Open Access Article
Open Access ArticleA design-based research approach to improving pedagogy in the teaching laboratory
Christine E.
Mundy
 *a,
Marietjie
Potgieter
*a,
Marietjie
Potgieter
 a and
Michael K.
Seery
a and
Michael K.
Seery
 b
b
aDepartment of Chemistry, University of Pretoria, Pretoria, Gauteng 0002, South Africa. E-mail: christine.mundy@up.ac.za
bCardiff Metropolitan University, Western Avenue, Cardiff, CF5 2YB, UK
First published on 18th October 2023
Abstract
The laboratory is a complex environment where the three levels of the chemistry triplet coincide. As the laboratory environment places a large demand on the working memory of students, cognitive load theory can address overload which causes barriers to learning. Breaking down barriers requires iterative phases of analysis/exploration, design/construction and evaluation/reflection over multiple cycles which are the hallmarks of design-based research. In a complex setting, managing change and redressing teaching approaches can be difficult to navigate. Design-based research incorporates iterative phases in which theory informs decision making. This paper uses the context of a laboratory exercise of emission spectra to illustrate how the cognitive load theory can be used in tandem with design-based research to support student learning in the exercise. Using this approach, it was possible to show how barriers to student understanding, including task demands and conceptual demands were supported through proposed approaches focusing on extraneous, intrinsic and ultimately germane cognitive load.
Introduction
Substantial effort is research and scholarship relating to laboratory teaching in higher education has been reported in the primary literature. This includes discussion about the purpose (Seery, 2020) and the extent of evidence (Bretz, 2019) for laboratory work, detailed analysis on the reported learning outcomes that accrue from laboratory teaching in the curriculum (Agustian et al., 2022), curriculum design models (Seery et al., 2019), and a plethora of scholarship-influenced approaches to transforming teaching and learning practice, especially in light of the COVID pandemic (Kelley, 2021). Yet there remains a sense that laboratory education continues to be a place where innovation and improvements to practice are needed. Hegarty-Hazel's statement to the effect that regardless of what is written in teaching documentation, the intentions the person running the laboratory class on the day will direct the goals and purpose of practical work remains prescient today as when it was written nearly four decades ago (Boud et al., 1986). This points to a substantial challenge and opportunity in laboratory education reform – the challenge is the ensuring of a consistency in pedagogic goals for laboratory work from a student perspective; the opportunity is that real and meaningful change can occur in cases where an instructor wishes to enact reform. In such cases, an instructor may be guided by their own epistemology or influenced by learning theory, and the challenge for reform is how they enact it.In this work we offer the perspective of our considerations of how we enacted our intentions for improvement in teaching approaches using design-based research as an overarching model for change. We demonstrate how we draw upon a learning theory that has influenced our design (cognitive load theory) in a particular learning context (the laboratory) and with a particular topic in chemistry (emission spectroscopy).
Design-based research
Design-based research has come to the forefront in educational research, and is being increasingly utilized (Anderson and Shattuck, 2012). Design-based research begins with the analysis of a problem followed by identifying relevant theory for the hypothesis of a solution in practice, iterative cycles of testing and refining the solution alongside reflections which enhance both practice and the theory (Reeves, 2006). This is depicted graphically in Fig. 1, as applied to the case under discussion in this work: addressing barriers in emission spectroscopy in laboratory contexts are combined with a guiding theoretical perspective (our choice being cognitive load theory, vide infra). As the intervention matures from cycle to cycle there is a constant interplay between theoretical understanding of cognitive load theory enriching practical insights alongside growth in the researchers’ theoretical understanding based on practical findings, which is consequently integrated in future cycles of implementation. The different colours used in Fig. 1 correspond to the elements of cognitive load being deployed in each cycle (see Fig. 4–6). Cycles 1 and 2 appear large and cycles 3 and 4 appear small in Fig. 1 to illustrate the relative size of the interventions: moving from large quantitative data sets to small qualitative data sets (see Phases, Cycles and Data Sources).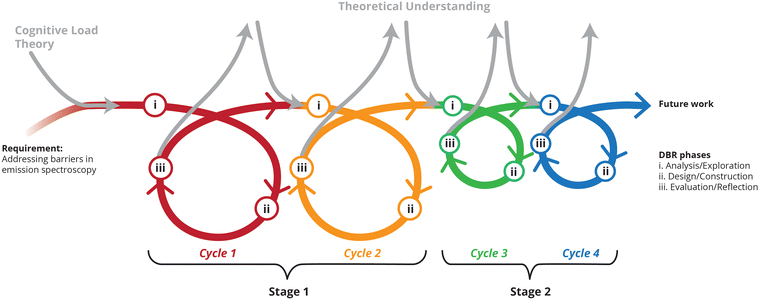 | ||
| Fig. 1 Stages, cycles and phases of design-based research (DBR) in this study, based on figures by Fraefel (2014) and Underwood (2021). | ||
In short, design-based research is a research methodology that seeks to understand how, when, and why educational innovations work in practice (Design-Based Research Collective, 2003).
Design-based research “occurs in the buzzing, blooming confusion of real-life settings where most learning actually occurs” (Barab and Squire, 2004, p. 4). The richness of a design-based research methodology adds to the further understanding of pedagogical observations in actual learning contexts, enhancing the utility of the findings for researchers and practitioners (Wang and Hannafin, 2005). For laboratory contexts, it surpasses classical methodologies that usually focus only on summative findings; instead its intention is situated in the formative process of uncovering barriers and supporting learning, making it particularly appropriate to the “buzzing, blooming confusion” of this teaching scenario.
In this article, we discuss the application of a design-based research approach reflects all of the five basic characteristics of design-based research as outlined by Wang and Hannafin (2005):
1. Pragmatic: the search for useful design principles and solutions to student barriers are well established in the topic and context of laboratory.
2. Grounded: relevant learning theory is available that has value to inform pedagogical approaches (in our case on the design-decisions were informed by the Information Processing Model and Cognitive Load Theory).
3. Interactive, iterative, and flexible: the primary researcher directly participated in the study and the structure of the laboratory exercise changed with time.
4. Integrative: capacity to invoke mixed method data collection tools as the need arose to gain insights at different stages.
5. Contextual: the complex laboratory setting included students from a variety of backgrounds, language proficiencies, and levels of exposure to laboratories.
There are three core processes or phases described in design-based research: analysis/exploration, design/construction and evaluation/reflection (McKenney and Reeves, 2012), (refer again to Fig. 1). Briefly, the first phase deals with a problem as it emerges, identifying and diagnosing the problem based on literature, context and goals. The second core phase is design/construction, in this phase a tentative solution is proposed through creating or revising a model or skeleton which informs well-considered design decisions. This phase grounds theory in the reality of the study and generates practical solutions to the problems faced. Finally, the evaluation/reflection phase balances various findings with critical reflections. This phase includes a formal reflection in which the chain of reasoning is assessed against the efficacy of the outcomes of the solution.
The three phases do not necessarily follow each other in a linear fashion and often there are iterative and flexile pathways between the phases (McKenney and Reeves, 2012). After the iterative phases of outlining design requirements, creating design propositions, making design decisions and critically reflecting on the design solution, the researcher arrives at design principles. Design principles set design-based research apart from action research in that they have theoretical and practical applicability outside the scope of the current study (Plomp and Nieveen, 2013).
The teaching context: learning about emission spectroscopy in the laboratory
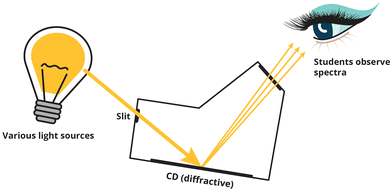
Emission spectroscopy is not a simple topic, and while it can be visually stimulating, it can result in cognitively challenging laboratory experiences. Emission spectroscopy sheds light on the quantized nature of the atom, which is necessary for students to fully understand key chemistry concepts such as bonding and hybridisation. In our context, a simplified and low-cost laboratory experiment, previously described by Mundy and Potgieter (2020) was used in the first-year general chemistry laboratory. Briefly, in this exercise students constructed small spectroscopes, called Mini Specs, and made observations of the diffraction of light from different light sources (see Fig. 2). Students recorded their observations and interpretation on guided report sheets which were marked as assessment of their learning (see Table 1).| Learning goal | Report sheet question |
|---|---|
| 1. Focusing component | What is the purpose of the slit in your Mini Spec? |
| 2. Diffractive component | What is the purpose of the piece of CD in your Mini Spec? |
| 3. Spectral line formation | Spectral lines are formed when: |
| 4. Line classification | When viewing artificial light sources you see emission lines with your Mini Spec. What is the significance of this finding? |
| 5. Intensity of lines | Some of the emission lines appear brighter than others. What is the significance of this finding? |
Before the introduction of the Mini Spec laboratory exercise, one week of lectures and tutorials dealt with emission spectroscopy, tied to this topic were three pre-existing learning goals (see Learning Goals 3–5). The introduction of a macroscopic or laboratory component to the curriculum necessitated competencies of its own, primarily understanding the basic functioning of a spectroscope (see Learning Goals 1–2). The defined learning goals for emission spectroscopy thus became:
1. Identifying the focusing component in the Mini Spec (L1)
2. Identifying the diffraction grating in the Mini Spec (L2)
3. Understanding how emission lines are formed (L3)
4. Classifying the type of emission spectra using the descriptors of continuous or discrete (L4)
5. Interpreting emission spectra as evidence of the quantized electronic structure of the atom (L5).
The learning goals above outline the essentials for understanding emission spectroscopy: when students did not achieve these goals, barriers were be proposed to be standing in the way of understanding. Barriers may be considered as places where the learning materials and interactions do not facilitate students’ obtaining or communicating their understanding of the learning goals.
In this paper we aim to utilise cognitive load theory to inform several cycles of design-based research to uncover and mitigate the barriers novice students face in foundational emission spectroscopy. The combination of design-based research and cognitive load theory provided a unique and robust lens which may be appropriate for other research interventions in the complex setting of the laboratory, or may simply guide more effective teaching and learning in the laboratory.
The guiding theory: cognitive load theory
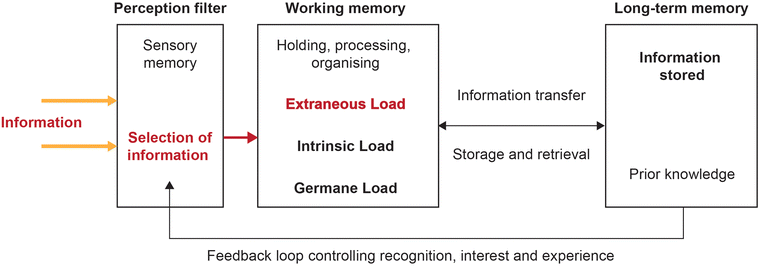
The hidden nature of the student thinking means that identifying learning barriers in emission spectroscopy required a robust theoretical lens which could provide potential explanations as to what was happening in the minds of the students and offer mechanisms by which we may overcome emergent barriers. Cognitive Load Theory emerges from an information processing perspective and deals with patterns of human thinking that are proposed to be universal (Johnstone, 1991, 2010). Johnstone's model includes both external stimuli information and the elements that the individual brings with them into a learning situation (see the incoming information on the left and the feedback loop from long-term memory on the right of Fig. 3 respectively). Information processing relies on holding, processing, and organizing information within the working memory. Additionally, sense-making requires an iterative process of retrieval and storage of knowledge in the long-term memory.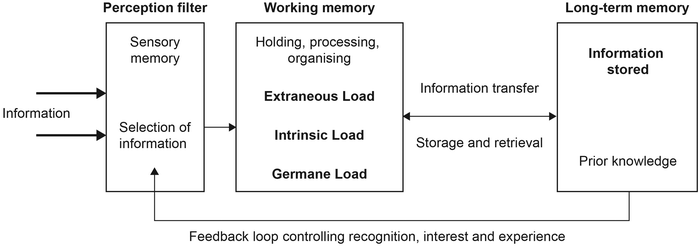 | ||
| Fig. 3 Modified information processing model used to highlight the aspects of cognitive load to be considered in working memory (extraneous, intrinsic, and germane). | ||
Cognitive load theory proposes three tenets that effect the efficiency of the working memory (Sweller, 1994). In Fig. 3, we have embedded extraneous, intrinsic and germane load as variable components in the original information processing model. Extraneous cognitive load hinders processing through the poor presentation or design of instructional materials (Paas et al., 2003). Intrinsic load is topic specific and refers to a topic's inherent complexity, difficulty and unfamiliarity (Paas et al., 2003). High extraneous or intrinsic cognitive load is disadvantageous to mental processing in the working memory, causing cognitive overload or breakdown. On the other hand, germane load is a positive component of cognitive load in that it represents the mental effort required for learning or the load caused by genuine learning processes of holding and organising information in the working memory (Van Merriënboer et al., 2006).
Through embedding the tenets of cognitive load theory into the information processing model, the researchers were able to ground hypotheses on potential barriers for students as they engaged in their learning in the laboratory setting.
We aim to achieve this by using cognitive load theory to frame barriers as instances contributing to cognitive overload in the mind of the student (to be discussed further in the next section). Barriers are therefore instances where the learning process was impeded in the mind of the student. These prompts then acted as action points to pursue in the subsequent cycle of DBR, and the discussion of the article will reflect on the overall process to elaborate why we found the combination of design-based research and cognitive load theory to be a powerful agent for change.
Research question
In this study we answer the following question: How can the design-based research be used to frame and enact meaningful pedagogic intervention?Research methods
Context
Design-based research requires a rich description of, and integration with, the context of the study. This study was set in a first-year general chemistry module which forms part of a BSc academic development programme. Academic development programmes, in the form extended or augmented programmes, have become prevalent in South Africa to facilitate access to tertiary education for students that would otherwise not qualify for admission by offering holistic development and support (Shay et al., 2016). Students on programmes such as these are mainly English second language (ESL) speakers with limited laboratory experience (Rollnick et al., 2001). It follows that the student body is diverse, with many students coming from previously disadvantaged backgrounds.Phases, cycles and data sources
This paper spans four iterative and transformative cycles of design-based research (refer again to Fig. 1). The three core phases of analysis/exploration, design/construction and evaluation/reflection were present within each cycle (see (i), (ii) and (iii) in Fig. 1). Across these cycles the numbers of participants and the data collection sources changed as the research matured. To delineate between the four cycles, two main stages can be envisioned for this study. The first stage had two cycles, one year apart, with large numbers of randomly self-enrolled participants from general chemistry, n = 443 and n = 405, respectively. All participants were enrolled in general chemistry at the time. Quantitative data was used to monitor students’ overall performance on their submitted report sheets and time spent building the Mini Spec. Stage 1 data also included structured observations by lecturing staff overseeing the laboratory.The second stage consisted of cycles 3 and 4. These cycles were far smaller in terms of the numbers of participants and hence appear smaller in Fig. 1 (n = 9 and n = 29). Participants from cycle 3 were volunteers who had already completed the general chemistry course in the previous year. Participants from cycle 4 were again currently enrolled in general chemistry. Participants for cycles 4 were chosen in a stratified and purposive way: laboratory demonstrators (graduate students) approached students to participate in the study according to the time it took for the students to complete the entire experiment. That is, clusters of students were approached on a daily basis centred on whether they completed the experiment very quickly, comfortably within the allocated three hours or took close to exceeding the time allowed.
In stage 2, the students’ report sheets represented a different data source: overall performance was not considered as in stage 1, instead students’ understanding was gauged per learning goal using an evaluative rubric. Coding was done according to the five relevant learning goals using codes of poor, partial and good. Two independent coders achieved an 83% agreement using the same rubric. Secondly, participating students were invited to engage in a structured post-lab collaborative discussion, recordings were made and transcribed with permission.
Ethical clearance was granted by the corresponding author's institution for this research (180000144). All interactions with the laboratory exercise provided students the opportunity to interact with spectroscopy in a hands-on manner that did not exist before this research.
Reporting on design-based research
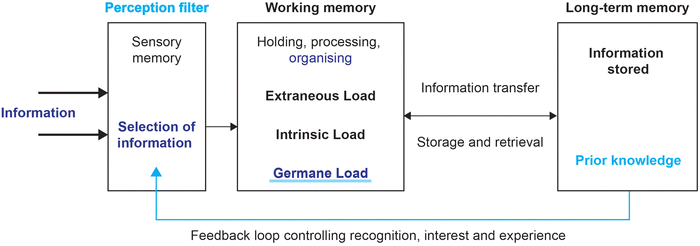
The three core phases of analysis/exploration, design/construction and evaluation/reflection were used to illustrate how the theoretical and methodological theories drove the understanding of student learning. As stated previously, the emphasis of design-based research is not just in the findings but on the processes taken to reach maturing theoretical insights and practical solutions. In this iterative reporting style, barriers to students’ understanding of emission spectroscopy will emerge from theoretical understanding, will be dealt with and the solutions will be reflected upon.Cycle 1 is presented in a comprehensive style, see italics, to illustrate the many cues used by a design-based researcher as they navigate the three main phases. Different elements of the information processing model with embedded tenets of cognitive load theory are used at different stages of the research. Colour coding of the elements in red, orange, green and two shades of blue (in Fig. 4–6) correspond with the colour of the cycles depicted in Fig. 1.
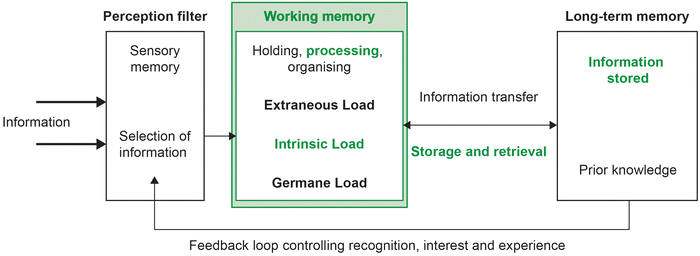 | ||
| Fig. 5 Using the information processing model to address barrier for students (focussing on reducing individual intrinsic load through a collective working memory). | ||
Stage 1
Therefore, the selection of a suitable spectroscope needed to balance the context of the study: low cost, due to limited resources, and sensitivity to cognitive load, so as to best prepare materials for students to engage with the programme. The mini spectroscope (Schwabacher, 1999) was selected as it was of minimal cost, and required everyday materials including a 1/16th of a CD, cardboard and adhesive. The everyday components of the Mini Spec, as we called it, should lower intrinsic cognitive load as students already have exposure to simple materials and appropriate psychomotor skills.
Design/construction: in this phase, the researcher constructs a potential solution: one of the simplest, and usually the first, method in the design of instructional materials is for an instructor to reduce extraneous load (see Fig. 4, Cycle 1's hypothesised cognitive pathway in red). By reducing extraneous load, processing capacity is increased in the working memory and the potential for transferring and enriching knowledge to and from the long-term memory is enhanced. Practical design-based decisions are implemented: extraneous cognitive load may be reduced by purposeful alterations by the instructor, in this case excess text and symbols were removed from the Mini Spec template. Additionally the construction instructions were simplified in terms of their length and literacy demands. In the laboratory, the reduction of extraneous load should result in students being able to allocate more cognitive resources to understanding the functioning of the Mini Spec and performing observations with the Mini Spec.
Evaluation/reflection: in this final phase, various findings are consolidated: staff observations revealed students’ enjoyment of the Mini Spec laboratory exercise, however, the construction of their Mini Specs remained challenging. Conclusions are drawn: the construction task remained demanding for students. Critical reflection of the design-based decision/solution: the construction of the Mini Spec was intended to be a simple process, however, it was clear that the construction process could be improved. Interplay between theory and practice: our use of the information processing model led to the hypothesis that extraneous load should be considered.
Design/construction: in cycle 2 a more sophisticated approach to reducing extraneous cognitive load was hypothesised by introduction two channels for incoming information (see Fig. 4, Cycle 2 also focuses on extraneous load, specifically the factor of dual incoming information on extraneous load in orange). Having multiple sources of information should allow the student to select the source of incoming information which they can process with the greatest ease and discard other “extra” information which would have increased extraneous load if it were the only incoming information available. Implementation of this design-decision meant that to aid students in constructing their Mini Specs, additional physical information was given in the form of construction references (Mini Spec templates in various stages of completion present throughout the lab) i.e. supplementary or redundant information was provided to help improve construction time.
Evaluation/reflection: students showed higher overall performance in the laboratory report sheet (M = 73.2%) as compared to those in cycle 1 (M = 68.9%, p = 0.0005) and construction times were statistically significantly improved with a p value of 0.003 (see Mundy and Potgieter (2020)). The latter finding supports the reverse redundancy effect: “The redundancy facilitation hypothesis predicts a reverse redundancy effect in scenarios where redundant material can support basic cognitive processing that is not yet automated in non-native speakers while minimizing extra cognitive load” (Mayer et al., 2014, p. 654). In fact, the researchers propose that the dual channels of incoming information were key in supporting ESL students’ completion of the task of construction and thereby improved overall performance. In summary, the alleviation of barriers through reducing extraneous load proposed in Stage 1 helped overcome the demands of the task and associated language demands placed on students.
Stage 2
Stage 2 saw a shift from trying to better instructional materials, to a desire to understand the intricacies surrounding common conceptual difficulties in the topic of emission spectroscopy. The data collection methods went from highly quantitative to richly qualitative.Intrinsic load is not as easy to manipulate for individual students. Collaborative cognitive load theory, as described by Kirschner et al. (2018), presented an opportunity to overcome instances where individual intrinsic load is too high. Collaboration leads to a shared or collective working memory which is greater than the working memory of any one individual (Kirschner et al., 2011, 2018). Language difficulties may also be part of the barrier ESL students’ face when communicating their understanding of emission spectroscopy. Language and processing thereof may utilise up to twenty percent of an ESL student's working memory (Johnstone and Selepeng, 2001).
Design/construction: in this phase of the study, the total score assigned in the report sheet (as in Stage 1) was not sufficient – items in the report sheets were analysed as a starting-point to gauge individual understanding of the five learning goals. However, the constraints of individual cognitive capacity may mask the true barriers associated with emission spectroscopy.
By placing individuals in a team or collaborative environment, a collective working memory will be created (see Fig. 5). This larger cognitive space increases the capacity for processing. The nature of collaborative transactions processed in a collective working memory allows understanding and meaning to ideally be jointly negotiated and more effectively communicated. A structured collaborative post-lab group work activity was introduced which allowed the researchers to partially compensate for the limitations of individual cognitive load. The collaborative activity mainly focused on learning goal 3 and 4 (see Appendix A).
As stated previously, participants in cycle 3 were volunteers who had already completed the general chemistry course in the previous year, and as such the introduction of the collaborative activity was low-risk for the random voluntary participants (n = 9).
Evaluation/reflection: participants’ understanding of the five learning goals was expressed on the individual report sheets (see Tables 1 and 2). From the coding process, it was clear the participants’ understanding was poorest when it came to learning goals 2 and 5. The diffractive purpose of the wedge of CD in the Mini Spec was either understood or misunderstood entirely, e.g. “The CD reflects light and emits colour”. The majority of participants’ clung to the common misconception for learning goal 5: linking the brightness of the line to the energy of the photon, not understanding that the brightness of a spectral line refers to the increased probability of a particular photon being released. These findings re-affirm common conceptual barriers faced by students in literature.
| Learning goal | Description | Poor understanding | Partial understanding | Good understanding |
|---|---|---|---|---|
| 1. | Focusing component | 3 | 2 | 4 |
| 2. | Diffractive component | 4 | 1 | 4 |
| 3. | Spectral line formation | 2 | 3 | 4 |
| 4. | Line classification | Data not sufficient for coding | ||
| 5. | Intensity of lines | 8 | 0 | 1 |
Participants’ understanding of learning goals 1 and 3 was limited but not as poor as for goals 2 and 5. In terms of spectral line formation (learning goal 3), participants were unclear as to whether or not it is the electron or the photon transitioning between energy levels. In terms of the purpose of the slit being to focus light, three participants attributed an incorrect purpose e.g. splitting light. Two participants showed partial understanding of the slit as the entrance point for light into the Mini Spec but did not elaborate on the focusing action of the slit on incoming light.
Data could not be coded according to the rubric for learning goal 4 due to the formulation of the question in the report sheet, however, the analysis revealed that participants need guidance when discussing the distinction between discrete and continuous spectra.
Analysis of the collaborative discussion gave a deeper perspective of the barriers faced by participants: language emerged as a barrier in the guise of simple, non-technical terminology, language, as a barrier will be discussed in greater detail in a proceeding article. For example: analysis of learning goal 3 in a collaborative environment revealed participants’ pre-occupation with the directionality of the word “jumps” in An electron “jumps” between energy levels. The identification of a language barrier through collaboration explained the poor understanding exhibited on an individual level whose cause would have remained indiscernible to the researchers.
A vignette from the collaborative discussion of group 2 (n = 4) shows how meaning was jointly negotiated and effectively communicated in the collaborative environment, resulting in an improved understanding of spectra classification:
Female 1: The most important observation from the spectrum of the energy saver globe is that… Only certain bands of light-coloured light are emitted
All: Agree
Female 1: Because, a full (continuous) spectrum of light is definitely not emitted, only certain colours
Female 2: Because it is energy-saving it shouldn’t, it would not be able to emit all the bands of spectrum of light. If it was not energy-saving it would emit all the bands.
In reflecting on the value of Cycle 3's remediation, the design-decision allowed participants to collectively overcome some of the demands associated with concepts in emission spectroscopy through the shared working memory's processing capacity., The reduction of intrinsic load in a collective working memory environment aligning with the grounds for the design-based decision that informed this action.
Scaffolding is an educational construct which explains and exemplifies the dichotomous nature of germane load. Mental effort must be put in to understand and navigate the scaffolding, but at the same time, the structure of the scaffolding makes organising and storage of information easier.
Design/construction: actions that support the activation of germane load were used in Cycle 4. This approach was multi-faceted to accommodate various approaches to scaffolding understanding for several conceptual difficulties (see Fig. 6, dark blue and light blue). For example, in an effort to scaffold understanding of the diffractive function of the wedge of CD, the words “like a prism” were added to the report sheet for L2 so that students can recall prior knowledge of prisms and assimilate the function of splitting light with that of the wedge of CD (see light blue). This action hypothesises that by linking to prior knowledge using a familiar concept, the perception filter can be activated and prepared allowing the information to enter the working memory where it is processed.
For L1, the report sheet was modified to include guided inquiry to scaffold student understanding the focussing purpose of the Mini Spec slit by adding the questions, “What would happen if the slit was too large? Or too small?” For L4, two extra columns were added to an observations table in the report sheet, guiding students to understand distinct spectral signatures from different types of light sources not only in terms of the colours emitted but whether the spectra were discrete or continuous. That is, extra information was given to for L1 and L4 (see dark blue).
Evaluation/reflection: the coding of students’ responses on their report sheet yielded positive results for L1–L3 with the majority of responses coded as exemplifying good understanding (see Table 3). There was improvement in L4 with most students reporting a partial understanding, for example “Natural light contains all of the spectrum while artificial lines do not contain all of the colours”. Students’ understanding of L5 was not improved by including the term brightness, suggesting that further research is necessary.
| Learning goal | Description | Poor understanding | Partial understanding | Good understanding |
|---|---|---|---|---|
| 1. | Focusing component | 2 | 16 | 11 |
| 2. | Diffractive component | 1 | 9 | 19 |
| 3. | Spectral line formation | 1 | 4 | 24 |
| 4. | Line classification | 4 | 20 | 5 |
| 5. | Intensity of lines | 19 | 5 | 5 |
When reflecting on the interplay of theory and practice in Cycle 4, it can be seen that there are different ways to scaffold student understanding based on the same theoretical premise. The researcher proposes that theory around germane load be enriched to include notions of applying (e.g. explicit scaffolding requiring mental effort for L1 and L4) versus inducing germane load (lowering mental effort required to understand L2, hence allowing ease of processing and understanding). That is, the dark blue approach could be that of applying germane load whereas the light blue could be regarded as inducing germane load (see Fig. 6).
Conclusion
This paper sets out to exemplify how cognitive load theory can be used in tandem with design-based research to inform student learning. Specifically, it was found that the incorporation of information processing model as a basis for actions to take to improve learning in each cycle led to the identification and breakdown of barriers to student learning.Each cycle centred on a tenet of cognitive load theory, peeling away barriers as the research moved from extraneous to intrinsic and finally to germane load. For this reason, the iterative cycles of design-based research were extremely important: the covert nature of the mind of students meant that barriers were discovered and broken down over time. That is, multi-faceted solutions in a complex setting could not be understood through only one iteration, further highlighting the effectiveness of the tandem of cognitive load theory with design-based research.
Furthermore, the nature of the methodology invites change through reflection and allows the researchers to document its effectiveness over time. We were able to manipulate and therefore support learning in the lab over iterative cycles which built on one another, and document the real change seen at each step using a pragmatic approach to data collection.
The timeline and scale of the frameworks allowed time for researchers’ insights on proposed approaches to mature concurrently with the study. Theory was incorporated at each phase and a new insight emerged with reference to the possibility of delineating germane load into applied and induced.
The current research into learning barriers remains on-going, for example, the poor understanding of L5 still requires the researchers to go through at least one more cycle of analysis/exploration, design/construction and evaluation/reflection.
Design-based research generates many findings and insights, which are difficult to communicate in a single article (McKenney and Reeves, 2021). Presenting individual articles or case studies can be a means to address this, but it omits the long-term vision and energies of the researcher. This paper represents a “how-to” in terms of presenting design-based research with exemplary essential findings for each cycle. In general, findings for other studies could be presented in a similar fashion.
The supply of maps or graphics showing the theoretical understanding used to inform designed-based decisions is novel and should be considered as standard practice for reporting in design-based research. This is because the proposed approaches acknowledge the theory and further speak to the scholarly nature of the design-based research study.
The advantages to the combination of cognitive load theory with design-based research should be considered by other chemistry educational practitioners and researchers in that both frameworks are so well aligned to understanding and unpacking complex topics in the laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the laboratory staff for their patience and assistance. We also wish to thank the students participated in this study. Finally, we thank The University of Pretoria's graphic design department with their expertise. The research was supported by the National Research Foundation of South Africa (Grant 111816).References
- Agustian H. Y., Finne L. T., Jørgensen J. T., Pederson M. I., Christiansen F. V., Gammelgaard B. and Nielsen, J. A., (2022). Learning outcomes of university chemistry teaching in laboratories: a systematic review of empirical literature, Rev. Educ., 10(2), e3360.
- Anderson T. and Shattuck J., (2012), Design-based research: a decade of progress in education research? Educ. Res., 41(1), 16–25.
- Barab S. and Squire K., (2004), Design-based research: putting a stake in the ground, J. Learn. Sci., 13(1), 1–14.
- Boud D., Hegarty-Hazel E. and Dunn J., (1986), Teaching in laboratories, Society for Research into Higher Education.
- Bretz S. L., (2019), Evidence for the importance of laboratory courses, J. Chem. Educ., 96(2), 193–195.
- Design-Based Research Collective, (2003), Design-based research: an emerging paradigm for educational inquiry. Educ. Res., 32(1), 5–8.
- Forbes P. B. and Nöthling J. A., (2014), Shedding light on spectrophotometry: the SpecUP educational spectrophotometer, South African J. Sci., 110(1–2), 1–5.
- Fraefel U., (2014), Professionalization of pre-service teachers through university-school partnerships, WERA Focal Meeting, Edinburgh. Retrieved from DOI:10.13140/RG.2.1.1979.5925.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assisted Learn., 7(2), 75–83.
- Johnstone A. H., (2010), You can’t get there from here, J. Chem. Educ., 87(1), 22–29.
- Johnstone A. H. and Selepeng D., (2001), A language problem revisited, Chem. Educ. Res. Practice Europe, 2(1), 19–29.
- Kelley E. W., (2021), LAB theory, HLAB pedagogy, and review of laboratory learning in chemistry during the COVID-19 pandemic, J. Chem. Educ., 98(8), 2496–2517.
- Kirschner F., Paas F. and Kirschner P. A., (2011), Task complexity as a driver for collaborative learning efficiency: the collective working-memory effect, Appl. Cognitive Psychol., 25(4), 615–624.
- Kirschner P. A., Sweller J., Kirschner F. and Zambrano J., (2018), From Cognitive Load Theory to Collaborative Cognitive Load Theory, Int. J. Comput.-Supported Collaborative Learn., 1–21.
- Mayer R. E., Lee H. and Peebles A., (2014), Multimedia Learning in a Second Language: A Cognitive Load Perspective, Appl. Cognitive Psychol., 28(5), 653–660.
- McKenney S. and Reeves T. C., (2012), Conducting educational design research, London: Routledge.
- McKenney S. and Reeves T. C., (2021), Educational design research: portraying, conducting, and enhancing productive scholarship, Med. Educ., 1, 82–92.
- Mundy C. and Potgieter M., (2020), Hands-On Spectroscopy: Inside and Outside the First-Year Laboratory, J. Chem. Educ., 97(6), 1549–1555.
- Paas F., Renkl A. and Sweller J., (2003), Cognitive load theory and instructional design: Recent developments, Educ. Psychol., 38(1), 1–4.
- Plomp T. and Nieveen N., (2013), Educational Design Research, Enschede: SLO, Netherlands Insitute for Curriculum Development.
- Reeves T. C., (2006), Design research from the technology perspective, in K. Gravemeijer and P. Cobb, ed., Design research from a learning design perspective. In Educational design research, Routledge, pp. 86–109.
- Rollnick M., Zwane S., Staskun M., Lotz S. and Green G., (2001), Improving pre-laboratory preparation of first year university chemistry students, Int. J. Sci. Educ., 23(10), 1053–1071.
- Schwabacher A., (1999), Mini Spectroscope. Retrieved 2019, from UWM Chemistry: https://stars.eng.usf.edu/scopeinstruct.pdf.
- Seery M. K., (2020), Establishing the laboratory as the place to learn how to do chemistry, J. Chem. Educ., 97(6), 1511–1514.
- Seery M. K., Agustian H. Y. and Zhang X., (2019), A framework for learning in the chemistry laboratory, Isr. J. Chem., 59(6–7), 546–553.
- Shay S., Wolff K. and Clarence-Fincham J., (2016), Curriculum reform in South Africa: more time for what? Critical Studies Teach. Learn., 4(1), 74–88.
- Sweller J., (1994), Cognitive load theory, learning difficulty, and instructional design, Learning and Instruction, 4(4), 295–312.
- Underwood S. M., (2021), Design-Based Implementation Research (DBIR): An Approach to Propagate a Transformed General Chemistry Curriculum across Multiple Institutions, J. Chem. Educ., 98(12), 3643–3655 DOI:10.1021/acs.jchemed.1c00723.
- Vanderveen J. R., Martin B. and Ooms K. J., (2013), Developing tools for undergraduate spectroscopy: an inexpensive visible light spectrometer, J. Chem. Educ., 90(7), 894–899.
- Van Merrienboer J. J. G. and Sweller J., (2005), Cognitive load theory and complex learning: Recent developments and future directions, Educ. Psychol. Rev., 17(2), 147–177.
- Van Merriënboer J. J., Kester L. and Paas F., (2006), Teaching complex rather than simple tasks: Balancing intrinsic and germane load to enhance transfer of learning, Appl. Cogn. Psychol., 20(3), 343–352.
- Wakabayashi F., (2008), Resolving spectral lines with a periscope-type DVD spectroscope, J. Chem. Educ., 85(6), 849.
- Wang F. and Hannafin M. J., (2005), Design-based research and technology-enhanced learning environments, Educ. Technol. Res. Dev., 53(4), 5–23.
| This journal is © The Royal Society of Chemistry 2024 |