 Open Access Article
Open Access ArticleFabrication of BiVO4/Ag2CrO4 heterojunction composites modified with graphene oxide for enhanced photoelectrochemical and photocatalytic performance†
Adewunmi Olufemi Oluwole a,
Tunde L. Yusuf
a,
Tunde L. Yusuf b,
Shepherd M. Tichapondwaa,
Michael O. Daramolaa and
Samuel A. Iwarere
b,
Shepherd M. Tichapondwaa,
Michael O. Daramolaa and
Samuel A. Iwarere *a
*a
aDepartment of Chemical Engineering, Faculty of Engineering, Built Environment and Information Technology, University of Pretoria, Hatfield, Pretoria 0002, South Africa. E-mail: samuel.iwarere@up.ac.za
bDepartment of Chemistry, Faculty of Natural and Agricultural Sciences, University of Pretoria, Hatfield, Pretoria 0002, South Africa
First published on 29th November 2024
Abstract
A novel GO/BiVO4/Ag2CrO4 heterojunction photocatalyst was prepared by depositing Ag2CrO4 on the highly active (040) facet of BiVO4, followed by incorporating graphene oxide (GO) through an in situ precipitation method. This synergistic modification of BiVO4 by Ag2CrO4 and GO results in excellent photocatalytic performance, with a degradation efficiency of 94.6% coupled with a maximum rate constant of 0.223 min−1, which is 2.40, 2.19 and 0.66 times higher than that of BiVO4, Ag2CrO4, and BiVO4/Ag2CrO4, respectively, for the degradation of ciprofloxacin (CIP) under visible light irradiation. The degradation efficiency of ciprofloxacin was evaluated using total organic carbon (TOC) analysis. Under investigated conditions, the GO/BiVO4/Ag2CrO4 photocatalyst achieved a TOC reduction of 63.4%. The enhanced photocatalytic performance is attributed to the beneficial role of GO in facilitating electron transport for photo-charge carrier migration, leading to strong interfacial coupling between BiVO4 and Ag2CrO4, which in turn promotes efficient charge separation and transfer. The physicochemical properties of the fabricated heterojunction photocatalysts were characterized using X-ray diffraction (XRD), field emission-scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM) coupled with energy-dispersive X-ray (EDX) analysis, Brunauer–Emmett–Teller (BET) analysis, Fourier-transform infrared (FTIR) spectroscopy, ultraviolet-visible diffuse reflectance spectroscopy (UV-DRS), and photoluminescence (PL) emission spectroscopy, while the photoelectrochemical properties of the fabricated photocatalyst were investigated through electrochemical impedance spectroscopy (EIS), Mott–Schottky plots, and photocurrent response analysis. The scavenging experiment was conducted to confirm the role of H+ and ·O2− in the photocatalytic degradation of ciprofloxacin, which aids in proposing probable degradation mechanism for ciprofloxacin under visible light irradiation. Hence, this study offers an effective strategy for fabricating heterojunction photocatalysts aimed at enhancing the photodegradation of pollutants in wastewater.
Introduction
Over the last decades, the pollution of the environment through the discharge of various organic, inorganic and pharmaceutical compounds has forced researchers to develop novel systems for the removal and degradation of such environmental pollutants.1,2 The increased consumption of pharmaceutical compounds, especially antibiotics for human and livestock disease treatment, can be attributed to the increase in world population.3–5 The presence of antibiotics in aquatic environments, even at low concentrations, may induce an imbalance in the aquatic ecosystem and human health through the proliferation of bacterial drug resistance.6–8 CIP is a member of the fluoroquinolone group of antibiotics that shows broad-spectrum antimicrobial activity.9 Nevertheless, owing to its high structural stability, CIP cannot be effectively metabolized in the human body, and as a result, its residues spread throughout the environment causing serious health problems because of its toxicities and high resistance to degradation as it can accumulate in the living organism within the environment.10,11 Over the last decades, researchers have utilized many techniques, including absorption, ozonolysis, electrolysis, membrane separation, photocatalysis, and microbial decomposition, for the removal of these pharmaceutical compounds from water.12–14 Among these techniques, photocatalysis has been proven to be a desirable approach for the degradation of pharmaceutical compounds in wastewater, which can be attributed to its high efficiency, high energy-conservation and low cost.15,16 It is important to note that the application of frequently used photocatalyst semiconductors, including TiO2, ZnO, Al2O3, SnO2, WO3, ZrO2, CuO, and MoS2, has limitations such as low adsorption capacity for hydrophobic pollutants and inefficiency in the utilization of abundant visible light under solar irradiation.11,17–19Bismuth-oxide-based semiconductors such as bismuth vanadate (BiVO4) have been widely studied in the field of visible-light photocatalysis as a result of their narrow bandgap (around 2.4 eV), good visible light absorption, stable photo-corrosion resistance, versatile structural and electronic properties and less expensiveness.12,19,20 Nevertheless, the photocatalytic efficiency of BiVO4 remains unsatisfactory for the degradation of pharmaceutical compounds in water. This is primarily due to its high recombination rate of photo-generated electron–hole pairs, low utilization efficiency of visible light, and weak surface adsorption properties.21–23 Moreover, despite the narrow band gap of BiVO4, its valence band (VB) and conduction band (CB) potentials are insufficiently positive or negative with its photogenerated electrons and holes lacking redox activity, and this will make the generation of free radicals such as hydroxyl radicals (·OH) or superoxide radicals (·O2−) difficult resulting in low photocatalytic efficiency in the degradation of pharmaceutical compounds.22,24 Therefore, many methods such as doping with other photocatalyst materials via modification have been initiated to improve the photocatalytic efficiency of BiVO4 composites where the synthesis of heterojunction photocatalysts is considered the most convenient and efficient technique to overcome the problems associated with severe photogenerated electron–hole recombination and poor redox capacity of pristine BiVO4. The exploration of silver-based semiconductor materials such as silver chromate (Ag2CrO4), a new visible-light-driven photocatalyst, has attracted attention recently. This is due to its high photocatalytic performance as a result of its small band gap at about 1.8 eV with an efficient electronic structure associated with the SPR effects of Ag0 and strong absorption in the visible-light field.25,26 However, just like many of the single semiconductors, the photocatalytic efficiency of Ag2CrO4 suffers from rapid electron–hole recombination. Additionally, Ag2CrO4 is slightly soluble in aqueous solutions. Its self-photo-corrosion and limited solubility, similar to what is observed in other Ag-containing photocatalysts, contribute to a reduction in the photocatalytic stability of Ag2CrO4.
Therefore, the hybridization of a single semiconductor with other semiconductors or metal oxides with a suitable band structure has proven to be an effective fabrication strategy to tackle the challenges associated with the separation of photoinduced electron–hole pairs.27,28 The development of heterojunctions between semiconductors such as BiVO4, SnO2, Ag2CrO4, CuO, ZnO and TiO2 has proven to be an effective and easy approach to facilitate the photo-generated charge separation efficiency and enhance the photocatalytic activity of these unitary semiconductors.26,28,29 In spite of the efficiency observed in the design of heterojunction composites via the combination of two or three nanostructure semiconductors, their photocatalytic efficiency still experiences some limitation as a result of the marginal absorbance in the visible region. Carbon-based materials such as activated carbon, graphene oxide, reduced graphene oxide and carbon nanotubes are good choices for efficient charge transfer.30,31 The conjunction of graphene oxide with semiconductors or metal oxides has demonstrated exceptional photocatalytic performance for the degradation of organic pollutants in aqueous matrices.32,33 This can be attributed to its high conductivity, provision of strong UV and visible-light absorption, and facilitated electron transport, which in turn inhibit charge recombination and thermal and mechanical stability.34–36 GO can act as a sacrificial electron acceptor in composites such as GO/BiVO4/Ag2CrO4. Due to its high electron mobility and the presence of electron-attracting oxygen functional groups, GO can accept and transport photoexcited electrons from Ag2CrO4. This minimizes the likelihood of Ag+ being reduced to Ag0, stabilizing the photocatalyst. By accepting electrons from Ag2CrO4, GO helps maintain effective charge separation, reducing electron–hole recombination. This prolonged lifetime of photogenerated holes enables sustained oxidation of pollutants, enhancing the overall photocatalytic efficiency of the system under visible light. The reduction of Ag+ to Ag0 within Ag2CrO4 during photocatalysis can degrade the material and reduce its stability. GO, by capturing these electrons, reduces the chance of Ag+ being converted into metallic Ag, thus minimizing photocorrosion and extending the operational lifetime of the composite.
In this research, a heterostructure composite of GO/BiVO4/Ag2CrO4 was synthesized, for the first time, by an in situ precipitation method. The electron sinks and plasmonic effects of GO and Ag2CrO4 facilitate the separation of photogenerated electrons and holes, thereby enhancing the photocatalytic degradation performance of ciprofloxacin under visible light irradiation. The structural, morphological, and optical properties of the GO/BiVO4/Ag2CrO4 nanostructures are reported herein.
Experimental
Materials
Chemicals and reagents including graphite powder (99.9%), sulfuric acid (H2SO4, 98%), potassium permanganate (KMnO4, 98.5%), hydrochloric acid (HCl, 35%), hydrogen peroxide (H2O2, 30%), nitric acid (HNO3, 68%), bismuth nitrate pentahydrate (Bi(NO3)3·5H2O, ≥99%), ammonium metavanadate (NH4VO3), tert-butyl alcohol (t-BuOH), benzoquinone (BQ), ethylenediaminetetraacetate salt (EDTA-2Na), NaOH (≥97%) and ciprofloxacin (>98% purity) were purchased from Sigma-Aldrich, South Africa. All chemicals are of fine analytical grade; hence, they were used without further purification.Synthesis of BiVO4
The monoclinic BiVO4 nanoparticle was prepared by a hydrothermal method in which stoichiometric amounts of bismuth nitrate pentahydrate (Bi(NO3)3·5H2O) and ammonium metavanadate (NH4VO3) were dispersed into 30 mL of 4 M HNO3 aqueous solution under ultrasonic treatment for 30 min, respectively. These two solutions were mixed under magnetic stirring for 1 h at room temperature. Solutions of NaOH and HNO3 were used to adjust the pH of the mixture to 7 under stirring for further 1 h. The stirred mixture was later transferred into a 100 mL Teflon-lined stainless autoclave and sealed. The autoclave was finally put into an oven and maintained at a temperature of 180 °C for 16 h, after which it was allowed to cool down to room temperature. The as-prepared BiVO4 samples were later centrifuged, washed with deionized water (D.I. H2O) and ethanol for several times and finally dried at 70 °C overnight.Preparation of GO
GO was synthesized by improved Hummers' method.37 A concentrated mixture of H2SO4 (184 mL) and HNO3 (48 mL) was added to 1 g of graphite powder and stirred at room temperature until the powder was fully dispersed. After cooling the mixed solution to around 20 °C in an ice bath, 6 g of potassium permanganate (KMnO4) was slowly added. The obtained mixed solution was heated in a water bath to 85 °C and maintained for 30 min until a bright yellow suspension was obtained. Then 10 mL of hydrogen peroxide (H2O2) was slowly added to the mixture and allowed to stir for 30 min. The obtained reactants were collected by centrifugation and washed with 10% of HCl and distilled water until the pH value reached 7.0. The reactant black precipitate was dried at 60 °C overnight to obtain GO.Preparation of Ag2CrO4
For the synthesis of Ag2CrO4, 1.5 g of AgNO3 was dissolved in 50 mL D.I. H2O and allowed to stir for 30 min while 0.7 g of K2CrO4 dissolved in 20 mL of D.I. H2O was added. The mixture was allowed to stir at room temperature for 1 h. Afterward, the mixed solution was poured into a 100 mL Teflon-lined autoclave and treated for 18 h at 180 °C in an oven. The resulting product after cooling of the autoclave to room temperature was obtained by centrifugation and washed with water and ethanol severally. The obtained Ag2CrO4 precipitate was later dried in an oven at 60 °C overnight.Preparation of GO/BiVO4/Ag2CrO4 nanocomposites
First, 0.01 g of the prepared graphene oxide nanosheets (GO) were dispersed in 50 mL D.I. H2O with 5 mL of methanol added to enhance the dispersion process and then ultrasonicated for 1 h in order for the prepared GO to be thoroughly dispersed, followed by the addition of 1.00 g of the synthesized BiVO4 under ultra-sonication treatment. Then, 0.178 g of AgNO3 and 0.201 g of polyvinylpyrrolidone were added to the above solution and allowed to vigorously stir for 1 h; afterward, 0.097 g of K2CrO4 dissolved in 20 mL of D.I. water was added in a dropwise manner. The resulting solution was further stirred for additional 2 h. The as-synthesized GO/BiVO4/Ag2CrO4 nanocomposites were centrifuged, washed thoroughly with distilled water and ethanol until no precipitate of the nanocomposites was seen in the decanted water, and finally dried at 70 °C overnight. The obtained composites of GO/BiVO4/Ag2CrO4 were later calcined at 550 °C for 3 h. This process was also used in the synthesis of BiVO4/Ag2CrO4 composites but without the introduction of graphene oxide (GO).Characterization
The crystalline-phase composition of the fabricated nanocomposites was determined using a PANalytical X'Pert Pro powder X-ray diffractometer with Cu-Kα radiation (λ = 1.789 Å) in θ–θ configuration. SEM equipped with EDS techniques (JEOL Scanning Microscope JSM-6400) was employed to perform imaging and elemental composition analysis, while the microstructure images of the fabricated nanocomposites were further analysed using a JOEL JEM 2100F, 200 kV transmission electron microscope (TEM). The specific surface area and pore distribution of the nanocomposites were investigated using a Brunauer–Emmett–Teller (BET) Micromeritics Tristar II 3020 Version 3.02 system after degassing overnight at 100 °C prior to analysis. Fourier-transform infrared (FT-IR) spectra of the samples were recorded using a PerkinElmer Spectrum One spectrometer with KBr pellets. The light absorption performance of the nanocomposites was evaluated using a UV-vis spectrophotometer (Agilent Technologies Cary 60 UV-vis, Malaysia) while an Autolab Potentiostat (PGSTAT204, Netherlands) workstation was used for electrochemical impedance spectroscopy (EIS), Mott–Schottky plots and photocurrent response analysis of the synthesized heterostructure composites. A Shimadzu TOC-V analyzer was used to quantitatively assess the extent of ciprofloxacin mineralization before and after the photocatalysis reaction.Photoelectrochemical experiments
The photoelectrochemical characteristics of the prepared nanocomposite samples were measured using a standard three-electrode cell in the presence of the fabricated anode, a platinum wire and Ag/AgCl (3.0 M KCl) to function as the working, counter and reference electrodes, respectively. The working electrode was prepared by using a fluorine-doped tin oxide (FTO) substrate coupled with a geometric area of 1.5 cm by 1.5 cm coated with 30 mg of the fabricated materials, while 5 wt% PVDF and 100 μL NMP serve as binders.38 Then 5 mM [Fe(CN)6]3−/[Fe(CN)6]4− in 0.1 M KCl was prepared for the analysis of the electrochemical impedance spectroscopic analysis with collected data analyzed using the Randles circuit model. The photocurrent density was obtained in 0.1 M Na2SO4, with an applied bias potential of 1.5 V relative to the Ag/AgCl reference electrode while the Mott–Schottky measurements were conducted under dark conditions using a 5 mM [Fe(CN)6]3−/[Fe(CN)6]4− solution in a 0.1 M KCl electrolyte.Results and discussion
X-ray diffraction of the synthesized samples was investigated to characterize their crystal-phase structure, and the patterns are shown in Fig. 1. The peaks of the prepared BiVO4 corresponded to the characteristic peaks of monoclinic BiVO4 (JCPDS: 14-0688) while the diffraction crystallinity peaks observed for pure Ag2CrO4 resulted from its orthorhombic structure (JCPDS no. 26-0952).39,40 The diffraction peaks observed for BiVO4/Ag2CrO4 are linked to BiVO4 and Ag2CrO4 with lower amplitudes. However, the diffraction pattern of pure GO shows the characteristic peak due to its interlayer stacks indexed to the (001) and (100) diffraction planes of its oxygen-containing functional group.41 The characteristic peaks of the fabricated GO/BiVO4/Ag2CrO4 heterojunction composites are similar to the binary composites of BiVO4/Ag2CrO4, which indicates that compositing GO and Ag2CrO4 did not significantly change the crystal morphology of BiVO4 with diffraction peaks of BiVO4 dominant due to its crystallinity. | ||
| Fig. 1 XRD of the fabricated BiVO4, Ag2CrO4, BiVO4/Ag2CrO4 and GO/BiVO4/Ag2CrO4 heterojunction photocatalysts. | ||
The crystal size of the fabricated nanocomposites was calculated according to the Scherrer formula S2.†42 As provided in Table 1, it can be seen that the introduction of Ag2CrO4 into BiVO4 caused a significant reduction in the crystallite size of BiVO4 from 44.72 nm to 32.68 nm with a slight improvement in the crystalline size of the GO/BiVO4/Ag2CrO4 heterojunction photocatalyst (36.16 nm), suggesting a better photocatalytic activity as it has been proven that photocatalysts with smaller particle sizes tend to exhibit better photocatalytic efficiency.43
| Photocatalyst material | Surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore diameter (nm) | Crystalline size (nm) |
|---|---|---|---|---|
| BiVO4 | 0.605 | 0.00036 | 13.80 | 44.72 |
| Ag2CrO4 | 0.814 | 0.0014 | 69.43 | 30.85 |
| GO | 1023.42 | 0.63 | 76.91 | 9.33 |
| BiVO4/Ag2CrO4 | 3.563 | 0.00074 | 27.61 | 32.68 |
| GO/BiVO4/Ag2CrO4 | 17.141 | 0.00081 | 37.36 | 36.16 |
From Fig. 2a, it can be seen that the surface architecture of the fabricated BiVO4 composites displayed a well-defined flat smooth surface with sharp edges and a decagonal shape from SEM analysis. Two facets are also observed in the morphology, which can be attributed to (040) and (110), while these facets are responsible for the transfer of charge carriers.44 The prepared Ag2CrO4 sample represents a cluster of cube-like nanostructures and particle-like microstructures. The SEM image of binary BiVO4/Ag2CrO4 shows a microsphere-type morphology, with the aggregation of the cube-like nanostructures of Ag2CrO4 on the surface of BiVO4 as they are seen to maintain their original morphology after the introduction of Ag2CrO4 nanoparticles, as it can reduce the charge carrier recombination rates, hence promoting the photocatalytic performance.45 As shown in Fig. 2e, the addition of GO into the BiVO4/Ag2CrO4 nanocomposites increases the space between nanostructures on the microspheres' surface, resulting in an enhanced specific surface area and generation of more active sites.
 | ||
| Fig. 2 SEM micrographs of (a) BiVO4, (b) Ag2CrO4, (c) BiVO4/Ag2CrO4, (d) GO, (e) GO/BiVO4/Ag2CrO4, and (f) EDS mapping of GO/BiVO4/Ag2CrO4 nanocomposites. | ||
Energy-dispersive spectrum (EDS) studies were used to reveal the chemical composition of the fabricated GO/BiVO4/Ag2CrO4 nanocomposite. Seven elements including C, Bi, O, N, V, Ag, and Cr were found on the surface of the fabricated GO/BiVO4/Ag2CrO4 nanocomposite, as shown in the EDS elemental mapping and composition in Fig. 3, proving that the nanocomposite is without impurity, which is consistent with the XRD results.
TEM analysis was used to provide further structural and crystal phase analyses of the fabricated GO/BiVO4/Ag2CrO4 nanocomposites. Fig. 4a indicates that the synthesized BiVO4 composites are made up of aggregated decagonal shapes of nanoparticles, which is consistent with the SEM result. The TEM image of the Ag2CrO4 composite shows the presence of dark regions in the Ag2CrO4 nanoparticles, which can be attributed to the overlapping within the nanoparticles. The Fig. 4c shows the integration of the dark nanoparticle of Ag2CrO4 on the surface of the spherical nanosheets of BiVO4, while the particles of Ag2CrO4 and the lamellar sheets of GO are spread over the surface of BiVO4 microspheres, as shown in Fig. 4e for the fabricated GO/BiVO4/Ag2CrO4 nanocomposite. The close interaction observed in the fabricated GO/BiVO4/Ag2CrO4 nanocomposite suggests the formation of heterojunction between GO, BiVO4 and Ag2CrO4 which is advantageous for the separation of charge carriers, thereby confirming the successful fabrication of the ternary GO/BiVO4/Ag2CrO4 nanocomposites.46
 | ||
| Fig. 4 TEM micrographs of (a) BiVO4, (b) Ag2CrO4, (c) BiVO4/Ag2CrO4, (d) GO and (e) GO/BiVO4/Ag2CrO4 nanocomposites. | ||
The surface functional groups present in the synthesized BiVO4, Ag2CrO4, GO, BiVO4/Ag2CrO4 and GO/BiVO4/Ag2CrO4 samples were investigated by FTIR spectroscopy, and the results are shown in Fig. 5. For BiVO4, the absorption peaks observed at 3436 cm−1 are assigned to the bending vibration of residual H2O molecules adsorbed on the surface of the synthesized BiVO4. This absorbed H2O molecule can be converted into hydroxyl radicals during the degradation process.47 The peak at 430 cm−1 is characteristic of the bending vibration of Bi–O, while the strong bands at 740 cm−1 are attributed to the monoclinic scheelite of BiVO4 due to the asymmetric stretching of the V–O bond. The bands at 1314, 1637, 2345 and 2926 cm−1 belong to the bending vibration of the residual NO3−, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–C and C–H bonds.48 For the FTIR spectra of Ag2CrO4, the prominent absorption band at 878 cm−1 is ascribed to the Cr–O stretching vibration in CrO42− while the absorption peak at 3438 cm−1 can be attributed to the O–H group stretching vibration.49 In the spectra of GO, the characteristic peaks at 1732, 1613, and 1109 cm−1 can be ascribed to carboxyl C
O, C–C and C–H bonds.48 For the FTIR spectra of Ag2CrO4, the prominent absorption band at 878 cm−1 is ascribed to the Cr–O stretching vibration in CrO42− while the absorption peak at 3438 cm−1 can be attributed to the O–H group stretching vibration.49 In the spectra of GO, the characteristic peaks at 1732, 1613, and 1109 cm−1 can be ascribed to carboxyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, carboxyl-OH stretching, and alkoxy C–O stretching with the strong absorption band at 3410 cm−1 attributed to the OH stretching vibration.50 The FTIR spectrum of the BiVO4/Ag2CrO4 and GO/BiVO4/Ag2CrO4 composites represents the overlap spectra of BiVO4, Ag2CrO4 and GO, which indicates that the synthetic process is successful.
O stretching, carboxyl-OH stretching, and alkoxy C–O stretching with the strong absorption band at 3410 cm−1 attributed to the OH stretching vibration.50 The FTIR spectrum of the BiVO4/Ag2CrO4 and GO/BiVO4/Ag2CrO4 composites represents the overlap spectra of BiVO4, Ag2CrO4 and GO, which indicates that the synthetic process is successful.
 | ||
| Fig. 5 Fourier transform infrared (FTIR) spectra of the fabricated BiVO4, Ag2CrO4, BiVO4/Ag2CrO4, GO and GO/BiVO4/Ag2CrO4 nanocomposites. | ||
The surface area, pore volume, and pore size distributions of the synthesized materials were analysed using the BET nitrogen adsorption–desorption isotherm. Fig. 6 shows the isotherms of the synthesized nanocomposites to exhibit an H3 hysteresis loop with a type IV isotherm, which designates the occurrence of mesopores in the synthesized materials, which can play a significant role in the degradation of organic pollutants in water.51
 | ||
| Fig. 6 Nitrogen sorption isotherms and the corresponding pore volume and diameter distribution of the fabricated nanocomposites. | ||
It was determined from the BET analysis that the surface area of BiVO4, Ag2CrO4 and GO is 0.605, 0.814 and 1023.42 cm2 g−1, respectively, whereas the surface area of BiVO4/Ag2CrO4 and GO/BiVO4/Ag2CrO4 is 3.563 and 17.141 cm2 g−1, which are greater than that of BiVO4 and Ag2CrO4. Thus, the GO/BiVO4/Ag2CrO4 nanocomposites conserve the surface texture of its components (BiVO4, Ag2CrO4 and GO) and the remarkable surface area offers a large number of vigorous sites to adsorb the organic contaminants, which may be advantageous in enhancing its photocatalytic activities. The values of pore volume and pore size distribution are highlighted in Table 1, and the synthesized nanocomposites of GO/BiVO4/Ag2CrO4 exhibit an improved pore volume and pore size when compared to the other materials due to the presence of GO.
Electrochemistry analysis
The photocatalytic performance of photocatalyst materials largely depends on the absorption of visible light; therefore, it is important to investigate the absorption ability of the synthesized materials using UV-vis diffuse reflectance spectrum (DRS), and the results are shown in Fig. 7. All the synthesized materials absorb photons in the visible region at 535 nm, 751 nm, 722 and 734 nm for BiVO4, Ag2CrO4, BiVO4/Ag2CrO4 and GO/BiVO4/Ag2CrO4 respectively. The shift in the absorption edge of GO/BiVO4/Ag2CrO4 towards the near-infrared region with increased absorption can be attributed to the interaction of BiVO4 with Ag2CrO4 and with the inclusion of GO via compositing, where GO functions as a matrix in enhancing light adsorption capacity in the visible light region with a redshift. The data obtained from the UV-DRS analyses were used to investigate the band gap energy of the synthesized nanocomposites using the Tauc equation, as given in eqn (1):| αhv = A(νh − Eg)n/2 | (1) |
Photoluminescence emission spectra analysis has proven to be one of the effective approaches in understanding the separation capacity of the photoinduced carriers of nanocomposite materials due to the direct result of recombination of the free carriers.53 Therefore, creating a correlation between the intensity of PL and the photocatalytic activities of the synthesized material as lower recombination of the photogenerated charge carrier originating from a lower PL intensity will result in enhanced photocatalytic activities and vice versa. The PL spectra of the synthesized BiVO4 catalyst show a strong emission peak at about 562 nm, as presented in Fig. 8a corresponding to the recombination of the hole formed from the hybrid orbitals of Bi 6s and O 2p, and the electron generated from the V 3d orbital.54 This suggests that BiVO4 possesses a lower separation efficiency of a photo-induced electron–hole, hence less photocatalytic performance. The fluorescence intensity of the nanocomposites of the GO/BiVO4/Ag2CrO4 sample was significantly weaker than that of the BiVO4, Ag2CrO4 and BiVO4/Ag2CrO4 catalysts, which shows the recombination restraint of the electron–hole pairs. The obtained result shows that the reduction in the recombination of the photogenerated charge carrier of the fabricated GO/BiVO4/Ag2CrO4 nanocomposites will prove useful during the photocatalytic degradation of organic pollutants in aqueous matrices.
The separation and transfer of electron–hole pairs of the fabricated nanocomposites were analyzed by electrochemical impedance spectroscopy (EIS). The radius of the arc in the Nyquist plot measurements provides information on the charge transfer process with the diameter of the arc, which is characteristic of the charge transfer resistance, where a smaller arc radius indicates higher efficiency in charge transfer.55 The findings for the fabricated nanocomposites are displayed in Fig. 8b, with the arc radius of GO/BiVO4/Ag2CrO4 is smaller than that of pure BiVO4, Ag2CrO4 and BiVO4/Ag2CrO4, indicating a faster separation and transfer of photogenerated carrier's nanocomposites. This implied that the fabricated GO/BiVO4/Ag2CrO4 nanocomposite possessed a greater charge separation efficiency coupled with the decrease in their band gap energy, hence better photocatalytic activity during visible light irradiation.
The transient photocurrent behaviour of the fabricated materials was investigated at 10 second intervals during ON–OFF cycles of xenon lamp irradiation by chronoamperometry with an applied potential of 1.5 V (see Fig. 9). These measurements allowed us to assess the transfer of photoinduced carriers and evaluate the photocatalytic performance of both pristine materials and heterostructures. Specifically, pristine BiVO4 and Ag2CrO4 exhibited maximum photocurrent responses of 0.037 and 0.051 mA cm−2, respectively.
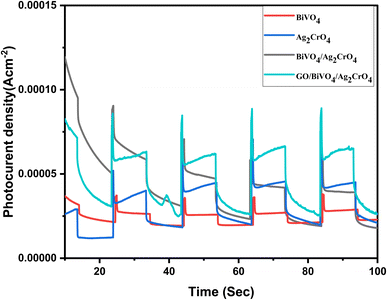 | ||
| Fig. 9 Photocurrent response plots of BiVO4, Ag2CrO4, BiVO4/Ag2CrO4, and GO/BiVO4/Ag2CrO4 nanocomposites. | ||
However, the fabricated heterostructures demonstrated significantly higher responses. Notably, the combination of BiVO4 and Ag2CrO4 enhanced carrier separation and facilitated charge transfer on the catalyst surface. In particular, the BiVO4/Ag2CrO4 heterostructure achieved a much higher response, with a recorded maximum photocurrent of 0.070 mA cm−2. However, upon the introduction of GO, the ternary composite GO/BiVO4/Ag2CrO4 exhibited a photocurrent maxima of 0.089 mA cm−2 which is indicative that the fabricated GO/BiVO4/Ag2CrO4 nanocomposite possesses a higher carrier separation efficiency.
Photocatalytic experiments
The photocatalytic activity of the fabricated nanocomposites was tested for the degradation of ciprofloxacin in synthetic water under visible light irradiation (200 W fluorescent lamps). This was done by dispensing 50 mg of each batch of the composites in 100 mL of 10 mg L−1 of ciprofloxacin solution, while the suspension was stirred in the darkness for 30 min to achieve the adsorption–desorption equilibrium between the ciprofloxacin solution and the photocatalyst. At every 20 min interval, 4 mL aliquot samples of the suspension was collected, centrifuged and filtered through a 0.45 μm Millipore filter before analysis using a UV-vis spectrophotometer (Biochrom, Cambridge, UK) to estimate the change in ciprofloxacin concentration at a wavelength of 274 nm. The calculation of the photodegradation percentage was determined using the following equation:
 | (2) |
Adsorption studies for the fabricated composites were carried out under the same condition. The adsorption capacity of the fabricated nanocomposites as presented in S4† shows that BiVO4, Ag2CrO4, GO, BiVO4/Ag2CrO4 and GO/BiVO4/Ag2CrO4 were able to adsorb 20.40, 23.04, 32.43, 40.13 and 45.40% of ciprofloxacin. The absorption ability of the synthesized nanocomposites can be associated with the specific surface area, as shown in Table 1.
The C/C0 value of the degradation efficiency of the fabricated photocatalyst is displayed in Fig. 10a with the degradation rate provided in Table 2. It can be seen that BiVO4, Ag2CrO4, BiVO4/Ag2CrO4 mixture and GO/BiVO4/Ag2CrO4 achieved 48.64, 55.87, 72.44, 59.03 and 94.56% for the degradation of ciprofloxacin after 120 min of irradiation under visible light. The enhancement in the photodegradation efficiency of GO/BiVO4/Ag2CrO4 can be attributed to the incorporation of GO into the fabricated BiVO4/Ag2CrO4 composites. This is due to the interaction of the GO sheet with the fabricated BiVO4/Ag2CrO4 via physical adsorption, increased surface area and electrostatic transfer, resulting in a reduction of the photogenerated electron–hole pair recombination coupled with the electron donation-mediation of surface plasmon resonance (SPR) supplied with the presence of Ag2CrO4, hence the improved photocatalytic efficiency of GO/BiVO4/Ag2CrO4 for the degradation of ciprofloxacin. This is in agreement with the analysis result obtained from UV-DSR, PL, EIS, and BET.
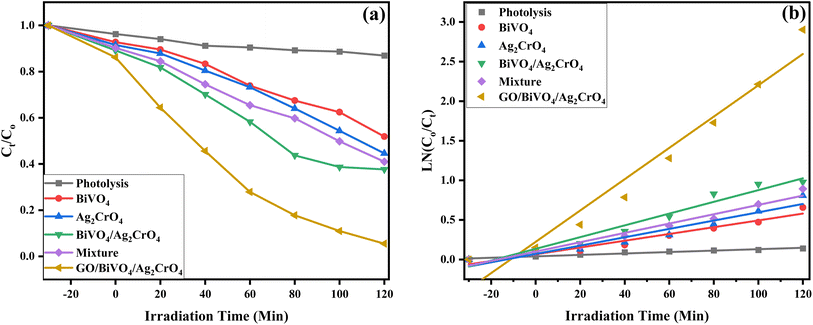 | ||
| Fig. 10 (a) Degradation plot of ciprofloxacin using the fabricated nanocomposites and (b) their corresponding kinetic plot. | ||
| Photocatalyst material | Degradation (%) | Rate constant k (min−1) | Coefficient R2 |
|---|---|---|---|
| Photolysis | 13.07 | 0.03953 | 0.95143 |
| BiVO4 | 48.64 | 0.06572 | 0.94804 |
| Ag2CrO4 | 55.87 | 0.06981 | 0.92836 |
| BiVO4/Ag2CrO4 | 72.44 | 0.13385 | 0.95564 |
| Mixture | 59.03 | 0.09959 | 0.96743 |
| GO/BiVO4/Ag2CrO4 | 94.56 | 0.22253 | 0.94652 |
The kinetics of the photocatalytic process was analyzed using the pseudo-first-order kinetic via the Langmuir–Hinshelwood model equation as follows:
| (−ln(C/C0) = kt) | (3) |
Fig. 10b shows that the rate constant of GO/BiVO4/Ag2CrO4 was 0.22253 min−1, which was 2.40, 2.19 and 0.66 times higher than that of BiVO4, Ag2CrO4, and BiVO4/Ag2CrO4 respectively while all the values of the coefficient R2 were greater than 0.93 (R2 > 0.93). The result proves that the improvement in the photocatalytic performance is a result of the loading of GO into the composites of BiVO4/Ag2CrO4. Furthermore, the degradation efficiency of ciprofloxacin was evaluated using total organic carbon (TOC) analysis. Under optimal conditions, the GO/BiVO4/Ag2CrO4 photocatalyst achieved a TOC reduction of 63.4% (Fig. S6†). The degradation of ciprofloxacin by the fabricated novel heterojunction GO/BiVO4/Ag2CrO4 photocatalyst was compared with previously reported studies on ciprofloxacin degradation with other photocatalysts, as provided in Table S2.† It can be seen that the GO/BiVO4/Ag2CrO4 photocatalyst fabricated in this study displayed better degradation efficiency at a shorter time when compared with other photocatalysts used in ciprofloxacin degradation.
Effect of catalyst dosage
Catalyst dosage was carried out in order to understand the concentration effect that GO/BiVO4/Ag2CrO4 will have on the degradation of ciprofloxacin where different weight ratios of GO/BiVO4/Ag2CrO4 were used, and their photodegradation performance was evaluated under visible light irradiation. Fig. 11a displays ciprofloxacin photodegradation at 30, 50, 70, and 100 mg weight to achieve 73.24, 94.51, 98.06 and 86.83%, respectively. It can be seen from the result that the optimum loading of the nanocomposites is 70 mg for efficient photodegradation of ciprofloxacin, which can be attributed to the increase in vacant active sites, while the decrease in the photocatalytic degradation of ciprofloxacin at 100 mg can be due to the light reflected by the catalyst particle, thereby preventing the entry of photons into the solution.56Effect of pH
Additionally, investigating the pH level of the reaction medium is one of the major parameters that affect the photocatalytic degradation efficiency of pharmaceutical compounds by influencing the chemical state and surface charge of the fabricated GO/BiVO4/Ag2CrO4 nanocomposites, resulting in electrostatic interaction between them.57 The point of zero charge (pHzpc) of the fabricated GO/BiVO4/Ag2CrO4 nanocomposites was analyzed to predict its interaction with the ciprofloxacin pollutant during the degradation process. The pHzpc of the fabricated photocatalyst was evaluated by using the pH drift method described in S4 in the ESI.†58 As shown in Fig. S4,† the pHzpc of the GO/BiVO4/Ag2CrO4 photocatalyst was calculated to be 6.08. Therefore, an experiment on the photodegradation of ciprofloxacin was carried out at different pH values of 2, 4, 6, 8 and 10 (Fig. 11b) with the percentage degradation of ciprofloxacin achieved at 30.34, 64.84, 96.51, 81.04 and 75.03% for pH 2, 4, 6, 8 and 10 respectively. The photocatalytic degradation of ciprofloxacin using the fabricated GO/BiVO4/Ag2CrO4 nanocomposites increases from pH 2 to 6 and then decreases. The least degradation of ciprofloxacin by the nanocomposites was observed at pH 2, which can be attributed to catalytic corrosion and electrostatic repulsion between the surface of GO/BiVO4/Ag2CrO4 and ciprofloxacin in the acidic medium.59 A higher degradation of ciprofloxacin (96.51%) was observed at pH 6, which indicates that the photocatalytic system is close to the pHpzc of the fabricated GO/BiVO4/Ag2CrO4 nanocomposites with the zwitterionic of ciprofloxacin at pKa1 6.16 and pKa2 8.23 leading to the minimum electrostatic repulsion between the nanocomposites and ciprofloxacin compounds.60Stability study
The stability of the fabricated nanocomposites for ciprofloxacin degradation was further investigated through four (4) cycling experiments. Four continuous degradation experiments were conducted to degrade ciprofloxacin without changing the nanocomposites. The experiment followed the same procedure of each measurement except for changing the ciprofloxacin solution for each run while the GO/BiVO4/Ag2CrO4 nanocomposite was re-collected by centrifugation, rinsed with D.I. H2O and dried for next cycle run. It can be seen from Fig. 12a that there is only a slight drop in the degradation performance of the nanocomposite after four cycles, which can be due to the loss of the catalyst during each round of catalyst collection.61 Overall, the obtained result indicated that the fabricated GO/BiVO4/Ag2CrO4 is stable, and it can be reusable in photocatalytic processes for the degradation of pharmaceutical compounds in aqueous matrices.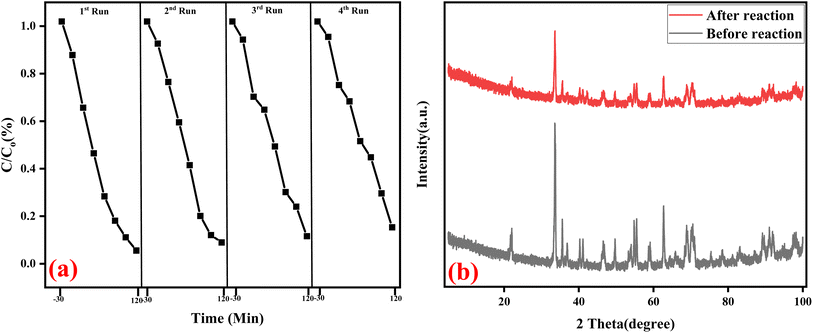 | ||
| Fig. 12 (a) Successive test run for the degradation of ciprofloxacin and (b) XRD patterns before and after reusing the GO/BiVO4/Ag2CrO4 nanocomposites. | ||
The XRD patterns of both fabricated GO/BiVO4/Ag2CrO4 and recycled GO/BiVO4/Ag2CrO4 nanocomposites are displayed in Fig. 12b, and it can be seen that a negligible variation in peak intensity between the fresh and recycled nanocomposites was observed, which confirms that the fabricated heterojunction nanocomposites possess a remarkable photostability during the photodegradation processes.62
Effect of radical scavenger experiments
Scavenger analyses were performed to determine the most reactive oxidation species in the degradation of ciprofloxacin under visible irradiation and also to provide addition information for the degradation mechanism. This was performed through the addition of 5 mM of ethylenediaminetetraacetate salt (EDTA-2Na for h+ radicals), t-butanol (t-BuOH for OH˙ radicals) and p-benzoquinone (p-BQE for ·O2 radicals).The results revealed a significant difference in the degradation of ciprofloxacin. Specifically, the use of a ·OH radical scavenger led to a decrease in photodegradation efficiency from 94.56% to 69.80%, while the ·O2− scavenger and hole scavenger caused a further reduction in the photodegradation of ciprofloxacin noted as 57.21 and 21.00%, respectively, as depicted in Fig. 13. The finding from the scavenging experiment shows that ·OH, ·O2− and h+ are the reactive species in the degradation of ciprofloxacin with H+ and ·O2− playing a major role in the degradation process when compared to OH radicals.
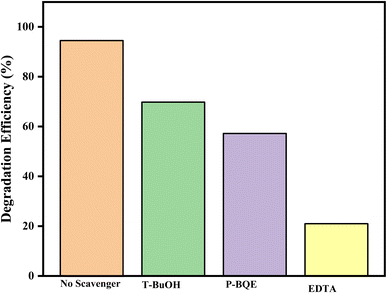 | ||
| Fig. 13 Detection of reactive species with GO/BiVO4/Ag2CrO4 composites during the ciprofloxacin degradation process. | ||
Photocatalytic degradation mechanisms
The probable photocatalytic mechanism for the degradation of ciprofloxacin using the fabricated heterojunction GO/BiVO4/Ag2CrO4 nanocomposite under visible-light irradiation is schematically illustrated, using the energy band gap results obtained from the UV-DSR analysis of BiVO4 and Ag2CrO4 composites (Fig. 7), and their corresponding VB and CB potentials were calculated using Butler and Ginley's equation63,64 as follows:| EVB = X − Ee + 0.5Eg | (4) |
| ECB = EVB − Eg | (5) |
Additionally, the Mott–Schottky (M–S) plots shown in Fig. 14 were employed to examine the electronic properties of BiVO4 and Ag2CrO4 electrodes. These plots allow for the determination of the flat band potential (Efb) and majority carrier density for each material. The M–S plot for BiVO4 and Ag2CrO4 exhibited a positive slope, indicating an n-type semiconducting behaviour. The Efb values derived from the x-axis intercept were 0.54 V and 0.32 V vs. the Ag/AgCl reference electrode for Ag2CrO4 and BiVO4, respectively. In order to convert these values to the standard hydrogen electrode (NHE) reference scale, the Nernst equation was applied: ENHE = EAg/AgCl + 0.197 (pH). This calculation resulted in Efb values of +0.74 V and 0.52 V vs. NHE for Ag2CrO4 and BiVO4, respectively. It is also important to consider the typical positioning of the flat band potential relative to the band edges in semiconductors. In n-type materials, the Efb value is generally located at about 0.1–0.3 V above the conduction band (CB) potential. Consequently, the ECB values for BiVO4 and Ag2CrO4 were determined to be 0.38 V and 0.51 V vs. NHE, respectively. These findings collectively clarify the band energy structures of BiVO4 and Ag2CrO4.
Therefore, on the basis of these results, the photocatalytic mechanism of the fabricated GO/BiVO4/Ag2CrO4 heterostructure composites is proposed in Fig. 15. The low energy bandgap of the heterostructure GO/BiVO4/Ag2CrO4 composites, as investigated using UV-DSR, was helpful in their excitation under visible light irradiation, resulting in reduced electron–hole recombination rates. The valence band and conduction band of the prepared BiVO4 and Ag2CrO4 are attached by GO due to their strong interfacial electrostatic interaction.
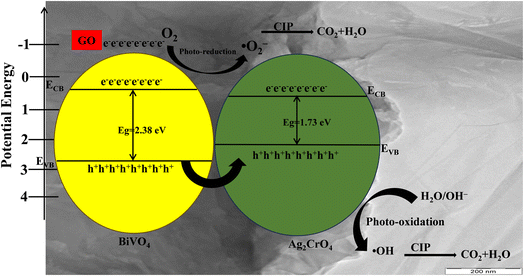 | ||
| Fig. 15 Possible mechanism for ciprofloxacin photodegradation by the GO/BiVO4/Ag2CrO4 photocatalyst. | ||
As shown in Fig. 15, under visible light irradiation, the electron (e−) on the valence band (VB) of BiVO4 migrated to the valence band (VB) of Ag2CrO4, while the holes (h+) on the VB of BiVO4 are rapidly migrated to the VB of Ag2CrO4 under the driving force of the internal electric field and strong interfacial electrostatic interaction provided by GO to achieve the rapid separation of photogenerated charge carriers. The CB potential of BiVO4 and Ag2CrO4 is more positive than the standard redox potential of O2/·O2− (−0.33 eV vs. NHE); in this instance, it is clear that the photogenerated electrons of BiVO4 and Ag2CrO4 in the CB lack sufficient potential to take part in the oxidation process. However, from the scavenger study, O2˙− was mostly responsible for the degradation of CIP where the incorporated GO can serve as an electron acceptor, storing photoexcited electrons generated by the semiconductor components (like BiVO4 or Ag2CrO4) in the composite. These stored electrons can be subsequently transferred to molecular oxygen adsorbed on the GO surface, generating superoxide radicals (O2˙−). By accepting electrons, GO prevents electron–hole recombination within the photocatalyst system. This leads to a higher number of available electrons that can transfer to oxygen, thereby indirectly boosting superoxide generation. The scavenger results can be justified by elucidating the function of GO in the reduction process, as reported in a previous study66 and explained as follows:
| GO + hv → GO* | (6) |
| 3O2 + GO* → 1O2 | (7) |
| 1O2 + GO* → ·O2− + GO+ | (8) |
Therefore, GO as an electron mediator increases the contribution of ·O2− in the photocatalytic activity of the GO/BiVO4/Ag2CrO4 photocatalyst. The VB potential of Ag2CrO4 is more positive than the standard redox potential of H2O/·OH (+1.99 eV vs. NHE), resulting in the oxidation of H2O by h+ to produce ·OH. It should be noted that the VB potential of BiVO4 is 2.73 eV, which is higher than the standard redox potential of OH−/·OH (2.4 eV vs. NHE) resulting in the oxidation of adsorbed H2O molecules by the photogenerated h+ in the VB to produce·OH. The proposed mechanism is consistent with the observation made from the scavenging experiment, as shown in Fig. 13, which shows that h+ and ·O2− plays a significant role in the degradation of ciprofloxacin when compared to OH radicals when using the fabricated GO/BiVO4/Ag2CrO4 heterostructure composites.
Conclusion
In summary, a novel heterojunction visible-light-driven GO/BiVO4/Ag2CrO4 photocatalyst has been successfully synthesized via an in situ precipitation technique and innovatively applied as a highly efficient system in the degradation of ciprofloxacin in simulated water. This excellent multifunctional photocatalytic performance of the fabricated GO/BiVO4/Ag2CrO4 photocatalyst may be attributed to the development of a nanohybrid junction between BiVO4 and Ag2CrO4 facilitated by the addition of GO in order to promote efficient charge transfer at their interface with improved separation of the photogenerated electrons and holes. The fabricated GO heterojunction photocatalyst also displayed remarkable stability compared to BiVO4 and Ag2CrO4, which may be due to the formation of nanohybrids. Based on the active species scavenging experiments and calculated energy band positions, the mechanism of the enhanced photocatalytic activity of the GO/BiVO4/Ag2CrO4 photocatalyst was discussed. In general, the BiVO4 hybridized with Ag2CrO4 and GO could be used for solving the problems associated with low photocatalytic performance, which will provide a promising way for environmental remediation.Data availability
The data supporting the findings of this research study are available from the corresponding author upon reasonable request. Additionally, ESI data† and materials have been included in this submission.Conflicts of interest
The authors declare no conflict of interest.References
- O. C. Olatunde, L. Sawunyama, T. L. Yusuf and D. C. Onwudiwe, J. Water Process Eng., 2024, 66, 105890 CrossRef.
- T. L. Yusuf, B. O. Orimolade, D. Masekela, B. Mamba and N. Mabuba, RSC Adv., 2022, 12, 26176–26191 RSC.
- D. Yadav, S. Rangabhashiyam, P. Verma, P. Singh, P. Devi, P. Kumar, C. M. Hussain, G. K. Gaurav and K. S. Kumar, Chemosphere, 2021, 272, 129492 CrossRef CAS PubMed.
- X. Hu, X. Hu, Q. Peng, L. Zhou, X. Tan, L. Jiang, C. Tang, H. Wang, S. Liu and Y. Wang, Chem. Eng. J., 2020, 380, 122366 CrossRef CAS.
- E. O. Omotola, A. O. Oluwole, P. O. Oladoye and O. S. Olatunji, Environ. Toxicol. Pharmacol., 2022, 91, 103831 CrossRef CAS PubMed.
- T. L. Yusuf, O. C. Olatunde, D. Masekela, N. Mabuba, D. C. Onwudiwe and S. Makgato, Ceram. Int., 2024, 50, 45581–45591 CrossRef CAS.
- T. An, H. Yang, G. Li, W. Song, W. J. Cooper and X. Nie, Appl. Catal., B, 2010, 94, 288–294 CrossRef CAS.
- X. Nie, X. Wang, J. Chen, V. Zitko and T. An, Environ. Toxicol. Chem., 2008, 27, 168–173 CrossRef CAS PubMed.
- T. L. Yusuf, B. O. Ojo, T. Mushiana, N. Mabuba, O. A. Arotiba and S. Makgato, Catal. Sci. Technol., 2024, 14, 6015–6026 RSC.
- R. Nejat, Heliyon, 2024, 10(16), e35829 CrossRef CAS PubMed.
- T. L. Yusuf, O. C. Olatunde, D. Masekela, K. D. Modibane, D. C. Onwudiwe and S. Makgato, ChemElectroChem, 2024, 11, e202400309 CrossRef CAS.
- F. Chen, Q. Yang, X. Li, G. Zeng, D. Wang, C. Niu, J. Zhao, H. An, T. Xie and Y. Deng, Appl. Catal., B, 2017, 200, 330–342 CrossRef CAS.
- B. Y. Balarabe, P. Maity, A. C. S. Teixeira and S. A. Iwarere, Inorg. Chem. Commun., 2023, 158, 111560 CrossRef CAS.
- P. Alfonso-Muniozguren, E. A. Serna-Galvis, M. Bussemaker, R. A. Torres-Palma and J. Lee, Ultrason. Sonochem., 2021, 76, 105656 CrossRef CAS PubMed.
- S. K. Tammina, B. K. Mandal and F. N. Khan, Environ. Technol., 2019, 13, 197–210 Search PubMed.
- S. A. Bhat, F. Zafar, A. H. Mondal, A. Kareem, A. U. Mirza, S. Khan, A. Mohammad, Q. M. R. Haq and N. Nishat, J. Iran. Chem. Soc., 2020, 17, 215–227 CrossRef CAS.
- I. El Saliby, L. Erdei, J.-H. Kim and H. K. Shon, Water Res., 2013, 47, 4115–4125 CrossRef CAS PubMed.
- H. A. A. Jamjoum, K. Umar, R. Adnan, M. R. Razali and M. N. Mohamad Ibrahim, Front. Chem., 2021, 9, 752276 CrossRef CAS PubMed.
- D. Masekela, N. C. Hintsho-Mbita, L. N. Dlamini, T. L. Yusuf and N. Mabuba, Mater. Today Commun., 2024, 38, 108500 CrossRef CAS.
- J. Luo, K. Wang, Y. Qiu, X. Zhou, X. Ning, L. Zhan and X. Zhou, J. Alloys Compd., 2024, 1008, 176572 CrossRef CAS.
- L. Zhong, L. Chen, X. Xie, Z. Qin and T. Su, Catalysts, 2024, 14, 722 CrossRef CAS.
- T. L. Yusuf, S. A. Ogundare, F. Opoku, O. A. Arotiba and N. Mabuba, J. Environ. Chem. Eng., 2023, 11, 110711 CrossRef CAS.
- T. L. Yusuf, S. A. Ogundare, F. Opoku and N. Mabuba, Surf. Interfaces, 2023, 36, 102534 CrossRef CAS.
- O. Monfort and G. Plesch, Environ. Sci. Pollut. Res., 2018, 25, 19362–19379 CrossRef CAS PubMed.
- J. Silva, N. A. Neto, M. Teodoro, A. Paiva, M. Bomio and F. Motta, J. Alloys Compd., 2022, 928, 167136 CrossRef CAS.
- B. Shanthi, K. Saravanan, C. Ravichandran, B. Venkatachalapathy, K. Sathiyanarayanan, N. Karthikeyan and R. Suresh, Mater. Lett., 2023, 351, 135010 CrossRef CAS.
- A. Nagar and S. Basu, Environ. Technol., 2021, 23, 101646 CAS.
- A. Yousefi, A. Nezamzadeh-Ejhieh and M. Mirmohammadi, Environ. Technol., 2021, 22, 101496 CAS.
- A. Yousefi, A. Nezamzadeh-Ejhieh and M. Mirmohammadi, Environ. Technol., 2021, 22, 101433 CAS.
- M. Liang, Y. Yu, Y. Wang and Y. Yu, J. Hazard. Mater., 2020, 391, 121016 CrossRef CAS PubMed.
- K. Wangkawong, W. Lanla, V. Supphachalonepol, D. Channei and B. Inceesungvorn, Inorg. Chem. Commun., 2024, 112260 CrossRef CAS.
- Y. Lin, R. Hong, H. Chen, D. Zhang and J. Xu, J. Nanomater., 2020, 2020, 4147357 Search PubMed.
- O. H. Abuzeyad, A. M. El-Khawaga, H. Tantawy and M. A. Elsayed, J. Mol. Struct., 2023, 135787 CrossRef CAS.
- X. Yu, J. Shi, L. Feng, C. Li and L. Wang, Appl. Surf. Sci., 2017, 396, 1775–1782 CrossRef CAS.
- S. Phanichphant, A. Nakaruk, K. Chansaenpak and D. Channei, Sci. Rep., 2019, 9, 16091 CrossRef PubMed.
- M. Piao, Y. Sun, Y. Wang and H. Teng, ChemistrySelect, 2022, 7, e202200182 CrossRef CAS.
- J. Chen, B. Yao, C. Li and G. Shi, Carbon, 2013, 64, 225–229 CrossRef CAS.
- T. Mohlala, T. L. Yusuf and N. Mabuba, J. Electroanal. Chem., 2023, 947, 117806 CrossRef CAS.
- L. Yi, H. Jiang, Y. Ma, R. Zhu, G. Zhang and Z. Ren, Chemosphere, 2024, 354, 141658 CrossRef CAS PubMed.
- N. Rajalakshmi, D. Barathi, S. Meyvel and P. Sathya, Inorg. Chem. Commun., 2021, 132, 108849 CrossRef CAS.
- O. A. Oyewo, S. Ramaila and L. Mavuru, Inorg. Chem. Commun., 2023, 151, 110601 CrossRef CAS.
- S. Mansour, R. Akkari, S. Ben Chaabene and M. Saïd Zina, Adv. Mater. Sci. Eng., 2020, 2020, 6505301 CrossRef.
- O. Oyegbeda, S. O. Akpotu and B. Moodley, J. Environ. Chem. Eng., 2024, 113447 CrossRef CAS.
- R. Li, F. Zhang, D. Wang, J. Yang, M. Li, J. Zhu, X. Zhou, H. Han and C. Li, Nat. Commun., 2013, 4, 1432 CrossRef PubMed.
- C. V. Reddy, A. Nagar, N. P. Shetti, I. N. Reddy, S. Basu, J. Shim and R. R. Kakarla, Chemosphere, 2023, 322, 138146 CrossRef CAS PubMed.
- Y. Wang, J. Sun, J. Li and X. Zhao, Langmuir, 2017, 33, 4694–4701 CrossRef CAS PubMed.
- S. Chen, D. Huang, G. Zeng, W. Xue, L. Lei, P. Xu, R. Deng, J. Li and M. Cheng, Chem. Eng. J., 2020, 382, 122840 CrossRef CAS.
- R. Kanagadurai, R. Sankar, G. Sivanesan, S. Srinivasan, R. Rajasekaran and R. Jayavel, Mater. Chem. Phys., 2008, 108, 170–175 CrossRef CAS.
- A. Malathy, V. Manikandan, S. Devanesan, K. Farhat, A. Priyadharsan, C. Ragavendran, S. Ragupathy, R. Ranjith and S. Sivakumar, Int. J. Biol. Macromol., 2023, 244, 125303 CrossRef CAS PubMed.
- A. O. Oluwole and O. S. Olatunji, Chem. Eng. J. Adv., 2022, 12, 100417 CrossRef CAS.
- P. Madhusudan, J. Ran, J. Zhang, J. Yu and G. Liu, Appl. Catal., B, 2011, 110, 286–295 CrossRef CAS.
- B. O. Orimolade and O. A. Arotiba, Sci. Rep., 2020, 10, 5348 CrossRef CAS PubMed.
- L. Zhang, Z. Dai, G. Zheng, Z. Yao and J. Mu, RSC Adv., 2018, 8, 10654–10664 RSC.
- A. N. Zulkifili, A. Fujiki and S. Kimijima, Appl. Sci., 2018, 8, 216 CrossRef.
- H. Li, Y. Sun, B. Cai, S. Gan, D. Han, L. Niu and T. Wu, Appl. Catal., B, 2015, 170, 206–214 CrossRef.
- A. Raja, P. Rajasekaran, K. Selvakumar, M. Arunpandian, K. Kaviyarasu, S. A. Bahadur and M. Swaminathan, Sep. Purif. Technol., 2020, 233, 115996 CrossRef CAS.
- A. V. Karim and A. Shriwastav, Chem. Eng. J., 2020, 392, 124853 CrossRef CAS.
- E. Cristiano, Y.-J. Hu, M. Sigfried, D. Kaplan and H. Nitsche, Clays Clay Miner., 2011, 59, 107–115 CrossRef CAS.
- A. Hassani, A. Khataee and S. Karaca, J. Mol. Catal. A: Chem., 2015, 409, 149–161 CrossRef CAS.
- W. D. Santos, M. M. Teixeira, I. R. Campos, R. B. de Lima, A. Mantilla, J. A. Osajima, A. S. de Menezes, D. Manzani, A. Rojas and A. C. Alcântara, Microporous Mesoporous Mater., 2023, 359, 112657 CrossRef CAS.
- J. Liu, L. Zhang, Y. Sun and Y. Luo, Nanomaterials, 2021, 11, 1104 CrossRef CAS PubMed.
- M. Joy, B. N. Nair, A. A. P. Mohamed, K. G. Warrier and U. N. S. Hareesh, Eur. J. Inorg. Chem., 2016, 3912–3920 CrossRef CAS.
- R. A. Senthil, S. Osman, J. Pan, A. Khan, V. Yang, T. R. Kumar, Y. Sun, Y. Lin, X. Liu and A. Manikandan, Colloids Surf., A, 2020, 586, 124079 CrossRef CAS.
- A. O. Oluwole and O. S. Olatunji, J. Water Process Eng., 2023, 54, 104045 CrossRef.
- M. Pirhashemi, S. Elhag, R. E. Adam, A. Habibi-Yangjeh, X. Liu, M. Willander and O. Nur, RSC Adv., 2019, 9, 7992–8001 RSC.
- H. Zhang, H. Azimi, M. Mahmoudian, M. Ebadi, R. Moradi, A. Shirmardi and R. Yousefi, J. Environ. Manage., 2024, 370, 122734 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07144a |
| This journal is © The Royal Society of Chemistry 2024 |





