Open Access Article
Open Access ArticleEvaluation of ferroelectricity in a distorted wurtzite-type structure of Sc-doped LiGaO2†
Sou Yasuhara *a,
Ayato Nakagawaa,
Kazuki Okamotob,
Takahisa Shiraishib,
Hiroshi Funakubo
*a,
Ayato Nakagawaa,
Kazuki Okamotob,
Takahisa Shiraishib,
Hiroshi Funakubo b,
Shintaro Yasui
b,
Shintaro Yasui cd,
Mitsuru Itoh
cd,
Mitsuru Itoh e,
Takaaki Tsurumia and
Takuya Hoshina
e,
Takaaki Tsurumia and
Takuya Hoshina a
a
aSchool of Materials and Chemical Technology, Tokyo Institute of Technology, 2-12-1 Ookayama, Meguro-ku, Tokyo 152-8550, Japan. E-mail: yasuhara.s.aa@m.titech.ac.jp
bSchool of Materials and Chemical Technology, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, Kanagawa 226-8501, Japan
cLaboratory for Zero-Carbon Energy, Tokyo Institute of Technology, 2-12-1 Ookayama, Meguro-ku, Tokyo 152-8550, Japan
dLaboratory for Materials and Structures, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, Kanagawa 226-8503, Japan
eOffice of Campus Management, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, Kanagawa 226-8501, Japan
First published on 2nd May 2024
Abstract
Since the discovery of ferroelectricity in a wurtzite-type structure, this structural type has gathered much attention as a next-generation ferroelectric material due to its high polarization value combined with its high breakdown strength. However, the main targets of wurtzite-type ferroelectrics have been limited thus far to simple nitride/oxide compounds. The investigation of new ferroelectric materials with wurtzite-type structures is important for understanding ferroelectricity in such structures. We therefore focus on β-LiGaO2 in this study. Although AlN and ZnO possess well-known wurtzite-type structures (P63mc), β-LiGaO2 has a distorted wurtzite-type structure (Pna21), and there are no reports of ferroelectricity in LiGaO2. In this study, we have revealed that LiGaO2 exhibits relatively high barrier height energy for polarization switching, however, Sc doping effectively reduces that energy. Then, we conducted thin film preparation and evaluation for Sc-doped LiGaO2 to observe its ferroelectric properties. We successfully observed ferroelectric behavior by using piezoresponse force microscopy measurements for LiGa0.8Sc0.2O2/SrRuO3/(111)SrTiO3.
Wurtzite-type ferroelectrics have attracted much attention since the discovery of Sc-doped AlN exhibiting a high polarization value of over 100 μC cm−2.1 The wurtzite-type structure was well known as a pyroelectric material due to its high energy barrier for polarization switching.2–4 In this structure, all cations occupy tetrahedral sites and pass through the oxygen-stuffed layer during polarization switching.5,6 Ferroelectric behaviors have been reported thus far in Sc-doped AlN,1,7 B-doped AlN,8,9 Sc-doped GaN,10,11 and Mg-doped ZnO.12 Chemical doping into a wurtzite-type structure reduces the barrier energy of polarization switching, resulting in ferroelectricity. In the case of Sc-doped AlN, the polarization switching barrier energy was calculated as 0.12 eV.13 In previous reports, strain effects for Sc-doped AlN,14,15 doping level dependence in Sc-doped AlN16 and ferroelectricity predictions of other new wurtzite-type material such as LaN,17 Mg2XN3 (X = Sb, Ta and Nb)18 and other complex materials19 are demonstrated by using first-principles calculations. Although wurtzite-type ferroelectric materials are candidates for next-generation ferroelectric materials, ferroelectric observations in such structures have been limited to simple oxides/nitrides with chemical doping due to the relatively high barrier energy for polarization switching.20 The limited selectivity of doping elements is attributed to the composition of simple oxides/nitrides, which consist of divalent/trivalent cations. In addition, there are limited options for new simple oxides/nitrides to be considered as ferroelectric materials.4,17–19 Therefore, we have tried to investigate complex oxide materials with wurtzite-type related structures.
We focus on β-LiGaO2, which exhibits a distorted wurtzite-type structure. The crystal structure of β-LiGaO2 falls within the orthorhombic system with a space group of Pna21, and spontaneous polarization takes place along the c-axis.21,22 A single crystal growth of β-LiGaO2 was reported to serve as a substrate on which to deposit GaN thin films.23,24 Due to the difference in ionic radii between Li+ and Ga3+,25 the tetrahedra are distorted compared to those in the wurtzite-type structure. Moreover, the selectivity in doping elements is improved because of the presence of monovalent and trivalent cations in the structure. NaGaO2, AgGaO2, and CuGaO2 are reported to possess the same structure as β-LiGaO2.26–28 However, it is difficult to treat NaGaO2 in air due to its high deliquescence. Besides, the synthesis of AgGaO2 and CuGaO2 requires NaGaO2 as a starting material. Therefore, we decided to investigate the ferroelectricity in β-LiGaO2. In this study, we carried out first-principles calculation and thin film preparation to evaluate a polarization switching of LiGaO2 system. First, we revealed that non-doped LiGaO2 showed relatively high barrier height energy for the polarization switching. Then, we carried out calculation to evaluate the effect of Ga-site substitution on LiGaO2. The results suggested that Sc-doping effectively reduces that energy. Finally, we prepared Sc-doped LiGaO2 epitaxial thin films and uncovered the probability of ferroelectricity using piezoresponse force microscopy (PFM).
We carried out density functional theory (DFT) calculations using the projector augmented wave (PAW) method29 as implemented in the VASP30,31 for structural optimizations. We utilized the modified Perdew–Burke–Ernzerhof generalized gradient approximation (PBEsol-GGA)32 as the exchange–correlation functional in our calculations. The k-point mesh was 8 × 8 × 8. The cutoff energy and convergence energy were 550 eV and 1.0 × 10−7 eV, respectively. Born effective charges were calculated using density functional perturbation theory (DFPT). The spontaneous polarization value was estimated as the product of Born effective charges and atomic displacements. The calculated crystal structure is depicted by using VESTA software.33
All thin films were fabricated via a pulsed laser deposition method with a fourth harmonic wavelength of Nd:YAG. First, we prepared a ceramics target of Li(Ga1−xScx)O2 (x = 0, 0.05, 0.15, 0.20). The starting materials of Li2CO3, Ga2O3, and Sc2O3 were weighed through stoichiometry and well ground with an agate pestle and mortar. The powder was calcined at 740 °C for 12 h. After the calcination, the powder was pelletized by using a cold isostatic press of 200 MPa, and the prepared pellets were sintered at 1100 °C for 2 h. We then deposited LiGaO2 film onto (111)SrTiO3 using the sintered target. The deposition conditions were a substrate temperature of 500 °C and an oxygen partial pressure of 10 mTorr. The crystal structure of prepared thin films was evaluated using HR-XRD (Smartlab, Rigaku). For the evaluation of ferroelectricity, a conductive SrRuO3 was deposited as a bottom electrode. The SrRuO3 film was deposited at the substrate temperature of 650 °C and oxygen partial pressure of 50 mTorr. The ferroelectricity of the prepared films was evaluated using PFM (MFP-3D, Asylum Research).
The crystal structure of β-LiGaO2 (Pna21) is shown in Fig. 1a. Using this polar structure as the reference, we created an intermediate structure representing polarization switching, where all cations moved in the [00−1] direction and occupied five-coordinate sites.3,14,16 Fig. 1b and c show the crystal structures of the polar and intermediate states, respectively, after the structural optimization. The difference in final energy per formula unit between the polar and intermediate structures is 0.64 eV per f.u. This value is quite high compared with the 0.35 eV per f.u. in the Sc-doped AlN cases,13 indicating that it is hard to observe ferroelectricity in non-doped LiGaO2. We then focused on Ga3+ site substitution by trivalent cations (Al3+, B3+, Sc3+). These three elements were selected in a viewpoint of ionic radii. The calculated unit cell has four Ga3+ sites. Replacing one Ga3+ site corresponds to a 25% doping level. The calculated structures with 25% Ga replaced LiGaO2 by Al, B, and Sc are shown in Fig. S1.† The barrier height energies of Al-, B-, and Sc-doped LiGaO2 are 0.55, 1.84, and 0.21 eV per f.u., respectively. These results suggest that Sc doping is particularly effective at reducing the barrier height energy for polarization switching.
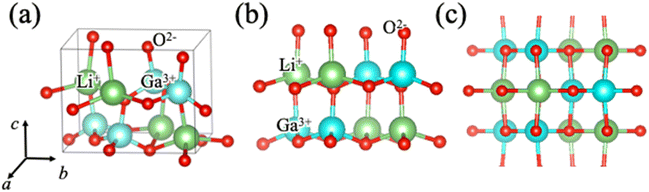 | ||
| Fig. 1 (a) Schematic illustration of LiGaO2 crystal structure. (b) The initial state of polarization switching and (c) the intermediate state of polarization switching in LiGaO2. | ||
We investigated the dependence of the Sc amount in the LiGaO2 structure by using first-principles calculations. The crystal parameters after the structural optimization with Sc-doped LiGaO2 (Sc-doped level: 0%, 25%, 50%, 75%, and 100%) are shown in Table 1, as are the average u-parameters. The u-parameter is the position of a cation-stuffed plane relative to an anion-stuffed plane along the polarization axis (c-axis) of a wurtzite-type structure. In a simple wurtzite-type structure, the structure is polar when the u-parameter is less than 0.5. In the case of a complex oxide with a wurtzite-type structure, there are more than two kinds of cations; thus, the cation-stuffed plane is not flat. We then evaluated the average u-parameters calculated for each cation site. The calculation results suggest that the u-parameters of the non-doped and 25% Sc-doped LiGaO2 are less than 0.5. On the other hand, the u-parameters remain stable at 0.5 with Sc contents exceeding 50% in Sc-doped LiGaO2. This result is attributed to the intermediate state of the polarization switching being more stable than the polar state. This result indicates that the intermediate state became stable as the Sc content increased in Sc-doped LiGaO2. The same phenomenon was reported in Sc-doped AlN, where 50% Sc-doped AlN shows almost zero polarization value.14 Besides, the c-axis length decreased with increasing Sc content in Sc-doped LiGaO2. The polarization values calculated by using the DFPT method are 91.4 μC cm−2 (non-doped LiGaO2) and 86.1 μC cm−2 (LiGa0.75Sc0.25O2). The predicted polarization value is comparable to that in 90.0 μC cm−2 for ZnO,5 and 90.6 μC cm−2 for Mg2NbN3.18 Then, we have tried to prepare LiGaO2 epitaxial thin films via a pulsed laser deposition method.
| Sc0% | Sc25% | Sc50% | Sc75% | Sc100% | |
|---|---|---|---|---|---|
| a (Å) | 5.394 | 5.509 | 5.922 | 6.022 | 6.131 |
| b (Å) | 6.373 | 6.444 | 6.962 | 7.044 | 7.104 |
| c (Å) | 5.022 | 5.007 | 4.171 | 4.184 | 4.196 |
| u (a.u.) | 0.382 | 0.386 | 0.5 | 0.5 | 0.5 |
Fig. 2a shows the out-of-plane XRD results for the prepared thin films. β-LiGaO2 (Pna21) 002 and 004 peaks were observed along the SrTiO3[111] without any impurity peaks. This result indicates that the β-LiGaO2 (Pna21)-oriented film is grown on the (111)SrTiO3 substrate along the c-axis. Fig. 2b shows the results of phi-scan measurements on LiGaO2 011 and SrTiO3 110 to examine the crystal structure in the in-plane direction. Although a three-fold rotation symmetry is observed in SrTiO3 110, a six-fold rotation is observed in LiGaO2 011 with a 30° peak shift from the SrTiO3 110 peaks. These results indicate that the in-plane relationship is LiGaO2[010]//SrTiO3[10−1], and the observed six-fold rotation symmetry of LiGaO2 011 appears to have originated from the three-fold rotation of (111)SrTiO3. The result of 2D reciprocal space mapping of prepared LiGaO2/(111)SrTiO3 thin film is shown in Fig. S2.† The relationship of crystal orientation between (001)LiGaO2 and (111)SrTiO3 was depicted in Fig. S3.† Along SrTiO3 [10−1] direction, LiGaO2 is well matched with a lattice mismatch of 2.7%. The relationship of LiGaO2/(111)SrTiO3 is in good agreement with that of ZnO/(111)SrTiO3,34 although the space groups of ZnO is different with that of LiGaO2 whereas the atomic alignment is same. There results indicated that (001)LiGaO2/(111)SrTiO3 epitaxial thin films were obtained. For electric measurements, LiGaO2/SrRuO3/(111)SrTiO3 thin films were prepared by using SrRuO3 as a bottom electrode. However, no ferroelectric behavior was observed via PFM (Fig. S4†) and P–E hysteresis measurements due to the relatively high barrier height energy of polarization switching in non-doped LiGaO2. Therefore, we focused on Sc doping to Ga3+ sites in LiGaO2.
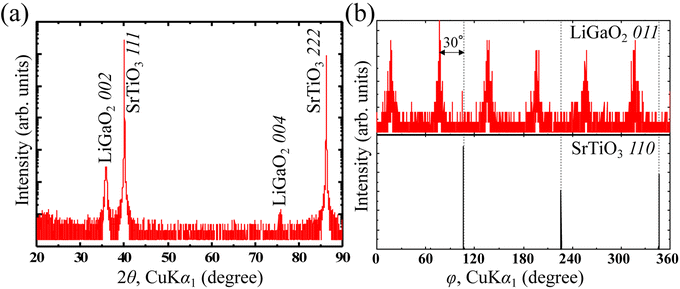 | ||
| Fig. 2 (a) Out-of-plane XRD profiles of LiGaO2/(111)SrTiO3. (b) Phi-scan XRD patterns of LiGaO2 011 and SrTiO3 110. | ||
Li(Ga1−xScx)O2 (x = 0.05, 0.15, 0.20) ceramics targets were sintered by a conventional solid state reaction to prepare Sc-doped LiGaO2 thin films. The XRD results of the prepared thin films are shown in Fig. 3a. LiGaO2 002 and SrRuO3 111c/222c peaks are observed along the out-of-plane direction, the same as in the non-doped LiGaO2 epitaxial thin film. The c-axis length calculated from the out-of-plane XRD results are plotted in Fig. 3b. The calculated c-axis value is also shown in Fig. 3b. The c-axis length monotonically decreased as Sc content increased in Sc-doped LiGaO2, indicating that Sc-doped LiGaO2 epitaxial thin films were obtained. The result also suggests that the solution limit of Sc into LiGaO2 epitaxial thin film is above 20%.
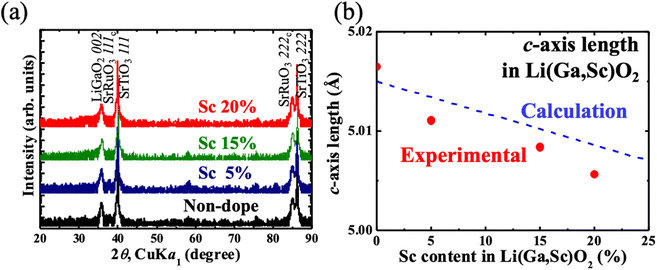 | ||
| Fig. 3 (a) Out-of-plane XRD profiles of LiGa1−xScxO2/(111)SrTiO3 (x = 0, 0.05, 0.15, 0.20). (b) The c-axis lengths of prepared thin films with calculated values (blue dashed line). | ||
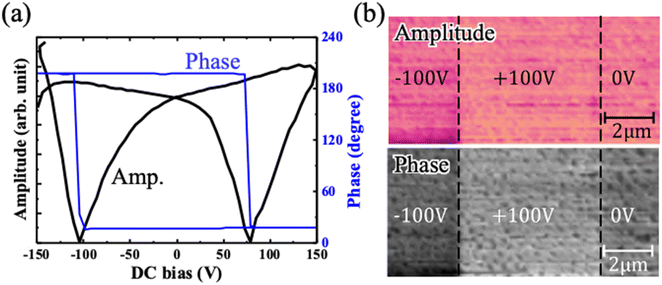
Finally, PFM results of LiGa0.8Sc0.2O2/SrRuO3/(111)SrTiO3 are shown in Fig. 4a. We can clearly see a ferroelectric butterfly-shape curve of the amplitude signal and a 180° phase hysteresis curve. Additional PFM results measured in several points are shown in Fig. S5.† The result of non-doped LiGaO2 epitaxial thin film is also shown in Fig. S4,† in which no ferroelectric behavior is observed. The results of written PFM images of phase and amplitude shown in Fig. 4b, which reflects a pre-imposed voltage before measurements. These findings suggest that the prepared thin film exhibits the potential for ferroelectric behavior. The frequency dependence of dielectric constant and dielectric loss of prepared sample is shown in Fig. S6.† The dielectric loss is less than 0.1 at 1 kHz. Unfortunately, we could not see a hysteresis loop in the P–E measurements because the prepared films were leaky during measurements applying high voltage. In our future studies, we intend to enhance the thin film quality by optimizing growth conditions. This will enable us to observe a P–E hysteresis loop, facilitating a direct evaluation of ferroelectricity in Sc-doped LiGaO2.
We investigated ferroelectricity in LiGaO2 by using calculation and preparation/evaluation of epitaxial thin films. We carried out first-principles calculations by using the VASP code for LiGaO2 and Sc-doped LiGaO2. The calculated results suggested that Sc doping reduces the barrier height energy of polarization switching in Sc-doped LiGaO2. Then, we have started to prepare LiGaO2 epitaxial thin film, and revealed that LiGaO2 was epitaxially grown on a (111)SrTiO3 substrate by a pulsed laser deposition method. The growth relationships between LiGaO2 (Pna21) and the SrTiO3 substrate are follows: (001)LiGaO2//(111)SrTiO3 and (010)LiGaO2//(10−1)SrTiO3. In this study, the pure LiGaO2 does not show any ferroelectric behavior by using P–E measurements and PFM. We then prepared Sc-doped LiGaO2 epitaxial thin films. We successfully observed ferroelectric behavior via PFM measurement for LiGa0.8Sc0.2O2/SrRuO3/(111)SrTiO3.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was partially supported by JSPS KAKENHI Grants-in-Aid for Research Activity Start-up (So. Y., 20K22549) and for Early-Career Scientists (So. Y., 22K14470), and by the Murata Science Foundation.References
- S. Fichtner, N. Wolff, F. Lofink, L. Kienle and B. Wagner, J. Appl. Phys., 2019, 125, 114103 CrossRef.
- A. Konishi, T. Ogawa, C. A. J. Fisher, A. Kuwabara, T. Shimizu, S. Yasui, M. Itoh and H. Moriwake, Appl. Phys. Lett., 2016, 109, 102903 CrossRef.
- Z. Liu, X. Wang, X. Ma, Y. Yang and D. Wu, Appl. Phys. Lett., 2023, 122, 122901 CrossRef CAS.
- H. Moriwake, R. Yokoi, A. Taguchi, T. Ogawa, C. A. J. Fisher, A. Kuwabara, Y. Sato, T. Shimizu, Y. Hamasaki, H. Takashima and M. Itoh, APL Mater., 2020, 8, 121102 CrossRef CAS.
- H. Moriwake, A. Konishi, T. Ogawa, K. Fujimura, C. A. J. Fisher, A. Kuwabara, T. Shimizu, S. Yasui and M. Itoh, Appl. Phys. Lett., 2014, 104, 242909 CrossRef.
- L. Li and M. Wu, ACS Nano, 2017, 11, 6382–6388 CrossRef CAS PubMed.
- K. H. Ye, G. Han, I. W. Yeu, C. S. Hwang and J.-H. Choi, Phys. Status Solidi RRL, 2021, 15, 2100009 CrossRef CAS.
- W. Zhu, J. Hayden, F. He, J.-I. Yang, P. Tipsawat, M. D. Hossain, J.-P. Maria and S. Trolier-Mckinstry, Appl. Phys. Lett., 2021, 119, 062901 CrossRef CAS.
- J. Hayden, M. D. Hossain, Y. Xiong, K. Ferri, W. Zhu, M. V. Imperatore, N. Giebink, S. Trolier-McKinstry, I. Dabo and J.-P. Maria, Phys. Rev. Mater., 2021, 5, 044412 CrossRef CAS.
- D. Wang, P. Wang, B. Wang and Z. Mi, Appl. Phys. Lett., 2021, 119, 11902 CrossRef.
- M. Uehara, R. Mizutani, S. Yasuoka, T. Shiraichi, T. Shimizu, H. Yamada, M. Akiyama and H. Funakubo, Appl. Phys. Lett., 2021, 119, 172901 CrossRef CAS.
- K. Ferri, S. S. Bachu, W. Zhu, M. Imperatore, J. Hayden, N. Alem, N. Giebink, S. Trolier-McKinstry and J.-P. Maria, J. Appl. Phys., 2021, 130, 044101 CrossRef CAS.
- H. Wang, N. Adamski, S. Mu and C. G. Van de Walle, J. Appl. Phys., 2021, 130, 104101 CrossRef CAS.
- S. Clima, C. Pashartis, J. Bizindavyi, S. R. C. McMitchell, M. Houssa, J. V. Houdt and G. Pourtois, Appl. Phys. Lett., 2021, 119, 172905 CrossRef CAS.
- D. F. Urban, O. Ambacher and C. Elsässer, Phys. Rev. B, 2021, 103, 115204 CrossRef CAS.
- K. Furuta, K. Hirata, S. A. Anggraini, M. Akiyama, M. Uehara and H. Yamada, J. Appl. Phys., 2021, 130, 024104 CrossRef CAS.
- A. J. E. Rowberg, S. Mu, M. W. Swift and C. G. Van de Walle, Phys. Rev. Mater., 2021, 5, 094602 CrossRef CAS.
- X.-Y. Chen, J.-L. Yang, L.-F. Chen, H.-K. Xu, J.-M. Chen, G.-X. Lai, X.-F. Xu, H. Ji, J.-J. Tang and Y.-J. Zhao, Phys. Chem. Chem. Phys., 2022, 24, 29570–29578 RSC.
- C.-W. Lee, N. U. Din, K. Yazawa, G. L Brennecka, A. Zakutayev and P. Gorai, ChemRxiv, 2023, preprint, DOI:10.26434/chemrxiv-2023-hf60w.
- Z. Liu, X. Wang, X. Ma, Y. Yang and D. Wu, Appl. Phys. Lett., 2023, 122, 122901 CrossRef CAS.
- M. Marezio, Acta Crystallogr., 1965, 18, 481 CrossRef CAS.
- C. A. Lenyk, M. S. Holston, B. E. Kananen, L. E. Halliburton and N. C. Giles, J. Appl. Phys., 2018, 124, 135702 CrossRef.
- C. Chen, C.-A. Li, S.-H. Yu and M. M. C. Chou, J. Cryst. Growth, 2014, 402, 325–329 CrossRef CAS.
- T. Ishii, Y. Tazoh and S. Miyazawa, J. Cryst. Growth, 1998, 186, 409–419 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., 1976, A32, 751–767 CrossRef CAS.
- I. Suzuki, A. Kakinuma, M. Uead and T. Omata, J. Cryst. Growth, 2018, 504, 26–30 CrossRef CAS.
- H. Nagatani, I. Suzuki, S. Takemura, T. Ohsawa, N. Ohashi, S. Fujimoto and T. Omata, AIP Adv., 2018, 8, 085203 CrossRef.
- I. Suzuki, H. Nagatani, M. Kita and T. Omata, Appl. Phys. Express, 2017, 10, 095501 CrossRef.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- J. P. Perdew, A. Ruzsinszky, G. I. Csonka, O. A. Vydrov, G. E. Scuseria, L. A. Constantin, X. Zhou and K. Burke, Phys. Rev. Lett., 2008, 100, 136406 CrossRef PubMed.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- Y. Zhan, J. Li, Y. Yin, W. Zhang and C. Jia, RSC Adv., 2019, 8, 37668–37674 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra02296c |
| This journal is © The Royal Society of Chemistry 2024 |