive charge, among those who cited each concept and with each representation. Students citing relative or absolute electronegativity, or uneven charge, identified the partial positive charge correct at a very high rate. Students with bond-line and ball-and-stick were more likely to cite resonance (Table 3), and citing resonance also corresponded to a high percent correct. Students with EPM who cited color also made correct predictions at a high rate.
When predicting the partial negative charge, students who cited relative or absolute electronegativity, or uneven charge also had a high percent for correctly predicting partial charge across the three representations (Table 6), except for students with bond-line citing uneven charge. While no students cited resonance with EPM, the infrequent use of resonance with bond-line and ball-and-stick corresponded with a low percent correct; since ethyl amine does not exhibit resonance, this process was not expected to generate accurate predictions. As with the positive charge, color was seldom used as the sole reason for assigning a partial negative charge and when used was productive for EPM.
RQ3. What is the relationship between representation and students’ classifying carbon as electrophilic and nitrogen as nucleophilic in nature?
As mentioned in the rationale, when students describe the first step in the mechanism after predicting partially charged atoms within the molecules, it is assumed they are using this knowledge in determining nucleophilicity and electrophilicity. Analysis of prompts 1 and 3 where students identified partially charged atoms and prompt 6 where they explained the first step in the mechanism showed that the correct predictions for partial positive and negative charges corresponded to the correct identification of an electrophile and nucleophile respectively while students were describing the first step. Among those who said carbon is partially positive 55.9% identified carbon as electrophilic; among those who did not identify carbon as partially positive 19.1% identified carbon as electrophilic (X2(1, N = 342) = 21.9, p < 0.05, Cohen's w = 0.25, medium effect). Among those who said nitrogen is partially negative 55.5% identified nitrogen as nucleophilic; among those who did not identify nitrogen as partially negative 36.6% identified nitrogen as nucleophilic (X2 (1, N = 342) = 5.2, df = 342,1, p < 0.05, Cohen's w = 0.12, small effect).
Table 7 shows the percentage of students among each set of representations that mentioned whether carbon is electrophilic, and nitrogen is nucleophilic, when students explained the first step in the mechanism between benzoyl chloride and ethyl amine. As before, the percentages represent the proportion of students from each representation whose response matched the description; that is 50.9% of the students with a bond-line structure mentioned carbon is electrophilic. The coding process included alternative phrasing that represented the same concept. Student responses were assigned “carbon is electrophilic” when the response identified either the carbonyl carbon, the carbon double-bonded to oxygen, or acyl chloride, as electron poor, has a partial positive charge, has a positive charge, or is an electrophile. Similarly, “nitrogen is nucleophilic” was assigned when student responses described nitrogen, amine, or ethyl amine as electron rich, has a partial negative charge, has a negative charge, or is a nucleophile.
Comparing the three representations, no difference was observed by representation in describing carbon as electrophilic (X2(2, N = 342) = 1.1, p > 0.05, Cohen's w = 0.06). Students with bond-line identified nitrogen as nucleophilic at a highest rate, followed by EPM, with ball-and-stick the lowest (X2(2, N = 342) = 7.6, p < 0.05, Cohen's w = 0.15, small effect). The 43.1% of students with ball-and stick that identified nitrogen as a nucleophile stands in contrast to the 87.1% of the ball-and-stick students who identified nitrogen as the location of the partially negative charge (Table 2). The difference in percentages may be a result of the explicit inclusion of electronegativity values in the survey while no explicit mention of nucleophilicity is included. Among students who predicted nitrogen as partially negative but did not identify nitrogen as nucleophilic, their responses varied. Responses described the nitrogen will be attracted to carbon due to opposite charges, for example, “the electronegativenitrogen is attracted to the carbon,” wherein they do not mention that the nitrogen is nucleophilic. Students also mentioned that chlorine is a good leaving group, “becausechlorine is a good leaving group. Therefore, once chlorine leaves the carbo cation is formed which theNitrogen will then attack.” Alternatively, they use the term “nucleophilic attack” without explicitly mentioning nitrogen as the nucleophile, “nucleophilic attackon the fairly positive carbon atom.” Thus responses were either vague in describing a nucleophile or mentioned alternative reaction pathways.
Discussion and implications
Students with EPMs were more likely to predict the partial negative charge correctly, while success rate in predicting the partial positive charge was independent of representations. Students with EPMs were also more likely to cite electronegativity or uneven distribution of electrons in their explanation, particularly compared to students with bond-line. The concept of electronegativity offers an explanatory rationale for partial positive and negative charges and may support students in identifying electrophilic and nucleophilic characteristics within a molecule (Frost et al., 2023; Yik et al., 2023). Partial charges are an implicit feature in bond-lines and ball-and-stick, and students need to access their prior knowledge in comparing electronegative values of atoms that share a bond and inferring molecular polarity from bond dipoles in exhibiting this concept. In contrast, EPMs use color variation among the spheres to make partial charges explicit. Using the cognitive theories in multimedia learning that posit students compare features of the representation to the referent and then access prior knowledge to fit into their internal process, EPMs use of color increases similarity to the referent and makes it easier to access electronegativity knowledge. Notably, only approximately 5% of students with EPM explained the location of partial charges based on color alone, suggesting that the strong majority of students mapped the representation (EPM) onto the referent (partial charges resulting from uneven electron distributions). This finding further supports that showing electron distribution explicitly can contribute to students’ mental models [internal process] about this concept since students seem to be focusing on explicit features more than implicit properties (Graulich et al., 2019). The results of this study push the field of work with representations and students’ learning mechanisms by offering evidence where students are supported to focus on the electron distributions. In the literature, students use of mechanistic reasoning and electron-pushing formalism are frequently studied and the take-away point is to allow students to understand that electron flow is from areas of high electron density to low electron density, which allows causal reasoning (Galloway et al., 2019; Kranz et al., 2023; Dood and Watts, 2023). Because EPMs make this implicit feature of electron distribution explicit, it can thus help students understanding electron-pushing formalism and making causal connection between electron density and why species interact.More students with bond-line were cued to using resonance both with benzoyl chloride and ethylamine. Benzoyl chloride cueing resonance is also found in other studies where acyl chlorides or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O are popular structures used to explain the concept of resonance (Watts et al., 2020; Brandfonbrener et al., 2021). In bond-line structures, lone pairs on oxygen in benzoyl chloride and nitrogen in ethyl amine were explicit, and the double bond in benzoyl chloride was explicit, which may have contributed to the higher citing of resonance. A review of PowerPoint slides used at the research setting to instruct students about resonance also showed predominant use of bond-line structures. While the tasks given within this study did not require resonance, for tasks where resonance is needed, students may benefit from using representations where electrons are explicit. Additionally, for instruction that uses representations where electrons are implicit, students may benefit from modeling how to infer resonance from these representations, likely through translation to representations where the lone pairs are explicit. Finally, it is worth noting that a proportion of students may use resonance when it is not applicable, as was done here with ethyl amine. Building instruction and assessment where students determine whether resonance is applicable may help students distinguish when to use this concept.
O are popular structures used to explain the concept of resonance (Watts et al., 2020; Brandfonbrener et al., 2021). In bond-line structures, lone pairs on oxygen in benzoyl chloride and nitrogen in ethyl amine were explicit, and the double bond in benzoyl chloride was explicit, which may have contributed to the higher citing of resonance. A review of PowerPoint slides used at the research setting to instruct students about resonance also showed predominant use of bond-line structures. While the tasks given within this study did not require resonance, for tasks where resonance is needed, students may benefit from using representations where electrons are explicit. Additionally, for instruction that uses representations where electrons are implicit, students may benefit from modeling how to infer resonance from these representations, likely through translation to representations where the lone pairs are explicit. Finally, it is worth noting that a proportion of students may use resonance when it is not applicable, as was done here with ethyl amine. Building instruction and assessment where students determine whether resonance is applicable may help students distinguish when to use this concept.
There are instances in the literature that describe interventions that made use of representations to improve understanding of topics in organic chemistry, such as, using chemical formula, skeletal, and bond-line structures to create concept maps to solve reaction mechanisms (Hermanns, 2020), showing symbolic, microscopic, and macroscopic representations generated through software during instruction (Mekwong and Chamrat, 2021; Springer, 2014) and curriculums to address misconceptions such as the spiral curriculum (O’Dwyer and Childs, 2014). This work can support these efforts by serving as a foundational evidence base for integrating representations within organic chemistry instruction. Owing to how EPMs cue an underlying concept in organic chemistry of electron rich/poor areas within a molecule (a concept that rationalizes why molecules interact), the implications for organic chemistry instructors is to incorporate EPMs when instructing about electron rich/poor areas. Based on cognitive theory students could use features in EPM to better recall concepts related to charge distribution such as electronegativity. The results suggest that instruction that makes use of EPM may find learning gains with students invoking foundational concepts to explain mechanisms and reactivity; however, this hypothesized relationship was not tested herein and would require future evaluation. Students could be trained on software such as Jmol to construct EPMs and use them in explaining molecule interactions.
The results also indicate that students may benefit in using representations such as ball-and-stick and EPMs in making predictions. Practicing chemists generate EPMs from experimental data and use the resulting model to detect high reactivity within a molecule. Examples of this are included in the popular journal Nature in which several articles were published using EPMs during the year this paper was written (Jain et al., 2023; Kang et al., 2023; Shalaby et al., 2023). Being able to interpret and utilize EPMs is becoming an essential skill of a practicing chemist and should be considered as a part of student training. A review of chemistry textbooks found that EPMs were frequently included but lacked conceptual support for students’ use of EPMs (Hinze et al., 2013). Beyond observing models during lectures or in textbooks, students should be given opportunities to interact with them (Kumi et al., 2013). This study calls for instructors to incorporate EPMs during instruction and demonstrate how to translate between EPMs and bond-line structures. Students can be guided and tasked with generating EPMs and explaining the charge distribution within a molecule or rationalizing reaction mechanisms.
Conclusion
There is a call to use more computer-generated EPMs as they have the ability to explicitly show uneven charge distribution that can be used to rationalize mechanisms (Sanderson, 1959; Shusterman and Shusterman, 1997; Fleming et al., 2000; Sanger and Badger, 2001; Hinze et al., 2013). This study offered evidence in support of that notion. It was found that more students with EPM correctly predicted partially negative atoms than students with the other representations. It was also found that more students with EPM cited electronegativity, a concept central to predicting charges. No difference was observed across representations in students predicting an electrophilic character; while representations did influence students identifying a nucleophilic character. Results of this study informed that EPMs have affordances in cueing electron rich/poor areas within molecules that could promote students identifying electrophilic and nucleophilic characteristics and also understand the why behind mechanisms, especially nucleophilic substitution on aromatic compounds. Abstractness of organic chemistry should not be an obstacle in understanding chemical reactivity (Friesen, 2008). As EPMs make the implicit property of uneven charge distribution explicit, they are likely to offer support to students in seeing attraction between oppositely charged parts of molecules leading to leaving groups leaving, substitution occurring, or acid/base reactions.Limitations
This study took place within one semester at one setting and cannot speak to generalizability but offers evidence of transferability by specifying what representations organic chemistry students work with in a research setting. Since students’ explanations were involved and interpreted to answer the research questions, hermeneutic considerations play a role as another researcher could have interpreted these data differently. Furthermore, as the study relied on single survey prompts for students to explain their processes, there was no opportunity to seek clarification. Thus, the results presented represent students’ initial explanations when receiving the prompt. To address this limitation, the potential for ambiguity in interpreting student responses was acknowledged in the results presented. The authors also acknowledge that students at the research setting were not assessed with ball-and-stick or EPM, thus interpretations of their explanations using these representations is how students are likely to perform without formal instruction and that future studies need to be conducted on how students with formal training perform. Also, the survey provided the students with electronegativity values which was likely to prime students to use the concept in explanations. Future work that explores the impact of providing differing information to students (e.g. omitting electronegativity values, adding a legend for EPMs) would be helpful in providing more insight into the role these factors play in student reasoning.Conflicts of interest
There are no conflicts to declare.Appendix
The survey prompts used in this study are presented in Table 8. The coding of student explanations for partial positive and negative charges are presented in Table 9 where pull is operationalized as an atom or element causing an action on electrons such that they are moving to one side. For example, pull on electrons, shift in electron density, or lone pairs moving between atoms. Features are operationalized as characteristics within a representation that can be explicit such as lone pairs in bond-line structure, or implicit such as negative charge of an atom in bond-line structure due to connectivity. Features include dipole, induction, polarity, connectivity, lone pairs or valence electrons, positive or negative charge of an atom, nucleophile, electrophile, color, type of bond such as covalent or pi bond, bonds breaking or forming, hydrogen bond. Partial positive or partial negative are not features.| a Specific survey indicates the representation the student was assigned to, for example, bond-line, ball-and-stick, or EPM. | ||
|---|---|---|
| Instructions as seen by the students; present at the top of each survey |

|
|
| Informed consent | ○ Yes, I consent to participate in the research study. | |
| ○ No, I DO NOT consent to participate in the research study. | ||
| Type | Prompt number | Survey question |
|---|---|---|
| Hotspot | Prompt 1 | Click on the atom that will experience a partial positive charge in the image below for benzoyl chloride (C6H5COCl). |
| [Insert representation for specific surveya from Fig. 1] | ||
| Essay | Prompt 2 | Please explain your prediction for the atom with the partial positive charge. |
| Hotspot | Prompt 3 | Click on the atom that will experience a partial negative charge in the image below for ethylamine (NH2CH2CH3). |
| [Insert representation for specific surveya from Fig. 1] | ||
| Essay | Prompt 4 | Please explain your prediction for the atom with the partial negative charge. |
| File-upload | Prompt 5 | Draw the mechanism using MARVIN JS. Once in the Marvin JS tab, go to gear symbol on the top horizontal toolbar and make sure “show lone pairs” is checked. Save as MRV file and upload. Keep the tab open to be used for the next question. Benzoyl chloride (C6H5COCl) and ethylamine (NH2CH2CH3). |
| [Insert representations for specific surveya from Fig. 1] | ||
| Essay | Prompt 6 | Please explain why the reactants benzoyl chloride (C6H5COCl) and ethylamine (NH2CH2CH3) interact the way they do in the first step of the mechanism you proposed. |
| [Insert representations for specific surveya from Fig. 1] | ||
| Multiple-choice | Prompt 7 | Predict the product for the reaction between benzoyl chloride (C6H5COCl) and ethylamine (NH2CH2CH3). |
| [Insert representations for specific surveya from Fig. 1] | ||
| ○ N-Ethyl benzamide (C6H5CONHCH2CH3) [insert representation for specific surveya from Fig. 2] | ||
| ○ N-Ethyl formide (HCONHCH2CH3) [insert representation for specific surveya from Fig. 2] | ||
| ○ Benzyl ethyl amine (C6H5CH2NHCH2CH3) [insert representation for specific surveya from Fig. 2] | ||
| ○ Propriophenone (C6H5COCH2CH3) [onsert representation for specific surveya from Fig. 2] |
| Code | Definition | Example quote (complete responses by students) |
|---|---|---|
| a Lower priority than other codes, but higher than “feature without pull on electrons”; exclusive, cannot occur with other codes. b Lower priority than all codes; exclusive, cannot occur with other codes. | ||
| Pull on electrons without mentioning any featurea | An atom is pulling on electrons or electrons are moving between atoms without mentioning or explaining any features such as electronegativity, resonance, dipole, induction or polarity | Nitrogen will pull electron density from the hydrogens and carbon that it's bonded to. |
| Feature without mentioning pull on electronsb | Features other than electronegativity or resonance are mentioned but response is missing electrons are being pulled. | Nitrogen has the stronger dipole moment so it holds the negative charge. |
| Absolute electronegativity and pull on electrons | Electronegativity is mentioned using words such as electronegative, high, very, more without comparing to another atom, most and electrons are being pulled | The Nitrogen atom is an electronegative atom meaning it will pull the electrons in the C-N bond towards itself resulting in a partial negative charge. |
| Absolute electronegativity without pull on electrons | Electronegativity is mentioned using words such as electronegative, high, very, more without comparing to another atom, most but response is missing electrons are being pulled | It is next to an electronegative oxygen and chlorine |
| Relative electronegativity and pull on electrons | Electronegativity is compared using words such as more than, higher, lower, or values are subtracted and electrons are being pulled. For this to code to be applied, the student needs to mention another atom or within the molecule | The nitrogen is more electronegative than hydrogen and carbon, therefore, pulling the shared electrons closer resulting in a partial negative charge. |
| Relative electronegativity without pull on electrons | Electronegativity is compared using words such as more than, higher, lower, or values are subtracted but response is missing electrons are being pulled. For this to code to be applied, the student needs to mention another atom or within the molecule | Oxygen and Chlorine are both more electronegative than the carbon atom |
| Resonance and pull on electrons | Resonance is mentioned and electrons or lone pairs are being pulled | This carbon will experience a partial positive charge during resonance after 2 electrons from the double bond are moved onto the oxygen . the oxygen atom will then experience a partial negative charge. |
| Resonance without pull on electrons | Resonance is mentioned but response is missing electrons or lone pairs are being pulled | If the compound undergoes resonance , the Oxygen will be negatively charged, allowing the carbon to be partially positive |
| Electron withdrawing group and pull on electrons | Electron withdrawing groups are mentioned and electrons are being pulled | The oxygen is an EWG , so it pulls electron density from the Cl. |
| Electron withdrawing groups without pull on electrons | Electron withdrawing groups are mentioned but response is missing electrons are being pulled | NH2 is an EWG |
| Electron donating groups without pull on electrons | Electron donating groups are mentioned but response is missing electrons are being pulled | NH2 is electron-donating and thus the C bonded to the N will experience a partial negative. |
| Code | Definition (student mentions…) | Example quote (complete responses by students) |
|---|---|---|
| a Exclusive with each other. b Exclusive with each other. c Exclusive with nucleophilic attack unclear, nucleophile attacks electrophile, nitrogen attacks carbon, and lone pair attacks. d Exclusive with each other. | ||
| Presence of element only | “Nitrogen” or “Carbon” | The nitrogen acts as the nucleophile and attacks the partial positive carbon electrophile, pushing the electrons from the double bond onto the oxygen |
| Presence of molecule only | “Amine”, “ethylamine”, “carbonyl”, “C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O”, “ketone”, “acyl chloride”, “acid chloride”, or “benzoyl chloride” O”, “ketone”, “acyl chloride”, “acid chloride”, or “benzoyl chloride” |
The reactants benzoyl chloride and ethylamine interact the way they do in the first step because there is a protonation step |
| Presence of element and molecule | “Nitrogen” or “Carbon”, and “amine”, “ethylamine”, “carbonyl”, “C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O”, “ketone”, “acyl chloride”, “acid chloride”, or “benzoyl chloride” O”, “ketone”, “acyl chloride”, “acid chloride”, or “benzoyl chloride” |
The nitrogen atom of ethylamine with its lone pair acts can act as a nucleophile, and the carbonyl carbon of benzoyl chloride is very electrophilic. The nitrogen nucleophile thus attacks the carbonyl carbon |
| Presence of neither element nor molecule | Neither “nitrogen” or “carbon”, nor “amine”, “ethylamine”, “carbonyl”, “C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O”, “ketone”, “acyl chloride”, “acid chloride”, or “benzoyl chloride” O”, “ketone”, “acyl chloride”, “acid chloride”, or “benzoyl chloride” |
The partial negative charge will nuc. attack the position of the partial positive charge |
| Nitrogen has a partial negative chargea | Nitrogen, amine, or ethyl amine exhibit a partial or slightly negative charge |
The
partial negative nitrogen
atom is attracted to the partial positive carbon atom in the acyl chloride group causing the lone pairs in the nitrogen to form a double bond with the carbon. This reaction forces to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond to break apart and makes the oxygen a negative ion O double bond to break apart and makes the oxygen a negative ion |
| Nitrogen has a charge that is uncleara | Nitrogen, amine, or ethylamine has a charge without implying partial | Net negative on Nitrogen and Net positive on carbon react. |
| Nitrogen is nucleophilic | Nitrogen, amine, or ethylamine is nucleophilic | The nitrogen is nucleophilic in nature so it attacks the carbonyl carbon |
| Nitrogen is electron rich | Nitrogen, amine, or ethylamine is electron rich | Since the nitrogen is partially negative and electron-rich , it will act as the nucleophile and attack the partially positive carbon of the carbonyl/acid chloride |
| Carbon has a partial positive chargeb | Carbon, carbonyl, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, ketone, acyl chloride, acid chloride. or benzoyl chloride has a partial positive charge O, ketone, acyl chloride, acid chloride. or benzoyl chloride has a partial positive charge |
The nitrogen acts as a nucleophile, attacking the partially positive carbon in the carbonyl of the benzoyl chloride, punching the electrons in the double bond up to the oxygen giving the oxygen a negative charge |
| Carbon has a charge that is unclearb | Carbon, carbonyl, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, ketone, acyl chloride, acid chloride. or benzoyl chloride has a charge without implying partial O, ketone, acyl chloride, acid chloride. or benzoyl chloride has a charge without implying partial |
The chloride is a good leaving group, so there is a + charge on the carbon , especially since the double bond on the oxygen breaks |
| Carbon is electrophilic | Carbon, carbonyl, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, ketone, acyl chloride, acid chloride. or benzoyl chloride is electrophilic O, ketone, acyl chloride, acid chloride. or benzoyl chloride is electrophilic |
The amine is nucleophilic and the carbonyl carbon is electrophilic |
| Carbon is electron poor | Carbon, carbonyl, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, ketone, acyl chloride, acid chloride. or benzoyl chloride is electron poor O, ketone, acyl chloride, acid chloride. or benzoyl chloride is electron poor |
The electron dense nitrogen will act as a nucleophile and attack the electron poor carbonyl carbon |
| Attraction of opposite charges | Positive and negative charges attract | The partial negative charge of the nitrogen is attracted to the partial positive charge of the carbon a part of the carbonyl, thus the nitrogen performs a nucleophilic attack on the carbon |
| Attack that is unclearc | Attack but no mention of nucleophile, nitrogen, or lone pair i.e. no specification of the agent attacking | The most electronegative atom of one molecule attacked the most positively charged atom of the other molecule |
| Nucleophilic attack that is uncleard | Nucleophilic attack but no identification of the attacking agent or the receiving agent. | Amines are very strong Lewis Bases (electron donors) which makes them nucleophilic. Thus, it will perform a nucleophilic attack as the first step in the mechanism |
| Nucleophile attacks electrophiled | Nucleophile attack on electrophile OR attack and identification of nucleophile and electrophile | the ethylamine would act as a nucleophile and attack the electrophile , which is the benzoyl chloride since it wishes to donate it's electrons (Electron donating group) |
| Nitrogen attacks carbon | Nitrogen, amine, or ethylamine attacking carbon, carbonyl, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, ketone, acyl chloride, acid chloride, or benzoyl chloride O, ketone, acyl chloride, acid chloride, or benzoyl chloride |
The first step was a nucleophilic attack. The nitrogen has a partial negative and the carbon has a partial positive. This allows for the nitrogen to attack the carbonyl group . |
| Lone pair attacks | Lone pair is making the attack | The lone pair on the partial negative nitrogen attacks the partially positive carbon on the other molecule. The pi bond on the oxygen then moves up and makes the oxygen negative |
| Chloride as leaving group | Chlorine leaves or gets kicked off | The benzoyl chloride and ethylamine interact the way they do in the first step of the mechanism because the chlorine serves as a leaving group and is used to add the ethylamine to the central molecule |
| Carbon has only four bonds | Carbon has only four bonds or cannot exceed four bonds | The carbon in the carbonyl group is extremely electrophilic. With the nitrogen acting as a nucleophile, it would make sense that it would attack that carbon. Due to the fact that carbon cannot have more than 4 bonds , the 2 electrons of the double of the carbonyl would move up to oxygen |
| Double bond to atom | Electrons of the double bond within carbonyl move onto the oxygen atom | The nitrogen acts as a nucleophile, attacking the partially positive carbon in the carbonyl of the benzoyl chloride, punching the electrons in the double bond up tot he oxygen giving the oxygen a negative charge |
Acknowledgements
This material is based upon work supported by the National Science Foundation under Grant No. 2142324. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. We would also like to thank the instructors for allowing data collection and the students for participating in this study. We would also like to thank the members of the Lewis Lab during the time of data analysis of the project for their input and constant support.References
- Anderson T. R., Schönborn K. J., du Plessis L., Gupthar A. S. and Hull T. L., (2013). Identifying and developing students’ ability to reason with concepts and representations in biology, Multiple Representations Biol. Educ., 19–38.
- Anzovino M. E. and Bretz S. L., (2015), Organic chemistry students' ideas about nucleophiles and electrophiles: the role of charges and mechanisms, Chem. Educ. Res. Pract., 16(4), 797–810.
- Anzovino M. E. and Bretz S. L., (2016), Organic chemistry students' fragmented ideas about the structure and function of nucleophiles and electrophiles: a concept map analysis, Chem. Educ. Res. Pract., 17(4), 1019–1029 10.1039/c6rp00111d.
- Bhattacharyya G., (2013), From Source to Sink: Mechanistic Reasoning Using the Electron-Pushing Formalism, J. Chem. Educ., 90(10), 1282–1289 DOI:10.1021/ed300765k.
- Bhattacharyya G. and Bodner G. M., (2005), “It Gets Me to the Product”: How Students Propose Organic Mechanisms, J. Chem. Educ., 82(9), 1402 DOI:10.1021/ed082p1402.
- Bhattacharyya G. and Harris M. S., (2018), Compromised Structures: Verbal Descriptions of Mechanism Diagrams, J. Chem. Educ., 95(3), 366–375 DOI:10.1021/acs.jchemed.7b00157.
- Brandfonbrener P. B., Watts F. M. and Shultz G. V., (2021), Organic Chemistry Students’ Written Descriptions and Explanations of Resonance and Its Influence on Reactivity, J. Chem. Educ., 98(11), 3431–3441 DOI:10.1021/acs.jchemed.1c00660.
- Cartrette D. P. and Mayo P. M., (2011), Students' understanding of acids/bases in organic chemistry contexts, Chem. Educ. Res. Pract., 12(1), 29–39 10.1039/c1rp90005f.
- Caspari I., Kranz D. and Graulich N., (2018), Resolving the complexity of organic chemistry students' reasoning through the lens of a mechanistic framework, Chem. Educ. Res. Pract., 19(4), 1117–1141 10.1039/c8rp00131f.
- Cohen J., (2013), Statistical Power Analysis for the Behavioral Sciences, Academic Press.
- Coleman A. B., Lorenzo K., McLamb F., Sanku A., Khan S. and Bozinovic G., (2023), Imagining, designing, and interpreting experiments: using quantitative assessment to improve instruction in scientific reasoning, Biochem. Mol. Biol. Educ., 51, 286–301.
- Cooper M. M., Kouyoumdjian H. and Underwood S. M., (2016), Investigating Students’ Reasoning about Acid–Base Reactions, J. Chem. Educ., 93(10), 1703–1712 DOI:10.1021/acs.jchemed.6b00417.
- Crandell O. M., Kouyoumdjian H., Underwood S. M. and Cooper M. M., (2019), Reasoning about Reactions in Organic Chemistry: Starting It in General Chemistry, J. Chem. Educ., 96(2), 213–226 DOI:10.1021/acs.jchemed.8b00784.
- Crandell O. M., Lockhart M. A. and Cooper, M. M., (2020), Arrows on the Page Are Not a Good Gauge: Evidence for the Importance of Causal Mechanistic Explanations about Nucleophilic Substitution in Organic Chemistry, J. Chem. Educ., 97(2), 313–327 DOI:10.1021/acs.jchemed.9b00815.
- Davidowitz B. and Rollnick M., (2011), What lies at the heart of good undergraduate teaching? A case study in organic chemistry, Chem. Educ. Res. Pract., 12(3), 355–366.
- Decocq V. and Bhattacharyya G., (2019), TMI (Too much information)! Effects of given information on organic chemistry students’ approaches to solving mechanism tasks, Chem. Educ. Res. Pract., 20(1), 213–228 10.1039/c8rp00214b.
- Domin D. S., Al-Masum M. and Mensah J., (2008), Students’ categorizations of organic compounds, Chem. Educ. Res. Pract., 9(2), 114–121.
- Dood A. J. and Watts F. M., (2022), Mechanistic Reasoning in Organic Chemistry: A Scoping Review of How Students Describe and Explain Mechanisms in the Chemistry Education Research Literature, J. Chem. Educ., 99, 2864–2876 DOI:10.1021/acs.jchemed.2c00313.
- Dood A. J. and Watts F. M., (2023), Students’ Strategies, Struggles, and Successes with Mechanism Problem Solving in Organic Chemistry: A Scoping Review of the Research Literature, J. Chem. Educ., 100(1), 53–68 DOI:10.1021/acs.jchemed.2c00572.
- Dood A. J., Dood J. C., de Arellano D. C. R., Fields K. B. and Raker J. R., (2020a), Analyzing explanations of substitution reactions using lexical analysis and logistic regression techniques, Chem. Educ. Res. Pract., 21(1), 267–286 10.1039/c9rp00148d.
- Dood A. J., Dood J. C., de Arellano D. C. R., Fields K. B. and Raker J. R., (2020b), Using the research literature to develop an adaptive intervention to improve student explanations of an SN1 reaction mechanism, J. Chem. Educ., 97(10), 3551–3562.
- Ealy J. B. and Hermanson J., (2006), Molecular images in organic chemistry: assessment of understanding in aromaticity, symmetry, spectroscopy, and shielding. J. Sci. Educ. Technol., 15, 59–68.
- Eckhard J., Rodemer M., Bernholt S. and Graulich N., (2022), What Do University Students Truly Learn When Watching Tutorial Videos in Organic Chemistry? An Exploratory Study Focusing on Mechanistic Reasoning, J. Chem. Educ., 99(6), 2231–2244 DOI:10.1021/acs.jchemed.2c00076.
- Farheen A. and Lewis S. E., (2021), The impact of representations of chemical bonding on students' predictions of chemical properties, Chem. Educ. Res. Pract., 22(4), 1035–1053 10.1039/d1rp00070e.
- Fleming S. A., Hart G. R. and Savage P. B., (2000), Molecular orbital animations for organic chemistry, J. Chem. Educ., 77(6), 790.
- Friesen J. B., (2008), Saying what you mean: teaching mechanisms in organic chemistry, J. Chem. Educ., 85(11), 1515.
- Frost, S. J., Yik B. J., Dood A. J., de Arellano D. C.-R., Fields K. B. and Raker J. R., (2023), Evaluating electrophile and nucleophile understanding: a large-scale study of learners’ explanations of reaction mechanisms, Chem. Educ. Res. Pract., 24(2), 706–722.
- Galloway K. R., Leung M. W. and Flynn A. B., (2019), Patterns of reactions: a card sort task to investigate students’ organization of organic chemistry reactions, Chem. Educ. Res. Pract., 20(1), 30–52 10.1039/c8rp00120k.
- Gentner D., (1983), Structure-mapping: a theoretical framework for analogy, Cognitive science, 7(2), 155–170.
- Given L. M., (2008), The Sage encyclopedia of qualitative research methods, Sage publications.
- Graulich N., (2015), The tip of the iceberg in organic chemistry classes: how do students deal with the invisible? Chem. Educ. Res. Pract., 16(1), 9–21.
- Graulich N., Hedtrich S. and Harzenetter R., (2019), Explicit versus implicit similarity – exploring relational conceptual understanding in organic chemistry, Chem. Educ. Res. Pract., 20(4), 924–936 10.1039/c9rp00054b.
- Grove N. P., Cooper M. M. and Rush K. M., (2012), Decorating with arrows: toward the development of representational competence in organic chemistry, J. Chem. Educ., 89(7), 844–849.
- Hand B. and Choi A., (2010), Examining the impact of student use of multiple modal representations in constructing arguments in organic chemistry laboratory classes, Res. Sci. Educ., 40, 29–44.
- Head J., Bucat R., Mocerino M. and Treagust D., (2005), Exploring students’ abilities to use two different styles of structural representation in organic chemistry, Canadian J. Sci., Math. Technol. Educ., 5, 133–152.
- Henderleiter J., Smart R., Anderson J. and Elian, O., (2001), How Do Organic Chemistry Students Understand and Apply Hydrogen Bonding? J. Chem. Educ., 78(8) 1126–1130 DOI:10.1021/ed078p1126.
- Hermanns J., (2020), Training OC: a new course concept for training the application of basic concepts in organic chemistry, J. Chem. Educ., 98(2), 374–384.
- Hinze S. R., Williamson V. M., Deslongchamps G., Shultz M. J., Williamson K. C. and Rapp, D. N., (2013), Textbook treatments of electrostatic potential maps in general and organic chemistry, J. Chem. Educ., 90(10), 1275–1281.
- Jain P., Satija J. and Sudandiradoss, C., (2023), Discovery of andrographolide hit analog as a potent cyclooxygenase-2 inhibitor through consensus MD-simulation, electrostatic potential energy simulation and ligand efficiency metrics, Sci. Rep., 13(1), 8147 DOI:10.1038/s41598-023-35192-7.
- Jones T., Romanov A., Pratt J. M. and Popova M., (2022), Multi-framework case study characterizing organic chemistry instructors’ approaches toward teaching about representations, Chem. Educ. Res. Pract., 23, 930–947 10.1039/d2rp00173j.
- Kang J., Park I., Shim J. H., Kim D. Y. and Um W., (2023), Prediction of stable radon fluoride molecules and geometry optimization using first-principles calculations, Sci. Rep., 13(1), 2898 DOI:10.1038/s41598-023-29313-5.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34(9), 949–968.
- Kozma R. and Russell J., (2005), Students Becoming Chemists: Developing Representationl Competence, Springer, Netherlands, pp. 121–145 DOI:10.1007/1-4020-3613-2_8.
- Kraft A., Strickland A. M. and Bhattacharyya G., (2010), Reasonable reasoning: multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11(4), 281–292.
- Kranz D., Schween M. and Graulich N., (2023), Patterns of reasoning - exploring the interplay of students' work with a scaffold and their conceptual knowledge in organic chemistry, Chem. Educ. Res. Pract., 24(2), 453–477 10.1039/d2rp00132b.
- Kumi B. C., Olimpo J. T., Bartlett F. and Dixon B. L., (2013), Evaluating the effectiveness of organic chemistry textbooks in promoting representational fluency and understanding of 2D–3D diagrammatic relationships, Chem. Educ. Res. Pract., 14(2), 177–187.
- Mayer R. E., (2005), Multimedia Learning: Guiding Visuospatial Thinking with Instructional Animation, Cambridge University Press.
- McClary L. and Talanquer V., (2011), College chemistry students' mental models of acids and acid strength, J. Res. Sci. Teach., 48(4), 396–413.
- Mekwong S. and Chamrat S., (2021), The development learning activities using three levels of chemical representation for enhance upper secondary students’ organic chemistry concepts, J. Phys.: Conf. Ser., 1835, 012027.
- O’Connor C. and Joffe H., (2020), Intercoder Reliability in Qualitative Research: Debates and Practical Guidelines, Int. J. Qualitative Methods, 19, 160940691989922 DOI:10.1177/1609406919899220.
- O’Dwyer A. and Childs P., (2014), Organic Chemistry in Action! Developing an Intervention Program for Introductory Organic Chemistry To Improve Learners’ Understanding, Interest, and Attitudes, J. Chem. Educ., 91(7), 987–993 DOI:10.1021/ed400538p.
- Offerdahl E. G., Arneson J. B. and Byrne N., (2017), Lighten the Load: Scaffolding Visual Literacy in Biochemistry and Molecular Biology, CBE Life Sci Educ, 16(1), es1 DOI:10.1187/cbe.16-06-0193.
- Olimpo J. T., Kumi B. C., Wroblewski R. and Dixon B. L., (2015), Examining the relationship between 2D diagrammatic conventions and students' success on representational translation tasks in organic chemistry, Chem. Educ. Res. Pract., 16(1), 143–153.
- Popova M. and Bretz S. L., (2018a), “It's Only the Major Product That We Care About in Organic Chemistry”: An Analysis of Students’ Annotations of Reaction Coordinate Diagrams, J. Chem. Educ., 95(7), 1086–1093.
- Popova M. and Bretz S. L., (2018b), Organic chemistry students’ challenges with coherence formation between reactions and reaction coordinate diagrams, Chem. Educ. Res. Pract., 19(3), 732–745.
- Popova M. and Jones T., (2021), Chemistry instructors’ intentions toward developing, teaching, and assessing student representational competence skills, Chem. Educ. Res. Pract., 22, 733–748 10.1039/d0rp00329h.
- Prain V. and Tytler R., (2012), Learning Through Constructing Representations in Science: a framework of representational construction affordances, Int. J. Sci. Educ., 34(17), 2751–2773 DOI:10.1080/09500693.2011.626462.
- Raker J. R. and Holme T. A., (2013), A historical analysis of the curriculum of organic chemistry using ACS exams as artifacts, J. Chem. Educ., 90(11), 1437–1442.
- Rau M. A., (2017), Conditions for the Effectiveness of Multiple Visual Representations in Enhancing STEM Learning, Educ. Psychol. Rev., 29(4), 717–761 DOI:10.1007/s10648-016-9365-3.
- Rodemer M., Eckhard J., Graulich N. and Bernholt S., (2021), Connecting explanations to representations: benefits of highlighting techniques in tutorial videos on students’ learning in organic chemistry, Int. J. Sci. Educ., 43(17), 2707–2728.
- Sanderson R., (1959), Models for demonstrating electronegativity and “partial charge”. J. Chem. Educ., 36(10), 507.
- Sanger M. J. and Badger S. M., (2001), Using computer-based visualization strategies to improve students' understanding of molecular polarity and miscibility, J. Chem. Educ., 78(10), 1412.
- Schnotz W., (2005), An integrated model of text and picture comprehension, Cambridge Handbook Multimedia Learning, 49(2005), 69.
- Schönborn K. J. and Anderson T. R., (2009), A Model of Factors Determining Students’ Ability to Interpret External Representations in Biochemistry, Int. J. Sci. Educ., 31(2), 193–232 DOI:10.1080/09500690701670535.
- Schönborn K. J. and Anderson T. R., (2010), Bridging the educational research-teaching practice gap, Biochem. Mol. Biol. Educ., 38(5), 347–354 DOI:10.1002/bmb.20436.
- Shalaby M. A., Fahim A. M. and Rizk S. A., (2023), Microwave-assisted synthesis, antioxidant activity, docking simulation, and DFT analysis of different heterocyclic compounds, Sci. Rep., 13(1), 4999 DOI:10.1038/s41598-023-31995-w.
- Shenton A. K., (2004), Strategies for ensuring trustworthiness in qualitative research projects, Educ. Information, 22, 63–75 DOI:10.3233/EFI-2004-22201.
- Shusterman A. J. and Shusterman G. P., (1997), Teaching Chemistry with Electron Density Models, J. Chem. Educ., 74(7), 771 DOI:10.1021/ed074p771.
- Smith D. K., (2023), Priority and Selectivity Rules To Help Students Predict Organic Reaction Mechanisms, J. Chem. Educ., 100, 1164–1178 DOI:10.1021/acs.jchemed.2c00950.
- Springer M. T., (2014), Improving students’ understanding of molecular structure through broad-based use of computer models in the undergraduate organic chemistry lecture, J. Chem. Educ., 91(8), 1162–1168.
- Stowe R. L. and Cooper M. M., (2017), Practicing What We Preach: Assessing “Critical Thinking” in Organic Chemistry, J. Chem. Educ., 94(12), 1852–1859 DOI:10.1021/acs.jchemed.7b00335.
- Strickland A. M., Kraft A. and Bhattacharyya G., (2010), What happens when representations fail to represent? Graduate students’ mental models of organic chemistry diagrams, Chem. Educ. Res. Pract., 11(4), 293–301 10.1039/c0rp90009e.
- Stull A. T., Hegarty M., Dixon B. and Stieff M., (2012), Representational Translation With Concrete Models in Organic Chemistry, Cognition Instruction, 30(4), 404–434 DOI:10.1080/07370008.2012.719956.
- Sunyono S., Leny Y. and Muslimin I., (2015), Supporting students in learning with multiple representation to improve student mental models on atomic structure concepts, Sci. Educ. Int., 26(2), 104–125.
- Taagepera M. and Noori S., (2000), Mapping students' thinking patterns in learning organic chemistry by the use of knowledge space theory, J. Chem. Educ., 77(9), 1224–1229 DOI:10.1021/ed077p1224.
- Talanquer V., (2018), Exploring Mechanistic Reasoning in Chemistry, Springer, Singapore, pp. 39–52 DOI:10.1007/978-981-10-5149-4_3.
- Talanquer V., (2022), The Complexity of Reasoning about and with Chemical Representations, JACS Au, 2, 2658–2669 DOI:10.1021/jacsau.2c00498.
- Ward L. W., Rotich F., Hoang J. and Popova M., (2022), Representational Competence Under the Magnifying Glass—The Interplay Between Student Reasoning Skills, Conceptual Understanding, and the Nature of Representations. Student Reasoning Org. Chem., pp. 36–56, ch. 3 10.1039/9781839167782-00036.
- Watts F. M., Schmidt-McCormack J. A., Wilhelm C. A., Karlin A., Sattar A., Thompson B. C., Gere A. R. and Shultz G. V., (2020), What students write about when students write about mechanisms: analysis of features present in students’ written descriptions of an organic reaction mechanism, Chem. Educ. Res. Pract., 21(4), 1148–1172.
- Watts F. M., Zaimi I., Kranz D., Graulich N. and Shultz G. V., (2021), Investigating students’ reasoning over time for case comparisons of acyl transfer reaction mechanisms, Chem. Educ. Res. Pract., 22(2), 364–381.
- Watts F. M., Park G. Y., Petterson M. N. and Shultz G. V., (2022), Considering alternative reaction mechanisms: students' use of multiple representations to reason about mechanisms for a writing-to-learn assignment, Chem. Educ. Res. Pract., 23(2) 486–507 10.1039/d1rp00301a.
- Webber D. M. and Flynn A. B., (2018), How Are Students Solving Familiar and Unfamiliar Organic Chemistry Mechanism Questions in a New Curriculum? J. Chem. Educ., 95(9), 1451–1467 DOI:10.1021/acs.jchemed.8b00158.
- Wright L. K., Cardenas J. J., Liang P. and Newman, D. L., (2017), Arrows in biology: lack of clarity and consistency points to confusion for learners, CBE—Life Sci. Educ., 17(1), ar6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6007777/pdf/cbe-17-ar6.pdf.
- Yik B. J., Dood A. J., Frost S. J., de Arellano D. C.-R., Fields K. B. and Raker J. R., (2023), Generalized rubric for level of explanation sophistication for nucleophiles in organic chemistry reaction mechanisms, Chem. Educ. Res. Pract., 24(1), 263–282.
- Zhou W., Xu Z. and Zhao J., (2023), A Novel Lewis Structure and Its Utilization in the Examination of Mechanisms of Organic Chemical Reactions, J. Chem. Educ., 100, 3694–3702.
| This journal is © The Royal Society of Chemistry 2024 |