Open Access Article
Open Access ArticleDevelopment of an electrochemical sensor modified with gold nanoparticles for detecting fumonisin B1 in packaged foods
Liyuan Zhao,
Longzhu Zhou,
Dieudonné M. Dansou,
Chaohua Tang,
Junmin Zhang,
Yuchang Qin* and
Yanan Yu *
*
State Key Laboratory of Animal Nutrition and Feeding, Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing, 100193, China
First published on 16th September 2024
Abstract
Fumonisin B1 (FB1) is naturally present in the environment and can easily contaminate packaged foods during processing, storage and transportation, thus posing a threat to human health. We have developed an enzyme-free FB1 detector for the detection of packaged foods, which provides rapid and sensitive detection of FB1 in food. Gold nanoparticles (AuNPs; 5–10 nm) were uniformly dispersed on screen-printed electrodes, which acted as an excellent catalytic oxidizer. The surface structure of the modified electrode was characterized using scanning electron microscope and X-ray photoelectron spectroscopy. Differential pulse voltammetry demonstrated a good linear electrochemical response towards FB1 at concentrations ranging from 1 ng L−1 to 1 mg L−1 with a detection limit of 0.08 ng L−1. We employed the AuNPs-SPE sensor to detect FB1-spiked packaged meat products achieving recovery rates ranging from 89.7% to 113.3%.
Introduction
According to the Food and Agriculture Organization (FAO), 25% of the world's food crops are affected by mycotoxin-producing fungi. In response to this threat, global efforts are underway to develop relevant standards.1 China has not yet established a maximum limit standard for fumonisins in foods, and there is no unified international standard for the maximum limit of fumonisins in foods. However, the Joint Expert Committee of the FAO and the World Health Organization (WHO) on Food Additives has set the daily maximum tolerable intake of total FB1 at 2 μg per kg per day.2 The European Union (EU) has established a maximum limit of 200 μg kg−1 for fumonisins in corn products for infants and young children, as stated in the food safety standards for fumonisin.3With the continuous improvement of people's quality of life, packaged meat and meat products have become an integral part of diets. However, contamination of packaged meat products with mycotoxins can originate from various sources and at all stages of the process, including harvesting, storage, transportation, and packaging, thereby attracting increasing attention.4,5 Several studies have demonstrated that mycotoxin levels in meat and meat products pose a threat to human health. Therefore, to ensure the quality and safety of meat, it is essential to develop a sensitive measurement method for detecting meat FB1.6–8
Several methods have been developed to detect the FB1 content in foods. Among them, high-performance liquid chromatography, mass spectrometry, and thin layer chromatography are the most common. However, these methods often involve complex, time consuming, and expensive sample pre-treatment steps.9 Sensors have emerged as a promising alternative for FB1 detection. While biosensors are susceptible to external environmental interference and immune crossover, compromising detection stability,10 electrochemical sensors offer a simple, portable, and sensitive detection tool that can quickly detect FB1 in foods at a low cost.
Screen-printed electrodes (SPEs) are commonly used in electrochemical sensors constructed on plastic or ceramic substrates through thick film deposition technology, allowing for simple, inexpensive, and fast on-site analyses with high reproducibility, sensitivity, and accuracy.11 Precious metals and transition metals, such as gold (Au), silver, platinum, and titanium, are often employed in electrode construction to enhance the electrical signal conductivity of SPEs. Precious metals offer stable chemical properties, adequate biocompatibility, and excellent conductivity. Gold nanoparticles (AuNPs) are particularly noteworthy in sensing applications due to their unique optical and electronic properties.12,13 The small size of gold nanoparticles exposes more surface groups, contributing to their excellent catalytic properties.14 Ion implantation is the process of ionizing atoms of a specified element, which, under the action of an electric field, achieve a high velocity and deposited onto the surface of a solid material. Ions can be uniformly embedded into the substrate surface to form stable metal particles at the nanometer level.15 Ion implantation is an effective surface modification technology for materials, where a small number of ions can change the conductivity of the main material by several orders of magnitude. This technology does not require any binders or chemicals, making it environmentally friendly and easy to perform.16,17
In this study, we used ion implantation to modify an SPE with AuNPs, thereby developing an AuNPs-SPE. Subsequently, we used this sensor to detect FB1 in food by differential pulse voltammetry (DPV).
Materials and methods
Chemicals and reagents
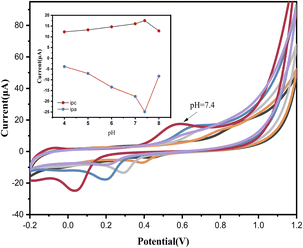
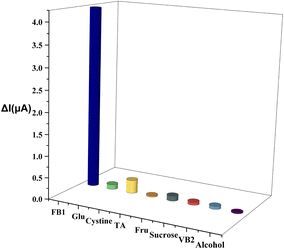
We obtained FB1 (99% purity) from Shanghai Yuanye Bio-Technology Co Ltd (Shanghai, China) and SPEs from Metrohm-Dropsens (Shanghai, China). We prepared 0.1 M phosphate buffer saline (PBS) of different pH values by mixing 0.1 M NaH2PO4 and Na2HPO4. A standard FB1 stock solution (100 mg mL−1) was prepared by dissolving FB1 in water. Possible interfering compounds, such as glutamic acid, cystine, tartaric acid, fructose, sucrose, and vitamin B2, were prepared at concentrations of 10 g L−1 in ultrapure water. Glutamic acid, cystine, and vitamin B2 were first dissolved in an alkaline solution. Before use, SPE was cleaned with ultrapure water and dried with N2 gas to prevent electrode oxidation. DPV and cyclic voltammetry were commonly used to characterize the electrochemical characteristics of SPEs. Comparatively, DPV is more sensitive than cyclic voltammetry; therefore, we used DPV to quantify FB1. The potential range was set from 0 to 0.8 V, and the scanning speed was 100 mV S−1. All experiments were conducted at room temperature.Instruments
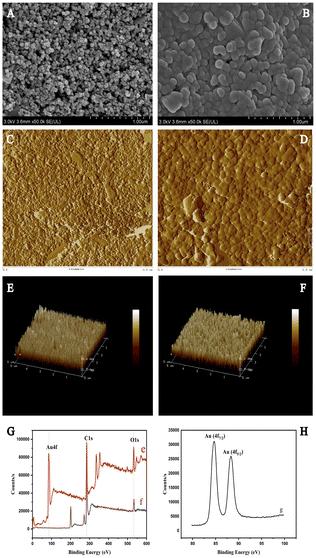
All electrochemical measurements were performed using a CHI660E electrochemical workstation (Chenhua Instrument Company, Shanghai, China) in a three-electrode system. The reference electrode and electric contacts were made of silver. The pH values of the buffer solutions were determined using a pH meter (Mettler Toledo Instrument Company, Shanghai, China). For the Au ion implantation process, the acceleration voltage was set at 10 keV, and the injection dose was 1 × 1017 ions cm−2. The surface morphology was observed using scanning electron microscopy (SEM; SU8020 microscope, Hitachi, Japan). X-ray photoelectron spectroscopy (XPS) was performed using a Thermo Escalab 250 XI (Thermo Scientific, Britain, Europe) with an Al Ka X-ray source.Preparation of real samples
We purchased packaged pork and beef separately from the local market. The samples were ground and mixed with 10 mL of 0.1 M PBS, the sonicated for 30 min, and subsequently centrifuged for 10 min at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g. Following the removal of meat fat, we diluted the supernatant 20× for electrochemical detection.
000×g. Following the removal of meat fat, we diluted the supernatant 20× for electrochemical detection.
Results
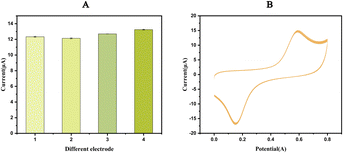
Validation of electrode modification
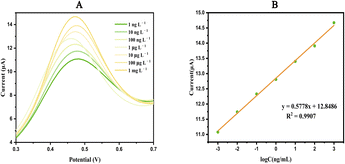
Detection ability of the sensor
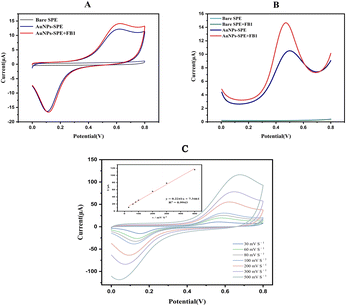
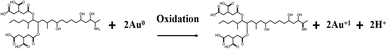
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C + 12.8486, R2 = 0.9907, where C is the concentration of FB1). Electron transfer is facilitated in the presence of FB1.26 The limit of detection (LOD) of FB1 was determined to be 0.08 ng L−1 based on the equation LOD = 3Sd/b, where Sd is the standard deviation of the electrochemical response of the blank solution for 30 cycles, and Fig. 4B is the slope of the calibration curve.
C + 12.8486, R2 = 0.9907, where C is the concentration of FB1). Electron transfer is facilitated in the presence of FB1.26 The limit of detection (LOD) of FB1 was determined to be 0.08 ng L−1 based on the equation LOD = 3Sd/b, where Sd is the standard deviation of the electrochemical response of the blank solution for 30 cycles, and Fig. 4B is the slope of the calibration curve.
| Samples | Spiked (μg L−1) | Detected (μg L−1) | Recovery% | RSD (n = 3)% |
|---|---|---|---|---|
| Pork | 1 | 1.13 ± 0.03 | 113.0 | 2.2 |
| 5 | 4.48 ± 0.11 | 89.7 | 2.4 | |
| 10 | 10.08 ± 0.20 | 100.8 | 2.0 | |
| Beef | 1 | 1.13 ± 0.05 | 113.3 | 4.5 |
| 5 | 5.04 ± 0.15 | 100.8 | 2.9 | |
| 10 | 11.96 ± 0.04 | 101.2 | 0.4 |
The detection performance of the proposed sensor was compared with other existing methods for FB1 detection, and the results were summarized in Table 2. Our sensor demonstrates excellent sensitivity. Additionally, most of the aptasensor and fluorescence methods need to be combined with immunoassays, which increase the complexity of the operation. In contrast, the electrochemical sensor is simple to operate and does not require exquisite equipment, which can meet the need of easy and practical monitoring of FB1.
| Method | Samples | Linear range | LOD | Ref. |
|---|---|---|---|---|
| Fluorescence resonance energy transfer | Maize | 0–3000 ng mL−1 | 14.42 ng mL−1 | 27 |
| Electrochemical aptasensor | Maize | 0.5–500 ng mL−1 | 0.14 ng mL−1 | 28 |
| Colorimetric sensor | Cereal | 3–200 ng mL−1 | 1.73 μg L−1 | 29 |
| Paper-based electrochemical aptasensor | Wheat | 50 fg mL−1–100 ng mL−1 | — | 30 |
| ELISA | Garlic | 0.25–0.50 mg L−1 | 0.25 mg L−1 | 31 |
| This work | Pork | 1 ng L−1–1 mg L−1 | 0.08 ng L−1 | — |
| Beef |
Discussion
It is well known that FB1 is harmful to animal and human health, often causing imperceptible contamination of packaged foods at various stages of production and distribution, As a result, residues of FB1 in packaged foods should be strictly controlled. The presence of FB1 in packaged meat products can be easily overlooked. Therefore, the development of a simple methodology for the detection of FB1 is of great value in food toxin detection.Many methods still suffer from low sensitivity, complexity of production, and are rarely considered for the detection of FB1 in packaged meat products. Traditional detection methods of FB1 include thin-layer chromatography, high-performance liquid chromatography, gas chromatography/mass spectrometry, and immunosorbent assays.32–34 These methods have several limitations, such as complex pre-treatment and the use of toxic solvents.35 In recent years, numerous new methods have emerged for detecting mycotoxins. However, few methods are available for detecting FB1 in foods, especially in meat. Electrochemical detection technology is a well-established technique for detection and analysis.
Our method offers high sensitivity and a low detection limit (LOD) which can be applied for a rapid detection of meat mycotoxins. AuNPs are commonly used as metal modification materials, which are easily synthesized, exhibit high reaction interface activity, reduce the energy barrier of electrochemical redox reactions, and demonstrate have adequate electrochemical performance.36 We modified a certain size of AuNPs on the SPE surface by ion implantation and characterized the surface morphology and microstructure of bare and AuNPs-SPE by SEM and found that AuNPs were uniformly and densely distributed on the SPE surface.
The CV response (Fig. 3A) and DPV response (Fig. 3B) of the bare SPE and AuNPs-SPE results indicate that the redox peak observed with the AuNPs-SPE was notably higher than that of the bare SPE, confirming that the AuNPs exerted a catalytic effect on the redox reaction of FB1. Ion implantation with AuNPs improved the electrochemical detection capability and optimized the detection conditions to improve the binding of FB1. Consequently, AuNPs-SPE demonstrated highly sensitive FB1 detection capabilities and can be used as an effective tool for detecting FB1 in packaged foods.
Conclusions
To conclude, AuNPs-SPEs do not require complex pre-processing steps. FB1 can be rapidly and sensitively quantified in packaged foods using DPV. AuNPs-SPEs has good stability, reproducibility, and specificity, with a detection limit of 0.08 ng L−1 for FB1. SPE was modified by ion implantation, which improved the conductivity of the electrode and provided a novel method for the detection of mycotoxins in packaged foods.Data availability
Data will be made available on request.Author contributions
Liyuan Zhao: validation, software, formal analysis, methodology, writing – original draft. Longzhu Zhou: investigation, validation. Dieudonné M. Dansou: writing – review & editing, validation. Chaohua Tang: resources, writing – review & editing. Junmin Zhang: writing – review & editing, funding acquisition, supervision. Yuchang Qin: supervision, funding acquisition. Yanan Yu: conceptualization, supervision, methodology, funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was supported by the National Natural Science Foundation of China (32102590), Chongqing Rongchang Agriculture and Animal Husbandry High Tech Industry Research and Development Project (cstc2020ngzx0005) and the Chinese Academy of Agricultural Science and Technology Innovation Project (ASTIP-IAS-12).Notes and references
- C. Levasseur-Garcia, Toxins, 2018, 10, 8 Search PubMed.
- G. S. Shephard, H.-M. Burger, J. P. Rheeder, J. F. Alberts and W. C. A. Gelderblom, Food Control, 2019, 97, 77–80 CrossRef CAS.
- L. Qu, L. Wang, H. Ji, Y. Fang, P. Lei, X. Zhang, L. Jin, D. Sun and H. Dong, Toxins, 2022, 14, 182 CrossRef CAS.
- J. Pleadin, D. Kovačević and N. Perši, Food Control, 2015, 57, 377–384 CrossRef CAS.
- S. M. Abd-Elghany and K. I. Sallam, Food Chem., 2015, 179, 253–256 CrossRef CAS PubMed.
- M. Sokolovic, M. Berendika, T. Amsel Zelenika, B. Simpraga and F. Krstulovic, Toxins, 2022, 14, 444 CrossRef CAS PubMed.
- A. R. Alaboudi, T. M. Osaili and G. Otoum, Food Control, 2022, 132, 108511 Search PubMed.
- C. Juan, S. Oueslati, J. Manes and H. Berrada, J. Food Sci., 2019, 84, 3885–3893 CrossRef CAS PubMed.
- X. Chen, H. Wu, X. Tang, Z. Zhang and P. Li, Electroanalysis, 2021, 35, 210022 Search PubMed.
- Y. Xiang, M. B. Camarada, Y. Wen, H. Wu, J. Chen, M. Li and X. Liao, Electrochim. Acta, 2018, 282, 490–498 CrossRef CAS.
- G. Paimard, E. Ghasali and M. Baeza, Chemosensors, 2023, 11, 113 CrossRef CAS.
- L. Tu, Gold Bull., 2022, 55, 169–185 CrossRef CAS.
- Y. Zhong, P. Liao, J. Kang, Q. Liu, S. Wang, S. Li, X. Liu and G. Li, J. Am. Chem. Soc., 2023, 145, 4659–4666 CrossRef CAS PubMed.
- Z. Wang, Y. Shao, Z. Zhu, J. Wang, X. Gao, J. Xie, Y. Wang, Q. Wu, Y. Shen and Y. Ding, Coord. Chem. Rev., 2023, 495, 215369 CrossRef CAS.
- D. Gupta and R. Kumar, Mater. Sci. Semicond. Process., 2023, 158, 107326 CrossRef CAS.
- S. D. Pravesh, D. Singh and M. Singh, Pramana, 2023, 97, 58 CrossRef.
- A. K. Manna, A. Kanjilal, D. Kanjilal and S. Varma, Nucl. Instrum. Methods Phys. Res., Sect. B, 2020, 474, 68–73 CrossRef CAS.
- R. El-Sayed, F. Ye, H. Asem, R. Ashour, W. Zheng, M. Muhammed and M. Hassan, Biochem. Biophys. Res. Commun., 2017, 491, 15–18 CrossRef CAS.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
- M. Keerthi, A. Kumar Panda, Y. H. Wang, X. Liu, J. H. He and R. J. Chung, Food Chem., 2022, 378, 132083 CrossRef CAS.
- F. Y. Kong, R. F. Li, L. Yao, Z. X. Wang, H. Y. Li, W. J. Wang and W. Wang, Nanotechnology, 2019, 30, 285502 CrossRef CAS PubMed.
- Y. Luo, F.-Y. Kong, C. Li, J.-J. Shi, W.-X. Lv and W. Wang, Sens. Actuators, B, 2016, 234, 625–632 CrossRef CAS.
- R. Devi, S. Gogoi, S. Barua, H. Sankar Dutta, M. Bordoloi and R. Khan, Food Chem., 2019, 276, 350–357 CrossRef CAS PubMed.
- A. R. Cardoso, F. T. C. Moreira, R. Fernandes and M. G. F. Sales, Biosens. Bioelectron., 2016, 80, 621–630 CrossRef CAS PubMed.
- K. Li, J. Cui, Q. Yang, S. Wang, R. Luo, A. Rodas-Gonzalez, P. Wei and L. Liu, Food Chem., 2023, 405, 134791 CrossRef CAS.
- Z. Han, Z. Tang, K. Jiang, Q. Huang, J. Meng, D. Nie and Z. Zhao, Biosens. Bioelectron., 2020, 150, 111894 CrossRef CAS.
- X. Zhao, J. Gao, Y. Song, J. Zhang and Q. Han, Sensors, 2022, 22, 8598 CrossRef CAS PubMed.
- B. Naghshbandi, M. Adabi, K. Pooshang Bagheri and H. Tavakolipour, J. Nanobiotechnol., 2023, 20, 534 CrossRef PubMed.
- X. Niu, H. He, H. Ran, Z. Wu, Y. Tang and Y. Wu, Food Chem., 2023, 429, 136903 CrossRef CAS.
- X. Zhang, Z. Li, L. Hong, X. Wang and J. Cao, J. Agric. Food Chem., 2023, 71, 19121–19128 CrossRef CAS.
- S. Tonti, M. Mandrioli, P. Nipoti, A. Pisi, T. G. Toschi and A. Prodi, J. Agric. Food Chem., 2017, 65, 7000–7005 CrossRef CAS.
- Q. Zhou, F. Li, L. Chen and D. Jiang, J. Food Sci., 2016, 81, T2886–T2890 CAS.
- J. He, B. Zhang, H. Zhang, L. L. Hao, T. Z. Ma, J. Wang and S. Y. Han, J. Food Sci., 2019, 84, 2688–2697 CrossRef CAS.
- M. Zhang, X. Guo and J. Wang, Biosens. Bioelectron., 2023, 224, 115077 CrossRef CAS PubMed.
- S. Z. Iqbal, S. Nisar, M. R. Asi and S. Jinap, Food Control, 2014, 43, 98–103 CrossRef CAS.
- Y. Ju, H. Zhang, J. Yu, S. Tong, N. Tian, Z. Wang, X. Wang, X. Su, X. Chu, J. Lin, Y. Ding, G. Li, F. Sheng and Y. Hou, ACS Nano, 2017, 11, 9239–9248 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |