Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metal free C–O bond cleavage: a new strategy for the synthesis of substituted oxazoles†
Shengwang Lia,
Guiqin Liua,
Zheyan Zhanga,
Ruiling Chen*b,
Haiying Tian*b,
Huifeng Wang*a and
Xiuling Chen *a
*a
aHubei Key Laboratory of Radiation Chemistry and Functional Materials, Hubei University of Science and Technology, Xianning 437100, China. E-mail: cxl828800@163.com; Fax: (+)86-715-8338007
bSchool of Pharmacy, Changzhi Medical College, Changzhi, 046000, China
First published on 4th September 2024
Abstract
A strategy for the efficient metal-free C–O bond cleavage of ester using amines for the synthesis of substituted oxazoles was developed for the first time. The synthesis proceeded smoothly under metal-free conditions, combining C–O bond cleavage as well as C–N and C–O bond formation in one pot to yield desired products in moderate to excellent yields, and accommodated a wide range of functional groups and substrates.
Introduction
The cleavage of strong C–O bonds and the transformation of biomass into valuable compounds have attracted the attention of many researchers.1,2 Currently, C–O bond cleavage is mainly concentrated on phenols, ethers, and alcohols.3–5 However, there are relatively few reports on C–O bond cleavage of esters under metal-free conditions.6 Therefore, the development of a new strategy for C–O bond cleavage of esters is a primary research endeavor sought by many in modern chemical science. Metal-free-catalyzed reactions are among the most magnetic synthetic methods.7 With our continued interest in C–O bond cleavage,8 we aim to develop new methods for C–O cleavage and transformation under metal-free conditions.Heterocyclic compounds play a crucial role in drug discovery and medicinal chemistry owing to their unique structural properties and biological activities.9–12 Five-membered heterocyclic compounds containing oxygen and nitrogen atoms, such as substituted oxazoles, are prominent heterocyclic structures. Substituted oxazole is a prominent five-membered heterocyclic structure that widely exists in plenty of natural products, drugs, and biological compounds and exhibits activity against diabetes, Gram-positive and Gram-negative bacterial infections, breast cancer, and pancreatic cancer. For example, oxaprozin, a substituted oxazole, is widely used to treat rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, cervical spondylosis, and periarthritis of the shoulder.9–12 The synthesis of substituted oxazole derivatives has attracted increasing attention owing to the unique properties of the oxazole moiety. Recently, a variety of highly efficient methods have been reported for the synthesis of substituted oxazoles, such as cyclization of benzylamines with 1,2-dicarbonyls, 1,3-dicarbonyls, α-bromo ketones, aldehydes, alkenes, chalcones, ketones,13–19 and other moieties.20 Although multitudinous efficient methods have been developed to prepare substituted oxazoles, the substrate scope of amines is mainly limited to benzylamine. Recently, Li's group developed a novel method for the preparation of substituted oxazoles via CO2/photoredox-cocatalyzed tandem oxidative cyclization of α-bromo ketones and amines. Alkyl-substituted amines were also tolerated in this reaction. However, since CO2 gas and eosin Y were used in the reaction system, the photocatalyst suffered from poor stability and was easily destroyed by light and oxidants, thus remaining in drug molecules. Additionally, the reaction rate was slow and required a longer exposure to light. Hence, the exploration of more efficient methods for the preparation of substituted oxazoles is in continuous demand.
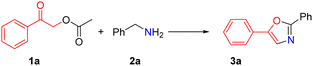
Herein, we developed a novel method for substituted oxazole synthesis through cyclization of substituted 2-oxo-2-phenylethyl acetate and amines via highly chemoselective C–O bond cleavage as well as C–N and C–O bond formation in one pot (eqn (1)). Compared with the existing methods, we expanded the substrate scope and developed a novel ester and alkyl amine as a substrate for the synthesis of substituted oxazole with moderate to good yields under metal-free conditions. This protocol offers an environmentally benign process and accommodates a broad substrate scope. Benzylamines bear electron-donating or electron-withdrawing groups and aliphatic amines to give the corresponding substituted oxazole compounds in good to excellent yields.
Results and discussion
2-Oxo-2-phenylethyl acetate (1a) and benzylamine (2a) served as model reactants for an initial optimization study. To optimize reaction conditions, our initial efforts focused on screening solvents in the presence of equivalent I2/K2CO3 systems (the results of the other bases are in Table S1†), and the results are summarized in Table 1. Use of various anhydrous solvents showed that the reaction does not occur in non-polar solvents such as 1,4-dioxane, toluene, chlorobenzene trace, 1,2-dichloroethane (Table 1, entries 1–4), and the reaction proceeds smoothly in polar solvents such as acetonitrile N,N-dimethyl formamide, dimethylsulfoxide, ethyl acetate (Table 1, entries 5–8). This implies that a relatively polar solvent is necessary to dissolve K2CO3 or a nucleophilic substitution reaction may be involved. The content of I2 and K2CO3 had a great influence on the reaction: in the absence of K2CO3 (0.4 mmol) and with 0.1 mmol of I2, compound 3a was not detected. Likewise, in the absence of I2 (0.4 mmol) and with 0.1 mmol of K2CO3, compound 3a was not detected (Table 1, entry 10). The best result was obtained by carrying out the reaction with 0.4 mmol K2CO3 and I2 (Table 1, entry 9). Moreover, 3a was not detected when K2CO3 and I2 were absent from the reaction system (Table 1, entries 11 and 12). Thus, both the presence and the amounts of I2 and K2CO3 are essential for the reaction. The present reaction also depends on temperature: product 3a was not detected when the reaction was carried out at room temperature (Table 1, entry 13), and only 35% yield of 3a was obtained at 50 °C (Table 1, entry 14). Upon raising the temperature to 110 °C, 3a with only 78% yield was obtained. This is probably because the high temperatures led to the side reactions of benzylamine (Table 1, entry 15). It was noteworthy that other bases such as Cs2CO3, KOH, and DBU were all ineffective (Table 1, entries 16–18). Thus, the optimized conditions for the synthesis of 3a can be defined as follows: substrate = 2-oxo-2-phenylethyl acetate (1a) (0.2 mmol), benzylamine (2a) (0.24 mmol), I2 and K2CO3 (0.4 mmol), and anhydrous ethyl acetate at 80 °C for 12 h.| Entry | Additive | Solvent | 3a%b |
|---|---|---|---|
| a Reaction conditions: 2-oxo-2-phenylethyl acetate (1a) (0.2 mmol), benzylamine (2a) (0.24 mmol), I2 (0.4 mmol), K2CO3 (0.4 mmol), solvent (2 mL), N2 in 25 mL Schlenk tube, 80 °C, 8 h.b Isolated yield.c 25 °C.d 50 °C.e 100 °C. | |||
| 1 | K2CO3/I2 | Dioxane | Trace |
| 2 | K2CO3/I2 | Toluene | Trace |
| 3 | K2CO3/I2 | Chlorobenzene | Trace |
| 4 | K2CO3/I2 | 1,2-Dichloroethane | Trace |
| 5 | K2CO3/I2 | Acetonitrile | 52% |
| 6 | K2CO3/I2 | N,N-dimethyl formamide | 61% |
| 7 | K2CO3/I2 | Dimethylsulfoxide | 44% |
| 8 | K2CO3/I2 | Ethyl acetate | 90% |
| 9 | K2CO3 (0.4)/I2 (0.2) | Ethyl acetate | Trace |
| K2CO3 (0.4)/I2 (0.3) | 32% | ||
| K2CO3 (0.4)/I2 (0.4) | 92% | ||
| 10 | K2CO3(0.2)/I2 (0.4) | Ethyl acetate | Trace |
| K2CO3 (0.3)/I2 (0.4) | 40% | ||
| K2CO3 (0.4)/I2 (0.4) | 92% | ||
| 11 | K2CO3 | Ethyl acetate | Trace |
| 12 | I2 | Ethyl acetate | Trace |
| 13c | K2CO3/I2 | Ethyl acetate | — |
| 14d | K2CO3/I2 | Ethyl acetate | 35% |
| 15e | K2CO3/I2 | Ethyl acetate | 78% |
| 16 | Cs2CO3/I2 | Ethyl acetate | 42% |
| 17 | KOH/I2 | Ethyl acetate | 31% |
| 18 | DBU/I2 | Ethyl acetate | Trace |
With the optimal reaction conditions established, we proceeded to investigate the substrate scope of the reaction by employing 2-oxo-2-phenylethyl acetate (1a) with a series of electronically diversified benzylamine and other primary amines (2a–2m), the results are shown in Table 2. It was found that benzylamine tolerates a wide range of functionalities, electron donating (CH3 and OCH3) and withdrawing groups (NO2, Br, and Cl), with 2-oxo-2-phenylethyl acetate to afford the corresponding substituted oxazoles (3a–3f). The substrate 2-naphthylmethylamine (2g) was also tolerated in the reaction condition, giving an excellent yield of 3g (85%). Moreover, 2h and 2i bearing a heterocycle moiety were employed as good substrates in the cyclization reaction with 1a, and substituted oxazoles 3h and 3i were isolated in 78% and 81% yields, respectively. In addition, no significant effect was observed for sterically demanding substrates 2j and 2k, which worked well in the reaction with 1a to give desired products 3j and 3k. We were delighted to find that aliphatic amines phenylethylamine and n-propylamine could be tolerated under our reaction conditions to give substituted oxazoles 3l and 3m in moderate yields. Thus, the present methodology shows a general applicability in the synthesis of substituted oxazoles.
The efficiency of this reaction was further investigated with substituted 2-oxo-2-phenylethyl acetate with benzylamine, as shown in Table 3. It was observed that 2-oxo-2-phenylethyl acetate with electron-rich and electron-deficient substituted groups could be cyclized by benzylamine (2a), giving the corresponding substituted oxazole products in moderate to good yields. For example, the reactions of 2-oxo-2-phenylethyl acetate with electron-rich groups (methyl and methoxy) at the para-position on the benzene ring proceeded well to give substituted oxazole products with a yield of 84–88%. Strong electron-deficient substituents such as -nitro, -trifluoromethyl, and -hydroxy could also react smoothly with benzylamine (2a) to give desired target products (3p–3r). It was found that reactions of halogen (F, Br, and Cl) substituted 2-oxo-2-phenylethyl acetate with 2a proceeded well and gave the desired oxazole derivatives 3s, 3t and 3u in 79%, 81% and 80% yields, respectively. Notably, a substituent at the ortho- or meta-position of the phenyl ring gave the corresponding product 3v and 3w with a yield of 81% and 74%, respectively. These results indicated that the steric effects did not affect the efficiency of the reactions. Interestingly, 2-cyclopropyl-2-oxoethyl acetate was also compatible with the present reaction system to give the desired oxazole product 3x in 69% yield, while alkyl-substituted bromoethyl ketones could not yield corresponding oxazole products in other studies,15d indicating that our reaction conditions have wide applicability.
 | (1) |
 | (2) |
 | (3) |
To get some information on the reaction mechanism, a control experiment was carried out as discussed below. When radical inhibitor 2,6-di-tert-butyl-4-methylphenol (BHT) was added to the reaction medium, 3a was not detected, which suggested that a free radical process was involved in the present reaction (eqn (1)). When 2-bromoacetophenone 1m replaced 2-oxo-2-phenylethyl acetate as the substrate, the trace product 3a was detected, suggesting that substituted bromoacetophenone is not an interim process in the present reaction system (eqn (2)). The 2-(benzylamino)-1-phenylethan-1-one 1n was used as a reactive material under the standard condition, 26% 3a was obtained proving that the reaction probably proceeded through imine intermediates (eqn (2)).
 | (4) |
To confirm the formation of by-products during the reaction, 2-oxo-2-phenylethyl 2-phenylacetate (1o) was used as a substrate. 3a was obtained in 88% yield (eqn (4)), and phenylacetic acid was detected in 81% yield, proving that C–O bond cleavage actually occurs in the reaction.
Based on the results described above and those of the previous reports, the possible pathway for the synthesis of substituted oxazoles is given in Scheme 1:15a,d K2CO3 promoted iodination to produce iodine intermediate 1aa; the SN2 reaction between 1aa and amines proceeded to produce the intermediate 1ab; and the decomposition of the alkyl carbonate via C–O bond cleavage led to the removal of an α-proton and the release of acetic acid to produce imine intermediate 1ac, followed by enolization of 1ac to obtain 1ad, intramolecular nucleophilic addition to produce 1ae, and iodine oxidation of 1ae to obtain substituted oxazole TM. Although 4 equiv. of acid was produced theoretically, 2 equiv. of K2CO3 was sufficient because its conjugated acid (HCO3−) was involved in the deprotonation process.
Conclusion
In summary, a strategy for the metal-free C–O bond cleavage of ester and functionalization for the synthesis of substituted oxazoles has been developed. C–O bond cleavage together with C–N and C–O bond formation are realized in one pot via iodine as the sole oxidant. The present findings not only provide a general and concise method for the preparation of substituted oxazoles but also open an avenue for the selective C–O bond cleavage of esters.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial supports by National Natural Science Foundation of China (22378102) and special fund for the Key Laboratory of Hubei Province (2022ZX04).References
- (a) J. Cornella, C. Zarate and R. Martin, Metal-catalyzed activation of ethers via C–O bond cleavage: a new strategy for molecular diversity, Chem. Soc. Rev., 2014, 43, 8081–8097 RSC; (b) B. Su, Z.-C. Cao and Z.-J. Shi, Exploration of earth-abundant transition metals (Fe, Co, and Ni) as catalysts in unreactive chemical bond activations, Acc. Chem. Res., 2015, 48, 886–896 CrossRef CAS PubMed; (c) E. J. Tollefson, L. E. Hanna and E. R. Jarvo, Stereospecific nickel-catalyzed cross-coupling reactions of benzylic ethers and esters, Acc. Chem. Res., 2015, 48, 2344–2353 CrossRef CAS PubMed.
- (a) T. Chen and L.-B. Han, Angew. Chem., Int. Ed., 2015, 54, 8600 CrossRef CAS PubMed; (b) H. Hikawa, Y. Matsuura, S. Kikkawa and I. Azumaya, Platinum(II)-catalyzed dehydrative C3-benzylation of electron-deficient indoles with benzyl alcohols, Org. Chem. Front., 2019, 6, 3150–3157 RSC.
- (a) J. Cornella, E. P. Jackson and R. Martin, Nickel-catalyzed enantioselective C–C bond formation through C–O cleavage in aryl esters, Angew. Chem., Int. Ed., 2015, 127, 4147–4150 CrossRef; (b) E. Koch, R. Takise, A. Studer, J. Yamaguchi and K. Itami, Ni-Catalyzed α-arylation of esters and amides with phenol derivatives, Chem. Commun., 2015, 51, 855 RSC; (c) T. Mukai, K. Hirano, T. Satoh and M. Miura, Palladium-catalyzed direct benzylation of azoles with benzyl carbonates, Org. Lett., 2010, 12, 1360–1363 CrossRef CAS PubMed; (d) J. Xiao, T. Chen and L.-B. Han, Nickel-catalyzed direct C–H/C–O cross couplings generating fluorobenzenes and heteroarenes, Org. Lett., 2015, 17, 812–815 CrossRef CAS PubMed.
- M. Tobisu and N. Chatani, Cross-couplings using aryl ethers via C–O bond activation enabled by nickel catalysts, Acc. Chem. Res., 2015, 48, 1717–1726 CrossRef CAS PubMed.
- Z. Qiu and C.-J. Li, Transformations of less-activated phenols and phenol derivatives via C–O cleavage, Chem. Rev., 2020, 120(18), 10454–10515 CrossRef CAS PubMed.
- For selected recent references using aryl esters as coupling partners, see: (a) R. Takise, K. Muto and J. Yamaguchi, Cross-coupling of aromatic esters and amides, Chem. Soc. Rev., 2017, 46, 5864–5888 RSC; (b) H. Zeng, Z. Qiu, A. Domínguez-Huerta, Z. Hearne, Z. Chen and C.-J. Li, An adventure in sustainable cross-coupling of phenols and derivatives via carbon–oxygen bond cleavage, ACS Catal., 2017, 7, 510–519 CrossRef CAS; (c) R. Takise, K. Muto, J. Yamaguchi and K. Itami, Nickel-catalyzed α-arylation of ketones with phenol derivatives, Angew. Chem., Int. Ed., 2014, 53, 6791 CrossRef CAS PubMed; (d) Y. Feng, Y. Wang, S. Zhao, D. Zhang, X. Li, H. Liu, Y. Dong and F. Sun, A practical ortho-acylation of aryl iodides enabled by moisture-insensitive activated esters via palladium/norbornene catalysis, Org. Chem. Front., 2020, 7, 3420–3426 RSC; (e) L. Meng, Y. Kamada, K. Muto, J. Yamaguchi and K. Itami, C–H alkenylation of azoles with enols and esters by nickel catalysis, Angew. Chem., Int. Ed., 2013, 52, 10048 CrossRef CAS PubMed; (f) Y. Song, X.-Y. Feng, J.-S. Chen, B. Carter, Z.-W. Xu and W.-B. Lin, Multistep engineering of synergistic catalysts in a metal–organic framework for tandem C–O bond cleavage, J. Am. Chem. Soc., 2020, 142, 4872–4882 CrossRef CAS PubMed; (g) H.-F. Chen, Y. Ye, W.-Q. Tong, J.-H. Fang and H.-G. Gong, Formation of allylated quaternary carbon centers via C–O/C–O bond fragmentation of oxalates and allyl carbonates, Chem. Commun., 2020, 56, 454–457 RSC.
- (a) A. Bhunia, S. R. Yetra and A. T. Biju, Recent advances in transition-metal-free carbon–carbon and carbon–heteroatom bond-forming reactions using arynes, Chem. Soc. Rev., 2012, 41, 3140–3152 RSC; (b) A. H. St Amant, C. P. Frazier, B. Newmeyer, K. R. Fruehauf and J. Read de Alaniz, Direct synthesis of anilines and nitrosobenzenes from phenols, Org. Biomol. Chem., 2016, 14, 5520–5524 RSC; (c) B. Das, N. Bhunia and M. Lingaiah, A simple and efficient metal-free synthesis of tetrasubstituted pyrroles by iodine-catalyzed four-component coupling reaction of aldehydes, amines, dialkyl acetylenedicarboxylates, and nitromethane, Synthesis, 2011, 21, 3471–3474 CrossRef.
- X. Chen, F. Ji, Y. Zhao, Y. Liu, Y. Zhou, T. Chen and S. F. Yin, Copper-catalyzed aerobic oxidative C (aryl)–OH bond functionalization of catechols with amines affording benzoxazoles, Adv. Synth. Catal., 2015, 357, 2924–2930 CrossRef CAS.
- (a) Z. Jin, Muscarine, imidazole, oxazole, and thiazole alkaloids, Nat. Prod. Rep., 2011, 28, 1143–1191 RSC; (b) V. S. C. Yeh, Muscarine, imidazole, oxazole, and thiazole alkaloids, Tetrahedron, 2004, 60, 11995–12042 CrossRef CAS; (c) P. Wipf, Synthetic studies of biologically active marine cyclopeptides, Chem. Rev., 1995, 95, 2115–2134 CrossRef CAS.
- I. J. Turchi and M. J. S. Dewar, Chemistry of Oxazoles, Chem. Rev., 1975, 75, 389–437 CrossRef CAS.
- S. Heng, K. R. Gryncel and E. R. Kantrowitz, A library of novel allosteric inhibitors against fructose 1,6-bisphosphatase, Bioorg. Med. Chem., 2009, 17, 3916–3922 CrossRef CAS PubMed.
- (a) A. Ibrar, I. Khan, N. Abbas, U. Farooqa and A. Khan, Transition-metal-free synthesis of oxazoles: valuable structural fragments in drug discovery, RSC Adv., 2016, 6, 93016–93047 RSC; (b) M. Elagawany, L. Maram and B. Elgendy, Synthesis of 3-aminoquinazolinones via a SnCl2-mediated ANRORC-like reductive rearrangement of 1,3,4-oxadiazoles, J. Org. Chem., 2023, 88, 17062–17068 CrossRef CAS PubMed.
- W. J. Xue, Q. Li, Y. P. Zhu, J. G. Wang and A. X. Wu, Convergent integration of two self-labor domino sequences: a novel method for the synthesis of oxazole derivatives from methyl ketones and benzoins, Chem. Commun., 2012, 48, 3485–3487 RSC.
- C. Wan, J. Zhang, S. Wang, J. Fan and Z. Wang, Facile synthesis of polysubstituted oxazoles via a copper-catalyzed tandem oxidative cyclization, Org. Lett., 2010, 12, 2338–2341 CrossRef CAS PubMed.
- (a) W. C. Gao, R. L. Wang and C. Zhang, Practical oxazole synthesis mediated by iodine from α-bromoketones and benzylamine derivatives, Org. Biomol. Chem., 2013, 11, 7123–7128 RSC; (b) C. Wan, L. Gao, Q. Wang, J. Zhang and Z. Wang, Simple and efficient preparation of 2,5-disubstituted oxazoles via a metal-free-catalyzed cascade cyclization, Org. Lett., 2010, 12, 3902–3905 CrossRef CAS PubMed; (c) T. Chatterjee, J. Y. Cho and E. J. Cho, Synthesis of substituted oxazoles by visible-light photocatalysis, J. Org. Chem., 2016, 81, 6995–7000 CrossRef CAS PubMed; (d) X. Zhang, Y. He, J. Li, R. Wang, L. Gu and G. Li, CO2/photoredox-cocatalyzed tandem oxidative cyclization of α-bromo ketones and amines to construct substituted oxazoles, J. Org. Chem., 2019, 84, 8225–8231 CrossRef CAS PubMed.
- (a) Z. Xu, C. Zhang and N. Jiao, Synthesis of oxazoles through copper-mediated aerobic oxidative dehydrogenative annulation and oxygenation of aldehydes and amines, Angew. Chem., Int. Ed., 2012, 51, 11367–11370 CrossRef CAS PubMed; (b) Y. F. Wang, H. Chen, X. Zhu and S. Chiba, Copper-catalyzed aerobic aliphatic C–H oxygenation directed by an amidine moiety, J. Am. Chem. Soc., 2012, 134, 11980–11983 CrossRef CAS PubMed.
- K. K. Toh, A. Biswas, Y. F. Wang, Y. Y. Tan and S. Chiba, Copper-mediated oxidative transformation of N-allyl enamine carboxylates toward synthesis of azaheterocycles, J. Am. Chem. Soc., 2014, 136, 6011–6020 CrossRef CAS PubMed.
- H. Jiang, H. Huang, H. Cao and C. Qi, TBHP/I2-mediated domino oxidative cyclization for one-pot synthesis of polysubstituted oxazoles, Org. Lett., 2010, 12, 5561–5563 CrossRef CAS PubMed.
- Z.-Q. Wang, W.-W. Zhang, L.-B. Gong, R.-Y. Tang, X.-H. Yang, Y. Liu and J.-H. Li, Copper-Catalyzed Intramolecular Oxidative 6-exo-trig Cyclization of 1,6-Enynes with H2O and O2, Angew. Chem., Int. Ed., 2011, 50, 8968–8973 CrossRef CAS PubMed.
- (a) S. Wu, F. Geng, J. Dong, L. Liu, L. Su and Y. Zhou, General and practical synthesis of naphtho[2,1-d]oxazoles from naphthols and amines, Org. Chem. Front., 2022, 9, 3828–3833 RSC; (b) J. Reddy, M. P. Ball-Jones and Dr P. W. Davies, Alkynyl thioethers in gold-catalyzed annulations to form oxazoles, Angew. Chem., Int. Ed., 2017, 56, 13310–13313 CrossRef PubMed; (c) T.-T. Zeng, J. Xuan, W. Ding, K. Wang, L.-Q. Lu and W.-J. Xiao, [3+2] cycloaddition/oxidative aromatization sequence via photoredox catalysis: one-pot synthesis of oxazoles from 2H-azirines and aldehydes, Org. Lett., 2015, 17(16), 4070–4073 CrossRef CAS PubMed; (d) U. D. Newar, S. Borra and R. A. Maurya, Visible-light 2,4-dinitrophenol-mediated photoannulation of α-azidochalcones into 2,5-diaryloxazoles, Org. Lett., 2022, 24, 4454–4458 CrossRef CAS PubMed; (e) M.-R. Li, Z.-Q. He, W. Zhao, Y. Yu, F. Huang and J. B. Baell, Photocatalytic benzylic C–H oxidation/cyclization of enaminones to the synthesis of polysubstituted oxazoles, J. Org. Chem., 2023, 88(13), 8257–8267 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra05122j |
| This journal is © The Royal Society of Chemistry 2024 |