Open Access Article
Open Access ArticleOne-step hydrothermal synthesis of a Ni3S2–FeMoO4 nanowire–nanosheet heterostructure array for synergistically boosted oxygen evolution reaction†
Han Chen‡
ab,
Jing Zhang‡bc,
Rui Wanbc,
Xiang Zhangbc,
Qijun Panbc,
Mingtao Li*d and
Bin Chen *bc
*bc
aInstitutes of Physical Science and Information Technology, School of Materials Science and Engineering, Anhui University, Hefei 230601, P. R. China
bKey Laboratory of Materials Physics, Anhui Key Laboratory of Nanomaterials and Nanotechnology, Institute of Solid State Physics, HFIPS, Chinese Academy of Sciences, 350 Shushanhu Road, Hefei, Anhui 230031, P. R. China. E-mail: bchen@issp.ac.cn
cUniversity of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, P. R. China
dSchool of Mechanical and Resource Engineering, Wuzhou University, Wuzhou 543002, China. E-mail: mtli@gxuwz.edu.cn
First published on 18th March 2024
Abstract
A key factor for boosting oxygen evolution reaction (OER) is the design of heterostructures with steerable defects and interfaces, which can optimize the surface electronic structures and achieve efficient water splitting to produce hydrogen fuel. Herein, we propose a novel one-step hydrothermal approach to fabricate hierarchical Ni3S2 nanowires with an S-doped FeMoO4 nanosheet heterostructure array in situ on Ni–Fe foam (NFF) as a self-standing electrode for synergistically boosted OER. The metalloid Ni3S2 nanowires with good conductivity support the FeMoO4 nanosheets and act as high-speed paths for the charge transfer. Numerous ultrathin S-doped FeMoO4 nanosheets are uniformly distributed on each Ni3S2 nanowire to form heterostructures with larger specific surface area and more revealable active sites, and a strong synergistic effect is created at the heterostructure interfaces to further promote the OER dynamics. Additionally, the NFF serves as the conductive support substrate and simultaneously provides the Ni and Fe sources for the self-growing Ni3S2–FeMoO4, leading to a structurally-integrated electrode with low contact resistance, fast mass transfer, and good stability. Therefore, the Ni3S2–FeMoO4/NFF electrode offers a low overpotential of 331 mV to achieve 500 mA cm−2 and long-term stability at 100 mA cm−2 level for more than 40 h. This work provides insight into the heterostructure of molybdate and sulfide, and a deep understanding of the significance of the synergism in OER operation.
Regarding the escalating energy crises, there is an urgent need to explore and develop clean and efficient energy conversion technologies.1 Electrochemical water splitting driven by renewable electricity has been widely regarded as an effective strategy to produce carbon-free hydrogen. The overall efficiency of water electrolysis is greatly hindered by the oxygen evolution reaction (OER) owing to its sluggish four-proton-couple electron transfer progress.2 Electrocatalysts can significantly decrease the energy barrier and accelerate OER operation. The benchmark electrocatalysts rely heavily on iridium and ruthenium-based oxides. However, their large-scale application is unfortunately limited due to their resource scarcity and expensive cost.3 Therefore, this issue inspires interest in exploring abundant non-precious metal electrocatalysts.
Currently, transition metal-based materials, including layered double hydroxides,4 oxides,5–7 phosphides,8 and sulfides,9–11 have been widely studied as alternative electrocatalysts. Among them, Ni3S2 has attracted increasing attention due to its high electrical conductivity and unique metallic feature.12 However, the relatively low intrinsic activity and poor stability of Ni3S2 limit the industrial application for water electrolysis. Numerous efforts have been devoted to heterostructure engineering by combining Ni3S2 with other active materials to further promote the OER activity.13 Recently, Mo-based oxides have been combined with Ni3S2 to obtain heterostructures, which could form strong synergistic effect to promote the OER activity and the electrochemical durability.14 For example, hierarchical MoOx/MoS2 decorated NiOx/Ni3S2 nanorods have been prepared by a hydrothermal reaction and surface reconfiguration strategy, which exhibits a remarkable OER performance and excellent stability.15 Nevertheless, these Ni3S2-based heterostructures were mostly fabricated by complex multi-steps of preparing Ni3S2 support matrix and then synthesizing the active materials on Ni3S2. Unfortunately, little attention has been paid to the remarkable achievement of obtaining heterostructures of nanowires/nanosheets through a one-step method. On the other hand, it is difficult to accurately control the heterostructure engineering due to the unsuitable lattice matching, resulting in limited interface and weak synergistic effect. Thus, the electrocatalytic activity of these Ni3S2-based heterostructures are still insufficient for industrial application that needs to be operated at a high current density more than 500 mA cm−2. Therefore, it is urgently desired to explore a rational and straightforward synthesis strategy to construct novel Ni3S2-based heterostructures for realizing industrial application of water electrolysis.
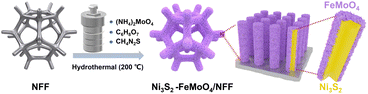
Herein, we design and fabricate a novel hierarchical heterostructure of Ni3S2 nanowires coated with S-doped FeMoO4 nanosheets in situ on nickel-iron foam (denoted as Ni3S2–FeMoO4/NFF) by an effective one-step hydrothermal strategy, which obviously reduced the difficulty of preparation and saved time out (Fig. 1). The Ni–Fe foam (NFF) not only serves as the conductive support substrate, but also simultaneously provides the Ni and Fe sources for the self-growing Ni3S2–FeMoO4 heterostructures, leading to a structurally-integrated electrode with low contact resistance and good mechanical stability. Importantly, the S-doped FeMoO4 ultrathin nanosheets have been successfully integrated with Ni3S2 nanowires to form heterostructures. In addition, the 3D hierarchical, self-supported, and binder-free nanowire–nanosheet architectural morphology endows the obtained electrode with abundant active sites, rapid bubbles release, and timely electrolyte diffusion. As a result, the Ni3S2–FeMoO4 heterostructures array electrode demonstrates a superb OER performance with ultralow potential and good long-time stability.
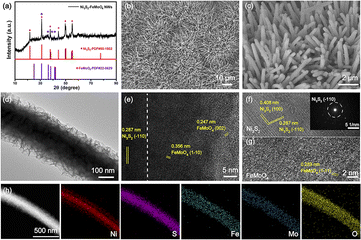
The typical Ni3S2–FeMoO4 nanowire–nanosheet array electrode was in situ synthesized on the NFF by a facile one-step hydrothermal method for 10 h. Scanning electron microscopy (SEM) shows that the pristine NFF consists of numerous interconnected skeletons with porous surface (Fig. S1†). XRD pattern reveals that three prominent characteristic peaks are assignable to the metallic NFF (PDF#38-0419), while the weak diffraction peaks adopted to Ni3S2 (PDF#85-1802) and FeMoO4 (PDF#22-0629) can be observed, indicating the Ni3S2–FeMoO4 is successfully grown on NFF (Fig. S2†). To eliminate the influence of NFF, XRD patterns of the catalysts powder peeled from NFF were employed to characterize the crystalline phases. As depicted in Fig. 2a, the distinct and sharp peaks can be assigned to the corresponding crystal planes of Ni3S2 and the other peaks are assigned to FeMoO4. SEM images unveil that the Ni3S2–FeMoO4 has a 3D coral-like nanowire architecture with average length and diameter of 15 μm and 300 nm, respectively (Fig. 2b and c). Transmission electron microscopy (TEM) in Fig. 2d illustrates numerous ultrathin nanosheets are uniformly distributed on each nanowire to form core–shell heterostructures. The high-resolution TEM (HRTEM) image obviously shows the heterointerface, where the left crystalline region is the nanowire and the right low-crystallinity region is the nanosheet (Fig. 2e). Notably, the obtained lattice fringes in left region exhibit the lattice distance of 0.287 nm, well aligned with the (−1 1 0) plane of Ni3S2, whereas the interplanar spacing of 0.247 nm and 0.356 nm correspond to the (0 0 2) and (1 −1 0) planes of FeMoO4 in right region. The enlarged HRTEM images further confirm that the hierarchical Ni3S2–FeMoO4 core–shell heterostructures have been successfully prepared (Fig. 2f and g). Additionally, the selected area electron diffraction (SAED) agrees well with Ni3S2, indicating the low crystallinity of FeMoO4 nanosheets. High-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) image reveals the Ni3S2–FeMoO4 more clearly (Fig. 2h). The corresponding elemental mapping images show the Ni and S elements predominantly localize in the core nanowire region, while Mo, O, and Fe elements are mainly distributed along the outer nanosheets region with slight S element, which further elucidates the compositional distribution of Ni3S2 nanowire and S-doped FeMoO4 nanosheet core–shell structure.
X-ray photoelectron spectroscopy (XPS) explored the elemental valence and electronic interactions on the Ni3S2–FeMoO4/NFF. The XPS full spectrum further demonstrates that Ni, Fe, Mo, O, and S are present in Ni3S2–FeMoO4 (Fig. S3a†). The Ni 2p spectrum is fitted in Fig. S3b,† the characteristic peaks at 855.5 eV (2p3/2) and 873.08 eV (2p1/2) are assigned to Ni2+, respectively, while the peaks at 856.61 eV (2p3/2) and 874.53 eV (2p1/2) are assigned to Ni3+. The S 2p XPS spectrum is shown in Fig. S3c,† and the peaks observed at 169.18 eV and 168.02 eV are attributed to the S 2p1/2 and 2p3/2 bimodal peaks, respectively, which are indicative of the sulfite (SO32−) species due to surface oxidation in air. Similarly, the peaks at 163.03 eV and 161.78 eV are assigned to the disulfide (S22−) species. It is shown that the reaction of S element with Ni produces S metallized metal.16 In Fig. S3d and e,† the Fe 2p and Mo 3d spectra further confirm the nanosheets are FeMoO4. In addition, O 1s XPS spectrum in Fig. S3f† consists of three characteristic peaks of metal–oxygen bond (530.14 eV), hydroxides bond (530.77 eV) and oxygen vacancy (531.55 eV), indicating the incorporation of S in the FeMoO4.17 All these results demonstrate the Ni3S2–FeMoO4 nanowire–nanosheet array successfully grow on the NFF by one-step hydrothermal method.
To explore the evolutionary process detail of morphology, the XRD patterns and SEM images of Ni3S2–FeMoO4 with different hydrothermal treatment times were displayed in Fig. S4 and S5,† respectively. The XRD results demonstrate that the electrocatalyst crystallized after 10 h. The morphology started from smooth and bulky nanowires structure for 4 h (Fig. S5a and b†). As the preparation time increased to 10 h, the coral-like structures of nanowires coated with numerous nanosheets could be observed on the NFF (Fig. S5c and d†). When the hydrothermal time further reach to 16 h, the nanowire–nanosheet structures continued to grow and became longer with a smaller diameter, ultimately resulting in collapsed network (Fig. S5e and f†). In addition, a contrast sample was prepared by the same condition except for using Ni foam (NF), which was denoted as NiMo-OS/NF.18 The SEM images of NiMo-OS/NF (Fig. S6†) display that numerous thin nanosheets were distributed across the NF substrate without nanowire–nanosheet structures. As shown in Fig. S7,† the XRD pattern reveals only three diffraction peaks for NF(PDF#70-0908), and no obvious peaks of Ni3S2 and FeMoO4 could be observed. This result verifies the Fe element in NFF played a vital role in the growth of Ni3S2–FeMoO4 nanowire–nanosheet heterostructures.
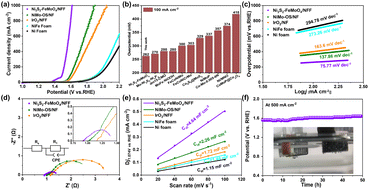
The OER performance was evaluated in 1 M KOH electrolyte, based on a traditional three-electrode system. Linear scan voltammetry (LSV) curves were recorded for Ni3S2–FeMoO4/NFF, NiMo-OS/NF, IrO2, NiFe foam, and Ni foam (Fig. 3a). Specifically, Ni3S2–FeMoO4/NFF electrode exhibits optimal OER activity, and offers ultralow overpotentials of only 262 mV and 331 mV to achieve 100 mA cm−2 and 500 mA cm−2, respectively. These performance metrics are notably superior to those of NiMo-OS/NF (366 mV and 513 mV) and IrO2/NFF (399 mV and 587 mV) to afford the same current densities. More importantly, a high current density of 1000 mA cm−2 can be achieved at a low overpotential of 382 mV for the Ni3S2–FeMoO4/NFF. Notably, the overpotential of Ni3S2–FeMoO4/NFF is obviously lower than most recently reported relevant electrocatalysts (Fig. 3b).19–28 Furthermore, the OER activity can be optimized by varying the hydrothermal time as detailed in Fig. S8.† The results show that the Ni3S2–FeMoO4/NFF for 10 h exhibits superior catalytic properties, which reveals that the unique nanowire–nanosheet heterostructure with larger surface area and more reactive sites could significantly improve the OER activity.
To further understand the OER kinetics, Tafel measurements were carried out. As shown in Fig. 3c, in a large current density region (near 100 mA cm−2), the Ni3S2–FeMoO4/NFF electrode offers a much lower Tafel slope of 75.77 mV dec−1 than those of NiMo-OS/NF (137.86 mV dec−1) and IrO2/NFF (163.6 mV dec−1), demonstrating the heterointerface of FeMoO4 nanosheets and Ni3S2 nanowires acquire the rapid OER dynamics. Additionally, electrochemical impedance spectroscopy (EIS) was measured to evaluate the charge transfer kinetics of these electrodes. As displayed in Fig. 3d, the Ni3S2–FeMoO4/NFF electrode possesses a smaller charge transfer resistance (Rct) value of 0.5 Ω than those of NiMo-OS/NF (1.5 Ω) and IrO2/NFF (2.2 Ω). Such obviously reduced Rct value shows the superior interfacial interactions were produced at the heterostructure interfaces, contributing to greatly promote the OER catalytic kinetics.29 Moreover, the Ni3S2–FeMoO4/NFF electrode shows a relatively lower series resistance (Rs) than other electrodes, indicating its excellent conductivity and reduced contact resistance (Fig. 3d inset). These results also reveal the nanocrystalline Ni3S2 nanowires with good conductivity not only support the FeMoO4 nanosheets but also serve as the high-speed path for charge transfer, which further improves the charge transfer efficiency.
In addition, electrochemical surface area (ECSA) was evaluated by the double layer capacitances (Cdl), which was obtained from the cyclic voltammetry (CV) at different scan rates (Fig. S9†). As illustrated in Fig. 3e, the Ni3S2–FeMoO4/NFF electrode shows a larger Cdl (4.64 mF cm−2) than NiMo-OS/NF (2.26 mF cm−2) and NFF (1.48 mF cm−2), indicating that the larger ECSA can be attributed to the 3D hierarchical nanowire–nanosheet heterostructures array, which is conducive to the adsorption of water molecules and the close contact with the electrolyte.30 Fig. S10† reveals the ECSA normalized LSV curve of Ni3S2–FeMoO4/NFF still possess the smallest overpotential value, demonstrating the Ni3S2–FeMoO4 heterostructure effectively enhances the intrinsic OER activity. We also measured the ECSA normalized LSV curve of Ni3S2–FeMoO4/NFF before and after OER stability test to evaluate the intrinsic OER activity for Ni3S2–FeMoO4/NFF. As observed in Fig. S11,† the value of Cdl becomes higher due to the dissolution of Mo and more active sites in Fig. S11c.† The ECSA normalized LSV curve of Ni3S2–FeMoO4/NFF shows that even the electrochemical active surface area increases after cycle stability test, the normalized current density trend remains consistent with that of Fig. S11d,† indicating the OER activity of Ni3S2–FeMoO4/NFF is not affected by the electrochemical active surface area.31 The electrochemical stability is indispensable for evaluating the OER catalytic performance. The chronopotentiometry measurements in Fig. S12† and 3f show that the Ni3S2–FeMoO4/NFF electrode almost remains a constant potential at 100 mA cm−2, and only a slight potential change even at 500 mA cm−2 for continuous test, demonstrating a remarkable OER stability in large current densities. In addition, it can be seen that the produced bubbles were very small and could timely detach from the Ni3S2–FeMoO4/NFF electrode into the electrolyte in Fig. 3f (inset).32 Thus the 3D self-supported nanowire–nanosheet architectural morphology endows the obtained electrode with abundant exposed active sites, rapid bubbles release, and timely electrolyte diffusion.
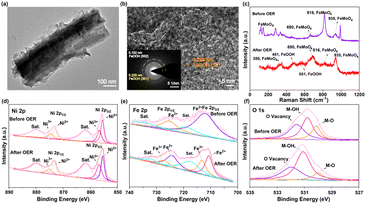
In order to further investigate the real active phase of Ni3S2–FeMoO4/NFF, the variation of structure, composition, and valence state were analysed after OER test. The SEM of Ni3S2–FeMoO4/NFF shows the hierarchical nanowire–nanosheet morphology is maintained well after OER test, demonstrating its excellent structural stability and corrosion resistance (Fig. S13†). As shown in Fig. S14,† the XRD spectra before and after OER test show that the characteristic peaks well index to Ni3S2 and no peak for FeMoO4 is observed, implying the S-doped FeMoO4 may reconstruct to amorphous phase after OER process. Meanwhile, the TEM image of Ni3S2–FeMoO4 post-OER uncovers that the density of nanosheets slightly decreases (Fig. 4a). As shown in Fig. 4b, the observed interplanar spacing of 0.229 nm is well index to the (3 0 1) plane of FeOOH, indicating the FeMoO4 surface was reconstructed to FeOOH after OER operation. In particular, the elemental mapping images (Fig. S15†) indicate that the Mo reduces while the Fe and O still distribute along the outer side, which agrees with the results of TEM/EDX in Table S1,† further certifying the surface reconstruction of FeMoO4. The Raman spectra of Ni3S2–FeMoO4/NFF before and after OER process were performed in Fig. 4c. Compared to the pre-electrode, the new Raman peaks at 481 cm−1 and 551 cm−1 could be coincided with FeOOH,33 indicating the presence of FeOOH after OER process. As observed in Fig. 4d and S16a,† the binding energies of Ni 2p and S 2p spectra have no apparent shift. Moreover, the Fe XPS spectrum shows the new peaks at 713.22 eV and 727.17 eV correspond to Fe3+ 2p3/2 and Fe3+ 2p1/2, respectively, further demonstrating that the part of Fe2+ are oxidized to Fe3+ (Fig. 4e). Besides, the Mo 3d XPS spectrum (Fig. S16b†) shows that the characteristic peak strength was weakened after OER test, indicating the loss of Mo and the formation of cationic vacancy, which can facilitate the formation of amorphous oxyhydroxide as highly active sites for OER. The O 1s spectrum shows the more concentration of oxygen vacancies (Fig. 4f), which can promote OER process.
In summary, we present a novel one-step hydrothermal strategy to fabricate hierarchical Ni3S2–FeMoO4 nanowire–nanosheet heterostructures array in situ on the NFF substrate. Numerous ultrathin S-doped FeMoO4 nanosheets are uniformly distributed on each Ni3S2 nanowire to form heterostructures, which offer a multitude of active sites, and leverage the multi-component interfaces to generate a synergistic effect for OER enhancement. The S-doped FeMoO4 facilitates the formation of oxygen vacancy and surface reconstruction. Therefore, the self-standing and binder-free Ni3S2–FeMoO4/NFF electrode delivers ultralow overpotentials of 262 mV and 331 mV to afford 100 and 500 mA cm−2, respectively, as well as good long-term durability at large current density of 500 mA cm−2 for 50 h. Overall, this study demonstrates that the heterostructured Ni3S2–FeMoO4/NFF markedly improves OER performance and open a novel approach for monolithic electrode preparation through autogenous in situ growth.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 52102324), the Hefei Institutes of Physical Science, Chinese Academy of Sciences Director's Fund (Grants No. BJPY2023B04, YZJJ-GGZX-2022-01, and YZJJ202102), and the Guangxi Natural Science Foundation (No. 2022GXNSFDA080002).References
- H. Ding, H. F. Liu, W. S. Chu, C. Z. Wu and Y. Xie, Chem. Rev., 2021, 121, 13174–13212 CrossRef CAS PubMed.
- H. A. Sun, X. M. Xu, H. Kim, W. Jung, W. Zhou and Z. P. Shao, Energy Environ. Mater., 2023, 6, e12441 CrossRef CAS.
- H. Y. Zhong, Q. Zhang, J. C. Yu, X. Zhang, C. Wu, Y. F. Ma, H. An, H. Wang, J. Zhang, X. P. Wang and J. M. Xue, Adv. Energy Mater., 2023, 13, 2301391 CrossRef CAS.
- B. Chen, Z. Zhang, S. Kim, M. Baek, D. Kim and K. Yong, Appl. Catal., B, 2019, 259(9), 118017 CrossRef CAS.
- J. Zhang, Y. Ye, B. Wei, F. Hu, L. T. Sui, H. W. Xiao, L. Q. Gui, J. Sun, B. B. He and L. Zhao, Appl. Catal., B, 2023, 330, 122661 CrossRef CAS.
- S. Hirai, T. Ohno, R. Uemura, T. Maruyama, M. Furunaka, R. Fukunaga, W. T. Chen, H. Suzuki, T. Matsuda and S. Yagi, J. Mater. Chem. A, 2019, 7, 15387–15394 RSC.
- S. Hirai, S. Yagi, W. T. Chen, F. C. Chou, N. Okazaki, T. Ohno, H. Suzuki and T. Matsuda, Adv. Sci., 2017, 4, 1700176 CrossRef PubMed.
- B. Chen, D. Kim, Z. Zhang, M. Lee and K. Yong, Chem. Eng. J., 2021, 422, 130533 CrossRef CAS.
- Y. Y. Chen, Y. Xu, S. Niu, J. Yan, Y. Y. Wu, F. K. Du, Y. Z. Zhao, Z. R. Zhu, Z. J. Jiang and X. C. Tan, Chem. Commun., 2021, 57, 4572–4575 RSC.
- J. F. Zhang, Y. C. Hu, D. L. Liu, Y. Yu and B. Zhang, Adv. Sci., 2017, 4, 1600343 CrossRef.
- L. H. Li, S. Hagiwara, C. Jiang, H. Kusaka, N. Watanabe, T. Fujita, F. Kuroda, A. Yamamoto, M. Miyakawa, T. Taniguchi, H. Hosono, M. Otani and T. Kondo, Chem. Eng. J., 2023, 471, 144489 CrossRef CAS.
- L. L. Feng, G. T. Yu, Y. Y. Wu, G. D. Li, H. Li, Y. H. Sun, T. Asefa, W. Chen and X. X. Zou, J. Am. Chem. Soc., 2015, 137, 14023–14026 CrossRef CAS.
- T. S. Song, H. Xue, J. Sun, N. K. Guo, J. W. Sun, Y. R. Hao and Q. Wang, Chem. Commun., 2022, 58, 9874–9877 RSC.
- J. L. Zhu, J. M. Qian, X. B. Peng, B. R. Xia and D. Q. Gao, Nano-Micro Lett., 2023, 15, 30 CrossRef CAS PubMed.
- P. L. Zhai, Y. X. Zhang, Y. Z. Wu, J. F. Gao, B. Zhang, S. Y. Cao, Y. T. Zhang, Z. W. Li, L. C. Sun and J. G. Hou, Nat. Commun., 2020, 11, 5462 CrossRef CAS PubMed.
- H. X. Wang, J. S. Ren, A. J. Wang, Q. Wang, W. Zhao and L. Zhao, Chem. Commun., 2022, 58, 9202–9205 RSC.
- F. L. Wang, X. Y. Zhang, J. C. Zhou, Z. N. Shi, B. Dong, J. Y. Xie, Y. W. Dong, J. F. Yu and Y. M. Chai, Inorg. Chem. Front., 2022, 9, 2068–2080 RSC.
- S. Chen, Z. Xiang, Z. Xiao, K.-P. Wang, Q. Zhang and L. Wang, Nano Res., 2023, 16, 6922–6932 CrossRef CAS.
- Y. J. Mei, Y. B. Feng, C. X. Zhang, Y. Zhang, Q. L. Qi and J. Hu, ACS Catal., 2022, 12, 10808–10817 CrossRef CAS.
- Y. S. Jin, S. L. Huang, X. Yue, H. Y. Du and P. K. Shen, ACS Catal., 2018, 8, 2359–2363 CrossRef CAS.
- H. Yin, H. Xiao, R. Qin, J. Chen, F. Tan, W. Zhang, J. Zhao, L. Zeng, Y. Hu, F. Pan, P. Lei, S. Yuan, L. Qian, Y. Su and Z. Zhang, ACS Appl. Mater. Interfaces, 2023, 15, 20100–20109 CrossRef CAS.
- X. Luo, P. X. Ji, P. Y. Wang, R. L. Cheng, D. Chen, C. Lin, J. A. Zhang, J. W. He, Z. H. Shi, N. Li, S. Q. Xiao and S. C. Mu, Adv. Energy Mater., 2020, 10, 1903891 CrossRef CAS.
- Y. Hao, G. Du, Y. Fan, L. Jia, D. Han, W. Zhao, Q. Su, S. Ding and B. Xu, ACS Appl. Mater. Interfaces, 2021, 13, 55263–55271 CrossRef CAS.
- X. Zhang, A. Wu, D. Wang, Y. Jiao, H. Yan, C. Jin, Y. Xie and C. Tian, Appl. Catal., B, 2023, 328, 122474 CrossRef CAS.
- V. Maheskumar, A. Min, C. J. Moon, R. A. Senthil and M. Y. Choi, Small Struct., 2023, 4, 2300212 CrossRef CAS.
- H. Roh, H. Jung, H. Choi, J. W. Han, T. Park, S. Kim and K. Yong, Appl. Catal., B, 2021, 297, 120434 CrossRef CAS.
- L. Yi, S. Xiao, Y. Wei, D. Li, R. Wang, S. Guo and W. Hu, Chem. Eng. J., 2023, 469, 144015 CrossRef CAS.
- M. Wang, Z. Wan, X. Meng, Z. Li, X. Ding, P. Li, C. Li, J.-G. Wang and Z. Li, Appl. Catal., B, 2022, 309, 121272 CrossRef CAS.
- W. H. Huang, X. M. Li, X. F. Yang, H. B. Zhang, F. Wang and J. Zhang, Chem. Commun., 2021, 57, 4847–4850 RSC.
- Q. Liu, L. S. Xie, Z. Liu, G. Du, A. M. Asiri and X. P. Sun, Chem. Commun., 2017, 53, 12446–12449 RSC.
- S. Hirai, S. Yagi, H. C. Oh, Y. Sato, W. Liu, E. P. Liu, W. T. Chen, A. Miura, M. Nagao, T. Ohno and T. Matsuda, RSC Adv., 2022, 12, 24427–24438 RSC.
- N. N. Zhou, R. Liu, X. W. Wu, Y. L. Ding, X. Zhang, S. Liang, C. H. Deng, G. C. Qin, Z. L. Huang and B. Chen, J. Power Sources, 2023, 574, 233163 CrossRef CAS.
- Y. Li, Y. Y. Wu, M. K. Yuan, H. R. Hao, Z. Lv, L. L. Xu and B. Wei, Appl. Catal., B, 2022, 318, 121825 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra01770f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |