Open Access Article
Open Access ArticleSynthesis of 1-aryl-2,3-diaroyl cyclopropanes from 1,3,5-triaryl-1,5-diketones and their transformation into E,E-1,4-diaryl-1,3-butadienes†
Kashpar John Britto,
Maniarasu Meenakshi and
Kannupal Srinivasan *
*
School of Chemistry, Bharathidasan University, Tiruchirappalli 620024, Tamil Nadu, India. E-mail: srinivasank@bdu.ac.in; Tel: +91-431-2407053
First published on 12th July 2024
Abstract
A new method for the synthesis of 1-aryl-2,3-diaroyl cyclopropanes has been developed by iodine/DBU-mediated cyclization of 1,3,5-triaryl-1,5-diketones. The alcohols derived by the reduction of these cyclopropanes, when treated with conc. HCl, afforded a series of 1,3-dienes through cyclopropyl ring-opening and subsequent fragmentation. Overall, the synthetic sequence represents a new non-Wittig methodology for the synthesis of 1,3-dienes from 1,5-diketones.
Introduction
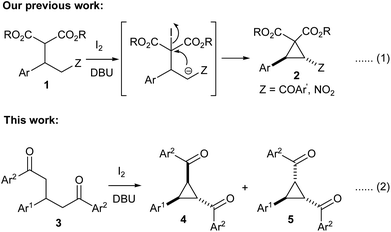
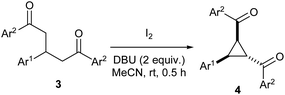
Cyclopropanes, especially those having electron donating and withdrawing groups in the vicinal position continue to receive a major focus in organic synthesis owing to their enormous synthetic potential.1 The inherent angle and torsional strains in the three-carbon ring system coupled with the presence of push–pull groups in adjacent positions bestow them with high reactivity. Such cyclopropanes undergo various transformations such as annulation,2 rearrangement3 and ring-opening4 reactions upon exposure to suitable reagents to yield a diverse range of products. Most of the methods used for the synthesis of donor–acceptor cyclopropanes fall into two major categories: (1) [2 + 1] cycloaddition of carbenes generated from diazo compounds or iodonium ylides to alkenes and (2) Michael-initiated ring closure strategy involving addition of nucleophiles to electrophilic alkenes followed by cyclization. Both methods have been extensively used for the access of various types of donor–acceptor cyclopropanes in both racemic and chiral forms.51,3,5-Triaryl-1,5-diketones are important building blocks for the synthesis of various heterocyclic compounds such as pyridines,6 thiophenes7 and pyrylium compounds.8 These 1,5-diketones could be easily prepared by the base-mediated Michael addition of aryl methyl ketones to chalcones in a one-pot or stepwise manner.9 However, the application of these 1,5-diketones for synthesis of carbocycles such as cyclopropanes has been scarcely investigated in the literature. To the best of our knowledge, there is only one report to effect the transformation using iodobenzene diacetate and the reaction yields cyclopropanes only in low yields with three other by-products.10 Our research group prepared aroyl/nitro substituted donor–acceptor cyclopropanes 2 by iodine/DBU mediated oxidative cyclization of Michael adducts of chalcones/nitrostyrenes with malonates 1 (Scheme 1, eqn. (1)).11 Noticing the presence of acidic protons in suitable positions in 1,3,5-triaryl-1,5-diketones 3 as well, we envisaged that they could be also subjected to a similar oxidative cyclization using iodine/DBU to obtain 1-aryl-2,3-diaroyl cyclopropanes 4/5 (Scheme 1, eqn. (2)). We herein present the results along with further transformation of the cyclopropanes into E,E-1,5-diaryl-1,3-dienes. It may be noted that this type of donor–acceptor cyclopropane has been mostly synthesized by employing sulphur ylides in the literature.12
Result and discussion
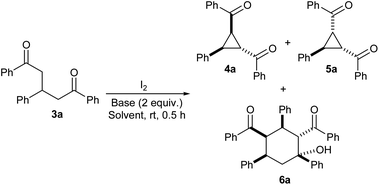
We began the study by identifying suitable reaction conditions for iodine-mediated oxidative cyclization of 1,3,5-triaryl-1,5-diketones 3, which were prepared as per literature reports.9 We selected diketone 3a as a model substrate and treated with iodine in the presence of two equiv. of DBU in DCM, as per our reported methods for the cyclization of Michael adducts of chalcones/nitrostyrenes.11 Pleasingly, it underwent the expected oxidative cyclization to afford two diasteromeric cyclopropanes 4a and 5a along with a cyclohexanol derivative 6a13 in 43, 37 and 7% yields, respectively (entry 1). With a view to synthesize one of the cyclopropane products in a better yield, we employed different bases such as Et3N, piperidine and DABCO in the reaction (entries 2–4). However, we could not see better outcomes with these bases as compared to DBU. Next, we examined the use of different solvents in the reaction (entries 5–10). We found better results with MeCN (entry 8) and hence it was selected as the solvent of choice for other reactions. In Table 1, it is interesting to note that the yield of 4a (in which the two aroyl groups are trans to each other) is always higher than that of 5a (in which the aroyl groups are cis) and hence we attribute the observed diastereoselectivity to the repulsion between the two aroyl groups.| S. no. | Reaction conditions | Yielda (%) | ||
|---|---|---|---|---|
| 4a | 5a | 6a | ||
| a Isolated yield.b When 1 equiv. of DBU was used, the yields of 4a, 5a and 6a were 52, 13 and 7%, respectively. | ||||
| 1 | DBU, DCM | 43 | 37 | 7 |
| 2 | Et3N, DCM | 52 | 10 | 13 |
| 3 | Piperidine, DCM | 62 | 9 | 14 |
| 4 | DABCO, DCM | 48 | 6 | 8 |
| 5 | DBU, CHCl3 | 35 | 7 | 5 |
| 6 | DBU, 1,2-DCE | 51 | 9 | 11 |
| 7 | DBU, toluene | 31 | 30 | 10 |
| 8 | DBU,b MeCN | 65 | 11 | 12 |
| 9 | DBU, THF | 61 | 9 | 10 |
| 10 | DBU, EtOH | 37 | 35 | 20 |
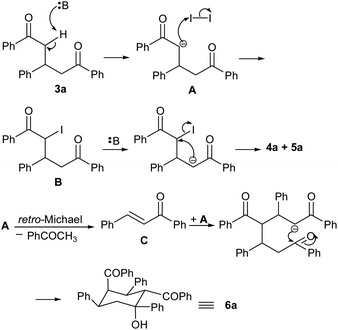
A plausible mechanism for the formation of different products in the transformation is outlined in Scheme 2. The base (DBU) removes one of the acidic protons in diketone 3a and the resulting carbanion A attacks iodine to give mono-iodinated intermediate B. Next, the base removes the remaining acidic proton in B and the anion so formed attacks the iodine-containing carbon in an intermolecular SN2 fashion to give diastereomeric cyclopropane products 4a and 5a. The carbanion A produced in the first step may also undergo retro-Michael reaction to give chalcone C via elimination of acetophenone. A subsequent tandem Michael addition/aldol reaction between A and C gives cyclohexanol 6a.
While investigating the reactivity of the major cyclopropane products 4 (discussed later), we found that the minor cyclopropane products 5 also give the same result. So, we next focused our attention only on synthesizing various derivatives of 4 (and not 5) and the results are summarized in Table 2. Initially, we employed diketones having different electron-donating, electron-withdrawing and halogen substituents on Ar1 and Ar2 (entries 1–13). Pleasingly, all the diketones afforded the respective cyclopropanes 4a–m in 56–68% yields. The reaction also tolerated the use of bulky 1-naphthyl and heteroaromatic, 2-thienyl as Ar1 or Ar2 and the corresponding cyclopropanes 4n–s are produced in 60–67% yields (entries 14–19).
| S. no. | Ar1, Ar2 (3) | Yield of 4a (%) |
|---|---|---|
| a Isolated yield. | ||
| 1 | Ph, Ph (3a) | 65 (4a) |
| 2 | Ph, 4-MeC6H4 (3b) | 59 (4b) |
| 3 | Ph, 4-MeOC6H4 (3c) | 63 (4c) |
| 4 | Ph, 4-BrC6H4 (3d) | 56 (4d) |
| 5 | 4-MeC6H4, Ph (3e) | 60 (4e) |
| 6 | 4-F3CC6H4, Ph (3f) | 57 (4f) |
| 7 | 2-MeC6H4, 4-MeC6H4 (3g) | 67 (4g) |
| 8 | 4-MeC6H4, 4-MeC6H4 (3h) | 68 (4h) |
| 9 | 4-tBuC6H4, 4-MeC6H4 (3i) | 67 (4i) |
| 10 | 4-ClC6H4, 4-MeC6H4 (3j) | 60 (4j) |
| 11 | 4-FC6H4, 4-MeC6H4 (3k) | 61 (4k) |
| 12 | 4-MeC6H4, 4-BrC6H4 (3l) | 58 (4l) |
| 13 | 4-MeC6H4, 4-ClC6H4 (3m) | 60 (4m) |
| 14 | 1-Naphthyl, Ph (3n) | 62 (4n) |
| 15 | 1-Naphthyl, 4-MeC6H4 (3o) | 67 (4o) |
| 16 | Ph, 2-thienyl (3p) | 61 (4p) |
| 17 | 4-MeC6H4, 2-thienyl (3q) | 60 (4q) |
| 18 | 2-Thienyl, Ph (3r) | 65 (4r) |
| 19 | 2-Thienyl, 4-MeC6H4 (3s) | 63 (4s) |
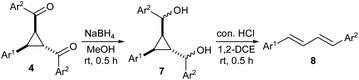
Next, we investigated the ring-opening reactions of cyclopropanes 4 with various Lewis acids such as AlCl3, SnCl4, TiCl4, BF3·OEt2, FeCl3, SnCl2, Cu(OTf)2, In(OTf)3, Sc(OTf)3, and Yb(OTf)3 and Brønsted acids such p-TsOH, TFA and con. HCl to identify the mode of cleavage of the cyclopropane ring. Unfortunately, none of the reagents could bring any change to cyclopropanes 4. So, we decided to reduce their keto group into alcoholic group and then attempt their ring-opening reactions.14 Accordingly, cyclopropanes 4 were subjected to reduction using NaBH4 in MeOH and the resulting diasteromeric mixtures of alcohols 7, after work-up, were treated as such without further purification with different Lewis/Brønsted acids. We found that the treatment of 7 with a few drops of con. HCl in 1,2-DCE yielded a series of 1,3-dienes 8 (Table 3; the structure of 8h was confirmed by X-ray crystallographic analysis15). The conversion could also be achieved by using one equiv. of AlCl3, Sc(OTf)3, Yb(OTf)3 or TFA with similar yields.16 It may be noted that in case of 8f, the strong electron withdrawing aryl group would destabilize the respective carbocation B (Scheme 3) and hence its formation would have been sluggish resulting in trace amount of the final product. We also observed that one of the diasteromeric cyclopropanes, 5a also produced the same 1,3-diene 8a in 86% yield when treated with con. HCl.
| S. no. | Ar1, Ar2 (4) | Yield of 8a (%) |
|---|---|---|
| a Isolated yield.b The diasteromeric cyclopropane, 5a also gave 8a in 86% yield. | ||
| 1 | Ph, Ph (4a) | 89 (8a)b |
| 2 | Ph, 4-MeC6H4 (4b) | 79 (8b) |
| 3 | Ph, 4-MeOC6H4 (4c) | 80 (8c) |
| 4 | Ph, 4-BrC6H4 (4d) | 75 (8d) |
| 5 | 4-MeC6H4, Ph (4e) | 82 (8e) |
| 6 | 4-F3CC6H4, Ph (4f) | Trace (8f) |
| 7 | 2-MeC6H4, 4-MeC6H4 (4g) | 87 (8g) |
| 8 | 4-MeC6H4, 4-MeC6H4 (4h) | 88 (8h) |
| 9 | 4-tBuC6H4, 4-MeC6H4 (4i) | 81 (8i) |
| 10 | 4-ClC6H4, 4-MeC6H4 (4j) | 80 (8j) |
| 11 | 4-FC6H4, 4-MeC6H4 (4k) | 83 (8k) |
| 12 | 4-MeC6H4, 4-BrC6H4 (4l) | 72 (8l) |
| 13 | 4-MeC6H4, 4-ClC6H4 (4m) | 82 (8m) |
| 14 | 1-Naphthyl, Ph (4n) | 87 (8n) |
| 15 | 1-Naphthyl, 4-MeC6H4 (4o) | 85 (8o) |
| 16 | Ph, 2-thienyl (4p) | Trace (8p) |
| 17 | 4-MeC6H4, 2-thienyl (4q) | 84 (8q) |
| 18 | 2-Thienyl, Ph (4r) | 80 (8r) |
| 19 | 2-Thienyl, 4-MeC6H4 (4s) | 87 (8s) |
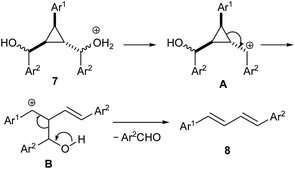
Mechanistically, the ring-opening reaction of cyclopropane alcohols 7 may take place as shown in Scheme 3. The protonation of hydroxyl group of alcohols 7 leads to the elimination of the hydroxyl group with formation of carbocation A. This triggers cyclopropane ring-opening to generate a new carbocation B, which undergoes fragmentation to yield 1,3-diene 8 with loss of arylaldehyde. We have previously observed such fragmentation with aroyl-substituted donor–acceptor cyclopropanes.11b
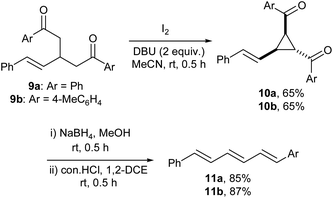
Finally, we extended the scope of the three-step transformation for diketones 9 having a styryl group with a view to obtain the respective trienes (Scheme 4). Pleasingly, diketones 9 gave the respective styryl cyclopropanes 10 when treated with iodine/DBU and the cyclopropanes furnished the corresponding trienes 11 when subjected to reduction followed by treatment with con. HCl.
1,3-Dienes are usually synthesized by (i) the Witting and related reactions, (ii) cross-coupling reactions, (iii) olefin metathesis and (iv) rearrangement/isomerisation reactions.17 Among them, the Wittig strategy is one of the most commonly employed methods. The present work represents a new non-Wittig strategy for the synthesis of 1,3-dienes and 1,3,5-trienes from 1,5-diketones.
Conclusions
In conclusion, we have synthesized a series of 1-aryl-2,3-diaroyl cyclopropanes by iodine/DBU-mediated cyclization of 1,3,5-triaryl-1,5-diketones. The cyclopropanes when subjected to reduction followed by treatment with con. HCl afforded E,E-1,5-diaryl-1,3-butadienes, through the formation of the respective alcohols, followed by cyclopropyl ring-opening promoted by elimination of a hydroxyl group and subsequent fragmentation. It was also possible to obtain few trienes by employing styryl-substituted cyclopropanes.Experimental section
General remarks. Melting points were determined by the open capillary tube method and are uncorrected. The IR spectra were recorded on an FT-IR spectrometer using ATR. The 1H and 13C NMR spectra were recorded on a 400 MHz NMR spectrometer. High resolution mass spectra (ESI) were recorded on a Q-TOF mass spectrometer. X-ray crystallographic data were collected on a CCD diffractometer using graphite-monochromated Mo-Kα radiation. Thin layer chromatography (TLC) was performed on pre-coated alumina sheets and detected under UV light. Silica gel (100–200 mesh) was used for column chromatography. 1,5-Diketones 3a–s and 9a–b were prepared as per a reported literature procedure9d and among the diketones, 3a, 3c–f, 3h, 3j, 3k, 3l, 3n, 3q and 9a are known compounds.18 Among the new compounds 3b, 3p and 3r had about 15–25% inseparable impurities and hence were taken as such to the next step.3-o-Tolyl-1,5-di-p-tolyl-pentane-1,5-dione (3g)
White solid. Yield: 2.83 g (70%); M. p. 90–92 °C; 1H NMR (400 MHz, CDCl3): δ 7.89 (d, J = 8.0 Hz, 4H), 7.32–7.27 (m, 5H), 7.21–7.11 (m, 3H), 4.37 (dd, J1 = 6.8 Hz, J2 = 11.0 Hz, 1H), 3.48 (dd, J1 = 7.0 Hz, J2 = 16.6 Hz, 2H), 3.36 (dd, J1 = 7.0 Hz, J2 = 16.6 Hz, 2H) 2.44 (s, 6H), 2.42 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 198.4, 143.9, 142.3, 136.0, 134.5, 131.9, 130.7, 130.3, 129.3, 128.3, 126.3, 126.2, 125.7, 44.6, 32.2, 21.7, 19.8 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H26O2, 371.2006; found: 371.2010.3-(4-tert-Butyl-phenyl)-1,5-di-p-tolyl-pentane-1,5-dione (3i)
White solid. Yield: 1.52 g (71%); M. p. 102–104 °C; 1H NMR (400 MHz, CDCl3): δ 7.76 (d, J = 8.4 Hz, 4H), 7.20–7.17 (m, 2H), 7.13 (t, J = 8.2 Hz, 6H), 3.35 (dd, J1 = 7.2 Hz, J2 = 16.4 Hz, 2H), 3.23 (dd, J1 = 7.0 Hz, J2 = 16.6 Hz, 2H) 2.31 (s, 6H), 1.19 (s, 9H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 198.5, 149.2, 143.8, 141.0, 134.5, 129.2, 128.3, 127.1, 125.5, 44.9, 36.8, 34.4, 31.4, 21.7 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C29H32O2, 413.2475; found: 413.2477.1,5-Bis-(4-chloro-phenyl)-3-p-tolyl-pentane-1,5-dione (3m)
White solid. Yield: 2.89 g (75%); M. p. 73–75 °C; 1H NMR (400 MHz, CDCl3): δ 7.80 (d, J = 8.4 Hz, 4H), 7.33 (d, J = 8.4 Hz, 4H), 7.03 (dd, J1 = 7.8 Hz, J2 = 22.2 Hz, 4H), 3.90 (t, J = 7.0 Hz, 1H), 3.36 (dd, J1 = 7.0 Hz, J2 = 16.6 Hz, 2H), 3.20 (dd, J1 = 7.0 Hz, J2 = 16.6 Hz, 2H), 2.21 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 197.5, 140.4, 139.6, 136.5, 135.2, 129.6, 129.4, 128.9, 127.2, 45.0, 36.9, 21.0 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H20Cl2O2, 411.0913; found: 411.0918.3-Naphthalen-1-yl-1,5-di-p-tolyl-pentane-1,5-dione (3o)
White solid. Yield: 2.73 g (71%); M. p. 107–109 °C; 1H NMR (400 MHz, CDCl3): δ 8.33 (d, J = 8.4 Hz, 1H), 7.93–7.90 (m, 5H), 7.77 (d, J = 8.0 Hz, 1H), 7.60–7.51 (m, 3H), 7.46 (t, J = 7.6 Hz, 1H), 7.27 (d, J = 8.0 Hz, 4H), 5.11 (t, J = 6.6 Hz, 1H), 3.66 (dd, J1 = 7.2 Hz, J2 = 16.8 Hz, 2H), 3.57 (dd, J1 = 6.0 Hz, J2 = 17.0 Hz, 2H), 2.44 (s, 6H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 198.4, 143.9, 140.3, 134.6, 134.2, 131.4, 129.3, 129.0, 128.3, 127.2, 126.3, 125.6, 125.4, 123.3, 44.4, 21.7 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C29H26O2, 407.2006; found: 407.2013.3-Thiophen-2-yl-1,5-di-p-tolyl-pentane-1,5-dione (3s)
White solid. Yield: 2.43 g (71%); M. p. 97–99 °C; 1H NMR (400 MHz, CDCl3): δ 7.78 (d, J = 8.0 Hz, 4H), 7.16 (d, J = 8.0 Hz, 4H), 7.01 (d, J = 4.4 Hz, 1H), 6.79 (d, J = 4.8 Hz, 2H), 4.33 (t, J = 6.8 Hz, 1H), 3.40 (dd, J1 = 6.8 Hz, J2 = 16.8 Hz, 2H), 3.30 (dd, J1 = 7.0 Hz, J2 = 16.6 Hz, 2H), 2.32 (s, 6H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 197.8, 147.7, 144.0, 134.4, 129.3, 128.3, 126.7, 124.2, 123.3, 45.6, 32.6, 21.7 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H22O2S, 363.1413; found: 363.1420.3-Styryl-1,5-di-p-tolyl-pentane-1,5-dione (9b)
Yellow solid. Yield: 2.79 g (78%); M. p. 70–72 °C; 1H NMR (400 MHz, CDCl3): δ 7.81 (d, J = 8.0 Hz, 3H), 7.22–7.15 (m, 9H), 7.13–7.08 (m, 1H), 6.34 (d, J = 16.0 Hz, 1H), 6.20 (dd, J1 = 8.2 Hz, J2 = 15.6 Hz, 1H), 3.52 (dd, J1 = 7.0 Hz, J2 = 13.8 Hz, 1H), 3.24 (dd, J1 = 6.6 Hz, J2 = 16.2 Hz, 2H), 3.08 (dd, J1 = 6.8 Hz, J2 = 16.0 Hz, 2H), 2.33 (s, 6H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 198.6, 143.9, 137.2, 134.6, 132.1, 130.3, 129.3, 128.4, 128.3, 127.2, 126.3, 43.4, 35.2, 21.7 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C27H26O2, 383.2006; found: 383.2008.General procedure for the synthesis of donor–acceptor cyclopropanes 4a–s and 10a–b
To a solution of 1,5-diketones 3 (3.0 mmol) in acetonitrile was added DBU (6.0 mmol) followed by iodine (3.0 mmol) and stirred for 0.5 h. The reaction mixture was quenched by aq. Na2S2O3 solution, diluted with water and extracted with ethyl acetate. The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel using EtOAc/hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19, v/v) to afford the pure cyclopropanes 4a–s and 10a–b.
19, v/v) to afford the pure cyclopropanes 4a–s and 10a–b.
((1R*,2S*,3S*)-3-phenylcyclopropane-1,2-diyl)bis(phenylmethanone) (4a):12c
White solid. Yield: 644 mg (65%); M. p. 120–122 °C; IR (KBr): v 1652, 1587, 1289, 1208, 1013, 733, 693 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.21 (d, J = 7.6 Hz, 2H), 8.04 (d, J = 7.6 Hz, 2H), 7.68 (t, J = 7.2 Hz, 1H), 7.61–7.56 (m, 3H), 7.51–7.48 (m, 2H), 7.32–7.20 (m, 5H), 4.31 (t, J = 5.6 Hz, 1H), 3.84 (dd, J1 = 4.8 Hz, J2 = 10.0 Hz, 1H), 3.62 (dd, J1 = 6.2 Hz, J2 = 10.2 Hz, 1H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 197.5, 193.8, 137.5, 137.1, 134.4, 133.6, 133.3, 128.83, 128.78, 128.7, 128.5, 128.4, 128.3, 127.3, 38.1, 37.5, 29.8 ppm.((1R*,2S*,3S*)-3-phenylcyclopropane-1,2-diyl)bis(p-tolylmethanone) (4b)
White solid. Yield: 632 mg (59%); M. p. 126–128 °C; 1H NMR (400 MHz, CDCl3): δ 7.99 (d, J = 8.0 Hz, 2H), 7.83 (d, J = 8.0 Hz, 2H), 7.25 (d, J = 8.0 Hz, 2H), 7.18–7.14 (m, 6H), 7.12–7.08 (m, 1H), 4.14 (t, J = 5.4 Hz, 1H), 3.67 (dd, J1 = 4.8 Hz, J2 = 10.0 Hz, 1H), 3.45 (dd, J1 = 6.0 Hz, J2 = 10.0 Hz, 1H), 2.37 (s, 3H), 2.32 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 197.1, 193.4, 144.5, 144.1, 135.1, 134.64, 134.61, 129.5, 129.3, 128.8, 128.6, 128.5, 128.2, 127.2, 37.8, 37.3, 29.6, 21.8, 21.7 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H23O2, 355.1693; found: 355.1706.((1R*,2S*,3S*)-3-phenylcyclopropane-1,2-diyl)bis((4-methoxy)phenylmethanone) (4c)
White solid. Yield: 731 mg (63%); M. p. 110–112 °C; 1H NMR (400 MHz, CDCl3): δ 8.08 (d, J = 8.4 Hz, 2H), 7.97–7.89 (m, 3H), 7.35 (d, J = 4.8 Hz, 1H), 7.14 (d, J = 6.8 Hz, 3H), 6.92 (d, J = 8.8 Hz, 2H), 6.83 (d, J = 8.4 Hz, 2H), 4.11–4.08 (m, 1H), 3.81 (s, 3H), 3.77 (s, 3H), 3.63 (dd, J1 = 4.8 Hz, J2 = 10.0 Hz, 1H), 3.42 (dd, J1 = 6.2 Hz, J2 = 9.8 Hz, 1H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 195.9, 192.3, 163.9, 163.6, 134.8, 132.3, 130.8, 130.75, 130.67, 130.2, 128.8, 128.5, 128.2, 127.1, 114.0, 113.8, 113.7, 55.6, 55.5, 37.5, 37.0, 29.4 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H23O4, 387.1591; found: 387.1599.((1R*,2S*,3S*)-3-phenylcyclopropane-1,2-diyl)bis((4-bromophenyl)methanone) (4d)
White solid. Yield: 810 mg (56%); M. p. 146–148 °C; 1H NMR (400 MHz, CDCl3): δ 8.05 (d, J = 8.4 Hz, 2H), 7.89 (d, J = 8.4 Hz, 2H), 7.71 (d, J = 8.4 Hz, 2H), 7.63 (d, J = 8.8 Hz, 2H), 7.30–7.23 (m, 5H), 4.22 (t, J = 5.4 Hz, 1H), 3.76 (dd, J1 = 4.0 Hz, J2 = 10.2 Hz, 1H), 3.59 (dd, J1 = 6.0 Hz, J2 = 10.0 Hz, 1H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 196.2, 192.6, 136.0, 135.7, 133.9, 132.2, 130.0, 129.8, 129.0, 128.7, 128.6, 128.4, 127.5, 38.2, 37.4, 29.6 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H17Br2O2, 482.9590; found: 482.9590.((1R*,2S*,3S*)-3-(p-tolyl)cyclopropane-1,2-diyl)bis(phenylmethanone) (4e):12c
White solid. Yield: 620 mg (60%); M. p. 139–141 °C; 1H NMR (400 MHz, CDCl3): δ 8.20 (d, J = 7.6 Hz, 2H), 8.04 (d, J = 7.6 Hz, 2H), 7.67 (t, J = 7.2 Hz, 1H), 7.61–7.55 (m, 3H), 7.49 (t, J = 7.6 Hz, 2H), 7.30 (s, 1H), 7.19 (d, J = 8.0 Hz, 2H), 7.07 (d, J = 8.0 Hz, 1H), 4.27 (t, J = 5.6 Hz, 1H), 3.81 (dd, J1 = 4.8 Hz, J2 = 10.0 Hz, 1H), 3.57 (dd, J1 = 6.2 Hz, J2 = 9.8 Hz, 1H), 2.29 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 197.5, 193.9, 137.6, 137.1, 136.9, 133.5, 133.2, 131.3, 129.0, 128.8, 128.63, 128.61, 128.5, 128.4, 38.0, 37.5, 30.0, 21.1 ppm.((1R*,2S*,3S*)-3-(4-(trifluoromethyl)phenyl)cyclopropane-1,2-diyl)bis(phenylmethanone) (4f)
White solid. Yield: 673 mg (57%); M. p. 110–112 °C; 1H NMR (400 MHz, CDCl3): δ 8.21 (d, J = 7.6 Hz, 2H), 8.04 (d, J = 7.2 Hz, 2H), 7.68 (t, J = 7.2 Hz, 1H), 7.63–7.57 (m, 3H), 7.55–7.49 (m, 4H), 7.44 (d, J = 8.0 Hz, 2H), 4.33 (t, J = 5.4 Hz, 1H), 3.87 (dd, J1 = 4.8 Hz, J2 = 10.0 Hz, 1H), 3.65 (dd, J1 = 6.2 Hz, J2 = 9.8 Hz, 1H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 196.8, 193.4, 138.6, 137.3, 136.9, 133.8, 133.6, 129.3, 129.2, 128.9, 128.8, 128.5, 128.4, 125.33, 125.29, 125.25, 125.22, 37.3, 37.1, 29.9 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H18F3O2, 395.1253; found: 395.1263.((1R*,2S*,3S*)-3-(o-tolyl)cyclopropane-1,2-diyl)bis(p-tolylmethanone) (4g)
White solid. Yield: 747 mg (67%); M. p. 130–132 °C; 1H NMR (400 MHz, CDCl3): δ 8.00 (d, J = 8.0 Hz, 2H), 7.85 (d, J = 8.0 Hz, 2H), 7.25 (d, J = 8.0 Hz, 2H), 7.19–7.16 (m, 3H), 7.08–7.02 (m, 2H), 6.98–6.96 (m, 1H), 4.14 (dd, J1 = 4.8 Hz, J2 = 6.0 Hz, 1H), 3.80 (dd, J1 = 4.6 Hz, J2 = 9.4 Hz, 1H), 3.37 (dd, J1 = 6.4 Hz, J2 = 9.6 Hz, 1H), 2.36 (s, 3H), 2.32 (s, 3H), 2.15 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 197.2, 193.2, 144.5, 144.1, 137.7, 134.8, 134.6, 132.7, 129.9, 129.5, 129.3, 128.9, 128.6, 128.5, 127.3, 125.6, 37.2, 36.0, 30.6, 21.8, 21.7, 19.8 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H25O2, 369.1849; found: 369.1868.((1R*,2S*,3S*)-3-(p-tolyl)cyclopropane-1,2-diyl)bis(p-tolylmethanone) (4h)
White solid. Yield: 750 mg (68%); M. p. 131–133 °C; 1H NMR (400 MHz, CDCl3): δ 7.97 (d, J = 8.0 Hz, 2H), 7.81 (d, J = 8.0 Hz, 2H), 7.22 (d, J = 8.0 Hz, 2H), 7.14 (d, J = 8.0 Hz, 2H), 7.04 (d, J = 7.6 Hz, 2H), 6.92 (d, J = 7.6 Hz, 2H), 4.18–4.09 (m, 1H), 3.64 (dd, J1 = 4.8 Hz, J2 = 10.0 Hz, 1H), 3.40 (dd, J1 = 6.2 Hz, J2 = 9.8 Hz, 1H), 2.34 (s, 3H), 2.29 (s, 3H), 2.15 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 197.2, 193.5, 144.4, 144.0, 136.8, 135.2, 134.7, 131.5, 129.5, 129.3, 129.0, 128.64, 128.62, 128.5, 37.7, 37.3, 34.7, 31.6, 29.8, 22.7, 21.8, 21.7, 21.1 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H25O2, 369.1849; found: 369.1853.((1R*,2S*,3S*)-3-(4-(tert-butyl)phenyl)cyclopropane-1,2-diyl)bis(p-tolylmethanone) (4i)
White solid. Yield: 832 mg (67%); M. p. 126–128 °C; 1H NMR (400 MHz, CDCl3): δ 7.97 (d, J = 8.0 Hz, 2H), 7.83 (d, J = 8.4 Hz, 2H), 7.23 (d, J = 8.0 Hz, 2H), 7.16–7.14 (m, 4H), 7.10 (d, J = 8.4 Hz, 2H), 4.13–4.10 (m, 1H), 3.66 (dd, J1 = 5.0 Hz, J2 = 9.8 Hz, 1H), 3.39 (dd, J1 = 6.0 Hz, J2 = 10.0 Hz, 1H), 2.35 (s, 3H), 2.30 (s, 3H), 1.16 (s, 9H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 197.2, 193.6, 149.9, 144.4, 144.0, 135.3, 134.7, 129.5, 129.3, 128.6, 128.5, 128.4, 125.2, 37.9, 37.4, 34.4, 31.3, 31.0, 30.0, 21.8, 21.7 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C29H31O2, 411.2319; found: 411.2320.((1R*,2S*,3S*)-3-(4-chlorophenyl)cyclopropane-1,2-diyl)bis(p-tolylmethanone) (4j)
White solid. Yield: 700 mg (60%); M. p. 137–139 °C; 1H NMR (400 MHz, CDCl3): δ 7.97 (d, J = 8.0 Hz, 2H), 7.81 (d, J = 8.4 Hz, 2H), 7.25 (d, J = 8.0 Hz, 2H), 7.19 (s, 2H), 7.10 (s, 4H), 4.09 (dd, J1 = 4.8 Hz, J2 = 6.0 Hz, 1H), 3.67–3.64 (m, 1H), 3.40 (dd, J1 = 6.0 Hz, J2 = 10.0 Hz, 1H), 2.37 (s, 3H), 2.33 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 196.7, 193.1, 144.6, 144.3, 135.0, 134.5, 133.2, 133.0, 130.1, 129.5, 129.4, 128.6, 128.5, 128.45, 128.42, 37.1, 36.8, 29.7, 21.73, 21.68 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H22ClO2, 389.1303; found: 389.1316.((1R*,2S*,3S*)-3-(4-fluorophenyl)cyclopropane-1,2-diyl)bis(p-tolylmethanone) (4k)
White solid. Yield: 680 mg (61%); M. p. 115–117 °C; 1H NMR (400 MHz, CDCl3): δ 7.97 (d, J = 8.0 Hz, 2H), 7.87–7.80 (m, 2H), 7.25 (d, J = 8.0 Hz, 2H), 7.17 (d, J = 8.0 Hz, 2H), 7.14–7.10 (m, 2H), 6.82 (t, J = 8.6 Hz, 2H), 4.10 (t, J = 5.4 Hz, 1H), 3.64 (dd, J1 = 4.6 Hz, J2 = 9.8 Hz, 1H), 3.41 (dd, J1 = 6.2 Hz, J2 = 9.8 Hz, 1H), 2.37 (d, J = 4.4 Hz, 3H), 2.31 (d, J = 7.2 Hz, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 196.8, 193.3, 163.1, 160.7, 144.6, 144.3, 135.1, 134.6, 130.4, 130.3, 130.1, 129.5, 129.4, 129.2, 128.6, 128.5, 128.4, 115.3, 115.1, 37.1, 36.8, 29.9, 21.73, 21.68 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H22FO2; 373.1598; found: 373.1606.((1R*,2S*,3S*)-3-(p-tolyl)cyclopropane-1,2-diyl)bis((4-bromophenyl)methanone) (4l)
White solid. Yield: 861 mg (58%); M. p. 157–159 °C; 1H NMR (400 MHz, CDCl3): δ 7.92 (d, J = 8.4 Hz, 2H), 7.77 (d, J = 8.4 Hz, 2H), 7.59 (d, J = 8.4 Hz, 2H), 7.50 (d, J = 8.8 Hz, 2H), 7.02 (d, J = 8.0 Hz, 2H), 6.94 (d, J = 8.0 Hz, 2H), 4.06 (t, J = 5.4 Hz, 1H), 3.62 (dd, J1 = 4.8 Hz, J2 = 10.0 Hz, 1H), 3.42 (dd, J1 = 6.2 Hz, J2 = 9.8 Hz, 1H), 2.18 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 196.3, 192.7, 137.2, 136.1, 135.8, 132.1, 132.0, 130.8, 130.0, 129.9, 129.1, 128.9, 128.6, 128.4, 38.1, 37.5, 29.7, 21.1 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H19Br2O2, 496.9746; found: 496.9748.((1R*,2S*,3S*)-3-(p-tolyl)cyclopropane-1,2-diyl)bis((4-chlorophenyl)methanone) (4m)
White solid. Yield: 735 mg (60%); M. p. 136–138 °C; 1H NMR (400 MHz, CDCl3): δ 8.13 (d, J = 8.4 Hz, 2H), 7.97 (d, J = 8.4 Hz, 2H), 7.54 (d, J = 8.4 Hz, 2H), 7.46 (d, J = 8.8 Hz, 2H), 7.15 (d, J = 8.4 Hz, 2H), 7.07 (d, J = 8.0 Hz, 2H), 4.21–4.18 (m, 1H), 3.75 (dd, J1 = 4.8 Hz, J2 = 10.0 Hz, 1H), 3.55 (dd, J1 = 6.0 Hz, J2 = 10.0 Hz, 1H), 2.30 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 196.1, 192.5, 140.1, 139.8, 137.2, 135.7, 135.4, 130.8, 129.9, 129.8, 129.2, 129.1, 129.0, 128.5, 38.1, 37.5, 29.7, 21.1 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H19Cl2O2, 409.0757; found: 409.0765.((1R*,2S*,3S*)-3-(naphthalene-1-yl)cyclopropane-1,2 diyl)bis(phenylmethanone) (4n):12c
White solid. Yield: 707 mg (62%); M. p. 121–122 °C; 1H NMR (400 MHz, CDCl3): δ 8.16–8.14 (m, 2H), 8.01 (d, J = 8.0 Hz, 1H), 7.89–7.87 (m, 2H), 7.72–7.65 (m, 2H), 7.60–7.56 (m, 1H), 7.51–7.39 (m, 4H), 7.36–7.30 (m, 4H), 7.29–7.24 (m, 1H), 4.31 (dd, J1 = 4.6 Hz, J2 = 6.2 Hz, 1H), 4.00 (dd, J1 = 4.8 Hz, J2 = 9.6 Hz, 1H), 3.89 (dd, J1 = 6.4 Hz, J2 = 9.6 Hz, 1H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 197.5, 193.6, 137.3, 137.1, 133.7, 133.5, 133.1, 132.7, 130.2, 128.9, 128.7, 128.6, 128.5, 128.4, 128.1, 126.9, 126.1, 125.7, 125.1, 123.7, 36.54, 36.50, 30.8 ppm.((1R*,2S*,3S*)-3-(naphthalene-1-yl)cyclopropane-1,2-diyl)bis(p-tolylmethanone) (4o)
White solid. Yield: 821 mg (67%); M. p. 128–130 °C; 1H NMR (400 MHz, CDCl3): 8.09–8.03 (m, 3H), 7.79 (d, J = 8.0 Hz, 2H), 7.70–7.68 (m, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.38–7.25 (m, 6H), 7.11 (d, J = 8.0 Hz, 2H), 4.27 (dd, J1 = 4.6 Hz, J2 = 6.2 Hz, 1H), 3.95 (dd, J1 = 4.8 Hz, J2 = 9.6 Hz, 1H), 3.82 (m, 1H), 2.37 (s, 3H), 2.28 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 197.1, 193.2, 144.6, 144.0, 135.0, 134.7, 133.6, 132.8, 130.5, 129.6, 129.2, 128.8, 128.7, 128.5, 128.1, 126.9, 126.1, 125.7, 125.1, 123.8, 36.4, 36.3, 30.7, 21.8, 21.7 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C29H25O2, 405.1849; found: 405.1857.((1R*,2S*,3S*)-3-phenylcyclopropane-1,2-diyl)bis(thiophen-2-ylmethanone) (4p)
White solid. Yield: 620 mg (61%); M. p. 146–148 °C; 1H NMR (400 MHz, CDCl3): δ 8.09 (d, J = 3.6 Hz, 1H), 7.93 (d, J = 3.6 Hz, 1H), 7.78 (d, J = 4.8 Hz, 1H), 7.67 (d, J = 4.8 Hz, 1H), 7.29 (d, J = 4.4 Hz, 4H), 7.27–7.24 (m, 2H), 7.20–7.18 (m, 1H), 4.08 (t, J = 5.4 Hz, 1H), 3.75 (dd, J1 = 4.6 Hz, J2 = 9.8 Hz, 1H), 3.54 (dd, J1 = 6.4 Hz, J2 = 10.0 Hz, 1H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 189.6, 186.0, 144.7, 144.2, 134.7, 134.12, 134.10, 130.0, 132.9, 128.6, 128.3, 127.4, 37.6, 36.8, 30.9 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H15O2S2, 339.0508; found: 339.0517.((1R*,2S*,3S*)-3-(p-tolyl)cyclopropane-1,2-diyl)bis(thiophen-2-ylmethanone) (4q)
White solid. Yield: 635 mg (60%); M. p. 156–158 °C; 1H NMR (400 MHz, CDCl3): δ 8.08 (d, J = 3.6 Hz, 1H), 7.92 (d, J = 3.6 Hz, 1H), 7.77 (d, J = 4.8 Hz, 1H), 7.66 (d, J = 5.2 Hz, 1H), 7.27–7.24 (m, 1H), 7.19–7.17 (m, 3H), 7.09 (d, J = 8.0 Hz, 2H), 4.05 (t, J = 5.4 Hz, 1H), 3.73 (dd, J1 = 4.8 Hz, J2 = 9.6 Hz, 1H), 3.51 (dd, J1 = 6.2 Hz, J2 = 9.8 Hz, 1H), 2.32 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 189.7, 186.2, 144.8, 144.2, 137.0, 134.7, 134.0, 133.0, 132.5, 131.0, 129.0, 128.8, 128.5, 128.3, 37.5, 36.8, 31.0, 21.2 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H17O2S2, 353.0664; found: 353.0673.((1R*,2S*,3R*)-3-(thiophene-2-yl)cyclopropane-1,2-diyl)bis(phenylmethanone) (4r)
White solid. Yield: 655 mg (65%); M. p. 116–118 °C; 1H NMR (400 MHz, CDCl3): δ 8.06 (d, J = 7.2 Hz, 2H), 7.94 (d, J = 7.2 Hz, 2H), 7.55 (d, J = 7.6 Hz, 1H), 7.45 (dd, J1 = 7.2 Hz, J2 = 14.8 Hz, 3H), 7.38 (t, J = 7.6 Hz, 2H), 6.99 (d, J = 5.2 Hz, 1H), 6.81 (d, J = 3.2 Hz, 1H), 6.78–6.76 (m, 1H), 4.13 (t, J = 5.4 Hz, 1H), 3.69 (dd, J1 = 5.2 Hz, J2 = 9.6 Hz, 1H), 3.53 (dd, J1 = 6.0 Hz, J2 = 9.6 Hz, 1H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 196.7, 193.2, 137.4, 137.3, 136.9, 133.7, 133.4, 128.8, 128.7, 128.5, 128.4, 126.8, 126.5, 124.7, 37.7, 32.2, 31.5 ppm. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C21H16O2SNa, 355.0763; found: 355.0766.((1R*,2S*,3R*)-3-(thiophene-2-yl)cyclopropane-1,2-diyl)bis(p-tolylmethanone) (4s)
White solid. Yield: 687 mg (63%); M. p. 140–142 °C; 1H NMR (400 MHz, CDCl3): δ 8.08 (d, J = 8.4 Hz, 2H), 7.97 (d, J = 8.4 Hz, 2H), 7.36 (d, J = 8.0 Hz, 2H), 7.31–7.30 (m, 2H), 7.11 (dd, J1 = 1.2 Hz, J2 = 5.2 Hz, 1H), 6.93–6.87 (m, 2H), 4.21 (t, J = 5.6 Hz, 1H), 3.77 (dd, J1 = 5.2 Hz, J2 = 9.6 Hz, 1H), 3.64–3.59 (m, 1H), 2.49 (s, 3H), 2.45 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 196.3, 192.8, 144.6, 144.2, 137.7, 134.9, 134.5, 129.5, 129.4, 128.7, 128.6, 126.8, 126.4, 124.6, 37.5, 31.9, 31.3, 21.8, 21.7 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H21O2S, 361.1257; found: 361.1256.((1R*,2S*,3R*)-3-((E)-styryl)cyclopropane-1,2-diyl)(bis-phenylmethanone) (10a)
Yellow oil. Yield: 686 mg (65%); IR (KBr): v 1658, 1589, 1331, 1215, 1011, 749, 692, 644 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.07 (t, J = 7.4 Hz, 4H), 7.61–7.55 (m, 3H), 7.49 (t, J = 7.4 Hz, 3H), 7.31 (d, J = 7.2 Hz, 2H), 7.27–7.23 (m, 2H), 7.19 (d, J = 7.2 Hz, 1H), 6.68 (d, J = 15.6 Hz, 1H), 6.24 (dd, J1 = 9.6 Hz, J2 = 15.6 Hz, 1H), 3.92 (t, J = 5.2 Hz, 1H), 3.85–3.81 (m, 1H), 3.06–3.00 (m, 1H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 196.7, 195.3, 137.5, 137.1, 136.6, 133.9, 133.54, 133.47, 128.8, 128.7, 128.6, 128.5, 127.7, 126.2, 123.8, 37.9, 35.7, 33.0 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H21O2, 353.1536; found: 353.1547.((1R*,2S*,3S*)-3-((E)-styryl)cyclopropane-1,2-diyl)bis(p-tolylmethanone) (10b)
Yellow oil. Yield: 748 mg (65%); 1H NMR (400 MHz, CDCl3): δ 8.03 (t, J = 7.8 Hz, 4H), 7.37–7.29 (m, 8H), 7.25–7.21 (m, 1H), 6.72 (d, J = 15.6 Hz, 1H), 6.30 (dd, J1 = 9.6 Hz, J2 = 15.6 Hz, 1H), 3.95 (t, J = 5.2 Hz, 1H), 3.85 (dd, J1 = 5.2 Hz, J2 = 9.2 Hz, 1H), 3.09–3.03 (m, 1H), 2.46 (d, J = 7.2 Hz, 6H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 196.4, 194.9, 144.4, 144.3, 136.7, 135.1, 134.6, 133.6, 129.43, 129.40, 128.6, 128.5, 127.5, 126.2, 124.1, 37.6, 35.5, 32.8, 21.73, 21.71 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C27H25O2, 381.1849; found: 381.1866.((1R*,2R*,3S*)-3-phenylcyclopropane-1,2-diyl)bis(phenylmethanone) (5a):12c
White solid. Yield: 108 mg (11%); M. p. 148–150 °C; IR (KBr): v 1678, 1588, 1211, 990, 750, 689 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.05–8.03 (m, 4H), 7.60–7.56 (m, 2H), 7.49–7.41 (m, 6H), 7.36 (d, J = 7.2 Hz, 3H), 3.60 (t, J = 6.0 Hz, 1H), 3.44 (d, J = 6.0 Hz, 2H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 194.3, 138.5, 137.2, 133.2, 128.9, 128.7, 128.6, 128.4, 127.3, 126.6, 36.94, 36.89, 31.3 ppm.((1S*,2S*,3R*,4S*,6S*)-4-hydroxy-2,4,6-triphenylcyclohexane-1,3-diyl)bis(phenylmethanone) (6a)
White solid. Yield: 117 mg (12%); M. p. 188–194 °C; IR (KBr): v 1653, 1588, 1290, 1209, 1013, 732, 693 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.00 (d, J = 7.6 Hz, 1H), 7.85 (d, J = 7.6 Hz, 2H), 7.61–7.47 (m, 3H), 7.34–7.30 (m, 3H), 7.26 (d, J = 7.2 Hz, 2H), 7.23 (d, J = 7.6 Hz, 1H), 7.17–7.09 (m, 8H), 7.05–7.01 (m, 3H), 6.87 (d, J = 7.2 Hz, 2H), 5.78 (d, J = 12.0 Hz, 1H), 5.28 (s, 1H), 4.43 (t, J = 4.4 Hz, 1H), 4.27–4.19 (m, 2H), 3.58–3.38 (m, 1H), 2.11 (dd, J1 = 3.2 Hz, J2 = 13.6 Hz, 1H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 208.3, 206.9, 146.9, 143.9, 141.7, 140.2, 139.3, 138.3, 137.0, 133.1, 132.9, 132.0, 128.8, 128.7, 128.6, 128.4, 128.3, 128.20, 128.19, 128.1, 127.83, 127.76, 127.7, 127.5, 127.4, 127.0, 126.7, 126.6, 125.2, 52.8, 50.2, 48.1, 44.9, 42.3, 38.4, 37.2 ppm. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C38H32O3Na, 559.2244; found: 559.2242.General procedure for the synthesis of 1,3-dienes 8a–s and 1,3,5-trienes 11a–b
To a solution of cyclopropane 4 (0.9 mmol) in MeOH (10 mL), was added NaBH4 (1.8 mmol) and the reaction mixture was stirred at room temperature for 0.5 h. After removal of the solvent under reduced pressure, the reaction mixture was extracted with ethyl acetate and the combined organic layers were washed with brine solution and dried over anhydrous Na2SO4. The removal of solvent under reduced pressure provided the crude cyclopropane alcohol as a mixture of diastereomers. The crude product (0.6 mmol) was then treated with con. HCl (five drops) in 1,2-DCE at room temperature for 0.5 h. The reaction mixture was extracted with DCM. The combined organic layers were washed with water and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and the crude product was purified by column chromatography using EtOAc/hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v) to afford 1,3-dienes 8a–s and 1,3,5-trienes 11a–b.
19 v/v) to afford 1,3-dienes 8a–s and 1,3,5-trienes 11a–b.
(1E,3E)-1, 4-diphenylbuta-1, 3-diene (8a):19
White solid. Yield: 176 mg (89%); M. p. 150–151 °C; IR (KBr, cm−1): v 1659, 1589, 1446, 1331, 1214, 1012, 747, 694, 645 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.36 (d, J = 7.6 Hz, 4H), 7.25 (t, J = 7.8 Hz, 4H), 7.17–7.14 (m, 2H), 6.92–6.84 (m, 2H), 6.63–6.56 (m, 2H) ppm. 13C {1H} NMR (100 MHz, CDCl3): 137.4, 132.9, 129.3, 128.7, 127.6, 126.4, ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C16H15, 207.1168; found: 207.1167 (The minor cyclopropane products 5 also give the same result).1-Methyl-4-((1E,3E)-4-phenylbuta-1, 3-dien-1-yl)benzene (8b)
White solid. Yield: 169 mg (79%); M. p. 152–154 °C; 1H NMR (400 MHz, CDCl3): δ 7.36 (d, J = 7.2 Hz, 2H), 7.26 (t, J = 8.6 Hz, 4H), 7.16 (q, J = 7.2 Hz, 1H), 7.07 (d, J = 7.6 Hz, 2H), 6.91–6.80 (m, 2H), 6.61–6.55 (m, 2H), 2.27 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 137.5, 134.6, 132.9, 132.3, 129.5, 129.4, 128.7, 128.4, 127.5, 126.3, 21.3 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C17H17, 221.1325; found: 221.1317.1-Methoxy-4-((1E,3E)-4-phenylbuta-1, 3-dien-1-yl)benzene (8c)
White solid. Yield: 187 mg (80%); M. p. 164–165 °C; 1H NMR (400 MHz, CDCl3): δ 7.35 (d, J = 7.6 Hz, 2H), 7.30 (d, J = 8.8 Hz, 2H), 7.24 (t, J = 7.6 Hz, 2H), 7.17–7.12 (m, 1H), 6.86 (dd, J1 = 10.4 Hz, J2 = 15.2 Hz, 1H), 6.79 (d, J = 8.4 Hz, 2H), 6.76–6.72 (m, 1H), 6.54 (d, J = 15.2 Hz, 2H), 3.73 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 159.3, 137.6, 132.5, 131.7, 130.2, 129.6, 128.7, 127.7, 127.4, 127.3, 126.3, 114.2, 55.3 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C17H17O, 237.1274; found: 237.1269. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C17H17O, 237.1274; found: 237.1269.1-Bromo-4-((1E,3E)-4-phenylbuta-1, 3-dien-1-yl)benzene (8d):19
White solid. Yield: 218 mg (75%); M. p. 188–190 °C; 1H NMR (400 MHz, CDCl3): δ 7.35 (d, J = 8.4 Hz, 4H), 7.26–7.15 (m, 5H), 6.87–6.80 (m, 2H), 6.63–6.45 (m, 2H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 137.2, 136.3, 133.6, 131.8, 131.5, 130.0, 128.9, 128.8, 127.9, 127.8, 126.5, 121.3 ppm.1-Methyl-4-((1E,3E)-4-phenylbuta-1, 3-dien-1-yl)benzene (8e)
White solid. Yield: 169 mg (82%); M. p. 152–154 °C; 1H NMR (400 MHz, CDCl3): δ 7.36 (d, J = 7.6 Hz, 2H), 7.27–7.23 (m, 4H), 7.17–7.15 (m, 2H), 7.10 (d, J = 8.0 Hz, 2H), 6.91–6.80 (m, 2H), 6.57 (d, J = 14.8 Hz, 1H), 2.27 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 137.54, 137.51, 134.6, 132.9, 132.3, 129.5, 129.4, 129.7, 128.7, 128.3, 127.5, 126.3, 21.3 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C17H17, 221.1325; found: 221.1321.1-Methyl-2-((1E,3E)-4-(p-tolyl)buta-1, 3-dien-1-yl)benzene (8g):20
White solid. Yield: 194 mg (87%); M. p. 105–106 °C; 1H NMR (400 MHz, CDCl3): δ 7.59 (d, J = 7.6 Hz, 1H), 7.40–7.37 (m, 3H), 7.21–7.18 (m, 5H), 6.92–6.90 (m, 2H), 6.69 (d, J = 14.8 Hz, 1H), 2.43 (s, 3H), 2.39 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 137.5, 136.3, 135.5, 134.6, 132.7, 130.51, 130.48, 129.4, 128.7, 127.4, 126.33, 126.27, 126.1, 125.0, 21.3, 19.9 ppm.(1E,3E)-1, 4-di-p-tolylbuta-1,3-diene (8h)
White solid. Yield: 198 mg (88%); M. p. 107–109 °C; 1H NMR (400 MHz, CDCl3): δ 7.38 (d, J = 8.0 Hz, 4H), 7.18 (d, J = 8.0 Hz, 4H), 6.99–6.91 (m, 2H), 6.70–6.63 (m, 2H), 2.39 (s, 6H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 137.4, 134.7, 132.3, 129.4, 128.5, 126.3, 21.3 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H19, 235.1481; found: 235.1486.1-(tert-butyl)-4-((1E,3E)-4-(p-tolyl)buta-1, 3-dien-1-yl)benzene (8i)
White solid. Yield: 201 mg (81%); M. p. 137–139 °C; 1H NMR (400 MHz, CDCl3): δ 7.31–7.24 (m, 6H), 7.05 (d, J = 8.0 Hz, 2H), 6.85–6.81 (m, 2H), 6.57–6.53 (m, 2H), 2.26 (s, 3H), 1.25 (s, 9H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 150.7, 137.4, 134.8, 132.3, 132.2, 129.4, 129.2, 128.8, 128.6, 126.3, 126.1, 126.0, 125.6, 34.7, 31.3, 21.3 ppm. HRMS (ESI-TOF) m/z: [M–Me + H]+ calcd for C21H25, 263.1794; found: 263.1794.1-Chloro-4-((1E,3E)-4-(p-tolyl)buta-1, 3-dien-1-yl)benzene (8j)
White solid. Yield: 188 mg (80%); M. p. 198–200 °C; 1H NMR (400 MHz, CDCl3): δ 7.29–7.26 (m, 4H), 7.23–7.20 (m, 2H), 7.07 (d, J = 8.0 Hz, 2H), 6.87–6.78 (m, 2H), 6.62–6.48 (m, 2H), 2.28 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 137.7, 136.0, 134.5, 133.5, 132.9, 130.8, 130.0, 129.4, 128.8, 128.0, 127.4, 126.4, 21.3 ppm. HRMS (ESI-TOF) m/z: [M]+ calcd for C17H15Cl, 254.0862; found: 254.0891.1-Fluoro-4-((1E,3E)-4-(p-tolyl)buta-1, 3-dien-1-yl)benzene (8k)
White solid. Yield: 187 mg (83%); M. p. 201–203 °C; 1H NMR (400 MHz, CDCl3): δ 7.32 (dd, J1 = 5.6 Hz, J2 = 8.8 Hz, 2H), 7.26 (d, J = 8.0 Hz, 2H), 7.07 (d, J = 8.0 Hz, 2H), 6.95 (t, J = 8.6 Hz, 2H), 6.86–6.75 (m, 2H), 6.60–6.49 (m, 2H), 2.28 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 137.6, 134.5, 132.9, 130.9, 129.4, 129.2, 128.5, 128.1, 127.8, 127.7, 126.33, 126.26, 115.7, 115.5, 21.3 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C17H16F, 239.1231; found: 239.1214.1-Bromo-4-((1E,3E)-4-(p-tolyl)buta-1, 3-dien-1-yl)benzene (8l)
White solid. Yield: 217 mg (72%); M. p. 197–199 °C; 1H NMR (400 MHz, CDCl3): δ 7.49 (d, J = 8.0 Hz, 2H), 7.39 (d, J = 8.0 Hz, 2H), 7.35–7.31 (m, 2H), 7.19 (d, J = 7.6 Hz, 2H), 7.01–6.90 (m, 2H), 6.73–6.59 (m, 2H), 2.40 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 137.8, 136.5, 134.4, 133.6, 131.8, 130.8, 130.2, 129.4, 128.0, 127.8, 126.4, 121.1, 21.3 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C17H16Br, 298.0357; found: 298.0343.1-Chloro-4-((1E,3E)-4-(p-tolyl)buta-1, 3-dien-1-yl)benzene (8m)
White solid. Yield: 203 mg (82%); M. p. 198–200 °C; 1H NMR (400 MHz, CDCl3): δ 7.29–7.25 (m, 4H), 7.22–7.18 (m, 2H), 7.07 (d, J = 7.6 Hz, 2H), 6.87–6.78 (m, 2H), 6.60–6.49 (m, 2H), 2.27 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 137.7, 136.0, 134.4, 133.5, 132.9, 130.8, 130.0, 129.4, 128.8, 128.0, 127.5, 126.4, 21.3 ppm. HRMS (ESI-TOF) m/z: [M]+ calcd for C17H15Cl, 254.0862; found: 254.0848.2-((1E,3E)-4-phenylbuta-1, 3-dien-1-yl)naphthalene (8n)
White solid. Yield: 199 mg (87%); M. p. 155–157 °C. 1H NMR (400 MHz, CDCl3): δ 8.12 (d, J = 8.4 Hz, 1H), 7.80–7.77 (m, 1H), 7.71 (d, J = 8.4 Hz, 1H), 7.66 (d, J = 7.2 Hz, 1H), 7.49–7.38 (m, 7H), 7.30–7.27 (m, 2H), 7.08–6.93 (m, 2H), 6.66 (d, J = 15.2 Hz, 1H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 133.8, 133.1, 132.0, 129.55, 129.46, 128.7, 128.6, 128.0, 127.7, 126.5, 126.1, 125.7, 123.6, 123.3 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H17, 257.1325; found: 257.1309.2-((1E,3E)-4-(p-tolyl)buta-1, 3-dien-1-yl)naphthalene (8o)
White solid. Yield: 208 mg (85%); M. p. 158–160 °C; 1H NMR (400 MHz, CDCl3): δ 8.08 (d, J = 8.0 Hz, 1H), 7.73 (d, J = 8.0 Hz, 1H), 7.65 (d, J = 8.0 Hz, 1H), 7.60 (d, J = 7.2 Hz, 1H), 7.42–7.33 (m, 4H), 7.28 (t, J = 7.6 Hz, 2H), 7.05 (d, J = 7.6 Hz, 2H), 6.96–6.87 (m, 2H), 6.58 (d, J = 14.4 Hz, 1H), 2.25 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 137.7, 134.8, 134.7, 133.9, 133.2, 132.2, 131.2, 129.5, 128.9, 128.72, 128.69, 128.0, 126.5, 126.1, 125.9, 125.8, 123.7, 123.3, 21.4 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H19, 271.1481; found: 271.1471.2-((1E,3E)-4-(p-tolyl)buta-1, 3-dien-1-yl)thiophene (8q)
White solid. Yield: 179 mg (84%); M. p. 166–168 °C; 1H NMR (400 MHz, CDCl3): δ 7.39 (d, J = 8.0 Hz, 2H), 7.22 (t, J = 8.0 Hz, 3H), 7.05 (d, J = 4.8 Hz, 2H), 6.93–6.82 (m, 3H), 6.68 (d, J = 14.8 Hz, 1H), 2.41 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 143.1, 137.6, 134.6, 132.7, 129.5, 129.3, 127.8, 127.7, 126.4, 125.8, 125.1, 124.3, 21.3 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C15H15S, 227.0889; found: 227.0889.2-((1E,3E)-4-phenylbuta-1, 3-dien-1-yl)thiophene (8r)
White solid. Yield: 161 mg (80%); M. p. 161–163 °C. 1H NMR (400 MHz, CDCl3): δ 7.34 (d, J = 7.6 Hz, 2H), 7.25 (t, J = 7.6 Hz, 2H), 7.19–7.13 (m, 2H), 7.09 (d, J = 4.8 Hz, 1H), 6.93–6.89 (m, 2H), 6.84–6.78 (m, 1H), 6.71 (s, 1H), 6.56 (d, J = 15.2 Hz, 1H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 142.9, 137.4, 132.7, 129.1, 128.73, 128.70, 127.7, 127.6, 126.4, 125.9, 125.7, 124.5 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C14H13S, 213.0732; found: 213.0723.2-((1E,3E)-4-(p-tolyl)buta-1, 3-dien-1-yl)thiophene (8s)
White solid. Yield: 189 mg (87%); M. p. 162–164 °C; 1H NMR (400 MHz, CDCl3): δ 7.25 (d, J = 8.0 Hz, 2H), 7.10–7.05 (m, 3H), 6.91–6.89 (m, 2H), 6.80–6.74 (m, 1H), 6.69 (d, J = 7.6 Hz, 2H), 6.54 (d, J = 14.8 Hz, 1H), 2.27 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 143.1, 137.6, 132.7, 129.4, 129.3, 127.8, 127.7, 126.3, 125.7, 125.1, 124.3, 21.3 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C15H15S, 227.0889; found: 227.0885.1-Phenyl-4-((1E,3E,5E)-6-phenylhexa-1, 3, 5-trien-1-yl)benzene (11a):21
Yellow solid. Yield: 182 mg (85%); M. p. 196–198 °C; IR (KBr): v 1655, 1588, 1289, 1209, 1006, 738, 692 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.48 (d, J = 7.6 Hz, 4H), 7.38 (t, J = 7.6 Hz, 5H), 7.31–7.27 (m, 2H), 7.04–6.92 (m, 2H), 6.66 (d, J = 15.6 Hz, 2H), 6.58 (dd, J1 = 2.8 Hz, J2 = 7.2 Hz, 1H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 137.4, 133.6, 132.9, 132.7, 130.13, 129.8, 129.3, 129.2, 128.7, 127.8, 127.6, 126.6, 126.4, 124.3 ppm.1-Methyl-4-((1E,3E,5E)-6-phenylhexa-1, 3, 5-trien-1-yl)benzene (11b)
White solid. Yield: 201 mg (87%); M. p. 186–188 °C; 1H NMR (400 MHz, CDCl3): δ 7.47 (d, J = 7.6 Hz, 2H), 7.39–7.35 (m, 4H), 7.27 (t, J = 7.4 Hz, 1H), 7.18 (d, J = 8.0 Hz, 2H), 6.97–6.86 (m, 2H), 6.65 (d, J = 3.2 Hz, 1H), 6.61 (d, J = 3.2 Hz, 1H), 6.56 (dd, J1 = 4.2 Hz, J2 = 4.6 Hz, 2H), 2.40 (s, 3H) ppm. 13C {1H} NMR (100 MHz, CDCl3): δ 137.53, 137.50, 134.7, 133.8, 133.1, 132.8, 132.4, 129.4, 129.3, 128.7, 128.2, 127.5, 126.4, 126.3, 21.3 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H19, 246.1409; found: 246.1412.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Science and Engineering Research Board (SERB) and MoE-RUSA 2.0, India for financial support and DST-FIST for instrumentation facilities at School of Chemistry, Bharathidasan University.Notes and references
- (a) H. U. Reissig and R. Zimmer, Chem. Rev., 2003, 103, 1151–1196 CrossRef CAS; (b) M. Yu and B. L Pagenkopf, Tetrahedron, 2005, 61, 321–347 CrossRef CAS; (c) M. Y. Melnikov, E. M. Budynina, O. A. Ivanova and I. V. Trushkov, Mendeleev Commun., 2011, 21, 293–301 CrossRef CAS; (d) T. F. Schneider, J. Kaschel and D. B. Werz, Angew. Chem., Int. Ed., 2014, 53, 5504–5523 CrossRef CAS; (e) H. K. Grover, M. R. Emmett and M. A. Kerr, Org. Biomol. Chem., 2015, 13, 655–671 RSC; (f) T. Selvi and K. Srinivasan, Isr. J. Chem., 2016, 56, 454–462 CrossRef CAS; (g) P. Singh, R. K. Varshnaya, R. Dey and P. Banerjee, Adv. Synth. Catal., 2020, 362, 1447–1484 CrossRef CAS; (h) Y. Xia, X. Liu and X. Feng, Angew. Chem., Int. Ed., 2021, 60, 9192–9204 CrossRef CAS; (i) A. Deepthi, C. B. Meenakshy and M. Mohan, Synthesis, 2023, 55, 3875–3894 CrossRef CAS; (j) Donor-Acceptor Cyclopropanes in Organic Synthesis, ed. P. Banerjee and A. T. Biju, Wiley-VCH, Weinheim, 2024 Search PubMed.
- (a) V. K. Yadav and V. Sriramurthy, Angew. Chem., Int. Ed., 2004, 43, 2669–2671 CrossRef CAS; (b) A. T. Parsons and J. S. Johnson, J. Am. Chem. Soc., 2009, 131, 3122–3123 CrossRef CAS PubMed; (c) G. Sathishkannan and K. Srinivasan, Org. Lett., 2011, 13, 6003–6005 CrossRef; (d) S. Chakrabarty, I. Chatterjee, B. Wibbeling, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2014, 53, 5964–5968 CrossRef CAS PubMed; (e) M. A. Zotova, R. A. Novikov, E. V. Shulishov and Y. V. Tomilov, J. Org. Chem., 2018, 83, 8193–8207 CrossRef CAS PubMed; (f) S. Nicolai and J. Waser, Angew. Chem., Int. Ed., 2022, 61, e202209006 CrossRef CAS.
- (a) J. Kaschel, T. F. Schneider, P. Schirmer, C. Maab, D. Stalke and D. B. Werz, Eur. J. Org Chem., 2013, 4539–4551 CrossRef CAS; (b) H. Chen, J. Zhang and D. Z. Wang, Org. Lett., 2015, 17, 2098–2101 CrossRef CAS PubMed; (c) S. Thangamalar, M. Thangamani and K. Srinivasan, Org. Biomol. Chem., 2022, 20, 3145–3153 RSC; (d) S. Jiru, Y. Fu and S. R. Wang, Org. Lett., 2023, 25, 555–559 CrossRef.
- (a) E. M. Budynina, K. L. Ivanov, I. D. Sorokin and M. Y. Melnikov, Synthesis, 2017, 49, 3035–3068 CrossRef CAS; (b) V. Pirenne, B. Muriel and J. Waser, Chem. Rev., 2021, 121, 227–263 CrossRef CAS.
- (a) Y. V. Tomilov, L. G. Menchikov, R. A. Novikov, O. A. Ivanova and I. V. Trushkov, Russ. Chem. Rev., 2018, 87, 201–250 CrossRef CAS; (b) P. Gopinath and S. Chandrasekaran, Curr. Org. Chem., 2019, 23, 276–312 CrossRef CAS.
- (a) J. X. Yang, X. T. Tao, C. X. Yuan, Y. X. Yan, L. Wang, Z. Liu, Y. Ren and M. H. Jiang, J. Am. Chem. Soc., 2005, 127, 3278–3279 CrossRef CAS PubMed; (b) M. B. Boroujeni, A. Hashemzadeh, M. T. Faroughi, A. Shaabani and M. M. Amini, RSC Adv., 2016, 6, 100195–100202 RSC.
- R. Chithiravel, K. Rajaguru, S. Muthusubramanian and N. Bhuvanesh, RSC Adv., 2015, 5, 86414–86420 RSC.
- (a) C. Müller, D. Wasserberg, J. J. Weemers, E. A. Pidko, S. Hoffmann, M. Lutz, A. L. Spek, S. C. Meskers, R. A. Janssen, R. A. van Santen and D. Vogt, Chem.–Eur. J., 2007, 13, 4548–4559 CrossRef; (b) T. T. El-Idreesy, Eur. J. Org Chem., 2012, 4515–4522 CrossRef CAS; (c) J. J. Koh, C. I. Lee, M. A. Ciulei, H. Han, P. K. Bhowmik, V. Kartazaev and S. K. Gayen, J. Mol. Struct., 2018, 1171, 458–465 CrossRef CAS.
- (a) Z. Li, G. Wen, L. He, J. Li, X. Jia and J. Yang, RSC Adv., 2015, 5, 52121–52125 RSC; (b) S. S. Kamble and G. S. Shankarling, ChemistrySelect, 2017, 2, 1917–1924 CrossRef CAS; (c) Z. Yin, C. Xiong, J. Guo, X. Hu, Z. Shan and V. Borovkov, Synlett, 2019, 30, 2143–2147 CrossRef CAS; (d) K. H. Asressu, C. K. Chan and C. C. Wang, ACS Omega, 2021, 6, 7296–7311 CrossRef CAS.
- T. V. Moskovkina, Zh. Org. Khim., 1989, 25, 502–507 CAS.
- (a) G. Sathishkannan and K. Srinivasan, Org. Lett., 2011, 13, 6002–6005 CrossRef CAS; (b) G. Sathishkannan and K. Srinivasan, Adv. Synth. Catal., 2014, 356, 729–735 CrossRef; (c) T. Selvi and K. Srinivasan, J. Org. Chem., 2014, 79, 3653–3658 CrossRef CAS PubMed.
- (a) D. Bhaskar Reddy, B. Venkataramana Reddy, T. Seshamma, N. Subba Reddy and M. V. Ramana Reddy, Synthesis, 1989, 4, 289–290 CrossRef; (b) G. D. McAllister, M. F. Oswald, R. J. Paxton, S. A. Raw and R. J. Taylor, Tetrahedron, 2006, 62, 6681–6694 CrossRef CAS; (c) W. Huang and L. L. Wang, J. Chem. Res., 2013, 37, 380–384 CrossRef CAS; (d) C. Lei, D. Zhu, V. I. T. Tangcueco and J. S. Zhou, Org. Lett., 2019, 21, 5817–5822 CrossRef CAS.
- X. Luo and Z. Shan, Tetrahedron Lett., 2006, 47, 5623–5627 CrossRef CAS.
- (a) A. W. Herriott, E. P. W. M. Olavarria and J. Jones, Org. Chem., 1968, 33, 3804–3808 CrossRef CAS; (b) B. Patro, H. Ila and H. Junjappa, Tetrahedron, 1992, 33, 809–812 CrossRef CAS.
- CCDC Number of 8h: 2338084.
- For synthesis of dienes and polyenes from cyclopropanes, see: (a) M. Thangamani and K. Srinivasan, J. Org. Chem., 2018, 83, 571–577 CrossRef CAS PubMed; (b) M. Mato, C. Carcia-Morales and A. M. Echavarren, ACS Catal., 2020, 10, 3564–3570 CrossRef CAS; (c) M. Garbo and C. Mazet, Org. Lett., 2022, 24, 752–756 CrossRef CAS.
- H. Rodriguez-Solla and R. G. Sonengas, Molecules, 2021, 26, 249 CrossRef.
- (a) F. Y. Zhang and E. J. Corey, Org. Lett., 2001, 3, 639–641 CrossRef CAS PubMed; (b) H. Takahashi, T. Arai and A. Yanagisawa, Synlett, 2006, 17, 2833–2835 Search PubMed; (c) R. K. Sodhi, S. Paul, V. K. Gupta and R. Kant, Tetrahedron Lett., 2015, 56, 1944–1948 CrossRef CAS; (d) Z. Yin, C. Xiong, J. Guo, X. Hu, Z. Shan and V. Borovkov, Synlett, 2019, 30, 2143–2147 CrossRef CAS; (e) B. Liu, J. Wang, Y. Pang, Z. Ge and R. Li, Tetrahedron, 2014, 70, 9240–9244 CrossRef CAS; (f) M. Mato, C. García-Morales and A. M. Echavarren, ACS Catal., 2020, 10, 3564–3570 CrossRef CAS.
- B. C. Ranu and S. Banerjee, Eur. J. Org Chem., 2006, 3012–3015 CrossRef CAS.
- E. Denmark and S. A. Tymonko, J. Am. Chem. Soc., 2005, 127, 8004–8005 CrossRef.
- M. Yamashita, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2010, 12, 592–595 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra of all products and X-ray structural information of 8h. CCDC 2338084. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ra02525c |
| This journal is © The Royal Society of Chemistry 2024 |