Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
One-pot synthesis of tetrahydropyrimidinecarboxamides enabling in vitro anticancer activity: a combinative study with clinically relevant brain-penetrant drugs†
Dipti B. Upadhyay‡
a,
Joaquina Nogales‡b,
Jaydeep A. Mokariya a,
Ruturajsinh M. Vala
a,
Ruturajsinh M. Vala a,
Vasudha Tandonb,
Sourav Banerjee
a,
Vasudha Tandonb,
Sourav Banerjee *b and
Hitendra M. Patel
*b and
Hitendra M. Patel *a
*a
aDepartment of Chemistry, Sardar Patel University, Vallabh Vidyanagar, Gujarat, India. E-mail: hm_patel@spuvvn.edu
bDivision of Cancer Research, School of Medicine, University of Dundee, Dundee DD1 9SY, UK. E-mail: s.y.banerjee@dundee.ac.uk
First published on 27th August 2024
Abstract
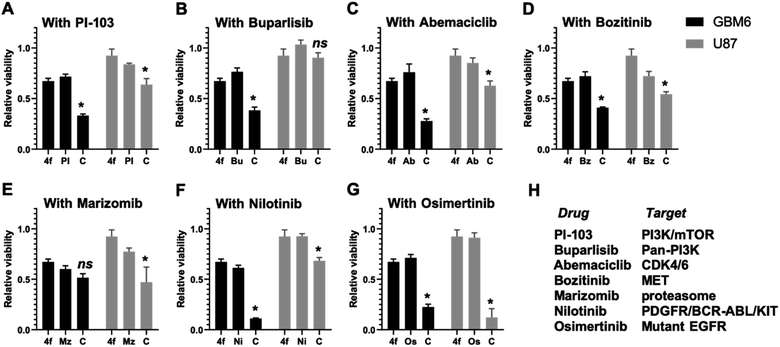
In this study, we describe a one-pot three-component synthesis of bioactive tetrahydopyrimidinecarboxamide derivatives employing lanthanum triflate as a catalyst. Out of the synthesized compounds, 4f had the most potent anti-cancer activity and impeded cell cycle progression effectively. Anti-cancer bioactivity was observed in 4f against liver, breast, and lung cancers as well as primary patient-derived glioblastoma cell lines. Compound 4f effectively inhibited the 3D neurosphere formation in primary patient-derived glioma stem cells. Specifically, 4f exhibited synergistic cytotoxicity with the EGFR inhibitor that is the clinical epidermal growth factor receptor inhibitor osimertinib. 4f does not exhibit anti-kinase activity and is cytostatic in nature, and further work is needed to understand the true molecular target of 4f and its derivatives. Through our current work, we establish a promising tetrahydopyrimidinecarboxamide-based lead compound with anti-cancer activity, which may exhibit potent anti-cancer activity in combination with specific clinically relevant small molecule kinase inhibitors.
1 Introduction
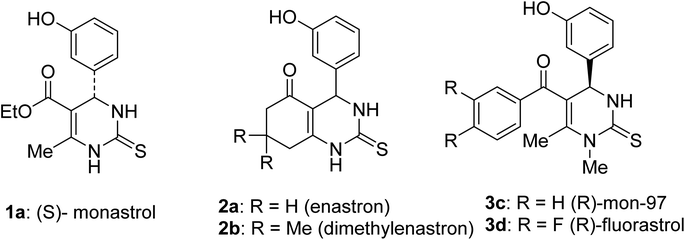
Dihydropyrimidinones (DHPMs) produced via the Biginelli reaction have received significant attention owing to their diverse biological applications, such as acetylcholinesterase inhibitors,1 HIV-1 replication inhibitors,2 urease inhibitors,3 calcium channel blockers,4 antimicrobial agents,5 antioxidant agents,6 nonsteroidal RORα agonists,7 anti-cancer cytotoxic agents,8 and antitubercular agents.9 From this DHPM family of compounds, monastrol emerged as a key lead compound.10,11 Monastrol has significant anticancer properties (Fig. 1) and prevents metastasis by impeding the movement of kinesin spindle protein Eg5,12 which associates with microtubules of the mitotic spindle13 and engages in intracellular transport and cell division.14 Dihydropyrimidine derivatives allosterically inhibit Eg5-mediated microtubule organisation and drive the anti-metastatic activities.15 Monastrol was first observed to cause cell cycle disruption in Xenopus models16 and later monastrol and its derivatives were reported to have robust anti-proliferative activities against cancer cells.17 Multiple research groups have since developed several DHPMs that exhibited antiproliferative properties against other human solid tumour cell lines (Fig. 1).In the current study, we wanted to utilise our long-standing successful strategy11,18–25 of utilising the Biginelli reaction to synthesize benzyloxy derivatives of DHPM to obtain new bioactive, anti-cancer compounds. The benzyloxyphenyl group has diverse medicinal properties, including antimicrobial,26 antitubercular,27 antifungal,28 and anticancer activities.11,29–32 Similarly, the incorporation of a 1,2,3-triazole moiety into various compounds has shown significant pharmacological potential, such as anticancer,33–38 antimicrobial,33,39 anti-inflammatory,40 antitubercular,41 anti-HIV,42 and antiviral effects.33 Studies have shown that 1,2,3-triazoles can exert anticancer effects through various mechanisms, such as enzyme inhibition and targeting receptor tyrosine kinases.43–48
The aim of this study was to utilise the Biginelli reaction to integrate the 1,4-disubstituted 1,2,3-triazole and benzyloxyphenyl group using a wide variety of catalysts like Lewis acids, salts, or ionic liquids to generate phenyloxymethyl-1,2,3-triazole derivatives with potentially distinct anticancer attributes. Furthermore, we employed lanthanum triflate as a catalyst that acts as a Lewis acid resulting in excellent yield over a short reaction period. From the series of compounds synthesized, we identified one derivative to have potent anti-proliferative effects across multiple solid tumour cell lines and effectively impede the cell cycle with a reduction in cancer stem cell numbers in primary patient-derived cancer cells. The derivative also induced cytotoxicity in combination with FDA-approved clinical drugs thus suggesting a novel paradigm in our efforts to utilise the Biginelli reaction and green catalysts to generate anti-cancer compounds.
2 Results and discussion
2.1 Chemistry
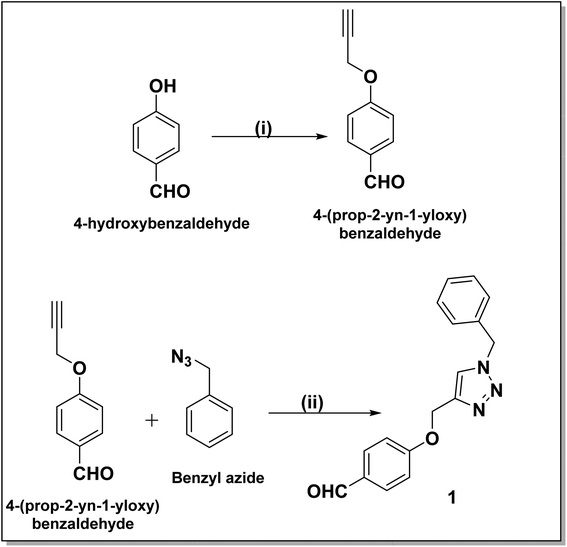
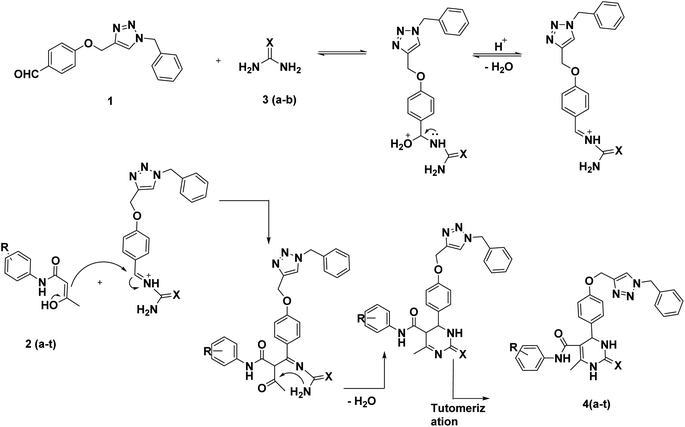
The synthesis of benzyl triazolyl methoxy derivative of DHPM was carried out by the multicomponent reaction of the 4-((1-benzyl-1H-1,2,3-triazole-4-yl)methoxy)benzaldehyde, different acetoacetanilide derivatives, and urea/thiourea. The inclusion of this aldehyde in the reaction is anticipated to enhance its biological applications. Compound 1 which is 4-((1-benzyl-1H-1,2,3-triazole-4-yl)methoxy)benzaldehyde, was prepared through a click reaction involving 4-(prop-2-yn-1-yloxy)benzaldehyde and benzyl azide, as illustrated in Scheme 1. The reaction was carried out by employing 10 mol% lanthanum triflate as a catalyst and under a heating environment at 100 °C for about 1–1.5 h, which resulted in good-to-excellent yields.Our initial study was carried out both in solvent-free conditions as well as under various solvents like ethanol, acetonitrile, ethylene dichloride, tetrahydrofuran, dimethylformamide, and water (Table 1). The effect of solvent amount and required time was investigated. Among the mentioned solvents, ethanol gave the best outcomes with an excellent yield. It was found that using more solvent caused the reaction to take 3.5 hours to complete under reflux conditions. However, when the minimum amount of ethanol (0.5 ml) was utilized at 100 °C, the reaction mixture solidified within the 35 min but remained slightly incomplete. Hence, we decided to use 0.5 ml more ethanol to the reaction mixture and stir for 25 minutes. This successfully completed the reaction, and we observed a high-yield conversion of DHPM. Having set optimized parameters, La(OTf)3-catalyzed 20 derivatives of dihydopyrimidones were synthesized by employing various substrates (Table 2). All the synthesized compounds were characterized by 1H nuclear magnetic resonance (NMR), 13C{1H}35 attached proton test,49 and either high-resolution mass spectra (HRMS) or liquid chromatography-mass spectrometry (LCMS) analysis (see ESI†).
| Entry | Solvent | Temp. | Time | Conversion relative to aldehydeb |
|---|---|---|---|---|
| a Abbreviations: DCE, 1,2-dichloroethane; DMF, dimethylformamide; TLC, thin-layer chromatography; THF, tetrahydrofuran. Reaction condition: 1 mmol 4-((1-benzyl-1H-1,2,3-triazole-4-yl)methoxy)benzaldehyde 1c, 1.1 mmol acetoacetanilide derivative 2c, 1.2 mmol urea 3, and 0.1 mmol La(OTf)3.b Observation from TLC analysis.c More than 0.5 ml solvent.d 0.5 ml solvent. | ||||
| 1 | CH3CNc | Reflux | 4 h | 100% |
| 2 | DCEc | Reflux | 4 h | Incomplete |
| 3 | THFc | Reflux | 4 h | Incomplete |
| 4 | DMFc | Reflux | 3 h | Mixture of product |
| 5 | Waterc | Reflux | 5 h | Incomplete |
| 6 | Ethanolc | Reflux | 3.5 h | 100% |
| 7 | Ethanold | 100 °C | 1 h | 100% |
| 8 | Solvent-free | 100 °C | 30 min | Incomplete |
| 9 | Catalyst-free | 100 °C | 30 min | Mixture of product |
| Entry | –R | X | Product | Time (h) | Yielda (%) |
|---|---|---|---|---|---|
| a Isolated yield. | |||||
| 1 | –H | O | 4a | 1 | 97 |
| 2 | 2-Cl | O | 4b | 1 | 89 |
| 3 | 3-Cl | O | 4c | 1 | 90 |
| 4 | 4-Cl | O | 4d | 1 | 91 |
| 5 | 4-F | O | 4e | 1.5 | 88 |
| 6 | 3-CF3 | O | 4f | 1.5 | 91 |
| 7 | 2-CH3 | O | 4g | 1 | 87 |
| 8 | 4-CH3 | O | 4h | 1 | 86 |
| 9 | 2-OCH3 | O | 4i | 1 | 91 |
| 10 | 4-OCH3 | O | 4j | 1 | 89 |
| 11 | 3,4-(CF3)2 | O | 4k | 1.5 | 79 |
| 12 | –H | S | 4l | 1 | 94 |
| 13 | 2-Cl | S | 4m | 1 | 92 |
| 14 | 3-Cl | S | 4n | 1 | 89 |
| 15 | 4-Cl | S | 4o | 1 | 95 |
| 16 | 4-F | S | 4p | 1.5 | 85 |
| 17 | 3-CF3 | S | 4q | 1.5 | 86 |
| 18 | 2-CH3 | S | 4r | 1 | 88 |
| 19 | 4-CH3 | S | 4s | 1 | 90 |
| 20 | 2-OCH3 | S | 4t | 1 | 88 |
After improving the reaction, the substrate scope for the La(OTf)3-catalyzed synthesis of dihydropymidine derivatives was investigated by changing the different acetoacetanilide derivatives. Substitution of the acetoacetanilides gave a slight variation in the time of reaction and yield of the product (Table 2). Compound 4a was obtained with an excellent 97% yield in one hour (Table 2, entry 1), while when 3,4-(CF3)2 substituted acetoacetanilide derivative was employed under the same reaction condition, a slightly decreased yield of 79% was observed (Table 2, entry 11) in 1.5 h. When chloro substituted acetoacetanilide derivatives were used as a substrate, a comparatively good yield was observed than the methyl-substituted acetoacetanilide derivatives (Table 2). In the case of trifluoro methyl substituted acetoacetanilide derivative, it takes 1.5 h to complete the reaction. Based on the above-discussed facts, it was observed that substitutions on the acetoacetanilide have shown great influence on the reaction time as well as in isolated yield (Scheme 2).
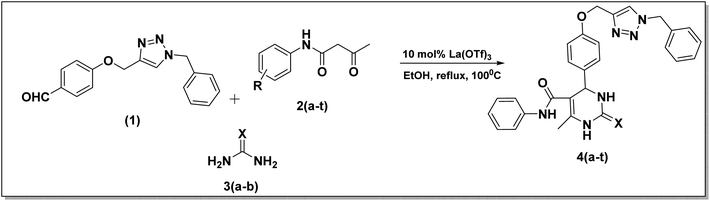 | ||
| Scheme 2 Multicomponent synthesis of dihydropyrimidinones from 4-((1-benzyl-1H-1,2,3-triazole-4-yl)methoxy)benzaldehyde 1, acetoacetenilide derivatives 2(a–t), and urea derivative 3(a–b). | ||
The Biginelli reaction is an example of an acid-catalysed three-component reaction. In the first step of the mechanism, the acid protonates the aldehyde. The nucleophilic NH2 group from urea then attacks the electrophilic aldehyde, leading to the formation of an N-acyliminium ion intermediate with the release of a water molecule. Next, the enol form of the β-keto amide attacks the N-acyliminium ion, resulting in the formation of a ureide intermediate. This intermediate subsequently converts into the final product (Scheme 3).
2.2 Biological evaluation
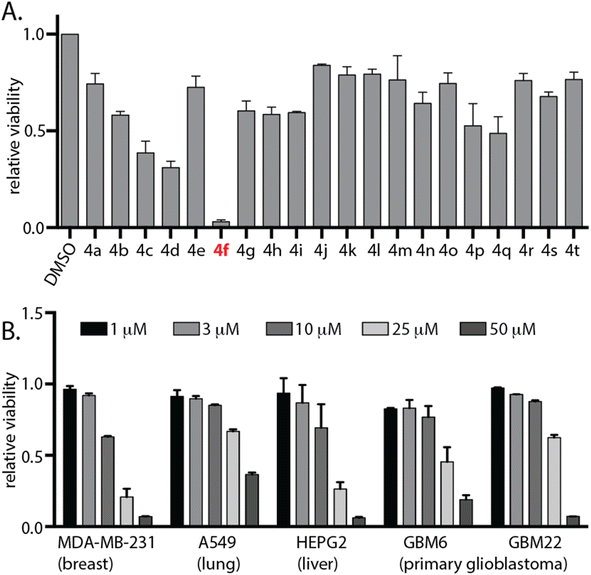 | ||
| Fig. 2 4f exhibits anti-cancer activity in diverse cancer cell lines. (A) Cell viability of a panel of DHPM derivatives 4a–t at 25 μM against human U87 cell line was assessed after 96 hours using the CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay kit. Viability of DMSO-treated cells was used as a control. Data are represented as fold viability of DMSO-treated control. (B) Indicated cell lines were treated with various doses of 4f for 72 hours and their viability was measured using CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay kit. Viability of DMSO-treated cells was used as control. Data are represented as fold viability of DMSO-treated control for each cell line with n = 3 biological replicates. See also ESI Fig. S1.† | ||
| Comp. | MW | RB | HBA | HBD | MR | TPSA | X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
NR | NC | NH | Atom |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Abbreviation: MW: molecular weight; RB: rotational bond; HBA: H-bond acceptor; HBD: H-bond donor; MR: molecular refractivity; TPSA: topological polar surface area; NR: no. of rings; NC: no. of carbon; NH: no. of heteroatoms. | |||||||||||||
| 4f | 562.54 | 10 | 8 | 3 | 151.21 | 110.17 | 3.74 | 4.91 | 3.32 | 5 | 29 | 12 | 66 |
| Lipinski filter | Ghose filter | Veber filter | Egan filter | Muegge filter |
|---|---|---|---|---|
| MW ≤ 500 | 160 ≤ MW ≤ 480 | RB ≤ 10 | W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ≤ 5.88 P ≤ 5.88 |
200 ≤ MW ≤ 600 |
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ≤ 4.15 P ≤ 4.15 |
−0.4 ≤ W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ≤ 5.6 P ≤ 5.6 |
TPSA ≤ 140 | TPSA ≤ 131.6 | −2X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ≤ 5; TPSA ≤ 150 P ≤ 5; TPSA ≤ 150 |
| HBA ≤ 10 | 40 ≤ MR ≤ 130 | NR ≤ 7; NC > 4; NH > 1; RB ≤ 15; HBA ≤ 10; HBD ≤ 5 | ||
| HBD ≤ 5 | 20 ≤ atoms ≤ 70 |
3 Conclusion
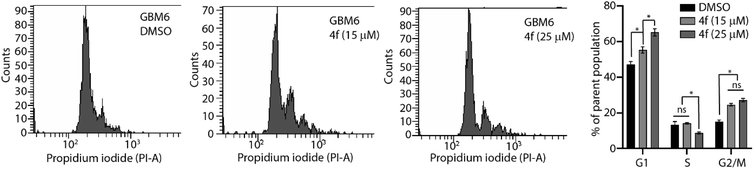
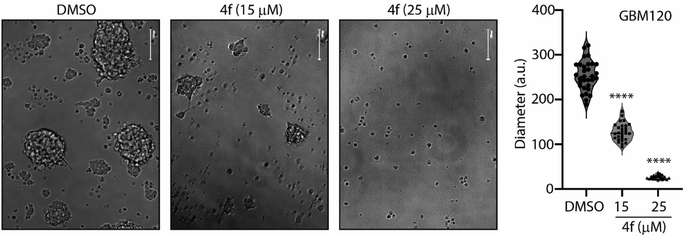
A series of benzyloxy derivatives of DHPM was synthesised using a multicomponent reaction. Lanthanum triflate catalyzed reaction gave good to excellent yield within 1–1.5 h. The advantages of this protocol include good to high yields, operational simplicity, simple filtration and needing no extraction or separation by column chromatography is necessary. Out of the series we identified 4f with the most potent anti-cancer activity in a diverse set of cancer cell lines including primary patient-derived cells. Furthermore, in combination with brain-penetrant small molecule kinase and proteasome inhibitors, 4f induced potent cytostatic activity and cell cycle impediment which attests for further medicinal chemistry and development of the benzyloxy derivatives of DHPM backbone. Interestingly, treating patient derived glioma stem 3D neurospheres with 4f significantly reduced neurosphere diameter (Fig. 5), this suggesting potent anti-glioma activity in stem-like organoid models. Furthermore, 4f exhibited a remarkable potency in combination with EGFR-mutant targeting osimertinib. Although kinase inhibitory activity (ESI Fig. S2†) or apoptosis (ESI Fig. S1†) were not observed for 4f, the data do suggest that a future in vivo bioavailable derivative of 4f could be used as a combination with osimertinib to target cancers with mutated/amplified EGFR. Both osimertinib and marizomib are covalent inhibitors and represent a paradigm shift toward cancer therapeutics. The ability of our drug to efficiently combine with covalent inhibitor provides an impetus toward further SAR and congener exploration to improve the DHPM backbone for clinical readiness. Compound 4f shows the most promising drug candidate due to its favorable drug-likeness and in silico ADMET properties. Therefore, this study establishes a novel synthetic scheme which will allow for the development of future clinically relevant anti-cancer molecules which can be used in combination with specific drugs targeting EGFR mutated cancers.4 Experimental
4.1 General
All chemicals were purchased from commercially available sources and used without further purification. Melting points were determined by the open capillary tube method and are uncorrected. 1H NMR and 13C{1H} NMR, HSQC, and HMBC spectral analysis were recorded on BRUKER AVANCE II 600 NMR Spectrometer equipped with cryogenic TCI probe using DMSO-d6 as the solvents. Abbreviation used for NMR signal: s = Singlet, d = Doublet, t = triplet, dd = double doublet m = Multiplet. The chemical shifts are expressed in parts per million and coupling constants (J) are provided in Hertz.4.2 General procedure for the synthesis of spiroxindoles 4(a–t)
A mixture of 1 mmol 4-((1-benzyl-1H-1,2,3-triazole-4-yl)methoxy)benzaldehyde 1(a–c), 1.1 mmol of acetoacetenilide derivatives 2(a–k), and 1.2 mmol urea derivative 3(a–b) was added to a 50 ml round bottom flask with 0.5 ml ethanol and 0.1 mmol (10 mol%) lanthanum triflate. It was stirred at 100 °C for 35 min. Within this time, the reaction mixture solidified or became sticky, and then 0.5 ml more ethanol was added and stirred for the required time, as mentioned in Table 2. Next, 5 ml more ethanol was added and cooled to room temperature. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was poured slowly into 20 ml ice-cold water with stirring and kept still until the precipitation of the product was completed. The crude product was filtered and washed with 20% aqueous solution of ethanol (5 ml × 3). Products were recrystallized from 6 ml ethanol.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 157.20 (C–O–), 165.23 (C
O), 157.20 (C–O–), 165.23 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (ESI-TOF) m/z calcd for C28H25ClN6O3 (M + H)+: 494.21, found: 495.25.
O); MS (ESI-TOF) m/z calcd for C28H25ClN6O3 (M + H)+: 494.21, found: 495.25.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 157.97 (C–O–), 165.73 (C
O), 157.97 (C–O–), 165.73 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (ESI-TOF) m/z calcd for C28H25ClN6O3 (M + H)+: 528.17, found: 529.03.
O); MS (ESI-TOF) m/z calcd for C28H25ClN6O3 (M + H)+: 528.17, found: 529.03.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 157.85 (–C–O–), 166.13 (C
O), 157.85 (–C–O–), 166.13 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (ESI-TOF) m/z calcd for C28H25ClN6O3 (M + H)+: 528.16, found: 529.01.
O); MS (ESI-TOF) m/z calcd for C28H25ClN6O3 (M + H)+: 528.16, found: 529.01.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 157.05 (C–O), 165.94 (C
O), 157.05 (C–O), 165.94 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (ESI-TOF) m/z calcd for C28H25ClN6O3 (M + H)+: 528.16, found: 529.00.
O); MS (ESI-TOF) m/z calcd for C28H25ClN6O3 (M + H)+: 528.16, found: 529.00.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 159.64 (C–O), 165.78 (C
O), 159.64 (C–O), 165.78 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (ESI-TOF) m/z calcd for C28H25FN6O3 (M + H)+: 512.19, found: 513.08.
O); MS (ESI-TOF) m/z calcd for C28H25FN6O3 (M + H)+: 512.19, found: 513.08.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 157.89 (C–O), 166.29 (C
O), 157.89 (C–O), 166.29 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (ESI-TOF) m/z calcd for C29H25F3N6O3 (M + H)+: 562.19, found: 563.01.
O); MS (ESI-TOF) m/z calcd for C29H25F3N6O3 (M + H)+: 562.19, found: 563.01.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 157.92 (–CH–), 165.72 (C
O), 157.92 (–CH–), 165.72 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (ESI-TOF) m/z calcd for C29H28N6O3 (M + H)+: 508.22, found: 509.10.
O); MS (ESI-TOF) m/z calcd for C29H28N6O3 (M + H)+: 508.22, found: 509.10.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 157.82 (C–O), 165.68 (C
O), 157.82 (C–O), 165.68 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (ESI-TOF) m/z calcd for C29H28N6O3 (M + H)+: 508.22, found: 509.10.
O); MS (ESI-TOF) m/z calcd for C29H28N6O3 (M + H)+: 508.22, found: 509.10.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 152.69 (C–O), 158.18 (C–O), 165.06 (C
O), 152.69 (C–O), 158.18 (C–O), 165.06 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).; MS (ESI-TOF) m/z calcd for C29H28N6O4 (M + H)+: 524.22, found: 525.03.
O).; MS (ESI-TOF) m/z calcd for C29H28N6O4 (M + H)+: 524.22, found: 525.03.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 155.67 (C–O), 157.82 (C–O), 168.47 (C
O), 155.67 (C–O), 157.82 (C–O), 168.47 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (ESI-TOF) m/z calcd for C29H28N6O4 (M + H)+: 524.22, found: 525.08.
O); MS (ESI-TOF) m/z calcd for C29H28N6O4 (M + H)+: 524.22, found: 525.08.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 165.53 (C–O), 174.38 (C
O), 165.53 (C–O), 174.38 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S); MS (ESI-TOF) m/z calcd for C28H26N6O2S (M + H)+: 445.1067, found: 445.1081.
S); MS (ESI-TOF) m/z calcd for C28H26N6O2S (M + H)+: 445.1067, found: 445.1081.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.87 (C–O), 173.68 (C
O), 164.87 (C–O), 173.68 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S); MS (ESI-TOF) m/z calcd for C28H25ClN6O2S (M + H)+: 510.18, found: 511.06.
S); MS (ESI-TOF) m/z calcd for C28H25ClN6O2S (M + H)+: 510.18, found: 511.06.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 165.79 (C–O), 174.42 (C
O), 165.79 (C–O), 174.42 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S); MS (ESI-TOF) m/z calcd for C28H25ClN6O2S (M + H)+: 544.14, found: 544.98.
S); MS (ESI-TOF) m/z calcd for C28H25ClN6O2S (M + H)+: 544.14, found: 544.98.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 165.61 (C–O), 174.41 (C
O), 165.61 (C–O), 174.41 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S); MS (ESI-TOF) m/z calcd for C28H25ClN6O2S (M + H)+: 544.14, found: 544.97.
S); MS (ESI-TOF) m/z calcd for C28H25ClN6O2S (M + H)+: 544.14, found: 544.97.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 165.44 (C–O), 174.40 (C
O), 165.44 (C–O), 174.40 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S); MS (ESI-TOF) m/z calcd for C28H25FN6O2S (M + H)+: 528.17, found: 529.02.
S); MS (ESI-TOF) m/z calcd for C28H25FN6O2S (M + H)+: 528.17, found: 529.02.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 165.97 (C–O), 174.43 (C
O), 165.97 (C–O), 174.43 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S); MS (ESI-TOF) m/z calcd for C29H25F3N6O2S (M + H)+: 578.17, found: 579.01.
S); MS (ESI-TOF) m/z calcd for C29H25F3N6O2S (M + H)+: 578.17, found: 579.01.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 165.46 (C–O), 174.55 (C
O), 165.46 (C–O), 174.55 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S); MS (ESI-TOF) m/z calcd for C29H28N6O2S (M + H)+: 524.20, found: 525.03.
S); MS (ESI-TOF) m/z calcd for C29H28N6O2S (M + H)+: 524.20, found: 525.03.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 165.31 (C–O), 174.36 (C
O), 165.31 (C–O), 174.36 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S); MS (ESI-TOF) m/z calcd for C29H28N6O2S (M + H)+: 524.19, found: 525.03.
S); MS (ESI-TOF) m/z calcd for C29H28N6O2S (M + H)+: 524.19, found: 525.03.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 158.36 (C–O), 164.83 (C–O), 174.02 (C
O), 158.36 (C–O), 164.83 (C–O), 174.02 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S); MS (ESI-TOF) m/z calcd for C29H28N6O3S (M + H)+: 540.19, found: 541.00.
S); MS (ESI-TOF) m/z calcd for C29H28N6O3S (M + H)+: 540.19, found: 541.00.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 158.09 (C–O), 165.08 (C–O), 174.34 (C
O), 158.09 (C–O), 165.08 (C–O), 174.34 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S); MS (ESI-TOF) m/z calcd for C29H28N6O3S (M + H)+: 540.19, found: 541.04.
S); MS (ESI-TOF) m/z calcd for C29H28N6O3S (M + H)+: 540.19, found: 541.04.4.3 Materials and methods
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
DBU, JN, RMV, SB, and HMP are named inventors on the patent no. 528662 awarded by the government of India on 18th March 2024 pertaining to these reported compounds. No other conflicts of interest reported.Acknowledgements
DBU, JAM, RMV and HMP are grateful to the department of chemistry, Sardar Patel University for providing lab facilities. DBU is thankful to Knowledge Consortium of Gujarat (KCG), Department of Education, Government of Gujarat, India for SHODH-Scheme of Developing High Quality Research (Student Ref No. 2021016413). JAM is grateful to CSIR, New Delhi, for a CSIR-SRF. SB is funded by the United Kingdom Research and Innovation Future Leader Fellowship MR/W008114/1. VT and SB are funded by the Ninewells Cancer Campaign Cancer Research grant.References
- S. Arunkhamkaew, A. Athipornchai, N. Apiratikul, A. Suksamrarn and V. Ajavakom, Novel racemic tetrahydrocurcuminoid dihydropyrimidinone analogues as potent acetylcholinesterase inhibitors, Bioorg. Med. Chem. Lett., 2013, 23(10), 2880–2882 CrossRef PubMed.
- J. Kim, C. Park, T. Ok, W. So, M. Jo, M. Seo, Y. Kim, J.-H. Sohn, Y. Park, M. K. Ju, J. Kim, S.-J. Han, T.-H. Kim, J. Cechetto, J. Nam, P. Sommer and Z. No, Discovery of 3,4-dihydropyrimidin-2(1H)-ones with inhibitory activity against HIV-1 replication, Bioorg. Med. Chem. Lett., 2012, 22(5), 2119–2124 CrossRef PubMed.
- S. Shamim, K. M. Khan, U. Salar, F. Ali, M. A. Lodhi, M. Taha, F. A. Khan, S. Ashraf, Z. Ul-Haq, M. Ali and S. Perveen, 5-Acetyl-6-methyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones: As potent urease inhibitors; synthesis, in vitro screening, and molecular modeling study, Bioorg. Chem., 2018, 76, 37–52 CrossRef PubMed.
- İ. S. Zorkun, S. Saraç, S. Çelebi and K. Erol, Synthesis of 4-aryl-3,4-dihydropyrimidin-2(1H)-thione derivatives as potential calcium channel blockers, Bioorg. Med. Chem., 2006, 14(24), 8582–8589 CrossRef PubMed.
- N. C. Desai, S. B. Joshi and K. A. Jadeja, A one-pot multicomponent Biginelli reaction for the preparation of novel pyrimidinthione derivatives as antimicrobial agents, J. Heterocycl. Chem., 2020, 57(2), 791–795 CrossRef CAS.
- H. A. Stefani, C. B. Oliveira, R. B. Almeida, C. M. P. Pereira, R. C. Braga, R. Cella, V. C. Borges, L. Savegnago and C. W. Nogueira, Dihydropyrimidin-(2H)-ones obtained by ultrasound irradiation: a new class of potential antioxidant agents, Eur. J. Med. Chem., 2006, 41(4), 513–518 CrossRef CAS.
- M. Dubernet, N. Duguet, L. Colliandre, C. Berini, S. Helleboid, M. Bourotte, M. Daillet, L. Maingot, S. Daix, J.-F. Delhomel, L. Morin-Allory, S. Routier and R. Walczak, Identification of New Nonsteroidal RORα Ligands; Related Structure–Activity Relationships and Docking Studies, ACS Med. Chem. Lett., 2013, 4(6), 504–508 CrossRef CAS PubMed.
- M. Sagha, F. Mousaei, M. Salahi and N. Razzaghi-Asl, Synthesis of new 2-aminothiazolyl/benzothiazolyl-based 3,4-dihydropyrimidinones and evaluation of their effects on adenocarcinoma gastric cell migration, Mol. Diversity, 2022, 26(2), 1039–1051 CrossRef CAS PubMed.
- N. C. Desai, A. R. Trivedi, H. V. Vaghani, H. C. Somani and K. A. Bhatt, Synthesis and biological evaluation of 1,3,4-oxadiazole bearing dihydropyrimidines as potential antitubercular agents, Med. Chem. Res., 2016, 25(2), 329–338 CrossRef CAS.
- S. J. Teague, A. M. Davis, P. D. Leeson and T. Oprea, The Design of Leadlike Combinatorial Libraries, Angew. Chem., Int. Ed., 1999, 38(24), 3743–3748 CrossRef.
- R. M. Vala, M. G. Sharma, D. M. Patel, A. Puerta, J. M. Padron, V. Ramkumar, R. L. Gardas and H. M. Patel, Synthesis and in vitro study of antiproliferative benzyloxy dihydropyrimidinones, Arch. Pharm., 2021, 354(6), 2000466 CrossRef.
- Z. Bidram, H. Sirous, G. A. Khodarahmi, F. Hassanzadeh, N. Dana, A. A. Hariri and M. Rostami, Monastrol derivatives: in silico and in vitro cytotoxicity assessments, Res. Pharm. Sci., 2020, 15(3), 249–262 CrossRef PubMed.
- A. Castillo and M. J. Justice, The kinesin related motor protein, Eg5, is essential for maintenance of pre-implantation embryogenesis, Biochem. Biophys. Res. Commun., 2007, 357(3), 694–699 CrossRef PubMed.
- N. Hirokawa, Y. Noda, Y. Tanaka and S. Niwa, Kinesin superfamily motor proteins and intracellular transport, Nat. Rev. Mol. Cell Biol., 2009, 10(10), 682–696 CrossRef CAS PubMed.
- I. Garcia-Saez, S. DeBonis, R. Lopez, F. Trucco, B. Rousseau, P. Thuéry and F. Kozielski, Structure of human Eg5 in complex with a new monastrol-based inhibitor bound in the R configuration, J. Biol. Chem., 2007, 282(13), 9740–9747 CrossRef CAS.
- T. U. Mayer, T. M. Kapoor, S. J. Haggarty, R. W. King, S. L. Schreiber and T. J. Mitchison, Small Molecule Inhibitor of Mitotic Spindle Bipolarity Identified in a Phenotype-Based Screen, Science, 1999, 286(5441), 971–974 CrossRef CAS.
- D. Russowsky, R. F. S. Canto, S. A. A. Sanches, M. G. M. D'Oca, Â. de Fátima, R. A. Pilli, L. K. Kohn, M. A. Antônio and J. E. de Carvalho, Synthesis and differential antiproliferative activity of Biginelli compounds against cancer cell lines: Monastrol, oxo-monastrol and oxygenated analogues, Bioorg. Chem., 2006, 34(4), 173–182 CrossRef CAS PubMed.
- V. Tandon, R. M. Vala, A. Chen, R. L. Sah, H. M. Patel, M. C. Pirrung and S. Banerjee, Syrbactin-class dual constitutive-and immuno-proteasome inhibitor TIR-199 impedes myeloma-mediated bone degeneration in vivo, Biosci. Rep., 2022, 42(2), BSR20212721 CrossRef CAS PubMed.
- S. G. Patel, R. M. Vala, P. J. Patel, D. B. Upadhyay, V. Ramkumar, R. L. Gardas and H. M. Patel, Synthesis, crystal structure and in silico studies of novel 2, 4-dimethoxy-tetrahydropyrimido [4, 5-b] quinolin-6 (7 H)-ones, RSC Adv., 2022, 12(29), 18806–18820 RSC.
- R. M. Vala, V. Tandon, L. G. Nicely, L. Guo, Y. Gu, S. Banerjee and H. M. Patel, Synthesis of N-(4-chlorophenyl) substituted pyrano[2,3-c]pyrazoles enabling PKBβ/AKT2 inhibitory and in vitro anti-glioma activity, Ann. Med., 2022, 54(1), 2549–2561 CrossRef PubMed.
- S. G. Patel, A. González-Bakker, R. M. Vala, P. J. Patel, A. Puerta, A. Malik, R. K. Sharma, J. M. Padrón and H. M. Patel, Microwave-assisted multicomponent synthesis of antiproliferative 2,4-dimethoxy-tetrahydropyrimido[4,5-b]quinolin-6(7H)-ones, RSC Adv., 2022, 12(47), 30404–30415 RSC.
- D. B. Upadhyay, J. A. Mokariya, P. J. Patel, S. G. Patel, A. Das, A. Nandi, J. Nogales, N. More, A. Kumar, D. P. Rajani, M. Narayan, J. Kumar, S. Banerjee, S. K. Sahoo and H. M. Patel, Indole clubbed 2,4-thiazolidinedione linked 1,2,3-triazole as a potent antimalarial and antibacterial agent against drug-resistant strain and molecular modeling studies, Arch. Pharm., 2024, e2300673 CrossRef.
- J. A. Mokariya, D. P. Rajani and M. P. Patel, 1,2,4-Triazole and benzimidazole fused dihydropyrimidine derivatives: Design, green synthesis, antibacterial, antitubercular, and antimalarial activities, Arch. Pharm., 2023, 356(4), e2200545 CrossRef.
- J. A. Mokariya, A. G. Kalola, P. Prasad and M. P. Patel, Simultaneous ultrasound- and microwave-assisted one-pot 'click' synthesis of 3-formyl-indole clubbed 1,2,3-triazole derivatives and their biological evaluation, Mol. Diversity, 2022, 26(2), 963–979 CrossRef CAS PubMed.
- D. P. Vala, R. M. Vala and H. M. Patel, Versatile Synthetic Platform for 1,2,3-Triazole Chemistry, ACS Omega, 2022, 7(42), 36945–36987 CrossRef CAS.
- N. Anand, K. K. G. Ramakrishna, M. P. Gupt, V. Chaturvedi, S. Singh, K. K. Srivastava, P. Sharma, N. Rai, R. Ramachandran and A. K. Dwivedi, Identification of 1-[4-benzyloxyphenyl-but-3-enyl]-1 H-azoles as new class of antitubercular and antimicrobial agents, ACS Med. Chem. Lett., 2013, 4(10), 958–963 CrossRef CAS PubMed.
- S. Emami, M. Kazemi-Najafabadi, S. Pashangzadeh, A. Foroumadi, M. A. Faramarzi, N. Samadi, M. Falahati, R. Fateh and M. Ashrafi-Khozani, Synthesis and Antifungal Activity of 1-[(2-Benzyloxy) Phenyl]-2-(Azol-1-yl) Ethanone Derivatives: Exploring the Scaffold Flexibility, Chem. Biol. Drug Des., 2011, 78(6), 979–987 CrossRef CAS.
- B. S. Kumar, A. Kumar, J. Singh, M. Hasanain, A. Singh, K. Fatima, D. K. Yadav, V. Shukla, S. Luqman and F. Khan, Synthesis of 2-alkoxy and 2-benzyloxy analogues of estradiol as anti-breast cancer agents through microtubule stabilization, Eur. J. Med. Chem., 2014, 86, 740–751 CrossRef PubMed.
- A. M. King, X.-F. Yang, Y. Wang, E. T. Dustrude, C. Barbosa, M. R. Due, A. D. Piekarz, S. M. Wilson, F. A. White and C. Salomé, Identification of the benzyloxyphenyl pharmacophore: a structural unit that promotes sodium channel slow inactivation, ACS Chem. Neurosci., 2012, 3(12), 1037–1049 CrossRef.
- X. Dou, D. Nath, H. Shin, E. Nurmemmedov, P. C. Bourne, J.-X. Ma and A. S. Duerfeldt, Evolution of a 4-benzyloxy-benzylamino chemotype to provide efficacious, potent, and isoform selective PPARα agonists as leads for retinal disorders, J. Med. Chem., 2020, 63(6), 2854–2876 CrossRef.
- S. T. Sudevan, T. M. Rangarajan, A. G. Al-Sehemi, A. S. Nair, V. P. Koyiparambath and B. Mathew, Revealing the role of the benzyloxy pharmacophore in the design of a new class of monoamine oxidase-B inhibitors, Arch. Pharm., 2022, 355(8), 2200084 CrossRef PubMed.
- D. B. Upadhyay, R. M. Vala, J. Nogales, S. Banerjee and H. M. Patel, A tetrahydopyrimidinecarboxamide derivatives, 2024, p. 528662 Search PubMed.
- M. M. Alam, 1,2,3-Triazole hybrids as anticancer agents: A review, Arch. Pharm., 2022, 355(1), 2100158 CrossRef PubMed.
- K. Lal and P. Yadav, Recent advancements in 1, 4-disubstituted 1H-1, 2, 3-triazoles as potential anticancer agents, Anti-Cancer Agents Med. Chem., 2018, 18(1), 21–37 CrossRef CAS PubMed.
- J. Akhtar, A. A. Khan, Z. Ali, R. Haider and M. Shahar Yar, Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities, Eur. J. Med. Chem., 2017, 125, 143–189 CrossRef CAS.
- T. Liang, X. Sun, W. Li, G. Hou and F. Gao, 1,2,3-Triazole-Containing Compounds as Anti–Lung Cancer Agents: Current Developments, Mechanisms of Action, and Structure–Activity Relationship, Front. Pharmacol, 2021, 12, 661173 CrossRef CAS PubMed.
- K. I. Slavova, L. T. Todorov, N. P. Belskaya, M. A. Palafox and I. P. Kostova, Developments in the Application of 1,2,3-Triazoles in Cancer Treatment, Recent Pat. Anti-Cancer Drug Discovery, 2020, 15(2), 92–112 CrossRef CAS PubMed.
- D. Veeranna, L. Ramdas, G. Ravi, S. Bujji, V. Thumma and J. Ramchander, Synthesis of 1,2,3-Triazole Tethered Indole Derivatives: Evaluation of Anticancer Activity and Molecular Docking Studies, ChemistrySelect, 2022, 7(29), e202201758 CrossRef CAS.
- J. A. Mokariya, R. C. Patel, D. P. Rajani and M. P. Patel, Synthesis of novel indole-oxindole clubbed 1, 2, 3-triazole hybrids: antimicrobial evaluation and molecular docking study, Res. Chem. Intermed., 2023, 49(7), 2933–2953 CrossRef CAS.
- S. Shafi, M. M. Alam, N. Mulakayala, C. Mulakayala, G. Vanaja, A. M. Kalle, R. Pallu and M. S. Alam, Synthesis of novel 2-mercapto benzothiazole and 1,2,3-triazole based bis-heterocycles: their anti-inflammatory and anti-nociceptive activities, Eur. J. Med. Chem., 2012, 49, 324–333 CrossRef PubMed.
- R. S. Keri, S. A. Patil, S. Budagumpi and B. M. Nagaraja, Triazole: A Promising Antitubercular Agent, Chem. Biol. Drug Des., 2015, 86(4), 410–423 CrossRef.
- Y. Tian, Z. Liu, J. Liu, B. Huang, D. Kang, H. Zhang, E. De Clercq, D. Daelemans, C. Pannecouque, K. H. Lee, C. H. Chen, P. Zhan and X. Liu, Targeting the entrance channel of NNIBP: Discovery of diarylnicotinamide 1,4-disubstituted 1,2,3-triazoles as novel HIV-1 NNRTIs with high potency against wild-type and E138K mutant virus, Eur. J. Med. Chem., 2018, 151, 339–350 CrossRef PubMed.
- R. Kumar, L. Vats, S. Bua, C. T. Supuran and P. K. Sharma, Design and synthesis of novel benzenesulfonamide containing 1,2,3-triazoles as potent human carbonic anhydrase isoforms I, II, IV and IX inhibitors, Eur. J. Med. Chem., 2018, 155, 545–551 CrossRef.
- D. Baraniak and J. Boryski, Triazole-Modified Nucleic Acids for the Application in Bioorganic and Medicinal Chemistry, Biomedicines, 2021, 9(6), 628 CrossRef CAS.
- A. Ben Ali, Y. El Bakri, C. H. Lai, J. Sebhaoui, L. El Ghayati, E. M. Essassi and J. T. Mague, Crystal structure, computational study and Hirshfeld surface analysis of ethyl (2S,3R)-3-(3-amino-1H-1,2,4-triazol-1-yl)-2-hy-droxy-3-phenyl-propano-ate, Acta Crystallogr., Sect. E: Crystallogr. Commun., 2019, 75(Pt 12), 1919–1924 CrossRef CAS.
- B. F. Abdel-Wahab, B. M. Kariuki, H. A. Mohamed, M. S. Bekheit, H. M. Awad and G. A. El-Hiti, Synthesis and anticancer activity of 3-(1-aryl-5-methyl-1H-1, 2, 3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehydes, J. Mol. Struct., 2023, 1294, 136528 CrossRef CAS.
- K. Sanphanya, S. K. Wattanapitayakul, S. Phowichit, V. V. Fokin and O. Vajragupta, Novel VEGFR-2 kinase inhibitors identified by the back-to-front approach, Bioorg. Med. Chem. Lett., 2013, 23(10), 2962–2967 CrossRef CAS.
- B. Banerji, K. Chandrasekhar, K. Sreenath, S. Roy, S. Nag and K. D. Saha, Synthesis of Triazole-Substituted Quinazoline Hybrids for Anticancer Activity and a Lead Compound as the EGFR Blocker and ROS Inducer Agent, ACS Omega, 2018, 3(11), 16134–16142 CrossRef PubMed.
- L. Bekaert, S. Valable, E. Lechapt-Zalcman, K. Ponte, S. Collet, J. M. Constans, G. Levallet, K. Bordji, E. Petit, P. Branger, E. Emery, A. Manrique, L. Barré, M. Bernaudin and J. S. Guillamo, [18F]-FMISO PET study of hypoxia in gliomas before surgery: correlation with molecular markers of hypoxia and angiogenesis, Eur. J. Nucl. Med. Mol. Imaging, 2017, 44(8), 1383–1392 CrossRef PubMed.
- L. G. Nicely, R. M. Vala, D. B. Upadhyay, J. Nogales, C. Chi, S. Banerjee and H. M. Patel, One-pot two-step catalytic synthesis of 6-amino-2-pyridone-3,5-dicarbonitriles enabling anti-cancer bioactivity, RSC Adv., 2022, 12(37), 23889–23897 RSC.
- V. Tandon, R. M. Vala, A. Chen, R. L. Sah, H. M. Patel, M. C. Pirrung and S. Banerjee, Syrbactin-class dual constitutive- and immuno-proteasome inhibitor TIR-199 impedes myeloma-mediated bone degeneration in vivo, Biosci. Rep., 2022, 42(2), BSR20212721 CrossRef.
- S. Banerjee, T. Wei, J. Wang, J. J. Lee, H. L. Gutierrez, O. Chapman, S. E. Wiley, J. E. Mayfield, V. Tandon, E. F. Juarez, L. Chavez, R. Liang, R. L. Sah, C. Costello, J. P. Mesirov, L. de la Vega, K. L. Cooper, J. E. Dixon, J. Xiao and X. Lei, Inhibition of dual-specificity tyrosine phosphorylation-regulated kinase 2 perturbs 26S proteasome-addicted neoplastic progression, Proc. Natl. Acad. Sci. U. S. A., 2019, 116(49), 24881–24891 CrossRef CAS PubMed.
- A. Keenlyside, T. Marples, Z. Gao, H. Hu, L. G. Nicely, J. Nogales, H. Li, L. Landgraf, A. Solth, A. Melzer, K. Hossain-Ibrahim, Z. Huang, S. Banerjee and J. Joseph, Development and optimisation of in vitro sonodynamic therapy for glioblastoma, Sci. Rep., 2023, 13(1), 20215 CrossRef PubMed.
- S. Banerjee, A. Zagorska, M. Deak, D. G. Campbell, A. R. Prescott and D. R. Alessi, Interplay between Polo kinase, LKB1-activated NUAK1 kinase, PP1 beta(MYPT1) phosphatase complex and the SCF beta TrCP E3 ubiquitin ligase, Biochem. J., 2014, 461, 233–245 CrossRef CAS.
- J. Bain, L. Plater, M. Elliott, N. Shpiro, C. J. Hastie, H. McLauchlan, I. Klevernic, J. S. Arthur, D. R. Alessi and P. Cohen, The selectivity of protein kinase inhibitors: a further update, Biochem. J., 2007, 408(3), 297–315 CrossRef CAS.
- S. Banerjee, S. J. Buhrlage, H.-T. Huang, X. Deng, W. Zhou, J. Wang, R. Traynor, A. R. Prescott, D. R. Alessi and N. S. Gray, Characterization of WZ4003 and HTH-01-015 as selective inhibitors of the LKB1-tumour-suppressor-activated NUAK kinases, Biochem. J., 2014, 457, 215–225 CrossRef CAS PubMed.
- A. Daina, O. Michielin and V. Zoete, SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules, Sci. Rep., 2017, 7(1), 42717 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra04171b |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |