Open Access Article
Open Access ArticlePhotoredox-catalyzed sulfonylation of diaryliodonium salts with DABSO and silyl enolates involving the insertion of SO2†
Shuoshuo Zhang‡
,
Qian Zhang‡,
Shuizhen Lin,
Xinkui Lin and
Xiaolei Huang *
*
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang 321004, China. E-mail: huangxl@zjnu.edu.cn
First published on 7th May 2024
Abstract
A versatile photoredox-catalyzed three-component sulfonylation of diaryliodonium salts with DABSO and silyl enolates involving the insertion of SO2 was developed. Moreover, by employing β-alkyl substituted silyl enolates as substrates, the sulfonylation would give α-alkyl substituted β-keto sulfones, which are difficult to accessed by previous method involving the insertion of SO2.
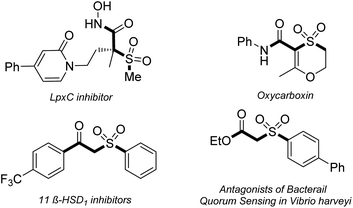
β-Keto sulfones are privileged frameworks in many pharmaceuticals and display remarkable biological activities and pharmacological properties such as anti-bacterial, anti-fungal, anti-hepatitis, and non-nucleoside inhibition (Fig. 1).1 Additionally, β-keto sulfones are utilized as versatile intermediate synthons in diverse synthetic transformations due to the simultaneous existence of multiple functional groups including carbonyl, sulfonyl and active methylene moieties.2 Therefore, there is high demand for developing efficient synthesis methods to construct β-keto sulfones.
Conventional approaches for the synthesis of β-keto sulfones include oxidation of 2-oxo-sulfides with strong oxidants3 and the sulfonylation of α-halo-ketones with preinstalled sulfonyl-containing segments,4 which have several drawbacks, such as the employment of poorly accessible and smelly organosulfur compounds as starting materials, limited substrate applicability, and harsh conditions. In recent years, sulfonylation involving the insertion of sulfur dioxide via a radical process has emerged as a powerful method to access sulfone derivatives,5 in which various SO2 surrogates, like DABSO, metabisulfites, rongalite reagents and SOgen, are employed instead of SO2 gas.6 Outstanding contributions to synthesize diverse β-keto sulfones from silyl enolates via sulfur dioxide insertion have been made by Wu's groups using aryldiazonium tetrafluoroborates,7 aryl/alkyl halides,8 and thianthrenium salts9 as active radical precursors in some cases. These transformations proceed with good tolerance of functional groups, easily enabling the incorporation of various sulfonyl skeletons into ketones.
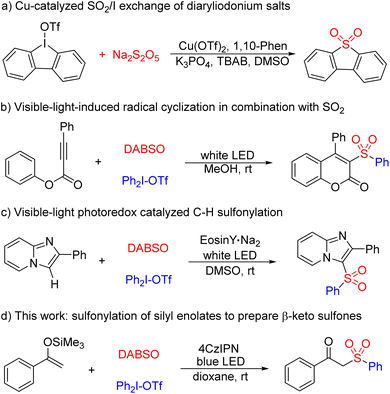
On the other hand, diaryliodonium salts can participate in various transformations as a type of versatile, easily available, non-toxic, environmentally, and air stable solid arylating reagent.10 So far, diaryliodonium salts as active radical precursors in multi-component sulfonylation reactions involving the insertion of sulfur dioxide has been developed initially. Jiang and coworkers reported a straightforward protocol for the synthesis of diverse functionalized diarylannulated sulfones through SO2/I exchange of iodonium(III) salts. In this reaction, the aryl radical generated from diaryliodonium salt was captured by Na2S2O5 to form a SO2 radical anion, which served as the key intermediate to realize the exchange strategy (Scheme 1a).11 In 2018, Manolikakes12 and Zhang13 demonstrated that visible-light-induced reduction of diaryliodonium salts would generate aryl radicals, which could undergo cascade cyclization in combination with SO2 for the synthesis of sulfonylated coumarins (Scheme 1b) and 3-arylsulfonylquinoline derivatives, respectively. Very recently, Piguel and coworkers established the first visible-light photoredox catalyzed C–H sulfonylation of imidazopyridines with diaryliodonium salts and DABSO for the straightforward synthesis of novel C-3 sulfonylated imidazoheterocycles (Scheme 1c).14 Herein, we would like to report a novel 4CzIPN-catalyzed three-component sulfonylation of diaryliodonium salts with DABSO and silyl enolates via a radical process involving SO2 insertion. This photoredox catalysis with the assistance of visible light represents a green and sustainable approach for the synthesis of β-keto sulfones (Scheme 1d).
Trimethyl((1-phenylvinyl)oxy)silane 1a, diphenyliodonium triflate 2a and 1,4-diazabicyclo[2.2.2]octane bis(sulfur dioxide) (DABSO) were selected as starting materials to optimize the sulfonylation conditions. Initially, irradiation of all three reaction partners in 1,4-dioxane with blue LED (25 W) at room temperature for 12 hours under N2 led to the desired β-keto sulfone 3a in 47% yield (Table 1, entry 1). Other SO2 surrogates such as K2S2O5, Na2S2O5, Na2S2O4 displayed lower activity (Table 1, entries 2–4). A variety of solvents were examined. 1,4-Dioxane proved to be superior to tetrahydrofuran (THF), acetonitrile, 1,2-dichloroethane (DCE), N,N-dimethylformamide (DMF) and toluene (Table 1, entries 1 and 5–10). When SO2-MeCN solution was used as SO2 source instead of DABSO, the reaction mixture in MeCN gave 3a in a slightly increased yield (Table 1, entries 6 and 7). Next, a range of experiments were carried out under various light sources such as blue LED (10 W, 25 W, 30 W), white LED (24 W), compact fluorescent lamp (CFL, 18 W), Kessil light (390 nm) and UV (600 W). As a result, 25 W of blue LED has the best performance (Table S1 in ESI†). Despite extending the time to 24 h, only 55% yield of 3a can be obtained (Table S1,† entry 8). The addition of photoredox catalysts was beneficial for the transformation. For example, the yield of 3a sharply rose to more than 80% when 2.0 mol% of either [Ir{dFCF3ppy}2(bpy)]PF6 or 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) was added (Table 1, entries 11 and 14). Considering cost and availability factors, 4CzIPN was chosen as the optimal photocatalyst. When the reaction was carried out under air atmosphere, 60% yield of 3a was generated (Table 1, entry 15). In addition, the three-component sulfonylation cannot proceed without light (Table 1, entry 16). In addition, the introduction of various bases, such as NaOtBu, NaOH, Na2CO3, NaHCO3 and NEt3, has caused the reaction yield to decrease to varying degrees (Table S2 in ESI†).
| Entry | [SO2] | PC | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (2.0 equiv.), 2a (0.1 mmol, 1.0 equiv.), [SO2] (2.0 equiv.), PC (2 mol%), and n-C12H26 (10 μL) in solvent (1.0 mL) at room temperature for 12 h, irradiation with 25 W blue LED, under N2.b GC yields.c Under air atmosphere.d No light. Ir-1 = [Ir{dFCF3ppy}2(bpy)]PF6, Ru-1 = [Ru(bpy)3]Cl2·6H2O. SO2 solution: SO2 solution in MeCN (∼7.9 M). | ||||
| 1 | DABSO | — | 1,4-Dioxane | 47 |
| 2 | K2S2O5 | — | 1,4-Dioxane | 35 |
| 3 | Na2S2O5 | — | 1,4-Dioxane | 24 |
| 4 | Na2S2O4 | — | 1,4-Dioxane | 0 |
| 5 | DABSO | — | THF | 34 |
| 6 | DABSO | — | MeCN | 45 |
| 7 | SO2 solution | — | MeCN | 50 |
| 8 | DABSO | — | DCE | 37 |
| 9 | DABSO | — | PhMe | 0 |
| 10 | DABSO | — | DMF | 0 |
| 11 | DABSO | Ir-1 | 1,4-Dioxane | 84 |
| 12 | DABSO | Ru-1 | 1,4-Dioxane | Trace |
| 13 | DABSO | Eosin Y | 1,4-Dioxane | 56 |
| 14 | DABSO | 4CzIPN | 1,4-Dioxane | 85 |
| 15c | DABSO | 4CzIPN | 1,4-Dioxane | 60 |
| 16d | DABSO | 4CzIPN | 1,4-Dioxane | 0 |
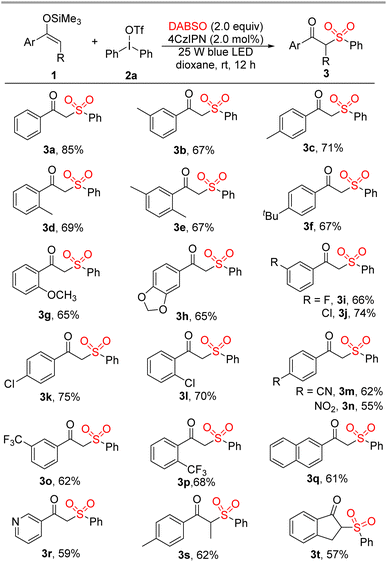
We investigated the scope of this three-component sulfonylation transformation with the optimal conditions in hand. The treatment of diverse silyl enolates 1 with diphenyliodonium triflate 2a and DABSO afforded the corresponding β-keto sulfone derivatives in moderate to good yields (Scheme 2). Silyl enolates possessing methyl and alkoxy groups on the phenyl ring could react with 2a and DABSO smoothly to give the desired products (3b–3h). Halogen atoms, especially chlorine, can be compatible under the optimal conditions (3i–3l), which revealed possible further transformations of the resulting halogenated products with other nucleophiles.15 Representative electron-withdrawing groups, such as cyano, nitro, and trifluoromethyl, were also tolerated in this three-component sulfonylation transformation under the optimal conditions (3m–3p). When trimethyl((1-(naphthalen-2-yl)vinyl)oxy)silane was treated with 2a and DABSO, the desired 3q was isolated in 61% yield. In addition, heterocyclic substituted silyl enolate could also be transformed to 3r in 59% yield. It is notable that α-substituted β-keto sulfones cannot be obtained from reported radical transformations involving the insertion of SO2.7–9 To our delight, β-alkyl substituted silyl enolates could work well in this three-component sulfonylation to give α-alkyl substituted β-keto sulfones 3s and 3t in acceptable yields.
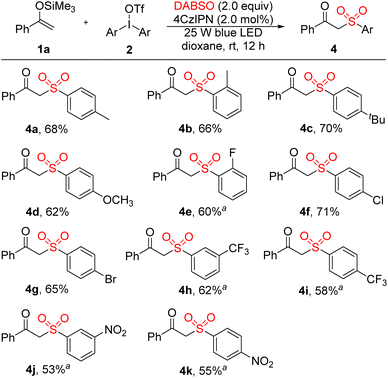
Subsequently the scope of diaryliodonium salts was examined. As shown in Scheme 3, both diaryliodonium triflates and diaryliodonium tetrafluoroborates were suitable substrates for this photocatalytic reaction. Diaryliodonium salts bearing different functional groups, containing electron-donating methyl (4a and 4b), tert-butyl (4c), methoxy (4d) and electron-withdrawing fluorine (4e), chlorine (4f), bromine (4g), trifluoromethyl (4h and 4i) and nitro (4j and 4k), were readily compatible in this 4CzIPN-catalyzed three-component sulfonylation.
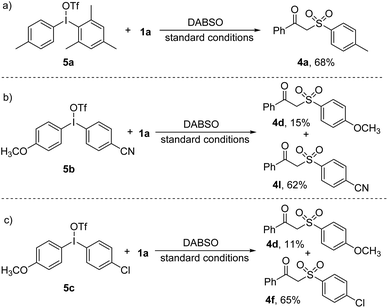
We next investigated the 4CzIPN-catalyzed three-component sulfonylation with unsymmetrical diaryliodonium salts. When unsymmetrical salt possessing a p-methylphenyl and a bulky mesityl group was used, only 4a was obtained in 68% via a selective transfer of the p-methylphenyl group (Scheme 4a). The reaction of 5b afforded methoxy substituted sulfone 4d and cyano substituted sulfone 4l in 15% and 62%, respectively (Scheme 4b). When unsymmetrical diaryliodonium salt 5c reacted with 1a and DABSO, the β-keto sulfone 4f was isolated as the main product in 65% with selective transfer of the electron-deficient p-chlorophenyl group over the electron-rich p-methoxyphenyl moiety (Scheme 4c). These results indicate that the electron-deficient aryl moiety is more easily reduced by photocatalyst to generate an aryl radical.
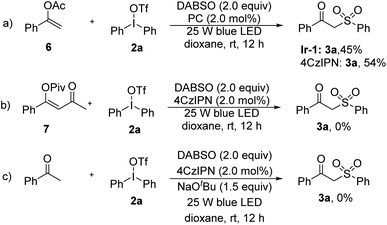
The scope of enolates in addition to silyl enolates was checked. 1-Phenylvinyl acetate 6 can afford the desired product in moderate yield in the presence of either Ir-1 or 4CzIPN (Scheme 5a), while 1,3-diketone derived enol ester 7 was not compatible (Scheme 5b). Under the standard conditions, the combination of acetophenone and sodium tert-butoxide was adopted, which can generate enolate in situ to replace the enol silane. As a result, the generation of 3a was not observed (Scheme 5c).
To gain more insight into the mechanism, the following radical inhibition experiments were performed. The standard sulfonylation transformation was completely restrained in the presence of 3.0 equiv. of 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO) (Scheme 6a). At the same time, the yield of 3a decreased to 47% when 3.0 equiv. of butylated hydroxytoluene (BHT) was added in the reaction system (Scheme 6b). These results implied that a radical process might be involved in the mechanism.
 | ||
| Scheme 6 Parallel control experiments: (a) radical inhibition experiment with TEMPO; (b) radical inhibition experiment with BHT. | ||
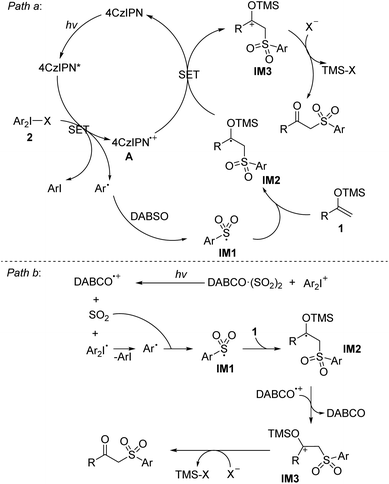
Based on the above observations and previous work, plausible mechanism of the photoredox-catalyzed sulfonylation involving the insertion of SO2 is proposed. For the pathway in the presence of photocatalyst (Scheme 7, path a):9 The photoexcited 4CzIPN* reduces diaryliodonium salt 2 through single electron transfer (SET) process to give an aryl radical and the oxidized photocatalyst A. Aryl radical is trapped by SO2 to generate the sulfonyl radical IM1. Then, the addition of sulfonyl radical IM1 into the double bond of silyl enolate 1 affords radical intermediate IM2, which can be oxidized to cation intermediate IM3 by photocatalyst A via another SET along with the regeneration of the photocatalyst 4CzIPN. Finally, cation intermediate IM3 undergoes desilylation with nucleophilic anion species to give the desired β-keto sulfone 3. For the pathway in the absence of photocatalyst (Scheme 7, path b):12 the interaction of iodonium salt with DABSO (DABCO·(SO2)2) would produce DABCO radical cation, dioxide, and diaryl iodine radical. Fragmentation of diaryl iodine radical furnishes an aryl radical, which is trapped by SO2 to generate the sulfonyl radical IM1. Then, the addition of sulfonyl radical IM1 into the double bond of silyl enolate 1 affords radical intermediate IM2, which can be oxidized to cation intermediate IM3 by DABCO radical cation. Finally, cation intermediate IM3 undergoes desilylation to give 3.
Conclusions
In summary, a versatile strategy for the synthesis of diverse β-keto sulfones via photoredox-catalyzed sulfonylation of diaryliodonium salts with DABSO and silyl enolates involving the insertion of SO2 has been established. In this reaction, blue-light-induced reduction of diaryliodonium salts afford the aryl radical, which would be trapped by SO2 to form sulfonyl radical as the key intermediate. This novel photoredox catalysis with the assistance of visible light represents a green and sustainable approach for the synthesis of β-keto sulfones and features wide substrates scope, good reactivity, and broad functional group tolerance. In addition, by employing β-alkyl substituted silyl enolates, this three-component sulfonylation would give α-alkyl substituted β-keto sulfones, which cannot be accessed by previous method involving the insertion of SO2.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 22101259) and the Zhejiang Provincial Natural Science Foundation of China (No. LQ22B020002).References
- (a) A. Markham and S. J. Keam, Drugs, 2018, 78, 1271–1276 CrossRef CAS PubMed; (b) E. H. Fleming, E. E. Ochoa, J. E. Nichols, M. K. O'Banion, A. R. Salkind and N. J. Roberts Jr, J. Med. Virol., 2018, 90, 26–33 CrossRef CAS PubMed; (c) J. Xiang, M. Ipek, V. Suri, M. Tam, Y. Xing, N. Huang, Y. Zhang, J. Tobin, T. S. Mansoura and J. McKew, Bioorg. Med. Chem., 2007, 15, 4396–4405 CrossRef CAS PubMed; (d) C. Curti, M. Laget, P. Vanelle, C. Curti, M. Laget, A. Ortiz Carle, A. Gellis and P. Vanelle, Eur. J. Med. Chem., 2007, 42, 880–884 CrossRef CAS PubMed; (e) J. Xiang, M. Ipek, V. Suri, W. Massefski, N. Pan, Y. Ge, M. Tam, Y. Xing, J. F. Tobin, X. Xu and S. Tam, Bioorg. Med. Chem. Lett., 2005, 15, 2865–2869 CrossRef CAS PubMed; (f) H. Peng, Y. Cheng, N. Ni, M. Li, G. Choudhary, H. T. Chou, C.-D. Lu, P. C. Tai and B. Wang, ChemMedChem, 2009, 4, 1457–1468 CrossRef CAS PubMed.
- (a) Y. M. Markitanov, V. M. Timoshenko and Y. G. Shermolo-vich, J. Sulfur Chem., 2014, 35, 188–236 CrossRef CAS; (b) O. O. Shyshkina, K. S. Popov, O. O. Gordivska, T. M. Tkachuk, N. V. Kovalenko, T. A. Volovnenko and Yu. M. Volovenko, Chem. Heterocycl. Compd., 2011, 47, 923–945 CrossRef CAS.
- (a) B. M. Trost and D. P. Curran, Tetrahedron Lett., 1981, 22, 1287–1290 CrossRef CAS; (b) A. K. Singh, R. Chawla, T. Keshari, V. K. Yadav and L. D. S. Yadav, Org. Biomol. Chem., 2014, 12, 8550–8554 RSC; (c) X.-J. Pan, J. Gao and G.-Q. Yuan, Tetrahedron, 2015, 71, 5525–5530 CrossRef CAS.
- (a) Y. Y. Xie and Z. C. Chen, Synth. Commun., 2001, 31, 3145–3149 CrossRef CAS; (b) Q. Lu, J. Zhang, G. Zhao, Y. Qi, H. Wang and A. Lei, J. Am. Chem. Soc., 2013, 135, 11481–11484 CrossRef CAS PubMed.
- (a) G. Liu, C.-B. Fan and J. Wu, Org. Biomol. Chem., 2015, 13, 1592–1599 RSC; (b) D. Zheng and J. Wu, Sulfur Dioxide Insertion Reactions for Organic Synthesis. Springer, Singapore, 2017, pp. 11–77 CrossRef; (c) G.-S. Qiu, K.-D. Zhou, J. Wu and L. Gao, Org. Chem. Front., 2018, 5, 691–705 RSC; (d) G.-S. Qiu, L.-F. Lai, J. Wu and J. Cheng, Chem. Commun., 2018, 54, 10405–10414 RSC; (e) K. Hofman, N. W. Liu and G. Manolikakes, Chem.–Eur. J., 2018, 24, 11852–11863 CrossRef CAS PubMed; (f) S.-Q. Ye, G.-S. Qiu and J. Wu, Chem. Commun., 2019, 55, 1013–1019 RSC; (g) S. Ye, X. Li, J. Wu and W. Xie, Eur. J. Org Chem., 2020, 2020, 1274–1287 CrossRef CAS; (h) D. Zeng, M. Wang, W.-P. Deng and X. Jiang, Org. Chem. Front., 2020, 7, 3956–3966 RSC; (i) S. P. Blum, K. Hofman, G. Manolikakes and S. R. Waldvogel, Chem. Commun., 2021, 57, 8236–8249 RSC; (j) E. L. S. de Souza and C. C. Oliveira, Eur. J. Org Chem., 2023, 26, e202300073 CrossRef CAS.
- (a) D. Zheng, J. Yu and J. Wu, Angew. Chem., Int. Ed., 2016, 55, 11925–11929 CrossRef CAS PubMed; (b) H. Wang, S. Sun and J. Cheng, Org. Lett., 2017, 19, 5844–5847 CrossRef CAS PubMed; (c) Y. Zong, Y. Lang, M. Yang, X. Li, X. Fan and J. Wu, Org. Lett., 2019, 21, 1935–1938 CrossRef CAS; (d) H. Xia, Y. An, X. Zeng and J. Wu, Org. Chem. Front., 2018, 5, 366–370 RSC; (e) X. Gong, M. Wang, J. Wu and S. Ye, Org. Lett., 2019, 21, 1156–1160 CrossRef CAS; (f) Y. Meng, M. Wang and X. Jiang, CCS Chem., 2021, 3, 17–24 CrossRef CAS; (g) M. Zhang, L. Liu, B. Wang, Y. Yang, Y. Liu, Z. Wang and Q. Wang, ACS Catal., 2023, 13, 11580–11588 CrossRef CAS; (h) H. Li, Y. Zhang and X. Zou, ACS Catal., 2024, 14, 3664–3674 CrossRef CAS.
- T. Liu, D. Zheng, Y. Ding, X. Fan and J. Wu, Chem.–Asian J., 2017, 12, 465–469 CrossRef CAS PubMed.
- X. Gong, Y. Ding, X. Fan and J. Wu, Adv. Synth. Catal., 2017, 359, 2999–3004 CrossRef CAS.
- F.-S. He, P. Bao, Z. Tang, F. Yu, W.-P. Deng and J. Wu, Org. Lett., 2022, 24, 2955–2960 CrossRef CAS PubMed.
- (a) J. Chen, H. Qu, J. Peng and C. Chen, Chin. J. Org. Chem., 2015, 35, 937–946 CrossRef CAS; (b) M. Sheng, D. Frurip and D. Gorman, J. Loss Prev. Process Ind., 2015, 38, 114–118 CrossRef CAS.
- M. Wang, S. Chen and X. Jiang, Org. Lett., 2017, 19, 4916–4919 CrossRef CAS PubMed.
- (a) Z. Chen, N.-W. Liu, M. Bolte, H. Rena and G. Manolikakes, Green Chem., 2018, 20, 3059–3070 RSC; (b) N.-W. Liu, Z. Chen, A. Herbert, H. Ren and G. Manolikakes, Eur. J. Org Chem., 2018, 2018, 5725–5734 CrossRef CAS; (c) A. M. Nair, I. Halder, S. Khan and C. M. R. Volla, Adv. Synth. Catal., 2020, 362, 224–229 CrossRef CAS.
- D. Sun, K. Yin and R. Zhang, Chem. Commun., 2018, 54, 1335–1338 RSC.
- C. Breton-Patient, D. Naud-Martin, F. Mahuteau-Betzer and S. Piguel, Eur. J. Org Chem., 2020, 2020, 6653–6660 CrossRef CAS.
- (a) C.-L. Sun and Z.-J. Shi, Chem. Rev., 2014, 114, 9219–9280 CrossRef CAS PubMed; (b) P. Ruiz-Castillo, L. Stephen and S. L. Buchwald, Chem. Rev., 2016, 116, 12564–12649 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, analytical data for products, NMR spectra of products. See DOI: https://doi.org/10.1039/d4ra02773f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |