Reconstructing perspectives: investigating how molecular geometry cards (MGCards) and molecular model building (MMB) disrupt students' alternative notions of molecular structure – a qualitative study
Erlina
 *a,
Dylan P.
Williams
*a,
Dylan P.
Williams
 b,
Chris
Cane
c,
Hairida
b,
Chris
Cane
c,
Hairida
 a,
Maria
Ulfah
a,
Maria
Ulfah
 a and
Azwa F.
Wafiq
a and
Azwa F.
Wafiq
 a
a
aChemistry Education Study Program, Faculty of Teacher Training and Education, Tanjungpura University, Kalimantan Barat, 78124, Indonesia. E-mail: erlina@fkip.untan.ac.id
bSchool of Chemistry, University of Birmingham, B15 2SQ, UK
cDepartment of Genetics and Genome Biology, University of Leicester, LE1 7RH, UK
First published on 30th May 2024
Abstract
The range of abstract concepts encountered when learning chemistry and the inability of students to make connections between the macroscopic, sub-microscopic, and symbolic representations, used in chemistry teaching, are believed to be the main reasons for students’ difficulty when learning chemistry. Prediction and determination of molecular geometry using the theory of valence shell electron pair repulsion (VSEPR) is a sample of the abstract concept that is hard to understand by students who learn chemistry. Students may comprehend these ideas better if the learning process is supplemented with cutting-edge, interactive learning aids. To address the conceptual difficulties that students encounter when learning how to predict the shapes of molecules, a card game (MGCards) has been developed which is supported by simple molecular model building (MMB). The card game allows students to work through the steps required to predict the shape of a molecule in an engaging format. The student learning process is supported by feedback at all stages (if students make a mistake, they receive hints that will help them in the next step of the game). Action research with qualitative methods has been used to design, develop, and evaluate the MGCards. The MGCards and MMB were piloted at the University of Leicester with year one Natural Sciences students and modified based on the feedback received. Both MGCards and MMB were then used as part of the first-year chemistry education programme at Tanjungpura University in Indonesia. The findings of students’ answer analysis (pre- and post-test) in both cycles showed that students had a better understanding after learning with MGCards and MMB. The positive feedback for MGCards and MMB confirmed that these resources were effective in delivering an engaging learning experience. The results suggest that MGCards and MMB play a significant role in enhancing students’ understanding while also keeping them engaged.
Introduction
Molecular geometry is an important topic studied in general chemistry courses because it plays a key role in determining the physical and chemical properties of compounds (Nicoll, 2001; Meyer, 2005; Dale, 2006). In addition to that, Burrows and Mooring (2015) as well as Duis (2011) argue that students can increase their knowledge of other chemistry topics like organic chemistry and biochemistry by studying the fundamentals of general chemistry. As a result, it is particularly important to master the fundamental basic chemistry ideas that underlie chemical systems, such as bonds, molecular structures, and reaction mechanisms and their features (Fensham, 1975). The construction of structures based on physical principles as well as the perception and interpretation of symbolic representations in chemical bond structures are required of students in the complicated subject of molecular structures (Taber and Coll, 2002; Nicoll, 2003). Gillespie (1997) therefore suggested that this concept should be included in general chemistry courses. Valence shell electron pair repulsion (VSEPR) theory is used to predict the geometry of simple molecules based on minimizing the electrostatic repulsion of electron pairs surrounding the central atom of the molecule.Previous studies have shown that some students find molecular structure determination difficult (Furió and Calatayud, 1996; Gabel, 1998; Birk and Kurtz, 1999; Nicoll, 2001; Coll and Treagust, 2003; Taber, 2003; Özmen, 2004; Pabuccu and Geban, 2006; Yilmaz and Dinçol Özgür, 2012).
Additionally, many researchers reported that students had alternate ideas and misconceptions related to this concept (Peterson et al., 1989; Furió and Calatayud, 1996; Purser, 1999; Nicoll, 2001; Nakiboğlu, 2003; Wu and Shah, 2004; Cokelez and Dumon, 2005; Meyer, 2005; Sarıkaya, 2007; Dhindsa and Treagust, 2009; Cooper et al., 2012; Wang and Barrow, 2013; Uyulgan et al., 2014; Burrows and Mooring, 2015). Nicoll (2001) reported that some first-year chemistry undergraduate students cannot explain the reason why the molecules adopt a certain geometry, such as bent for water molecules. According to Harrison and Treagust (1996), misconceptions about the form of molecules were present among grade 12 students (age 16 or 17), with 25% of students (N = 84) believing that the molecular shape is only a result of the repulsion between the bonding electron pairs. The remaining 22% of students, however, asserted that simply the repulsion between the nonbonding electron pairs is responsible for the structure of molecules.
According to Sumarni (2010), a similar issue was also noted in Indonesia, where 74.2% of first-year chemistry education students at the Semarang State University (UNNES), Indonesia, in the academic year 2009–10 reported having misconceptions about how to draw molecules. Students frequently overlook how the lone pair electrons surrounding the main atom affect the structure of molecules. In the first author's experience teaching a pre-service General Chemistry course, it has been observed that many students have trouble correctly predicting the structures of molecules. Many students try to memorize the shapes of examples used in school, but this can be difficult if they don't understand the factors that influence molecular geometry (Harrison and Treagust, 1996). For example, lone pairs of electrons can affect the shape of an object, so students might not be able to predict the shape of ammonia if they ignore these lone pair electrons (Furió and Calatayud, 1996). Students who don't learn the procedure frequently overlook the quantity of substituents surrounding the core atom. A common misconception among students is that molecules with similar formulae will take on similar forms. For example, when asked to guess the forms of ammonia (NH3) and boron trichloride (BCl3), some pupils believe they are both trigonal planar. This is an incorrect answer as ammonia has a central atom in group 15 of the periodic table (whereas boron trichloride has a central atom in group 13 of the periodic table); thus, the central atom is surrounded by a single (non-bonding) pair of electrons. As a result, the molecule takes on a trigonal pyramidal shape, and the H–N–H bond angles are less than what would be expected for a structure with four electron-bonding pairs revolving around the central atom.
Students need to develop the ability to visualise molecules, including the central atom's substituents, as bonded and lone pairs of electrons to determine molecular geometries. Jones and Kelly (2015) suggested that visualization can improve students’ understanding of abstract concepts in chemistry. Visualization plays an important role in understanding chemical concepts such as chemical bonding, molecular shape determination, and intermolecular forces. The movement and interaction of atoms and molecules can be illustrated through visualisation and modelling tools, providing more precise and educational images at the molecular (or particulate) level (Jones and Kelly, 2015). This study included a straightforward molecular model-building (MMB) activity, based on the work of Jones and Kelly, that distinguished between bonding pairs and lone pairs of electrons. The study investigated how this activity affected students' ability to dispel common misconceptions.
Literature review
Gamification in education
According to a recent academic review, gamification in education has been a trending focus of research since 2011 (Ofosu-Ampong, 2020). Gamification is perceived to be effective in enhancing user engagement and motivation. Although gamification mechanisms are varied, previous research has identified different functional ways gamification can be implemented in educational settings. These include, but are not limited to, using gamification to foster students' intrinsic motivation, and using gaming principles to improve the penetration of educational learning materials (Krath et al., 2021).Gamification plays a significant role in education by enhancing student engagement, motivation, and learning outcomes (Rivera and Garden, 2021). It leverages game elements and mechanics to create an interactive and immersive learning environment that captures students' attention and encourages active participation.
One important role of gamification is increased engagement. By incorporating game elements such as challenges, rewards, and competition, gamification makes learning more enjoyable and interactive, leading to higher student engagement (Garris et al., 2002). This increased engagement can result in improved information retention and understanding of the material.
Gamification also enhances student motivation. Through the use of clear goals, immediate feedback, and a sense of progression, gamification taps into intrinsic motivation, encouraging students to actively pursue learning goals and overcome challenges (Ofosu-Ampong, 2020). By integrating elements like levels, badges, and leaderboards, gamification provides extrinsic motivators that can further promote student engagement and drive.
Another role of gamification is the reinforcement of learning concepts. Games provide a dynamic and interactive environment where students can apply their knowledge, make decisions, and observe the outcomes practically and engagingly (McCarthy, 2021). This hands-on experience allows students to reinforce and solidify their understanding of complex concepts.
Additionally, gamification fosters the development of critical thinking and problem-solving skills. Many educational games and gamified activities require students to solve problems, make decisions, and think critically to progress (Rivera and Garden, 2021). This promotes the development of essential skills that are valuable both inside and outside the classroom.
Furthermore, gamification promotes collaboration and social interaction among students. Multiplayer games or gamified activities create opportunities for teamwork, communication, and knowledge sharing, fostering a supportive and cooperative learning environment (Garris et al., 2002). Students can learn from each other, collaborate on tasks, and engage in healthy competition, which enhances their overall learning experience.
In summary, gamification in education plays a crucial role in increasing student engagement, motivation, and learning outcomes. By leveraging game elements, it creates an interactive and immersive learning environment that enhances student participation, reinforces learning concepts, develops critical thinking skills, and promotes collaboration among students.
Research in the field of gamification in chemistry education has gained attention in recent years. One study implemented a gamification strategy in an undergraduate introductory organic chemistry course to enhance student engagement and motivation. This strategy included elements such as attendance, punctuality, game-based applications, board games, knowledge tournaments, video classes, and group tasks (da Silva Júnior et al., 2022).
Another study explored the benefits of game-based learning in chemistry classrooms. It highlighted that game-based learning, which promotes hands-on and minds-on activities, has emerged as an effective instructional approach in chemistry education (Byusa et al., 2022).
Kalogiannakis et al. (2021) conducted a systematic literature review exploring the utilization of gamification in science education, encompassing chemistry. The study underscored the importance of incorporating gamification strategies to boost engagement and improve learning efficacy. Furthermore, it emphasised the integration of technology within science curriculums to amplify motivation and foster scientific reasoning. Further research can be found in the field, such as studies focusing on the development and evaluation of specific chemistry learning games (Lutfi et al., 2023).
The role of visualisation in learning chemistry
The term visualisation is used to name a representation, to point out the process of generating a graphical representation, or as a synonym for visual imagery (Vavra et al., 2011). The representation is generally visual, either still images or dynamic animations, two- or three-dimensional, but can also refer to tactile representations, for example, physical models, or audio representations, such as increasing volume to simulate increasing energy (Jones and Kelly, 2015). According to Bishop (1989) visualisation can refer to the product, object or visual image or the process, activity, or skills.In chemistry, visualisation has numerous applications. Visuospatial skills are highly needed to comprehend chemistry concepts. The importance of learners’ differences and the role of visualisation in reducing how much students have to remember in chemistry were highlighted by Wu and Shah (2004). Moreover, they claim that visualisation provides multiple representations of the same information, which enables “students to visualise the connections between representations and relevant concepts”. Visual representations have several important functions in chemistry: to make connections visible, to present the dynamic and interactive nature of chemistry, to promote the transformation between two-dimensional and three-dimensional thinking, and to reduce how much students need to remember by making the information explicit.
The models are forms of representation, especially at the microscopic level. According to Justi and Gilbert (2002) a model is a representation of a phenomenon, an object, or a notion: for instance, the use of a Molymod set to depict the structure of water molecules. The use of a model in the classroom could improve students’ understanding of chemistry concepts, as in the study conducted by Rogers et al. (2000), who used a concrete model for teaching electrochemistry by using two boxes joined together, polystyrene balls of the same size to describe atoms and ions and marbles to delineate valence electrons. The outcome significantly promoted students’ understanding of electrochemistry concepts.
Another type of model used in chemistry is one that makes use of computer animation. This approach was first developed by Merrill and Ridgway (1969) by using space-filling models and animation followed by Smith (1970), Davies and Moore (1976), Smith et al. (1985), Greenbowe (1994), Tasker (1994), Kozma and Russell (1997), Tasker and Dalton (2006), and Akaygun and Jones (2013).
Johnstone (1993) suggested that chemistry was challenging for novice students to learn largely due to it being a complex tangle of three levels of representations: macroscopic level of the visible and laboratory aspects, symbolic level of chemical symbols, formulas, and mathematics and the sub-microscopic level of the interaction of atoms, molecules, and ions. The reason for this suggestion is that experts could move easily between these three levels while novices had difficulty understanding one level, let alone connecting to the other two levels. Based on this reason, many instructors thought that students would master chemistry if they were assisted in learning how the three levels related. Some studies were conducted to emphasise the connection between macroscopic, sub-microscopic, and symbolic levels using animation. Animations provide students with an explanation of the macroscopic and sub-microscopic phenomena, which can have a powerful effect in transforming how students make sense of chemistry (Jones and Kelly, 2015).
A study on the effect of computer animations was conducted by Williamson and Abraham (1995). In this study, they developed eight animations on gases, liquids, and solids, including gas behaviour, phase transitions, intermolecular forces, and London dispersion forces. The animations were used in six lectures. Some of these animations were interactive so that students were able to alter the variable, such as pressure or temperature, to see how it affected the gas. The researchers used the animation in two treatment situations: as a supplement in large-group lectures and as both the lecture supplement and an assigned individual activity in a computer laboratory. Both of these treatment groups received significantly higher conceptual understanding scores in a test than did the control group. Similar findings were found for a second topic (reaction chemistry), which used five animations in four lectures. Based on these outcomes, Williamson and Abraham (1995) concluded that the animations led to increased understanding and that students developed mental models of particulate behaviour that were more like those of experts.
Yezierski and Birk (2006) used the Vischem animations developed by Tasker to study the effect of viewing animations on students' learning. In this study, they used Vischem animations of water in different phases: liquid, solid, vapor, and changing phases from solid to liquid to investigate how animations affected students’ misconceptions related to the particulate nature of matter. Their findings demonstrated that students who viewed the animations performed better in the test of understanding. Therefore, they recommended using class time to discuss and interpret the animations to the macroscopic phenomena they observed.
Based on the findings, it can be concluded that visualisation is the key to understanding chemistry. Visualisation plays an important role in constructing students’ conceptual understanding of chemistry concepts. Therefore, visualisation was highly recommended to be used in teaching the abstract concept of chemistry.
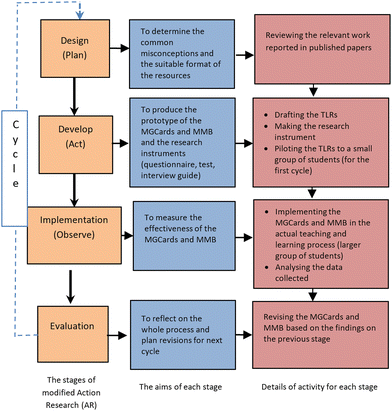
The development of the resources
The development process of the resources (in this study resources refer to molecular geometry cards or MGCards and molecular model building or MMB) consisted of two stages: design and development (see Fig. 1). The design stage started with a literature review that informed the eventual structure of the MGCards and MMB. | ||
| Fig. 1 The procedures of the development process of the MGCards and MMB that consist of two steps: design and development. | ||
The findings from the review unfolded that the determination of molecular geometry or the shape of the molecule is challenging for some students; hence they held misconceptions (Furió and Calatayud, 1996; Gabel, 1998; Birk and Kurtz, 1999; Nicoll, 2001; Coll and Treagust, 2003; Taber, 2003; Özmen, 2004; Pabuccu and Geban, 2006; Yilmaz and Dinçol Özgür, 2012). Considering the findings of the review, it was decided that the topic presented in the teaching and learning resource is how to predict the shape of a molecule based on VSEPR theory, and the format is a card game supported with molecular model building activity.
The next step was to develop a plan for the MGCards and MMB. Developing a plan allows the researcher to audit all relevant aspects of the topic and to arrange all of them in a way that creates a meaningful student experience. The plan directly informed the structure of the card game and the model-building activity. The first step was designing the cards in terms of the presentation, the structure of the cards, and the content of the cards (what and how the questions will be presented), also combining the molecular model building activity and the procedures of the activity. After the plan had been defined, the process of drafting a prototype of the MGCards and MMB began. The game structure must reflect the structure defined in the plan. The procedure of the implementation process is shown in Fig. 1.
The MGCards resources were collected together into a game pack (see Fig. 2). All of the necessary game cards (as well as a set of rules) and the modelling pieces necessary for the complementary MMB activity can be found in Appendix A. The wrong answer cards provide participants with hints to help them have another attempt at the questions. The details of the rules and the format of the game along with a downloadable version have been previously published (Erlina et al., 2018).
 | ||
| Fig. 2 (a) A set of the MGCards consist of the cards, periodic table, and worksheet; (b) example sets of the MMB kit with the coloured polystyrene balls, map pins, and cocktail sticks. | ||
As explained above, the MGCards are accompanied by a worksheet that participants use to record the steps used to predict the shape of the molecule based on their experiences of playing the game. This step is included to allow participants to reflect on the geometry determination they have worked through in the game. This step was also used as a means of evaluating the impact of the game on the students’ understanding of the topic.
The MGCards game was supported by the complementary molecular model building (MMB) activity. The MMB activity asked students to build models of the molecules they had just determined the shape of (in the MGCards game) using different size polystyrene balls (to represent atoms), cocktail sticks (to represent bonds), and map pins (to represent lone pair electrons) as shown in Fig. 3. To allow the players to differentiate between the central atom and its substituents, the polystyrene balls were painted in different colours.
An additional requirement for this project was to keep costs to a minimum for the user (so schools without access to significant amounts of funding can still use the game). To achieve this, a simple modelling kit was created using cheap materials. The kit developed for this game allows lone pairs of electrons to be represented by placing the map pins in the regions of space they occupy. Students can therefore visualize how lone pairs of electrons around the central atom influence the shapes of molecules as well as the magnitude of the bond angles. Participants are asked to assemble the MMB at the end of each round of the game to reinforce the key learning points from the activity and to help them visualise the three-dimensional structure of the molecule they have been working on.
The purpose and research questions
The research aims specifically to develop MGCards and MMB to enhance students’ understanding of molecular geometry based on VSEPR theory. Furthermore, the research investigates the impact of the implementation of the MGCards and MMB on students’ understanding. The following questions guide the present study.• What is the impact of the MGCards and MMB on student learning?
• To what extent do the targeted MGCards and MMB provide engaging learning experiences to students?
Methods
Research methods
This project is based on the principles of action research. Action research (AR) is used to facilitate the development of teaching and learning resources. Cohen et al. (2018) defined action research (AR) as “a small-scale intervention in the functioning of the real world and a close examination of the effects of such intervention”. Meanwhile, Bassey (as reviewed by Costello, 2011) defined action research as “a method to understand, to evaluate and to change the educational practice”. The focus of this study is to address students’ alternative conceptions of the core ideas of chemistry by developing and implementing the MGCards and MMB. The development and implementation process would make a change to students’ alternative conceptions. Thus, action research is suited to this study.Action research in this project adapted qualitative approaches as suggested by several authors (Patton, 1990; Costello, 2011; McNiff and Whitehead, 2011; Stringer, 2014). In this study, the “change” is students’ understanding of the molecular geometry presented in the MGCards and MMB.
There are four stages in action research, namely planning, action, observation, and reflection. These four steps in this research are modified into design, development, implementation, and evaluation. The modified four stages are design (planning), development (action), implementation (observation), and reflection (evaluation). The details of each stage can be seen in Fig. 4.
Participants
The participants in this study were divided into two groups for the pilot study and the implementation of the MGCards and MMB activities. The pilot study included 13 first-year students from the Natural Sciences Department at the University of Leicester. In addition, 33 and 82 students participated in the first and second cycle, respectively. The study focused on the first-year cohort of the Chemistry Education program in the Department of Mathematics and Science Education at Universitas Tanjungpura, Indonesia. Students of both pilot and experimental studies were of the same age. All participants volunteered to be involved in this study.Data collection
Four methods were used to collect data in this project: questionnaires, tests to measure students’ understanding before and after using the MGCards and MMB (pre-test and post-test), interviews, and observations.Qualitative data collection methods in this study involved document analyses, interviews, observation, and audio-visual recordings. Document analysis, in this study, refers to the module syllabus and module textbooks, students’ answers to the pre-test and post-test as well as the worksheet.
Interviews were conducted before and after the presentation of MGCards and MMB. Students who were involved in the interview process were volunteers. All interviewees who were involved in the study were excited to share their experiences after learning with the MGCards and MMB. The interviews were conducted in students’ free time for their convenience.
Data analyses
Data collected through observation, video recording, and interviews were analysed using the NVivo 11 software package. NVivo is qualitative data analysis software produced by QSR International for qualitative data analysis (Hilal and Alabri, 2013). The NVivo software package was also used in the coding process. NVivo also ensures easy, effective, and efficient coding which makes retrieval easier (Bazeley and Jackson, 2013). For instance, NVivo can open and link a paragraph from one source to another paragraph in either the same or another source and retrieve it with less effort. This task could have been very time-consuming if using manual coding.Triangulation
The type of triangulation used in this research is methodological triangulation. Methodological triangulation refers to the use of more than one method for gathering data (Denzin, 1978; Patton, 2002). The multiple methods of data collection used in this research are questionnaires, tests, interviews, and observation. By using these approaches, the validity and reliability of this research will be maximised.Ethical approval
All data collected within this study were approved before commencing the research by The Research Ethics, Governance, and Integrity Offices of University of Leicester. We also got the approval from the Universitas Tanjungpura. Appropriate consent was obtained from students as required.Results and discussion
The context of the research
The MGCards and MMB activities were implemented in the classroom at the same time that the topic was taught in the course. Thus, the activities were implemented as integral parts of teaching in the module as suggested in qualitative research for keeping the natural setting of the research (Denzin and Lincoln, 2013). The way the lecturer taught the topic was changed to make it similar to the way the activity works. The number of students for each class is listed in Appendix B.A pilot study of the MGCards and MMB
Before the implementation of the MGCards and MMB, the pilot study was administered. This pilot was conducted to gain an initial insight into the impact of the MGCards and MMB and to identify any issues that would need to be addressed ahead of the full research phase. The pilot study was conducted at the University of Leicester with 13 students from the first-year cohort of the Natural Sciences undergraduate degree program. All participants voluntarily agreed to take part in the study. The study took place at the end of a general chemistry PBL session. At the time of the pilot, students had just been introduced to the topics of chemical bonding, shapes of molecules, and the principles of reactivity. In the pilot phase, participants were asked to organise themselves into small groups (two groups of four students and one group of five students) to do the activity. This pilot of the activity lasted approximately 30 minutes which included completion of the evaluation questionnaire.To ensure that the pilot study findings were transferable to the actual study, this study implemented the consistent methodology. The research design, including instructional materials, delivery methods, and assessment techniques, was kept consistent between the pilot study and the actual study. This consistency facilitated accurate extrapolation of the results from one context to the other. Moreover, the potential confounding variables were controlled by ensuring that participants in both groups were exposed to the same conditions, such as session duration, timing, and location. This uniformity allowed for a direct comparison of the results from the pilot and experimental groups.
Evaluation of the pilot was conducted through a two-section questionnaire. Section one of the questionnaire consisted of eight statements about the presentations and contents of the MGCards and MMB. Participants were asked to state their level of agreement with these statements on a Likert scale with five options (strongly disagree, disagree, neutral, agree, and strongly agree). Section two consisted of open-ended questions that asked participants to suggest improvements that could be made to the activity and state which part of the MGCards game they found least useful. The details of the Likert statements used in section one and the open-ended questions used in section two can be found in Appendix C.
During the pilot, the students’ activities and interactions during the activity were observed and recorded by taking notes. A questionnaire was used to gather comments and suggestions. After a chemistry lesson, the MGCards were used. Three groups played the game, with each group having four or five students. All students received a survey after the exercise. The result of the pilot study was that all of the students thought the activity was enjoyable, especially while putting the molecular model together.
Observations made during the exercise indicate that the activity cards were not fully effective when used in groups of four or five students, since only two or three of the students were engaged. As a result, pairs of students played the activity card during the evaluation. The size and colour of the cards were the only two things that needed to be changed based on students’ suggestions. Based on this feedback, the MGCards were changed to be bigger and more colourful.
Research question 1: what is the impact of the MGCards and MMB on student learning?
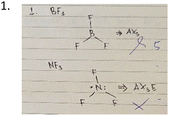
This section discussed students’ conceptual understanding in the first and second cycle based on individual interviews in the first and second cycle.NF3 (trigonal pyramidal) and BF3 (trigonal planar) adopt a different shape as NF3 has 1 lone pair electron around the central atom while BF3 has no lone pair electrons. Based on VSEPR theory, the electrons (lone pair and bond pair electron/s) will repel each other to get a stable position. The repulsion will affect the shape and bond angle of the molecule. Based on the results, 12 students (N = 13) answered that the shape of both molecules is trigonal planar and 12 of the interviewees were unable to support their answers with scientifically valid explanations. Only one interviewee successfully determined the shapes of both molecules, but this student was unable to provide a scientifically correct justification for her answers. Eleven of the students’ explanations were related to the similarity in the formulae of the two structures. There was an assumption that this similarity indicated that both molecules would adopt the same shape. One interviewee stated that BF3 was linear and that NH3 was trigonal planar. Both of these answers are incorrect and the prediction of a linear structure for BF3 implies that the student hadn’t engaged with the principles of VSEPR theory. This interviewee was unable to provide a scientific rationale for his answer (“I don’t know, it just popped out of my head”). The same interviewee also said that he had no memory of learning this topic in high school, but that he may have encountered it when doing private study. This topic is compulsory for high school as part of the chemistry curriculum in Indonesia. None of the interviewees successfully demonstrated an understanding of the effect of lone pair electron/s on the shape of a molecule.
When they were asked about why the molecules adopt a certain shape, students responded by saying “I don’t even know the reason why the molecules have a different shape…all I know is they have a different shape because they (molecules) have a different formula.” Another student's explanation is: “……although I’ve read about the VSEPR theory in my chemistry textbook, I don’t understand the relation between repulsion with the electron and the shape of the molecule.” One student gave an interesting quotation which is “I used to memorize all the shapes of molecules given in the textbook.” Based on the explanations given, it could be concluded that all students did not have a proper understanding of the topic. Students seem to memorize the shape of the molecule given in the textbook without understanding the reason why that shape is adopted. This may be due to the teaching and learning processes emphasizing the name of the shape but not the reasons why the molecule adopts a given shape.
The reason why students have a poor understanding was then revealed in the interviews; most of them said that they had not studied the topic in high school or their teacher never explained the topic although this concept was mandated in the high school curriculum to be taught. Some of the students’ arguments are presented below.
“…. I never learned about the concept (shape of a molecule based on VSEPR theory) when I was in high school as my teachers never explained about this.” Another student said, “My chemistry teacher only asked us (high school students) to read the topic in the textbook…perhaps because too many concepts to be taught while the time is limited”. Another student said “I don’t understand at all about the concept even though I’ve read the topic in a text-book when I was in high school”. This response implies that the teacher did not explain the concepts due to the limited time which resulted in students only learning the concept by reading the textbook by himself/herself. Based on the interviewees' responses it is clear why all of them do not have a decent understanding of the topic. The findings of the student interviews are consistent with students’ very low pre-test scores.
The findings from the analysis of the pre-test and post-test data were supported by the outcomes of a series of interviews conducted after the intervention. The increase in the average score following the intervention suggested that the students’ understanding of the concept may have improved. Individual interviews were conducted with 13 volunteer participants. To verify this, the post-intervention interview included a section where interviewees had to explain to the interviewer how they determined the structure of molecules given in the pre-test. The questions in the interview were directly based on the students’ answers in the post-test. Interviewees successfully determined the different shapes of BCl3 and NH3 and correctly explained why they were different. A summary of the key findings of the interviews can be found in Appendix F. It can be concluded that the MGCards and MMB learning resources were effective in enhancing students’ understanding of the concepts that were tested.
The apparent improvement in students’ understanding was also scrutinized by analysing students’ answers to the pre-test and post-test questions. Both tests comprise of 3 questions. A summary of students’ answers for both tests is tabulated in Tables 1–3.
28 (out of 33) students drew the Lewis structures of the molecules without providing any further explanation as shown in the examples in Table 1. Some students explained the geometry without drawing the Lewis structure or the shapes of the molecules. Drawing the Lewis structure is the way the topic is taught in Indonesia. Some students started their answers by determining the valence electron configurations of the bonded atoms (as shown in post-test answer 2). 30 (out of 33) students drew a Lewis structure for the molecule even though the question did not ask them to do that. This suggests that students think the Lewis structure is a representation of the shape of the molecule. Students were not used to drawing the three-dimensional representations of molecular geometry (as shown in Table 1). They tended to draw two-dimensional representations of the structures (as shown in Table 1). Students also gave an appropriate explanation and drew the lone pair around the central atom in ammonia, but still ultimately drew molecular geometries that looked the same (e.g. pre-test answer 2 in Table 1).
In the post-test, 25 out of 33 students correctly explained why both molecules adopt different shapes. Students seemed to have a better understanding of the post-test and drew the Lewis structure to show the shape of both molecules, although they did not give a full explanation including full electron counts. Additionally, they also drew the molecular geometry and stated the name of the shape.
Table 2 presents examples of students’ answers to question two for both the pre-test and post-test. Some answers only state the name of the shape without further explanation (electron configuration, Lewis structure or the geometry of the molecule). Students’ answers to the post-test tend to demonstrate a better understanding of the process as they include relevant valence electron configuration and Lewis structures and mention the name of the shape (no. 2 of students’ post-test answers). Even though students’ post-test answers were not fully correct, as they only provided the Lewis structure to illustrate the shape of the molecules, they provided the correct name of the shape.
Based on Table 3, the answers for question no 2 of the pre-test were categorised into three types. The first type is the correct answer with further explanation. Students presented diagrams to differentiate the shapes of the molecules. Students who adopt this type of answer demonstrate an understanding of the concepts, although they only draw the Lewis structure to show the shape of the molecule. The second type of answer only provides an incorrect explanation without including diagrams. Students proposed the number of atoms bonded affects the shape of the molecule as the reason why CH4, NH3, and H2O have different bond angles, which is not fully correct, as the repulsion between lone pair electrons and the bond pair electrons is considered to affect the bond angles as well as the shape of the molecules. The third type is not answering the question. The last 2 types of students’ answers describe students’ lack of understanding of the concepts for predicting the shape of the molecule.
The three types of answers given in the post-test represent students’ understanding of the concepts of the shape of molecules. All types of student's responses to the post-test have shown that they made use of the electron configuration and Lewis structure to support their answers. Students also gave further explanation which is correct, although they did not mention the magnitude of repulsion between lone pairs in their explanation. It appears that some students fail to appreciate the significance of the relative magnitudes of repulsion of electron pairs (i.e. lone pair-lone pair > lone pair-bond pair > bond pair-bond pair).
Second cycle
Based on the students' responses in the interview, most of them showed a similar trend to those in the first cycle of interviews. Over half of those participants agreed that the shape of both molecules is trigonal planar. The answer is half correct as the shape of NH3 is trigonal pyramidal. A third of them claimed that the shape is trigonal planar and trigonal pyramidal which is the correct answer. The rest declared linear and trigonal planar which is incorrect. Each of them could not provide the correct explanation, even those who came up with the correct answer. The explanations given by students are “I don’t know why they have a different shape” or “I don’t understand, I thought they have the same shape as both molecules have a similar formula.” Other students declared “I just know that they have a different shape.” Students’ responses to this question demonstrated that they don’t understand the effect of lone pair/s electrons around the central atom on the shape of molecules and bond angle.The interview also exposed the reason why students do not understand the topic as the majority of them implied that the topic was never explained by their teacher. Despite the high school curriculum covering this concept to be taught, the teacher did not explain the topic to students. Students proposed some arguments related to it. “… My teacher never explains the topic, so I thought the topic was not important enough to be learned.” Another student came up with “She (chemistry teacher) asked us (high school students) to just read the topic in the textbook.” One student suggested, “I read the topic in the textbook when I was in high school, but honestly, I don’t understand.” Even though the topic is incorporated in the chemistry high school curriculum, chemistry teachers seem to disdain teaching the topic to students. As a consequence, students have a lack of understanding of the concept. Both interviews’ results described students’ understanding before and after learning with the MGCards and MMB. All of them could provide the correct shape of the molecule given along with the reason in the post-interviews. Students could explain the different shapes of BCl3 molecules and NH3 molecules although they possess a similar formula. A summary of students’ responses to pre- and post-interviews is presented in Table 5. Based on the table, it can be concluded that MGCards and MMB could enhance students’ understanding of the concepts. Moreover, the improvement in students’ understanding was also observed by analysing students’ answers in the pre-test and post-test. Both tests comprise of 3 questions. A summary of students' answers for both tests is tabulated in Tables 4–6.
For the pre-test, there was one student who answered the question correctly, and he/she also provided the correct explanation. Meanwhile, some students did not answer the question or provide any further explanation. Some of them also started the answer with electron configuration like students’ answer no 1 in the pre-test. Interestingly, one student (example no 3 of the pre-test in Appendix C) wrote the number of lone pair and bond pair electrons correctly to answer the question, even though without electron configuration.
For the post-test, the majority of students gave a further correct explanation of why both molecules adapt different shapes. Students seemed to have a better understanding of the post-test as they had already drawn the correct shape for both molecules. Moreover, they had also drawn the shape after the Lewis structure and put the correct name of the shape.
Table 5 describes the type of students’ answers to question no 2 of the pre-tests. The first type is the correct answer. This type of answer shows students’ understanding of the concepts. He/she already used the electron configuration and drew the Lewis structure to answer the question. Meanwhile, the second type is the incomplete and incorrect answer. He/she only drew the Lewis structure to show the bond pair/s electrons between the atoms. For the last type, he/she only wrote the name of the shape (the shapes for NF3 and BF3 were wrong). The second and third type of answers show students’ lack of understanding of these concepts.
Based on Table 5, there are two types of students’ answers for the post-test. Both types are correct, except for the shape of GaI3 for which they drew the wrong Lewis structure (the answer should be trigonal planar not T-shaped) but the second type provided a further explanation. He/she added the steps used to predict the shape of the molecule. Both types used the electron configuration and Lewis structure. It is noted that no students answered using this type in the pre-test. These 2 types of students’ answers show that students have a proper understanding related to the concept.
Students have various explanations to answer the third question of the pre-test. The first answer is the correct answer. He/she also used the diagram to support the explanation. However, for the last three answers students provided incorrect explanations, even some of them did not answer the question. Again, the incorrect explanation indicates students’ poor understanding of the concepts.
On the contrary, students could provide a proper explanation to answer this question. Students also use the diagram to support their explanation. It is interesting that students even use the terms “repulsion” and “repel” which cannot be found in the pre-test. Based on this answer, it can be concluded that students have an adequate understanding of the concepts.
A few themes emerged from the qualitative analysis of students’ interviews about how the MGCards and MMB contribute to students' understanding. A summary of the themes is presented in Table 7.
| Question: In what ways did the MGCards and MMB help you understand the concept? | |
|---|---|
| First cycle | Second cycle |
| • Answering the questions in the cards | • Doing the whole activity from start to finish helped students understand how to predict the shape of the molecule |
| • Assembling the molecular model | • Assembling the molecular model is the best part of the activity, as I (the student) can see the ‘real’ shape of the molecule |
| • Combining the MGCards with the MMB | • Assembling the molecular model helped me understand the effect of lone pair electron/s around the central atom on the shape of the molecule |
| • I can see the shape of the molecule after assembling the molecular model | |
In both cycles, students emphasised that using the MGCards and MMB was very helpful, as the activity of assembling the molecular model allowed them to see the ‘real’ shape of the molecule. In addition to that, students also stated that the assembly activity contributed to their understanding of the influence of the lone pair/s electrons on the shapes of molecules. As suggested by Rogers et al. (2000), models provide representations of scientific concepts that can make the ideas more understandable to learners. Modelling requires the user to make links between the model and the reality that is being modelled (Chittleborough and Treagust, 2009). The use of concrete models to promote students’ conceptual understanding at the microscopic level was also effective in correcting students’ misconceptions (Rogers et al., 2000). Models are useful tools in learning science that can be used to improve explanations, generate discussion, make predictions, provide visual representations of abstract concepts and generate mental models (Treagust et al., 2003). Thus, it explained why the MGCards and MMB had helped students understand the concepts.
Moreover, the three levels of representations embedded in the MGCards and MMB addressed one of the main causes of the difficulty of learning chemistry, that is the abstract concepts at the basis of chemistry and the integration of the three levels of representations (macroscopic, symbolic, and sub-microscopic). Therefore, the MGCards and MMB facilitated students to visualise abstract concepts. As reported by Vavra et al. (2011) visualisation is an effective tool of teaching to promote learning and understanding and also to aid in analysis and problem-solving. A combination of visual and text-based explanations can enable students to access the information so that students can develop a proper understanding.
Research question 2: to what extent do the targeted MGCards and MMB provide engaging learning experiences to students?
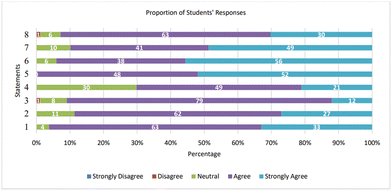
One of the objectives of the study was to create an engaging learning experience for students. The effectivity of the MGCards and MMB was measured based on students’ responses to the questionnaire, interview, and the researcher's observations during the activity. The details of students’ responses in the first and second cycles can be seen in Fig. 5 and 6. Both figures illustrate the responses of students to the first section of the questionnaire. The 8 statements presented in the questionnaire are: 1 = the card-game instructions are clear and understandable; 2 = answering the questions in the card-game helped me understand the topic; 3 = I understand how to predict the shape of the molecule after playing the game; 4 = the questions in the card-game are challenging; 5 = I enjoyed playing the card-game; 6 = the presentation of the card-game is interesting; 7 = making the molecular model using polystyrene balls and cocktail sticks was useful; 8 = making the molecular models helped me understand the influence of lone pairs of electrons on the shapes of molecules.First cycle
The majority of students, comprising 85% who strongly agreed and the remaining who agreed, found the rules of the game clear and easy to follow, indicating high satisfaction. The engaging nature of the MGCards, the presentations, and the usefulness of the simple molecular model were also noted by students. Furthermore, 100% of students (N = 33) responded that they either agree or strongly agree that the instructions were easy to follow; 100% of them enjoyed doing the activity; and 100% of students reported that they either agree or strongly agree that the molecular model could help them visualize the shape of molecules. As shown in Fig. 5, the vast majority of students also responded that they agree or strongly agree with each statement except for the statement ‘the questions in the MGCards are challenging’ with only 3% of students disagreeing with this statement.The notion of enjoyment of playing the MGCards game and assembling simple molecular models shown in the questionnaire responses was also a theme raised by students in the post-intervention interview (e.g. “It's really fun, I didn’t feel bored at all. I hope this activity could be implemented to other topics as well” and “I enjoy the whole activity”). Some student interview responses referred to the benefits of playing the game in their conceptual understanding “The activity really helps me to grasp the concept and writing the steps in the worksheet also supports my understanding”.
The molecular models used in the activity helped students to visualize the shape of the molecules; thus students understand the effect of lone pair electrons around the central atom on the shape of the molecules. This statement was supported by students’ responses to the post-intervention interview (e.g. “I know how to predict the shape of the molecule after playing the game, as I can see the ‘real’ shape by assembling the molecular model” and “Having the map pin to show the effect of lone pair electron surround the central atom to the shape of the molecule was a great idea, as I can visualize how it works”). In addition, the MGCards and the MMB support the integration of three levels of representations in the study of chemistry. The evidence that supported this assertion was the students’ responses in the post-intervention interview (e.g. “by assembling the molecular model, I can understand why the molecule of BF3adopt the shape of a trigonal planar and NF3adopt a shape of trigonal pyramidal as putting the map pin visualize the lone pair around the central atom”).
Table 8 presents an overview of student feedback on the MGCards and MMB activities. It is interesting to note that almost half of the participants stated that the whole experience of playing the MGCards and assembling the molecular models was the most useful part of the activity. Interestingly, for the last two statements 100% of them agreed that there is no part of the activity that is less useful and no changes were needed to be made to the MGCards including the molecular model. Thus, it can be concluded that the participants believe that the MGCards and MMB activities are effective ways of supporting their learning of this topic.
| Statements | Percentage | |
|---|---|---|
| Which part of the games is the most useful? | The cards/questions that lead to the correct shape | 24.2 |
| Writing the steps how to predict the shape of molecules in the end of the game on the worksheet | 6.1 | |
| Making the molecular models by assembling the polystyrene balls, map pins and cocktail sticks | 24.2 | |
| The whole experience of playing the MGCards including assembling the molecular model | 45.5 | |
| Which part of the games is less useful? | None | 100 |
| What changes do you want to make related to the MGCards and MMB? | None | 100 |
Second cycle
Fig. 6 depicts the proportion of students’ responses to the first section of the questionnaire. The majority of students either agree or strongly agree with all items. The most notable is item no 5 with 52% and 48% stating that they strongly agree and agree that the activity was enjoyable. The clarity of the instruction and the good presentation of the MGCards are also regarded by students with 96% and 94% respectively agreeing or strongly agreeing. Answering the questions and assembling the molecular model could elevate their understanding is noticed by students with 90% and 89% of them agreeing or strongly agreeing to the item. Students’ responses to item no 4 are quite different as 70% of them agree or strongly agree while 30% of them chose neutral to the item. Overall, students showed a positive response toward the MGCards and MMB.In section two, more than half of students (52.4%) stated that the whole experience of playing MGCards and assembling the simple molecular model was the most useful part of the activity. 100% of them agree that there is no less useful part of the activity and express that no changes need to be made to the activity. The details of students’ responses to each item can be found in Table 9.
| Statements | Percentage | |
|---|---|---|
| Part of the games that was the most useful | The cards/questions | 6.1 |
| Formulating the steps how to predict the shape of molecule on the worksheet | 4.9 | |
| Making molecular models by assembling polystyrene balls, map pins and cocktail sticks | 36.6 | |
| The whole experience of playing the MGCards including assembling the molecular model | 52.4 | |
| Less useful | None | 100 |
| Changes | None | 100 |
In the post-interviews, students clarify that they enjoy doing the activity and constructing the simple molecular model as well. Here are some of the students’ quotations: “I usually get bored in the class but today was great fun.” “I love assembling the molecular model as it helps me visualize the effect of the lone pair/s electrons on the shape of the molecules.” “I love the process of going back to the previous card if we choose the wrong answer.” “I thought the idea of providing the hint was brilliant.” The findings confirm that students enjoy the activity of playing MGCards and building simple molecular models.
The majority of students in both cycles commented on their enjoyment of doing the activity (such as answering the questions in the MGCards and assembling the MMB). Students also showed enthusiasm when carrying out the activity (based on the researcher's observations). They even suggested applying the card game to other topics and mentioned how fun the activity was in the interview. The main reason, as revealed in the interviews, was that the activity of assembling the molecular model (using MGCards and MMB) allowed students to visualize the actual shape of the molecules. All these findings indicated that the MGCards and MMB effectively raised students’ engagement in learning Molecular Geometry topics.
Presenting the MGCards and MMB in the activity form contributed to enhancing students’ understanding and engaging students in learning chemistry. The card formats accommodated students with different learning styles (auditory, visual, verbal, and kinesthetic). As claimed by Csapo and Hayen (2006) students vary in the way they process and understand information. Thus, Sarasin (1999) argued that providing suitable approaches to students’ learning styles is the key to effective teaching. Moreover, Dunn et al. (1995) found that students’ average grade points increased when teacher and student learning styles were more closely matched.
Conclusions
In conclusion, implementing the MGCards and MMB activities alongside traditional teaching methods proved to be an effective approach to enhancing students' understanding of molecular geometry concepts. Integrating these activities into the classroom allowed for a natural and immersive learning experience, aligning with qualitative research principles while maintaining the authenticity of the educational environment.The pilot study conducted before the full research phase provided valuable insights into the impact of the activities and highlighted areas for improvement, such as the need for larger and more colourful cards. Subsequent cycles of the research demonstrated significant progress in students' conceptual understanding, particularly regarding the relationship between molecular structures and the presence of lone pair electrons.
Through individual interviews, it became evident that many students initially struggled to grasp key concepts due to inadequate prior instruction or lack of emphasis on the topic in the high school curriculum. However, the implementation of the MGCards and MMB activities led to notable improvements in student's ability to predict molecular shapes and articulate their understanding of underlying principles.
The qualitative analysis of students' responses revealed a high level of engagement and enjoyment with the activities. Students appreciated the clarity of instructions, found the tasks challenging yet enjoyable, and valued the opportunity to visualize molecular structures through hands-on activities. The integration of the three levels of representations (macroscopic, symbolic, and sub-microscopic) facilitated deeper conceptual understanding and helped address students' misconceptions.
Overall, the MGCards and MMB activities proved effective in providing engaging learning experiences and enhancing students' comprehension of molecular geometry concepts. By catering to diverse learning styles and promoting active participation, these activities contributed to a more holistic and effective approach to chemistry education.
Limitations
The limitation of this study is that the methods used to develop the MGCards and MMB in this study may not be directly transferable to other topics of science, but the findings on how students respond to MGCards and MMB may well be transferable. In addition to that, the design of the study, i.e., the pre-post intervention study design, is subject to limitations because learning gains are expected following an educational task. Given the participants' limited prior knowledge of molecular shapes, it is understandable that introducing the concept during the course resulted in improved comprehension, as evidenced by the post-test responses. However, the influence of different teaching methods is still in question. To determine if intervention cycles yield greater learning gains than traditional lecturing, further research comparing these approaches is necessary. Also, the process of generating and evaluating the impact of MGCards and MMB described in this paper can be applied in a much broader context making this approach accessible to educators in chemistry and other disciplines.Implications for research
The resources developed in this study were based on students’ common alternative conceptions of core ideas in General Chemistry. The evaluation of the impact of the MGCards and MMB on student learning at the General Chemistry course level was conducted. With minor modifications, the MGCards and MMB in this study may be transferable from the context of undergraduate students to high school or secondary school chemistry teaching or from a chemical context to one suitable for a different degree programme (e.g. Biochemistry, Organic Chemistry, etc.) or be adapted for use in other countries (e.g. by alignment with the local curriculum and/or translation). The topic and activity of MGCards and MMB can be modified and expanded.There is potential to identify students’ alternative conceptions for other concepts in chemistry or other subjects and use this information as the basis for developing other resources. Further research focusing on combatting pre-existing student and perhaps even school–teacher misconceptions could lead to the development of new teaching and learning resources for chemistry or indeed for other STEM subjects, to improve students’ understanding.
The control–experiment classes, one class learning with the MGCards and MMB while the other class learning without the MGCards and MMB, should be designed to investigate the impact of the resources based on multiple representations to improve students’ conceptual understanding of core concepts and to challenge students’ alternative conceptions in chemistry or other subjects.
One particularly interesting area would be to investigate the self-awareness of students about their learning (e.g. recognising conceptual shifts, etc.). Understanding students’ self-awareness could help teachers to design suitable teaching and learning processes to enable students to focus their attention better on their studies.
Finally, integrating the three levels of chemistry representations in the teaching and learning resources should be considered by other researchers when designing and developing the resources for other topics, especially if they are aiming to address students’ alternative conceptions since the three levels of representations are believed as one of the major contributors to students’ difficulty in learning chemistry.
Any conclusions that are made as a result of this research are valid for this particular study, situation, and time. The extrapolation of the conclusions to other situations can be only hypothesised or proposed and are not necessarily validated by this study.
Conflicts of interest
There are no conflicts to declare.Appendices
Appendix A
Contents of the activity aCards for the example of NF3| Type of the card | Content of the cards | Number of card(s) |
|---|---|---|
| Introductory cards | Cover, rules, learning objectives, a brief explanation of VSEPR theory, important terms, sample molecules | 1–6 |
| Questions cards | What is the central atom in this example? | 7 |
| What is the total number of electrons that N has? | 8 | |
| How many valence electrons does N have? | 11 | |
| What is the total number of electrons that F has? | 14 | |
| How many valence electrons does F have? | 18 | |
| How many electrons are used by each F atom to make bonds with the central atom? | 20 | |
| What is the total number of valence electron pairs? | 22 | |
| How many bond pairs (BP) and lone pairs (LP) are there around the central atom? | 26 | |
| What is the Lewis structure of NF3? | 28 | |
| Based on your Lewis structure, what is the shape of the molecule? | 31, 33 | |
| Correct answer cards | Questions | Answer given at the start of the next question and card 35 |
| Wrong answer cards | Feedback and hints | 9, 10, 12, 13, 15, 16, 17, 19, 21, 23, 24, 25, 27, 29, 30, 32, 34, 36 |
The table was adapted from the published paper in the Journal of Chemical Education (Erlina et al., 2018).
Appendix B
Number of students in each class during the second cycle of research| Classes | Number of students | Number of pairs/groups |
|---|---|---|
| Chemistry education A1 | 31 | 14 pairs and 1 group of 3 students |
| Chemistry education A2 | 30 | 15 pairs |
| Chemistry education A3 | 21 | 9 pairs and 1 group of 3 students |
Appendix C
Statements and questions presented in the questionnaire| No | Statements and questions | |
|---|---|---|
| Section 1 (Likert scale) | Section 2 (open-ended questions) | |
| 1 | The card-game instructions are clear and understandable | Which parts of the card game were most useful? |
| 2 | Answering the questions in the card-game helped me understand the topic | Which parts of the card game were less useful? |
| 3 | I understand how to predict the shape of molecule after playing the game | What changes would you like us to make to the card game? |
| 4 | The questions in the card-game are challenging | |
| 5 | I enjoyed playing the card-game | |
| 6 | The presentation of the card-game is interesting | |
| 7 | Making the molecular model using polystyrene balls and cocktail sticks was useful | |
| 8 | Making the molecular models helped me understand the influence of lone pairs of electrons on the shapes of molecules | |
Appendix D
The structure of the pre-intervention interview| Themes | Key questions | Prompts | Duration |
|---|---|---|---|
| Personal data | How would you describe yourself? | • Name | 5–10 minutes |
| • Term/class | |||
| • Place of origin | |||
| • Previous high school | |||
| Learning style | How do you typically learn? | • Preferred learning style | |
| • Preferred learning resources | |||
| Difficulties in learning chemistry | What obstacles are you facing in learning chemistry? | • Terminologies and concepts | |
| • Teaching methods | |||
| • Learning resources | |||
| • How to deal with the obstacles | |||
| Contents | What concepts do you learn throughout chemistry course? | • Scope of content | 15–20 minutes |
| • Sequence of concepts | |||
| • Expectations of learning chemistry | |||
| • Different experiences with learning chemistry in high school | |||
| Concepts | How would you describe your understanding about the shapes of molecules? | • Concept of VSEPR theory, electron configuration, central atom, substituent, lone pair, bond pair, repulsion | |
| Based on your answer on the pre-test, what are the shapes of molecule BF3 and NF3 | Student's understanding of the concepts of VSEPR theory | ||
| Reason | Why do those molecules adopt the shapes you have stated? | ||
| Responses | What types of learning resources do you use to support your learning of the subject | Type(s) of learning resources (textbook, internet/website, video, lecture's note, etc.) | 5 minutes |
Appendix E
The structure of the post-intervention interview| Themes | Key questions | Prompts | Duration |
|---|---|---|---|
| Concepts | How would you describe your learning experience of shape of molecule with the MGCards and MMB? | • Concepts of shape of molecule | 15 minutes |
| • The game-activity | |||
| • Assembling the MMB | |||
| Based on your answer on the post-test, what is the shape for BF3 and NF3? | Concept of VSEPR theory, electron configuration, central atom, substituent, lone pair, bond pair, repulsion | ||
| Reason | Why those molecules (BF3 and NF3) adopt a certain shape? | ||
| Response | In what ways do the MGCards and MMB help you to understand the concept of shape of molecule? | • Which part of the MGCards and MMB's activity is the most useful part? | 15 minutes |
| • Which part is less favourite | |||
| What will you do to improve MGCards and MMB to support you in learning the concept of shape of molecule? | • Technical aspects | ||
| • Content presentation | |||
| How the following task-activity help you to understand the concept of shape of molecule? | Kind of difficulty in doing the activity |
Appendix F
The summary of types of students’ responses to the pre-intervention and post-intervention interviews| Students’ understanding before playing the game | Students’ understanding after playing the game | ||
|---|---|---|---|
| Responses | Given reasons | Responses | Given reasons |
| Students who determined correct shape of BF3 but incorrect shape of NF3 | Both molecules have a similar formula therefore their shape must be the same | No student gave an incorrect answer | — |
| Students who gave correct shape of both BF3 and NF3 molecules | Interviewee wasn’t able to justify their answer. The shape had been memorised from the content in a chemistry textbook | All students gave the correct answer for both molecules | As NH3 has a lone pair of electrons and three bond pairs around the central atom it must be trigonal pyramidal. BCl3 has no lone pair and just three bond pairs therefore its shape is trigonal planar |
Acknowledgements
This research has been supported by The Ministry of Research, Technology, and Higher Education (KEMENRISTEKDIKTI) Indonesia. We extend our gratitude to the University of Leicester, UK and Tanjungpura University, Indonesia along with our colleagues and the students who participated in this study.References
- Akaygun S. and Jones L. L., (2013), Research-based Design and Development of a Simulation of Liquid–vapor Equilibrium, Chem. Educ. Res. Pract., 14(3), 324–344 10.1039/C3RP00002H.
- Bazeley P. and Jackson K., (2013), Qualitative data analysis with Nvivo, Sage Publications Limited.
- Birk J. P. and Kurtz M. J., (1999), Effect of Experience on Retention and Elimination of Misconceptions about Molecular Structure and Bonding, J. Chem. Educ., 76(1), 124 DOI:10.1021/ed076p124.
- Bishop A. J., (1989), Review of research on visualization in mathematics education, Focus Learn. Problems Mathematics, 11(1), 7–16.
- Burrows N. L. and Mooring S. R., (2015), Using concept mapping to uncover students’ knowledge structures of chemical bonding concepts, Chem. Educ. Res. Pract., 16(1), 53–66 10.1039/C4RP00180J.
- Byusa E., Kampire E. and Mwesigye A. R., (2022), Game-based Learning Approach on Students’ Motivation and Understanding of Chemistry Concepts: A Systematic Review of Literature, Heliyon, 8(5) DOI:10.1016/j.heliyon.2022.e09541.
- Chittleborough G. D. and Treagust D. F., (2009), Why Models are Advantageous to Learning Science, Educación Química, 20(1), 12–17 DOI:10.1016/S0187-893X(18)30003-X.
- Cohen L., Manion L. and Morrison K., (2018), Research Methods in Education, Routledge DOI:10.4324/9781315456539.
- Cokelez A. and Dumon A., (2005), Atom and molecule: upper secondary school French students’ representations in long-term memory, Chem. Educ. Res. Pract., 6(3), 119–135 10.1039/B4RP90005G.
- Coll R. K. and Treagust D. F., (2003), Investigation of secondary school, undergraduate, and graduate learners’ mental models of ionic bonding, J. Res. Sci. Teach., 40(5), 464–486 DOI:10.1002/tea.10085.
- Cooper M. M., Underwood S. M. and Hilley C. Z., (2012), Development and validation of the implicit information from Lewis structures instrument (IILSI): do students connect structures with properties? Chem. Educ. Res. Pract., 13(3), 195–200 10.1039/C2RP00010E.
- Costello P. J., (2011), Effective action research: Developing reflective thinking and practice, Bloomsbury Publishing.
- Csapo N. and Hayen R., (2006), The Role of Learning Stylesh in the Teaching/Learning Process, Issues In Information Systems, 7(1), 129–133 DOI:10.48009/1_iis_2006_129-133.
- Dale G. L., (2006), Student construction of small molecule models using Spartan model to explore polarity, The University of Southern Mississippi, USA.
- da Silva Júnior J. N., de Castro G. L., Melo Leite Junior A. J., Monteiro A. J. and Alexandre F. S. O., (2022), Gamification of an Entire Introductory Organic Chemistry Course: A Strategy to Enhance the Students’ Engagement. J. Chem. Educ., 99(2), 678–687 DOI:10.1021/acs.jchemed.1c00766.
- Davies W. G. and Moore J. W., (1976), Illustration of Some Consequences of The Indistinguishability of Electrons: Use of Computer-Generated Dot-Density Diagrams, J. Chem. Educ., 53, 426.
- Denzin N. K., (1978), Triangulation: A case for methodological evaluation and combination, Sociological methods.
- Denzin N. K. and Lincoln Y. S., (2013), The sage handbook of qualitative research, Sage: Sage Publications, Inc.
- Dhindsa H. S. and Treagust D. F., (2009), Conceptual understanding of Bruneian tertiary students: Chemical bonding and structure, Brunei Int. J. Sci. Math. Educ., 1(1), 33–51.
- Duis J. M., (2011), Organic Chemistry Educators’ Perspectives on Fundamental Concepts and Misconceptions: An Exploratory Study, J. Chem. Educ., 88(3), 346–350 DOI:10.1021/ed1007266.
- Dunn R., Griggs S. A., Olson J., Beasley M. and Gorman B. S., (1995), A Meta-Analytic Validation of the Dunn and Dunn Model of Learning-Style Preferences, J. Educ. Res., 88(6), 353–362 DOI:10.1080/00220671.1995.9941181.
- Erlina E., Cane C. and Williams D. P., (2018), Prediction! the VSEPR Game: Using Cards and Molecular Model Building to Actively Enhance Students’ Understanding of Molecular Geometry, J. Chem. Educ., 95(6), 991–995 DOI:10.1021/acs.jchemed.7b00687.
- Fensham F. C., (1975), Mėdînâ in Ezra and Nehemiah, Vetus Testamentum, 25(4), 795–797 DOI:10.1163/156853375X00412.
- Furió C. and Calatayud M. L., (1996), Difficulties with the Geometry and Polarity of Molecules: Beyond Misconceptions, J. Chem. Educ., 73(1), 36 DOI:10.1021/ed073p36.
- Gabel D., (1998), The complexity of chemistry and implications for teaching. International Handbook of Science Education. 1, Springer.
- Garris R., Ahlers R. and Driskell J. E., (2002), Games, Motivation, and Learning: A Research and Practice Model, Simulation Gaming, 33(4), 441–467 DOI:10.1177/1046878102238607.
- Gillespie R. J., (1997), The Great Ideas of Chemistry, J. Chem. Educ., 74(7), 862 DOI:10.1021/ed074p862.
- Greenbowe T. J., (1994), An Interactive Multimedia Software Program for Exploring Electrochemical Cells, J. Chem. Educ., 71(7), 555 DOI:10.1021/ed071p555.
- Harrison A. G. and Treagust D. F., (1996), Secondary students’ mental models of atoms and molecules: implications for teaching chemistry. Sci. Educ., 80(5), 509–534 DOI:10.1002/(SICI)1098-237X(199609)80:5
![[double bond splayed right]](https://www.rsc.org/images/entities/char_e00a.gif) 509::AID-SCE2
509::AID-SCE2![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) 3.0.CO;2-F.
3.0.CO;2-F. - Hilal A. H. and Alabri S. S., (2013), Using NVivo for data analysis in qualitative research, Int. Interdisciplinary J. Educ., 2(2), 181–186.
- Johnstone A. H., (1993), The Development of Chemistry Teaching: A Changing Response to Changing Demand, J. Chem. Educ., 70(9), 701 DOI:10.1021/ed070p701.
- Jones L. L. and Kelly R. M., (2015), Visualization: The Key to Understanding Chemistry Concepts, in M. V. Orna (ed.) Sputnik to Smartphones: A Half-Century of Chemistry Education, American Chemical Society, pp. 121–140 DOI:10.1021/bk-2015-1208.
- Justi R. S. and Gilbert J. K., (2002), Science teachers’ knowledge about and attitudes towards the use of models and modelling in learning science, Int. J. Sci. Educ., 24(12), 1273–1292 DOI:10.1080/09500690210163198.
- Kalogiannakis M., Papadakis S. and Zourmpakis A.-I., (2021), Gamification in Science Education. A Systematic Review of the Literature, Educ. Sci., 11(1), 22 DOI:10.3390/educsci11010022.
- Kozma R. B. and Russell J., (1997), Multimedia and Understanding: Expert and Novice Responses to Different Representations of Chemical Phenomena, J. Res. Sci. Teach., 34(9), 949–968 DOI:10.1002/(SICI)1098-2736(199711)34:93.3.CO;2-F.
- Krath J., Schürmann L. and von Korflesch H. F. O., (2021), Revealing the Theoretical Basis of Gamification: A Systematic Review and Analysis of Theory in Research on Gamification, Serious Games and Game-based Learning, Comput. Human Behavior, 125, 106963 DOI:10.1016/j.chb.2021.106963.
- Lutfi A., Aftinia F. and Permani B. E., (2023), Gamification: Game as A Medium for Learning Chemistry to Motivate and Increase Retention of Student Learning Outcomes, J. Technol. Sci. Educ., 13(1), 193 DOI:10.3926/jotse.1842.
- McCarthy J., (2021), Using Gamification to Ignite Student Learning, George Lucas Educational Foundation.
- McNiff J. and Whitehead J., (2011), All you need to know about action research, 2nd edn, SAGE Publications.
- Merrill R. J. and Ridgway D. W., (1969), The CHEMStudy Story, Freeman.
- Meyer P. G., (2005), A study of how precursor key concepts for organic chemistry success are understood by general chemistry students, Western Michigan University.
- Nakiboğlu C., (2003), Instructional Misconceptions of Turkish Prospective Chemistry Teachers About Atomic Orbitals and Hybridization, Chem. Educ. Res. Pract., 4(2), 171–188 10.1039/b2rp90043b.
- Nicoll G., (2001), A Report of Undergraduates’ Bonding Misconceptions, Int. J. Sci. Educ., 23(7), 707–730 DOI:10.1080/09500690010025012.
- Nicoll G., (2003), A Qualitative Investigation of Undergraduate Chemistry Students’ Macroscopic Interpretations of the Submicroscopic Structures of Molecules, J. Chem. Educ., 80(2), 205 DOI:10.1021/ed080p205.
- Ofosu-Ampong K., (2020), The Shift to Gamification in Education: A Review on Dominant Issues, J. Educ. Technol. Syst., 49(1), 113–137 DOI:10.1177/0047239520917629.
- Özmen H., (2004), Some student misconceptions in chemistry: a literature review of chemical bonding, J. Sci. Educ. Technol., 13(2), 147–159.
- Pabuccu A. and Geban O., (2006), Remediating Misconceptions Concerning Chemical Bonding through Conceptual Change Text, Hacettepe Univ. J. Educ., 30, 184–192.
- Patton M. Q., (1990), Qualitative evaluation and research methods, SAGE Publications, Inc.
- Patton M. Q., (2002), Qualitative research and evaluation methods, Thousand Oaks.
- Peterson R. F., Treagust D. F. and Garnett P., (1989), Development and application of a diagnostic instrument to evaluate grade-11 and -12 students’ concepts of covalent bonding and structure following a course of instruction, J. Res. Sci. Teach., 26(4), 301–314 DOI:10.1002/tea.3660260404.
- Purser G. H., (1999), Lewis Structures Are Models for Predicting Molecular Structure, Not Electron. Struct., 76(7), 1013–1018.
- Rivera E. S. and Garden C. L. P., (2021), Gamification for Student Engagement: A Framework, J. Further Higher Educ., 45(7), 999–1012 DOI:10.1080/0309877X.2021.1875201.
- Rogers F., Huddle P. A. and White M. D., (2000), Using a teaching model to correct known misconceptions in electrochemistry, J. Cemical Educ., 77(1), 104.
- Sarasin A., (1999), The molecular pathways of ultraviolet-induced carcinogenesis, Mutation Res., 428(1–2), 5–10 DOI:10.1016/S1383-5742(99)00025-3.
- Sarıkaya M., (2007), Making the molecular models from easily obtainable materials, Turkish J. Educ. Sci., 5(3), 513–537.
- Smith S. G., (1970), The Use of Computers in the Teaching of Organic Chemistry, J. Chem. Educ., 47(9), 608 DOI:10.1021/ed047p608.
- Smith S. G., Chabay R. and Kean E., (1985), Falcon Software: Wellesley, MA. This software has now been combined with the Exploing Chemistry multimedia series into Rogers, E,; Stovall, I,; Jones, L,; Chabay, R,; Kean, E., Fundamentals of Chemistry, 2000, at https://www.chem.uiuc.edu/webFunChem/GenChemTutorials.htm (accessed January, 7, 2022).
- Stringer E. T., (2014), Action Research, Thousand Oak.
- Sumarni W., (2010), Penerapan Learning Cycle Approach sebagai Upaya Meminimalisasi Miskonsepsi Mahasiswa pada Materi Struktur Molekul, J. Penelitian Pendidikan, 27(2) DOI:10.15294/jpp.v27i2.177.
- Taber K. S., (2003), Mediating mental models of metals: acknowledging the priority of the learner's prior learning, Sci. Educ., 87(5), 732–758 DOI:10.1002/sce.10079.
- Taber K. S. and Coll R., (2002), Chemical bonding, in J. K. Gilbert et al., (ed.) Chemical education: Research-based practice, Kluwer Academic Publishers BV.
- Tasker R., (1994), Vischem. https://vischem.com.au, (Accessed June. 3, 2022).
- Tasker R. and Dalton R., (2006), Research into Practice: Visualisation of the Molecular World Using Animations, Chem. Educ. Res. Pract., 7(2), 141–159 10.1039/B5RP90020D.
- Treagust D., Chittleborough G. and Mamiala T., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25(11), 1353–1368 DOI:10.1080/0950069032000070306.
- Uyulgan M. A., Akkuzu N. and Alpat Ş., (2014), Assessing the students’ understanding related to molecular geometry using a two-tier diagnostic test, J. Baltic Sci. Educ., 13(6), 839–855 DOI:10.33225/jbse/14.13.839.
- Vavra K. L., Janjic-Watrich V., Loerke K., Phillips L. M., Norris S. P. and Macnab J., (2011), Visualization in Science Education, Alberta Sci. Educ. J., 41(1), 22–30.
- Wang C. Y. and Barrow L. H., (2013), Exploring conceptual frameworks of models of atomic structures and periodic variations, chemical bonding, and molecular shape and polarity: a comparison of undergraduate general chemistry students with high and low levels of content knowledge, Chem. Educ. Res. Practice, 14(1), 130–146 10.1039/c2rp20116j.
- Williamson V. M. and Abraham M. R., (1995), The Effects of Computer Animation on the Particulate Mental Models of College Chemistry Students, J. Res. Sci. Teach., 32(5), 521–534 DOI:10.1002/tea.3660320508.
- Wu H.-K. and Shah P., (2004), Exploring Visuospatial Thinking in Chemistry Learning, Sci. Educ., 88(3), 465–492 DOI:10.1002/sce.10126.
- Yezierski E. J. and Birk J. P., (2006), Misconceptions about the Particulate Nature of Matter. Using Animations To Close the Gender Gap, J. Chem. Educ., 83(6), 954 DOI:10.1021/ed083p954.
- Yilmaz A. and Dinçol Özgür S., (2012), The effect of generative multimedia learning environment on teacher candidates’ achievement, attitude and retention according to their learning styles, Hacettepe Egitim Dergisi, 42, 441–452.
| This journal is © The Royal Society of Chemistry 2024 |