Open Access Article
Open Access ArticleAn innovative Schiff-base colorimetric chemosensor for the selective detection of Cu2+ ions and its applications†
Ram Kumarab,
Bholey Singh *c,
Parveen Gahlyana,
Abhishek Vermad,
Mamta Bhandarie,
Rita Kakkar
*c,
Parveen Gahlyana,
Abhishek Vermad,
Mamta Bhandarie,
Rita Kakkar e and
Balaram Pani
e and
Balaram Pani *b
*b
aDepartment of Chemistry, University of Delhi, Delhi 110007, India
bDepartment of Chemistry, Bhaskaracharya College of Applied Sciences, University of Delhi, New Delhi 110075, India. E-mail: balarampani63@gmail.com
cDepartment of Chemistry, Swami Shraddhanand College, University of Delhi, Delhi 110036, India. E-mail: bholeysingh@ss.du.ac.in
dDepartment of Chemistry, Kirori Mal College, University of Delhi, Delhi 110007, India
eComputational Chemistry Group, Department of Chemistry, University of Delhi, Delhi 110007, India
First published on 22nd July 2024
Abstract
A novel Schiff base moiety, (E)-4-(1-hydrazonoethyl)benzene-1,3-diol (2), and 2,4-dihydroxybenzaldehyde were condensed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio to generate 4-((E)-1-(((Z)-2,4dihydroxybenzylidene)hydrazono)ethyl)benzene-1,3-diol (L), which was then characterized using high-resolution mass spectrometry (HRMS), 1H-NMR, 13C NMR, and single-crystal XRD techniques. UV-vis absorbance measurements were used to determine whether the Schiff base could detect the cupric ions more effectively than the other transition metal ions. When Cu2+ ions were involved, a new band was observed at 462 nm. From the Job plot, the binding stoichiometry for the anticipated L
1 molar ratio to generate 4-((E)-1-(((Z)-2,4dihydroxybenzylidene)hydrazono)ethyl)benzene-1,3-diol (L), which was then characterized using high-resolution mass spectrometry (HRMS), 1H-NMR, 13C NMR, and single-crystal XRD techniques. UV-vis absorbance measurements were used to determine whether the Schiff base could detect the cupric ions more effectively than the other transition metal ions. When Cu2+ ions were involved, a new band was observed at 462 nm. From the Job plot, the binding stoichiometry for the anticipated L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu2+ partnership is determined to be 1
Cu2+ partnership is determined to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. For the purpose of validating structural correlations and absorption data, DFT simulations were performed. Further, docking studies for L indicated high binding affinity for human hemoglobin, providing vital information about the ligand's favorable binding locations inside hemoglobin binding sites and the consequent interactions with HHb. The binding coefficient and limit of detection were found to be 3.02 × 104 M−1 and 42.09 nM, respectively. Reversibility of the complex was seen upon the addition of EDTA to the L–Cu2+ solution, and a colorimetric variation simulating the “INHIBIT” molecular logic gate was seen upon the addition of Cu2+ and EDTA to L. Furthermore, the chemosensor's potential application in the detection of Cu2+ in the solid state by chemosensor L also confirms its usefulness in real-world applications emphasizing its versatility and practical utility.
1. For the purpose of validating structural correlations and absorption data, DFT simulations were performed. Further, docking studies for L indicated high binding affinity for human hemoglobin, providing vital information about the ligand's favorable binding locations inside hemoglobin binding sites and the consequent interactions with HHb. The binding coefficient and limit of detection were found to be 3.02 × 104 M−1 and 42.09 nM, respectively. Reversibility of the complex was seen upon the addition of EDTA to the L–Cu2+ solution, and a colorimetric variation simulating the “INHIBIT” molecular logic gate was seen upon the addition of Cu2+ and EDTA to L. Furthermore, the chemosensor's potential application in the detection of Cu2+ in the solid state by chemosensor L also confirms its usefulness in real-world applications emphasizing its versatility and practical utility.
1. Introduction
Cu2+ is a vital trace element for human health and plays an important role in the production of several enzymes, cell metabolism, and other biochemical processes. However, excessive Cu2+ has harmful effects on human health and the environment, particularly the amplifying effect of the biological chain, which can be enriched multiple times, eventually entering the human body1,2 and causing damage to the central nervous system including Alzheimer's, Parkinson's, and prion diseases.3 Therefore, the detection of Cu2+ is important for environmental monitoring and healthcare issues. Cu2+ levels in drinking water should be less than 31.5 M, according to the World Health Organization (WHO).4–6 A number of conventional techniques have been developed over the years to identify metal ions, including inductively coupled plasma spectroscopy, atomic absorption spectroscopy, and inductively coupled plasma mass spectrometry.7–10 In addition to these approaches, impedimetric sensing and electrochemical monitoring have also been mentioned in recent publications on the detection of Cu2+.11–14 These techniques provide precise results for the detection of metal ions; however, they have significant practical limitations owing to the high cost of equipment, difficulty of the procedures, and length of inspections.15–17 There are several important reasons for the importance of detecting copper. It may also be noted that copper is considered an “economically critical mineral” due to its importance for a variety of important technologies.18–20 Having low-cost, sensitive, selective, and portable sensors for characterizing copper could therefore be useful for both prospecting and downstream process monitoring during copper production, especially from unconventional sources such as industrial waste.21,22However, there is an urgent need to develop quick, trustworthy, inexpensive, and straightforward measurement techniques, including electrochemical and optical sensing methods, for practical applications, particularly rapid on-site detection of metal ions. In the present investigation, we have created a Schiff base moiety based on 2,4-dihydroxy benzaldehyde that acts as a colorimetric chemosensor. We employed different types of metal ions as analytes to demonstrate how this technique can be used to selectively determine Cu2+. The synthesized novel chemosensor L displayed remarkable sensitivity and selectivity for Cu2+ ions over other metals forming a stable combination with Cu2+ ions, resulting in a visible color shift making it a promising tool for environmental monitoring and analytical applications. Additionally, the findings from the computational and practical investigations contribute to a greater scientific understanding of protein–ligand interactions, opening the way for advances in domains such as pharmacology, biochemistry, and molecular biology.
2. Experimental
2.1 General information, materials and methods
For synthesis and spectroscopic investigations, exclusively reagent-grade chemicals and solvents were used. The following ingredients were purchased from Spectrochem Pvt. Ltd (India): 1-(2,4-dihydroxyphenyl)ethan-1-one, hydrazine hydrate, ethanol, and 2,4-dihydroxy benzaldehyde. Further purification was not required to perform the reaction. To conduct spectroscopic studies, stock solutions of metal ions were prepared in double-distilled water. Chloride salts of Cu2+, Co2+, Cr3+, Cs+, Fe2+, Fe3+, In3+, K+, Li+, Mg2+, Na+, Ni2+, Zn2+, Hg2+, Mn2+, and Cd2+, nitrate salts of Ag+, Pb2+ and sulphate salt of Al3+ were obtained from Spectrochem Pvt. Ltd and were utilized as received. Column chromatography (silica gel, 100–200 mesh) with methanol–chloroform as the solvent system was used to purify the chemicals. Thin-layer chromatography (Merck silica gel 60F 254) was used to monitor the completion of the reaction. Fourier transform infrared (FT-IR) spectra were obtained using a SHIMADZU IR Affinity 1S spectrophotometer. A JNM-EXCP 400 (JEOL, USA) spectrophotometer was used to record nuclear magnetic resonance (NMR) spectra at room temperature while operating at 400 MHz and 100 MHz. A single-sided transparent glass capillary tube was used to record the melting point of the compound using a Buchi M-560 melting point device. An Agilent Technologies G6530AA (LC-HRMS-Q-ROF) spectrometer was used to record high-resolution mass spectrometry (HRMS) spectra. A Rigaku Oxford diffraction instrument was used for crystal analysis. A Cary Series UV-visible spectrophotometer was used for UV-visible investigations using a 1 cm quartz cuvette. The pH was measured using a digital pH meter.2.2 Synthesis
2.3 Single crystal X-ray diffraction analysis
Fine crystals of ligand L were grown by slow evaporation of the saturated mother solvent methanol, which was layered with hexane at room temperature for SC-XRD studies. Good quality needle-shaped transparent crystals were obtained and exposed to X-rays on a Bruker diffractometer employing graphite monochromatized Mo Kα radiation (λ = 0.71073 Å) at a temperature of 107 K. The crystal data were reduced using the CrysAlis Pro software available with the diffractometer. Further, least-squares refinement after the introduction of anisotropic displacement parameters yielded the R values mentioned in Table S1 (ESI).† The structure was solved by direct methods using SHELXT 2018/2 (ref. 23) and refined using the full-matrix least-squares method on Olex2.refine 1.5, and SHELXL-2016/4. All calculations were performed using the OLEX2 package of crystallographic programs.24 For molecular graphics, the Mercury program (2022.3.0) was used.25 The selected cell parameters are listed in Table S1 (ESI).†2.4 UV-visible studies
UV-visible studies have been carried out to investigate the binding behavior of L towards various metal ions. An initial stock solution of L (1 mM) was prepared in acetonitrile, and stock solutions of ethylenediaminetetraacetic acid (EDTA) and metal ions (Ag+, Cu2+, Al3+, Co2+, Cr3+, Cs+, Fe2+, Fe3+, In3+, K+, Li+, Mg2+, Na+, Ni2+, Pb2+, Zn2+, Hg2+, Mn2+, and Cd2+) (10 mM) were prepared in double-distilled water. Chemosensor L solution (10 μM) was prepared in CH3CN for binding studies. In a cuvette, 2.97 mL of L (10 μM) was combined with 30 μL of the metal ion stock solution. The UV-visible spectra of probe L were recorded in the presence and absence of various metal ions.2.5 Selectivity
Competitive studies were carried out by adding a variety of metal ions to a solution of Cu2+ to evaluate the potential interference by other metal ions in the detection of Cu2+. By mixing equal parts of L and metal salts in a quartz cuvette, solutions were created to monitor UV-visible changes.2.6 Binding and stoichiometric calculations
Benesi–Hildebrand (B–H) and Job's plots, respectively, were used to calculate the binding constant (Ka) for the formation of the complexes and the stoichiometry of the complex of Cu2+ with chemosensor L. Eqn (1) was used to determine the binding constant from the slope of the B–H plot.
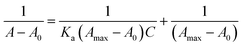 | (1) |
Eqn (2) was used to determine the limit of detection (LOD) of Cu2+.
 | (2) |
2.7 Computational methods
Molecular docking studies were employed to analyze the binding interactions between ligand L and hemoglobin (Hb) to elucidate potential therapeutic implications or physiological effects. Hemoglobin is a tetrameric protein found in red blood cells (RBCs) that transports oxygen from the lungs to tissues.26 Understanding how they bind to different ligands is critical for drug development. In this study, we used molecular docking to investigate the probable binding of ligand L to Hb. The three-dimensional crystal structure of human hemoglobin (PDB ID: 2d60) was retrieved from the protein databank. Any water molecules, co-crystallized ligands, or ions were eliminated and hydrogen atoms that were absent were added. Molecular docking assessments were performed for L with human hemoglobin using AutoDock Vina software.27 Grid parameters were chosen to include probable binding locations on the hemoglobin structure. PYMOL and BIOVIA Discovery Studio 2021 were employed to visualize the protein–ligand complex, and the conformer with the lowest binding energy was chosen for diagrammatic representation.Quantum chemical calculations are valuable tools for predicting equilibrium geometries of metal–ligand complexes. Density functional theory (DFT) calculations were performed using the B3LYP functional.17–20 All atoms were described using the 6-31G+(d,p) basis set, whereas heavy atoms were treated using the LanL2DZ basis set.21–24 The ligand and its complex were geometrically optimized in vacuo with respect to energy using the Gaussian 09 W suite,25 followed by harmonic frequency calculations to confirm the nature of the stationary points.
3. Result and discussion
3.1 Synthesis
The colorimetric sensor was prepared by stirring a solution of (E)-4-(1-hydrazonoethyl)benzene-1,3-diol and 2,4-dihydroxybenzaldehyde in ethanol (Scheme 1). To generate final product as sensor L, the reaction mixture was refluxed for 6 h. The stated procedure was used to generate the precursors 2 of the final product.28 The spectroscopic methods that such as 1H NMR, 13C NMR, IR, and HRMS were used to validate the existence of the final product and its predecessors.3.2 UV-vis spectral analysis
In order to acquire a more comprehensive understanding of the detecting capabilities of chemosensor L, we performed investigations such as UV-visible titration. At a concentration of 10, the UV-visible absorption spectrum of L was examined in acetonitrile solution and the pH of the solution was 7.5. The chemosensor didn't show any visible bands when 10 equivalent of metal ions (Ag+, Cu2+, Al3+, Co2+, Cr3+, Cs+, Fe2+, Fe3+, In3+, K+, Li+, Mg2+, Na+, Ni2+, Pb2+, Zn2+, Hg2+, Mn2+, and Cd2+) were introduced to the solution of L. The only exception to this was the Cu2+ case and new band emerged at 462 nm, which is shown in Fig. 1b.3.3 Selectivity
Equal concentrations of Cu2+ and other metal ions were used to check the selectivity, and competitive experiments were carried out to investigate the interference of other metal ions in the detection of Cu2+, as shown in Fig. 2. The color changed from colorless to yellow when Cu2+ was added to a solution of L, which already contained other metal ions, and the experiment revealed that the UV-visible spectrum of L contained Cu2+. | ||
| Fig. 2 3D representation of the selectivity of L (10 μM, CH3CN) for Cu2+ in the presence of different metal ions at a wavelength of 462 nm. | ||
3.4 pH Analysis
The colorimetric response of L was investigated as a function of pH in the presence and absence of Cu2+. 1 M sodium hydroxide (NaOH) and 1 M hydrochloric acid (HCl) solutions were added to the mixture to regulate pH. As shown in Fig. 3, protonation at pH 3 prevented the detection of Cu2+. The sensing capacity of L for Cu2+ increased with pH and then became nearly constant at pH 8. Therefore, it can be concluded that the chemosensor is appropriate for detecting Cu2+ under physiological pH conditions.3.5 Time dependent UV-vis spectra of L and L + Cu2+
To examine the UV-visible absorbance response of the chemosensors L and L + Cu2+ at 462 nm with regard to time, a time-dependent investigation of L was also carried out. When 1 equivalent of Cu2+ ions were added to the solution, the absorbance of L (10 μM) at 462 nm increased within a few seconds and remained constant throughout the time, as can be observed in Fig. 4. Therefore, probe L can be used for the rapid and precise detection of Cu2+ ions.3.6 Binding stoichiometry ratio analysis
To better understand the binding affinity of chemosensor L for Cu2+, a UV-visible titration experiment was conducted in CH3CN at pH 7.5. As Cu2+ was gradually added to the solution of L, as shown in Fig. 5a, the absorbance intensity steadily increased in the UV-Vis spectra. Furthermore, the binding constant and stoichiometry of the complex were determined using Job's and Benesi–Hildebrand (B–H) plots, respectively. At a 0.5 mole ratio of L to Cu2+, the maximum was observed in the Job's plot (Fig. 5b). Thus, L and Cu2+ have a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry and this binding stoichiometry of the complex was further confirmed by the linearity of the B–H curve (Fig. 5c), with an R2 value of 0.99428. Using the Benesi–Hildebrand plot, it was calculated that the binding constant (Ka) of L for Cu2+ was 3.02 × 104 M−1. The limit of detection (LOD) of chemosensor L for Cu2+ is 42.09 nM (Table S4 ESI†). This suggests that chemosensor L can be utilized to detect Cu2+ quantitatively, even at nano-level concentrations. In addition to this, we conducted the infrared spectroscopy experiments also to validate the L–Cu2+ complex formation as shown in Fig. S7.† It is clearly visible in the IR spectrum that –C
1 binding stoichiometry and this binding stoichiometry of the complex was further confirmed by the linearity of the B–H curve (Fig. 5c), with an R2 value of 0.99428. Using the Benesi–Hildebrand plot, it was calculated that the binding constant (Ka) of L for Cu2+ was 3.02 × 104 M−1. The limit of detection (LOD) of chemosensor L for Cu2+ is 42.09 nM (Table S4 ESI†). This suggests that chemosensor L can be utilized to detect Cu2+ quantitatively, even at nano-level concentrations. In addition to this, we conducted the infrared spectroscopy experiments also to validate the L–Cu2+ complex formation as shown in Fig. S7.† It is clearly visible in the IR spectrum that –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching frequency at 1662 cm−1 in free ligand is shifted to 1645 cm−1 in the L–Cu2+ complex and the –OH band at 3321 cm−1 in the uncomplexed L has been slightly shifted to 3310 cm−1 in the complex with increased of broadness. The IR frequencies changes validate the complex formation through –C
N stretching frequency at 1662 cm−1 in free ligand is shifted to 1645 cm−1 in the L–Cu2+ complex and the –OH band at 3321 cm−1 in the uncomplexed L has been slightly shifted to 3310 cm−1 in the complex with increased of broadness. The IR frequencies changes validate the complex formation through –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and phenolic –OH groups.
N and phenolic –OH groups.
 | ||
| Fig. 5 (a) Absorbance spectra of L (10 μM, CH3CN, pH 7.5) upon the addition of Cu2+ metal ions. (b) Job plot of L for Cu2+. (c) B–H plot of L for Cu2+. | ||
Sensor L shows outstanding sensing ability for Cu2+ ions as compared to many reported studies, as shown in Table 2.
3.7 Single crystal X-ray diffraction analysis
The crystal structure of ligand L was determined by the single-crystal X-ray diffraction method, which revealed that the molecular structure of the ligand consisted of two dihydroxy aryl rings that were skewed and coordinated with the next carbon atoms at 121.56°and 121.79°, respectively, and supported by the vertically appended methyl group with an imine functional group at an angle of 116.92°. This confirms the planar structure of the compound, which is clearly shown in Fig. 6, where the bond distances of adjacent atoms from the dihydroxy aryl ring to carbon atoms lie in the range 1.443(2)–1.463(2) Å, whereas the inter- and intra-molecular hydrogen and covalent bond lengths lie close to (1.888–2.682 Å). The fundamental building blocks of crystal engineering are weaker coordinating connections, which are crucial. Interestingly, the crystal structures of ligand L in the current study showed a variety of non-covalent interactions, including a network of intermolecular H-bonding and π–π stacking configurations (Fig. 7–9). These non-covalent interactions are responsible for different biological applications, such as interactions with hemoglobin protein and metal sensing, where molecules interact in the form of loose aggregates to form metal complexes, as confirmed by molecular docking studies and DFT calculations. | ||
| Fig. 6 Molecular structures (a and b) of ligand L. H atoms are omitted from b for clarity. C, gray; N = blue, O, red; H, light gray. | ||
3.8 Computational results
3.8.1.1 Ligand (L). The optimized geometry of the ligand is shown in Fig. 10. It is planar and has Cs symmetry with a 1A′ ground state.
The highest occupied and lowest unoccupied molecular orbitals (HOMO and LUMO, respectively) are shown in Fig. 11. These are π and π* orbitals, respectively, both of which have a′′ symmetry.
3.8.1.2 Cu2+ complex. The optimized structure of the complex is shown in Fig. 12. Similar to the ligand, the complex is planar (Cs symmetry), with the Cu2+ ion bound to three atoms: two oxygens of the deprotonated hydroxyl groups and an imine nitrogen atom, forming six- and seven-membered rings. The ground state is a doublet.
The HOMO and LUMO of the α and β orbitals are shown in Fig. 13. Although the HOMO is MO#84 for the α-spin state and MO#83 for the β-spin state, the two HOMOs are similar for the two spin states and have similar energies.
The HOMOs are also similar to those of the ligand in terms of energy and appearance, but the LUMO energies are lowered upon complexation with Cu2+, indicating that the electron affinity increases. The LUMO of the β orbitals has a different appearance and involves the d orbitals of the metal ion. It is also symmetric (a′) as opposed to the other frontier orbitals.
The Mulliken spin densities are distributed in the central portion of the complex, mainly on the Cu2+ ion (0.500), two oxygen atoms (0.147 and 0.132), and imine nitrogen (0.158), to which the metal ion is attached (Fig. 6 ESI†).
The decrease in the HOMO–LUMO gap upon complexation explains the appearance of a band in the visible region upon complexation with Cu2+. TD-DFT calculations were performed for the ligand and complex. The results for the first six excited states of ligand L are presented in (Table S2 ESI†).
The observation of these two peaks is in agreement with the experimental observation of the two peaks at 304 and 370 nm (Fig. 4 ESI†). Both transitions are π–π* transitions.
The computed spectrum after complexation is shown in (Fig. 5 in ESI†), and selected transitions for which the oscillator strength is nonzero are given in (Table S3 ESI†).
The highest value of oscillator strength was calculated for transition#10 at 444 nm, in agreement with the experimental results. This transition is the HOMO → LUMO transition of the α-electrons.29–33 We propose a plausible mechanism for the binding of Cu2+ to L (Scheme 2).
 | ||
| Fig. 14 (a and b): 2-D representation of binding interaction of L with human hemoglobin (PDB ID: 2d60). | ||
 | ||
| Fig. 15 3-D representation of binding interaction of L with human hemoglobin (PDB ID: 2d60). | ||
4. Practical applications
4.1 Detection of Cu2+ ions in solid media using L
The solid-state detection of Cu2+ was performed to assess the practical usefulness of L. A 100 μM solution of L in acetonitrile was mixed with 300 mg of silica gel (100–200 mesh size) for this experiment, the solution of L was evaporated under vacuum to produce colorless silica gel. The resulting colorless silica gel was then administered a dose of Cu2+. Subsequently, the water was evaporated, leaving behind yellow silica gel. This demonstrates the colorimetric detection of L towards Cu2+ in a solid medium, which can be clearly seen in Fig. 16. The chemosensor L binds to the silica gel through physical adsorption. Silica gel possesses a remarkable surface area and porous structure that enables the adsorption of chemosensor L molecules onto its surface. This interaction is mainly influenced by the attractive forces between molecules and the bonding between different functional groups. The binding stability of chemosensor L on silica gel is more than enough for practical colorimetric detection under laboratory conditions. However, to ensure long-term stability and adaptability to different environmental conditions. | ||
| Fig. 16 Silica gel showing the colorimetric response of L (100 μM in acetonitrile) towards Cu2+ ions in a solid medium. | ||
4.2 Application of strip filters
This experiment employing paper strips is a crucial application of chemosensor L because it can detect ions without the use of any technical methods. As a result, it speeds up the detection and simplifies the recognition process. A strip of Whatman filter paper was dipped into an acetonitrile solution of L and air-dried to conduct this test. The coated paper strips were submerged at various concentration of Cu2+ ions in aqueous solution. The hue shifted from light yellow to dark yellow as the quantity of Cu2+ increased, as observed by the naked eye (Fig. 17). This technique is very helpful for on-site detection of ambient condition. | ||
| Fig. 17 Strip dipped in acetonitrile solution of L with increasing conc. of Cu2+ (0 to 100 ppm from left to right). | ||
4.3 Detection of Cu2+ in real water sample
An investigation into the practicability of using the L as a probe to detect Cu2+ in real water samples was carried out in order to obtain the results of the feasibility study. In order to conduct this investigation, samples of water from the Ganga river, the Yamuna river, and the tap water were utilised. Before starting with the analysis of the target analyte (Cu2+) at the most appropriate pH conditions, the water samples were first filtered in order to remove any particles that were suspended in the water. There is a preferential binding of the probe L with the Cu2+ ions that are present in the river water samples. This result illustrates that it is possible for us to determine the presence of copper in the sample in a short amount of time (Fig. 18).Further, the effectiveness of the probe L was confirmed by testing it on actual samples (tap water) using the spike and recovery method. The samples were intentionally contaminated with different quantities of Cu2+ ions and then analysed using the newly developed technique. The experiment was repeated up to three times, and the obtained results in the actual samples demonstrated good recognition and recovery of Cu2+ ions. This is confirmed by the Table 1, which authenticate its practical and visual relevance for the recognition of Cu2+ ions in genuine samples.
| Sample | Cu2+ ions spiked (μM) | Cu2+ ions found (n = 3)* | Recovery of Cu2+ ions added (%) |
|---|---|---|---|
| a *n = Number of trials. | |||
| Tap water | 5 | 4.93 | 98.6 |
| 10 | 10.10 | 101 | |
| 15 | 14.97 | 99.8 | |
| 20 | 19.89 | 99.45 | |
4.4 Reversibility and molecular logic gate behavior of chemosensor L
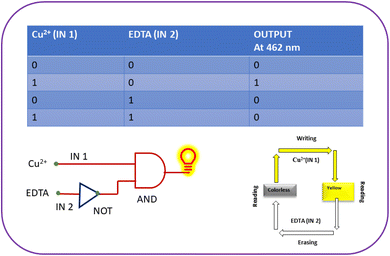
Another quality that makes chemosensors attractive for practical applications is their recyclability. EDTA was added to the L–Cu2+ solution to investigate the L recyclability. Owing to the reversible interaction between the L–Cu2+ complex and EDTA, the absorbance intensity changed when EDTA (0–1 equiv.) was added to the L–Cu2+ solution. EDTA is an example of a metal ion chelator. It can form a complex with Cu2+ and easily eliminate it from the complex when introduced into the L–Cu2+ solution. With the addition of EDTA, the hue changed from yellow to colorless, which could also be observed with the naked eye. The addition of 1 equiv. of EDTA completely bleached the hue of the L–Cu2+ solution. Following the addition of 0–1 equivalents of EDTA, the shift in the UV-vis absorbance spectra also revealed a decrease in the intensity of the complex band, as shown in Fig. 19.An interesting application of chemosensors has been observed in the fields of molecular keypad locks, information storage devices, and molecular logic gates. The absorbance reversible switching process of chemosensor L can be used for the construction of a molecular logic gate. An ‘INHIBIT’ molecular logic gate is constructed by using two logic operations of NOT and functions. This is highly important because of its non-commutative behavior, that is, its output signal is inhibited by only one type of input. Therefore, we have used ‘INHIBIT’ logic gate to explain the practical utility of chemosenor L on addition of metal ion Cu2+ and EDTA.
Chemical information is encoded into optical signals by an “INHIBIT” logic gate. The absorbance response at 462 nm was used as the output, and two chemical inputs, Cu2+ and EDTA, were designated IN 1 and IN 2, respectively.
As shown in Fig. 20, the truth table was created based on the absorbance data using binary digits as input. In this approach, Cu2+ and EDTA are represented as ‘1’ and ‘0’, respectively, depending on whether they are chemical inputs. Strong absorbance intensity during the signaling process was designated as ‘1’ for output and weak absorbance intensity as ‘0’ for output. The other three input combinations, namely IN 1 = 0′ and IN 2 = 0′, IN 1 = 0′ and IN 2 = 1, IN 1 = 1, and IN 2 = 1, exhibited weak absorbance and 0 was the output. The input combination IN 1 = 1 and IN 2 = 0 indicates a strong absorbance intensity and ‘1’ in the output.
The construction of the “INHIBIT” logic gate for the reversible chemosensor L in the presence of Cu2+ and the absence of EDTA was thus demonstrated by the above information, which was seen by the UV-vis absorbance response.
To build memory devices, IN 1 (Cu2+) and IN 2 (EDTA) were employed as two inputs. The system writes and stores binary state 1 when IN 1 = 1 and IN 2 = 0. The system memorizes binary state 1 and writes when it receives IN 1 (Cu2+), which exhibits strong absorbance intensity. However, when IN 2 (EDTA) is present, it erases the data and remembers the binary state 0 or low absorbance. To illustrate the “write-read-erase-read” functions of a sequential logic circuit, we applied a binary-logic function, as illustrated in Fig. 20 and 21.
 | ||
| Fig. 21 The reversible and reproducible the absorbance switch controlled by alternating addition of Cu2+ and EDTA into the probe (L) solution. | ||
5. Conclusions
The Schiff base L was found to be a successful probe for cupric ion identification in contrast to the other metal ions. The absorption experiments revealed that the synthesized chemosensor L had a greater affinity for cupric ions exhibiting a binding constant of 3.022 × 104 M−1 and limit of detection to be 42.09 nM. To validate the experimental results, computational studies were performed. In addition, L replicates the INHIBIT molecular logic gate and demonstrates reversible switching for Cu2+ upon the addition of EDTA to the solution of L–Cu2+, confirming that this chemosensor is reusable. The chemosensor demonstrated its practical utility by successfully detecting Cu2+ ions in both solid state and filter paper strips. DFT computations revealed that the interaction of L with Cu2+ resulted in a robust complex, where the Cu2+ ions interacted with three atoms of the ligand L. The coordination of the Cu2+ ion with the nitrogen atom of the imine group and two oxygen atoms of the hydroxyl group elucidated the complex's characteristics. Further, the docking studies for ligand L revealed a remarkable binding affinity with human hemoglobin, revealing information on its favorable binding positions within hemoglobin binding sites and the subsequent interactions with HHb. These findings highlight ligand L as a prospective candidate for further investigations in the design of novel therapeutic strategies and development for hemoglobin-related diseases.Data availability
The data supporting this article have been included in the ESI.† This CCDC for L: 2296396 contains ESI† crystallographic data for ligand in this study.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
Ram Kumar is grateful to Bhaskaracharya College of Applied Sciences, University of Delhi for providing research facilities, University of Delhi for financial support and the University Science Instrumentation Centre (USIC) for characterization facilities and also very grateful to single crystal operator Sanjay sir. All the authors are thankful to the Department of Chemistry, University of Delhi.References
- J. J. DiNicolantonio, D. Mangan and J. H. O'Keefe, Open heart, 2018, 5, e000784 CrossRef
.
- H. Tapiero, D. M. Townsend and K. D. Tew, Biomed. Pharmacother., 2003, 57, 386–398 CrossRef CAS
.
- E. Gaggelli, H. Kozlowski, D. Valensin and G. Valensin, Chem. Rev., 2006, 106, 1995–2044 CrossRef CAS PubMed
.
- A. M. Elkhatat, M. Soliman, R. Ismail, S. Ahmed, N. Abounahia, S. Mubashir, S. Fouladi and M. Khraisheh, Bull. Natl. Res. Cent., 2021, 45, 1–18 CrossRef
.
- S. Bayindir, A. S. Hussein, F. Lafzi and M. Toprak, J. Mol. Liq., 2023, 382, 121939 CrossRef CAS
.
- F.-C. Ho, Y.-J. Huang, C.-C. Weng, C.-H. Wu, Y.-K. Li, J. I. Wu and H.-C. Lin, ACS Appl. Mater. Interfaces, 2020, 12, 53257–53273 CrossRef CAS
.
- F. Wang, R. Nandhakumar, J. H. Moon, K. M. Kim, J. Y. Lee and J. Yoon, Inorg. Chem., 2011, 50, 2240–2245 CrossRef CAS PubMed
.
- E. Manandhar, J. H. Broome, J. Myrick, W. Lagrone, P. J. Cragg and K. J. Wallace, Chem. Commun., 2011, 47, 8796–8798 RSC
.
- R. Kumar, B. Singh, P. Gahlyan, R. Kumar and B. Pani, J. Mol. Struct., 2023, 135859 CrossRef CAS
.
- X. Wang, G. Liu, Y. Qi, Y. Yuan, J. Gao, X. Luo and T. Yang, Anal. Chem., 2019, 91, 12006–12013 CrossRef CAS PubMed
.
- M. T. Feroze, D. Doonyapisut, B. Kim and C.-H. Chung, Korean J. Chem. Eng., 2023, 40, 1014–1022 CrossRef CAS
.
- R. Cai, C. A. Shoukat, C. Zhang, X. Gao, H. Li, J. Chen, Y. Ji and X. Wu, Analyst, 2023, 148, 3306–3311 RSC
.
- M. H. Chua, H. Zhou, Q. Zhu, B. Z. Tang and J. W. Xu, Mater. Chem. Front., 2021, 5, 659–708 RSC
.
- R. Kumar, B. Singh, S. S. Pandey and B. Pani, SynOpen, 2023, 7, 703–717 CrossRef CAS
.
- P. V Mane, P. Patil, A. A. Mahishi, M. Kigga, M. P. Bhat, K.-H. Lee and M. Kurkuri, Heliyon, 2023, 9(6) DOI:10.1016/j.heliyon.2023.e16600
.
- P. Sengupta, A. Ganguly and A. Bose, Spectrochim. Acta, Part A, 2018, 198, 204–211 CrossRef CAS PubMed
.
- P. Patil, M. Prasad, M. P. Bhat, M. G. Gatti, S. Kabiri, T. Altalhi, H.-Y. Jung, D. Losic and M. Kurkuri, Chem. Eng. J., 2017, 327, 725–733 CrossRef CAS
.
- A. Beylot and J. Villeneuve, Resour. Policy, 2015, 44, 161–165 CrossRef
.
- C.-L. Huang, M. Xu, S. Cui, Z. Li, H. Fang and P. Wang, Resour., Conserv. Recycl., 2020, 161, 104861 CrossRef
.
- M. Mróz, Energies, 2022, 15, 560 CrossRef
.
- D. Peukert, C. Xu and P. Dowd, Minerals, 2022, 12, 1364 CrossRef CAS
.
- S. E. Crawford, P. R. Ohodnicki and J. P. Baltrus, J. Mater. Chem. C, 2020, 8, 7975–8006 RSC
.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed
.
- O. V Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef
.
- C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler and J. V. D. Streek, J. Appl. Crystallogr., 2006, 39, 453–457 CrossRef CAS
.
- S. Yu, in Blood Substitutes and Oxygen Biotherapeutics, Springer, 2022, pp. 45–51 Search PubMed
.
- O. Trott and A. J. Olson, J. Comput. Chem., 2010, 31, 455–461 CrossRef CAS
.
- A. I. Said, N. I. Georgiev and V. B. Bojinov, J. Photochem. Photobiol., A, 2015, 311, 16–24 CrossRef CAS
.
- R. Bauernschmitt and R. Ahlrichs, Chem. Phys. Lett., 1996, 256, 454–464 CrossRef CAS
.
- M. E. Casida, C. Jamorski, K. C. Casida and D. R. Salahub, J. Chem. Phys., 1998, 108, 4439–4449 CrossRef CAS
.
- R. E. Stratmann, G. E. Scuseria and M. J. Frisch, J. Chem. Phys., 1998, 109, 8218–8224 CrossRef CAS
.
- G. Scalmani, M. J. Frisch, B. Mennucci, J. Tomasi, R. Cammi and V. Barone, J. Chem. Phys., 2006, 124 DOI:10.1063/1.2173258
.
- F. Furche and R. Ahlrichs, J. Chem. Phys., 2002, 117, 7433–7447 CrossRef CAS
.
- K. M. Trevino, B. K. Tautges, R. Kapre, F. C. Franco Jr, V. W. Or, E. I. Balmond, J. T. Shaw, J. Garcia and A. Y. Louie, ACS Omega, 2021, 6, 10776–10789 CrossRef CAS PubMed
.
- Z. Aydin and M. Keles, ChemistrySelect, 2020, 5, 7375–7381 CrossRef CAS
.
- Y. Liu, L. Wang, C. Guo and Y. Hou, Tetrahedron Lett., 2018, 59, 3930–3933 CrossRef CAS
.
- P. Sengupta, A. Ganguly and A. Bose, Spectrochim. Acta, Part A, 2018, 198, 204–211 CrossRef CAS
.
- K. Rajaswathi, M. Jayanthi, R. Rajmohan, V. Anbazhagan and P. Vairaprakash, Spectrochim. Acta, Part A, 2019, 212, 308–314 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2296396. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ra03097d |
| This journal is © The Royal Society of Chemistry 2024 |