Open Access Article
Open Access ArticleCascade reaction for bio-polyol synthesis from sunflower oil over a W/ZSM-5 zeolite catalyst for the fabrication of a bio-polyurethane-based porous biocomposite with high oil uptake
Phan Huy Hoang *a and
Hoang Tien Datb
*a and
Hoang Tien Datb
aSchool of Chemistry & Life Science, Hanoi University of Science & Technology, No.1 Dai Co Viet Street, Hanoi, Vietnam. E-mail: hoang.phanhuy@hust.edu.vn
bResearch Institute of Pulp & Paper Industry, No.59 Vu Trong Phung, Hanoi, Vietnam
First published on 2nd July 2024
Abstract
A W/ZSM-5 zeolite was successfully prepared by incorporating tungsten transition metal into a zeolite structure using a conventional impregnation method. The as-obtained W/ZSM-5 zeolite was characterized using several characterization techniques such as XRD, IR and SEM-EDS. The catalyst was then applied to a cascade, single-batch reaction to synthesize bio-polyol from sunflower oils using H2O2 in isopropanol solvent. The obtained results indicated that the W/ZSM-5 zeolite had high catalytic efficiency in the epoxidation of the double bond of vegetable oil and the epoxy ring opening reaction to form bio-polyol. The effect of different reaction conditions on bio-polyol synthesis, such as the dosage of the catalyst and reaction time, were investigated. Bio-polyol was obtained from sunflower oil with a hydroxyl number of 160 mg KOH per g and functionality of 2.9 OH groups per mol. The as-synthesized sunflower oil-based polyol was used to replace fossil-based polyol in the fabrication of a bio-polyurethane-based composite with high oil uptake capacity. The oil adsorption capacity of the porous polyurethane-corn stalk composite was relatively high, up to 15.07 g g−1. In comparison with neat polyurethane and lignocellulosic materials, the new porous bio-composite had higher oil uptake capacity.
Introduction
Recently, the utilization of bio-based renewable resources for the production of useful materials, platform chemicals and fuel has grown rapidly. Bio-based resources offer many advantages such as environmental compatibility, economic feasibility and renewability as compared to the traditional petrochemical source.1–4 Bio-polyols are polyols of biological origin or based on bio-compounds and are an attractive alternative for polyols derived from petrochemicals in the preparation of polyurethanes and polyesters. Because they are renewable, less costly and more ecofriendly, they have drawn considerable attention.5–7 Vegetable oils are widely studied as a source of bio-polyols as they are cheap, environment friendly, readily available and easy to process. Polyols derived from vegetable oils are found to be a promising replacement for petroleum-derived polyols.5,8–10Many methods have been studied for synthesizing polyols from vegetable oils by introducing hydroxyl groups into the fatty acid chains at the carbon–carbon double bond position. In general, polyols are obtained from unsaturated vegetable oils through a two-step conversion: by epoxidation of vegetable oils, followed by ring opening of the epoxy group; or by hydroformylation and then hydrogenation; or by ozonolysis of the double bond and subsequent hydrogenation.2 The synthesis of bio-polyols through epoxidation of vegetable oils, followed by ring opening of epoxy groups, is often used because of simple reaction conditions and equipment. The epoxidation of vegetable oils, followed by oxirane ring opening, is one of the most important reactions to generate various polyols.11 In this process, to increase reaction speed and efficiency, many types of catalysts are used. Among them, there have been several reports on using transition tungsten metal-based catalysts, such as peroxo-tris(cetylpyridinium)12-tungstophosphate,12 polyoxotungstate/poly(divinylbenzene),13 Na2WO4/H2WO4,14 and a tungsten–bishydroxamic acid complex.15 However, these processes are still complicated because they are two-step reaction processes. Developing methods to convert vegetable oil into bio-polyols through one step/one pot reaction can bring many benefits to help shorten the reaction time and save chemicals. In this scene, bio-polyols can be synthesized by the hydroxylation of vegetable oils in one conversion step using a mixture of hydrogen peroxide (H2O2) and formic acid (HCOOH). By changing the reaction conditions, it is possible to obtain bio-polyols with different hydroxyl indexes suitable for different polyurethane applications.2,16 Bio-polyol was also prepared in one conversion step by the direct hydroxylation of vegetable oil in the presence of OsO4 as the catalyst and N-methylmorpholine N-oxide (NMO) as the oxidant.17 Nevertheless, the use of formic acid or NMO oxidant/OsO4 catalyst has effects on the environment as well as human health because it is a relatively toxic chemical. Furthermore, this homogeneous catalytic reaction will cause difficulties in the separation and recovery of the catalyst and reactants, leading to high costs. Therefore, studying the one-step reaction for bio-polyol synthesis using environment-friendly heterogeneous catalysts with high catalytic efficiency will be a highly scientific and practical research direction.
Zeolite catalysts are solid catalytic materials that are widely used in the chemical industry with typical properties such as molecular sieve structure, high catalytic activity and selectivity, large surface area and environmental friendliness.18,19 The catalytic activity of zeolites can be easily improved by adding highly active metals to their structure. Some of these catalysts are highly active in the double bond oxidation of oils, fats or alkenes.20–23 Besides, the ZSM-5 zeolite catalyst is also active in the epoxy ring opening reaction using the alcohol reagent under mild reaction conditions.24 The combination of tungsten-based catalyst and H2O2 as the oxidant has been well-known as a classic system for the epoxidation of double bond in olefins or vegetable oil. To the best of our knowledge, there were no examples for the use of the catalytic system of tungsten-loaded ZSM-5 zeolite for the preparation of vegetable oil-based polyol. We were, therefore, motivated that the W/ZSM-5 zeolite could be fabricated by fully taking the advantages that are offered by both highly catalytic tungsten metal for epoxidation and zeolite catalyst for epoxidation and epoxy ring opening. Such a synthesis could give us a new type of catalyst particles with easy separation and recycling with high catalytic activity for a new, sequential and single-batch reaction system for the synthesis of bio-polyol from vegetable oil using the reagent H2O2 in isopropanol solvent.
Furthermore, these bio-polyols can be used to replace a part of fossil polyol components along with isocyanate to fabricate polyurethane foam, an effective oil sorbent in oil-spill treatment. Very recently, using lignocellulose fillers such as plant fibers proved to be an effective way to alter the structure of the sorbent material, thus enhancing the oil adsorption capacity.25–27 Corn stalk is an agricultural residue produced in large quantities after harvesting in Vietnam. The current main method to handle post-harvest corn stalks is burning, which creates smoke that pollutes the air and surrounding environment. Finding an appropriate method for utilizing corn stover to create value-added materials while also treating agricultural waste will bring great benefits to the agriculture sector and environment. Therefore, in this study, corn stalks were pre-treated and used as the filler material for the preparation of PU-corn biomass bio-composite from vegetable oil-based bio-polyurethane and corn stalk for application in oil–water separation processes.
In this study, we present a “green” and cascade reaction for the synthesis of bio-polyol from vegetable oil using W/ZSM-5 zeolite catalyst. Notably, this process is a one-step synthesis method to achieve traditional two-step methods, including epoxidation and then hydroxylation. First, W/ZSM-5 zeolite was prepared by the impregnation method and then applied to a sequential, single-batch reaction to synthesize bio-polyol from sunflower oils using H2O2 in isopropanol solvent. W/ZSM-5 zeolite would catalyze the epoxidation of sunflower oils in the presence of H2O2 and consecutively catalyze the epoxy ring opening in the presence of alcohol to achieve bio-polyol. The sunflower oil-based bio-polyol was then used to replace petro-polyol in bio-polyurethane-based porous bio-composite with high oil adsorption capacity. Typically, the resulting bio-polyol was mixed with petro-polyol and corn stalk (CS) fibers and consequently mixed with isocyanate to fabricate the porous PU-CS bio-composite. This new porous material showed high efficiency in oil/water separation from oil-contaminated water systems.
Materials and methods
Preparation of W/ZSM-5 zeolite
ZSM-5 zeolite was synthesized by the hydrothermal method using hexadecyl trimethyl ammonium bromide (C16H33(CH3)3NBr, CTAB-Sigma) as a mesogenous template following the reported literature.20,28 In typical synthesis, sodium aluminate (NaAlO2-Sigma), solution of tetrapropylammonium hydroxide (TPAOH, 25% aqueous solution-Sigma) and potassium hydroxide were added to deionized water to get a homogeneous solution. Then, tetraethyl orthosilicate (TEOS-Sigma) and CTAB were added to the aforementioned solution to prepare a solution with a mole ratio of the composition TEOS/TPAOH/NaAlO2/KOH/H2O/CTAB = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05
1.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.85
0.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64
64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.45. The obtained synthetic solution was hydrothermally treated at 170 °C for 24 h in an autoclave. Then, the synthesized particles were collected by centrifuging, washed with deionized water (DI) water by three repetitions, and dried at 100 °C for several hours. Finally, it was calcined in air at 550 °C for 6 h to obtain the mesoporous ZSM-5 zeolite.
0.45. The obtained synthetic solution was hydrothermally treated at 170 °C for 24 h in an autoclave. Then, the synthesized particles were collected by centrifuging, washed with deionized water (DI) water by three repetitions, and dried at 100 °C for several hours. Finally, it was calcined in air at 550 °C for 6 h to obtain the mesoporous ZSM-5 zeolite.
To extend the catalytic activity of the ZSM-5 zeolite, W/ZSM-5 catalyst was prepared by incorporating tungsten transition metals into the zeolite matrix by the wetness impregnation method.20,21 The as-synthesized mesoporous ZSM-5 particles were added into ammonium metatungstate (99.99%-Sigma) dissolved in ammonia solution and stirred at 80 °C for several hours. Then, the solid material was dried in an oven and calcined at 550 °C for 4 h under air atmosphere to get W/ZSM-5 zeolite particles.
Synthesis of bio-polyol over the W/ZSM-5 catalyst
Vegetable oil sample, H2O2 (Sigma) and isopropanol solvent were added to the flask and stirred well by a magnetic stirrer until the reactants were well mixed. W/ZSM-5 catalyst was then added to the above solution and stirred. The reaction system in the flask was heated up to the temperature of 60 °C with stirring and kept for a certain reaction time (3–6 h). After the reaction, the zeolite catalyst was separated by centrifugation. Then, the reaction mixture was transferred into a separatory funnel and DI water was added to this mixture. The funnel was used to separate the upper layer of the reaction mixture. The funnel was shaken and left aside for a while to allow the most possible separation of the bio-polyol from the mixture. The bio-polyol product was achieved and washed with a washing solvent of water and acetone (50/50 wt%) three times. Then, the excess acetone and water was vacuum distilled with a rotary distillation apparatus. The as-obtained product was completely dried with anhydrous Na2SO4 (Sigma) and anhydrous silica in a desiccator for several hours. The produced bio-polyol was analyzed to determine the hydroxyl number OH#, iodine value and characterized by nuclear magnetic resonance (NMR) to confirm the chemical structure. The hydroxylation process was repeated twice, and the variance was recorded.Fabrication of porous polyurethane-corn stalk fiber bio-composite
Corn stalk (CS) fiber was prepared by cutting the corn stalk, grinding and sieving to select the samples in different sieve sizes of 1 mm. To enhance the oil adsorption capacity of the CS fiber, the CS samples were treated by hot water at 170 °C for 2 h25 and then dried and stored in a sealed zip bag at room temperature for further experiment.The as-synthesized bio-polyol from sunflower oil was used to replace a part of petrochemical polyol in preparation of polyurethane (PU) foam. PU-CS porous bio-composite was fabricated by the combination of bio-polyurethane foam and corn stalk fiber following a method developed by our own group.5 20% Petrochemical polyol in total polyol was replaced by bio-polyol, and this mixture of petrochemical polyol and bio-polyol was stirred vigorously for 3 minutes. Then, CS fiber (20 wt% over PU foam) was added to aforementioned mixture of polyol and stirred well for 5 minutes. To this mixture, 4,4-diphenylmethane diisocyanate (p-MDI, average molar mass equal to 340.6 g mol−1, and average% NCO equal to about 30 – Sigma) with molar ratio [NCO]/[OH] = 1.1 was added and stirred well for about 10–20 seconds. This mixture was poured into a mold cup for polymer curing to form the foam. At the end of the curing process, the porous bio-composite was removed from the mold and cut into small pieces for further experiments.
Characterization
X-ray diffraction (XRD) data were obtained on a Rigaku D/max lllC (3 kW) with a θ/θ goniometer equipped with a Cu KR radiation generator. Scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM/EDS) was performed using a JSM-7000F, JEOL, Japan. Brunauer–Emmett–Teller (BET) analysis was used to determine the surface areas of the samples. Fourier transform infrared (FT-IR) absorption spectra were recorded at room temperature on a Nicolet Nexus 670 FT-IR spectrometer. Nuclear magnetic resonance (NMR) analysis was performed on a 600 MHZ Bruker Advance 500 high-resolution magnetic resonance spectrometer (USA). The solvent used was deuterated chloroform (CDCl3), the standard signal being that of tetramethylsilane (TMS). The hydroxyl number of polyol was determined according to AOAC 965:32 standard based on the acetylation of free hydroxyl radicals of polyol with anhydride acetic in pyridine solvent.5 After the reaction, the unreacted acetic anhydride was converted to acetic acid and titrated with KOH. Iodine index was determined according to ISO 3961:2013 by Wijs method using 0.1 N Na2S2O3 standard solution and starch as indicator.2To determine the oil adsorption capacity of the porous adsorbent, the experiment was conducted based on ASTM F726-99 – Standard Test Method for Sorbent Performance of Adsorbents for use on Crude Oil and Related Spills.27 Typically, 80 mL of water and 20 mL of oil were poured into a 100 mL beaker. 0.5 g of the as-prepared adsorbent material was gently placed onto the oil surface of the beaker for different periods of time of 30, 60, 90 and 120 min. The sorbent material was then separated and treated under vacuum to remove water from the surface for about 5 minutes before weighing to calculate the adsorption capacity.
The adsorption capacity was calculated as the ratio of adsorbed amount of oil to initial dry weight of the sorbent, as shown in the following equation and given in units of g oil per g dry sorbent.5
| Oil sorption capacity (g g−1) = (St − So)/St |
The oil adsorption capacity determination was repeated twice, and the variance was recorded.
Results and discussion
W/ZSM-5 zeolite synthesis and characterization
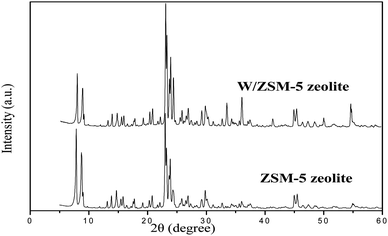
The XRD patterns of mesoporous ZSM-5 and W/ZSM-5 zeolite particles are shown in Fig. 1. It is seen that the as-obtained zeolite samples showed highly crystallinity of the MFI-type zeolitic structure with well-defined diffraction peaks. There was no presence of other non-zeolitic phases, indicating the purity of the as-prepared zeolite samples.Moreover, there were no alterations of the structure or crystallinity of the W/ZSM-5 zeolite, which was also confirmed by XRD analysis. Zeolitic structures were not affected by the doping of tungsten transition metals using the wetness impregnation method. The results also showed that no diffraction peaks of W-containing crystalline phases were observed, representing the low amount and high dispersion of tungsten species over the zeolite matrix. This result was explained due to the fact that the overlap of the XRD peak of WO3 coincided with the peak of ZSM-5 zeolite at 2 theta of 23–25°, 28° and 33°.
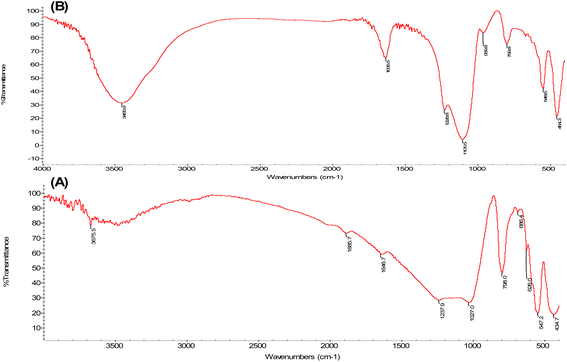
The IR spectrum of ZSM-5 zeolite showed the typical bands of ZSM-5 zeolite type, as seen from Fig. 2A. The vibrations of tetrahedrons (TO) with the band at 434 cm−1 and vibrations of the secondary building units with the band at 547 cm−1 were observed.20,29 The asymmetric stretching of framework Si–O–Si or Si–O–Al bonds with the band at 1027 cm−1 was also observed.29 As seen from Fig. 2B, the bands of the W/ZSM-5 zeolite sample demonstrated a shift in the frequency, suggesting that the modified zeolite catalysts experienced a change in the number of formed structural bonds after the impregnation of the tungsten species. The IR spectrum of the W/ZSM-5 sample (Fig. 2B) showed the small absorption bands at about 960 cm−1 and 690 cm−1, which were attributed to the stretching of W–O bonds of WO3.30,31 It indicated that tungsten metal was successfully loaded into the ZSM-5 zeolite matrix.
The W/ZSM-5 zeolite was characterized by scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM/EDS), and the results are shown in Fig. 3.
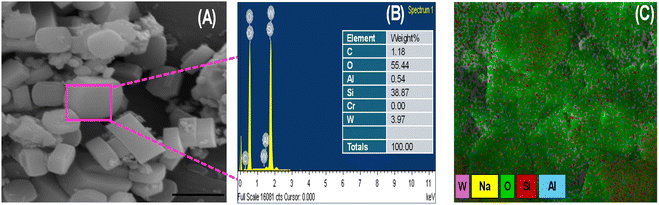 | ||
| Fig. 3 (A) SEM image, (B) elemental compositions and (C) elemental distribution of W/ZSM-5 zeolite obtained by EDS analysis. | ||
The SEM image of the zeolite (Fig. 3A) confirmed that the W/ZSM-5 zeolite had high crystallinity and uniformity. It can be seen that the zeolite particles exhibited a typical hexagonal morphology characteristic of MFI-type zeolites with an average diameter of about 500 nm. It is seen from EDS data (Fig. 3B) that the W species were successfully incorporated in the lattice of the ZSM-5 zeolite in addition to the presence of Si, Al, O in neat ZSM-5 zeolite. About 3.4 wt% W element was dispersed in W/ZSM-5 zeolite particles. It can be seen from the distribution of all the elements, especially the W element observed in Fig. 3C, that W has been successfully and effectively incorporated to the zeolite structure with high dispersion. Encouraged by such modified ZSM-5 zeolite with a crystallized framework and transition metal incorporation, outstanding catalytic properties were expected especially when dealing with epoxidation and consecutive epoxy ring opening.
Bio-polyol synthesis over W/ZSM-5 zeolite
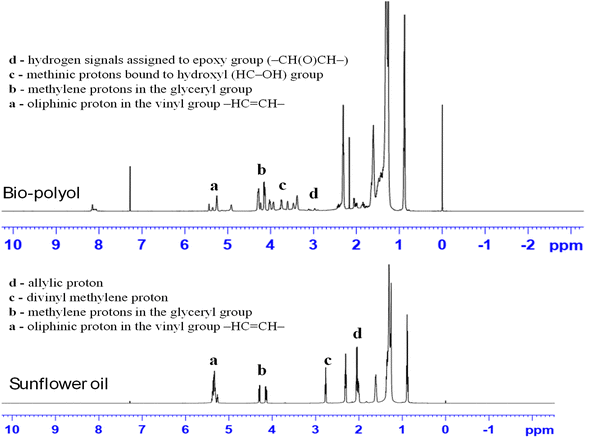
In order to investigate the potential in the one pot reaction of epoxidation and epoxy ring opening, W/ZSM-5 zeolite was applied for cascade, single-batch reaction to synthesize bio-polyol from sunflower oils using H2O2 in isopropanol solvent.The reactions were conducted at the reaction conditions as follows: 25 mL of sunflower oil was oxidized by 19 mL of H2O2 catalyzed by 1.25 g W/ZSM-5 zeolite catalyst (corresponding to the molar ratio of double bond/H2O2 = 1/10) at 60 °C for 5 h. The hydroxyl group in the structure of the sunflower oil-based polyol product was confirmed by nuclear magnetic resonance spectroscopy analysis (1H NMR), and the results are shown in Fig. 4.
As seen from the NMR spectra in Fig. 4, the 1H-NMR spectrum of the as-obtained bio-polyol (Fig. 4B) showed the peaks at δ 3.4–4.2 ppm corresponding to the signature of methine protons bound to the hydroxyl (HC–OH) group.2,32 While the peak at δ 5.2–5.4 ppm corresponding to the proton olefinic of the double bond and the peak at δ 2.7–2.8 ppm and δ 2 ppm corresponding to the divinyl methylene proton and allyl methylene protons, respectively, were observed to decline sharply in intensity or almost disappeared compared to these peaks of sunflower oil (Fig. 4A). This indicated that the bio-polyol was successfully synthesized from sunflower oil by one-pot cascade synthesis using H2O2 as the oxidant and W/ZSM-5 zeolite as the catalyst. Only the signal corresponding to the methine proton of glyceryl (ROOCH2)2CH(OOR) still existed.
The other important peaks of sunflower oil as well as bio-polyol were also observed from the NMR spectrum such as methylene protons in the glyceryl group in the δ 4.1–4.3 ppm region, α-methylene protons attached to the carbonyl group in the δ 2.3 ppm region, β-methylene protons on the carbonyl group at δ ∼1.6 ppm, methylene protons at the saturated carbon atom at δ 1.3–1.4 ppm; protons in the methyl groups at δ ∼0.9 ppm.2,9,32 Moreover, the hydrogen signal assigned to epoxy group (–CH(O)CH–) at 2.8–3.2 ppm was also observed with very low intensity in bio-polyol.
The iodine value and hydroxyl number of original sunflower oil and as-synthesized polyol were also determined. The hydroxyl number and iodine value of original sunflower oil was 8.08 mg KOH per g and 121 cgI2/100 g, respectively. After the reaction, the hydroxyl number of the as-synthesized bio-polyol increased sharply to 136.8 mg KOH per g while the iodine index of the bio-polyol obtained from sunflower oil was determined by chemical titration to be almost zero (following the Wijs method). These results once again confirm the conversion of the double bond and the formation of the hydroxyl group. It can be concluded that bio-polyol was successfully synthesized by the hydroxylation of sunflower oil in cascade one-step conversion of sunflower oil through epoxidation and then the epoxy ring opening process. In order to estimate the effect of catalyst dosage on bio-polyol synthesis, the amount of catalyst was varied while other conditions were kept constant, and the achieved results are shown in Table 1.
| Catalyst dosage (%) | Hydroxyl number of bio-polyol (mg KOH per g) |
|---|---|
| a Reaction condition: at 60 °C for 5 h. | |
| 0 | 12.1 ± 0.1 |
| 2.5 | 98.3 ± 0.4 |
| 5 | 136.8 ± 0.5 |
| 7.5 | 155.6 ± 0.5 |
| 10 | 158.9 ± 0.5 |
It can be seen that the contribution of the non-catalyzed reactions is negligible since only a low number of hydroxyl of polyol (about 12.1 mg KOH per g) was obtained in the absence of the catalyst. While it was found that the catalyst dosage was of great importance for the synthesis of bio-polyol when the catalytic dosage increased, the hydroxyl number of bio-polyol increased significantly. The result indicated that W/ZSM-5 zeolite has good catalytic activity in the hydroxylation of sunflower oil to form bio-polyol. It can be explained that in the presence of hydrogen peroxide, transition metals may be potentially oxidized to form tungsten peroxo-complex, an active intermediate, making hydrogen peroxide much more accessible to the interaction with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds. This complex is believed to be responsible for catalyzing the oxidation of unsaturated fatty acids to form epoxide product.20,33 The epoxide was then subjected to ring-opening reaction catalyzed by W/ZSM-5 catalyst in the presence of isopropanol to synthesize bio-polyol thanks to the acid sites of the ZSM-5 zeolite.24 Moreover, when the catalyst dosage increased from 7.5% to 10%, the hydroxyl number of the resulting bio-polyol increased slightly. Because when an excessive amount of catalyst was used, the aggregation of the catalyst particles in the solution occurred, causing the loss of activity of the catalyst. The aggregated particles settled down to the bottom of the reactor and could not contact the reactant. In addition, when the amount of the used catalyst was large, there was the possibility of side reactions, resulting in the loss of epoxy and low hydroxyl number of polyol. Based on these results, the catalyst dosage of 7.5% was chosen as suitable for bio-polyol synthesis.
C double bonds. This complex is believed to be responsible for catalyzing the oxidation of unsaturated fatty acids to form epoxide product.20,33 The epoxide was then subjected to ring-opening reaction catalyzed by W/ZSM-5 catalyst in the presence of isopropanol to synthesize bio-polyol thanks to the acid sites of the ZSM-5 zeolite.24 Moreover, when the catalyst dosage increased from 7.5% to 10%, the hydroxyl number of the resulting bio-polyol increased slightly. Because when an excessive amount of catalyst was used, the aggregation of the catalyst particles in the solution occurred, causing the loss of activity of the catalyst. The aggregated particles settled down to the bottom of the reactor and could not contact the reactant. In addition, when the amount of the used catalyst was large, there was the possibility of side reactions, resulting in the loss of epoxy and low hydroxyl number of polyol. Based on these results, the catalyst dosage of 7.5% was chosen as suitable for bio-polyol synthesis.
Another study was conducted to investigate the influence of reaction time on the synthesis of polyol from sunflower oil. The results shown in Table 2 indicate that the reaction time significantly affected the hydroxyl number of the product.
| Reaction time (h) | Hydroxyl number of bio-polyol (mg KOH per g) |
|---|---|
| a Reaction condition: at 60 °C and catalyst dosage of 7.5%. | |
| 3 | 92.7 ± 0.4 |
| 4 | 129.2 ± 0.5 |
| 5 | 155.6 ± 0.5 |
| 6 | 156.4 ± 0.5 |
When the reaction time was increased from 3 h to 5 h, the hydroxyl number of the product increased significantly. However, on prolonging the reaction time (from 5 h to 6 h), the hydroxyl number did not increase. It can be explained that for a longer reaction time, the side reaction can also occur where the hydroxyl groups in the newly formed polyol product react with the epoxy ring under H+ catalyst to form dimer or trimer by-products.2,34 Thus, the suitable reaction time for polyol synthesis was found to be 5 h.
The sunflower-based bio-polyol was achieved with hydroxyl number OH# ≈ 160 mg KOH per g, function f ≈ 2.9 OH groups per mol at chosen suitable reaction conditions. The actual hydroxyl number of the bio-polyol produced in our reaction system is less than that required by theory and by a process using H2O2 with HCOOH as the catalyst;2 it is also lower than what would be obtained through the reaction using H2O2 and H2SO4 catalyst32 but greater than that obtained in the case catalyzed by acetic acid with H2O2.8 The lower hydroxyl number can be attributed to the heterogeneous reaction using W/ZSM-5 zeolite particles. However, the obtained result are reasonable and with these above characteristics; the as-synthesized bio-polyol product can be applied for the preparation of PU foam.
Fabrication of porous PU-CS bio-composite and adsorption test
In order to investigate the potential application of vegetable oil-based bio-polyol, a part of bio-polyol was used to replace fossil-based polyols in polyol part (component A) for the preparation of polyurethane. The ratio of NCO/OH = 1.1, the amount of catalyst was 0.2 parts by weight (pbw), surfactant was 0.35 pbw and water as blowing agent was 4 (pbw). Moreover, fillers were added to the PU matrix to improve the oil adsorption capacity of PU foam. In this work, pretreated corn stalk (CS), which was treated by hot water, was used as a filler to fabricate the porous PU-CS composite with enhanced oil uptake. This combination will take advantage of two types of natural and synthetic adsorbents to create a new material that has both economic and technical advantages.By replacing 20% fossil-polyol with the as-synthesized bio-polyol and adding 20% treated corn stalk fiber with 1 mm length, the porous PU-CS bio-composite showed improved oil uptake compared to neat PU (without adding corn stalk) and only corn stalk fiber, as seen in Table 3. PU-100 sample made from 100% fossil-polyol showed a high oil uptake compared to neat PU, made from 20% bio-polyol and 80% fossil-polyol, and showed much lower oil uptake than the PU-CS composite. This phenomenon can explain that when using bio-polyol to replace the petrochemical polyol component, the density of PU foam increased (the porosity of PU foam decreases), resulting in a lower oil adsorption capacity. The oil uptake capacity of PU-CS composite was over three times higher than that of neat PU foam from the mixture of fossil-polyol and bio-polyol without filler, about three time higher than that of PU-10 and about five times higher than that of treated corn stalk. It can be explained that when corn stalk biomass was added to the foam during the polyurethane forming process, the membranes (cell walls) of PU matrix become less stable, leading to the creation of an open-cell structure of the porous composite rather than the closed-cell structure of original polyurethane foam.2 The open cell structure allows the oil to pass easily between the cells in the porous adsorbent instead of being impeded by the cell walls as before. Thus, the oil uptake capacity of the new porous bio-composite would be enhanced.
| Sample | Oil adsorption capacity at different adsorption times (g g−1) | |||
|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | |
| a PU foam made from 80% fossil polyol and 20% bio-polyol (replacing 20% fossil-polyol by the as-synthesized bio-polyol).b PU foam made from 100% fossil polyol. | ||||
| Neat PUa | 2.43 ± 0.1 | 3.09 ± 0.1 | 3.47 ± 0.1 | 4.26 ± 0.1 |
| Treated corn stalk | 1.56 ± 0.1 | 1.98 ± 0.1 | 2.65 ± 0.1 | 3.15 ± 0.1 |
| PU-CS composite | 9.08 ± 0.2 | 11.53 ± 0.1 | 13.14 ± 0.1 | 15.07 ± 0.1 |
| PU-100b | 2.68 ± 0.1 | 3.40 ± 0.1 | 3.76 ± 0.1 | 4.55 ± 0.1 |
Conclusion
In summary, we synthesized W/ZSM-5 zeolite and applied it as a catalyst for the cascade reaction to synthesize bio-polyol by consequently combining the catalytic epoxidation of sunflower oil and epoxy ring opening. Metal-loaded ZSM-5 zeolite was prepared by incorporating tungsten metal into zeolite matrix using the impregnation method. This W/ZSM-5 zeolite showed high catalytic activities in a sequential, single-batch reaction to synthesize bio-polyol from sunflower oils using H2O2 in isopropanol solvent. The obtained results indicated that W/ZSM-5 zeolite was able to catalyze the epoxidation of double bond in vegetable oil and then epoxy ring opening reaction to form bio-polyol. The suitable conditions for bio-polyol synthesis were found to be: molar ratio of H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil of 5
oil of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 60 °C for 5 h with catalyst dosage of 7.5%. Under these conditions, the as-synthesized bio-polyol had the hydroxyl number OH# ≈ 160 mg KOH per g, function f ≈ 2.9 OH groups per mol. The bio-polyol was then used to substitute petrochemical polyol in the fabrication of porous bio-polyurethane-based composite with high oil adsorption capacity. Replacing bio-polyol and adding biomass filler in the polyurethane matrix can change the structure and enhance the oil absorption, increase the recovery and regeneration, and reduce the usage of fossil materials. The advantages offered by this new porous bio-composite make it a potential sorbent material for oil-spill treatment. This opens up a new route for processing large quantities of oil on a pilot scale that must be studied in subsequent experiments.
1; 60 °C for 5 h with catalyst dosage of 7.5%. Under these conditions, the as-synthesized bio-polyol had the hydroxyl number OH# ≈ 160 mg KOH per g, function f ≈ 2.9 OH groups per mol. The bio-polyol was then used to substitute petrochemical polyol in the fabrication of porous bio-polyurethane-based composite with high oil adsorption capacity. Replacing bio-polyol and adding biomass filler in the polyurethane matrix can change the structure and enhance the oil absorption, increase the recovery and regeneration, and reduce the usage of fossil materials. The advantages offered by this new porous bio-composite make it a potential sorbent material for oil-spill treatment. This opens up a new route for processing large quantities of oil on a pilot scale that must be studied in subsequent experiments.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.Conflicts of interest
There are no conflicts to declare.References
- U. Biermann, W. Friedt, S. Lang, W. Lühs, G. Machmüller, J. O. Metzger, M. R. Mark, H. J. Schäfer and M. P. Schneider, New Syntheses with Oils and Fats as Renewable Raw Materials for the Chemical Industry, Angew. Chem., Int. Ed., 2000, 39, 2206–2224, DOI:10.1002/9783527619849.ch25.
- P. H. Hoang, N. M. Dat and B. Q. Hung, One-Pot Synthesis of Bio-Polyol from Vegetable Oil for Preparation of Bio-Polyurethane Based Porous Sorbent with High Oil Adsorption Capacity, J. Appl. Polym. Sci., 2023, 1–9, DOI:10.1002/app.54332.
- X. Tong, Y. Ma and Y. Li, Applied Catalysis A : General Biomass into Chemicals : Conversion of Sugars to Furan Derivatives by Catalytic Processes, Appl. Catal., A, 2010, 385, 1–13, DOI:10.1016/j.apcata.2010.06.049.
- I. K. M. Yu and D. C. W. Tsang, Bioresource Technology Conversion of Biomass to Hydroxymethylfurfural : A Review of Catalytic Systems and Underlying Mechanisms, Bioresour. Technol., 2017, 238, 716–732, DOI:10.1016/j.biortech.2017.04.026.
- T. D. Cuong, L. Q. Dien and P. H. Hoang, Preparation of Bio-Based Porous Material with High Oil Adsorption Capacity from Bio-Polyurethane and Sugarcane Bagasse, RSC Adv., 2024, 14, 6938–6947, 10.1039/d4ra00469h.
- C. Akshatha, V. Siji, J. A. Jyothy G Vijayan and T. N. P. Gopi, Highly Efficient Removal of Rhodamine B Dye Using Nanocomposites Made from Cotton Seed Oil-Based Polyurethane and Silylated Nanocellulose, J. Polym. Environ., 2022, 30, 4999–5011 CrossRef.
- A. Fridrihsone, F. Romagnoli, V. Kirsanovs and U. Cabulis, Life Cycle Assessment of Vegetable Oil Based Polyols for Polyurethane Production, J. Cleaner Prod., 2020, 266, 121403, DOI:10.1016/j.jclepro.2020.121403.
- J. G. Vijayan, A. Chandrashekar, J. Ag and T. N. Prabhu, Polyurethane and Its Composites Derived from Bio-Sources: Synthesis , Characterization and Adsorption Studies, Polym. Polym. Compos., 2022, 30, 1–18, DOI:10.1177/09673911221110347.
- P. Alagi, Y. J. Choi and S. C. Hong, Preparation of Vegetable Oil-Based Polyols with Controlled Hydroxyl Functionalities for Thermoplastic Polyurethane, Eur. Polym. J., 2016, 78, 46–60, DOI:10.1016/j.eurpolymj.2016.03.003.
- A. Prociak, M. Kurańska, U. Cabulis, J. Ryszkowska, M. Leszczyńska, K. Uram and M. Kirpluks, Effect of Bio-Polyols with Different Chemical Structures on Foaming of Polyurethane Systems and Foam Properties, Ind. Crops Prod., 2018, 120, 262–270, DOI:10.1016/j.indcrop.2018.04.046.
- C. Zhang, S. A. Madbouly and M. R. Kessler, Biobased Polyurethanes Prepared from Different Vegetable Oils, ACS Appl. Mater. Interfaces, 2015, 7, 1226–1233, DOI:10.1021/am5071333.
- S. E. Turnwald, M. A. Lorier, N. Zealand, L. J. Wright, N. Zealand, M. R. Mucalo and N. Zealand, Oleic Acid Oxidation Using Hydrogen Peroxide in Conjunction with Transition Metal Catalysis, J. Mater. Sci. Lett., 1998, 17, 1305–1307 CrossRef CAS.
- S. P. Maradur, C. Jo, D. Choi, K. Kim and R. Ryoo, Mesoporous Polymeric Support Retaining High Catalytic Activity of Polyoxotungstate for Liquid-Phase Olefin Epoxidation Using H2O2, ChemCatChem, 2011, 3, 1435–1438, DOI:10.1002/cctc.201100045.
- P. U. Maheswari, P. De Hoog, R. Hage, P. Gamez and J. Reedijk, A Na2WO4/H2WO4-Based Highly Efficient Biphasic Catalyst towards Alkene Epoxidation, Using Dihydrogen Peroxide as Oxidant, Adv. Synth. Catal., 2005, 347, 1759–1764, DOI:10.1002/adsc.200505133.
- C. Wang and H. Yamamoto, Tungsten-Catalyzed Asymmetric Epoxidation of Allylic and Homoallylic Alcohols with Hydrogen Peroxide, J. Am. Chem. Soc., 2014, 136, 1222–1225, DOI:10.1021/ja411379e.
- R. De Vasconcelos Vieira Lopes, N. P. D. Loureiro, A. P. T. Pezzin, A. C. M. Gomes, I. S. Resck and M. J. A. Sales, Synthesis of Polyols and Polyurethanes from Vegetable Oils-Kinetic and Characterization, J. Polym. Res., 2013, 20, 238, DOI:10.1007/s10965-013-0238-x.
- L. J. Sun, C. Yao, H. F. Zheng and J. Lin, A Novel Direct Synthesis of Polyol from Soybean Oil, Chin. Chem. Lett., 2012, 23, 919–922, DOI:10.1016/j.cclet.2012.05.030.
- C. S. Cundy and P. A. Cox, The Hydrothermal Synthesis of Zeolites: History and Development from the Earliest Days to the Present Time, Chem. Rev., 2003, 103, 663–701, DOI:10.1021/cr020060i.
- P. H. Hoang and N. M. Dat, Study on Using Cellulose Derivatives as Pore Directing Agent for Preparation of Hierarchical ZSM-5 Zeolite Catalyst, Adv. Powder Technol., 2021, 32, 3927–3933, DOI:10.1016/j.apt.2021.09.003.
- P. H. Hoang, N. T. Nhung and L. Q. Dien, Synthesis of Mesoporous Cr/ZSM-5 and W-Cr/ZSM-5 Zeolite Catalysts for Oxidation of Unsaturated Fatty Acid, AIP Adv., 2017, 7, 105311–105318, DOI:10.1063/1.4986310.
- C. Saux and L. B. Pierella, Studies on Styrene Selective Oxidation to Benzaldehyde Catalyzed by Cr-ZSM-5: Reaction Parameters Effects and Kinetics, Appl. Catal., A, 2011, 400, 117–121, DOI:10.1016/j.apcata.2011.04.021.
- P. H. Hoang, B. Van Don and N. H. Chung, Cleavage of Double Bond Using Metal-Loaded ZSM-5 Zeolite Catalysts for Renewable Biochemical Application, Can. J. Chem. Eng., 2019, 97, 1086–1091, DOI:10.1002/cjce.23307.
- J. S. Reddy, U. R. Khire, P. Ratnasamy and R. B. Mitra, Cleavage of the Carbon-Carbon Double Bond over Zeolites Using Hydrogen Peroxide, J. Chem. Soc. Chem. Commun., 1992, 202, 1234–1235, 10.1039/C39920001234.
- H. Ogawa, Y. Miyamoto, T. Fujigaki and T. Chihara, Ring-Opening of 1,2-Epoxyalkane with Alcohols over H-ZSM-5 in Liquid Phase, Catal. Lett., 1996, 40, 253–255, DOI:10.1007/BF00815291.
- P. H. Hoang, H. T. Dat, T. D. Cuong and L. Q. Dien, Pretreatment of Coir Lignocellulose for Preparation of a Porous Coir-Polyurethane Composite with High Oil Adsorption Capacity, RSC Adv., 2022, 12, 14976–14985, 10.1039/d2ra01349e.
- L. S. Martins, N. C. Zanini, L. S. Maia, A. G. Souza, R. F. S. Barbosa, D. S. Rosa and D. R. Mulinari, Crude Oil and S500 Diesel Removal from Seawater by Polyurethane Composites Reinforced with Palm Fiber Residues, Chemosphere, 2021, 267, 129288, DOI:10.1016/j.chemosphere.2020.129288.
- O. S. H. Santos, M. Coelho da Silva, V. R. Silva, W. N. Mussel and M. I. Yoshida, Polyurethane Foam Impregnated with Lignin as a Filler for the Removal of Crude Oil from Contaminated Water, J. Hazard. Mater., 2017, 324, 406–413, DOI:10.1016/j.jhazmat.2016.11.004.
- P. H. Hoang and L. Q. Dien, Synthesis of Magnetically Recyclable ZSM-5 Zeolite for Styrene Epoxide Rearrangement Reaction, Chem. Eng. J., 2015, 262, 140–145, DOI:10.1016/j.cej.2014.09.092.
- N. A. S. Amin and D. D. Anggoro, Characterization and Activity of Cr, Cu and Ga Modified ZSM-5 for Direct Conversion of Methane to Liquid Hydrocarbons, J. Nat. Gas Chem., 2003, 12, 123–134 CAS.
- S. Bai, K. Zhang, L. Wang, J. Sun, R. Luo, D. Li and A. Chen, Synthesis Mechanism and Gas-Sensing Application of Nanosheet-Assembled Tungsten Oxide Microspheres, J. Mater. Chem. A, 2014, 2, 7927–7934, 10.1039/c4ta00053f.
- S. N. gora. Eroi, A. S. Ello, D. Diabaté and D. B. Ossonon, Heterogeneous WO3/H2O2 System for Degradation of Indigo Carmin Dye from Aqueous Solution, S. Afr. J. Chem. Eng., 2021, 37, 53–60, DOI:10.1016/j.sajce.2021.03.009.
- M. Borowicz, J. Paciorek-Sadowska and M. Isbrandt, Synthesis and Application of New Bio-Polyols Based on Mustard Oil for the Production of Selected Polyurethane Materials, Ind. Crops Prod., 2020, 155, 112831, DOI:10.1016/j.indcrop.2020.112831.
- H. Noureddini and M. Kanabur, Liquid-Phase Catalytic Oxidation of Unsaturated Fatty Acids, JAOCS (J. Am. Oil Chem. Soc.), 1999, 76, 305–312, DOI:10.1007/s11746-999-0236-7.
- A. S. A. Hazmi, M. M. Aung, L. C. Abdullah, M. Z. Salleh and M. H. Mahmood, Producing Jatropha Oil-Based Polyol via Epoxidation and Ring Opening, Ind. Crops Prod., 2013, 50, 563–567, DOI:10.1016/j.indcrop.2013.08.003.
| This journal is © The Royal Society of Chemistry 2024 |