Open Access Article
Open Access ArticleIntricacies of CO2 removal from mixed gases and biogas using polysulfone/ZIF-8 mixed matrix membranes – part 1: experimental†
Shweta Negi and
Akkihebbal K. Suresh *
*
Department of Chemical Engineering, IIT Bombay, Powai, Mumbai 400076, India. E-mail: aksuresh@iitb.ac.in
First published on 24th September 2024
Abstract
In this work, we explore the potential of polysulfone/ZIF-8 mixed matrix membranes (MMMs) for the enrichment of biogas to biomethane. To this end, we present data for these MMMs on permeability and selectivity as function of pressure and feed composition, at different loadings of ZIF-8. Specifically, we study dense polysulfone membranes prepared by solvent evaporation, with a ZIF-8 loading in the range 0.5–5 wt% for separation of CO2 from artificial mixtures of CO2 and CH4, and also biogas from an operating plant. The MMMs with 1 wt% filler loading gave the highest enhancement in permeability and selectivity, of 56.8% and 41% respectively, as compared to pure PSF membranes. At higher loadings, a tendency for the ZIF-8 particles to agglomerate was seen, which may compromise the ability of the filler to improve membrane performance. With mixed gases, increases in CO2 permeability of about 8 to 34% were observed depending on the gas composition, the enhancement being the higher, the lower the CO2 content. For biogas, permeability and selectivity of the 1% ZIF-8 loaded MMMs were found to be 14.6% and 39.64% lesser respectively than the pure gas values. The study thus throws light on the differences in membrane performance with mixtures as compared to ideal values obtained with pure gases and hence underlines the importance of lab-scale testing of the membranes with actual gas mixtures in the intended applications.
1. Introduction
Renewable gases like biogas and landfill gas are being positioned as alternatives to conventional fuels such as natural gas due to their zero carbon footprint and ease of availability.1,2 While these gases have CH4 as a major component, the presence of significant amounts of CO2 lowers their calorific value and hampers their use as an efficient fuel. Various technologies, such as absorption, adsorption, cryogenic separations and membrane separations, are therefore used for separation of CO2.3–6 In particular, membrane separation has been successfully employed for CO2/CH4 separations in the past few decades. Compared to other techniques, it has a simple configuration, requires low maintenance and has low energy demands;7,8 it can also be scaled up easily without loss of efficiency.9 Early research on gas separations was mostly on dense polymeric membranes as they were easy to fabricate and gave a good idea of permeability and selectivity of the polymer used. The ease of membrane formation and its good performance for separation of gases, resulted in vigorous research in this area and an empirical, inverse correlation between permeability and selectivity was shown by Robeson in 1991, based on data available at the time; this came to be regarded as an “upper bound”10 for membranes. Such data for CO2/CH4 separations showed glassy polymeric membranes to possess high permeability and selectivity. Subsequent efforts directed to improve membrane performance resulted in a shift in upper bound in 2008 (ref. 11) for most gas pairs including CO2/CH4.Among materials regarded as the most promising for crossing the Robeson bounds are mixed matrix membranes (MMMs), which have suitable fillers embedded in a pure polymer matrix. MMMs exploit the permeability of both the pure polymer and filler material, resulting in an enhanced permeability. The most important aspect with MMMs is the selection of a suitable filler – it should (a) be compatible with the polymer in order to avoid undesirable features like void formation or rigidification at the polymer–particle interface and (b) assist in the separation, either based on selective affinity or size-exclusion because of pore size. The first report on MMMs dates to 1973 when Paul and Kemp12 developed membranes using PDMS and 5A zeolites and studied the effect on diffusion time lag. For CO2/CH4 separation, the most used fillers are zeolites, metal nanoparticles, silica-based fillers, carbon molecular sieves and metal–organic frameworks (MOFs). These have been discussed in detail in various reviews.13,14 MOFs consist of inorganic parts connected with organic linkers to form a porous framework with pore size tunability, high adsorption capacity and surface area. The organic part present in the MOFs provide for a good interaction between polymer and fillers. The MOFs that are found to show good performance for CO2/CH4 separation are ZIFs (Zeolitic Imidazolate Frameworks) that include ZIF-7, ZIF-8, ZIF-11, ZIF-90, ZIF-71, ZIF-108, ZIF-302; UiO (University of Oslo)-66 and MILs (Materials Institute Lavoisier) that include MIL-53(Al), MIL-68(Al), MIL-101(Cr), and MIL-125(Al).15 Sorribas et al. (2014) used silica–ZIF-8 spheres as filler in a polysulfone matrix and found that the CO2 permeability increased by 300% compared to that of pure membranes.16 Nuhnen et al. 2020 synthesized polyimide membranes with MIL-101(Cr) and MOF-199 as fillers and found that both the selectivity and permeability were higher than the pure membranes.17 Ahmed et al. (2018) used UiO-66 as filler in 6-FDA-DAM polyimide membranes. There has also been interest in amine-functionalised MOFs as fillers in MMMs.18 Thus, Rodenas et al. (2014) used NH2-MIL-53(Al) and NH2-MIL-101(Al) for development of MMMs within PSF polymer matrix and found an increase in both the selectivity and CO2 permeability.19 Wu et al. have used a dual interface engineering approach that led to better compatibilization polymer–particle interface resulting in increased permeability and selectivity for CO2/CH4 separations.20 The high permeability and selectivity in MOF containing MMMs is mainly due to the fact that the adsorption capacity of the MOF for CO2 is higher than that for CH4. In some MOFs, size sieving is also responsible for increased selectivity, because the pore size of MOF can be chosen to be between the kinetic diameters of the gases to be separated.
ZIF-8 is a MOF that has good permeability and selectivity for CO2/CH4 separations. The high CO2 permeability in ZIF-8 is due to its higher affinity for CO2 than CH4; also, its pore size of 3.4 A is in between the kinetic diameters of CO2 (3.3 A) and CH4 (3.8 A). There are several studies in literature on the use of ZIF-8 as a filler in MMMs in both asymmetric and dense membranes. Table 1 summarises the studies on ZIF-8 as a filler in MMMs for CO2/CH4 separation. In each case, the table compares the result with the filler with that for the neat polymer. An increase in selectivity for the MMM is reported in all these cases; while in some cases, this increase is at the cost of a decrease in permeability, in others, both permeability and selectivity have been seen to increase.
| Reference | Polymer | ZIF-8 loading (weight%) | CO2 permeability (barrer) | CO2/CH4 selectivity |
|---|---|---|---|---|
a All the data is for flat sheet membranes using pure gases unless indicated otherwise.b Volume%.c Permeance in GPU.d Mixed gas (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50-CO2 50-CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH4).e Hollow fiber. CH4).e Hollow fiber. |
||||
| Bushell et al. 2013 (ref. 21) | PIM-1 | 0 | 4390 | 14.2 |
| 28b | 4270 | 18.6 | ||
| Nordin et al. 2014 (ref. 22) | Polysulfone | 0 | 25.7c | 19.43 |
| 5 | 15.60c | 28.50 | ||
| Nordin et al. 2015 (ref. 23) | Polysulfone | 0 | 21.2c | 19.43 |
| 0.5 | 29.22c | 23.16 | ||
| Ahmad et al. 2018 (ref. 18) | 6FDA-bisP polyimide | 0 | 35.3d | 25.6 |
| 17 | 47.7d | 29.1 | ||
| Khan et al. 2020 (ref. 24) | Polysulfonee | 0 | 38.58 | 20.14 |
| 0.5 | 47.75 | 25.70 | ||
| Sasikumar et al. 2021 (ref. 25) | Polysulfonee | 0 | 29.64 | 13.78 |
| 0.5 | 41.15 | 22.25 | ||
An analysis done on MMMs shows that the intent of the studies was to establish ZIF-8 nanoparticles as an efficient candidate to enhance the membrane permeability, selectivity or both. The experimentation in these studies has usually been limited to one pressure, and to pure gases.21–23,25,26 While Ahmad et al. (2018) do report studies on equimolar mixture of CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH4, a comparison of performance with pure gases is not available. There is thus a research gap on the performance MMMs with mixed gas feeds. The literature on pure polysulfone membranes suggests that competitive sorption effects could be important in the case of mixed gases with the permeabilities being lower than with the pure components.26,27 The present study is undertaken to bridge this gap.
CH4, a comparison of performance with pure gases is not available. There is thus a research gap on the performance MMMs with mixed gas feeds. The literature on pure polysulfone membranes suggests that competitive sorption effects could be important in the case of mixed gases with the permeabilities being lower than with the pure components.26,27 The present study is undertaken to bridge this gap.
The present study is targeted towards determining the gas separation properties of pure PSF and PSF/ZIF-8 MMMs using pure gases as well as gas mixtures, over a range of pressures. Mixtures of CH4 and CO2 in different ratios are used as a feed to MMMs and a comparison is made with the pure PSF membranes. Real biogas from an operating biogas plant is also used to establish the efficacy of MMMs in separation of CO2 from biogas. It is hoped that such a study would enable a rational assessment of the potential of PSF/ZIF-8 MMMs for biogas (or landfill gas) enrichment.
2. Experimental
2.1 Materials used
Polysulfone (P-3500) (Solvay) and polydimethylsiloxane (PDMS) – Sylgard (Sigma-Aldrich), zinc nitrate hexahydrate (Sigma-Aldrich), methyl imidazole (MeIM, Sigma-Aldrich), N-methyl pyrrolidone (NMP, Sigma-Aldrich), tetrahydrofuran (Sigma-Aldrich), ethanol (Sigma-Aldrich), chloroform (CHCl3) and water were used in the synthesis of membranes. Polysulfone was dried for 24 hours before use in a hot air oven. All the other chemicals were used as received without further purification.2.2 ZIF-8 preparation
ZIF-8 was prepared using the method reported in ref. 28. Typically, 1.46 g of Zn(NO3)2·6H2O was dissolved in 100 ml of methanol under constant stirring. The resulting solution was added to a solution of 3.24 g of MeIM in 100 ml methanol, and the mixture kept stirred for 1 hour. The resulting milky white solution was centrifuged and washed with methanol 3 times to ensure complete removal of unreacted solute, followed by drying at 70 °C for 12 hours. The resulting particles were ground in a mortar and pestle and stored in a desiccator for further use.2.3 Membrane preparation
2.4 Characterization
The morphology of pure polysulfone as well as MMMs were determined using a JEOL-JSM 7600F FEGSEM after sputter coating the samples with platinum. EDS was also carried out in the same equipment for elemental mapping across the membrane surface. The samples were prepared by cutting a small piece from the membrane with a sharp blade in a single cut. NETZSCH STA Luxx analyser was used for determining the thermal stability of membranes. A known quantity of synthesized nanoparticles was placed in a Teflon pan and heated from 30 °C to 1000 °C at a heating rate of 10 °C min−1.
3. Results and discussion
3.1 Filler characterization
The XRD pattern of ZIF-8 is shown in Fig. 1a. The characteristic peaks for ZIF-8 are present at 2θ values of 7.3, 10.35, 12.7, 14.8, 16.4, 18, 22.1, 23.9 and 26.5, and are well in agreement with the simulated ZIF-8 peaks and with the literature.28,31,32 The FEGSEM micrograph for ZIF-8 is shown Fig. 1b where the rhombic dodecahedron morphology of the ZIF-8 particles is clearly visible. A similar particle shape for ZIF-8 has been reported in the literature.31,33,34 The formation of phase-pure ZIF-8 may therefore be concluded. The particle size of synthesized ZIF-8 is around 40 nm. Since the dense membranes have thickness of 25–30 microns, the filler particles are easily accommodated within the membrane thickness. TGA analysis of ZIF-8 was done from 29 °C to 1000 °C in a flow of nitrogen. The weight loss profile with temperature is shown in Fig. S2 (ESI†). Thermal degradation of ZIF-8 starts at around 350 °C and continues till 600 °C after which the weight becomes constant at around 30% of the initial weight, that corresponds to ZnO. Similar behaviour has also been reported in literature.25 | ||
| Fig. 1 Characterization of ZIF-8 (a) X-ray diffraction peaks (b) scanning electron microscope micrograph. | ||
The sorption isotherm using N2 gas in Fig. S3 (ESI†) shows a type I isotherm with a hysteresis around a relative pressure of 1 indicating that the sample is microporous. The surface area is 1176 m2 g−1 (BET method) and average pore volume is 0.706 cm3 g−1 and of 0.452 cm3 g−1. Cravillon et al. have reported BET surface area of 960 m2 g−1 and micropore volume of 0.36 cm3 g−1.28 Other literature reports the values that are in the range of what is observed experimentally in this study.35 It should be noted that any difference that are present can be attributed to different synthesis and pretreatment conditions involved in the analysis. This high surface area and microporosity suggests that ZIF-8 can enhance the gas sorption when used in the polymer matrix. The sorption isotherm with CO2 in Fig. S4† show that ZIF-8 has an adsorption capacity of 16.37 cm3 g−1 for CO2 at a pressure of around 1 bar (temperature 298 K). This affinity for CO2 suggests that ZIF-8 is suitable as a filler in mixed matrix membranes to enhance the separation properties.
3.2 Membrane characterization
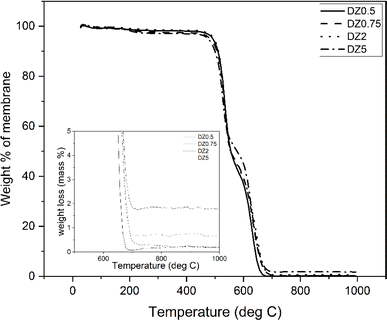
Thermal degradation behaviour of MMMs is shown in Fig. 2 from room temperature (29 °C) to 1000 °C in a flow of oxygen. It was observed that the membranes are stable till a temperature of 450 °C, the temperature at which degradation of polysulfone begins; all the polymer is lost by 600 °C. It can be observed in the inset of Fig. 2 that the residual weight of membrane increases with increase in loading of the filler.The ZIF-8 content in the membrane was estimated from the residual weight in the TGA experiments and the results are listed in Table 2. It was observed that the membranes show a loading close to the experimental loading.
| Membrane | Loading (weight%) | |||
|---|---|---|---|---|
| Experimental | TGA | EDS (whole) | EDS (agglomerate) | |
| DZ0.5 | 0.50 | 0.44 | 0.49 | — |
| D0.75 | 0.75 | 0.54 | 0.70 | — |
| DZ2 | 2.00 | 1.91 | 2.56 | 4.17 |
| DZ5 | 5.00 | 5.06 | 7.33 | 8.07 |
Surface images of the dense membranes are shown in Fig. 3 for the membranes DZ0.75, DZ1, DZ2 and DZ5. The membranes show a homogeneous surface and no defects are visible on the membrane. A significant agglomeration is seen for membranes DZ2 and DZ5 (Fig. 3c and d respectively). Agglomeration of particles in dense membranes have also been reported in the literature for MMMs18,36 and it may prove as a hindrance to gas transport through the membranes as the surface area available for gas permeation decreases.
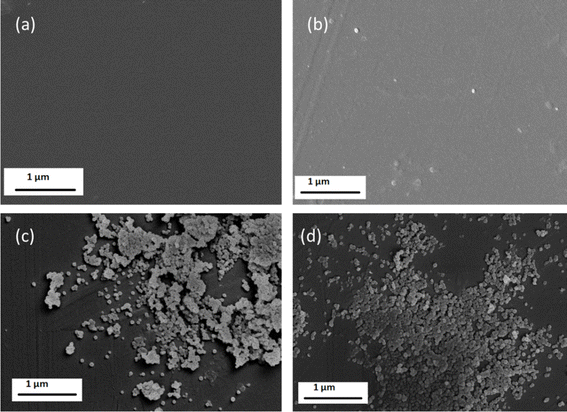 | ||
| Fig. 3 SEM micrographs for dense PSF/ZIF-8 mixed matrix membranes (a) DZ0.75 (b) DZ1 (c) DZ2 (d) DZ5. | ||
To check for the presence of any interfacial voids at polymer–particle interfaces, imaging was done for the DZ5 membrane that has the highest loading of ZIF-8. The image, shown in Fig. S4 (ESI†), shows no interfacial defects that could result in non-selective transport in the membranes. The characteristic shape of ZIF-8 particles is also discernible in this image.
Fig. 4 shows the elemental analysis of the membranes, carried out in EDS mode of FESEM. The membranes at lower loadings show a comparatively homogenous dispersion of particles whereas at higher loadings some agglomeration is visible as observed in earlier micrographs also. A small peak of Zn in the spectra is visible that increases in size with increase in ZIF-8 loading.
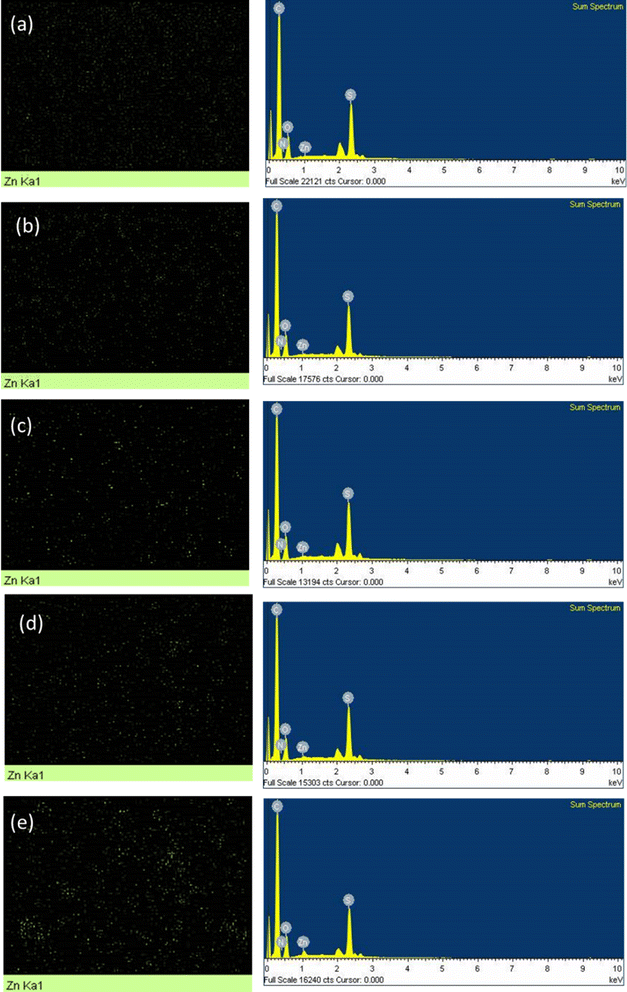 | ||
| Fig. 4 EDS analysis showing an increase in Zn content with ZIF-8 loading for PSF/ZIF-8 MMMs (a) DZ0.5 (b) DZ0.75 (c) DZ1 (d) DZ2 (e) DZ5. | ||
The percentage loading (weight% of ZIF-8 in membrane matrix) was also calculated from elemental analysis (EDS) at higher loadings (where such quantification was possible) and it was found that the calculated values are close to the loading as per the recipe employed, as shown in Table 2.
Cross-sectional SEM micrographs were also used to ascertain the distribution of ZIF-8 across the thickness of the MMMs and also to check for agglomeration tendencies. For this purpose, the membrane samples were sandwiched between two layers of resin, microtomed and observed under the microscope. The micrographs are shown in Fig. 5, and show a tendency towards agglomeration at ZIF-8 loading of higher than 0.75%.
Elemental mapping was done for the membranes DZ2 (see Fig. 6) and DZ5 (see Fig. 7) as the higher loading in these membranes makes detection possible by EDS analysis. It is observed that, while the ZIF-8 particles are distributed throughout the thickness, some agglomeration is visible at several locations. The amount of ZIF-8 calculated is around 4 wt%, and 8 wt%, which is higher than the actual loading of 2 wt% and 5 wt% in the membranes respectively. This ZIF-8 loading on the membrane surface for DZ2 and DZ5 membrane was 2.56 wt% and 7.33 wt%, much less than in the cross-section. The agglomeration of particles is one of the major problems that has been known to deteriorate membrane permeability due to blocked passages for the gas transport.
 | ||
| Fig. 6 Cross sectional micrographs of DZ2 membrane showing agglomeration and EDS analysis showing ZIF-8 particles agglomeration (red circles). | ||
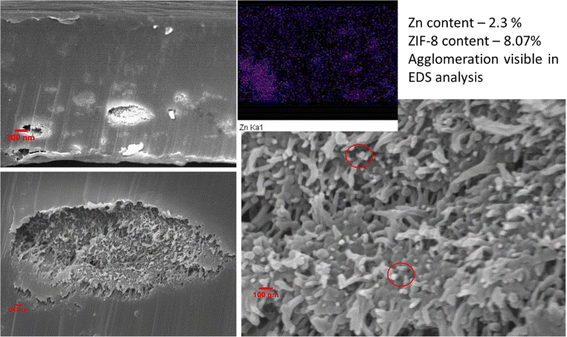 | ||
| Fig. 7 Cross sectional micrographs of DZ5 membrane showing agglomeration and EDS analysis showing ZIF-8 particles agglomeration (red circles). | ||
3.3 Gas permeation studies
The permeation behaviour of PSF as well as PSF/ZIF-8 MMMs were studied over a feed pressure range of 2 to 6 bar for pure and mixed gases. Membrane performance was also studied with raw biogas feed, with composition: CH4 (45%), CO2 (44%), N2 (10%) and O2 (∼3%), H2S (800 ppm). The biogas composition analysis was done in COMBIMASS® portable gas analyser. Since the analyser is not equipped for N2 detection, it was done using gas chromatography. In this section, we discuss the results.As Fig. 8 shows (also see the inset), the CO2 permeability values for all MMMs are higher than those for pure PSF membranes, indicating that ZIF-8 particles enhance the CO2 transport through the membranes. This enhancement is attributed to the affinity of ZIF-8 for CO2; researchers have also invoked, as an explanation for the increase in permeability, the pore size of ZIF-8 (3.4 Å), which is higher than the kinetic diameter of CO2 molecule (3.3 Å).23,41 However, as the inset shows clearly, the permeability increase for CO2 with loading starts to decrease beyond 1%. The highest increase in permeability is observed for DZ1 membrane, and is 56.8% compared to pure PSF. At loadings higher than 1 wt%, a decrease in membranes permeability is observed. The agglomeration of ZIF-8 particles on the membrane surface results in blockage of channels for gas flow thereby reducing the permeability.
Fig. 9 shows similar data for methane permeation through pure PSF and mixed matrix membranes. An increase in permeability of methane is also observed for MMMs (although less than for CO2), ranging from 3 to 35% for different loadings. While the transport of CH4 through the pores of ZIF-8 is restricted via size sieving due to its larger diameter, the modest increase observed may be because of some tendency of CH4 to adsorb on ZIF-8.42,43 In this case also, the permeability goes through a maximum, but at a lower loading (0.75%) than in the case of CO2. This may be because methane is larger in size than CO2, because of which its permeability reduces even for 1 wt% loading where very less agglomeration is seen.
Fig. 10 shows the selectivity values of the dense pure PSF and PSF/ZIF-8 MMMs as a function of feed gas pressure. The selectivity of the MMMs is higher than that of pure PSF membranes, and generally increases with increase in ZIF-8 loading, suggesting that ZIF-8 not only has the potential of improving the CO2 permeability but also CO2/CH4 selectivity. The highest increase of 42% in selectivity is observed for the membrane DZ1 whereas for DZ5 membrane the selectivity increases by 36%.
Fig. 11 and 12 show the variation in permeability for gas mixtures of different compositions, along with biogas, at different pressures for CO2 and CH4 respectively. For each composition, it is observed that the membrane permeability decreases with the increase in pressure that is expected phenomena for glassy polymers as explained in Section 3.3.1.
 | ||
Fig. 11 CO2 permeability of pure PSF membranes for mixed gas (CH4 and CO2) in different ratios (shown in x-axis are CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 ratio). Pure gas results are also shown for comparison. CO2 ratio). Pure gas results are also shown for comparison. | ||
 | ||
Fig. 12 CH4 permeability of pure PSF membranes for mixed gas (CH4 and CO2) in different ratios (shown in x-axis are CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 ratio). Pure gas results are also shown for comparison. CO2 ratio). Pure gas results are also shown for comparison. | ||
A comparison between permeabilities at particular pressure sheds light into how the membrane performance changes with composition of the gas. The results indicate an increase in CO2 permeability with a decrease in CO2 content of the feed gas at the same total pressure. The increase in permeability for feed gas containing 50%, 40%, 30% and 10% CO2 are 12%, 14.24%, 22.4% and 32.4% respectively, compared to pure gas values. This may seem as an advantage in the stagewise enrichment of the mixture, since the leaner the mixtures get in CO2, the faster does the latter permeate, but a much lower increment of 2.99% in CO2 permeability for biogas is recorded (compared to what is expected from made-up mixtures under similar conditions). For CH4, the permeability is higher in mixed gases than for pure gas and decreases with a decrease in methane content of the gas. The increase in CH4 permeability for feed gas containing 50%, 40%, 30% and 10% CO2 is 29%, 22.8%, 17.14% and 10.47% respectively. Here, biogas shows a little lower increment of 3% in CH4 permeability (lower than that seen for a made-up mixture with similar composition). The minor components of biogas may contribute to a reduction in permeance of the major constituents, depending on their affinity to the polymer membrane.
These findings are of obvious relevance to the design of membrane cascades for biogas enrichment, in which the permeate streams are progressively treated for recovery of methane while the retentate streams are treated for further enrichment of methane.
Glassy polymeric membranes are expected to show a competitive sorption effect where the presence of other components in the mixture decreases the availability of sorption sites for a given gas, thus reducing its permeability. To analyse the effect of competition, the permeabilities for CO2 and CH4 are plotted against their respective partial pressures in Fig. 13 and 14 respectively. It is observed that, for the same overall driving force for transport, CO2 permeability is the lesser, the lesser its content in the mixture, with CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 having 90
CO2 having 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 composition showing the least permeability. This cannot be explained as a partial pressure effect by the tenets of dual model sorption and can only be explained as the effect of competition – the passage of CO2 through the membranes is hindered by the presence of methane. However, the results for methane, shown in Fig. 14, show much less of an effect of competition; no significant change is seen in methane permeability for the same feed partial pressure, in mixtures as compared to pure gas.
10 composition showing the least permeability. This cannot be explained as a partial pressure effect by the tenets of dual model sorption and can only be explained as the effect of competition – the passage of CO2 through the membranes is hindered by the presence of methane. However, the results for methane, shown in Fig. 14, show much less of an effect of competition; no significant change is seen in methane permeability for the same feed partial pressure, in mixtures as compared to pure gas.
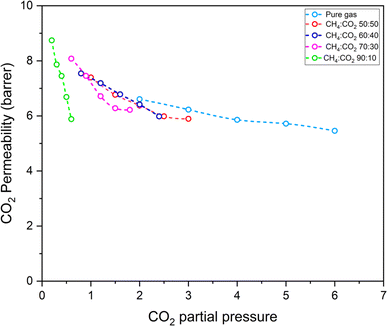 | ||
| Fig. 13 CO2 permeability of pure PSF membranes for mixed gas (CH4 and CO2) in different ratios. Pure gas results are also shown for comparison. | ||
 | ||
| Fig. 14 CH4 permeability of pure PSF membranes for mixed gas (CH4 and CO2) in different ratios. Pure gas results are also shown for comparison. | ||
The selectivity values of pure PSF membranes for mixed gases is shown in Fig. 15. The selectivity values of mixed gas with CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 of 70
CO2 of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and 90
30 and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 is higher than the pure gas selectivity due to a greater increase in CO2 permeance than that of CH4 whereas the selectivity of CH4
10 is higher than the pure gas selectivity due to a greater increase in CO2 permeance than that of CH4 whereas the selectivity of CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 of 60
CO2 of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and 50
40 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 is lower than the pure gas values. The highest selectivity is observed at a pressure of 4 bar for CH4
50 is lower than the pure gas values. The highest selectivity is observed at a pressure of 4 bar for CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 of 90
CO2 of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 mixed gas. For biogas, the selectivity values of the membranes are lower than the ideal selectivity and mixed gas selectivity due to a higher increase in CH4 permeability as compared to CO2 permeability. This study suggests that polysulfone membranes suffer a loss in selectivity and permeability with gas mixtures as compared to the pure component values (even more so with biogas).
10 mixed gas. For biogas, the selectivity values of the membranes are lower than the ideal selectivity and mixed gas selectivity due to a higher increase in CH4 permeability as compared to CO2 permeability. This study suggests that polysulfone membranes suffer a loss in selectivity and permeability with gas mixtures as compared to the pure component values (even more so with biogas).
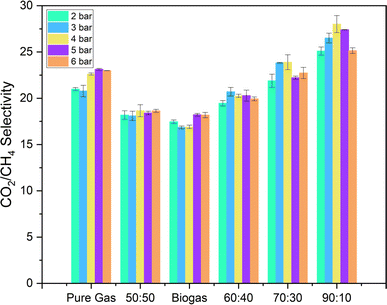 | ||
Fig. 15 CO2/CH4 selectivity of pure PSF membranes for mixed gas (CH4 and CO2) in different ratios (shown in x-axis are CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 ratio). Pure gas results are also shown for comparison. CO2 ratio). Pure gas results are also shown for comparison. | ||
The behaviour of MMMs with mixed gas feeds will now be discussed further. Fig. 16 shows the CO2 permeability values for DZ1 membrane. As in the case of pure PSF membranes, CO2 permeability increases with decrease in CO2 content in the feed at the same total pressure. The increase in permeability for feed gas containing 50%, 40%, 30% and 10% CO2 are 7.97%, 14.49%, 20.1% and 34.07% respectively, compared to pure gas values. For biogas, the CO2 permeability was 14.26% lower than with pure gas values and 20.58% lower than artificial gas mixture with similar CO2 composition. While this behaviour is qualitatively similar to what was observed for pure PSF membrane, the MMM shows higher permeability than pure PSF membranes at all compositions. Thus, the enhancement in CO2 permeability of DZ1 membranes from pure PSF membranes for feed gas containing 50%, 40%, 30% and 10% CO2 is 51.75%, 57.82%, 54.4% and 59.39% respectively at a total feed pressure of 2 bar, while at the same pressure, the CO2 permeability increased by 30.6% for biogas. The lowering of permeability for biogas indicates competitive sorption effects by the other (minor) components present.
 | ||
Fig. 16 CO2 permeability of DZ1 MMMs for mixed gas (CH4 and CO2) in different ratios (shown in x-axis are CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 ratio). Pure gas results are also shown for comparison. CO2 ratio). Pure gas results are also shown for comparison. | ||
For CH4 permeation in DZ1 membrane, as shown in Fig. 17, permeability values in mixtures are higher than for pure methane. The increase in CH4 permeability for feed gas containing 50%, 40%, 30% and 10% CO2 is 23.73%, 15.22%, 12.49% and 23.27% respectively. For biogas, an increase of 42.06% from the pure gas values and 14.81% from the artificial mixture with similar composition is observed. The increase in CH4 permeability of DZ1 membranes from pure PSF membranes for feed gas containing 50%, 40%, 30% and 10% CO2 is 6.13%, 3.81%, 6.31% and 23.53% respectively at 2 bar total feed pressure. With biogas, the CH4 permeability shows an increase of 53.31% as compared to pure PSF membranes. As the CH4 content increases, the permeability values decrease with CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 in 50
CO2 in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio showing the highest CH4 permeability, this behaviour being the same as for pure PSF membranes. The CH4 permeability in the case of biogas shows the highest increment from all the mixed gas values.
50 ratio showing the highest CH4 permeability, this behaviour being the same as for pure PSF membranes. The CH4 permeability in the case of biogas shows the highest increment from all the mixed gas values.
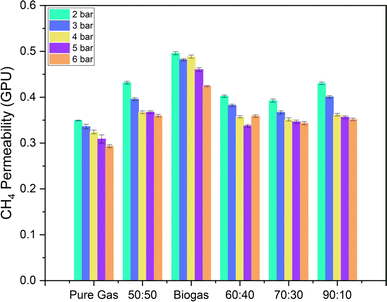 | ||
Fig. 17 CH4 permeability of DZ1 MMMs for mixed gas (CH4 and CO2) in different ratios (shown in x-axis are CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 ratio). Pure gas results are also shown for comparison. CO2 ratio). Pure gas results are also shown for comparison. | ||
For biogas, a higher decrease in CO2 permeability and increase in CH4 permeability from the pure gas values is observed as compared to pure PSF membranes. The presence of ZIF-8 in the MMMs may be responsible for such behaviour. The surface degradation of ZIF-8 on exposure to acidic gases like H2S has been reported in the literature42,44 with the development of cavities in some cases.43 A small increase in CO2 and CH4 uptake is also reported for ZIF-8 nanoparticles in the presence of H2S.42 However, a decrease in the diffusivity of CO2 is also reported due to development of a surface barrier that is created in ZIF-8 on exposure to acidic gases such as H2S. It appears that this combined effect on diffusivity and uptake is responsible for the decrease in CO2 permeability. Since ZIF-8 does not allow passage of CH4 an increased uptake results in increased permeability.
Selectivity values of DZ1 membranes for mixed gases is shown in Fig. 18. The selectivity values of mixed gas with CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 of 70
CO2 of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and 90
30 and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 is higher than the pure gas selectivity due to the greater increase in CO2 permeability than that of CH4, whereas the selectivity for 60
10 is higher than the pure gas selectivity due to the greater increase in CO2 permeability than that of CH4, whereas the selectivity for 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and 50
40 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mixtures is lower than the ideal selectivity with pure gases. A larger decrease in selectivity is seen for biogas, due to lower CO2 permeability and higher methane permeability. The selectivity values for MMMs is higher than for pure polysulfone membrane at all pressures studied. This is because of the higher increase in CO2 permeability as compared to methane due to presence of ZIF-8 in mixed matrix membranes.
50 mixtures is lower than the ideal selectivity with pure gases. A larger decrease in selectivity is seen for biogas, due to lower CO2 permeability and higher methane permeability. The selectivity values for MMMs is higher than for pure polysulfone membrane at all pressures studied. This is because of the higher increase in CO2 permeability as compared to methane due to presence of ZIF-8 in mixed matrix membranes.
 | ||
Fig. 18 CO2/CH4 selectivity of DZ1 MMMs for mixed gas (CH4 and CO2) in different ratios (shown in x-axis are CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 ratio). Pure gas results are also shown for comparison. CO2 ratio). Pure gas results are also shown for comparison. | ||
DZ5 membranes were also studied with the same gas mixtures as DZ1 MMMs. The trends of permeability from pure gas to mixed gas in mixed matrix membranes were the same as DZ1 membranes. The variation of CO2 permeability, CH4 permeability and CO2/CH4 selectivity for DZ5 membranes are shown in Fig. 19–21.
 | ||
Fig. 19 CO2 permeability of DZ5 MMMs for mixed gas (CH4 and CO2) in different ratios (shown in x-axis are CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 ratio). Pure gas results are also shown for comparison. CO2 ratio). Pure gas results are also shown for comparison. | ||
 | ||
Fig. 20 CO2 permeability of DZ5 MMMs for mixed gas (CH4 and CO2) in different ratios (shown in x-axis are CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 ratio). Pure gas results are also shown for comparison. CO2 ratio). Pure gas results are also shown for comparison. | ||
 | ||
| Fig. 21 CO2/CH4 selectivity of DZ5 MMMs for mixed gas (CH4 and CO2) in different ratios. Pure gas results are also shown for comparison. | ||
Though the DZ5 membranes showed considerable enhancement in permeability as compared with the pure polysulfone membranes for both pure and mixed gases, the CO2 and CH4 permeability were lower for DZ5 membranes as compared to DZ1 membranes. This is due to the agglomeration of particles at higher loading that can be seen in scanning electron micrographs (see Fig. 6). This agglomeration of particles leads to blockage of channels thereby restricting the amount of gas that should pass through them if there is no agglomeration. The selectivity values are similar to the DZ1 membrane; this is the result of similar decreases in CO2 and CH4 permeability.
4. Conclusions
While mixed matrix membranes for gas separations have been considered for over a decade now, the research has mostly been focused on development of novel fillers that show potential for enhancement of membrane permeability and selectivity, based on pure gas experiments. The present work is focused on mixed matrix membranes with ZIF-8 as a filler at various loadings, with the efficacy of the membranes being studied for separation of mixed gases (of different proportions of CO2 and CH4), as well as biogas, in addition to pure gas. MMMs generally show a higher permeability and CO2/CH4 selectivity, the highest increments being 56.8% and 41% respectively for the membrane with 1 wt% loading of ZIF-8. A decrease in performance at higher loadings (while these were still superior to pure PSF) is possibly because of the tendency of the filler particles to agglomerate. With gas mixtures too, MMMs were found to show increased permeability and selectivity than the pure PSF membranes for all gaseous mixture. With the change in feed gas composition, it was observed that the CO2 permeability increased with the increase in CO2 content of the feed gas. The effect of gas composition on permeability, at the same feed partial pressure, indicates significant competitive effects for CO2, while the same are negligible in the case of CH4. These results have obvious implications in staged enrichment of gas mixtures, as the mixture composition changes from one stage to the next.With biogas as feed, these membranes showed a 14.6% decrease in CO2 permeability and a 39.64% decrease in ideal selectivity from the as compared to pure gas values. These values are much lower for those observed with binary mixture containing equivalent ratio of CO2 and CH4. This difference in behaviour has been attributed to the presence of H2S in the real biogas; H2S is known to degrade the ZIF-8 structure thereby reducing the CO2 permeability.
These studies suggest that the performance of ZIF-8 MMMs is superior to pure polysulfone membranes. However, a loss of both permeability and selectivity is observed when binary gas mixtures are used. Their performance suffers further when biogas is used as a feed as compared to pure gases. With an increase in filler loading, agglomeration tends to take place thereby restricting further enhancements in permeability and selectivity. The use of ZIF-8 as filler in MMMs for biogas enrichment would require methods for reducing agglomeration at higher loadings so that full potential of ZIF-8 can be exploited.
The work highlights the potential of ZIF-8 in improving the permeability as well as selectivity of pure polysulfone membranes for removal of carbon dioxide from gas mixtures, and in particular for the upgradation of biogas to biomethane. The data on mixtures, such as generated in this paper is of obvious importance in the design of membrane cascades. In a forthcoming part of the study, we examine the behaviour of dense MMMs in the light of known theories of membrane and effective medium transport.
Data availability
The data recorded in this manuscript is available on request.Conflicts of interest
There are no conflicts to declare.References
- M. Miltner, A. Makaruk and M. Harasek, Review on available biogas upgrading technologies and innovations towards advanced solutions, J. Clean. Prod., 2017, 161, 1329–1337 CrossRef CAS.
- A. I. Adnan, M. Y. Ong, S. Nomanbhay, K. W. Chew and P. L. Show, Technologies for biogas upgrading to biomethane: a review, Bioengineering, 2019, 6(4), 1–23 CrossRef.
- E. Ryckebosch, M. Drouillon and H. Vervaeren, Techniques for transformation of biogas to biomethane, Biomass Bioenergy, 2011, 35(5), 1633–1645 CrossRef CAS.
- D. Andriani, A. Wresta, T. D. Atmaja and A. Saepudin, A review on optimization production and upgrading biogas through CO 2 removal using various techniques, Appl. Biochem. Biotechnol., 2014, 172(4), 1909–1928 CrossRef CAS.
- L. Yang, X. Ge, C. Wan, F. Yu and Y. Li, Progress and perspectives in converting biogas to transportation fuels, Renew. Sustain. Energy Rev., 2014, 40, 1133–1152 CrossRef CAS.
- Q. Sun, H. Li, J. Yan, L. Liu, Z. Yu and X. Yu, Selection of appropriate biogas upgrading technology-a review of biogas cleaning, upgrading and utilisation, Renew. Sustain. Energy Rev., 2015, 51, 521–532 CrossRef CAS.
- V. Vrbová and K. Ciahotný, Upgrading Biogas to Biomethane Using Membrane Separation, Energy Fuels, 2017, 31(9), 9393–9401 CrossRef.
- R. Kadam and N. L. Panwar, Recent advancement in biogas enrichment and its applications, Renew. Sustain. Energy Rev., 2017, 73, 892–903 CrossRef CAS.
- R. W. Baker, Future directions of membrane gas separation technology, Ind. Eng. Chem. Res., 2002, 41(6), 1393–1411 CrossRef CAS.
- L. M. Robeson, Correlation of separation factor versus permeability for polymeric membranes, J. Membr. Sci., 1991, 62(2), 165–185 CrossRef CAS.
- L. M. Robeson, The upper bound revisited, J. Membr. Sci., 2008, 320(1–2), 390–400 CrossRef CAS.
- D. R. Paul and D. R. Kemp, The diffusion time lag in polymer membranes containing adsorptive fillers, J. Polym. Sci., Polym. Symp., 1973, 93(41), 79–93 CrossRef.
- M. Vinoba, M. Bhagiyalakshmi, Y. Alqaheem, A. A. Alomair, A. Pérez and M. S. Rana, Recent progress of fillers in mixed matrix membranes for CO2separation: a review, Sep. Purif. Technol., 2017, 188, 431–450 CrossRef CAS.
- M. Rezakazemi, A. Ebadi Amooghin, M. M. Montazer-Rahmati, A. F. Ismail and T. Matsuura, State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): an overview on current status and future directions, Prog. Polym. Sci., 2014, 39(5), 817–861 CrossRef CAS.
- M. Vinoba, M. Bhagiyalakshmi, Y. Alqaheem, A. A. Alomair, A. Pérez and M. S. Rana, Recent progress of fillers in mixed matrix membranes for CO2 separation: a review, Sep. Purif. Technol., 2017, 188, 431–450 CrossRef CAS.
- S. Sorribas, B. Zornoza, C. Téllez and J. Coronas, Mixed matrix membranes comprising silica-(ZIF-8) core-shell spheres with ordered meso-microporosity for natural- and bio-gas upgrading, J. Membr. Sci., 2014, 452, 184–192 CrossRef CAS.
- A. Nuhnen, M. Klopotowski, H. B. Tanh Jeazet, S. Sorribas, B. Zornoza and C. Téllez, et al., High performance MIL-101(Cr)@6FDA-: M PD and MOF-199@6FDA- m PD mixed-matrix membranes for CO2/CH4 separation, Dalton Trans., 2020, 49(6), 1822–1829 RSC.
- M. Z. Ahmad, V. Martin-Gil, V. Perfilov, P. Sysel and V. Fila, Investigation of a new co-polyimide, 6FDA-bisP and its ZIF-8 mixed matrix membranes for CO2/CH4 separation, Sep. Purif. Technol., 2018, 207, 523–534 CrossRef CAS.
- T. Rodenas, M. V. Dalen, P. Serra-Crespo, F. Kapteijn and J. Gascon, Mixed matrix membranes based on NH2-functionalized MIL-type MOFs: influence of structural and operational parameters on the CO2/CH4 separation performance, Microporous Mesoporous Mater., 2014, 35–42 CrossRef CAS.
- C. Wu, K. Zhang, H. Wang, Y. Fan, S. Zhang and S. He, et al., Enhancing the gas separation selectivity of mixed-matrix membranes using a dual-interfacial engineering approach, J. Am. Chem. Soc., 2020, 142(43), 18503–18512 CrossRef CAS PubMed.
- A. F. Bushell, M. P. Attfield, C. R. Mason, P. M. Budd, Y. Yampolskii and L. Starannikova, et al., Gas permeation parameters of mixed matrix membranes based on the polymer of intrinsic microporosity PIM-1 and the zeolitic imidazolate framework ZIF-8, J. Membr. Sci., 2013, 427, 48–62 CrossRef CAS.
- N. A. H. M. Nordin, A. F. Ismail, A. Mustafa, R. S. Murali and T. Matsuura, The impact of ZIF-8 particle size and heat treatment on CO2/CH4 separation using asymmetric mixed matrix membrane, RSC Adv., 2014, 4(94), 52530–52541 RSC.
- N. A. H. M. Nordin, A. F. Ismail, A. Mustafa, R. S. Murali and T. Matsuura, Utilizing low ZIF-8 loading for an asymmetric PSf/ZIF-8 mixed matrix membrane for CO 2/CH 4 separation, RSC Adv., 2015, 5(38), 30206–30215 RSC.
- I. U. Khan, M. H. D. Othman, A. Jilani, A. F. Ismail, H. Hashim and J. Jaafar, et al., ZIF-8 based polysulfone hollow fiber membranes for natural gas purification, Polym. Test., 2020, 84, 106415 CrossRef.
- B. Sasikumar, S. Bisht, G. Arthanareeswaran, A. F. Ismail and M. H. D. Othman, Performance of polysulfone hollow fiber membranes encompassing ZIF-8, SiO2/ZIF-8, and amine-modified SiO2/ZIF-8 nanofillers for CO2/CH4 and CO2/N2 gas separation, Sep. Purif. Technol., 2021, 264, 118471 CrossRef CAS.
- H. J. Kim and S. I. Hong, The sorption and permeation of CO2 and CH4 for dimethylated polysulfone membrane, Korean J. Chem. Eng., 1997, 14(3), 168–174 CrossRef CAS.
- K. Ghosal, R. T. Chern, B. D. Freeman, W. H. Daly and I. I. Negulescu, Effect of basic substituents on gas sorption and permeation in polysulfone, Macromolecules, 1996, 29(12), 4360–4369 CrossRef CAS.
- M. Wiebcke, J. Cravillon, S. Münzer, S. J. Lohmeier, A. Feldhoff and K. Huber, et al., Rapid Room-Temperature Synthesis and Characterization of Nanocrystals of a Prototypical Zeolitic Imidazolate Framework, Chem. Mater., 2009, 21(8), 1410–1412 CrossRef.
- S. Negi and A. K. Suresh, Thermodynamic Aspects of Membrane Synthesis: Application to Biogas Enrichment, Ind. Eng. Chem. Res., 2023, 62(37), 15120–15135 CrossRef CAS.
- O. C. David, D. Gorri, A. Urtiaga and I. Ortiz, Mixed gas separation study for the hydrogen recovery from H2/CO/N2/CO2 post combustion mixtures using a Matrimid membrane, J. Membr. Sci., 2011, 378(1–2), 359–368 CrossRef CAS.
- Z. Si, D. Cai, S. Li, G. Li, Z. Wang and P. Qin, A high-efficiency diffusion process in carbonized ZIF-8 incorporated mixed matrix membrane for n-butanol recovery, Sep. Purif. Technol., 2019, 221, 286–293 CrossRef CAS.
- K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang and F. J. Uribe-Romo, et al., Exceptional chemical and thermal stability of zeolitic imidazolate frameworks, Proc. Natl. Acad. Sci. U. S. A., 2006, 103(27), 10186–10191 CrossRef CAS.
- N. A. H. M. Nordin, A. F. Ismail and A. Mustafa, Synthesis and preparation of asymmetric PSf/ZIF-8 mixed matrix membrane for CO2/CH4 separation, J. Teknol., 2014, 69(9), 73–76 Search PubMed.
- M. Balçık, S. B. Tantekin-Ersolmaz and M. G. Ahunbay, Interfacial analysis of mixed-matrix membranes under exposure to high-pressure CO2, J. Membr. Sci., 2020, 607, 118147 CrossRef.
- Y. Zhang, Y. Jia, M. Li and L. Hou, Influence of the 2-methylimidazole/zinc nitrate hexahydrate molar ratio on the synthesis of zeolitic imidazolate framework-8 crystals at room temperature, Sci. Rep., 2018, 8(1), 1–7 Search PubMed.
- N. Jusoh, Y. F. Yeong, K. K. Lau and A. M. Shariff, Transport properties of mixed matrix membranes encompassing zeolitic imidazolate framework 8 (ZIF-8) nanofiller and 6FDA-durene polymer: optimization of process variables for the separation of CO2from CH4, J. Cleaner Prod., 2017, 149, 80–95 CrossRef CAS.
- W. J. Koros, D. R. Paul and A. A. Rocha, Carbon Dioxide Sorption and Transport in Polycar bonate, J. Polym. Sci., 1976, 14, 687–702 CAS.
- W. J. Koros, Model for Sorption of Mixed Gases in Glassy Polymers, J. Polym. Sci., Part A-2, 1980, 18(5), 981–992 CAS.
- W. J. Koros, R. T. Chern, V. Stannett and H. B. Hopfenberg, Model for Permeation of Mixed Gases and Vapors in Glassy Polymers, J. Polym. Sci., Part A-2, 1981, 19(10), 1513–1530 CAS.
- G. Liu, Y. Labreche, V. Chernikova, O. Shekhah, C. Zhang and Y. Belmabkhout, et al., Zeolite-like MOF nanocrystals incorporated 6FDA-polyimide mixed-matrix membranes for CO2/CH4 separation, J. Membr. Sci., 2018, 565, 186–193 CrossRef CAS.
- M. J. C. Ordoñez, K. J. Balkus, J. P. Ferraris and I. H. Musselman, Molecular sieving realized with ZIF-8/Matrimid® mixed-matrix membranes, J. Membr. Sci., 2010, 361(1–2), 28–37 CrossRef.
- A. Dutta, N. Tymińska, G. Zhu, J. Collins, R. P. Lively and J. R. Schmidt, et al., Influence of Hydrogen Sulfide Exposure on the Transport and Structural Properties of the Metal-Organic Framework ZIF-8, J. Phys. Chem. C, 2018, 122(13), 7278–7287 CrossRef CAS.
- S. Reljic, A. Broto-Ribas, C. Cuadrado-Collados, E. O. Jardim, D. Maspoch and I. Imaz, et al. - 2020 - Reljic - Structural Deterioration of Well-Faceted MOFs upon H2S Exposure and its Effect in the Adsorption Performance, Chem.–Eur. J., 2020, 17110–17119 CrossRef CAS.
- C. Han, C. Zhang, N. Tymińska, J. R. Schmidt and D. S. Sholl, Insights into the Stability of Zeolitic Imidazolate Frameworks in Humid Acidic Environments from First-Principles Calculations, J. Phys. Chem. C, 2018, 122(8), 4339–4348 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra04477k |
| This journal is © The Royal Society of Chemistry 2024 |