Open Access Article
Open Access ArticleAn ethyl cellulose novel biodegradable flexible substrate material for sustainable screen-printing
Elena Palmieri†
a,
Rocco Cancelliere† *b,
Francesco Maita*a,
Laura Micheli
*b,
Francesco Maita*a,
Laura Micheli b and
Luca Maioloa
b and
Luca Maioloa
aIstituto per la Microelettronica e i Microsistemi, Consiglio Nazionale delle Ricerche, Via del Fosso del Cavaliere 100, Rome 00133, Italy. E-mail: francesco.maita@cnr.it
bDepartment of Chemical Science and Technologies, University of Rome Tor Vergata, Via della Ricerca Scientifica 1, Rome 00133, Italy. E-mail: rocco.cancelliere@uniroma2.it
First published on 6th June 2024
Abstract
We introduce an innovative solution to reduce plastic dependence in flexible electronics: a biodegradable, water-resistant, and flexible cellulose-based substrate for crafting electrochemical printed platforms. This sustainable material based on ethyl cellulose (EC) serves as an eco-friendly alternative to PET in screen printing, boasting superior water resistance compared to other biodegradable options. Our study evaluates the performance of carbon-based screen-printed electrodes (SPEs) fabricated on conventional PET, recycled PET (r-PET), and (EC)-based materials. Electrochemical characterization reveals that EC-SPEs exhibit comparable analytical performance to both P-SPEs and rP-SPEs, as evidenced by similar limits of detection (LOD), limits of quantification (LOQ), and reproducibility values for all the analytes tested (ferro-ferricyanide, hexaammineruthenium chloride, uric acid, and hydroquinone). This finding underscores the potential of our cellulose-based substrate to match the performance of conventional PET-based electrodes. Moreover, the scalability and low-energy requirements of our fabrication process highlight the potential of this material to revolutionize eco-conscious manufacturing. By offering a sustainable alternative without compromising performance, our cellulose-based substrate paves the way for greener practices in flexible electronics production.
Introduction
Screen printing has emerged as a prominent method for producing flexible electronics, owing to its versatility across various substrates, cost-effectiveness, and scalability for large-scale manufacturing. Notably, it facilitates rapid prototyping and customisation, although it faces challenges such as lower resolution compared to photolithography, and variations in layer thickness affecting device performance.1–5 Despite these limitations, screen printing remains significant in flexible electronics, balancing accessibility, and feasibility, especially in applications like wearable technology and sensors.Several polymeric substrates are used in flexible electronics due to their compatibility with screen printing. Examples6–12 include polyethene terephthalate (PET), polyethene naphthalate (PEN), and polyimide (PI). The quest for alternative materials in screen printing aims to replace these conventional substrates since they come from non-renewable sources. Biodegradable polymers like polylactic acid (PLA)13,14 and cellulose-based materials offer eco-friendly alternatives15–17 to used-to-date polymeric substrates. PLA, sourced from renewable materials such as maise starch, demonstrates excellent printability and biodegradability. Meanwhile, paper-based screen-printed electrodes have seen extensive application in recent decades. Indeed, paper-based electrodes have demonstrated performance comparable to that of PET electrodes in sensing diverse analytes.18 Nevertheless, challenges persist in their utilisation. For instance, paper requires a wax coating to facilitate the printing process19 and to delimit the electrode and the reaction zones, potentially impacting electrode recyclability. Occasionally, the employment of non-environmentally friendly chemicals or procedures becomes necessary to enhance the processability of the substrate.20 Moreover, its sensitivity to water poses limitations on certain applications21,22 and raises concerns regarding electrode storage in uncontrolled environments. Despite these hurdles, the versatility and sustainability of paper-based electrodes offer promising avenues for future development in flexible23 electronics, although PET is still the most commonly used substrate. In this regard, to address the growing environmental concerns associated with disposable plastic-based devices, a recent proposal has been to utilise recycled PET for manufacturing screen-printed electrodes (SPEs). This initiative aligns with the objectives outlined in the European Union's “European Strategy for Plastics in a Circular Economy,” which aims to ensure that by 2030, all plastic packaging introduced to the EU market will be either easily reusable or recyclable at minimal cost.24
In this quest for greener solutions, we introduce ethyl cellulose (EC),25,26 a bio-friendly option, as a game-changer for creating transparent, flexible and water-resistant substrates for electrochemical printed platforms. This material is suitable for eco-conscious manufacturing methods like roll-to-roll processes and printing. Plus, it can be exploited for the fabrication of devices using advanced techniques like photolithography,25,26 paving the way for its industrial use. Moreover, the one-step room temperature process makes EC a good candidate as a flexible electronic substrate material since it reduces further the energy needed to fabricate bendable devices, and the substrates can withstand up to 2500 bending cycles.26 The reduction of the environmental impact associated with traditional substrates would contribute to a more sustainable approach to the use of disposable devices. Therefore, we exploited this novel material for the fabrication of screen-printed electrodes, and we compared the performances of SPEs fabricated on EC (EC-SPE), commercial PET (P-SPE), and recycled PET (rP-SPE).
Experimental
Materials and methods
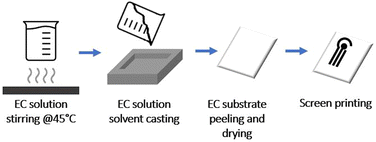
Ethyl cellulose (EC) with 48–49.5% ethoxy content was purchased from Sigma. All the solvents used were purchased from Sigma. Sodium nitrite, hydroquinone, L-ascorbic acid, uric acid, and potassium chloride were supplied by Sigma-Aldrich (Steinheim, Germany). Ferrocyanide and potassium ferricyanide were supplied from Fluka Chemie, Sigma-Aldrich (Buchs, Switzerland). The buffer solutions were 0.05 M phosphate-buffered saline (PBS) + 0.1 M KCl with a pH of 7.4 and 0.05 M carbonate buffer (CB) with a pH of 9.0.EC dispersion was prepared by dissolving it in ethanol and stirring at 45 °C until dissolution. The EC substrate is prepared at room temperature via solvent casting method from a 10% w ethanol-based dispersion. The full procedure and characterisation of the film are reported in a previous work24 and summarised in Fig. 1.
The in-house production of SPEs was conducted using a 245 DEK high-performance, multi-purpose, precision screen-printing machine. The working electrode (WE) with a geometric area of approximately 0.07 cm2 and the counter electrodes (CEs) were printed using graphite-based ink (Elettrodag 421 cured at 80 °C), while the reference electrode (RE) was printed using silver ink (cured at 80 °C).
UV-vis spectroscopy was conducted utilizing a PerkinElmer Lambda 35 UV/vis spectrometer, capturing spectra ranging from 1100 to 200 nm with a resolution of 4 nm.
FTIR-ATR characterization was carried out using a ThermoScientific Nicolet Summit spectrometer in absorbance mode. Samples were analyzed in attenuated total reflectance mode (ATR), utilizing a diamond crystal as a reflection accessory. The spectra were recorded from 3700 to 500 cm−1 with a resolution of 2 cm−1.
Optical images and profilometry characterization were conducted in collaboration with SIMITECNO SRL using the Interferometer TopMap Micro.View+ Polytec.
Results and discussion
Characterisation of the material
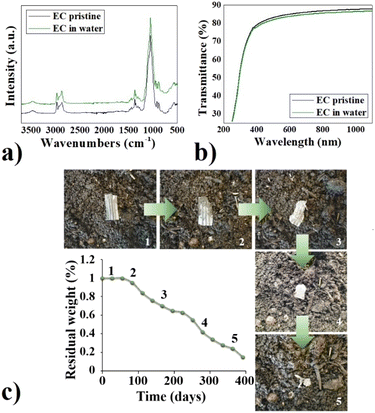
The chemical stability of the novel EC-based material and its mechanical performances were extensively examined in a prior study,26 and we gained insights into its limitations and advantages compared to other biomaterials and conventional petroleum-based materials. The clear benefit of EC over typical PET substrates lies in its biodegradability. What sets EC apart from other biodegradable cellulose-based options like paper is its exceptional water resistance, allowing for prolonged analyte measurements in aqueous environments without compromising electrode functionality, even when stored or subjected to uncontrolled environmental conditions. Remarkably, the material retains its integrity when immersed in water or exposed to sunlight, even after a few years, despite being biodegradable. To validate that the EC films we obtained exhibit properties consistent with those reported in the literature,27,28 EC samples were submerged in distilled water and placed on a windowsill, subjecting them to natural environmental conditions, in order to simulate and highlight uncontrolled storage conditions. Periodically, the EC samples were taken out of the water, dried with compressed air, and then monitored for weight changes. Additionally, they underwent FTIR-ATR and UV-vis characterization to assess any potential degradation. Regarding weight monitoring, no fluctuations in weight were observed, while FTIR-ATR and UV-vis spectra of the substrate before and after being immersed in water for one year are reported respectively in Fig. 2a and b. The FTIR-ATR spectra reported in Fig. 2a revealed the features reassumed in Table 1.29,30| Wavenumber (cm−1) | Attribution |
|---|---|
| 1050 | C–O–C vibration of pyranose ring |
| 2900 and 137 | –CH stretching and deformation vibrations |
| 1200–1500 | –CH bending |
| 500–800 | –OH out-of-plane bending |
| 1650 | –OH bending of absorbed water molecules |
| 3400 | –OH groups stretching |
Notably, there were no significant differences observed between the spectra before and after treatment with water. No signs of cellulose degradation or oxidation were detected. Furthermore, there was no alteration observed in the band at 1650 cm−1, indicative of –OH bending from absorbed water molecules, in the water-treated sample, and no variation occurred to the band at around 3500 cm−1 of the hydroxyl groups. UV-vis transmission analysis showed that no significant variation in the transmittance of the EC substrate was observed after water treatment (Fig. 2b). Otherwise, if the cellulose is susceptible to water, swelling phenomena and changes in transparency and colour could happen.31 Despite its stability in water, it is biodegradable. Its biodegradability was monitored via periodical weight measurements of EC samples placed in common soil and compost. It required more than a year to degrade in soil (Fig. 2c), while shorter time if properly composted (3 to 10 months, depending on the compositing conditions).
From this perspective, this cellulose-based material exhibits comparable behaviour to PLA, one of the most prevalent bioplastics utilized in various industries such as biomedical, textile, and packaging.32,33
The morphology of the substrates used for the fabrication of the SPEs, i.e. PET, r-PET and EC, underwent characterisation through electronic and optical microscopy. Optical images and profilometry characterization are reported in Fig. 3. While the morphologies of the three substrates exhibit similarities, some distinctions are noticeable. The electrode border is notably sharper for the PET substrate. Conversely, both EC and r-PET display borders with minor defects and less distinct margins, probably due to the different substrate wettability.
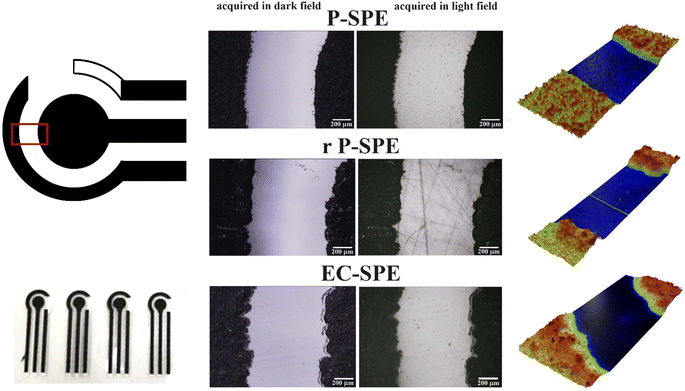 | ||
| Fig. 3 Optical images, picture and 3D mapping of SPE printed on commercial PET (P-SPE), recycled PET (rP-SPE) and EC (EC-SPE). | ||
Additionally, r-PET exhibits evident signs of surface irregularities due to its recycled packaging nature, whereas the morphologies of the other two substrates remain uniform. Furthermore, the roughness of the different materials was examined through profilometry analysis. The Sq (root mean square height) values, obtained from mapping a 0.2 cm2 area of each substrate, are 0.26 μm for r-PET, 0.13 μm for PET, and 0.1 μm for EC, while the Sa (arithmetical mean height) values are 0.17 μm for r-PET, 0.09 μm for PET, and 0.07 μm for EC. This demonstrates that our cellulose-based substrate possesses a roughness level comparable to commercial PET.
Electrochemical characterisation of the electrodes
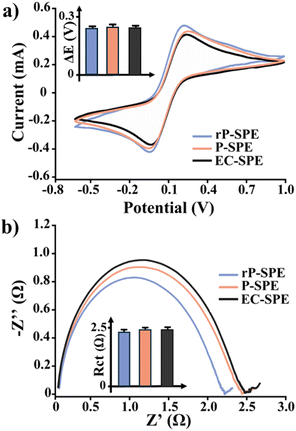
An extensive electrochemical characterisation was performed to verify the performance of the developed electrodes. To that end, the background current of P-SPEs, rP-SPE and EC-SPE was measured through chronoamperometry (0.4 V the oxidation peak of the Fe2+, 180 s) in 50 mM KCl solution. The recorded currents were 30 ± 4 nA for P-SPEs, 35 ± 5 nA for rP-SPEs, and 36 ± 4 nA for EC-SPEs (n = 6 electrodes for each electrode type), respectively.After that, the signal-to-noise ratios (S/N) corresponding to the current measured in the presence (10 mM Fe(CN)63−/4−) and the absence (50 mM KCl, the one previously measured) of a redox probe were evaluated. S/N of 228 ± 19, 225 ± 20, and 230 ± 20 was obtained for P-SPEs, rP-SPEs, and EC-SPEs, respectively. With these first outcomes acquired, it is feasible to highlight significant comparability for all the platforms investigated. The electron transport and diffusivity processes at the electrode interface of EC-SPEs were studied using Electrochemical Impedance spectroscopy (EIS) and Cyclic Voltammetry (CV) with a 10 mM solution of Fe(CN)63−/4− as an electroactive probe. Fig. 4a and b display the relative CVs and Nyquist plot.
Initial visual inspection revealed that both the voltammetry and impedimetric analysis produced comparable results in terms of peak current (Ip), peak-to-peak separation (ΔE) and charge transfer resistance (Rct). The numerical values of each electroanalytical parameter are provided in Table 2.
| Fe(CN)63−/4− | P-SPE | rP-SPE | EC-SPE |
|---|---|---|---|
| Ipa/Ipc | 0.9 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 |
| ΔE (V) | 0.24 ± 0.02 | 0.27 ± 0.02 | 0.26 ± 0.02 |
| D0 × 10−6 (cm2 s−1) | 1.9 ± 0.3 | 1.5 ± 0.3 | 1.6 ± 0.3 |
| Rct (Ω) | 2.2 ± 0.2 | 2.4 ± 0.2 | 2.4 ± 0.2 |
| W (Kσ) | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 |
Initially, the diffusional process at the electrode/electrolyte interface was investigated. As previously detailed in our prior research, the diffusion coefficient (D0) may be estimated by utilising the Randless Sevcik equation and the current peak (Ip) measured at a constant concentration of a redox probe (10 mM Fe(CN)63−/4−).34 D0 is the mean value of the diffusion coefficient for the oxidation process (Dox) of Fe2+ and the redox process of Fe3+ (Dred), determined from the cathodic current peak (Ipc). The D0 values shown in Table 2 indicate that P-SPE, rP-SPE, and EC-SPE exhibited comparable diffusional behaviours. Moreover, comparing these results with the D0 value reported in the literature by Konopka and McDuffy reveals a similar planar diffusion-controlled mechanism (about 10−6 magnitude) in the oxidation/reduction processes of Fe(CN)63−/4−.24 This behaviour is supported by the impedimetric results indicated by the W values obtained, which are the same for P-SPEs, rP-SPEs, and EC-SPEs. Afterwards, the electron transfer process was studied by comparing the current peak ratio (Ipa/Ipc), ΔE, and Rct. For a reversible couple such as Fe(CN)63−/4, the Ipa/Ipc is equal to one. The Ipa/Ipc ratios in Table 2 exhibit a strictly comparable behaviour for all the platforms evaluated. This is confirmed by ΔE values, which are always higher than 59 mV, thus suggesting a sluggish electron exchange.35 This criterion is crucial for recognising Nernstian behaviour and calculating the number of electrons transferred. All platforms in this instance can be classified as a “quasi-reversible” system.36 This phenomenon is characteristic of graphite-based screen-printed platforms. Many studies in the past decade have suggested modifying these platforms with nanomaterials. Our group achieved important results in this regard, by proposing multiwall nanotubes (MWNTs), graphene and biochar-modified SPEs for (bio)sensing purposes.8,37
After that, an in-depth investigation of the analytical performances of EC-SPE was conducted. Parameters such as limit of detection (LOD), limit of quantification (LOQ), sensitivity and reproducibility (RSD%) were evaluated by analysing electrochemical reversible and non-reversible redox probes. Ferro-ferricyanide, hexaammineruthenium chloride Ru(NH3)6Cl, uric acid (UA), and hydroquinone (HQ) were utilised as probes, with square wave voltammetry (SWV) employed as the analytical method. The comparative outcomes are presented in Table 3.
| P-SPE | rP-SPE | EC-SPE | |
|---|---|---|---|
| Fe(CN)63−/4− | |||
| LOD (μM) | 9.4 | 10.7 | 10.3 |
| LOQ (μM) | 28.4 | 35.2 | 34.3 |
| Sensitivity (mA/M cm2) | 40 | 42.4 | 41.3 |
| Reproducibility (RSD%) | 9 | 10 | 10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Ru(NH3)6Cl | |||
| LOD (μM) | 15.5 | 13.6 | 16.3 |
| LOQ (μM) | 51.4 | 47.4 | 53.8 |
| Sensitivity (mA/M cm2) | 61.7 | 53.3 | 66.4 |
| Reproducibility (RSD%) | 10 | 10 | 11 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| UA | |||
| LOD (μM) | 7.1 | 6.8 | 7.0 |
| LOQ (μM) | 22.6 | 21.2 | 21.7 |
| Sensitivity (mA/M cm2) | 44.3 | 37.4 | 42.1 |
| Reproducibility (RSD%) | 9 | 10 | 9 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| HQ | |||
| LOD (μM) | 8.8 | 9.5 | 10.1 |
| LOQ (μM) | 27.4 | 29.6 | 33.4 |
| Sensitivity (mA/M cm2) | 40.6 | 41.8 | 46.3 |
| Reproducibility (RSD%) | 10 | 10 | 10 |
These results demonstrate that EC-SPEs have identical analytical performance to P-SPEs and rP-SPEs, as evidenced by the comparable LOD, LOQ, and reproducibility values observed. Reproducibility was assessed by analysing six distinct electrodes from each substrate, with similar relative standard deviations (RSD%): 10% for P-SPEs, 11% for rP-SPEs, and 10% for EC-SPEs.
Conclusions
Based on the data presented, it can be inferred that the newly developed biodegradable material utilizing ethyl cellulose (EC) is well-suited as a substrate for fabricating screen-printed electrodes (SPEs). The observed sensing performance is on par with that of SPEs printed on PET and recycled PET substrates. Additionally, the production process for EC-based substrates occurs at low temperatures and allows for standardization and scalability to produce, for instance, A4-sized sheets. These sheets could be a viable alternative to the commercially used sheets for fabricating PET electrodes. This flexible, transparent material maintains stability over a year but can biodegrade when composted correctly. This finding offers hope for substantially reducing the environmental impact of disposable devices, extending beyond screen-printed electrodes to encompass all devices produced through screen printing on flexible PET-like substrates. We reached results comparable to those on PET in terms of LOD, LOQ, sensitivity and reproducibility for all the analytes tested (ferro-ferricyanide, hexaammineruthenium chloride, uric acid, and hydroquinone). By guaranteeing consistent performance, it sets the stage for the development of greener and more effective electronic devices. This innovation addresses the pressing need for environmentally friendly materials and demonstrates the feasibility of transitioning towards more sustainable manufacturing processes.Author contributions
Conceptualization – R. Cancelliere, E. Palmieri; data curation – R. Cancelliere, E. Palmieri; investigation – R. Cancelliere, E. Palmieri; methodology- E. Palmieri, R. Cancelliere; supervision – R. Cancelliere, L. Maiolo, F. Maita, L. Micheli; validation – L. Maiolo, F. Maita, L. Micheli; writing – original draft – E. Palmieri, R. Cancelliere; writing – review & editing – E. Palmieri, R. Cancelliere, L. Maiolo, F. Maita, L. Micheli.Conflicts of interest
There are no conflicts to declare.Notes and references
- L. Maiolo, A. Pecora, F. Maita, A. Minotti, E. Zampetti, S. Pantalei, A. Macagnano, A. Bearzotti, D. Ricci and G. Fortunato, Sens. Actuators, B, 2012, 179, 114 CrossRef.
- D.-H. Kim, Nature, 2008, 320, 507 CAS.
- T. Someya, Z. Bao and G. G. Malliaras, Nature, 2016, 540, 379 CrossRef CAS PubMed.
- P. Sodkrathok, C. Karuwan, W. Kamsong, A. Tuantranont and M. Amatatongchai, Talanta, 2023, 262, 124695 CrossRef CAS PubMed.
- S. Nur Ashakirin, M. H. M. Zaid, M. A. S. M. Haniff, A. Masood and M. F. Mohd Razip Wee, Measurement, 2023, 210, 112502 CrossRef.
- M. Li, D. W. Li, G. Xiu and Y. T. Long, Curr. Opin. Electrochem., 2017, 3, 137 CrossRef CAS.
- A. Muhammad, R. Hajian, N. A. Yusof, N. Shams, J. Abdullah, P. M. Woi and H. Garmestani, RSC Adv., 2018, 8, 2714 RSC.
- R. Cancelliere, A. Di Tinno, A. Cataldo, S. Bellucci and L. Micheli, Biosensors, 2022, 12, 2 CrossRef CAS PubMed.
- Q. Li, J. Zhang, Q. Li, G. Li, X. Tian, Z. Luo, F. Qiao, X. Wu and J. Zhang, Front. Mater., 2019, 5, 1 CrossRef.
- X. Gong, K. Huang, Y. H. Wu and X. S. Zhang, Sens. Actuators, A, 2022, 345, 113821 CrossRef CAS.
- T. Pandhi, C. Cornwell, K. Fujimoto, P. Barnes, J. Cox, H. Xiong, P. H. Davis, H. Subbaraman, J. E. Koehne and D. Estrada, RSC Adv., 2020, 10, 38205 RSC.
- S. Park, S. Ban, N. Zavanelli, A. E. Bunn, S. Kwon, H. R. Lim, W. H. Yeo and J. H. Kim, ACS Appl. Mater. Interfaces, 2023, 15(1), 2092–2103 CrossRef CAS PubMed.
- P. S. Sfragano, S. Laschi and I. Palchetti, Front. Chem., 2020, 8, 1 CrossRef PubMed.
- R. M. Cardoso, D. P. Rocha, R. G. Rocha, J. S. Stefano, R. A. B. Silva, E. M. Richter and R. A. A. Muñoz, Anal. Chim. Acta, 2020, 1132, 10 CrossRef CAS PubMed.
- E. Jansson, J. Lyytikäinen, P. Tanninen, K. Eiroma, V. Leminen, K. Immonen and L. Hakola, Materials, 2022, 15, 957 CrossRef CAS PubMed.
- C. M. Silveira, T. Monteiro and M. G. Almeida, Biosensors, 2016, 6, 1 CrossRef.
- S. Agate, M. Joyce, L. Lucia and L. Pal, Carbohydr. Polym., 2018, 198, 249 CrossRef CAS PubMed.
- N. Ruecha, R. Rangkupan, N. Rodthongkum and O. Chailapakul, Biosens. Bioelectron., 2014, 52, 13 CrossRef CAS PubMed.
- S. Cinti, M. Basso, D. Moscone and F. Arduini, Anal. Chim. Acta, 2017, 960, 123 CrossRef CAS PubMed.
- S. Kang, Z. Li, J. Li, H. Wei, Y. Guo, H. Li, P. Yan and H. Wu, Polymers, 2023, 15, 1 Search PubMed.
- A. Hauke, P. Simmers, Y. R. Ojha, B. D. Cameron, R. Ballweg, T. Zhang, N. Twine, M. Brothers, E. Gomez and J. Heikenfeld, Lab Chip, 2018, 18, 3750 RSC.
- E. C. Rama and M. T. F. Abedul, Biosensors, 2021, 11, 1 Search PubMed.
- H. Eynaki, M. A. Kiani and H. Golmohammadi, Nanoscale, 2020, 12, 18409 RSC.
- R. Cancelliere, G. Rea, L. Severini, L. Cerri, G. Leo, E. Paialunga, P. Mantegazza, C. Mazzuca and L. Micheli, Green Chem., 2023, 25, 6774 RSC.
- E. Palmieri, F. Maita, A. Pellegrino, G. Avola, M. Distefano and L. Maiolo, IEEE Int. Work. Metrol. Agric. For. MetroAgriFor 2023 – Proc. 2023, 2023, p. 763 Search PubMed.
- E. Palmieri, L. Maiolo, I. Lucarini, A. D. Fattorini, E. Tamburri, S. Orlanducci, R. Calarco and F. Maita, Adv. Mater. Technol., 2024, 9, 1 Search PubMed.
- T. Vasuphat, K. Tharnthip, D. Donraporn, J. Kittisak, P. Winita, S. Montira, S. Runglawan, M. Kiattikhun, R. Pornchai, T. Pratchaya, S. Yottha, A. Sittipong, D. Alan B. and W. Patnarin, Int. J. Biol. Macromol., 2023, 244, 125390 CrossRef PubMed.
- B. Das, P. Chokkalingam, P. Masilamani and S. Shukla, Int. J. Mol. Sci., 2023, 24, 2757 CrossRef CAS PubMed.
- J. Łojewska, P. Miśkowiec, T. Łojewski and L. M. Proniewicz, Polym. Degrad. Stab., 2005, 88, 512 CrossRef.
- X. Su, Z. Yang, K. B. Tan, J. Chen, J. Huang and Q. Li, Carbohydr. Polym., 2020, 241, 116259 CrossRef CAS.
- A. Etale, A. J. Onyianta, S. R. Turner and S. J. Eichhorn, Chem. Rev., 2023, 123(5), 2016–2048 CrossRef CAS PubMed.
- S. Wang, Q. Shen, C. Guo and H. Guo, Membranes, 2021, 11, 915 CrossRef CAS PubMed.
- S. Sousa, A. Costa, A. Silva and R. Simões, Polymers, 2019, 11, 1 CrossRef.
- R. Cancelliere, A. Di Tinno, A. Cataldo, S. Bellucci, S. Kumbhat and L. Micheli, Microchem. J., 2023, 191, 108868 CrossRef CAS.
- R. Cancelliere, T. Cosio, E. Campione, M. Corvino, M. P. D'Amico, L. Micheli, E. Signori and G. Contini, Front. Chem., 2023, 11, 1 Search PubMed.
- D. Diamond, Analytical Electrochemistry, 1996 Search PubMed.
- R. Cancelliere, A. Di Tinno, A. M. Di Lellis, G. Contini, L. Micheli and E. Signori, Biosens. Bioelectron., 2022, 213, 114467 CrossRef CAS PubMed.
Footnote |
| † These authors equally contributed to the paper. |
| This journal is © The Royal Society of Chemistry 2024 |