Open Access Article
Open Access ArticleTpBD/UiO-66-NH2 micro–mesoporous hybrid material as a stationary phase for open tubular capillary electrochromatography†
Lidi Gaoab,
Xinran Qua,
Shuang Menga,
Mo Chena,
Yuxin Hea,
Fuquan Zhaoa,
Hongtao Chuab,
Shili Qin *ab and
Fenglong Jin*c
*ab and
Fenglong Jin*c
aCollege of Chemistry and Chemical Engineering, Qiqihar University, Qiqihar 161006, China. E-mail: qinshili1103@163.com
bHeilongjiang Industrial Hemp Processing Technology Innovation Center, Qiqihar University, Qiqihar 161006, China
cTechnology Innovation Center of Industrial Hemp for State Market Regulation, Qiqihar University, Qiqihar 161006, China
First published on 3rd September 2024
Abstract
The excellent stability of covalent organic frameworks (COFs) and the diversity of metal organic frameworks (MOFs) make MOF/COF hybrid materials promising candidates for chromatographic stationary phases. In this paper, a TpBD/UiO-66-NH2 hybrid material was synthesized through a Schiff-base reaction between TpBD COFs and UiO-66-NH2 MOFs; characterized using Fourier-transform infrared spectroscopy, X-ray diffraction, and X-ray photoelectron spectroscopy; and bonded to a capillary to prepare a TpBD/UiO-66-NH2-bonded open tubular capillary electrochromatography (OT-CEC) column. Results suggested that the hybrid material had the crystal morphology of a single COF and MOF, a micro–mesoporous structure, and good thermal stability. The inner surface of the OT-CEC column was tightly and uniformly distributed with the stationary phase (∼1.5 μm). The baseline separation of 13 amino acids and three families (4 acidic antibiotics, 4 preservatives and 6 sulfonamides) of emerging pollutant mixtures was achieved due to the synergistic effect of TpBD and UiO-66-NH2 in the stationary phase. The OT-CEC column showed good reproducibility and stability with relative standard deviations of migration time and resolutions in the range of 1.17–3.93% and 1.79–4.31%, respectively.
1 Introduction
Separation of many complex components with similar structures or properties in a sample is one of the frontiers of analytical chemistry.1 The abuse of emerging pollutants such as antibiotics and preservatives has caused a large number of pollutants to be released into the environment, which poses a threat to human beings and various other organisms.2,3 At present, although there are many methods for detecting emerging pollutants, including enzyme-linked immunosorbent assay (ELISA), gas chromatography-mass spectrometry (GC-MS), high performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), capillary electrophoresis (CE) and capillary electrochromatography (CEC),4–6 it is rare and challenging to establish a simple, rapid and universal simultaneous separation method for complex emerging pollutants.CEC has a stationary phase to retain and distribute analytes and an electroosmotic flow (EOF) driven by an electric field, resulting in high selectivity, high efficiency and low sample consumption,7 and its application is becoming increasingly widespread in the domains of separation and analysis. In particular, open tubular CEC (OT-CEC) has been widely employed due to its advantages of simple preparation, low back pressure and no bubble generation. However, disadvantages of a low phase ratio and low column capacity hinder its wide application.8,9 Therefore, the development of new porous OT-CEC stationary phases with good stability and many active sites becomes an optimal solution.
Porous organic framework materials, such as covalent organic frameworks (COFs) or metal organic frameworks (MOFs), have been used as stationary phases for OT-CEC and demonstrate good separation performance due to their large specific surface area, rich and adjustable pore environments and many active sites from organic ligands and/or unsaturated metal centers.8,9 However, it is not easy to select suitable MOFs to construct the stationary phase of OT-CEC because most MOFs will collapse in aqueous solution or upon contact with certain solvents.10 Although a few MOFs exhibit adequate stability, their active sites are insufficient for the effective separation of complex systems.11 In contrast, COFs themselves generally supply enough active sites such as –OH, –CHO, and –COOH or through easy surface modification, and their stability is strong, even stronger than that of some MOFs with higher stability.12 However, the crystallinity of COFs is somewhat inferior to that of MOFs.
Therefore, the crystal structure of COFs should be improved to enhance the stability of MOFs via the construction of MOF/COF hybrid material and obtain various excellent performances (Fig. 1).13,14 In 2016, Fu et al.15 first reported that ZIF-8 MOF was grown on COF-300 to prepare hybrid material membranes, which demonstrated higher separation selectivity for H2/CO2 than the original individual MOF or COF membranes. Wang's research group16,17 studied cotton fiber and corncob functionalized by UiO-66-NH2 MOF@TpBD COF as extractant and adsorbent for the removal of bisphenols from water samples and enhancing the adsorption of sulfonamides and its metabolites, respectively. Both showed good adsorption capacity. Recently, Pang et al.18 developed the core–shell MOF@COF (NH2-UiO-66@TpBD-COF) hybrids. The hybrids showed superior catalytic performance in the visible light-driven oxidative coupling of amines to imines in air. However, to date, there have been no reports on the use of MOF/COF hybrid materials as a stationary phase for CEC.
TpBD COF, as an imine COF, has the excellent characteristics of good chemical stability, easy modification, –NH2, –OH, –CHO active groups as well as good separation performance.19,20 The high specific surface area, excellent crystallization performance, good porosity and unsaturated metal sites of UiO-66-NH2 play important roles in adsorption and separation.16–18,21 The TpBD/UiO-66-NH2 hybrid material will demonstrate excellent separation performance and wide application range as the stationary phase due to their synergistic effect, such as more active sites, high specific surface area and hierarchical pore structure in CEC. In this paper, TpBD/UiO-66-NH2 hybrid material was synthesized through the Schiff-base reaction between TpBD COF and UiO-66-NH2 MOF. TpBD/UiO-66-NH2-bonded OT-CEC column was prepared using TpBD/UiO-66-NH2 hybrid material as the stationary phase for the first time and was applied for the simultaneous separation of 13 amino acids and three families (4 acidic antibiotics, 4 neutral preservatives and 6 amphoteric sulfonamides) of emerging pollutant mixtures.
2 Materials and methods
2.1 Chemicals and materials
Phloroglucinol (Tp), benzidine (BD), 3-aminopropyl-triethoxysilane (APTES) and glutaraldehyde (50%) (v/v) were purchased from Aladdin Industrial Co. (Shanghai, China). 50% glutaraldehyde was diluted with ultrapure water (18.2 MΩ cm) and adjusted by 1 mol L−1 NaOH solution to obtain a glutaraldehyde solution (2%) (v/v) with pH = 11. Anhydrous zirconium chloride (ZrCl4) and 2-aminoterephthalic acid (BDC-NH2) were obtained from Beijing InnoChem Science & Technology Co., Ltd (Beijing, China). Glacial acetic acid, thiourea, benzene, toluene and o-xylene were purchased from Tianjin Kemiou Reagent Co., Ltd (Tianjin, China). Amino acids (glutamic acid (Glu), aspartic acid (Asp), serine (Ser), alanine (Ala), valine (Val), threonine (Thr), isoleucine (Ile), leucine (Leu), methionine (Met), tryptophan (Trp) and phenylalanine (Phe), arginine (Arg) and histidine (His)) were obtained from Sinopharm Chemical Reagent Ltd (Shanghai, China). Preservatives (methyl-4-hydroxybenzoate (MHB), ethyl-4-hydroxybenzoate (EHB), propyl-4-hydroxybenzoate (PHB), butyl-4-hydroxybenzoate (BHB)) were ordered from Jiuding Chemical Co. (Shanghai, China). Sulfonamides (sulfamerazine (SM1), sulfadimethoxine (SDM), sulfamethazine (SM2), sulfamethoxazole (SMZ), sulfisoxazole (SIZ), sulfathiazole (ST)) and acidic antibiotics (metronidazole (Mnz), chloramphenicol (Cpl), tetracycline hydrochloride (Tet), chlortetracycline hydrochloride (Ctc)) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China) (Fig. S1†). The solvents used in CEC were chromatographic grade. Fused silica capillaries (75 μm i.d. and 375 μm o.d.) were from Yongnian Fiber Factory (Handan, China).2.2 Instruments
All CEC separations were conducted on a CE system equipped with a diode array detector (Agilent 7100, Waldbronn, Germany). The surface morphology and elemental distribution were done by an S-4300 scanning electron microscope (SEM) equipped with an energy-dispersive X-ray spectrometer (EDS) (Hitachi, Japan). The morphology of TpBD/UiO-66-NH2 was also observed by an H-7650 transmission electron microscope (TEM) (Hitachi, Japan). X-ray photoelectron spectroscopy (XPS) characterization was performed using an ESCALAB 250 photoelectron spectrometer (ThermoFisher Scientific, USA). Fourier-transform infrared (FT-IR) spectra were obtained by an FT-IR spectrometer (Spectra PE Co, USA). X-ray diffraction (XRD) spectra were collected with a Bruker D8 diffraction instrument (Karlsruhe, Germany). Nitrogen adsorption/desorption isotherms were examined on a surface area analyzer (Micromeritics ASAP, USA). Thermogravimetric (TG) measurement was performed using an STA 449F5 (Netzsch, Germany) device. The zeta potential was measured with a Nano ZS 90 zeta potential analyzer (Malvern, UK).2.3 Preparation of TpBD/UiO-66-NH2-bonded OT-CEC column
The TpBD/UiO-66-NH2-bonded OT-CEC column was obtained by modifying UiO-66-NH2 to the TpBD-bonded OT-CEC column. The column preparation followed a four-step process: (i) pretreatment of the capillary column, (ii) activation of aldehyde groups, (iii) linkage of TpBD according to our previous report,20 as well as (iv) modification of UiO-66-NH2. In the last step, 38.8 mg UiO-66-NH2 was dispersed in 4 mL DMF, then the suspension was filled into the TpBD-bonded OT-CEC column, which was maintained in a water bath at 80 °C for 24 h by sealing both the ends. The modification procedure was repeated two times (Fig. 2). The OT-CEC column was flush with DMF and methanol to remove the unreacted substances in the capillary. The TpBD/UiO-66-NH2-bonded OT-CEC columns were obtained.2.4 Synthesis of TpBD/UiO-66-NH2
TpBD/UiO-66-NH2 was synthesized by the solvothermal method for FT-IR, XRD, SEM, EDS, XPS, TEM, TG, nitrogen adsorption/desorption and zeta-potential characterization or determination as the stationary phase of the TpBD/UiO-66-NH2-bonded OT-CEC column. TpBD and UiO-66-NH2 were synthesized according to the previous reports with minor modification, and their hybridization synthesis is represented in Fig. S2.†21,22 27.8 mg TpBD was dissolved in 20 mL DMF, in which 38.8 mg UiO-66-NH2 was added. The mixture was refluxed for 6 h at 80 °C under nitrogen atmosphere.22,23 The resultant product was centrifuged, after which the clear supernatant was decanted. Subsequently, the precipitate, TpBD/UiO-66-NH2, was sequentially washed three times with DMF and methanol and finally dried at 60 °C for 12 h in vacuum.2.5 CEC conditions
The total length of the as-prepared TpBD/UiO-66-NH2-bonded OT-CEC column was 40.0 cm, and the effective length was 26.5 cm. The ammonium acetate buffer with a pH range of 6.0 to 10.0 was chosen for detection. All the sample solutions were filtered by a 0.45 μm filter membrane. The injection pressure was 50 mbar × 3 s, and the instrument was operated at room temperature (no more than 25 °C).3 Results and discussion
3.1 Preparation conditions for the TpBD/UiO-66-NH2-bonded OT-CEC column
To achieve the strong synergistic effect and optimal separation performance, it was significantly important to obtain the optimal bonding amount of UiO-66-NH2 in the stationary phase under the condition of maintaining the crystallinity of TpBD and UiO-66-NH2. Based on our previous study,20 the main influence factors of UiO-66-NH2 bonded to TpBD-bonded OT-CEC column were investigated including reaction temperature, reaction time and UiO-66-NH2 dosage by the orthogonal test (Table 1). Column efficiency was used as the evaluation index with benzene as the analyte.| Experiment no. | Factors | Column efficiency (plates per m) | ||
|---|---|---|---|---|
| Reaction temperature (°C) | Reaction time (h) | UiO-66-NH2 dosage (mg) | ||
| A | B | C | ||
| 1 | 60 | 12 | 12.9 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 904 904 |
| 2 | 60 | 24 | 25.9 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 560 |
| 3 | 60 | 48 | 38.8 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 639 639 |
| 4 | 80 | 12 | 25.9 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 779 779 |
| 5 | 80 | 24 | 38.8 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 745 745 |
| 6 | 80 | 48 | 12.9 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 187 187 |
| 7 | 100 | 12 | 38.8 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 401 401 |
| 8 | 100 | 24 | 12.9 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190 190 |
| 9 | 100 | 48 | 25.9 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 611 611 |
| T1j | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 103 103 |
91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 084 084 |
83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 291 291 |
|
| T2j | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 711 711 |
96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706 706 |
91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
|
| T3j | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 202 202 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 437 437 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 785 785 |
|
| Rj | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 608 608 |
6269 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 494 494 |
|
The data showed that the UiO-66-NH2 dosage, reaction temperature and reaction time all had a significant effect on the column efficiency, and the influence intensity was in the order UiO-66-NH2 dosage > reaction temperature > reaction time (RC > RA > RB). The maximum column efficiency (37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 745 plates per m) was achieved under the conditions of reacting for 24 h at 80 °C with 38.8 mg UiO-66-NH2 (experiment no. 5). Meanwhile, the stationary phases of the MOF/COF hybrid materials containing three different dosages of UiO-66-NH2 were characterized by XRD. As shown in Fig. 3, the hybrid materials contained a strong characteristic diffraction peak of TpBD COF at 3.3°, indicating the existence of TpBD COF with its original crystal structure in the hybrid materials.24 The diffraction peaks at 7.36° and 8.50° proved that UiO-66-NH2 was successfully introduced into the hybrid materials.25 However, the position of the diffraction peaks in the hybrid materials, such as 25.68° and 30.66°, slightly changed compared with that of UiO-66-NH2, and their intensity and sharpness all reduced, probably because the bonding of TpBD and UiO-66-NH2 led to their poor crystallinity. Furthermore, the diffraction peaks of UiO-66-NH2 in the hybrid materials became strong with the increasing UiO-66-NH2 dosage. However, when exceeding 38.8 mg UiO-66-NH2, the viscosity of the suspension was too large to be poured into the capillary column. Therefore, the optimal preparation conditions selected were same as that of experiment no. 5.
745 plates per m) was achieved under the conditions of reacting for 24 h at 80 °C with 38.8 mg UiO-66-NH2 (experiment no. 5). Meanwhile, the stationary phases of the MOF/COF hybrid materials containing three different dosages of UiO-66-NH2 were characterized by XRD. As shown in Fig. 3, the hybrid materials contained a strong characteristic diffraction peak of TpBD COF at 3.3°, indicating the existence of TpBD COF with its original crystal structure in the hybrid materials.24 The diffraction peaks at 7.36° and 8.50° proved that UiO-66-NH2 was successfully introduced into the hybrid materials.25 However, the position of the diffraction peaks in the hybrid materials, such as 25.68° and 30.66°, slightly changed compared with that of UiO-66-NH2, and their intensity and sharpness all reduced, probably because the bonding of TpBD and UiO-66-NH2 led to their poor crystallinity. Furthermore, the diffraction peaks of UiO-66-NH2 in the hybrid materials became strong with the increasing UiO-66-NH2 dosage. However, when exceeding 38.8 mg UiO-66-NH2, the viscosity of the suspension was too large to be poured into the capillary column. Therefore, the optimal preparation conditions selected were same as that of experiment no. 5.
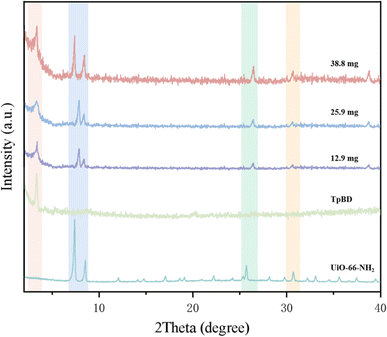 | ||
| Fig. 3 XRD spectra of TpBD/UiO-66-NH2 (experimental conditions: TpBD dosage, 27.8 mg; reaction temperature, 80 °C; reaction time, 24 h), TpBD and UiO-66-NH2. | ||
3.2 Characterization of TpBD/UiO-66-NH2
Fig. 4 shows the FT-IR spectra of TpBD, UiO-66-NH2 and TpBD/UiO-66-NH2. TpBD/UiO-66-NH2 contained almost all of the characteristic absorption peaks from UiO-66-NH2 and TpBD. The Zr–O–Zr bending vibration peak was at 764 cm−1. The N–H symmetric and antisymmetric stretching vibration peaks in the amino groups were at 3364 cm−1 and 3452 cm−1 corresponding to the peaks at 3361 cm−1 and 3453 cm−1 of UiO-66-NH2. The C–N stretching vibration bands in the range of 1260–1280 cm−1 corresponded to the bands at 1250 cm−1 of UiO-66-NH2 and 1283 cm−1 of TpBD. The symmetric and antisymmetric stretching vibration peaks of carboxyl groups in the BDC-NH2 ligand appeared at 1390 cm−1 and 1450 cm−1, which were red-shifted from the peaks at 1380 cm−1 and 1430 cm−1 for UiO-66-NH2.26,27 In addition, the wider absorption band in the range of 1580–1650 cm−1 indicated the overlapping vibration peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of the benzene ring skeleton in TpBD and the reaction between –COOH in the ligand and Zr4+ in UiO-66-NH2.28 Although most of the absorption displacements were slightly changed compared with TpBD and UiO-66-NH2, they confirmed the successful synthesis of the TpBD/UiO-66-NH2 hybrid material.
C of the benzene ring skeleton in TpBD and the reaction between –COOH in the ligand and Zr4+ in UiO-66-NH2.28 Although most of the absorption displacements were slightly changed compared with TpBD and UiO-66-NH2, they confirmed the successful synthesis of the TpBD/UiO-66-NH2 hybrid material.
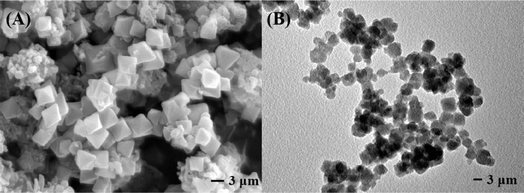
The stacking and overlapping of spherical TpBD and octahedral UiO-66-NH2 are observed in Fig. 5A and B. The chemical composition of TpBD/UiO-66-NH2 was determined by EDS elemental mapping (Fig. S3A–D).† The results showed that there were uniformly distributed O, C, N and Zr elements on the hybrid material, which further proved that the interaction between TpBD and UiO-66-NH2 was chemical bonding.
The elemental composition and chemical state of TpBD/UiO-66-NH2 were analyzed by XPS (Fig. 6). The full-scan spectrum of TpBD/UiO-66-NH2 indicated the presence of carbon (C), nitrogen (N), oxygen (O) and zirconium (Zr) in TpBD/UiO-66-NH2 (Fig. 6A), which was in line with the EDS elemental mapping results. The high-resolution spectra of the deconvoluted C 1s and N 1s (Fig. 6B and C) displayed the characteristic energy peaks of TpBD29 and that of deconvoluted Zr 3d as proof of the existence of UiO-66-NH2 in TpBD/UiO-66-NH2 was shown in Fig. 6D.17
 | ||
| Fig. 6 XPS curves (A), high resolution C 1s (B), N 1s (C) and Zr 3d (D) XPS spectra of TpBD, UiO-66-NH2 and TpBD/UiO-66-NH2. | ||
The C 1s spectrum of TpBD/UiO-66-NH2 was divided into five peaks corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (287.19 eV), C
O (287.19 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (285.59 eV), C–N (284.80 eV), C
N (285.59 eV), C–N (284.80 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (283.93 eV) and C–C (283.18 eV), respectively. Among them, the binding energy of C
C (283.93 eV) and C–C (283.18 eV), respectively. Among them, the binding energy of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N of the hybrid material was not much different from that in TpBD because the C
N of the hybrid material was not much different from that in TpBD because the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N of both was produced by the Schiff base reaction. The positive binding energy shift of C
N of both was produced by the Schiff base reaction. The positive binding energy shift of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (+0.74 eV) was due to the introduction of additional C
O (+0.74 eV) was due to the introduction of additional C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of carboxyl groups from UiO-66-NH2.
O of carboxyl groups from UiO-66-NH2.
The N 1s spectrum of TpBD/UiO-66-NH2 was composed of two peaks, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and N–C. The binding energy of N–C (397.91 eV) was obviously lowered by 1.61 eV and 0.37 eV compared with those of TpBD (399.52 eV) and UiO-66-NH2 (398.28 eV), which suggested that there was chemical bonding between the aldehyde groups from Tp in TpBD and the amino groups in UiO-66-NH2.30 The results further indicated that UiO-66-NH2 was successfully bonded to TpBD. In addition, the bonding ratio of each TpBD six-membered ring to the UiO-66-NH2 octahedral unit was about 5
C and N–C. The binding energy of N–C (397.91 eV) was obviously lowered by 1.61 eV and 0.37 eV compared with those of TpBD (399.52 eV) and UiO-66-NH2 (398.28 eV), which suggested that there was chemical bonding between the aldehyde groups from Tp in TpBD and the amino groups in UiO-66-NH2.30 The results further indicated that UiO-66-NH2 was successfully bonded to TpBD. In addition, the bonding ratio of each TpBD six-membered ring to the UiO-66-NH2 octahedral unit was about 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in TpBD/UiO-66-NH2, according to the calculation of the XPS data (Table S1†).31
1 in TpBD/UiO-66-NH2, according to the calculation of the XPS data (Table S1†).31
TpBD/UiO-66-NH2 had a large specific surface area (340.04 m2 g−1) and exhibited structural characteristics of the micropores and mesopores (the main pore size of 1.18–2.65 nm) due to the hybrid formation of TpBD with mesopores and UiO-66-NH2 with micropores32,33 (Fig. 7), which could provide more active sites for the small molecular analytes. The specific surface area and average pore volume of TpBD/UiO-66-NH2 were smaller than those of TpBD or UiO-66-NH2, indicating that partly intersected pore channels might exist (Table S2†).
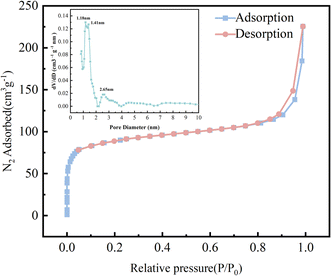 | ||
| Fig. 7 N2 adsorption–desorption isotherms of TpBD/UiO-66-NH2 at 77.3 K ((inset): pore size distribution). | ||
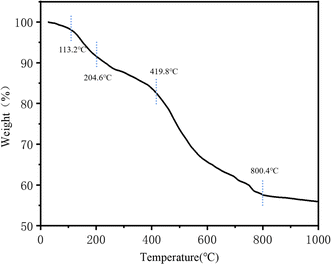
The thermal stability of TpBD/UiO-66-NH2 was evaluated by the TGA curve (Fig. 8). When the temperature was raised to 113.2–204.6 °C, the weight loss was due to the presence of solvents (water, DMF and methanol) adsorbed on the surface of the material. The weight loss at 204.6–419.8 °C was mainly from the dehydroxylation of Zr6 clusters in the hybrid material.34 The rapid decomposition of various ligands of TpBD/UiO-66-NH2 contributed to the weight loss after 419.8 °C.34,35 Only ZrO2 was left when the hybrid material was heated to 800.4 °C. The results showed that the hybrid material had good thermal stability and could be used as the stationary phase of the OT-CEC column.
Zeta potential can reflect the surface charge of TpBD/UiO-66-NH2, which is very important for analyzing the separation mechanism. The change in the zeta potential with pH values indicated that the surface of TpBD/UiO-66-NH2 was negatively charged (Fig. S4†). Although a small amount of amino groups existed in the form of NH3+ under acidic conditions, the dissociation of the large amount of Zr–OH into Zr–O− made the surface of TpBD/UiO-66-NH2 negatively charged. There were more negative charges with increasing pH, which was mainly attributed to the ionization of carboxyl groups from UiO-66-NH2 and phenolic hydroxyl groups from the TpBD of TpBD/UiO-66-NH2 in sequence.
3.3 Characterization of TpBD/UiO-66-NH2-bonded OT-CEC column
Compared with the bare capillary column (Fig. 9A), it was observed that the stationary phase (∼1.5 μm) was tightly and uniformly distributed in the TpBD/UiO-66-NH2-bonded OT-CEC column (Fig. 9B and C), which proved that TpBD/UiO-66-NH2 was successfully bonded to the inner wall of the capillary. Spherical-like TpBD, octahedral UiO-66-NH2 as well as their overlapping were clearly visible (Fig. 9D); however, their size was smaller than that of the TpBD/UiO-66-NH2 hybrid material (Fig. 5), likely due to the microenvironment in the capillary. It was anticipated that these hybrid crystal structures would effectively improve the separation performance of the OT-CEC column. | ||
| Fig. 9 SEM images of the cross section of the bare capillary column (A) and the TpBD/UiO-66-NH2-bonded OT-CEC column (B–D). | ||
3.4 EOF
EOF is the essential driving force for the separation process of the CEC method. The EOF of the TpBD/UiO-66-NH2-bonded OT-CEC column was investigated in the buffer pH range of 4.0–10.0 using thiourea as a neutral marker (Fig. S5†). EOF increased with the increase in the pH values, maintaining the same direction as that observed in the bare capillary column, which were due to the negative surface charges of the stationary phase in the TpBD/UiO-66-NH2-bonded OT-CEC column. However, the EOF of the TpBD/UiO-66-NH2-bonded OT-CEC column was smaller than that of the bare capillary column at the same pH. The reason may be that the dissociation of Zr–OH, carboxyl groups and phenolic hydroxyl groups of EOF produced in the bonded column was weaker than that of the silanol groups in the bare capillary column. However, the appropriate decrease in the EOF can increase the retention time of the analyte in the column and enhance the interaction between the analyte and the stationary phase to improve the separation efficiency.3.5 Reproducibility and stability
The reproducibility and stability of the TpBD/UiO-66-NH2-bonded OT-CEC column were evaluated using MHB, EHB, PHB, and BHB as the model analytes. Fig. S6† shows the chromatograms of MHB, EHB, PHB, and BHB after 1, 100 and 200 runs. It can be seen that there was no significant change in the migration time and peak shape after 200 runs. The relative standard deviations (RSDs) of the migration time for intra-day, inter-day and inter-column were 1.17–3.93%, and those of their resolutions were 1.79–4.31%, respectively (Table S3†). The results showed that the bonded OT-CEC column had good stability and satisfactory reproducibility.3.6 Separation performance of the TpBD/UiO-66-NH2-bonded OT-CEC column
The separation performance of the TpBD/UiO-66-NH2-bonded OT-CEC column was investigated using 13 amino acids as template analytes, including 2 acidic (Glu and Asp), 9 neutral (Ser, Ala, Val, Thr, Ile, Leu, Met, Trp and Phe) and 2 basic (Arg and His) amino acids. Under the optimal separation conditions (Fig. S7A–C†), the simultaneous baseline separation of 13 amino acids was achieved within 8.1 min with Rs = 1.63–10.35 and separation factor (α) = 1.06–1.35 (Fig. 10A and Table 2).| Analytes | pI or pKa (pKa1/pKa2) | Molecular volumea (Å3) | Property | Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
Molecular mass | Optimal pH | Rs | α |
|---|---|---|---|---|---|---|---|---|
| a Molecular volume (Å3) from Chemdraw 3D 20.0. | ||||||||
| Arg | 10.76 (2.17/9.67) | 5.7 × 7.5 × 10.3 | Basic polar | −4.50 | 174.20 | 8 | — | — |
| His | 7.59 (2.49/9.04) | 5.7 × 7.0 × 6.9 | −3.20 | 155.16 | 4.11 | 1.09 | ||
| Ser | 5.68 (1.14/9.43) | 4.9 × 6.4 × 7.5 | Neutral polar | −0.80 | 105.09 | 5.77 | 1.35 | |
| Thr | 6.16 (2.19/9.36) | 5.3 × 6.0 × 7.6 | −0.70 | 119.12 | 3.41 | 1.21 | ||
| Ala | 6.00 (2.19/9.67) | 6.9 × 5.7 × 5.1 | Neutral nonpolar | 1.80 | 89.09 | 4.97 | 1.32 | |
| Val | 5.96 (2.32/9.61) | 7.5 × 6.3 × 5.6 | 4.20 | 117.15 | 3.41 | 1.28 | ||
| Ile | 6.02 (2.41/10.22) | 9.1 × 6.3 × 5.8 | 4.50 | 131.17 | 6.71 | 1.21 | ||
| Leu | 5.98 (2.28/9.82) | 9.2 × 6.9 × 5.3 | 3.80 | 131.18 | 4.85 | 1.12 | ||
| Met | 5.74 (2.25/10.06) | 5.0 × 7.1 × 10.8 | 1.90 | 149.21 | 3.79 | 1.11 | ||
| Trp | 5.89 (2.09/10.01) | 13.0 × 7.9 × 4.7 | −0.90 | 204.23 | 2.14 | 1.06 | ||
| Phe | 5.48 (2.09/10.43) | 11.3 × 6.9 × 5.3 | 2.80 | 165.19 | 1.63 | 1.09 | ||
| Glu | 3.22 (2.58/9.24) | 5.4 × 7.1 × 10.3 | Acidic polar | −3.50 | 147.13 | 3.22 | 1.14 | |
| Asp | 2.77 (2.07/8.18) | 8.9 × 6.9 × 5.1 | −3.50 | 132.12 | 10.35 | 1.12 | ||
| Mnz | 2.62 | 10.0 × 7.3 × 4.0 | Acidic polar | −0.02 | 171.15 | 9 | — | — |
| Cpl | 11.03 | 14.5 × 8.9 × 6.1 | 1.14 | 323.13 | 2.44 | 1.32 | ||
| Tet | 3.30 | 14.8 × 10.3 × 8.8 | 3.00 | 480.90 | 2.38 | 1.16 | ||
| Ctc | 3.30 | 14.7 × 9.5 × 7.7 | 1.94 | 497.33 | 2.45 | 1.15 | ||
| MHB | 8.50 | 10.8 × 6.7 × 4.0 | Neutral polar | 1.98 | 152.15 | 9 | — | — |
| EHB | 8.35 | 12.3 × 6.9 × 4.0 | 2.47 | 166.17 | 7.21 | 1.52 | ||
| PHB | 7.91 | 13.4 × 7.0 × 4.0 | 2.80 | 180.20 | 8.86 | 1.41 | ||
| BHB | 8.50 | 14.8 × 7.1 × 4.0 | 3.57 | 194.23 | 6.22 | 1.25 | ||
| SM1 | 2.17 | 11.9 × 9.0 × 7.1 | Amphoteric polar | — | 264.30 | 9 | — | — |
| SDM | 2.04 | 13.5 × 9.4 × 7.0 | — | 310.30 | 3.68 | 1.23 | ||
| SM2 | 2.01 | 12.8 × 9.1 × 7.6 | — | 278.33 | 6.69 | 1.16 | ||
| ST | 7.20 | 11.6 × 8.5 × 5.9 | — | 255.32 | 3.79 | 1.18 | ||
| SIZ | 1.95 | 11.3 × 9.9 × 6.1 | — | 267.30 | 2.99 | 1.12 | ||
| SMZ | 1.97 | 11.0 × 9.1 × 6.3 | — | 253.28 | 4.04 | 1.11 | ||
The separation of the 13 amino acids was mainly dependent on their pI values. Briefly, the basic amino acids like Arg were firstly eluted, followed by the neutral amino acids, and the last elution was that of the acidic Glu and Asp, which was consistent with the migration rule of the analytes in the CEC separation system (i.e., cation > neutral molecule > anion) because Arg had positive charge (pI > pH), neutral amino acids had slight negative charge (pI < pH) and the acidic ones (pI ≪ pH) were highly negatively charged at the optimal pH 8. The retention time of His with the part negative charge (pI < pH) was similar to those of the neutral ones, although it is a basic amino acid. In addition, π–π interaction between the imidazole ring of His and the benzene ring of the ligands from TpBD and UiO-66-NH2 in the stationary phase also prolonged its retention time. The same mechanism also clarified the last elution of Trp and Phe with the benzene ring as the neutral amino acids. The other neutral ones with polar (Ser and Thr) or nonpolar (Ala, Val, Ile, Leu, Met) side chains migrated according to the size of the side chain, from small to large. It was noticeable that Ile and Leu were isomers; their complete separation should be attributed to the steric hindrance effect between the analytes and the COF and MOF with the micro–mesoporous structures in the stationary phase.
In the case of the acidic antibiotics, the preservatives and the sulfonamides investigated, three families of the analytes simultaneously reached base-line separation under the broad CEC experimental conditions with buffer pH values of 8–10, buffer concentration of 20–25 mmol L−1 and separation voltage of 15–20 kV (Fig. S8–S10†), and there was very short analysis time of 4.38 min for the sulfonamides. The separation mechanism including hydrogen bond, π–π interaction, molecular sieve effect, electrophoretic mobility, and dipole–dipole interaction between the analytes and the COF and/or MOF in the stationary phase played dominant roles in their separation.
For the acidic antibiotics, their migration order was Mnz, Cpl, Tet and Ctc, which was mainly attributed to the molecular sieve effect of the TpBD/UiO-66-NH2 hybrid material with hierarchical pore structures, i.e., the migration time of the analyte was shorter with the smaller molecular mass (Fig. 10B). In addition, it was noticeable that the longest retention time for Ctc maybe due to the more hydroxyl groups and the strong polarity of the chlorine element (Cl) on Ctc, which have stronger hydrogen bonds and n–π interaction with the stationary phase.36
For the neutral preservatives, because the effective electrophoretic mobility gradually decreased with the increased length of the alkyl chain and the dipole–dipole interaction between the preservatives and the stationary phase gradually strengthened with the increasing molecular mass and/or Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P in the order MHB < EHB < PHB < and BHB (Table 2), their migration order was MHB < EHB < PHB < BHB (Fig. 10C).
P in the order MHB < EHB < PHB < and BHB (Table 2), their migration order was MHB < EHB < PHB < BHB (Fig. 10C).
The baseline separation of 6 amphoteric sulfonamides was successfully realized in a very short migration time interval (2.37 min), which was because they had the same aniline and sulfanilamide groups. Their efficient separation mainly depended on the differences of the various interactions between the substituted heterocyclic moieties, including pyridyl-, pyrimidyl-, thiazolyl-, isoxazolyl-, and the stationary phase. Generally, the migration times of the analytes with a five-membered heterocyclic ring were longer than those of the ones with a six-membered ring, which might be due to the π-bonds formed between the former and because the benzene ring of the ligands from TpBD and UiO-66-NH2 in the stationary phase were stronger.37 Among the analytes with a five-membered heterocyclic ring (SM1, SDM and SM2), because of more N, O atoms with strong electronegativity in the SDM and SM2 molecules, they easily formed more and stronger hydrogen bonds with –NH2, –OH, and –COOH from TpBD and/or UiO-66-NH2 in the stationary phase, resulting in the elution of both after SM1. However, the longer migration time of SM2 than SDM was due to a molecular sieve and/or stereo-hindrance effect from the larger size of –O–CH3 than –CH3-substituted in the pyrimidine ring. The same mechanisms were also applicable to the sulfonamides with a six-membered ring. Therefore, the migration order of the sulfonamides was SM1, SDM, SM2, ST, SIZ and SMZ (Fig. 10D).
To evaluate the separation performances of the TpBD/UiO-66-NH2-bonded OT-CEC column, three families of the analytes were also separated with the TpBD- and UiO-66-NH2-bonded OT-CEC column under the same experimental conditions (Fig. S11 and Table S4†). The results indicated that all the analytes achieved base-line separation in the TpBD/UiO-66-NH2-bonded OT-CEC column and the TpBD-bonded OT-CEC column, but only the neutral preservatives (MHB, EHB, PHB, and BHB) were partly separated in the UiO-66-NH2-bonded OT-CEC column. It could be seen that TpBD in the stationary phase of the TpBD/UiO-66-NH2-bonded OT-CEC column played a dominant role in the separation of the analytes. Although the TpBD-bonded OT-CEC column had excellent separation performance for all the analytes, a significant decrease in the analysis time for the acidic antibiotics (from 6.87 min to 6.43 min) and the amphoteric sulfonamides (from 5.88 min to 4.38 min) as well as a great increase in the resolutions (more than 2-fold) for the neutral preservatives were observed in the TpBD/UiO-66-NH2-bonded OT-CEC column. It was obvious that UiO-66-NH2 in the stationary phase enhanced the separation efficiency of the TpBD/UiO-66-NH2-bonded OT-CEC column. Compared with UiO-66-NH2- and/or TpBD-bonded OT-CEC column, the as-prepared hybrid OT-CEC column in this work has superior separation ability and broader application.
3.7 Comparison with previous studies
There are only a small number of studies on MOF or COF used as the chromatographic stationary phases and even fewer are applied for CEC. In this study, the TpBD/UiO-66-NH2-bonded OT-CEC column with micro–mesoporous structures was prepared and exhibits universality for the analytes' types and properties. This is the first report on the construction of a MOF/COF hybrid material for the separation of many kinds of complex emerging pollutants by CEC. A total of 4 articles regarding OT-CEC were collected from Web of Science by searching the keywords “TpBD” or “UiO-66” and “stationary phase”. As shown in Table 3, certain separation performances of the OT-CEC column prepared in this work were comparable to those reported like Rs and analyte types; meanwhile, those of the others were superior. The separation performances of TpBD and/or UiO-66-NH2-bonded OT-CEC column were compared in detail with the TpBD/UiO-66-NH2-bonded OT-CEC column. In this work, 6 amphoteric sulfonamides realized fast baseline separation within 4.38 min, which was faster than the TpBD-bonded OT-CEC column (approaching 8.0 min). The Rs of the same families of analytes (like preservatives and sulfonamides) obtained on the TpBD/UiO-66-NH2-bonded OT-CEC column were all more than those on the TpBD bonded OT-CEC column. A total of 13 amino acids (acidic, neutral and basic) were simultaneously separated under broad experimental conditions. However, at most only 6 and 3 analytes were separated by the TpBD and UiO-66-NH2-bonded OT-CEC columns, respectively. Furthermore, the harsh separation conditions were necessary, for example, three kinds of separation medium (Tris–HCl, borax, citrate) were applied for the separation of the target analytes by the UiO-66-NH2-bonded OT-CEC column.38 Only ammonium acetate buffer as the separation medium was simple and environment-friendly in this work. Thus, the application of the MOF/COF hybrid material in the separation field is very effective and has a good application prospect, which is especially suitable for the separation and analysis of different analytes.| COF or MOF in stationary phase | Separation methods | Analyte types (numbers) | Analysis time (min) | Rs | Separation system | Ref. |
|---|---|---|---|---|---|---|
| a IMER is represented by immobilized enzyme micro reactor. | ||||||
| TpBD (COF) | OT-CEC | Alkylbenzenes (5), parabens (4), sulfonamides (6), benzoic acids (4) | 4.5–6.1 | 2.79–9.30 | Phosphate buffer | 33 |
| UiO-66-NH2 (MOF) | OT-CEC | Chlorobenzenes (3), phenoxyacids (3), nitrophenols (3), other phenols (3) | 4.8–6.0 | — | Tris–HCl, borax buffer, citrate buffer + methanol | 38 |
| UiO-66-NH2 (MOF) | IMER-CECa | Chlorobenzenes (3), alkaloids (3), nonsteroidal anti-inflammatory drugs (3) | 8.5–17.6 | — | Phosphate buffer (or + acetonitrile) | 39 |
| NHP-UiO-66 (MOF) | HPLC | Substituted benzenes (5), chlorobenzenes (3), polycyclic aromatic hydrocarbons (5), nucleosides (4), polypeptides (4), proteins (4) | 8.8–14.0 | 1.54–12.28 | Phosphate buffer + acetonitrile | 40 |
| TpBD/UiO-66-NH2 (MOF/COF) | OT-CEC | Amino acids (13), parabens (4), sulfonamides (6), acidic antibiotics (4) | 4.38–8.1 | 1.63–10.35 | Acetate buffer | This work |
4 Conclusions
In summary, we firstly reported an MOF/COF hybrid material (TpBD/UiO-66-NH2) as the stationary phase for the separation of emerging pollutant mixtures by OT-CEC. The TpBD/UiO-66-NH2-bonded OT-CEC column showed excellent separation performance for the analytes investigated including 13 amino acids, 4 antibiotics, 4 preservatives and 6 sulfonamides. All analytes achieved baseline separation within 8.1 min due to the synergistic effect between COF and MOF. The separation mechanism was primarily attributed to the combined action of hydrogen bonding, π–π interaction, molecular sieve effect, electrophoretic mobility, dipole–dipole interaction, and so on between the analytes and the stationary phase. Besides, the OT-CEC column had good stability and reproducibility with RSDs of migration time and the resolutions in the range of 1.17–3.93% and 1.79–4.31%, respectively. These results demonstrate the feasibility of introducing MOF/COF into the chromatographic column for the separation of a complex sample system, although the TpBD/UiO-66-NH2-bonded OT-CEC column preparation was tedious and time consuming. In the future, more MOF/COF in CEC separation will be established for the separation of different families of analytes, especially focusing on the applicability to real samples.Ethical statement
This article does not contain any studies with human or animal subjects.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Lidi Gao: conceptualization, supervision, project administration, funding acquisition, writing – original draft, writing – review & editing. Xinran Qu: conceptualization, investigation, methodology, formal analysis, data curation, visualization, validation, writing – original draft. Shuang Meng: investigation, validation, visualization. Mo Chen: investigation, visualization. Yuxin He: investigation, visualization. Fuquan Zhao: investigation, visualization. Hongtao Chu: investigation, visualization. Shili Qin: investigation, methodology, supervision. Fenglong Jin: conceptualization, methodology, supervision.Conflicts of interest
The authors declare no competing interests.Acknowledgements
We gratefully acknowledge the support of Fundamental Research Funds in Heilongjiang Provincial Universities in 2022 (No. 145209501), Qiqihar University Graduate Innovation Project Foundation in 2022 (No. YJSCX2022002) and Qiqihar University College Students' Innovation and Entrepreneurship Training Program Funded Project in 2023 (No. x202310232003).References
- G. Karina, E. Sofía, G. Rafael, A. Mariel, M. Georgia, P. Lizeth, E. Juan, M. Hafiz and P. Roberto, MethodsX, 2023, 10, 102160 CrossRef PubMed.
- P. Chaturvedi, P. Shukla, B. S. Giri, P. Chowdhary, R. Chandra, P. Gupta and A. Pandey, Environ. Res., 2021, 194, 110664 CrossRef CAS PubMed.
- X. Zhao, Y. Zheng, S. Y. Hu, W. H. Qiu, J. P. Jiang, C. Z. Gao, J. Z. Xiong, H. Y. Lu and F. Quan, J. Hazard. Mater., 2021, 411, 125047 CrossRef CAS PubMed.
- N. F. Zhu, K. J. Yuan, D. H. Xiong, F. X. Ai, K. Zeng, B. Y. Zhao, Z. Zhang and H. J. Zhao, Chem. Eng. J., 2023, 462, 142129 CrossRef CAS.
- C. Wang, D. Zhu, J. Zhang and Y. X. Du, Pharm. Biomed., 2022, 215, 114777 CrossRef CAS PubMed.
- V. K. Vashistha, S. Sethi, A. Mittal, D. K. Das, R. Pullabhotla, R. Bala and S. Yadav, Environ. Monit. Assess., 2024, 196, 153 CrossRef CAS PubMed.
- G. Gübitz and M. G. Schmid, Electrophoresis, 2004, 25, 3981–3996 CrossRef PubMed.
- X. Wang, X. Y. Hu, Y. T. Shao, L. Peng, Q. Q. Zhang, T. H. Zhou, Y. H. Xiang and N. S. Ye, Microchim. Acta, 2019, 18, 1–8 Search PubMed.
- Z. X. Fei, M. Zhang, J. H. Zhang and L. M. Yuan, Anal. Chim. Acta, 2014, 830, 49–55 CrossRef CAS PubMed.
- L. Feng, K. Y. Wang, G. S. Day, M. R. Ryder and H. C. Zhou, Chem. Rev., 2020, 120, 13087–13133 CrossRef CAS PubMed.
- C. Caratelli, J. Hajek, F. G. Cirujano, M. Waroquier, F. Xamena and V. Van Speybroeck, J. Catal., 2017, 352, 401–414 CrossRef CAS.
- F. Haase and B. V. Lotsch, Chem. Soc. Rev., 2020, 49, 8469–8500 RSC.
- Y. Deng, Y. Wang, X. Xiao, B. J. Saucedo, Z. J. Zhu, M. S. Xie, X. R. Xu, K. Yao, Y. L. Zhai, Z. Zhang and J. Chen, Small, 2022, 18, 2202928 CrossRef CAS PubMed.
- X. H. Ma, J. S. Kang, Y. W. Wu, C. H. Pang, S. H. Li, J. P. Li, Y. H. Xiong, J. H. Luo, M. Y. Wang and Z. Xu, TrAC, Trends Anal. Chem., 2022, 157, 116793 CrossRef CAS.
- J. R. Fu, S. Das, G. L. Xing, T. Ben, V. Valtchev and S. L. Qiu, J. Am. Chem. Soc., 2016, 138, 7673–7680 CrossRef CAS PubMed.
- X. L. Xu, N. Zhang, Y. Gao, T. Bao and S. C. Wang, J. Environ. Chem. Eng., 2022, 10, 107072 CrossRef CAS.
- N. Zhang, T. Bao, Y. Gao, X. L. Xu and S. C. Wang, Appl. Surf. Sci., 2022, 580, 152285 CrossRef CAS.
- J. Pang, W. J. Chen, J. T. Hu, J. Cheng, M. Q. Tang, Z. W. Liu and R. Tan, Catal. Sci. Technol., 2023, 13, 6198–6210 RSC.
- Q. D. Yu, Y. P. Wu, W. M. Zhang, W. D. Ma, J. Wang, H. Chen, Q. Q. Ding and L. Zhang, Talanta, 2022, 243, 123380 CrossRef CAS PubMed.
- L. D. Gao, X. Zhao, S. L. Qin, Q. Dong, X. F. Hu and H. T. Chu, Chirality, 2022, 34, 537–549 CrossRef CAS PubMed.
- X. Q. Zhang, Q. Han and M. Y. Ding, RSC Adv., 2015, 5, 1043–1050 RSC.
- I. Pakamore, J. Rousseau, C. Rousseau, E. Monflier and P. A. Szilágyi, Green Chem., 2018, 20, 5292–5298 RSC.
- G. Lu, C. L. Cui, W. N. Zhang, Y. Y. Liu and F. W. Huo, Chem.–Asian J., 2013, 8, 69–72 CrossRef CAS PubMed.
- B. P. Biswal, S. Chandra, S. Kandambeth, B. Lukose, T. Heine and R. Banerjeet, J. Am. Chem. Soc., 2013, 135, 5328–5331 CrossRef CAS PubMed.
- Y. Cao, H. M. Zhang, F. J. Song, T. Huang, J. Y. Ji, Q. Zhong, W. Chu and Q. Xu, Materials, 2018, 11, 589 CrossRef PubMed.
- P. Khandel, S. K. Shahi, D. K. Soni, R. K. Yadaw and L. Kanwar, Nano Convergence, 2018, 5, 1–17 CrossRef PubMed.
- A. Khatri and P. S. Rana, Bull. Mater. Sci., 2019, 42, 141 CrossRef.
- Z. H. Li, M. Q. Yang, X. T. Shen, H. T. Zhu and B. H. Li, Int. J. Environ. Res. Public Health, 2023, 20, 1393 CrossRef CAS PubMed.
- G. D. Fan, J. J. Zhan, J. Luo, J. Y. Lin, F. S. Qu, B. H. Du, Y. F. You and Z. S. Yan, J. Hazard. Mater., 2021, 404, 124062 CrossRef CAS PubMed.
- S. F. Du, K. J. Lin, S. K. Malladi, Y. X. Lu, S. H. Sun and Q. Xu, Sci. Rep., 2014, 4, 6439 CrossRef CAS PubMed.
- P. Deria, W. Bury, J. T. Hupp and O. K. Farha, Chem. Commun., 2014, 50, 1965–1968 RSC.
- H. Zhang, X. B. Shi, J. L. Li, P. Kumar and B. Liu, Nanomaterials, 2019, 9, 1283 CrossRef CAS PubMed.
- L. D. Gao, Q. Dong, X. Zhao, X. F. Hu, H. T. Chu, R. J. Lv and S. L. Qin, Processes, 2022, 10, 843 CrossRef CAS.
- M. Taddei, P. V. Dau, S. M. Cohen, M. Ranocchiari, J. A. van Bokhoven, F. Costantino, S. Sabatini and R. Vivani, Dalton Trans., 2015, 44, 14019–14026 RSC.
- W. T. Li, Z. J. Hu, J. Meng, X. Zhang, W. Gao, M. L. Chen and J. H. Wang, J. Hazard. Mater., 2021, 411, 125021 CrossRef CAS PubMed.
- S. P. Gao, B. Q. Xu, X. Y. Zheng, X. Wan, X. L. Zhang, G. M. Wu and Z. Y. Cong, Atmos. Res., 2021, 256, 105597 CrossRef.
- J. Hou, J. Yan, F. S. Zhang, Q. Zhao, H. Y. Chen, Y. Q. Zhang, G. J. Li, Y. Li and L. Ding, Food Chem., 2014, 150, 58–64 CrossRef CAS PubMed.
- P. X. Tang, R. Wang and Z. L. Chen, Electrophoresis, 2018, 39, 2619–2625 CrossRef CAS PubMed.
- R. Liu, G. Y. Yi, B. A. Ji, X. Q. Liu, Y. Q. Gu, Z. N. Xia and Q. F. Fu, Anal. Chem., 2022, 94, 6540–6547 CrossRef CAS PubMed.
- B. A. Ji, G. Y. Yi, K. L. Zhang, Y. H. Zhang, Y. Q. Gui, D. Gao, J. Zeng, L. J. Wang, Z. N. Xia and Q. F. Fu, Anal. Chem., 2020, 92, 15655–15662 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra05097e |
| This journal is © The Royal Society of Chemistry 2024 |