Open Access Article
Open Access ArticlePreparation, structure and application of polysaccharides from Poria cocos
Laiqing Deng and
Gangliang Huang *
*
Key Laboratory of Carbohydrate Science and Engineering, Chongqing Normal University, Chongqing 401331, China. E-mail: huangdoctor226@163.com
First published on 30th September 2024
Abstract
Poria cocos polysaccharides (PCPs) are fungal polysaccharides derived from the traditional Chinese medicine Poria cocos. They are considered an important active ingredient for their pharmacological activity. Herein, the extraction, separation and purification, structure, and application of PCPs are reviewed. Additional research is necessary to fully understand the advanced structure of PCPs, which has implications for their structure–activity relationship. Their application mostly involves the medical industry, with less involvement in other fields. This article highlights the current research status on PCPs in the above-mentioned areas and some problems that need to be solved in future research. Additionally, it points the way for further studies on PCPs in the hopes that they will be more widely and realistically used in various industries.
1. Introduction
Poria cocos, an important and therapeutic fungus belonging to the Poria family, is commonly found in southern and southwestern China. It is a saprophytic fungus that causes wood degradation. It can parasitize citrus, eucalyptus, and oak trees.1 P. cocos is used for clinical purposes in a wide range of chemical compositions, and it is also considered a functional herbal food.2 In traditional Chinese medicine (TCM), P. cocos is frequently used in conjunction with other herbs to treat a variety of diseases.3 For example, the DangGui-Shaoyao-San formula has been demonstrated to improve cognitive dysfunction in Alzheimer's disease patients.3 In Asian nations, including China, Japan, and South Korea, P. cocos has been used for medical treatment for several centuries.4,5 P. cocos is mainly sold as a nutritional health product that is easy to obtain and widely added to beverages, including soup, tea, and alcoholic beverages, for mixed consumption.6 Conventional Chinese medicine practitioners often use P. cocos to treat kidney disease,7 chronic gastritis,6 phlegm, swelling, restlessness, intestinal damage8 and other diseases as a result of its diuretic,9 sedative, and spleen-strengthening abilities. Its considered to be one of the highest ranked medicines in a variety of medical books on herbal medicine.10 P. cocos is traditionally considered by the Chinese to be a beneficial drug with life-extending properties,11 and most of its main active ingredients have been intensively studied.12 Triterpenes,13 polysaccharides,14 and steroid chemicals9 are among the many and rich chemical components of P. cocos. Aside from potassium salts, histidine, choline, and amino acids, there are other secondary compounds,15–17 The multiple pharmaceutical activities of P. cocos are related to the presence of several triterpenes and polysaccharides.18P. cocos polysaccharides (PCPs) are the most prevalent, helpful, and plentiful part of the fungus, making up around 90% of its fungal nuclei.19 Several components of PCPs have significant differences in their monosaccharide composition and molecular weight. PCPs can be divided into water-soluble polysaccharides and alkali-soluble polysaccharides according to their solubility; water-soluble polysaccharides are the main active ingredients of P. cocos used in clinical medicine. P. cocos alkali-soluble polysaccharides have almost no bioactivity, and chemical modification to improve their bioactivity and means of application are a main focus of research today.20 Studies have shown that alkali-soluble PCPs prevents the growth of cancer cells and affects the activity of a range of enzymes involved in apoptosis and senescence in living organisms.21 Pharmacological researchers have shown a variety of medicinal activities of PCP,22,23 such as immune regulation,24,25 anti-inflammatory effects,26,27 antioxidant effects,28 anti-tumor effects,21 hepatoprotective effects,29–31 and kidney protection,32 In addition, it is also one of the main ingredients in health supplements, such as P. cocos polysaccharide oral solution.11 The biological activity and mechanism of action of PCPs have been widely studied by researchers for many years, but their potential applications still need to be developed. Recent relevant literature on the application of PCPs in food packaging and feed additives indicates that PCPs are being investigated for use in industries other than the healthcare industry. With the continuous improvement of polysaccharide extraction, isolation and purification, structural identification, and other techniques, the study of PCPs is becoming more and more comprehensive; their structures and mechanisms of bioactivity are becoming more and more accurate; their application is expanding; and the past reviews may need new and additional descriptions. Herein, the extraction, isolation, purification, structure and application of PCPs are reviewed and summarized.
2. Preparation of PCPs
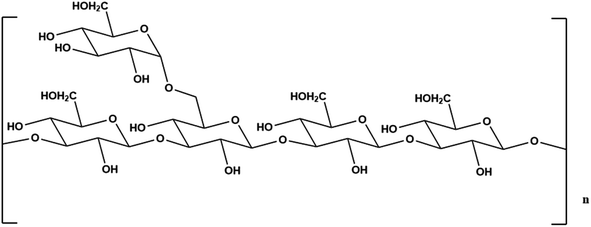
Nowadays, polysaccharides are primarily obtained from natural materials, and the majority of our research on polysaccharides begins with extraction. PCPs can be classified into two categories, extracellular polysaccharides and intracellular polysaccharides, according to different extraction locations. Extracellular polysaccharides are those obtained through liquid fermentation, while intracellular polysaccharides are those found in the fruiting bodies and mycelium.33,34 According to the solubility of PCPs in different solvents, they can be divided into water-soluble PCPs, which are biologically active, and alkali-soluble PCPs, which are mostly biologically inactive. The structure of an alkali-soluble polysaccharide is shown in Fig. 1, and it is mainly composed of linear β-(1 → 3)-D-glucan, containing a small amount of β-(1 → 6) glycosidic side chains, and its conformation is generally a triple helix conformation. There are three main methods used by researchers to extract PCPs: solvent extraction, enzyme-assisted extraction, and physically assisted extraction. In addition to the above extraction methods, deep eutectic solvent (DES) extraction and others are available in the recent literature. Table 1 summarizes the advantages and disadvantages of different PCP extraction methods. In order to obtain more PCPs, researchers sometimes use multiple extraction methods to extract polysaccharides at the same time, for example, using both enzyme extraction and physically assisted extraction to extract polysaccharides. The research on the extraction methods of PCPs is relatively mature, and future researchers should focus on improving the extraction methods or discovering new, high-yield, and cost-effective extraction methods.| Extraction methods | Advantages | Disadvantages | Extraction rate | Reference | |
|---|---|---|---|---|---|
| a Annotation: supercritical fluid (SCF); deep eutectic solvent (DES); not detected (—). | |||||
| Solvent method | Water extraction method | Low cost and easy to operate | Long extraction times and low yields | 5.40% | 35 |
| Alkaline extraction | High polysaccharide yield and low cost, easy to operate | Alkali may disrupt polysaccharide structure | — | 36 | |
| Physically assisted extraction method | Ultrasound-assisted extraction | Simple operation, fast extraction rate and good applicability | May disrupt polysaccharide structure, leading to unpredictable loss of activity | 1.38% | 1 |
| Microwave-assisted extraction | Short extraction time, low power consumption and high polysaccharide activity obtained | May disrupt polysaccharide structure, leading to unpredictable loss of activity | 9.95% | 37 | |
| SCF extraction | Low temperature, high efficiency and high penetration | Costly and cumbersome to operate | 3.209% | 38 | |
| Enzyme-assisted extraction | High yield, easy to operate and green | Expensive, enzymes have the potential to degrade polysaccharides | 12.8% | 39 | |
| DES extraction | Low cost, simple preparation, biodegradable, well-defined structure and low toxicity | May lead to disruption of the complex structure of polysaccharides and some DESs with high viscosity and toxicity | 46.24% | 35 and 40 | |
Crude PCPs extracted directly from P. cocos usually contain impurities such as proteins, oligosaccharides and pigments, which may have an impact on the biological activity and structure, thus affecting the structural analysis of PCPs and experiments on their activity.37,41 Removal of these impurities is needed to avoid affecting the studies.37 The process of isolating and purifying PCPs is essentially similar to that for other naturally occurring polysaccharides;37,38 it primarily entails the elimination of lipids, proteins, and pigments as well as the separation of PCPs with varying molecular weights and physical characteristics. Commonly used protein removal methods include hydrochloric acid, ellagic acid, trichloroacetic acid, and the Sevag method.42 The most commonly used of these is the Sevag method,37 in which polysaccharides are mixed with Sevag reagent43 (n-butanol and chloroform in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 or 1
5 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 by volume), and the mixture is centrifuged after being shaken. This removes proteins from the polysaccharides. Based on the principle of similar solubility, a common method of PCP degreasing used by researchers is refluxing using a Soxhlet extractor and an organic solvent such as ethanol. Lipid impurities that are not soluble in the organic solvent will be separated from the PCPs. Common methods of removing pigments in the laboratory include H2O2 decolorization, activated carbon decolorization, and large-pore adsorption resin methods.
4 by volume), and the mixture is centrifuged after being shaken. This removes proteins from the polysaccharides. Based on the principle of similar solubility, a common method of PCP degreasing used by researchers is refluxing using a Soxhlet extractor and an organic solvent such as ethanol. Lipid impurities that are not soluble in the organic solvent will be separated from the PCPs. Common methods of removing pigments in the laboratory include H2O2 decolorization, activated carbon decolorization, and large-pore adsorption resin methods.
Common polysaccharide separation and purification methods include partition precipitation, salt precipitation, quaternary ammonium precipitation, metal complexation, and column chromatography. Column chromatography is now commonly used by researchers to isolate and purify polysaccharides due to its ease of operation and wide selection for polysaccharide purification.41 Gel chromatography columns39,44 and ion exchange chromatography45,46 are commonly used for the separation and purification of PCPs.
2.1 Solvent extraction
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, giving a PCP yield of 82.3%.36 The advantage is that the experiments can be carried out at low temperatures with higher yields. The disadvantage is that alkaline and high-temperature environments may destroy the structure of PCPs, and care must be taken when using this method.
1, giving a PCP yield of 82.3%.36 The advantage is that the experiments can be carried out at low temperatures with higher yields. The disadvantage is that alkaline and high-temperature environments may destroy the structure of PCPs, and care must be taken when using this method.2.2 Physically assisted extraction method
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, microwave power 600 W. Under these conditions, the average yield of PCP was 7.22%, which was not only much higher than that of the conventional reflux extraction, but the time was also substantially decreased.52 This extraction method is rapid and the polysaccharides obtained are highly active; the downside is low yield. In addition, microwaves may cause structural damage to polysaccharides, leading to loss of their biological activity.
30, microwave power 600 W. Under these conditions, the average yield of PCP was 7.22%, which was not only much higher than that of the conventional reflux extraction, but the time was also substantially decreased.52 This extraction method is rapid and the polysaccharides obtained are highly active; the downside is low yield. In addition, microwaves may cause structural damage to polysaccharides, leading to loss of their biological activity.2.3 Enzyme-assisted extraction
The enzymatic extraction method still relies on the use of appropriate enzymes to disrupt the structure of the P. cocos mycelium and increase the solubility of the polysaccharides in the solvent. It is the focus of current research on polysaccharide extraction methods.54 Tang et al.39 isolated β-glucosidase enzyme from fermented Aspergillus niger HS-5 using an enzymatic extraction method; it was used to assist in the extraction of PCPs. Response surface methods and single-factor trials were used to identify the ideal enzymatic hydrolysis conditions for PCPs, as follows: hydrolysis temperature of 60 °C, time of 120 minutes, pH of 5.0, and volume of 20 mL. Under these conditions, the extraction rate of PCP can reach up to 12.8%.39 Khaskheli et al.55 used an enzyme extraction method to extract PCP. They found that a liquid/feed ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, temperature of 40 °C, time of 3.0 hours, and pH of 5 were the ideal extraction parameters. The maximum percentage of crude polysaccharides extracted was 4.14%.55 Compared with the traditional extraction technology, it has the advantages of high extraction rate, good extraction activity, low energy consumption and investment cost, high reproducibility in a short period of time, simple operation and green environmental protection. However, enzymes may still degrade polysaccharides during the extraction process, reducing the yield and affecting the results. In addition, the high cost of the enzyme extraction method is also a problem, making it unsuitable for large-scale production of PCPs.56
50, temperature of 40 °C, time of 3.0 hours, and pH of 5 were the ideal extraction parameters. The maximum percentage of crude polysaccharides extracted was 4.14%.55 Compared with the traditional extraction technology, it has the advantages of high extraction rate, good extraction activity, low energy consumption and investment cost, high reproducibility in a short period of time, simple operation and green environmental protection. However, enzymes may still degrade polysaccharides during the extraction process, reducing the yield and affecting the results. In addition, the high cost of the enzyme extraction method is also a problem, making it unsuitable for large-scale production of PCPs.56
2.4 Deep eutectic solvent (DES) extraction
Polysaccharides have a number of drawbacks as a result of their typical solvent extraction process, including low extraction rates, structural degradation, toxicity toward experimenters, and the operational environment. To address these issues, a novel solvent is becoming more and more necessary. DESs are very beneficial for PCP extraction, which is facilitated by linking hydrogen bonded acceptors and donors. Zhang et al.35 used DESs, a novel green extractant, to successfully extract PCPs. They discovered that a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 between choline and oxalic acid, an extraction temperature of 100 °C, and a 15 minutes extraction period were the optimal extraction conditions. After six cycles, the PCP extraction rate was 38.40%, indicating that DESs can be reused and have commercial value. The yield was significantly higher than that of the traditional hot water extraction, reaching as high as 46.24%. The outcomes of the experiment demonstrated that DESs are efficient solvents for extracting polysaccharides from P. cocos.35 Guo et al.40 employed the novel ternary DESs. The polysaccharide yield of this method was 55.02% under the optimized conditions of 300 mesh sieve, 30 mL g−1 liquid–solid ratio, 300 W mean fluorescence intensity ratio (MFIR) and 80 °C circulating air temperature. The yield was significantly higher compared to the traditional extraction of PCPs with hot water solvent and alkaline water.40 DESs can be regarded as the best method for PCP extraction at present due to its low cost, simple preparation, definable structure and biodegradability. The disadvantage is that DESs may also lead to the disruption of the complex structure of polysaccharides, resulting in macromolecular decomposition, and should be handled with caution.44
2 between choline and oxalic acid, an extraction temperature of 100 °C, and a 15 minutes extraction period were the optimal extraction conditions. After six cycles, the PCP extraction rate was 38.40%, indicating that DESs can be reused and have commercial value. The yield was significantly higher than that of the traditional hot water extraction, reaching as high as 46.24%. The outcomes of the experiment demonstrated that DESs are efficient solvents for extracting polysaccharides from P. cocos.35 Guo et al.40 employed the novel ternary DESs. The polysaccharide yield of this method was 55.02% under the optimized conditions of 300 mesh sieve, 30 mL g−1 liquid–solid ratio, 300 W mean fluorescence intensity ratio (MFIR) and 80 °C circulating air temperature. The yield was significantly higher compared to the traditional extraction of PCPs with hot water solvent and alkaline water.40 DESs can be regarded as the best method for PCP extraction at present due to its low cost, simple preparation, definable structure and biodegradability. The disadvantage is that DESs may also lead to the disruption of the complex structure of polysaccharides, resulting in macromolecular decomposition, and should be handled with caution.44
3. Structure, structure–activity relationship and techniques for the structural analysis of PCPs
The structure of polysaccharides is strongly associated with their biological activity.57 Structural research is significant for understanding the bioactive expression process and conformational relationships of polysaccharides. PCPs, considered complex macromolecules in chemical research, are difficult to finely obtain at multiple levels of their structure.58 As a heteropolysaccharide, PCPs comprise a blend of many types of polysaccharides, and distinct polysaccharide fractions can be produced by employing various extraction techniques.59 Based on the literature, it is inferred that the main component of PCPs is a water-insoluble polysaccharide that is a linear (1 → 3)-β-D-glucan,52 which acts as a nonspecific bioregulator and contains approximately more than 700 β-(1 → 3) glycosidic bonds.60,61 PCPs are mainly composed of ribose (Rib), arabinose (Ara), xylose (Xyl), mannose (Man), glucose (Glc) and galactose (Gal), of which Glc has the highest content, followed by Man content.62,63 Their monosaccharide component is mainly D-glucose. β-Glucan is the primary component of PCPs.64 Its structure has a β-(1 → 3)-linked glucose main chain and β-(1 → 6)-linked glucose side chains.12 At present, the research on the structure of PCPs is almost exclusively concerned with their molecular mass and monosaccharide composition, and their structural parameters and spatial configurations, such as functional groups and glycosidic bonding, need to be further investigated.52The chain conformation has an effect on the activity of PCPs. Many β-glucans in nature have a triple helix conformation, such as lentinan and split-leaf polysaccharides.65 Generally speaking, when polysaccharides have a triple-helical structure, their antitumor activity is stronger compared to other conformations.66 However, the β-glucan in P. cocos does not have a triple helix conformation, but exists in an irregular line group conformation, resulting in the aggregation of polysaccharides with relatively large molecular weights and that are insoluble in water. These characteristics make it difficult for PCPs to express biological activity. Researchers found that carboxymethyl P. cocos polysaccharides (CMP) with triple-helical conformation showed good immunomodulatory ability and hypothesized that altering the structure of alkali-soluble PCPs could lead to better pharmacological activity.67 Huang et al.68 found that a semi-rigid chain form had better anti-cancer activity compared to its natural counterpart, which has a flexible chain.68
Relative molecular mass has a significant effect on the activity of PCPs. Some researchers have found that PCPs with relatively small molecular weight show better biological activity and speculate that because of their relatively small molecular weight, they can more easily enter cells and express activity. Zhang et al.69 found that PCPs with a relatively low molecular mass were more capable of exhibiting better anti-tumor activity in vitro.69 Tang et al.48 degraded alkali-soluble PCPs to obtain degraded polysaccharides with low relative molecular mass, increased solubility and improved antioxidant activity.48
Past studies have shown that the primary structures of PCPs are better understood through existing techniques. Most experimenters have chosen methylation analysis (MA), high-performance liquid chromatography (HPLC), nuclear magnetic resonance (NMR), ion chromatography (IC), gel permeation chromatography (GPC), infrared spectroscopy (IR) and other methods to analyze the primary structure of PCPs.70 However, due to the complex structure of polysaccharides, it is difficult to analyze the high-level structure of PCPs by existing analytical techniques, which affects the functional understanding of PCPs. Referring to the analytical methods of other polysaccharides, it is hypothesized that the advanced structure of polysaccharides can be investigated using atomic force microscopy (AFM) in subsequent experiments.71 Based on the above methods of polysaccharide analysis, a variety of PCPs were found to have different structures, some of which were characterized as shown in Table 2. Regarding the determination of PCP content, most experimenters chose the phenol-sulfuric acid method.
| Compound name | Extraction method | Methods of analysis | Carbohydrate composition | Molecular weight (kDa) | Structural characteristic | Reference |
|---|---|---|---|---|---|---|
| a Methylation analysis (MA); high-performance liquid chromatography (HPLC); nuclear magnetic resonance (NMR); ion chromatography (IC); gel permeation chromatography (GPC); infrared spectroscopy (IR); gas chromatography (GS); high-performance gel permeation chromatography (HPGPC); atomic force microscopy (AFM); capillary electrophoresis (CE); Fourier transform infrared spectroscopy (FT-IR); high-performance size exclusion chromatography (HPSEC); gas chromatography-mass spectrometry (GC-MS); ultraviolet spectra (UV); scanning electron microscope (SEM); gas chromatography (GC); size exclusion chromatograph (SEC); laser light scattering (LLS); size exclusion chromatography-laser light scattering (SEC-LLS); ribose (Rib); arabinose (Ara); xylose (Xyl); mannose (Man); glucose (Glc); galactose (Gal); not detected (/). | ||||||
| ac-PCM0 | — | IR, NMR, GC | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fuc Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ara = 43.00 Ara = 43.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27.40 27.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27.20 27.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 1.00 |
32.9 | — | 69 |
| PCP-1 | DES extraction | GC-MS, HPGPC, FT-IR, NMR | Glc | 3.2 | (1 → 3)-β-D-glucan | 44 |
| PCP-II | Water extraction | HPGPC, CE | Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal = 10.00 Gal = 10.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.30 16.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.60 1.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62.90 62.90 |
29.0 | — | 72 |
| PCP-E | Enzyme-assisted extraction | HPLC, FT-IR, GPC | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ara = 1.98 Ara = 1.98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.36 0.36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81.72 81.72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.93 15.93 |
10.6 | — | 37 |
| PCP-U | Ultrasound-assisted extraction | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ara = 2.18 Ara = 2.18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.36 2.36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 87.27 87.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.18 8.18 |
21.2 | — | ||
| PCP-H | Hot water extraction | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ara = 0.92 Ara = 0.92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.18 0.18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86.88 86.88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.01 12.01 |
21.5 | — | ||
| PCP-M | Microwave-assisted extraction | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ara = 4.02 Ara = 4.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.93 4.93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 79.48 79.48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.57 11.57 |
15.1 | — | ||
| PCP-1 | Alkali extraction | HPSEC, HPLC, IR | Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 0.02 Glc = 0.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 1.00 |
2.33 | Degraded polysaccharides | 48 |
| PCP-2 | Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 0.01 Glc = 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 1.00 |
3.20 | Degraded polysaccharides | |||
| PCP-3 | Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 0.03 Glc = 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 1.00 |
2.85 | Degraded polysaccharides | |||
| PCP | Hot water extraction | GC-MS, FT-IR | Rib![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ara Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xyl Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal = 1.49 Gal = 1.49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.17 1.17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.62 0.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.34 10.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86.39 86.39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.31 1.31 |
— | (1 → 3)-β-D-glucan | 62 |
| PCP-1 | Hot water extraction | — | Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal = 1.00 Gal = 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.81 1.81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.27 0.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.27 7.27 |
30 | — | 73 |
| EPS-0M | Hot water extraction | HPSEC, UV, FT-IR, SEM, IC | Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fuc Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Rha = 17.30 Rha = 17.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46.30 46.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.90 19.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.70 8.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.00 5.00 |
1.77 | — | 59 |
| EPS-0.1M | Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fuc Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Rha = 11.50 Rha = 11.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46.50 46.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21.90 21.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.70 10.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.60 5.60 |
2.01 | — | |||
| IPS-0M | Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fuc Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Rha = 79.70 Rha = 79.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.90 8.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.50 5.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.70 1.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.10 3.10 |
0.03 | — | |||
| IPS-0.1M | Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fuc Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Rha = 50.30 Rha = 50.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20.90 20.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.10 16.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.00 6.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.00 4.00 |
4.97 | — | |||
| PCAPS1 | Alkali extraction | HPGPC, FT-IR, GC-MS, NMR | Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xyl Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fuc = 44.80 Fuc = 44.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28.70 28.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.20 12.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.70 7.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.20 6.20 |
11.5 | 1,3-Linked D-glucan backbone with 1,4-residue and 1,6-residue branches | 74 |
| PCPP-1A | — | — | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GA GA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fuc = 10.93 Fuc = 10.93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 235.76 235.76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26.99 26.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.56 6.56 |
45.38 | — | 14 |
| PCP | — | IC | Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ara Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D-glucosamine hydrochloride D-glucosamine hydrochloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xyl Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man = 1.00 Man = 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.20 1.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.50 2.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.40 5.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 83.90 83.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.80 1.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.20 4.20 |
— | — | 75 |
| PCP | Hot water extraction | HPGPC, HPLC, FT-IR, SEM, UV-vis | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fuc = 13.50 Fuc = 13.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.00 33.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.30 40.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.20 13.20 |
18.67 | 1,6-α-D-Galp, 1,2,6-α-D-Glcp, and T-α-D-Manp | 76 |
| SEPCP | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fuc = 2.10 Fuc = 2.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90.30 90.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.80 5.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.80 1.80 |
6.52 | 1,3-β-D-Glcp backbone and T-β-D-Glcp | |||
| PCP | Hot water extraction | IC, SEM, AFM, FT-IR, GC-MS | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D-glucosamine hydrochloride D-glucosamine hydrochloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fuc = 15.31 Fuc = 15.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.97 0.97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28.72 28.72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31.63 31.63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23.37 23.37 |
11.583 | may be t-Gal(p), 6-Gal(p) and 2,6-Gal(p) | 77 |
| Pi-PCM0 | — | FT-IR, NMR, GC | Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xyl Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 2.50 Glc = 2.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.50 1.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70.60 70.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18.50 18.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.00 7.00 |
64.6 | (1,3)-α-D-glucan | 78 |
| Pi-PCM1 | 0.9% NaCl extraction | Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ara Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xy Xy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 10.90 Glc = 10.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.80 2.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23.60 23.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36.50 36.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.20 25.20 |
304 | (1,3)-α-D-glucan | ||
| Pi-PCM2 | Hot water extraction | Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 1.90 Glc = 1.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29.60 29.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38.90 38.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29.70 29.70 |
1030 | β-D-galactofuranan, (1 → 3)-α-D-glucan and mannan | ||
| Pi-PCM3-I | 0.5 M NaOH extraction | Glc | 149 | Linear (1,3)-α-D-glucan | ||
| Pi-PCM3-II | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 10.90 Glc = 10.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21.00 21.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 68.10 68.10 |
452 | (1,3)-α-D-glucan | |||
| Pi-PCM4-I | 88% formic acid extraction | Glc | 2010 | Linear (1,3)-α-D-glucan | ||
| Pi-PCM4-II | Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 45.60 Glc = 45.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54.40 54.40 |
4360 | (1,3)-β-D-glucopyranoside and β-D-galactofuranoside | |||
| PCS0 | Alkaline extraction | SEC, GC-MS | Glc | 251 | (1, 3)-β-D-glucans | 79 |
| PCS10 | Glc | 104 | (1, 3)-β-D-glucans | |||
| PCS90 | Glc | 43 | (1, 3)-β-D-glucans | |||
| PC-I | — | NMR | Myo-inositol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sorbitol Sorbitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fuc Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) galactosamine galactosamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucosamine glucosamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man = :13.80 Man = :13.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.29 6.29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35.60 35.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55.90 55.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67.90 67.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47.20 47.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77.4 77.4 |
610.7 | — | 80 |
| PC-II | Myo-inositol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sorbitol Sorbitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fuc Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GS GS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man = 6.26 Man = 6.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.14 3.14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44.40 44.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.94 3.94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 159.00 159.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.94 0.94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.50 16.50 |
40.7 | 1,3-α-D-Gal main skeleton and 1,6-α-D-Gal side chains | |||
| PC-III | — | 7.9 | — | |||
| PC-IV | Myo-inositol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fuc Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man = 9.98 Man = 9.98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.10 33.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35.10 35.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.69 3.69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.80 3.80 |
1.6 | — | |||
| PC-V | Myo-inositol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sorbitol Sorbitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fuc Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) galactosamine galactosamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucosamine glucosamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man = 18.50 Man = 18.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.67 0.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17.80 17.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.22 8.22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.31 6.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.00 11.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.25 2.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.36 5.36 |
0.3 | — | |||
| PCWPW | Hot water extraction | HPGPC, IR, HPLC | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fuc = 36.90 Fuc = 36.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.30 7.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.48 40.48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.33 15.33 |
37.154 | — | 19 |
| PCWPS | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fuc = 30.07 Fuc = 30.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.60 16.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41.47 41.47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.10 10.10 |
186.209 | — | |||
| IDF | — | GC, SEM | Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucosamine glucosamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) uronic acid = 86.80 uronic acid = 86.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.53 1.53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.95 1.95 |
— | — | 81 |
| SDF | Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucosamine glucosamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) uronic acid = 6.71 uronic acid = 6.71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.01 1.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.10 13.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.31 5.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.27 1.27 |
— | — | |||
| wb-PCM0 | — | FT-IR, SEC-LLS, LLS, NMR, GC | Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xyl Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 6.10 Glc = 6.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.90 3.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.40 11.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.90 5.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71.70 71.70 |
144 | Mainly (1 → 3)-α-D-glucans, containing other sugar units such as β-linked D-mannose and β-linked D-galactose | 34 |
| wb-PCM1 | 0.9% NaCl extraction | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 7.70 Glc = 7.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.20 19.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73.10 73.10 |
333 | — | ||
| wb-PCM2 | Hot water extraction | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 0.90 Glc = 0.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.30 1.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95.90 95.90 |
— | — | ||
| wb-PCM3-I | 0.5 M NaOH extraction | Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 1.00 Glc = 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.20 2.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95.60 95.60 |
231 | (1 → 3)-α-D-glucans | ||
| wb-PCM3-II | Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xyl Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 2.60 Glc = 2.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.00 2.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.20 1.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.00 2.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 91.40 91.40 |
113 | (1,3)-β-D-glucan | |||
| wb-PCM4-I | 88% formic acid extraction | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 5.80 Glc = 5.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 94.10 94.10 |
1323 | (1,3)-β-D-glucan | ||
| wb-PCM4-II | Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 23.90 Glc = 23.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 76.10 76.10 |
1211 | (1,3)-β-D-glucan | |||
| wc-PCM0 | — | Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ara Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xyl Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 4.10 Glc = 4.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.00 3.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.50 2.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61.70 61.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.00 15.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.70 13.70 |
92 | Mainly comprised of α-D-mannose residues with branching | ||
| wc-PCM1 | 0.9% NaCl extraction | Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 10.50 Glc = 10.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24.50 24.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27.50 27.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37.50 37.50 |
262 | — | ||
| wc-PCM2 | Hot water extraction | Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 3.40 Glc = 3.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.50 12.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.40 13.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70.70 70.70 |
892 | — | ||
| wc-PCM3-I | 0.5 M NaOH extraction | Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 6.40 Glc = 6.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.70 16.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 76.90 76.90 |
545 | (1 → 3)-α-D-glucans | ||
| wc-PCM3-II | Glc | 89 | (1,3)-β-D-glucan | |||
| wc-PCM4-I | 88% formic acid extraction | — | — | (1,3)-β-D-glucan | ||
| wc-PCM4-II | — | — | (1,3)-β-D-glucan | |||
| wc-PCM0 | Hot water extraction | FT-IR, NMR, SEC-LLS, GC | Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ara Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xyl Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 4.10 Glc = 4.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.00 3.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.50 2.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61.70 61.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.00 15.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.70 13.70 |
92 | — | 82 |
| wc-PCM1 | Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 10.50 Glc = 10.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24.50 24.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27.50 27.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37.50 37.50 |
262 | — | |||
| wc-PCM2 | Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 3.40 Glc = 3.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.50 12.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.40 13.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70.70 70.70 |
892 | (1 → 3)-α-D-glucans | |||
| wb-PCM0 | Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xyl Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 6.10 Glc = 6.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.90 3.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.40 11.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.90 5.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71.70 71.70 |
144 | — | |||
| wb-PCM1 | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 7.70 Glc = 7.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.20 19.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73.10 73.10 |
333 | — | |||
| wb-PCM2 | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 0.90 Glc = 0.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.30 1.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95.90 95.90 |
— | (1 → 3)-α-D-glucans | |||
| ac-PCM0 | Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ara Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 1.40 Glc = 1.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43.00 43.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27.40 27.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27.20 27.20 |
101 | — | |||
| ac-PCM1 | Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 4.50 Glc = 4.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.80 15.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23.90 23.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53.40 53.40 |
125 | — | |||
| ac-PCM2 | Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 0.80 Glc = 0.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.10 19.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29.70 29.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51.40 51.40 |
170 | — | |||
| ab-PCM0 | Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xyl Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 9.20 Glc = 9.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.10 11.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21.50 21.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.70 12.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45.40 45.40 |
93 | — | |||
| ab-PCM1 | Fuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ara Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xyl Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 7.90 Glc = 7.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.00 4.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.60 2.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.50 10.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27.60 27.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47.30 47.30 |
57 | — | |||
| ab-PCM2 | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 5.60 Glc = 5.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.10 13.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81.20 81.20 |
— | — | |||
4. Application of PCPs
The application of PCPs is still mainly related to the medical industry, with a small number of applications in the aquaculture industry.6,83–86 PCPs have rich and effective pharmacological activities on the one hand, and on the other hand, they have the advantages of being nontoxic, easy to chemically modify, and chemically stable; they can be made into a series of medicines, vaccines, and other healthcare products; they can also be used as a drug carrier material, such as nanoparticles and hydrogels.4.1 Application of PCPs as drugs
Among today's treatments, the application of polysaccharides, a natural substance, as a form of medication is seen as a very promising therapeutic approach.87 The natural fungal polysaccharides derived from P. cocos, PCPs and their derivatives, contain intricate structures and a variety of biological functions. They are used in many different medical applications, including as immune modulators, antioxidants, antibacterial agents, and anticancer medication. However, the clinical application of PCPs is still uncommon; it is mainly regarded as an anticancer drug and an immunomodulator. Nowadays, in China, a Chinese patented medicine based on PCPs88 known as “Poria cocos polysaccharides oral liquid” is used as a health supplement that can strengthen the spleen, invigorate qi, and prevent aging. It promotes tumor cell apoptosis and inhibits tumor growth by decreasing the content of Bcl-2 protein and increasing the content of caspase-3 and caspase-9 protein.88 Fucose-containing mannoglucan polysaccharide (FMGP) in P. cocos greatly inhibits the migration of human lung cancer cell line CL1-5 cells by reducing TGFβRI production and FAK and AKT activation. This implies that FMGP may find application in the production of anticancer medications.89Additionally, PCPs have been demonstrated efficacious in the treatment of certain inflammatory diseases, including enteritis and prostatitis. Through an investigation into PCPs' mechanism of action in treating chronic nonbacterial prostatitis (CNP), it was discovered that PCPs can treat CNP through a number of different mechanisms, including the improvement of histologic damage in the inflammatory prostate, inhibition of TNF-α, IL-2, IL-8, and androgen production, and modulation of the intestinal microbiota.88 In particular, modulation of the gut microbiota provides a therapeutic target for the treatment of CNP.6 PCPs inhibit mRNA expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, and by regulating the intestinal microbiota, PCPs are protective against colitis.90
Oral administration of water-insoluble PCPs to mice with alcoholic hepatic steatosis (ALD) effectively ameliorated inflammatory damage and fat accumulation by modulating the gut microbiota and inhibiting ethanol-induced fungal overgrowth, and the results of the above experiments suggest that it is an effective treatment for and alleviates ALD.91 Studies have shown that PCPs can prevent atherosclerosis (AS) by inhibiting the elevation of serum inflammatory mediators and lipid levels in high-fat mice, and it is speculated that PCPs can be applied to future drugs for the prevention and treatment of atherosclerosis.27 PCPs exhibit potential for the treatment of depression in the pharmaceutical industry by inhibiting ConA-stimulated T cell proliferation and suppressing LPS-induced B cell proliferation.19
In comparison to veterinary rabies vaccine adjuvant aldehyde hydrogel, PCP-II in combination with rCVS-11-G enhances and promotes the elevation of virus-neutralizing antibody (VNA) titers in mice. Moreover, PCP-II activated multiple immune cells in the lymph nodes and blood, and induced proliferation of splenic T-lymphocytes and high levels of cytokine secretion from splenic cells. These results indicate that PCP-II has good adjuvant activity and is an excellent candidate for inactivated rabies vaccine.92 PCP-II increased the level of antigen-specific antibodies in mice immunized with influenza vaccine and significantly increased the titer of anti-hepatitis B surface antigen (HBsAg) antibodies and produced stronger and longer-lasting immunity. The experimental results indicate that PCP-II is an effective vaccine adjuvant worthy of research and development.93
In terms of application, PCPs not only rely on their own good drug activity to make drugs and functional foods, but studies have also shown that they can be used in combination with other drugs to play a better role. It is assumed that the synergistic effect with other drugs changes their structural characteristics to increase their activity. Low amounts of lotus root oligomeric procyanidins (LSPC) showed significant synergistic effects with CMP to modulate antimicrobial activity. It is hypothesized that the mixture can be applied as a natural antimicrobial agent.94 This offers fresh concepts for using PCPs in pharmaceutical development.
4.2 The application of PCPs as a drug carrier
Drug carriers are capable of altering the distribution of drugs in the body, controlling the rate of drug release, and delivering drugs to target organs.95 Since they are a naturally occurring polymer with stable chemical properties, facile chemical modification, and inexpensive processing costs, polysaccharides are frequently employed in the creation of tailored drug carriers.96 Polysaccharides play a significant role in drug delivery systems because of their biocompatibility, which allows drug molecules to be encapsulated in their interstitial spaces to provide controlled release of cargo drug molecules.85 The release kinetics of PCP-based nanoparticles, which were most similar to the Higuchi model, indicated diffusion-controlled release of SA (salicylic acid) in phosphate buffer (pH = 7.4). The experimental results suggested that PCPs have promising applications in the field of nanocarriers.97 When polysaccharides are used in the manufacture of pharmaceutical carriers, they have special physical and chemical properties such as high osmotic pressure, high viscosity, and water absorption, and they easily form gels.87 A hydrophilic spherical cellulose-based hydrogel was prepared by cross-linking water-soluble carboxymethyl pachyman (CMP) with biocompatible and biodegradable epichlorohydrin (ECH), which can be used to release model protein drugs in a controllable manner and to ensure the stability and activity of protein drugs. The experimental results showed that the substance could be used as a carrier for the delivery of protein drugs.984.3 Application of PCPs as feed additives
PCPs can be used as a feed additive in animal husbandry to treat livestock diseases, improve livestock growth and reproduction, and increase productivity.99 PCPs have a variety of potent pharmacological activities and can be added as a feed probiotic with the aim of enhancing host growth and immune function. PCPs used as pig feed additive significantly and linearly improved the growth of weaned piglets, and also effectively improved the cellular immunity of weaned piglets and regulated and improved the microbiota in their cecum. The above results demonstrate the potential of PCPs as a feed additive in the pig industry.100 PCPs were also used as a feed additive for spotted sea bass, which increased fish growth and antioxidant capacity, but they had less of an impact on the fish's lipid metabolism and nonspecific immunity. The ideal PCP addition level for spotted sea bass feed was 1.4 g kg−1. For aquaculture to improve the metabolic problems and control diseases in aquatic animals, PCP adoption as a food supplement is essential. This implies that there are potential benefits to using PCP in spotted sea bass aquaculture.1014.4 Application of PCPs as a food packaging material
Compared with traditional plastic packaging materials, new packaging materials developed based on polysaccharides are more environmentally friendly in terms of reducing dependence on fossil fuels and carbon emissions, due to the fact that polysaccharides, as polymeric carbohydrates, have the advantages of being nontoxic to organisms and compatible, as well as being biodegradable.102 The PCP-preparation of silver nanoparticles (AgNPs)/chitosan (CS) membranes fabricated from PCPs became more regular and more dense due to noncovalent interactions and significantly maintained the quality and prolonged the shelf life of strawberries. Moreover, the membrane is not toxic to the neighboring. The above results indicate that the PCP-AgNPs/CS films are environmentally friendly and have the potential to be used in current packaging materials.1035. Conclusion and prospects
PCPs are the main active ingredient in P. cocos. Years of research have shown that the extraction and purification of PCPs still face difficulties and low yields, and further improvements are needed by researchers. The most recent method related to the extraction of PCPs is the DES extraction method, which deserves continued research and improvement due to its high efficiency in extracting PCPs and its nontoxicity and ease of use, etc., among other advantages. Most of the purification methods for PCPs use column chromatography. Most of the information about the structure of PCPs remains on the molecular weight and monosaccharide composition of the polysaccharides. The advanced structures of PCPs still need to be studied. This may require innovations in polysaccharide structure analysis techniques to be realized. Future studies on PCPs should use AFM or NMR to study the advanced structures. It is hoped that more studies on the advanced structures of PCPs will be carried out in the future to help understand the link between structure and activity of PCPs and to increase the application of PCPs. Currently, PCPs are used as drugs (vaccine, clinical drug, and nutraceutical), functional food, drug carriers, feed additives and in other applications. It is hoped that there will be more clinical applications involving PCPs.Data availability
Data sharing is not applicable to this article, as no datasets were generated or analysed during the current study.Conflicts of interest
There are no conflicts to declare.References
- X. Chen, Q. Tang, Y. Chen, W. Wang and S. Li, Simultaneous extraction of polysaccharides from Poria cocos by ultrasonic technique and its inhibitory activities against oxidative injury in rats with cervical cancer, Carbohydr. Polym., 2010, 79, 409–413 CrossRef CAS.
- H. Xiao, X. F. Wang, B. Y. Yang, D. Q. Dou and H. X. Kuang, Immunoenhancing constituents of Poria cocos, Int. J. Pharmacol., 2015, 11, 463–469 Search PubMed.
- X. Fu, Q. Wang, Z. Wang, H. Kuang and P. Jiang, Danggui-Shaoyao-San: New Hope for Alzheimer's Disease, Aging Dis., 2015, 7, 502–513 CrossRef PubMed.
- L. Yang, J. Tang, J. J. Chen, A. Y. Peng, Q. M. Wang, L. Q. Rao, H. Yang, X. W. Zhang, H. Z. Yang, C. Zhang and G. P. Peng, Transcriptome analysis of three cultivars of Poria cocos reveals genes related to the biosynthesis of polysaccharides, J. Asian Nat. Prod. Res., 2019, 21, 462–475 CrossRef CAS PubMed.
- J. L. Ríos, Chemical Constituents and Pharmacological Properties of Poria cocos, Planta Med., 2011, 77, 681–691 CrossRef PubMed.
- J. S. Liu, L. Liu, G. W. Zhang and X. C. Peng, Poria cocos polysaccharides attenuate chronic nonbacterial prostatitis by targeting the gut microbiota: Comparative study of Poria cocos polysaccharides and finasteride in treating chronic prostatitis, Int. J. Biol. Macromol., 2021, 189, 346–355 CrossRef CAS.
- S. M. Lee, Y. J. Lee, J. J. Yoon, D. G. Kang and H. S. Lee, Effect of Poria cocos on Puromycin Aminonucleoside-Induced Nephrotic Syndrome in Rats, J. Evidence-Based Complementary Altern. Med., 2014, 2014, 570420 CrossRef.
- Y. T. Zou, J. Zhou, C. Y. Wu, W. Zhang, H. Shen, J. D. Xu, Y. Q. Zhang, F. Long and S. L. Li, Protective effects of Poria cocos and its components against cisplatin-induced intestinal injury, J. Ethnopharmacol., 2021, 269, 113722 CrossRef CAS.
- K. H. Lai, M. C. Lu, Y. C. Du, M. El-Shazly, T. Y. Wu, Y. M. Hsu, A. Henz, J. C. Yang, A. Backlund, F. R. Chang and Y. C. Wu, Cytotoxic Lanostanoids from Poria cocos, J. Nat. Prod., 2016, 79, 2805–2813 CrossRef CAS.
- L. L. Liu, Y. N. Wang, C. Tan, X. Y. Han and D. Q. Dou, Substance basis of warm nature of Poria cocos, Chin. Herb. Med., 2020, 12, 316–325 Search PubMed.
- J. Lu, J. Tian, L. Zhou, L. Meng, S. Chen, C. Ma, J. Wang, Z. Liu, C. Li and W. Kang, Phytochemistry and biological activities of Poria, J. Chem., 2021, 2021, 6659775 Search PubMed.
- A. Nie, Y. Chao, X. Zhang, W. Jia, Z. Zhou and C. Zhu, Phytochemistry and Pharmacological Activities of Wolfiporia cocos (F.A.Wolf) Ryvarden & Gilb, Front. Pharmacol, 2020, 11, 505249 Search PubMed.
- T. Tai, A. Akahori and T. Shingu, Triterpenes Of Poria-Cocos, Phytochemistry, 1993, 32, 1239–1244 Search PubMed.
- C. Q. Zheng, Y. W. Shao, L. M. Hao, Y. L. Shi, J. Q. Zhu, C. C. Zhao, Q. W. Jiang, J. J. Yi and J. K. Lu, Extraction, characterization, and biological activities of a polysaccharide from Poria cocos peels, J. Food Process. Preserv., 2022, 46, e16821 Search PubMed.
- Y. C. Sun, Biological activities and potential health benefits of polysaccharides from Poria cocos and their derivatives, Int. J. Biol. Macromol., 2014, 68, 131–134 CrossRef CAS.
- Y. Lai, H. Deng, Q. Fang, L. Ma, H. Lei, X. Guo, Y. Chen and C. Song, Water-Insoluble Polysaccharide Extracted from Poria cocos Alleviates Antibiotic-Associated Diarrhea Based on Regulating the Gut Microbiota in Mice, Foods, 2023, 12 Search PubMed.
- P. F. Yang, T. Hua, D. Wang, Z. W. Zhao, G. L. Xi and Z. F. Chen, Phytochemical and chemotaxonomic study of Poria cocos (Schw.) Wolf, Biochem. Syst. Ecol., 2019, 83, 54–56 CrossRef CAS.
- Y. Z. Wang, J. Zhang, Y. L. Zhao, T. Li, T. Shen, J. Q. Li, W. Y. Li and H. G. Liu, Mycology, cultivation, traditional uses, phytochemistry and pharmacology of Wolfiporia cocos (Schwein.) Ryvarden et Gilb.: A review, J. Ethnopharmacol., 2013, 147, 265–276 CrossRef CAS.
- W. Zhang, L. Chen, P. Li, J. Zhao and J. Duan, Antidepressant and immunosuppressive activities of two polysaccharides from Poria cocos (Schw.) Wolf, Int. J. Biol. Macromol., 2018, 120, 1696–1704 CrossRef CAS.
- F. M. Zhang, H. Zheng, T. Zheng, P. Xu, Y. Xu, Y. X. Cao, F. Jia, Y. Q. Zeng, Y. B. Fan, K. He, X. W. Dai, F. F. Hou and Y. Yang, Adsorption, in vitro digestion and human gut microbiota regulation characteristics of three Poria cocos polysaccharides, Food Sci. Hum. Wellness, 2024, 13, 1685–1697 CrossRef CAS.
- H. Y. Jiang and D. M. Zhong, Inhibitory Effect of Poria cocos Polysaccharides on Proliferation, Migration, and Invasion of Lung Cancer Cells, A549, Curr. Top. Nutraceutical Res., 2022, 20, 147–152 Search PubMed.
- J. Wang, D. Zheng, F. Huang, A. Zhao, J. Kuang, Z. Ren, T. Chen, J. Lei, J. Lin, X. Wang, W. Jia, G. Xie and X. Zheng, Theabrownin and Poria cocos Polysaccharide Improve Lipid Metabolism via Modulation of Bile Acid and Fatty Acid Metabolism, Front. Pharmacol, 2022, 13, 875549 CrossRef CAS PubMed.
- X. B. Zhou, Y. X. Zhang, Y. Q. Jiang, C. X. Zhou and Y. Ling, Poria cocos polysaccharide attenuates damage of nervus in Alzheimer's disease rat model induced by D-galactose and aluminum trichloride, Neuroreport, 2021, 32, 727–737 CrossRef CAS PubMed.
- Y. Tu, X. Luo, D. Liu, H. Li, H. Xia, C. Ma, D. Zhang, Y. Yang, X. Pan, T. Wang, Y. Xia, H. Dan, P. You and X. Ye, Extracts of Poria cocos improve functional dyspepsia via regulating brain-gut peptides, immunity and repairing of gastrointestinal mucosa, Phytomedicine, 2022, 95, 153875 CrossRef CAS PubMed.
- Y. W. Pu, Z. J. Liu, H. Tian and Y. X. Bao, The immunomodulatory effect of Poria cocos polysaccharides is mediated by the Ca2+/PKC/p38/NF-κB signaling pathway in macrophages, Int. Immunopharmacol., 2019, 72, 252–257 CrossRef CAS PubMed.
- X. Liu, X. Wang, X. Xu and X. Zhang, Purification, antitumor and anti-inflammation activities of an alkali-soluble and carboxymethyl polysaccharide CMP33 from Poria cocos, Int. J. Biol. Macromol., 2019, 127, 39–47 CrossRef CAS PubMed.
- W. F. Li, J. J. Yu, J. M. Zhao, X. Xiao, W. Q. Li, L. L. Zang, J. B. Yu, H. J. Liu and X. F. Niu, Poria cocos polysaccharides reduces high-fat diet-induced arteriosclerosis in ApoE-/- mice by inhibiting inflammation, Phytother. Res., 2021, 35, 2220–2229 CrossRef CAS PubMed.
- Y. Wu, D. Li, H. Wang and X. Wan, Protective Effect of Poria Cocos Polysaccharides on Fecal Peritonitis-Induced Sepsis in Mice Through Inhibition of Oxidative Stress, Inflammation, Apoptosis, and Reduction of Treg Cells, Front. Microbiol., 2022, 13, 887949 CrossRef PubMed.
- K. Wu, C. Guo, B. Yang, X. Wu and W. Wang, Antihepatotoxic benefits of Poria cocos polysaccharides on acetaminophen-lesioned livers in vivo and in vitro, J. Cell. Biochem., 2019, 120, 7482–7488 CrossRef CAS PubMed.
- Y. Cheng, Y. Xie, J. C. Ge, L. Wang, D. Y. Peng, N. J. Yu, Y. Zhang, Y. H. Jiang, J. P. Luo and W. D. Chen, Structural characterization and hepatoprotective activity of a galactoglucan from Poria cocos, Carbohydr. Polym., 2021, 263, 117979 CrossRef CAS PubMed.
- H. Ye, S. Ma, Z. Qiu, S. Huang, G. Deng, Y. Li, S. Xu, M. Yang, H. Shi, C. Wu, M. Li, J. Zhang, F. Zhang, M. Qin, H. Huang, Z. Zeng, M. Wang, Y. Chen, H. Lin, Z. Gao, M. Cai, Y. Song, S. Gong and L. Gao, Poria cocos polysaccharides rescue pyroptosis-driven gut vascular barrier disruption in order to alleviates non-alcoholic steatohepatitis, J. Ethnopharmacol., 2022, 296, 115457 CrossRef CAS PubMed.
- K. Wu, J. L. Fan, X. Y. Huang, X. M. Wu and C. Guo, Hepatoprotective effects exerted by Poria Cocos polysaccharides against acetaminophen-induced liver injury in mice, Int. J. Biol. Macromol., 2018, 114, 137–142 CrossRef CAS PubMed.
- S. Niu, L. M. Hao, S. X. Zhao, F. F. Wang and C. H. Du, Optimization of Liquid Fermentation for Mycelial Growth and Extracellular Polysaccharide Production by Poria cosos, Adv. Mater. Res., 2012, 554, 962–968 Search PubMed.
- Y. Jin, L. Zhang, L. Chen, Y. Chen, P. C. Cheung and L. Chen, Effect of culture media on the chemical and physical characteristics of polysaccharides isolated from Poria cocos mycelia, Carbohydr. Res., 2003, 338, 1507–1515 CrossRef CAS PubMed.
- W. Zhang, S. Cheng, X. Zhai, J. Sun, X. Hu, H. Pei and G. Chen, Green and efficient extraction of polysaccharides from Poria cocos FA Wolf by deep eutectic solvent, Nat. Prod. Commun., 2020, 15, 1934578X19900708 CAS.
- S. Chen, H. Zhang, L. Yang, S. Zhang and H. Jiang, Optimization of Ultrasonic-Assisted Extraction Conditions for Bioactive Components and Antioxidant Activity of Poria cocos (Schw.) Wolf by an RSM-ANN-GA Hybrid Approach, Foods, 2023, 12(3), 619 CrossRef CAS PubMed.
- G. Huang and B. Lin, Preparation for shaddock skin polysaccharide derivatives by response surface method, Sci. Rep., 2024, 14(1), 14054 CrossRef CAS PubMed.
- T. Xu, H. Zhang, S. Wang, Z. Xiang, H. Kong, Q. Xue, M. He, X. Yu, Y. Li, D. Sun, P. Gao and Z. Cong, A review on the advances in the extraction methods and structure elucidation of Poria cocos polysaccharide and its pharmacological activities and drug carrier applications, Int. J. Biol. Macromol., 2022, 217, 536–551 CrossRef CAS PubMed.
- Z. Tang, G. Huang and H. Huang, Ultrasonic-assisted extraction, analysis and properties of purple mangosteen scarfskin polysaccharide and its acetylated derivative, Ultrason. Sonochem., 2024, 109, 107010 CrossRef CAS PubMed.
- Y. Y. Guo, Y. S. Li, Z. C. Li, W. T. Yan, P. Chen and S. Yao, Extraction assisted by far infrared radiation and hot air circulation with deep eutectic solvent for bioactive polysaccharides from Poria cocos (Schw.) wolf, Green Chem., 2021, 23, 7170–7192 RSC.
- H. Wang and G. Huang, Extraction, purification, structural modification, activities and application of polysaccharides from different parts of mulberry, Food Funct., 2024, 15(8), 3939–3958 RSC.
- G. Huang, F. Chen, W. Yang and H. Huang, Preparation, deproteination and comparison of bioactive polysaccharides, Trends Food Sci. Technol., 2021, 109, 564–568 CrossRef CAS.
- Z. Song, G. Huang and H. Huang, The ultrasonic-assisted enzymatic extraction, characteristics and antioxidant activities of lychee nuclear polysaccharide, Ultrason. Sonochem., 2024, 110, 107038 CrossRef CAS PubMed.
- Z. Tang and G. Huang, Antioxidant activity of polysaccharide from Garcinia mangostana rind and their derivatives, BMC Complementary Med. Ther., 2024, 24(1), 283 CrossRef CAS PubMed.
- X. J. Jia, L. S. Ma, P. Li, M. W. Chen and C. W. He, Prospects of Poria cocos polysaccharides: Isolation process, structural features and bioactivities, Trends Food Sci. Technol., 2016, 54, 52–62 CrossRef CAS.
- K. Y. Lee and Y. J. Jeon, Polysaccharide isolated from Poria cocos sclerotium induces NF-kappaB/Rel activation and iNOS expression in murine macrophages, Int. Immunopharmacol., 2003, 3, 1353–1362 CrossRef CAS PubMed.
- Y. Zhang, J. Huang, M. Sun, Y. Duan, L. Wang, N. Yu, D. Peng, W. Chen and Y. Wang, Preparation, characterization, antioxidant and antianemia activities of Poria cocos polysaccharide iron (III) complex, Heliyon, 2023, 9, e12819 CrossRef CAS PubMed.
- J. Tang, J. Nie, D. P. Li, W. J. Zhu, S. P. Zhang, F. Ma, Q. Sun, J. Song, Y. L. Zheng and P. Chen, Characterization and antioxidant activities of degraded polysaccharides from Poria cocos sclerotium, Carbohydr. Polym., 2014, 105, 121–126 CrossRef CAS PubMed.
- J. W. Jeong, H. H. Lee, M. H. Han, G. Y. Kim, S. H. Hong, C. Park and Y. H. Choi, Ethanol extract of Poria cocos reduces the production of inflammatory mediators by suppressing the NF-kappaB signaling pathway in lipopolysaccharide-stimulated RAW 264.7 macrophages, BMC Complementary Altern. Med., 2014, 14, 101 CrossRef PubMed.
- Y. Wang, G. Huang and H. Huang, Ultrasonic/enzymatic extraction, characteristics and comparison of leechee peel polysaccharide, Ultrason. Sonochem., 2024, 108, 106948 CrossRef CAS PubMed.
- C. H. Chan, R. Yusoff, G. C. Ngoh and F. W. L. Kung, Microwave-assisted extractions of active ingredients from plants, J. Chromatogr. A, 2011, 1218, 6213–6225 CrossRef CAS PubMed.
- M. Zhao, Z. Guan, N. Tang and Y. Cheng, The differences between the water- and alkaline-soluble Poria cocos polysaccharide: A review, Int. J. Biol. Macromol., 2023, 235, 123925 CrossRef CAS PubMed.
- R. da Silva, T. A. P. Rocha-Santos and A. C. Duarte, Supercritical fluid extraction of bioactive compounds, TrAC, Trends Anal. Chem., 2016, 76, 40–51 CrossRef CAS.
- Y. Li, C. P. Zhu, X. C. Zhai, Y. Zhang, Z. Duan and J. R. Sun, Optimization of enzyme assisted extraction of polysaccharides from pomegranate peel by response surface methodology and their anti-oxidant potential, Chin. Herb. Med., 2018, 10, 416–423 Search PubMed.
- A. A. Khaskheli, S. G. Khaskheli, Y. Liu, S. A. Sheikh, Y.-F. Wang, A. H. Soomro, X. Tian, M. A. Homaida and W. Huang, Optimization of enzyme assisted extraction of polysaccharides from Poria cocos, J. Med. Plants Res., 2017, 11, 331–337 CrossRef CAS.
- S. S. Nadar, P. Rao and V. K. Rathod, Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review, Food Res. Int., 2018, 108, 309–330 CrossRef CAS PubMed.
- H. L. Huang and G. L. Huang, Extraction, separation, modification, structural characterization, and antioxidant activity of plant polysaccharides, Chem. Biol. Drug Des., 2020, 96, 1209–1222 CrossRef CAS PubMed.
- Y. Yang, J. Ji, L. Di, J. Li, L. Hu, H. Qiao, L. Wang and Y. Feng, Resource, chemical structure and activity of natural polysaccharides against alcoholic liver damages, Carbohydr. Polym., 2020, 241, 116355 CrossRef CAS PubMed.
- Y. R. Li, S. T. Liu, Q. Gan, J. Zhang, N. Chen, C. F. Han, W. J. Geng, B. X. Wang, N. Han, S. R. Jia and P. P. Han, Four polysaccharides isolated from Poria cocos mycelium and fermentation broth supernatant possess different activities on regulating immune response, Int. J. Biol. Macromol., 2023, 226, 935–945 CrossRef CAS PubMed.
- S. Warsi and W. Whelan, Structure of pachyman, the polysaccharide component of Poria-cocos wolf, 1957, 1573.
- Y. Wang, M. Zhang, D. Ruan, A. S. Shashkov, M. Kilcoyne, A. V. Savage and L. Zhang, Chemical components and molecular mass of six polysaccharides isolated from the sclerotium of Poria cocos, Carbohydr. Res., 2004, 339, 327–334 CrossRef CAS PubMed.
- K. RuiDian, L. ShunFa, C. Yi, J. ChuRong and S. QiaGuang, Analysis of chemical composition of polysaccharides from Poria cocos Wolf and its anti-tumor activity by NMR spectroscopy, Carbohydr. Polym., 2010, 80, 31–34 CrossRef.
- L. X. Zhu, X. Wang, S. C. Li, E. R. Qi, J. Meng, K. Y. C. Lam, X. P. Dong, J. Xu, H. B. Chen and Z. Z. Zhao, Qualitative and quantitative characterization of carbohydrate profiles in three different parts of Poria cocos, J. Pharm. Biomed. Anal., 2020, 179 Search PubMed.
- Y. Zhao, N. Wang, S. F. Pang and Y. H. Zhang, In-situ micro-FTIR spectroscopic observation on the hydration process of Poria cocos, Spectrochim. Acta, Part A, 2016, 164, 61–66 CrossRef CAS PubMed.
- Y. Meng, F. Lyu, X. Xu and L. Zhang, Recent advances in chain conformation and bioactivities of triple-helix polysaccharides, Biomacromolecules, 2020, 21, 1653–1677 CrossRef CAS PubMed.
- Z. Yu, G. Ming, W. Kaiping, C. Zhixiang, D. Liquan, L. Jingyu and Z. Fang, Structure, chain conformation and antitumor activity of a novel polysaccharide from Lentinus edodes, Fitoterapia, 2010, 81, 1163–1170 CrossRef PubMed.
- F. Liu, Y. Liu, X. Feng, S. A. Ibrahim and W. Huang, Structure characterization and in vitro immunomodulatory activities of carboxymethyl pachymaran, Int. J. Biol. Macromol., 2021, 178, 94–103 CrossRef CAS PubMed.
- Q. L. Huang and L. N. Zhang, Preparation, chain conformation and anti-tumor activities of water-soluble phosphated (1 → 3)-α-D-glucan from Poria cocos mycelia, Carbohydr. Polym., 2011, 83, 1363–1369 CrossRef CAS.
- L. Zhang, L. Chen, X. Xu, F. Zeng and P. C. Cheung, Effect of molecular mass on antitumor activity of heteropolysaccharide from Poria cocos, Biosci., Biotechnol., Biochem., 2005, 69, 631–634 CrossRef CAS PubMed.
- Y. Ren, Y. Bai, Z. Zhang, W. Cai and A. Del Rio Flores, The preparation and structure analysis methods of natural polysaccharides of plants and fungi: A review of recent development, Molecules, 2019, 24, 3122 CrossRef CAS PubMed.
- A. Zdunek, P. M. Pieczywek and J. Cybulska, The primary, secondary, and structures of higher levels of pectin polysaccharides, Compr. Rev. Food Sci. Food Saf., 2021, 20, 1101–1117 CrossRef CAS PubMed.
- Y. Wu, S. Li, H. Li, C. Zhao, H. Ma, X. Zhao, J. Wu, K. Liu, J. Shan and Y. Wang, Effect of a polysaccharide from Poria cocos on humoral response in mice immunized by H1N1 influenza and HBsAg vaccines, Int. J. Biol. Macromol., 2016, 91, 248–257 CrossRef CAS PubMed.
- K. Liu, Y. Yin, J. Zhang, X. Zai, R. Li, H. Ma, J. Xu, J. Shan and W. Chen, Polysaccharide PCP-I isolated from Poria cocos enhances the immunogenicity and protection of an anthrax protective antigen-based vaccine, Hum. Vaccines Immunother., 2020, 16, 1699–1707 CrossRef CAS PubMed.
- J. Q. He, J. W. Lu, L. M. Zhan, D. P. Zheng, Y. D. Wang, J. X. Meng, P. Li, J. Z. Zhao and W. X. Zhang, An Alkali-extracted polysaccharide from Poria cocos activates RAW264.7 macrophages via NF-KB signaling pathway, Arabian J. Chem., 2023, 16, 104592 CrossRef CAS.
- Y. Y. Tan, S. R. Yue, A. P. Lu, L. Zhang, G. Ji, B. C. Liu and R. R. Wang, The improvement of nonalcoholic steatohepatitis by Poria cocos polysaccharides associated with gut microbiota and NF-κB/CCL3/CCR1 axis, Phytomedicine, 2022, 103 Search PubMed.
- J. Liang, M. Zhao, S. Xie, D. Peng, M. An, Y. Chen, P. Li and B. Du, Effect of steam explosion pretreatment on polysaccharide isolated from Poria cocos: Structure and immunostimulatory activity, J. Food Biochem., 2022, 46, e14355 CAS.
- Y. Duan, J. Huang, M. Sun, Y. Jiang, S. Wang, L. Wang, N. Yu, D. Peng, Y. Wang, W. Chen and Y. Zhang, Poria cocos polysaccharide improves intestinal barrier function and maintains intestinal homeostasis in mice, Int. J. Biol. Macromol., 2023, 249, 125953 CrossRef CAS PubMed.
- Q. Huang, Y. Jin, L. Zhang, P. C. K. Cheung and J. F. Kennedy, Structure, molecular size and antitumor activities of polysaccharides from Poria cocos mycelia produced in fermenter, Carbohydr. Polym., 2007, 70, 324–333 CrossRef CAS.
- J. Chen, L. Chen, S. Lin, C. Liu and P. C. K. Cheung, Preparation and structural characterization of a partially depolymerized beta-glucan obtained from Poria cocos sclerotium by ultrasonic treatment, Food Hydrocolloids, 2015, 46, 1–9 CrossRef.
- M.-K. Lu, J.-J. Cheng, C.-Y. Lin and C.-C. Chang, Purification, structural elucidation, and anti-inflammatory effect of a water-soluble 1,6-branched 1,3-α-D-galactan from cultured mycelia of Poria cocos, Food Chem., 2010, 118, 349–356 CrossRef CAS.
- K.-H. Wong, P. C. Cheung and J.-Z. Wu, Biochemical and microstructural characteristics of insoluble and soluble dietary fiber prepared from mushroom sclerotia of Pleurotus tuber-regium, Polyporus rhinocerus, and Wolfiporia cocos, J. Agric. Food Chem., 2003, 51, 7197–7202 CrossRef CAS PubMed.
- Y. Jin, L. Zhang, M. Zhang, L. Chen, P. C. Cheung, V. E. Oi and Y. Lin, Antitumor activities of heteropolysaccharides of Poria cocos mycelia from different strains and culture media, Carbohydr. Res., 2003, 338, 1517–1521 CrossRef CAS PubMed.
- J. Zhang, D. Shu, X. Cheng, T. Tian, K. Xiao, D. Zhang and J. Yang, Effect of plant polysaccharides (Poria cocos and Astragalus polysaccharides) on immune responses and intestinal microbiota of Dabry's sturgeons, Biosci. Microbiota, Food Health, 2023, 42, 243–253 CrossRef CAS PubMed.
- Y. Hu, T. Yang and X. Hu, Novel polysaccharides-based nanoparticle carriers prepared by polyelectrolyte complexation for protein drug delivery, Polym. Bull., 2012, 68, 1183–1199 CrossRef CAS.
- S. Huang and G. Huang, The utilization of quantum dot labeling as a burgeoning technique in the field of biological imaging, RSC Adv., 2024, 14(29), 20884–20897 RSC.
- M. Khvostov, T. Tolstikova, S. Borisov and A. Dushkin, Application of natural polysaccharides in pharmaceutics, Russ. J. Bioorg. Chem., 2019, 45, 438–450 CrossRef CAS.
- H. M. Shi, J. C. Li, J. Yu, H. Li, G. L. Huang and T. Zhang, Extraction, purification and antioxidant activity of polysaccharides from different parts of Hibiscus manihot L, J. Mol. Struct., 2024, 1295, 136598 CrossRef CAS.
- C. Y. Shi, Q. H. Ma, M. Y. Ren, D. D. Liang, Q. T. Yu and J. B. Luo, Antitumorpharmacological mechanism of the oral liquid of Poriacocos polysaccharide, J. Ethnopharmacol., 2017, 209, 24–31 CrossRef CAS PubMed.
- T. Y. Lin, M. K. Lu and C. C. Chang, Structural identification of a fucose-containing 1,3-β-mannoglucan from Poria cocos and its anti-lung cancer CL1-5 cells migration via inhibition of TGRβR-mediated signaling, Int. J. Biol. Macromol., 2020, 157, 311–318 CrossRef CAS PubMed.
- K. Lan, H. Yang, J. Zheng, H. Hu, T. Zhu, X. Zou, B. Hu and H. Liu, Poria cocos oligosaccharides ameliorate dextran sodium sulfate-induced colitis mice by regulating gut microbiota dysbiosis, Food Funct., 2023, 14, 857–873 RSC.
- S. Sun, K. Wang, L. Sun, B. Cheng, S. Qiao, H. Dai, W. Shi, J. Ma and H. Liu, Therapeutic manipulation of gut microbiota by polysaccharides of Wolfiporia cocos reveals the contribution of the gut fungi-induced PGE2 to alcoholic hepatic steatosis, Gut Microbes, 2020, 12, 1830693 CrossRef PubMed.
- W. Zhang, N. Cheng, Y. Wang, X. Zheng, Y. Zhao, H. Wang, C. Wang, Q. Han, Y. Gao and J. Shan, Adjuvant activity of PCP-II, a polysaccharide from Poria cocos, on a whole killed rabies vaccine, Virus Res., 2019, 270, 197638 CrossRef CAS PubMed.
- Y. Wu, S. Li, H. Li, C. Zhao, H. Ma, X. Zhao, J. Wu, K. Liu, J. Shan and Y. Wang, Effect of a polysaccharide from Poria cocos on humoral response in mice immunized by H1N1 influenza and HBsAg vaccines, Int. J. Biol. Macromol., 2016, 91, 248–257 CrossRef CAS PubMed.
- J. Wang, M. Bie, W. Zhou, B. Xie and Z. Sun, Interaction between carboxymethyl pachyman and lotus seedpod oligomeric procyanidins with superior synergistic antibacterial activity, Carbohydr. Polym., 2019, 212, 11–20 CrossRef CAS PubMed.
- C. A. García-González, M. Alnaief and I. Smirnova, Polysaccharide-based aerogels-Promising biodegradable carriers for drug delivery systems, Carbohydr. Polym., 2011, 86, 1425–1438 CrossRef.
- J. Nai, C. Zhang, H. Shao, B. Li, H. Li, L. Gao, M. Dai, L. Zhu and H. Sheng, Extraction, structure, pharmacological activities and drug carrier applications of Angelica sinensis polysaccharide, Int. J. Biol. Macromol., 2021, 183, 2337–2353 CrossRef CAS PubMed.
- J. Zhang, H. Shao, Y. Xiao, Y. Zhu and S. Liang, Preparation and evaluation in vitro of salicylic acid-pachyman nanoparticles, J. Wuhan Univ. Technol. Mater. Sci. Ed., 2011, 26, 606–610 CrossRef CAS.
- Y. Xiao, W. Xu, Q. Zhu, B. Yan, D. Yang, J. Yang, X. He, S. Liang and X. Hu, Preparation and characterization of a novel pachyman-based pharmaceutical aid. II: A pH-sensitive, biodegradable and biocompatible hydrogel for controlled release of protein drugs, Carbohydr. Polym., 2009, 77, 612–620 CrossRef CAS.
- J. Zhang, D. Shu, X. Cheng, T. Tian, K. Xiao, D. Zhang and J. Yang, Effect of plant polysaccharides (Poria cocos and Astragalus polysaccharides) on immune responses and intestinal microbiota of Dabry's sturgeons, Biosci. Microbiota, Food Health, 2023, 42, 243–253 CrossRef CAS PubMed.
- J. Zhang, H. Wang, S. Meng, C. Zhang, L. Guo and Z. Miao, The Effects of Poria cocos Polysaccharides on Growth Performance, Immunity, and Cecal Microflora Composition of Weaned Piglets, Animals, 2024, 14(7), 1121 CrossRef PubMed.
- J. Lu, Z. F. Huang, Y. L. Ye, A. L. Xu and Z. B. Li, Effects of Poria cocos polysaccharide on growth performance, physiological parameters, and lipid metabolism of spotted sea bass Lateolabrax maculatus, J. Oceanol. Limnol., 2024, 42, 316–331 CrossRef CAS.
- A. Nešić, G. Cabrera-Barjas, S. Dimitrijević-Branković, S. Davidović, N. Radovanović and C. Delattre, Prospect of polysaccharide-based materials as advanced food packaging, Molecules, 2019, 25, 135 CrossRef PubMed.
- X. Yang, Y. Niu, Y. Fan, T. Zheng and J. Fan, Green synthesis of Poria cocos polysaccharides-silver nanoparticles and their applications in food packaging, Int. J. Biol. Macromol., 2024, 131928 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |