Open Access Article
Open Access ArticleSyntheses and biological activities of calix[4]resorcinarene derivatives modified by sulfonic acid and sulfonamides†
Xiaodan Ye,
Qing Wang,
Meng Sun,
Lusi Chen,
Aiquan Jia and
Qianfeng Zhang *
*
Insitute of Molecular Engineering and Applied Chemistry, Anhui University of Technology, 59 Hudong Road, Ma'anshan, Anhui 243002, P. R. China. E-mail: zhangqf@ahut.edu.cn
First published on 12th August 2024
Abstract
Functionalization of C-propyl-resorcinolcalix[4]arene (1a) and C-iso-butyl-resorcinolcalix[4]arene (1b) with sodium sulfite and formaldehyde solution gave two corresponding sulfonatomethylated calix[4]resorcinarenes 2a/b. Further modification of 2a/b with different primary amines afforded three calix[4]resorcinarene sulfonamides 3a/b and 4c. Antibacterial and antitumor tests were performed on the starting calix[4]resorcinarenes and their sulfonic acid and sulfonamide derivatives. The results showed that in terms of antimicrobial activity calix[4]resorcinarenes and their derivatives showed bacteriostatic activity against both Escherichia coli and Staphylococcus aureus. Of which compound 1b was the most effective against Escherichia coli with a MIC value of 6.25 mg mL; compound 2b was the most effective against Staphylococcus aureus with a MIC value of 3.12 mg mL−1. In terms of antitumor activity, calix[4]resorcinarenes and their derivatives showed inhibitory effects on the three tumor cells selected for the experiment. Among them, the survival rate of A549 was 76.03% in the presence of 40 μM 1b, and the survival rates of HepG2 and MDA-MB-321 were 28.66% and 65.39% in the presence of 40 μM 2b, respectively.
Introduction
Calixarenes have attracted much interest from researchers because of their unique structures and properties. Due to their multiple modification sites, various functional groups can be easily introduced to the upper or lower edge, such as amino acids, amides, amines, alcohols, esters, aldehydes and alkyl derivatives.1–4 Calixarenes, especially their water-soluble derivatives,5 have the advantage of having no significant cytotoxicity or immunogenicity in biochemistry.6,7 The molecules carried by their upper and lower edges may also interact with proteins and nucleic acids to modulate a variety of enzyme activities, cancer cell proliferation, and metabolic pathways,8–11 which are increasingly being applied in the design and synthesis of biologically active compounds.12 Among them, the calixarene derivatives are used as drug carriers, which have certain inhibitory activities against bacteria, fungi, and cancer cells. In addition to this, organic substances such as amides, sulfonamides, guanidines, amino acids, thioureas, and other specific moieties have been verified to have a wide range of biological activities such as antiviral, antibacterial, and antitumor activities,13–16 which can be used as intermediates in the synthesis of therapeutic drugs. This gives reason to believe that calixarenes modified with nitrogen-containing organics may have future applications in bioactivity. Reports show that calixarenes and their nitrogen-containing derivatives have achieved good results in antibacterial and antitumor applications.17–21 For example, Kashapov's group synthesized a series of aminoglucosyl calix[4]resorcinarene derivatives with different chemical group compositions at the lower edge, and the biological activity exhibited by these macrocyclic compounds with biocompatible fragments was, in some respects, the first to reveal that the observed hemolytic and antimicrobial activity was dependent on the lipophilicity of the calix[4]resorcinarene structure.19Sulfonamide compounds exhibit a broad spectrum of biological activities, antibacterial mechanisms (Wood-Fields theory)22,23 and antitumor effects.24 Moreover, they serve as a carbonic anhydrase inhibitor,25 cyclooxygenase inhibitor,25 microtubule inhibitor,26 and folate-dependent enzyme inhibitor27 for inhibiting tumor proliferation. Though research on calix[4]resorcinarene and its derivatives has taken an important direction in nanotechnology, separation science, and other areas in the past few decades, its role in biological activity should not be underestimated. There are few reports on calix[4]resorcinarene and its derivatives synthesized from resorcinol, and even fewer studies on biological properties. Therefore, it is of great significance to synthesize calix[4]resorcinarenes and their derivatives modified with a series of biologically active amines containing sulfonamide groups that promote the bioactivity of the compounds and explore the changes in their bioactivity. Our group has been studying calix[4]resorcinol for many years with some results in the host–guest chemistry and the organic template for preparation of gold nanoparticles.28,29 At the same time, we have found that calix[4]resorcinarene often exists as a drug carrier and the few articles that have studied its synthesis have been linked by amide bonds. This paper focuses on the synthesis of methylsulfonamide calix[4]resorcinarene derivatives and initially explores their antibacterial and antitumor activities.
Experimental section
Materials and methods
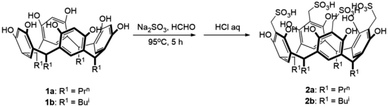
Compounds 1a/b30,31 and 2a/b32–34 were prepared according to literature methods. Both Escherichia coli and Staphylococcus aureus were LB medium, and the conditions were under 37 °C for 24 hours. Human umbilical vein endothelial cell line (HUVEC), human lung adenocarcinoma cell line (A549), hepatocellular carcinoma cell line (HepG2), and human breast cancer cell line (MDA-MB-231) were selected to evaluate the antitumor activity of the compounds. Their culture conditions were HUVEC was cultured in 89% DMEM, 10% FBS, and 1% PS for 24 h at 37 °C in an incubator containing 5% CO2; the culture conditions for HepG2 and A549 were in 89% 1640, 10% FBS, and 1% PS in a 37 °C incubator containing 5% CO2 for 24 hours; MDA-MB-231 was cultured in 89% L-15, 10% FBS and 1% PS for 24 h at 37 °C under 100% air. 1H NMR was determined by a Bruker AVANCE 400 MHz fully digitized nuclear magnetic resonance spectrometer with chemical shifts (δ, ppm) referenced to the reference SiMe4. Infrared spectra were recorded on a PerkinElmer 16 PC FT-IR spectrophotometer with use of pressed KBr pellets in the region of 400–4000 cm−1. Elemental analysis was determined by a PerkinElmer 2400 CHN analyzer. In vitro cellular OD values were determined by Biotek EPOCH2 enzyme labelling assay.Syntheses of 3a/b and 4c
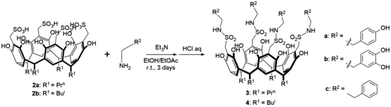
Compound 3a was synthesized by the following procedure: to the ethanol-ethyl acetate (50%) solution of tyramine hydrochloride (638 mg, 3.6 mmol) was added 0.79 mL Et3N at 0–5 °C and the mixture was stirred for half an hour. After returning to room temperature, 2a (240 mg, 0.23 mmol) was added and the reaction mixture was stirred under a nitrogen atmosphere for 3 days. The mixture was filtered and the pH of filtrate was adjusted to weak acidity with 1 M HCl, then the solution was cooled at 0–5 °C to give powder of 3a. Compounds 3b and 4c were synthesized similarly to compound 3a.The microbiological tests of compounds 1a/b, 2a/b, 3a/b, and 4c
The antimicrobial activities of compounds 1a/b, 2a/b, 3a/b, and 4c were studied with the cultures used for testing: Gram-positive bacteria Staphylococcus aureus CMCC(B) 26003 and Gram-negative bacteria Escherichia coli ATCC 25922 according to published procedures.35 Whatman No. 3 filter paper of 6 mm diameter was soaked in the prepared diluent to obtain the drug paper sheets required for the experiment and the inhibitory properties were studied by observing the size of the inhibitory circles produced by diffusion of the drug sheets on agar plates. An aqueous solution of kanamycin at 1.56 mg mL−1 was used as a positive control, sterile water and DMSO were used as blank controls, and the results showed that the above solvents had no significant inhibition effect on the bacteria involved in this experiment. Solutions of 1a/b, 2a/b, 3a/b, and 4c with a concentration of 200 mg mL−1 were prepared and the antibacterial activity is shown in Table 1. The MIC was defined as the minimum compound concentration that inhibited the growth of the corresponding test microorganism and the corresponding values are listed in Table S2.†| Minimum inhibitory concentration (MIC) in mg mL−1 | |||
|---|---|---|---|
| Serial number | Compounds | E. coli ATCC 25922 | S. aureus CMCC(B) 26003 |
| 1 | 1a | 100 | >200 |
| 2 | 1b | 6.25 | 12.5 |
| 3 | 2a | 12.5 | 6.25 |
| 4 | 2b | 12.5 | 3.12 |
| 5 | 3a | 200 | >200 |
| 6 | 3b | >200 | >200 |
| 7 | 4c | 25.0 | 12.5 |
HUVEC cells, MDA-MB-231, A549 and HePG2 cells used for in vitro toxicity testing were used as subjects. Anti-tumor properties were indicated through the survival rate of cells in each group detected by MTT assay, cells without any treatment were used as a blank control, and anti-tumor drug cisplatin was used as a positive control. The cells were cultured at 5% CO2, 37 °C for 24 h. The cells were incubated in 5% CO2 at 37 °C for 24 hours, then the medium was removed and 200 μL of medium containing different drugs was added to each well to continue treating the cells for 48 hours (final drug concentration 40 μM). After removing the medium and rinsing lightly with PBS 2 times, 200 μL of medium and 20 μL of MTT solution (final concentration of 0.5 mg mL−1) were added to each well, and after staining for 4 h, the liquid was removed, the absorbance value at 570 nm was measured by an enzyme marker after adding 150 μL of DMSO.
Results and discussion
Synthesis
Compounds 1a/b and 2a/b were prepared in high yields (83–90%) according to literature methods (Scheme 1).30–34 1H NMR and IR spectra of compounds 1a/b and 2a/b were shown in Fig. S1–S8.† The chemical shift of phenol OH protons appeared at about 8.95 ppm in compounds 1a/b as a singlet, which moved downfield to 9.73 ppm in compounds 2a/b by introducing –CH2SO3H groups at C-2 positions. Compounds 3a/b and 4c were synthesized by condensation of two sulfonic functionalized calix[4]resorcinarenes 2a/b and three organic amines in moderate yields (61–69%) (Scheme 2). Generally, the solubility of sulfonamides derivatives of calix[4]resorcinarenes became poor compared with sulfonic derivatives in common organic solvents. 1H NMR and IR spectra of compounds 3a/b and 4c were shown in Fig. S9–S14.† The linking –NHCH2CH2–Ar protons were observed in the range of 2.69–3.05 ppm in 1H NMR spectrum of compound 3b in Fig. S11.† Meanwhile, the typical medium vibration peaks at about 3250 and 1170 cm−1 for –NHSO2– groups were observed in the IR spectra of these sulfonamide compounds.Anti-microbial activity
The calix[4]resorcinarenes and their derivatives were tested for their biological activity based on their phenol structures (Table S2†).36,37 From the experimental results of compounds 1a/b, it can be seen that the longer the calix[4]resorcinarene R-chain, the better the inhibition effect on E. coli, but the effect on Staphylococcus aureus appears to be opposite to that of E. coli. Comparison of the circles of inhibition produced by compounds 2a/b reveals that the change of the R group has a much smaller influence on its antimicrobial activity than that of the group modified at the C-2 position. Whereas the compounds synthesized herein, i.e., after modification of the calixarene hydrocarbons with sulfonic acid groups at the C-2 position of the parent hydrocarbons, 2a/b not only showed good inhibitory effects on Gram-positive S. aureus, but also on Gram-negative E. coli. In 4c and 3a/b, the fewer hydroxyl groups on the benzene ring of R2, the better the antibacterial effect, indicating that hydroxyl groups negatively regulate the antibacterial effect. Modifying the sulfonic acid group at the C-2 position could significantly improve the inhibitory effect on Gram-positive S. aureus without compromising the inhibitory activity against Gram-negative E. coli. Comparing 4c with 2b, 4c with the sulfonamide active fragment exhibited a stronger inhibitory effect against both Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus, yet 2b demonstrated an even better antibacterial effect. In the reported literature,38,39 the synthesized calixarenes either inhibited only Gram-positive bacteria or inhibited both Gram-negative and positive bacteria but with average effect. In a word, calix[4]resorcinarenes and their derivatives exhibited better inhibition against S. aureus than E. coli. Compounds 2a/b showed good inhibitory effect against S. aureus (MIC = 6.25 mg mL−1, MIC = 3.12 mg mL−1), while compound 1b showed the best inhibitory effect against E. coli (MIC = 6.25 mg mL−1).Anti-tumor activity
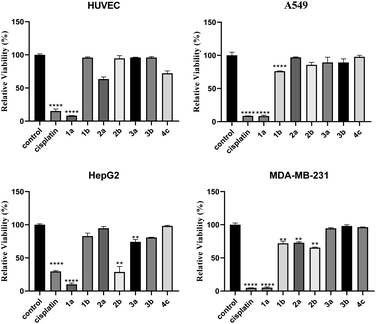
As shown in Fig. 1, by comparing the cell survival rate against HUVEC cells in the presence of compounds 1a/b and 2a/b, it can be found that the compounds with R as isobutyl are much less toxic to HUVEC than propyl, and the survival rate of HUVEC in the presence of compounds 3a/b and 4c modified by organic amines is even not less than 90%. Generally, these compounds have minimal damage to normal cells, their inhibitory effects on three types of tumor cells in vitro proliferation A549, HepG2, and MDA-MB-321 are performed. It can be found that calix[4]resorcinarene raw material 1a showed high inhibitory properties against cancer cells at a concentration of 40 mM, compound 1b showed strong inhibitory activity against the three strains of cancer cells selected for the experiments (cell survival rate: A549: 76.03%; HepG2: 82.86%; MDA-MB-321: 71.90%). Modification of the sulfonic acid group improved the inhibition of cancer cells HepG2 and MDA-MB-231, for example, compound 2b showed a decrease in relative survival to 28.66% for HepG2 cells. The tumor cell inhibitory effects of compounds modifying the sulfonamide structure varied depending on the R and amine groups: modification of compound 2a to compounds 3a/b effectively improved the inhibitory effects on A549 and HepG2. As can be seen from Fig. 1, both calix[4]resorcinarene raw materials and their sulfonic acid derivatives showed good inhibitory activity against MDA-MB-231, with cell survival rates ranging from 5.09% to 72.83, but the introduction of amide groups resulted in the increase of the survival rate of the MDA-MB-231 cells up to 96.31%. The inhibitory effect of sulfonic acid-modified compound 2b on HepG2 was comparable to cisplatin and significantly stronger than that of the other two sulfonamide derivatives with the same R2 group. Compared to previous studies,40 a series of compounds exhibited a more effective inhibitory effect on HepG2, with 2b being the most effective. The possible reasons for this result may be that the introduction of the sulfonamide group disrupts the hydroxyl structure of the sulfonic acid, which may have tumor inhibitory activity.Conclusions
A series of supramolecular compounds were synthesized from calix[4]resorcinarenes by sequential modification of sulfonic acid group and sulfonamide structures. Their antibacterial and antitumor activities were tested by disk diffusion test and MTT assay, respectively. The results showed that compound 1b had a significant inhibitory effect on the growth of Escherichia coli; compound 2b had a significant inhibitory effect on the growth of Staphylococcus aureus. The results of the relevant anti-tumor experiments suggested that compound 1b showed the highest cytotoxicity on A549, and the best inhibitory effect was observed on HepG2 and MDA-MB-321 with compound 2b. Generally, the sulfonamide derivatives lack strong antibacterial and anti-tumor properties possibly due to the introduction of the sulfonamide group disrupts the important hydroxyl group of the sulfonic acid structure.Conflicts of interest
There are no conflicts to declare.Data availability
The data can be obtained upon request from the corresponding author.Acknowledgements
This project was supported by National Natural Science Foundation of China (90922008).References
- S. Karakurt, T. F. Kellici, T. Mavromoustakos, A. G. Tzakos and M. Yilmaz, Curr. Org. Chem., 2016, 20, 1043–1057 CrossRef CAS.
- E. Akceylan, A. Uyanik, S. Eymur, O. Sahin and M. Yilmaz, Appl. Catal. Gen., 2015, 499, 205–212 CrossRef CAS.
- A. Uyanik, M. Bayrakci, S. Eymur and M. Yilmaz, Tetrahedron, 2014, 70, 9307–9313 CrossRef CAS.
- S. Eymur, E. Akceylan, O. Sahin, A. Uyanik and M. Yilmaz, Tetrahedron, 2014, 70, 4471–4477 CrossRef CAS.
- F. Perret, A. N. Lazar and A. W. Coleman, Chem. Commun., 2006, 42, 2425–2438 RSC.
- M. A. Blaskovich, Q. Lin, F. L. Delarue, J. Sun, H. S. Park, D. Coppola, A. D. Hamilton and S. M. Sebti, Nat. Biotechnol., 2000, 18, 1065–1070 CrossRef CAS PubMed.
- K. Wang, D.-S. Guo, H.-Q. Zhang, D. Li, X.-L. Zheng and Y. Liu, J. Med. Chem., 2009, 52, 6402–6412 CrossRef CAS PubMed.
- E. Ozyilmaz, M. Bayrakci and M. Yilmaz, Bioorg. Chem., 2016, 65, 1–8 CrossRef CAS.
- A. Yousaf, S. A. Hamid, N. M. Bunnori and A. Ishola, Therapy, 2015, 9, 2831–2838 CAS.
- P. L. Padnya, E. A. Andreyko, O. A. Mostovaya, I. K. Rizvanov and I. I. Stoikov, Org. Biomol. Chem., 2015, 13, 5894–5904 RSC.
- M. Durmaz, M. Yilmaz and A. Sirit, Org. Biomol. Chem., 2011, 9, 571–580 RSC.
- F. N. Pur, Mol. Divers., 2016, 20, 781–787 CrossRef PubMed.
- M. Y. Fosso, Y. Li and S. Garneau-Tsodikova, MedChemComm, 2014, 5, 1075–1091 RSC.
- J. L. Houghton, K. D. Green, W. Chen and S. Garneau-Tsodikova, ChemBioChem, 2010, 11, 880–902 CrossRef CAS PubMed.
- C. Santini, M. Pellei, V. Gandin, M. Porchia, F. Tisato and C. Marzano, Chem. Rev., 2013, 114, 815–862 CrossRef PubMed.
- J. A. Lessa, M. A. Soares, R. G. Dos Santos, I. C. Mendes, L. B. Salum, H. N. Daghestani, A. D. Andricopulo, B. W. Day and A. Vogt, BioMetals, 2013, 26, 151–165 CrossRef CAS PubMed.
- M. J. Colston, H. C. Hailes, E. Stavropoulos, A. C. Hervé, G. Hervé, K. J. Goodworth, A. M. Hill, P. Jenner, P. D. Hart and R. E. Tascon, Infect. Immun., 2004, 72, 6318–6323 CrossRef CAS PubMed.
- S. F. Alshahateeta, S. A. Al-Trawneha, W. A. Al-Zereinib and S. S. Al-Sarhana, Jordan J. Chem., 2014, 9, 170–186 CrossRef.
- R. Kashapov, Y. Razuvayeva, A. Ziganshina, T. Sergeeva, S. Lukashenko, A. Sapunova, A. Voloshina, N. Kashapova, I. Nizameev, V. Salnikov, S. Ziganshina, B. Gareev and L. Zakharova, Inorg. Chem., 2020, 59, 18276–18286 CrossRef CAS.
- L. An, L.-L. Han, Y.-G. Zheng, X.-N. Peng, Y.-S. Xue, X.-K. Gu, J. Sun and C.-G. Yan, Eur. J. Med. Chem., 2016, 123, 21–30 CrossRef CAS PubMed.
- L. An, C. Wang, Y.-G. Zheng, J.-D. Liu and T.-H. Huang, Eur. J. Med. Chem., 2021, 210, 112984 CrossRef CAS.
- S. Kumar Verma, R. Verma, F. Xue, P. Kumar Thakur, Y. R. Girish and K. P. Rakesh, Bioorg. Chem., 2020, 105, 104400 CrossRef CAS PubMed.
- A. Ovung and J. Bhattacharyya, Biophys. Rev., 2021, 13, 259–272 CrossRef CAS PubMed.
- S. Hao, X. Cheng, X. Wang, R. An, H. Xu, M. Guo, C. Li, Y. Wang, Z. Hou and C. Guo, Bioorg. Chem., 2020, 104, 104237 CrossRef CAS PubMed.
- A. A. Abdel-Aziz, A. Angeli, A. S. El-Azab, M. E. A. Hammouda, M. A. El-Sherbeny and C. T. Supuran, Bioorg. Chem., 2019, 84, 260–268 CrossRef CAS PubMed.
- Z. L. Liu, W. Tian, Y. Wang, S. Kuang, X. M. Luo and Q. Yu, Acta Pharmacol. Sin., 2012, 33, 261–270 CrossRef CAS PubMed.
- S. L. Kordus and A. D. Baughn, Medchemcomm, 2019, 10, 880–895 RSC.
- X.-M. Zhou, Q. Wang, M. Sun, J.-L. Liu, A.-Q. Jia and Q.-F. Zhang, J. Incl. Phenom. Macrocycl. Chem., 2023, 103, 289–299 CrossRef CAS.
- J.-L. Liu, B.-B. Zhang, A.-Q. Jia, Z.-F. Xin and Q.-F. Zhang, J. Incl. Phenom. Macrocycl. Chem., 2021, 99, 79–86 CrossRef CAS.
- A. Vollbrecht, I. Neda, H. Thönnessen, P. C. Jones, R. K. Harris, L. A. Crowe and R. Schmutzler, Chem. Ber., 1997, 130, 1715–1720 CrossRef CAS.
- L. M. Tunstad, J. A. Tucker, E. Dalcanale, J. Weiser, J. A. Bryant, J. C. Sherman, R. C. Helgeson, C. B. Knobler and D. J. Cram, J. Org. Chem., 1989, 54, 1305–1312 CrossRef CAS.
- E. Kh. Kazakova, N. A. Makarova, A. U. Ziganshina, L. A. Muslinkina, A. A. Muslinkina and W. D. Habicher, Tetrahedron Lett., 2000, 41, 10111–10115 CrossRef CAS.
- E. Sanabria, M. Á. Esteso, A. Pérez-Redondo, E. Vargas and M. Maldonado, Molecules, 2015, 20, 9915–9928 CrossRef CAS.
- E. Sanabira, M. Á. Esteso, E. Vargas and M. Maldonado, J. Mol. Liq., 2018, 254, 391–397 CrossRef.
- A. U. Hassan, S. H. Sumrra, M. A. Raza, M. Zubair, M. N. Zafar, E. U. Mughal, M. F. Nazar, A. Irfan, M. Imran and M. A. Assiri, Appl. Organomet. Chem., 2021, 35, e6054–e6070 CrossRef CAS.
- U. Panchal, K. Modi, M. Panchal, V. Mehtaet and V. K. Jain, Chinese. J. Catal., 2016, 37, 250–257 CrossRef CAS.
- T. Modjinoua, D. L. Versace, S. Abbad-Andaloussi, V. Langlois and E. Renarda, Mater. Today, 2017, 12, 19–28 Search PubMed.
- H. M. Abosadiya, S. A. Hasbullah, M. M. Mackeen, S. C. Low, N. Ibrahim, M. Koketsu and B. M. Yamin, Molecules, 2013, 18, 13369–13384 CrossRef CAS.
- N. Kushwaha, R. K. Saini and S. K. S. Kushwaha, Int. J. ChemTech Res., 2011, 3, 203–209 CAS.
- G. H. Elgemeie, R. A. Azzam and R. E. Elsayed, Med. Chem. Res., 2019, 28, 1099–1131 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra04426f |
| This journal is © The Royal Society of Chemistry 2024 |