Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis, biological evaluation, and molecular docking of novel ferulic acid derivatives containing a 1,3,4-oxadiazole thioether and trifluoromethyl pyrimidine skeleton†
Jiansong Ana,
Nianjuan Pana,
Chunyi Liua,
Haijiang Chena,
Qiang Feia,
Xiuhai Gan b and
Wenneng Wu
b and
Wenneng Wu *a
*a
aSchool of Food Science and Engineering, Guiyang University, Guiyang 550005, China. E-mail: wuwenneng123@126.com
bNational Key Laboratory of Green Pesticide, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University, Guiyang 550025, China
First published on 20th May 2024
Abstract
In this study, 24 novel ferulic acid derivatives containing 1,3,4-oxadiazole thioether and trifluoromethyl pyrimidine were designed and synthesized. Bioactivity assay showed that some of the target compounds exhibited moderate to good antifungal activity against Botryosphaeria dothidea BD), Phomopsis sp. (PS), Botrytis cinerea (BC), Fusarium spp. (FS), Fusarium graminearum (FG), and Colletotrichum sp. (CS). Especially, compound 6f demonstrated superior antifungal activity against Phomopsis sp., with an EC50 value of 12.64 μg mL−1, outperforming pyrimethanil (35.16 μg mL−1) and hymexazol (27.01 μg mL−1). Meanwhile, compound 6p showed strong antibacterial activity against X. axonopodis pv. citri (XAC) in vitro, with an inhibition ratio of 85.76%, which was higher than thiodiazole copper's 76.59% at 100 μg mL−1. Furthermore, molecular docking simulations elucidated that compound 6f engaged in hydrogen bonding with the succinate dehydrogenase (SDH) enzyme at SER-17, SER-39, ARG-14 and ARG-43 sites, clarifying its mode of action. This study highlights the potential of these novel ferulic acid derivatives as promising agents for controlling fungal and bacterial threats to plant health. To the best of our knowledge, this study represents the first report on the antifungal and antibacterial properties of ferulic acid derivatives containing 1,3,4-oxadiazole thioether and trifluoromethyl pyrimidine skeleton.
1. Introduction
Microbe-related diseases have posed a persistent threat to the healthy growth of crops, as well as to the overall quality and safety of agricultural produce.1 While pesticides remain a crucial component in the arsenal against these diseases in modern farming practices, their indiscriminate and prolonged use has given rise to two significant challenges.2–4 Firstly, it has led to the rapid development of resistance among pathogens, rendering many pesticides less effective. Secondly, the environmental pollution caused by these chemicals has become a cause for serious concern. In light of these issues, the quest for novel, highly effective, and environmentally benign antimicrobes has become an urgent priority.Natural products are not only diverse and bioactive, but also have a unique mechanism of action, easy degradation and good environmental compatibility, which has made them important precursors for the development of chemical pesticides.5–8 Among them, ferulic acid is a natural plant metabolite that is usually found in Ferula assa-foetida L., Ligusticum chuanxiong Hort., Catalpa ovata G. Don9 and so on. Ferulic acid is a phenylpropanoid natural product containing a phenolic hydroxyl group and has a great variety of pharmacological activities, such as herbicidal,10,11 fungicidal,12,13 antiviral,14,15 and bactericidal16–18 activities. Therefore, ferulic acid derivatives are a class of lead compounds designed and synthesized for new pesticides.
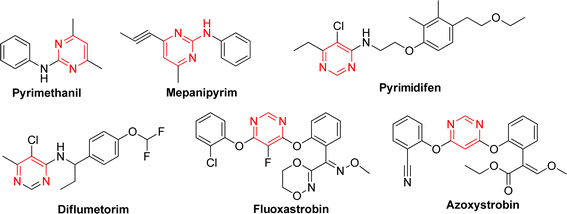
Heterocyclic compounds have developed rapidly due to their high efficiency, low toxicity and broad-spectrum biological activity. Those compounds were not only widely used in the medical field, able to treat various diseases, but also in other fields such as agriculture, chemical industry and other applications. Among them, 1,3,4-oxadiazole stands out for its remarkable activity, selectivity, and low toxicity. In particularly, their thioether derivatives have found widespread applications in both medicine and pest control. 1,3,4-Oxadiazole thioether exhibits a broad spectrum of bioactivities, including insecticidal,19,20 fungicidal,21,22 herbicidal,23 antibacterial,24,25 and antiviral26 properties, as documented in various studies. In addition, pyrimidine was a crucial class of substances that play a significant role in various life activities, being prevalent in both human and other biological organisms. Due to their versatility, pyrimidine compounds found extensive applications in medicine and pesticides, with certain compounds even evolving into commercially viable fungicides (Fig. 1). Notably in recent years, a significant amount of studies had shown that pyrimidine derivatives have antifungal,22,27 antibacterial,28 insecticidal,29 herbicidal,30 antiviral31 properties, and so on. Especially trifluoromethyl pyrimidine derivatives exhibited excellent antifungal activity in our previous research.32–38
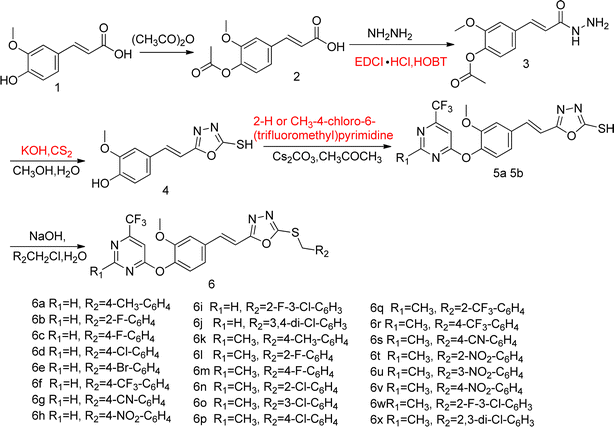
Therefore, it is anticipated that the amalgamation of natural product ferulic acid with 1,3,4-oxadiazole thioether and pyrimidine moieties could lead to developing potent and eco-friendly antifungal and antibacterial agents. Currently, a range of ferulic acid derivatives incorporating 1,3,4-oxadiazole thioether and trifluoromethyl pyrimidine frameworks (Fig. 2) have been devised and synthesized. Subsequently, their antifungal and antibacterial efficacies were being assessed.
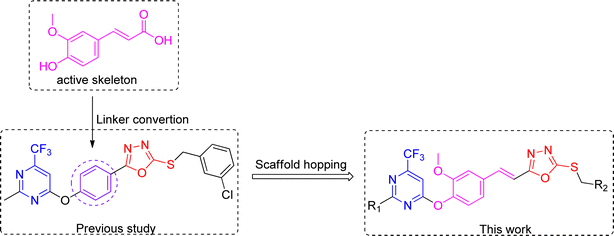 | ||
| Fig. 2 Molecular design for ferulic acid derivatives containing 1,3,4-oxadiazole thioether and trifluoromethyl pyrimidine rings. | ||
2. Materials and methods
2.1. Instruments and chemicals
Chemical reagents were sourced from Aladdin Reagent and Energy Chemical, both located in Shanghai, China. The melting points were measured with the aid of an XT-4 binocular microscope (Shanghai Electrophysics Optical Instrument Co., LTD.) NMR spectra, including 1H and 13C, were acquired on a Bruker 600 spectrometer. The title compounds were analyzed using a Thermo Scientific Q-Exactive instrument to acquire high-resolution mass spectrometry (HRMS) data.2.2. Chemical synthesis
(E)-4-(2-(5-Mercapto-1,3,4-oxadiazol-2-yl)vinyl)-2-methoxyphenol (4). White solid; yield 80.5%; m.p. 219.2–220.4 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.05 (s, Ph–OH), 7.48 (d, 1H, J = 16.20 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.31 (s, 1H, Ph–H), 7.08 (d, 1H, J = 7.60 Hz, Ph–H), 7.08 (d, 1H, J = 16.20 Hz, –CH
CH–), 7.31 (s, 1H, Ph–H), 7.08 (d, 1H, J = 7.60 Hz, Ph–H), 7.08 (d, 1H, J = 16.20 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.84 (s, 1H, J = 7.45 Hz, Ph–H), 3.79 (s, 3H, CH3O–).
CH–), 6.84 (s, 1H, J = 7.45 Hz, Ph–H), 3.79 (s, 3H, CH3O–).
(E)-5-(3-Methoxy-4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazole-2-thiol (5a). White solid; yield 48.1%; m.p. 172.4–174.1 °C; 1H NMR (600 MHz, DMSO-d6) δ: 8.93 (s, 1H, pyrimidine-H), 7.82 (s, 1H, pyrimidine-H), 7.52 (d, 1H, J = 16.64 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.30 (d, 1H, J = 1.56 Hz, Ph–H), 7.08 (dd, 1H, J1 = 8.16 Hz, J2 = 1.56 Hz, Ph–H), 7.06 (d, 1H, J = 16.64 Hz, –CH
CH–), 7.30 (d, 1H, J = 1.56 Hz, Ph–H), 7.08 (dd, 1H, J1 = 8.16 Hz, J2 = 1.56 Hz, Ph–H), 7.06 (d, 1H, J = 16.64 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.84 (s, 1H, J = 8.1 Hz, Ph–H), 3.80 (s, 3H, CH3O–).
CH–), 6.84 (s, 1H, J = 8.1 Hz, Ph–H), 3.80 (s, 3H, CH3O–).
(E)-5-(3-Methoxy-4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazole-2-thiol (5b). White solid; yield 50.8%; m.p. 160.7–163.5 °C; 1H NMR (600 MHz, DMSO-d6) δ: 7.62 (s, 1H, pyrimidine-H), 7.54 (d, 1H, J = 16.32 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.30 (d, 1H, J = 1.56 Hz, Ph–H), 7.08 (dd, 1H, J1 = 8.16 Hz, J2 = 1.56 Hz, Ph–H), 7.04 (d, 1H, J = 16.38 Hz, –CH
CH–), 7.30 (d, 1H, J = 1.56 Hz, Ph–H), 7.08 (dd, 1H, J1 = 8.16 Hz, J2 = 1.56 Hz, Ph–H), 7.04 (d, 1H, J = 16.38 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.84 (s, 1H, J = 8.1 Hz, Ph–H), 3.86 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–).
CH–), 6.84 (s, 1H, J = 8.1 Hz, Ph–H), 3.86 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–).
(E)-2-(3-Methoxy-4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-5-((4-methylbenzyl)thio)-1,3,4-oxadiazole (6a). White solid; yield 60.8%; m.p. 120.4–122.5 °C; 1H NMR (DMSO-d6, 600 MHz) δ: 8.95 (s, 1H, pyrimidine-H), 7.84 (s, 1H, pyrimidine-H), 7.67 (d, 1H, J = 1.2 Hz, Ph–H), 7.59 (d, 1H, J = 16.8 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.44 (d, 1H, J = 16.8 Hz, –CH
CH–), 7.44 (d, 1H, J = 16.8 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.41 (d, 1H, J = 1.2 Hz, Ph–H), 7.38 (d, 2H, J = 7.8 Hz, Ph–H), 7.33 (d, 1H, J = 8.4 Hz, Ph–H), 7.17 (d, 1H, J = 7.8 Hz, Ph–H), 4.54 (s, 2H, –CH2–), 3.79 (s, 3H, CH3O–), 2.28 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.25, 165.66, 163.21, 159.82, 156.15 (q, J = 35.55 Hz), 151.62, 141.85, 138.50, 137.62, 134.62, 133.80, 129.64, 129.46, 123.52, 122.01, 121.80 (q, J = 273.3 Hz), 112.25, 110.61, 106.10, 56.55, 36.18, 21.18. HRMS (ESI) calcd for C24H19F3N4O3SNa [M + Na]+: 523.10052, found: 523.10222.
CH–), 7.41 (d, 1H, J = 1.2 Hz, Ph–H), 7.38 (d, 2H, J = 7.8 Hz, Ph–H), 7.33 (d, 1H, J = 8.4 Hz, Ph–H), 7.17 (d, 1H, J = 7.8 Hz, Ph–H), 4.54 (s, 2H, –CH2–), 3.79 (s, 3H, CH3O–), 2.28 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.25, 165.66, 163.21, 159.82, 156.15 (q, J = 35.55 Hz), 151.62, 141.85, 138.50, 137.62, 134.62, 133.80, 129.64, 129.46, 123.52, 122.01, 121.80 (q, J = 273.3 Hz), 112.25, 110.61, 106.10, 56.55, 36.18, 21.18. HRMS (ESI) calcd for C24H19F3N4O3SNa [M + Na]+: 523.10052, found: 523.10222.
(E)-2-((2-Fluorobenzyl)thio)-5-(3-methoxy-4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazole (6b). White solid; yield 50.4%; m.p. 101.3–103.7 °C; 1H NMR (DMSO-d6, 600 MHz) δ: 8.95 (s, 1H, pyrimidine-H), 7.83 (s, 1H, pyrimidine-H), 7.67 (d, 1H, J = 1.2 Hz, Ph–H), 7.59 (d, 1H, J = 16.8 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.57 (s, 1H, Ph–H), 7.45 (d, 1H, J = 16.2 Hz, –CH
CH–), 7.57 (s, 1H, Ph–H), 7.45 (d, 1H, J = 16.2 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.41–7.37 (m, 2H, Ph–H), 7.38 (d, 2H, J = 7.8 Hz, Ph–H), 7.33 (d, 1H, J = 8.4 Hz, Ph–H), 7.26 (t, 1H, J = 9.0 Hz, Ph–H), 7.22 (t, 1H, J = 7.8 Hz, Ph–H), 4.60 (s, 2H, –CH2–), 3.80 (s, 3H, CH3O–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.24, 165.90, 162.66, 161.71 (d, J = 244.95 Hz), 159.81, 156.16 (q, J = 35.4 Hz), 151.63, 141.89, 138.64, 134.57, 131.96 (d, J = 2.85 Hz), 130.79 (d, J = 7.8 Hz), 125.10 (d, J = 3.0 Hz), 123.98 (d, J = 14.55 Hz), 123.51, 122.06, 121.80 (q, J = 273.0 Hz), 116.08 (d, J = 20.85 Hz), 112.21 (d, J = 243.45 Hz), 106.09, 56.54, 30.27. HRMS (ESI) calcd for C23H16F4N4O3SNa [M + Na]+: 527.07611, found: 527.07715.
CH–), 7.41–7.37 (m, 2H, Ph–H), 7.38 (d, 2H, J = 7.8 Hz, Ph–H), 7.33 (d, 1H, J = 8.4 Hz, Ph–H), 7.26 (t, 1H, J = 9.0 Hz, Ph–H), 7.22 (t, 1H, J = 7.8 Hz, Ph–H), 4.60 (s, 2H, –CH2–), 3.80 (s, 3H, CH3O–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.24, 165.90, 162.66, 161.71 (d, J = 244.95 Hz), 159.81, 156.16 (q, J = 35.4 Hz), 151.63, 141.89, 138.64, 134.57, 131.96 (d, J = 2.85 Hz), 130.79 (d, J = 7.8 Hz), 125.10 (d, J = 3.0 Hz), 123.98 (d, J = 14.55 Hz), 123.51, 122.06, 121.80 (q, J = 273.0 Hz), 116.08 (d, J = 20.85 Hz), 112.21 (d, J = 243.45 Hz), 106.09, 56.54, 30.27. HRMS (ESI) calcd for C23H16F4N4O3SNa [M + Na]+: 527.07611, found: 527.07715.
(E)-2-((4-Fluorobenzyl)thio)-5-(3-methoxy-4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazole (6c). White solid; yield 72.8%; m.p. 110.2–112.1 °C; 1H NMR (CDCl3-d, 600 MHz) δ: 8.87 (s, 1H, pyrimidine-H), 7.48–7.44 (m, 3H, Ph–H and –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.34 (s, 1H, Ph–H), 7.22 (d, 3H, J = 7.52 Hz, Ph–H and pyrimidine-H), 7.08 (t, 2H, J = 8.6 Hz, Ph–H), 7.03 (d, 1H, J = 16.4 Hz, –CH
CH–), 7.34 (s, 1H, Ph–H), 7.22 (d, 3H, J = 7.52 Hz, Ph–H and pyrimidine-H), 7.08 (t, 2H, J = 8.6 Hz, Ph–H), 7.03 (d, 1H, J = 16.4 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 4.52 (s, 2H, –CH2–), 3.84 (s, 3H, CH3O–); 13C NMR (CDCl3-d, 150 MHz) δ: 170.02, 165.28, 163.50, 162.35 (d, J = 245.88 Hz), 157.30 (q, J = 36.0 Hz), 151.54, 141.90, 137.73, 134.27, 131.43 (d, J = 2.35 Hz), 130.94 (d, J = 8.25 Hz), 123.16, 121.25 (q, J = 273.17 Hz), 115.84 (d, J = 21.57 Hz), 113.03, 110.26, 105.17, 55.89, 36.05; HRMS (ESI) calcd for C23H16F4N4O3SNa [M + Na]+: 527.07581, found: 527.07715.
CH–), 4.52 (s, 2H, –CH2–), 3.84 (s, 3H, CH3O–); 13C NMR (CDCl3-d, 150 MHz) δ: 170.02, 165.28, 163.50, 162.35 (d, J = 245.88 Hz), 157.30 (q, J = 36.0 Hz), 151.54, 141.90, 137.73, 134.27, 131.43 (d, J = 2.35 Hz), 130.94 (d, J = 8.25 Hz), 123.16, 121.25 (q, J = 273.17 Hz), 115.84 (d, J = 21.57 Hz), 113.03, 110.26, 105.17, 55.89, 36.05; HRMS (ESI) calcd for C23H16F4N4O3SNa [M + Na]+: 527.07581, found: 527.07715.
(E)-2-((4-Chlorobenzyl)thio)-5-(3-methoxy-4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazole (6d). White solid; yield 58.8%; m.p. 106.8–108.3 °C; 1H NMR (CDCl3-d, 600 MHz) δ: 8.87 (s, 1H, pyrimidine-H), 7.48 (d, 1H, J = 16.4 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.44 (d, 2H, J = 6.3 Hz, Ph–H), 7.36–7.34 (m, 3H, Ph–H), 7.29 (s, 1H, Ph–H), 7.22 (d, 3H, J = 8.4 Hz, Ph–H and pyrimidine-H), 7.02 (d, 1H, J = 16.4 Hz, –CH
CH–), 7.44 (d, 2H, J = 6.3 Hz, Ph–H), 7.36–7.34 (m, 3H, Ph–H), 7.29 (s, 1H, Ph–H), 7.22 (d, 3H, J = 8.4 Hz, Ph–H and pyrimidine-H), 7.02 (d, 1H, J = 16.4 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 4.50 (s, 2H, –CH2–), 3.84 (s, 3H, CH3O–); 13C NMR (CDCl3-d, 150 MHz) δ: 170.02, 165.32, 163.36, 159.29, 155.54 (q, J = 36.62 Hz), 151.54, 141.91, 137.77, 134.26, 134.20, 134.13, 130.52, 129.01, 123.17, 122.04, 121.79 (q, J = 270.1 Hz), 120.87, 112.02, 110.24, 105.15, 56.89, 36.05. HRMS (ESI) calcd for C23H16ClF3N4O3SNa [M + Na]+: 543.04608, found: 543.04759. HRMS (ESI) calcd for C23H15ClF4N4O3SNa [M + Na]+: 561.03674, found: 561.03817.
CH–), 4.50 (s, 2H, –CH2–), 3.84 (s, 3H, CH3O–); 13C NMR (CDCl3-d, 150 MHz) δ: 170.02, 165.32, 163.36, 159.29, 155.54 (q, J = 36.62 Hz), 151.54, 141.91, 137.77, 134.26, 134.20, 134.13, 130.52, 129.01, 123.17, 122.04, 121.79 (q, J = 270.1 Hz), 120.87, 112.02, 110.24, 105.15, 56.89, 36.05. HRMS (ESI) calcd for C23H16ClF3N4O3SNa [M + Na]+: 543.04608, found: 543.04759. HRMS (ESI) calcd for C23H15ClF4N4O3SNa [M + Na]+: 561.03674, found: 561.03817.
(E)-2-((4-Bromobenzyl)thio)-5-(3-methoxy-4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazole (6e). White solid; yield 42.6%; m.p. 107.1–109.5 °C; 1H NMR (CDCl3-d, 600 MHz) δ: 8.86 (s, 1H, pyrimidine-H), 7.60 (s, 1H, Ph–H), 7.48 (d, 1H, J = 16.4 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.44 (d, 1H, J = 7.9 Hz, Ph–H), 7.36–7.34 (d, 2H, J = 8.6 Hz, Ph–H), 7.29 (s, 1H, Ph–H), 7.22 (d, 3H, J = 8.6 Hz, Ph–H and pyrimidine-H), 7.02 (d, 1H, J = 16.4 Hz, –CH
CH–), 7.44 (d, 1H, J = 7.9 Hz, Ph–H), 7.36–7.34 (d, 2H, J = 8.6 Hz, Ph–H), 7.29 (s, 1H, Ph–H), 7.22 (d, 3H, J = 8.6 Hz, Ph–H and pyrimidine-H), 7.02 (d, 1H, J = 16.4 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 4.47 (s, 2H, –CH2–), 3.84 (s, 3H, CH3O–); 13C NMR (CDCl3-d, 150 MHz) δ: 170.02, 165.45, 162.98, 159.28, 157.30 (q, J = 35.78 Hz), 151.55, 141.94, 137.92, 136.07, 134.21, 132.84, 132.39, 131.03, 130.74, 128.52, 123.18, 121.25 (q, J = 273.21 Hz), 120.88, 111.08, 110.16, 105.13, 56.90, 35.45. HRMS (ESI) calcd for C23H16F3BrN4O3S Na [M + Na]+: 586.99542, found: 586.99708.
CH–), 4.47 (s, 2H, –CH2–), 3.84 (s, 3H, CH3O–); 13C NMR (CDCl3-d, 150 MHz) δ: 170.02, 165.45, 162.98, 159.28, 157.30 (q, J = 35.78 Hz), 151.55, 141.94, 137.92, 136.07, 134.21, 132.84, 132.39, 131.03, 130.74, 128.52, 123.18, 121.25 (q, J = 273.21 Hz), 120.88, 111.08, 110.16, 105.13, 56.90, 35.45. HRMS (ESI) calcd for C23H16F3BrN4O3S Na [M + Na]+: 586.99542, found: 586.99708.
(E)-2-(3-Methoxy-4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-5-((4-(trifluoromethyl)benzyl)thio)-1,3,4-oxadiazole (6f). White solid; yield 71.3%; m.p. 97.3–99.7 °C; 1H NMR (DMSO-d6, 600 MHz) δ: 8.95 (s, 1H, pyrimidine-H), 7.83 (s, 1H, pyrimidine-H), 7.75–7.72 (m, 4H, Ph–H), 7.66 (d, 1H, J = 1.2 Hz), 7.57 (d, 1H, J = 16.8 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.44 (d, 1H, J = 16.8 Hz, –CH
CH–), 7.44 (d, 1H, J = 16.8 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.41 (dd, 1H, J1 = 6.6 Hz, J2 = 1.8 Hz, Ph–H), 7.33 (d, 1H, J = 8.4 Hz, Ph–H), 4.67 (s, 2H, –CH2–), 3.79 (s, 3H, CH3O–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.24, 165.81, 162.92, 159.81, 156.15 (q, J = 34.95 Hz), 151.62, 142.25, 141.87, 138.59, 134.57, 130.34, 128.81 (q, J = 31.5 Hz), 125.92 (q, J = 31.6 Hz), 125.53 (q, J = 270.3 Hz), 123.50, 121.80 (q, J = 272.1 Hz), 112.23, 110.56, 106.09, 56.53, 35.52. HRMS (ESI) calcd for C24H16F6N4O3SNa [M + Na]+: 577.07251, found: 577.07395.
CH–), 7.41 (dd, 1H, J1 = 6.6 Hz, J2 = 1.8 Hz, Ph–H), 7.33 (d, 1H, J = 8.4 Hz, Ph–H), 4.67 (s, 2H, –CH2–), 3.79 (s, 3H, CH3O–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.24, 165.81, 162.92, 159.81, 156.15 (q, J = 34.95 Hz), 151.62, 142.25, 141.87, 138.59, 134.57, 130.34, 128.81 (q, J = 31.5 Hz), 125.92 (q, J = 31.6 Hz), 125.53 (q, J = 270.3 Hz), 123.50, 121.80 (q, J = 272.1 Hz), 112.23, 110.56, 106.09, 56.53, 35.52. HRMS (ESI) calcd for C24H16F6N4O3SNa [M + Na]+: 577.07251, found: 577.07395.
(E)-4-(((5-(3-Methoxy-4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazol-2-yl)thio)methyl)benzonitrile (6g). White solid; yield 63.2%; m.p. 135.5–137.6 °C; 1H NMR (DMSO-d6, 600 MHz) δ: 8.94 (s, 1H, pyrimidine-H), 7.85 (d, 2H, J = 7.8 Hz, Ph–H), 7.84 (s, 1H, pyrimidine-H), 7.71 (d, 2H, J = 7.8 Hz, Ph–H), 7.66 (d, 1H, J = 1.8 Hz, Ph–H), 7.56 (d, 1H, J = 16.2 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.43 (d, 1H, J = 16.8 Hz, –CH
CH–), 7.43 (d, 1H, J = 16.8 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.39 (d, 1H, J = 1.8 Hz), 7.33 (d, 1H, J = 8.4 Hz, Ph–H), 4.65 (s, 2H, –CH2–), 3.79 (s, 3H, CH3O); 13C NMR (DMSO-d6, 150 MHz) δ: 170.24, 165.83, 162.83, 159.81, 156.27 (q, J = 34.65 Hz), 151.62, 143.25, 141.88, 138.64, 134.56, 132.94, 130.52, 123.51, 122.04, 121.80 (q, J = 272.7 Hz), 119.11, 112.24, 110.93, 110.54, 106.09, 56.55, 35.67. HRMS (ESI) calcd for C24H16F3N5O3S Na [M + Na]+: 534.08032, found: 534.08182.
CH–), 7.39 (d, 1H, J = 1.8 Hz), 7.33 (d, 1H, J = 8.4 Hz, Ph–H), 4.65 (s, 2H, –CH2–), 3.79 (s, 3H, CH3O); 13C NMR (DMSO-d6, 150 MHz) δ: 170.24, 165.83, 162.83, 159.81, 156.27 (q, J = 34.65 Hz), 151.62, 143.25, 141.88, 138.64, 134.56, 132.94, 130.52, 123.51, 122.04, 121.80 (q, J = 272.7 Hz), 119.11, 112.24, 110.93, 110.54, 106.09, 56.55, 35.67. HRMS (ESI) calcd for C24H16F3N5O3S Na [M + Na]+: 534.08032, found: 534.08182.
(E)-2-(3-Methoxy-4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-5-((4-nitrobenzyl)thio)-1,3,4-oxadiazole (6h). White solid; yield 70.2%; m.p. 141.6–143.4 °C; 1H NMR (CDCl3-d, 600 MHz) δ: 8.85 (s, 1H, pyrimidine-H), 8.22 (d, 1H, J = 8.8 Hz, Ph–H), 7.70 (d, 2H, J = 8.7 Hz, Ph–H), 7.48 (d, 1H, J = 16.4 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.33 (s, 1H, Ph–H), 7.21 (d, 3H, J = 7.0 Hz, Ph–H and pyrimidine-H), 7.0 (d, 1H, J = 16.4 Hz, –CH
CH–), 7.33 (s, 1H, Ph–H), 7.21 (d, 3H, J = 7.0 Hz, Ph–H and pyrimidine-H), 7.0 (d, 1H, J = 16.4 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 4.58 (s, 2H, –CH2–), 3.83 (s, 3H, CH3O–); 13C NMR (CDCl3-d, 150 MHz) δ: 170.00, 165.57, 162.72, 159.26, 156.26 (q, J = 36.0 Hz), 151.56, 147.66, 143.37, 141.98, 138.07, 134.14, 130.10, 123.94, 123.18, 121.25 (q, J = 273.08 Hz), 120.89, 111.08, 110.03, 105.17, 56.90, 35.62. HRMS (ESI) calcd for C23H17F3N5O5S Na [M + Na]+: 554.12427, found: 554.07165.
CH–), 4.58 (s, 2H, –CH2–), 3.83 (s, 3H, CH3O–); 13C NMR (CDCl3-d, 150 MHz) δ: 170.00, 165.57, 162.72, 159.26, 156.26 (q, J = 36.0 Hz), 151.56, 147.66, 143.37, 141.98, 138.07, 134.14, 130.10, 123.94, 123.18, 121.25 (q, J = 273.08 Hz), 120.89, 111.08, 110.03, 105.17, 56.90, 35.62. HRMS (ESI) calcd for C23H17F3N5O5S Na [M + Na]+: 554.12427, found: 554.07165.
(E)-2-((3-Chloro-2-fluorobenzyl)thio)-5-(3-methoxy-4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazole (6i). White solid; yield 50.4%; m.p. 135.5–137.6 °C; 1H NMR (CDCl3-d, 600 MHz) δ: 8.86 (s, 1H, pyrimidine-H), 7.52–7.48 (m, 1H, Ph–H), 7.49 (d, 1H, J = 16.4 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.40–7.34 (m, 1H, Ph–H), 7.34 (s, 1H, Ph–H), 7.24–7.20 (m, 3H, Ph–H and pyrimidine-H), 7.02 (d, 1H, J = 16.4 Hz, –CH
CH–), 7.40–7.34 (m, 1H, Ph–H), 7.34 (s, 1H, Ph–H), 7.24–7.20 (m, 3H, Ph–H and pyrimidine-H), 7.02 (d, 1H, J = 16.4 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 4.56 (s, 2H, –CH2–), 3.84 (s, 3H, CH3O–); 13C NMR (CDCl3-d, 150 MHz) δ: 170.02, 165.45, 163.14, 159.28, 157.33 (d, J = 249.11 Hz), 157.28 (q, J = 36.02 Hz), 151.54, 141.92, 138.88, 134.24, 130.70, 129.67 (d, J = 2.48 Hz), 125.09 (d, J = 14.26 Hz), 124.74 (d, J = 4.70 Hz), 123.16, 121.46 (d, J = 17.45 Hz), 121.25 (q, J = 273.11 Hz), 120.87, 111.07, 110.17, 105.17, 56.89, 29.93. HRMS (ESI) calcd for C23H15ClF4N4O3SNa [M + Na]+: 561.03674, found: 561.03817.
CH–), 4.56 (s, 2H, –CH2–), 3.84 (s, 3H, CH3O–); 13C NMR (CDCl3-d, 150 MHz) δ: 170.02, 165.45, 163.14, 159.28, 157.33 (d, J = 249.11 Hz), 157.28 (q, J = 36.02 Hz), 151.54, 141.92, 138.88, 134.24, 130.70, 129.67 (d, J = 2.48 Hz), 125.09 (d, J = 14.26 Hz), 124.74 (d, J = 4.70 Hz), 123.16, 121.46 (d, J = 17.45 Hz), 121.25 (q, J = 273.11 Hz), 120.87, 111.07, 110.17, 105.17, 56.89, 29.93. HRMS (ESI) calcd for C23H15ClF4N4O3SNa [M + Na]+: 561.03674, found: 561.03817.
(E)-2-((3,4-Dichlorobenzyl)thio)-5-(3-methoxy-4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazole (6j). White solid; yield 71.5%; m.p. 92.5–94.7 °C; 1H NMR (DMSO-d6, 600 MHz) δ: 8.95 (s, 1H, pyrimidine-H), 7.84 (s, 1H, Ph–H), 7.78 (d, 1H, J = 1.86 Hz, Ph–H), 7.71 (d, 1H, J = 16.38 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.64–7.62 (m, 2H, Ph–H and pyrimidine-H), 7.50–7.47 (m, 2H, Ph–H and –CH
CH–), 7.64–7.62 (m, 2H, Ph–H and pyrimidine-H), 7.50–7.47 (m, 2H, Ph–H and –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.37 (dd, 1H, J1 = 6.72 Hz, J2 = 1.56 Hz, Ph–H), 7.32 (d, 1H, J = 8.16 Hz, Ph–H), 4.63 (s, 2H, –CH2–), 3.79 (s, 3H, CH3O–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.26, 168.41, 163.65, 159.84, 156.13 (q, J = 35.26 Hz), 151.58, 141.54, 138.62, 138.59, 135.06, 131.54, 131.46, 131.19, 130.73, 129.92, 123.49, 121.81 (d, J = 273.26 Hz), 121.62, 118.59, 112.00, 106.12, 56.50, 36.40. HRMS (ESI) calcd for C23H15Cl2F3N4O3SNa [M + Na]+: 577.00702, found: 577.00862.
CH–), 7.37 (dd, 1H, J1 = 6.72 Hz, J2 = 1.56 Hz, Ph–H), 7.32 (d, 1H, J = 8.16 Hz, Ph–H), 4.63 (s, 2H, –CH2–), 3.79 (s, 3H, CH3O–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.26, 168.41, 163.65, 159.84, 156.13 (q, J = 35.26 Hz), 151.58, 141.54, 138.62, 138.59, 135.06, 131.54, 131.46, 131.19, 130.73, 129.92, 123.49, 121.81 (d, J = 273.26 Hz), 121.62, 118.59, 112.00, 106.12, 56.50, 36.40. HRMS (ESI) calcd for C23H15Cl2F3N4O3SNa [M + Na]+: 577.00702, found: 577.00862.
(E)-2-(3-Methoxy-4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-5-((4-methylbenzyl)thio)-1,3,4-oxadiazole (6k). White solid; yield 46.4%; m.p. 134.0–135.2 °C; 1H NMR (DMSO-d6, 600 MHz) δ: 7.68 (d, 1H, J = 1.8 Hz, Ph–H), 7.68 (d, 1H, J = 16.2 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.55 (s, 1H, pyrimidine-H), 7.44 (d, 1H, J = 16.2 Hz, –CH
CH–), 7.55 (s, 1H, pyrimidine-H), 7.44 (d, 1H, J = 16.2 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.41 (dd, 1H, J1 = 6.0 Hz, J2 = 1.8 Hz, Ph–H), 7.38 (d, 1H, J = 8.4 Hz, Ph–H), 7.31 (d, 1H, J = 7.8 Hz, Ph–H), 7.18 (d, 1H, J = 7.8 Hz, Ph–H), 4.54 (s, 2H, –CH2–), 3.80 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–), 2.28 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.20, 169.72, 165.67, 163.20, 156.27 (q, J = 34.95 Hz), 151.67, 141.91, 138.50, 137.62, 134.45, 133.81, 129.64, 129.46, 123.50, 122.08, 121.83 (q, J = 273.75 Hz), 112.40, 110.55, 102.84, 56.60, 36.18, 25.88, 21.18.
CH–), 7.41 (dd, 1H, J1 = 6.0 Hz, J2 = 1.8 Hz, Ph–H), 7.38 (d, 1H, J = 8.4 Hz, Ph–H), 7.31 (d, 1H, J = 7.8 Hz, Ph–H), 7.18 (d, 1H, J = 7.8 Hz, Ph–H), 4.54 (s, 2H, –CH2–), 3.80 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–), 2.28 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.20, 169.72, 165.67, 163.20, 156.27 (q, J = 34.95 Hz), 151.67, 141.91, 138.50, 137.62, 134.45, 133.81, 129.64, 129.46, 123.50, 122.08, 121.83 (q, J = 273.75 Hz), 112.40, 110.55, 102.84, 56.60, 36.18, 25.88, 21.18.
(E)-2-((2-Fluorobenzyl)thio)-5-(3-methoxy-4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazole (6l). White solid; yield 65.2%; m.p. 115.6–117.1 °C; 1H NMR (DMSO-d6, 600 MHz) δ: 7.68 (d, 1H, J = 1.2 Hz, Ph–H), 7.59–7.54 (m, 3H, Ph–H and pyrimidine-H), 7.45 (d, 1H, J = 16.2 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.41–7.37 (m, 2H, Ph–H), 7.32 (d, 1H, J = 8.4 Hz, Ph–H), 7.26 (t, 1H, J = 9.6 Hz, Ph–H), 7.22 (t, 1H, J = 7.2 Hz, Ph–H), 4.60 (s, 2H, –CH2–), 3.80 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.20, 169.72, 165.92, 162.65, 161.70 (d, J = 241.95 Hz), 156.27 (q, J = 34.65 Hz), 151.67, 141.94, 138.66, 134.40, 131.97 (d, J = 3.3 Hz), 130.80 (d, J = 8.25 Hz), 125.12 (d, J = 3.45 Hz), 123.99 (d, J = 13.95 Hz), 123.51, 122.13, 121.82 (q, J = 272.7 Hz), 116.09 (d, J = 15.6 Hz), 112.36, 110.53, 102.84, 56.60, 35.30, 25.87. HRMS (ESI) calcd for C24H18F4N4O3SNa [M + Na]+: 541.09280, found: 541.09161.
CH–), 7.41–7.37 (m, 2H, Ph–H), 7.32 (d, 1H, J = 8.4 Hz, Ph–H), 7.26 (t, 1H, J = 9.6 Hz, Ph–H), 7.22 (t, 1H, J = 7.2 Hz, Ph–H), 4.60 (s, 2H, –CH2–), 3.80 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.20, 169.72, 165.92, 162.65, 161.70 (d, J = 241.95 Hz), 156.27 (q, J = 34.65 Hz), 151.67, 141.94, 138.66, 134.40, 131.97 (d, J = 3.3 Hz), 130.80 (d, J = 8.25 Hz), 125.12 (d, J = 3.45 Hz), 123.99 (d, J = 13.95 Hz), 123.51, 122.13, 121.82 (q, J = 272.7 Hz), 116.09 (d, J = 15.6 Hz), 112.36, 110.53, 102.84, 56.60, 35.30, 25.87. HRMS (ESI) calcd for C24H18F4N4O3SNa [M + Na]+: 541.09280, found: 541.09161.
(E)-2-((4-Fluorobenzyl)thio)-5-(3-methoxy-4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazole (6m). White solid; yield 55.8%; m.p.113.2–115.8 °C. 1H NMR (DMSO-d6, 600 MHz) δ: 7.67 (d, 1H, J = 1.8 Hz, Ph–H), 7.58 (d, 1H, J = 16.2 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.55 (t, 3H, J = 3.6 Hz, Ph–H and pyrimidine-H), 7.44 (d, 1H, J = 16.2 Hz, –CH
CH–), 7.55 (t, 3H, J = 3.6 Hz, Ph–H and pyrimidine-H), 7.44 (d, 1H, J = 16.2 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.40 (dd, 1H, J1 = 6.6 Hz, J2 = 1.2 Hz, Ph–H), 7.31 (d, 1H, J = 8.4 Hz, Ph–H), 7.18 (t, 2H, J = 9.0 Hz, Ph–H), 4.58 (s, 2H, –CH2–), 3.80 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.20, 169.72, 165.73, 163.08, 162.93, 161.31, 156.27 (q, J = 34.80 Hz), 151.66, 141.92, 138.54, 134.43, 133.36, 131.68, 131.62, 123.49, 122.08, 121.82 (q, J = 273.15 Hz), 115.95, 115.81, 112.38, 110.52, 102.82, 56.59, 35.49, 25.87.
CH–), 7.40 (dd, 1H, J1 = 6.6 Hz, J2 = 1.2 Hz, Ph–H), 7.31 (d, 1H, J = 8.4 Hz, Ph–H), 7.18 (t, 2H, J = 9.0 Hz, Ph–H), 4.58 (s, 2H, –CH2–), 3.80 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.20, 169.72, 165.73, 163.08, 162.93, 161.31, 156.27 (q, J = 34.80 Hz), 151.66, 141.92, 138.54, 134.43, 133.36, 131.68, 131.62, 123.49, 122.08, 121.82 (q, J = 273.15 Hz), 115.95, 115.81, 112.38, 110.52, 102.82, 56.59, 35.49, 25.87.
(E)-2-((2-Chlorobenzyl)thio)-5-(3-methoxy-4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazole (6n). White solid; yield 76.8%; m.p. 207.6–209.3 °C; 1H NMR (DMSO-d6, 600 MHz) δ: 7.68 (s, 1H, Ph–H), 7.65 (d, 1H, J = 7.32 Hz, Ph–H), 7.59 (d, 1H, J = 16.5 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.55–7.52 (m, 2H, Ph–H and pyrimidine-H), 7.46 (d, 1H, J = 16.38 Hz, –CH
CH–), 7.55–7.52 (m, 2H, Ph–H and pyrimidine-H), 7.46 (d, 1H, J = 16.38 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.39–7.34 (m, 3H, Ph–H), 7.32 (d, 1H, J = 8.16 Hz, Ph–H), 4.66 (s, 2H, –CH2–), 3.80 (s, 3H, CH3O–), 2.51 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.19, 169.72, 165.94, 162.65, 156.25 (q, J = 34.77 Hz), 151.66, 138.68, 134.40, 134.27, 133.81, 130.09, 130.50, 130.13, 127.99, 123.51, 122.15, 121.83 (q, J = 273.09 Hz), 112.34, 110.52, 102.85, 56.59, 34.59, 25.88. HRMS (ESI) calcd for C24H18ClF3N4O3SNa [M + Na]+: 557.06324, found: 557.06189.
CH–), 7.39–7.34 (m, 3H, Ph–H), 7.32 (d, 1H, J = 8.16 Hz, Ph–H), 4.66 (s, 2H, –CH2–), 3.80 (s, 3H, CH3O–), 2.51 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.19, 169.72, 165.94, 162.65, 156.25 (q, J = 34.77 Hz), 151.66, 138.68, 134.40, 134.27, 133.81, 130.09, 130.50, 130.13, 127.99, 123.51, 122.15, 121.83 (q, J = 273.09 Hz), 112.34, 110.52, 102.85, 56.59, 34.59, 25.88. HRMS (ESI) calcd for C24H18ClF3N4O3SNa [M + Na]+: 557.06324, found: 557.06189.
(E)-2-((3-Chlorobenzyl)thio)-5-(3-methoxy-4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazole (6o). White solid; yield 75.7%; m.p. 95.5–96.7 °C; 1H NMR (DMSO-d6, 600 MHz) δ: 7.67 (d, 1H, J = 1.32 Hz, Ph–H), 7.60 (s, 1H, Ph–H), 7.57 (d, 1H, J = 16.5 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.54 (s, 1H, Ph–H), 7.48 (d, 1H, J = 7.32 Hz, Ph–H), 7.44 (s, 1H, pyrimidine-H), 7.41–7.35 (m, 4H, Ph–H and –CH
CH–), 7.54 (s, 1H, Ph–H), 7.48 (d, 1H, J = 7.32 Hz, Ph–H), 7.44 (s, 1H, pyrimidine-H), 7.41–7.35 (m, 4H, Ph–H and –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.32 (d, 1H, J = 8.16 Hz, Ph–H), 4.58 (s, 2H, –CH2–), 3.80 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.21, 169.74, 165.81, 162.99, 156.28 (q, J = 34.71 Hz), 151.68, 141.94, 139.85, 138.59, 134.43, 133.48, 130.92, 129.41, 128.26, 128.20, 123.66, 122.11, 121.84 (q, J = 272.80 Hz), 112.37, 110.53, 102.84, 56.60, 35.51, 25.88. HRMS (ESI) calcd for C24H18ClF3N4O3SNa [M + Na]+: 557.06324, found: 557.06165.
CH–), 7.32 (d, 1H, J = 8.16 Hz, Ph–H), 4.58 (s, 2H, –CH2–), 3.80 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.21, 169.74, 165.81, 162.99, 156.28 (q, J = 34.71 Hz), 151.68, 141.94, 139.85, 138.59, 134.43, 133.48, 130.92, 129.41, 128.26, 128.20, 123.66, 122.11, 121.84 (q, J = 272.80 Hz), 112.37, 110.53, 102.84, 56.60, 35.51, 25.88. HRMS (ESI) calcd for C24H18ClF3N4O3SNa [M + Na]+: 557.06324, found: 557.06165.
(E)-2-((4-Chlorobenzyl)thio)-5-(3-methoxy-4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazole (6p). White solid; yield 57.8%; m.p. 127.4–129.2 °C; 1H NMR (CDCl3-d, 600 MHz) δ: 7.48 (d, 1H, J = 15.56 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.44 (d, 1H, J = 7.4 Hz, Ph–H), 7.35 (d, 1H, J = 8.16 Hz, Ph–H), 7.20 (d, 3H, J = 5.8 Hz, Ph–H), 7.05 (s, 1H, pyrimidine-H), 7.01 (d, 1H, J = 16.4 Hz, –CH
CH–), 7.44 (d, 1H, J = 7.4 Hz, Ph–H), 7.35 (d, 1H, J = 8.16 Hz, Ph–H), 7.20 (d, 3H, J = 5.8 Hz, Ph–H), 7.05 (s, 1H, pyrimidine-H), 7.01 (d, 1H, J = 16.4 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 4.50 (s, 2H, –CH2–), 3.83 (s, 3H, CH3O–), 2.63 (s, 3H, CH3–); 13C NMR (CDCl3-d, 150 MHz) δ: 169.99, 169.90, 165.37, 163.32, 157.31 (q, J = 35.42 Hz), 151.69, 142.19, 137.88, 1134.22, 134.12, 133.87, 130.52, 129.01, 123.25, 121.35 (q, J = 273.08 Hz), 120.89, 111.11, 110.03, 101.54, 55.93, 36.06, 25.79. HRMS (ESI) calcd for C24H18ClF3N4O3SNa [M + Na]+: 557.06324, found: 557.06140.
CH–), 4.50 (s, 2H, –CH2–), 3.83 (s, 3H, CH3O–), 2.63 (s, 3H, CH3–); 13C NMR (CDCl3-d, 150 MHz) δ: 169.99, 169.90, 165.37, 163.32, 157.31 (q, J = 35.42 Hz), 151.69, 142.19, 137.88, 1134.22, 134.12, 133.87, 130.52, 129.01, 123.25, 121.35 (q, J = 273.08 Hz), 120.89, 111.11, 110.03, 101.54, 55.93, 36.06, 25.79. HRMS (ESI) calcd for C24H18ClF3N4O3SNa [M + Na]+: 557.06324, found: 557.06140.
(E)-2-(3-Methoxy-4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-5-((2-(trifluoromethyl)benzyl)thio)-1,3,4-oxadiazole (6q). White solid; yield 66.5%; m.p. 95.5–97.3 °C; 1H NMR (DMSO-d6, 600 MHz) δ: 7.81 (t, 2H, J = 7.8 Hz, Ph–H), 7.72 (t, 1H, J = 7.8 Hz, Ph–H), 7.67 (d, 1H, J = 1.2 Hz, Ph–H), 7.58–7.52 (m, 3H, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–, Ph–H and pyrimidine-H), 7.45 (d, 1H, J = 16.2 Hz, –CH
CH–, Ph–H and pyrimidine-H), 7.45 (d, 1H, J = 16.2 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.39 (dd, 1H, J1 = 6.6 Hz, J2 = 1.8 Hz, Ph–H), 7.31 (d, 1H, J = 8.4 Hz, Ph–H), 4.73 (s, 2H, –CH2–), 3.79 (s, 3H, CH3O–), 2.49 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.18, 169.72, 165.98, 162.52, 156.27 (q, J = 34.65 Hz), 151.67, 141.96, 138.70, 134.70, 134.36, 133.55, 132.54, 129.25, 127.95 (q, J = 29.55 Hz), 126.86, 125.62 (q, J = 272.4 Hz), 123.49, 122.12, 121.82 (q, J = 273.0 Hz), 112.35, 110.48, 102.78, 56.57, 33.53, 25.83. HRMS (ESI) calcd for C25H18F6N4O3SNa [M + Na]+: 591.08960, found: 591.08826.
CH–), 7.39 (dd, 1H, J1 = 6.6 Hz, J2 = 1.8 Hz, Ph–H), 7.31 (d, 1H, J = 8.4 Hz, Ph–H), 4.73 (s, 2H, –CH2–), 3.79 (s, 3H, CH3O–), 2.49 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.18, 169.72, 165.98, 162.52, 156.27 (q, J = 34.65 Hz), 151.67, 141.96, 138.70, 134.70, 134.36, 133.55, 132.54, 129.25, 127.95 (q, J = 29.55 Hz), 126.86, 125.62 (q, J = 272.4 Hz), 123.49, 122.12, 121.82 (q, J = 273.0 Hz), 112.35, 110.48, 102.78, 56.57, 33.53, 25.83. HRMS (ESI) calcd for C25H18F6N4O3SNa [M + Na]+: 591.08960, found: 591.08826.
(E)-2-(3-Methoxy-4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-5-((4-(trifluoromethyl)benzyl)thio)-1,3,4-oxadiazole (6r). White solid; yield 47.8%; m.p. 116.3–117.9 °C; 1H NMR (DMSO-d6, 600 MHz) δ: 7.75–7.72 (m, 4H, Ph–H), 7.67 (d, 1H, J = 1.8 Hz, Ph–H), 7.56 (d, 1H, J = 16.2 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.53 (s, 1H, pyrimidine-H), 7.44 (d, 1H, J = 16.2 Hz, –CH
CH–), 7.53 (s, 1H, pyrimidine-H), 7.44 (d, 1H, J = 16.2 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.39 (dd, 1H, J1 = 6.6 Hz, J2 = 1.8 Hz, Ph–H), 7.31 (d, 1H, J = 8.4 Hz, Ph–H), 4.67 (s, 2H, –CH2–), 3.79 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.19, 169.71, 165.82, 162.91, 156.28 (q, J = 34.80 Hz), 151.66, 142.25, 141.93, 138.59, 134.40, 130.34, 128.82 (q, J = 31.65 Hz), 125.88, 125.53 (q, J = 270.6 Hz), 123.48, 122.08, 121.82 (q, J = 272.7 Hz), 112.37, 110.49, 102.81, 56.57, 35.53, 25.87. HRMS (ESI) calcd for C25H18F6N4O3SNa [M + Na]+: 591.08960, found: 591.08832.
CH–), 7.39 (dd, 1H, J1 = 6.6 Hz, J2 = 1.8 Hz, Ph–H), 7.31 (d, 1H, J = 8.4 Hz, Ph–H), 4.67 (s, 2H, –CH2–), 3.79 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.19, 169.71, 165.82, 162.91, 156.28 (q, J = 34.80 Hz), 151.66, 142.25, 141.93, 138.59, 134.40, 130.34, 128.82 (q, J = 31.65 Hz), 125.88, 125.53 (q, J = 270.6 Hz), 123.48, 122.08, 121.82 (q, J = 272.7 Hz), 112.37, 110.49, 102.81, 56.57, 35.53, 25.87. HRMS (ESI) calcd for C25H18F6N4O3SNa [M + Na]+: 591.08960, found: 591.08832.
(E)-4-(((5-(3-Methoxy-4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazol-2-yl)thio)methyl)benzonitrile (6s). White solid; yield 52.1%; m.p. 132.9–134.7 °C; 1H NMR (DMSO-d6, 600 MHz) δ: 7.85 (d, 2H, J = 7.8 Hz, Ph–H), 7.71 (d, 2H, J = 8.4 Hz, Ph–H), 7.66 (d, 1H, J = 1.2 Hz, Ph–H), 7.56 (d, 1H, J = 16.2 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.54 (s, 1H, pyrimidine-H), 7.43 (d, 1H, J = 16.8 Hz, –CH
CH–), 7.54 (s, 1H, pyrimidine-H), 7.43 (d, 1H, J = 16.8 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.40 (dd, 1H, J1 = 6.6 Hz, J2 = 1.8 Hz, Ph–H), 7.31 (d, 1H, J = 8.4 Hz, Ph–H), 7.26 (t, 1H, J = 8.4 Hz, Ph–H), 4.65 (s, 2H, –CH2–), 3.79 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.19, 169.71, 165.85, 162.82, 156.27 (q, J = 34.65 Hz), 151.66, 143.24, 141.93, 138.64, 134.39, 132.94, 130.52, 123.49, 122.10, 121.82 (q, J = 273.45 Hz), 119.10, 112.38, 110.93, 110.48, 102.83, 56.59, 35.67, 25.87. HRMS (ESI) calcd for C25H18F3N5O3SNa [M + Na]+: 548.09747, found: 548.09570.
CH–), 7.40 (dd, 1H, J1 = 6.6 Hz, J2 = 1.8 Hz, Ph–H), 7.31 (d, 1H, J = 8.4 Hz, Ph–H), 7.26 (t, 1H, J = 8.4 Hz, Ph–H), 4.65 (s, 2H, –CH2–), 3.79 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.19, 169.71, 165.85, 162.82, 156.27 (q, J = 34.65 Hz), 151.66, 143.24, 141.93, 138.64, 134.39, 132.94, 130.52, 123.49, 122.10, 121.82 (q, J = 273.45 Hz), 119.10, 112.38, 110.93, 110.48, 102.83, 56.59, 35.67, 25.87. HRMS (ESI) calcd for C25H18F3N5O3SNa [M + Na]+: 548.09747, found: 548.09570.
(E)-2-(3-Methoxy-4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-5-((2-nitrobenzyl)thio)-1,3,4-oxadiazole (6t). White solid; yield 73.9%; m.p. 112.6–114.5 °C; 1H NMR (CDCl3-d, 600 MHz) δ: 8.19 (d, 1H, J = 8.16 Hz, Ph–H), 7.91 (d, 1H, J = 7.68 Hz, Ph–H), 7.66 (t, 1H, J = 7.52 Hz, Ph–H), 7.55 (t, 1H, J = 8.16 Hz, Ph–H), 7.50 (d, 1H, J = 16.4 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.22–7.18 (m, 3H, Ph–H), 7.04 (s, 1H, pyrimidine-H), 7.00 (d, 1H, J = 16.4 Hz, –CH
CH–), 7.22–7.18 (m, 3H, Ph–H), 7.04 (s, 1H, pyrimidine-H), 7.00 (d, 1H, J = 16.4 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 4.88 (s, 2H, –CH2–), 3.83 (s, 3H, CH3O–), 2.62 (s, 3H, CH3–); 13C NMR (CDCl3-d, 150 MHz) δ: 169.98, 169.90, 165.50, 163.90, 157.29 (q, J = 35.45 Hz), 151.67, 147.68, 142.18, 137.93, 133.98, 133.85, 133.14, 132.61, 129.40, 125.64, 123.24, 121.35 (q, J = 273.06 Hz), 120.86, 112.16, 109.92, 101.52, 55.93, 34.26, 25.79. HRMS (ESI) calcd for C24H18F3N5O5S Na [M + Na]+: 568.08730, found: 568.08624.
CH–), 4.88 (s, 2H, –CH2–), 3.83 (s, 3H, CH3O–), 2.62 (s, 3H, CH3–); 13C NMR (CDCl3-d, 150 MHz) δ: 169.98, 169.90, 165.50, 163.90, 157.29 (q, J = 35.45 Hz), 151.67, 147.68, 142.18, 137.93, 133.98, 133.85, 133.14, 132.61, 129.40, 125.64, 123.24, 121.35 (q, J = 273.06 Hz), 120.86, 112.16, 109.92, 101.52, 55.93, 34.26, 25.79. HRMS (ESI) calcd for C24H18F3N5O5S Na [M + Na]+: 568.08730, found: 568.08624.
(E)-2-(3-Methoxy-4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-5-((3-nitrobenzyl)thio)-1,3,4-oxadiazole (6u). White solid; yield 68.2%; m.p. 135.8–137.1 °C; 1H NMR (CDCl3-d, 600 MHz) δ: 8.39 (t, 1H, J = 1.48 Hz, Ph–H), 8.21 (dd, 1H, J1 = 6.88 Hz, J2 = 1.4 Hz, Ph–H), 7.90 (d, 1H, J = 7.68 Hz, Ph–H), 7.59 (t, 1H, J = 7.92 Hz, Ph–H), 7.50 (d, 1H, J = 16.4 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.28 (s, 1H, Ph–H), 7.23–7.18 (m, 2H, Ph–H), 7.05 (s, 1H, pyrimidine-H), 7.02 (d, 1H, J = 16.4 Hz, –CH
CH–), 7.28 (s, 1H, Ph–H), 7.23–7.18 (m, 2H, Ph–H), 7.05 (s, 1H, pyrimidine-H), 7.02 (d, 1H, J = 16.4 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 4.61 (s, 2H, –CH2–), 3.84 (s, 3H, CH3O–), 2.63 (s, 3H, CH3–); 13C NMR (CDCl3-d, 150 MHz) δ: 169.99, 169.89, 165.60, 162.70, 157.31 (q, J = 35.48 Hz), 151.70, 148.49, 142.24, 138.15, 138.11, 135.28, 133.78, 129.79, 124.02, 123.27, 123.14, 121.35 (q, J = 272.82 Hz), 120.88, 112.20, 109.88, 101.55, 55.94, 35.66, 25.79. HRMS (ESI) calcd for C24H18F3N5O5S Na [M + Na]+: 568.08730, found: 568.08612.
CH–), 4.61 (s, 2H, –CH2–), 3.84 (s, 3H, CH3O–), 2.63 (s, 3H, CH3–); 13C NMR (CDCl3-d, 150 MHz) δ: 169.99, 169.89, 165.60, 162.70, 157.31 (q, J = 35.48 Hz), 151.70, 148.49, 142.24, 138.15, 138.11, 135.28, 133.78, 129.79, 124.02, 123.27, 123.14, 121.35 (q, J = 272.82 Hz), 120.88, 112.20, 109.88, 101.55, 55.94, 35.66, 25.79. HRMS (ESI) calcd for C24H18F3N5O5S Na [M + Na]+: 568.08730, found: 568.08612.
(E)-2-(3-Methoxy-4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-5-((4-nitrobenzyl)thio)-1,3,4-oxadiazole (6v). White solid; yield 44.9%; m.p. 133.6–135.7 °C; 1H NMR (CDCl3-d, 600 MHz) δ: 8.24 (d, 1H, J = 8.6 Hz, Ph–H), 7.70 (d, 2H, J = 8.6 Hz, Ph–H), 7.48 (d, 1H, J = 16.4 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.19–7.18 (m, 3H, Ph–H), 7.05 (s, 1H, pyrimidine-H), 7.01 (d, 1H, J = 16.4 Hz, –CH
CH–), 7.19–7.18 (m, 3H, Ph–H), 7.05 (s, 1H, pyrimidine-H), 7.01 (d, 1H, J = 16.4 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 4.59 (s, 2H, –CH2–), 3.83 (s, 3H, CH3O–), 2.62 (s, 3H, CH3–); 13C NMR (CDCl3-d, 150 MHz, 150 MHz) δ: 169.98, 169.88, 165.62, 162.68, 157.30 (q, J = 35.48 Hz), 151.70, 147.69, 143.34, 142.27, 138.17, 133.75, 130.10, 123.97, 123.27, 121.35 (q, J = 273.12 Hz), 120.92, 112.12, 109.83, 101.56, 55.93, 35.65, 25.79. HRMS (ESI) calcd for C24H18F3N5O5S Na [M + Na]+: 568.08730, found: 568.08582.
CH–), 4.59 (s, 2H, –CH2–), 3.83 (s, 3H, CH3O–), 2.62 (s, 3H, CH3–); 13C NMR (CDCl3-d, 150 MHz, 150 MHz) δ: 169.98, 169.88, 165.62, 162.68, 157.30 (q, J = 35.48 Hz), 151.70, 147.69, 143.34, 142.27, 138.17, 133.75, 130.10, 123.97, 123.27, 121.35 (q, J = 273.12 Hz), 120.92, 112.12, 109.83, 101.56, 55.93, 35.65, 25.79. HRMS (ESI) calcd for C24H18F3N5O5S Na [M + Na]+: 568.08730, found: 568.08582.
(E)-2-((3-Chloro-2-fluorobenzyl)thio)-5-(3-methoxy-4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazole (6w). White solid; yield 58.4%; m.p. 115.7–117.1 °C; 1H NMR (DMSO-d6, 600 MHz) δ: 7.67 (d, 1H, J = 1.8 Hz, Ph–H), 7.57–7.54 (m, 4H, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–, Ph–H and pyrimidine-H), 7.45 (d, 1H, J = 16.8 Hz, –CH
CH–, Ph–H and pyrimidine-H), 7.45 (d, 1H, J = 16.8 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.45 (dd, 1H, J1 = 6.6 Hz, J2 = 1.2 Hz, Ph–H), 7.32 (d, 1H, J = 7.8 Hz, Ph–H), 7.26 (t, 1H, J = 8.4 Hz, Ph–H), 4.63 (s, 2H, –CH2–), 3.80 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.19, 169.72, 165.99, 162.43, 156.40 (d, J = 247.65 Hz), 156.27 (q, J = 34.65 Hz), 151.26, 151.67, 141.96, 138.71, 134.37, 130.87, 130.76, 126.37 (d, J = 14.1 Hz), 125.96 (d, J = 4.2 Hz), 123.51, 122.14, 121.82 (q, J = 273.15 Hz), 120.31 (d, J = 17.25 Hz), 112.35, 110.51, 102.84, 56.60, 30.18, 25.87. HRMS (ESI) calcd for C24H17ClF4N4O3SNa [M + Na]+: 575.05382, found: 575.05261.
CH–), 7.45 (dd, 1H, J1 = 6.6 Hz, J2 = 1.2 Hz, Ph–H), 7.32 (d, 1H, J = 7.8 Hz, Ph–H), 7.26 (t, 1H, J = 8.4 Hz, Ph–H), 4.63 (s, 2H, –CH2–), 3.80 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.19, 169.72, 165.99, 162.43, 156.40 (d, J = 247.65 Hz), 156.27 (q, J = 34.65 Hz), 151.26, 151.67, 141.96, 138.71, 134.37, 130.87, 130.76, 126.37 (d, J = 14.1 Hz), 125.96 (d, J = 4.2 Hz), 123.51, 122.14, 121.82 (q, J = 273.15 Hz), 120.31 (d, J = 17.25 Hz), 112.35, 110.51, 102.84, 56.60, 30.18, 25.87. HRMS (ESI) calcd for C24H17ClF4N4O3SNa [M + Na]+: 575.05382, found: 575.05261.
(E)-2-((2,3-Dichlorobenzyl)thio)-5-(3-methoxy-4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)styryl)-1,3,4-oxadiazole (6x). White solid; yield 51.7%; m.p. 122.8–125.0 °C; 1H NMR (DMSO-d6, 600 MHz) δ: 7.68 (s, 1H, Ph–H), 7.64 (t, 2H, J = 6.6 Hz, Ph–H), 7.58 (d, 2H, J = 16.8 Hz, –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– and pyrimidine-H), 7.45 (d, 1H, J = 16.8 Hz, –CH
CH– and pyrimidine-H), 7.45 (d, 1H, J = 16.8 Hz, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.38 (t, 2H, J = 7.8 Hz, Ph–H), 7.31 (d, 1H, J = 8.4 Hz, Ph–H), 4.71 (s, 2H, –CH2–), 3.80 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.20, 169.72, 166.01, 162.45, 156.27 (q, J = 34.5 Hz), 151.67, 141.96, 138.75, 137.09, 134.39, 132.63, 131.88, 130.81, 130.67, 128.83, 123.52, 122.16, 121.83 (q, J = 273.45 Hz), 112.38, 110.51, 102.85, 56.61, 35.39, 25.88. HRMS (ESI) calcd for C24H17Cl2F3N4O3SNa [M + Na]+: 591.02427, found: 591.12203.
CH–), 7.38 (t, 2H, J = 7.8 Hz, Ph–H), 7.31 (d, 1H, J = 8.4 Hz, Ph–H), 4.71 (s, 2H, –CH2–), 3.80 (s, 3H, CH3O–), 2.50 (s, 3H, CH3–); 13C NMR (DMSO-d6, 150 MHz) δ: 170.20, 169.72, 166.01, 162.45, 156.27 (q, J = 34.5 Hz), 151.67, 141.96, 138.75, 137.09, 134.39, 132.63, 131.88, 130.81, 130.67, 128.83, 123.52, 122.16, 121.83 (q, J = 273.45 Hz), 112.38, 110.51, 102.85, 56.61, 35.39, 25.88. HRMS (ESI) calcd for C24H17Cl2F3N4O3SNa [M + Na]+: 591.02427, found: 591.12203.
2.3. In vitro antifungal activity test
The antifungal activities of compounds 6a–6x were evaluated in vitro against a range of fungal pathogens, including Botryosphaeria dothidea (BD), Phomopsis sp. (PS), Botrytis cinerea (BC), Fusarium spp. (FS), Fusarium graminearum (FG), and Colletotrichum sp. (CS), utilizing a previously reported methodology.36–38 The target compound (5 mg) was dissolved in 1 mL of DMSO and then added into PDA plates at a final working concentration of 50 μg mL−1. Mycelial discs, measuring 0.4 cm in diameter, were carefully placed at the center of each plate under sterile conditions. These plates were then incubated at a constant temperature of 28 °C for 3–4 days. For accurate comparison, DMSO, pyrimethanil and hymexazol at equal concentrations were used respectively as negative controls and positive controls. The inhibition rate (I) was determined using the following formula, where C represents the fungal diameter on the untreated PDA plate (control), while T denotes the fungal diameter on the treated PDA plate (test sample). This method provides a quantitative measure of the antifungal activity of the target compounds.| Inhibition rate I (%) = (C − T)/(C − 0.4) × 100 |
2.4. In vitro antibacterial activity test39
The antibacterial efficacy of compounds 6a–6y was examined in vitro against three significant plant pathogens: Xanthomonas oryzae pv. oryzicola (XOO), X. axonopodis pv. citri (XAC), and Pseudomonas syringae pv. actinidiae (Psa). Following a standardized procedure, each compound (3.75 mg) was dissolved in DMSO (150 μL) and subsequently diluted with a 0.1% aqueous Tween solution to formulate test solutions. These solutions were then combined with nutrient broth (NB) to attain final concentrations of 100 and 50 μg mL−1. Bacterial suspensions in NB (40 μL) were introduced into the test tubes, which were then incubated at 30 °C with continuous shaking at 180 rpm for 48 hours. Bacterial proliferation was assessed spectrophotometrically using a Multiskan Sky1530 spectrophotometer, relying on OD595 values within the 0.6 to 0.8 range to identify the logarithmic growth phase. DMSO and thiodiazole copper served as the negative and positive controls, respectively. The percentage inhibition (I%) was calculated based on adjusted turbidity readings from both treated and untreated NB samples.| Inhibition rate I (%) = (C − T)/C × 100 |
2.5. Molecular docking
The enzyme SDH plays a crucial role in the Krebs cycle, making it a potential target for developing antifungal agents known as SDHIs. To understand its mechanisms and target interactions, we studied the binding patterns between SDH and the highly effective compound 6f using Discovery Studio 2.5 software. This molecular docking analysis provided insights into how compound 6f interacts with SDH, following methodologies outlined in previous studies.37,383. Result and discussion
3.1. Chemistry
Utilizing ferulic acid as the initial material, the title compounds 6a–6x were crafted via acetylation, condensation, cyclization, etherification, and thioetherification reactions, affording yields ranging from 42.5% to 84.6%. The chemical structures of these compounds were unambiguously verified using 1H NMR, 13C NMR, and HRMS techniques. In the case of compound 6b, its 1H NMR spectrum exhibited two distinctive singlets at 7.84 and 7.67 ppm, confirming the presence of a hydrogen atom within the pyrimidine moiety. Additionally, the characteristic twin peaks observed at 7.59 and 7.45 ppm correspond to the two hydrogens present on the –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– group. Moreover, the molecular weight of compound 6b was precisely determined using HRMS, revealing the presence of [M + Na]+ ions with an m/z value of 527.07715. This information not only complements the structural elucidation but also provides further evidence for the successful synthesis and characterization of the target compounds. The spectra of 1H NMR and 13C NMR for compounds 6a–6x are shown in ESI.†
CH– group. Moreover, the molecular weight of compound 6b was precisely determined using HRMS, revealing the presence of [M + Na]+ ions with an m/z value of 527.07715. This information not only complements the structural elucidation but also provides further evidence for the successful synthesis and characterization of the target compounds. The spectra of 1H NMR and 13C NMR for compounds 6a–6x are shown in ESI.†
3.2. In vitro antifungal activity test
As shown in Table 1, the target compounds 6a–6y exhibited certain in vitro antifungal activities against BD (4.61–52.87%), PS (16.90–91.61%), BC (21.05–57.93%), FS (8.31–75.60%), FG (6.32–54.98%), and CS (20.87–58.64%). Among them, the inhibitory rates of compounds 6c, 6f, 6g and 6h against PS were 80.33%, 91.61%, 84.49%, and 89.10% respectively, higher than that of pyrimethanil (77.17%). Additionally, compounds 6c and 6d demonstrated moderate inhibition rates of 75.6% and 72.16% against FS respectively, which were superior to hymexazol (60.31%).| Inhibition rate (%) | ||||||
|---|---|---|---|---|---|---|
| BD | PS | BC | FS | FG | CS | |
| a Average of three replicates. | ||||||
| 6a | 5.31 ± 1.67 | 57.35 ± 2.36 | 23.22 ± 2.45 | 17.58 ± 1.12 | 24.59 ± 2.57 | 38.95 ± 1.08 |
| 6b | 5.69 ± 1.17 | 70.85 ± 1.39 | 32.11 ± 2.37 | 59.71 ± 1.16 | 22.30 ± 1.26 | 34.04 ± 1.16 |
| 6c | 12.73 ± 1.39 | 80.83 ± 2.44 | 38.15 ± 2.29 | 75.60 ± 0.82 | 31.58 ± 2.63 | 58.64 ± 1.60 |
| 6d | 52.87 ± 1.95 | 57.68 ± 2.29 | 30.02 ± 1.24 | 72.16 ± 1.41 | 26.48 ± 1.61 | 50.30 ± 1.67 |
| 6e | 12.39 ± 1.81 | 47.85 ± 1.43 | 27.11 ± 2.39 | 57.12 ± 1.22 | 16.51 ± 3.40 | 28.51 ± 2.29 |
| 6f | 23.15 ± 1.56 | 91.61 ± 3.12 | 31.27 ± 1.93 | 67.24 ± 1.24 | 18.19 ± 1.83 | 28.51 ± 1.16 |
| 6g | 7.64 ± 1.92 | 84.49 ± 3.20 | 35.02 ± 2.21 | 52.73 ± 1.36 | 17.45 ± 2.27 | 21.91 ± 2.85 |
| 6h | 12.20 ± 1.12 | 89.10 ± 2.36 | 20.44 ± 1.31 | 48.77 ± 2.34 | 22.49 ± 1.73 | 22.98 ± 1.29 |
| 6i | 5.73 ± 2.85 | 68.02 ± 1.80 | 57.93 ± 2.35 | 8.72 ± 1.17 | 12.85 ± 1.66 | 23.83 ± 1.25 |
| 6j | 16.77 ± 1.50 | 25.46 ± 1.72 | 47.10 ± 3.30 | 16.01 ± 1.16 | 21.60 ± 1.43 | 48.30 ± 2.42 |
| 6k | 7.93 ± 2.12 | 49.98 ± 1.22 | 21.05 ± 1.59 | 13.34 ± 1.15 | 49.56 ± 1.52 | 37.99 ± 1.38 |
| 6l | 16.90 ± 2.13 | 52.26 ± 1.32 | 35.02 ± 1.93 | 17.47 ± 2.16 | 20.38 ± 1.69 | 23.83 ± 1.51 |
| 6m | 7.59 ± 2.35 | 62.62 ± 2.28 | 20.86 ± 1.95 | 18.35 ± 1.18 | 54.98 ± 1.21 | 35.85 ± 2.29 |
| 6n | 11.40 ± 1.27 | 16.90 ± 1.12 | 24.81 ± 1.72 | 26.01 ± 1.72 | 18.39 ± 1.15 | 26.17 ± 1.71 |
| 6o | 17.99 ± 2.47 | 34.12 ± 1.09 | 27.10 ± 1.79 | 43.36 ± 0.86 | 30.77 ± 2.01 | 28.51 ± 1.08 |
| 6p | 23.50 ± 3.75 | 43.50 ± 1.30 | 43.50 ± 2.07 | 53.50 ± 1.59 | 33.50 ± 3.21 | 45.50 ± 1.38 |
| 6q | 12.22 ± 1.24 | 56.33 ± 1.05 | 25.44 ± 1.78 | 26.47 ± 2.21 | 6.32 ± 1.99 | 28.51 ± 2.23 |
| 6r | 18.23 ± 2.42 | 68.23 ± 1.42 | 29.19 ± 1.40 | 37.07 ± 1.32 | 15.75 ± 1.13 | 30.43 ± 1.08 |
| 6s | 15.24 ± 1.92 | 64.31 ± 3.20 | 25.44 ± 2.21 | 37.83 ± 1.36 | 10.73 ± 2.27 | 27.02 ± 2.85 |
| 6t | 24.94 ± 2.15 | 48.29 ± 1.26 | 20.86 ± 1.27 | 8.31 ± 1.92 | 20.31 ± 1.15 | 20.87 ± 2.42 |
| 6u | 32.39 ± 1.37 | 58.47 ± 1.28 | 36.27 ± 2.43 | 16.77 ± 1.53 | 23.45 ± 1.05 | 30.64 ± 1.28 |
| 6v | 41.57 ± 1.29 | 68.64 ± 1.26 | 45.86 ± 2.16 | 30.32 ± 1.30 | 31.84 ± 1.82 | 42.77 ± 1.16 |
| 6w | 12.04 ± 2.53 | 48.72 ± 2.12 | 42.11 ± 1.66 | 11.83 ± 1.11 | 14.56 ± 2.37 | 27.23 ± 1.95 |
| 6x | 4.61 ± 0.95 | 27.72 ± 1.23 | 33.47 ± 3.37 | 30.95 ± 3.76 | 16.51 ± 0.95 | 35.32 ± 1.77 |
| Hymexazol | 34.55 ± 1.08 | 47.09 ± 1.69 | 71.26 ± 4.43 | 60.31 ± 1.40 | 59.97 ± 1.38 | 30.83 ± 1.13 |
| Pyrimethanil | 87.16 ± 1.54 | 86.56 ± 1.76 | 81.99 ± 2.34 | 39.38 ± 1.68 | 26.89 ± 0.94 | 65.12 ± 1.54 |
The EC50 values of some target compounds against Phomopsis sp. and Fusarium spp. were determined and the results are shown in Table 2. The results presented in Table 2 demonstrate that compounds 6c, 6f, 6g, and 6h exhibited superior antifungal activity against Phomopsis sp., with EC50 values of 20.57, 12.64, 26.19 and 21.55 μg mL−1 respectively, outperforming pyrimethanil (35.16 μg mL−1) and hymexazol (27.01 μg mL−1). Meanwhile, compounds 6c and 6d displayed excellent antifungal activity against Fusarium spp., with EC50 values of 21.35 and 27.46 μg mL−1, respectively, which were better than hymexazol (30.79 μg mL−1).
| Compd | PS | FS | ||
|---|---|---|---|---|
| Regression eq. | EC50 (μg mL−1) | Regression eq. | EC50 (μg mL−1) | |
| a Average of three replicates. | ||||
| 6c | y = 1.244x + 4.332 | 20.57 ± 2.43 | y = 0.873x + 3.452 | 21.35 ± 1.51 |
| 6d | — | — | y = 1.057x + 3.685 | 27.46 ± 1.28 |
| 6f | y = 0.935x + 3.146 | 12.64 ± 1.15 | — | — |
| 6g | y = 1.3950x + 3.853 | 26.19 ± 2.04 | — | — |
| 6h | y = 0.972x + 3.192 | 21.55 ± 1.66 | — | — |
| Hymexazol | y = 0.744x + 3.933 | 27.01 ± 1.34 | y = 1.184x + 3.238 | 30.79 ± 1.72 |
| Pyrimethanil | y = 1.654x + 4.248 | 35.16 ± 1.95 | — | — |
3.3. In vitro antibacterial activity test
The in vitro antibacterial activities of compounds 6a–6x were assessed against plant pathogens XOO, XAC, and PSA using turbidimeter tests. Table 3 summarizes the preliminary results, revealing that some test compounds showed moderate antibacterial activity in comparison to Thiodiazole copper. Notably, compounds 6c, 6h, and 6p exhibited exceptional inhibitory activity against XAC, achieving inhibitory rates higher than the commercial control drug at both 100 μg mL−1 and 50 μg mL−1. Specifically, compounds 6c, 6h, and 6p achieved inhibitory rates of 79.33%, 71.93%, and 85.76%, respectively, compared to 76.59% for thiodiazole copper at 100 μg mL−1. Compounds 6c, 6n, and 6p demonstrated significant inhibitory activity with rates of 63.26%, 50.46%, and 57.85%, respectively, surpassing the 48.01% rate of thiodiazole copper at 50 μg mL−1. The other compounds displayed varying degrees of antibacterial activity against the tested pathogens.| Compounds | Inhibition rate (%) | |||||
|---|---|---|---|---|---|---|
| XOO | XAC | PSA | ||||
| 100 μg mL−1 | 50 μg mL−1 | 100 μg mL−1 | 50 μg mL−1 | 100 μg mL−1 | 50 μg mL−1 | |
| a Average of three replicates. | ||||||
| 6a | 18.93 ± 2.63 | 9.18 ± 1.71 | 34.41 ± 1.56 | 17.71 ± 3.36 | 16.53 ± 2.11 | 7.38 ± 2.20 |
| 6b | 40.21 ± 3.71 | 18.54 ± 2.59 | 13.13 ± 1.34 | 10.06 ± 0.78 | 11.24 ± 2.31 | 8.31 ± 1.12 |
| 6c | 26.40 ± 3.93 | 16.81 ± 3.41 | 79.33 ± 2.54 | 63.26 ± 1.56 | 8.49 ± 4.95 | 5.50 ± 4.30 |
| 6d | 14.88 ± 4.36 | 0 | 48.34 ± 2.87 | 43.56 ± 3.80 | 9.39 ± 1.49 | 0 |
| 6e | 22.04 ± 2.65 | 4.84 ± 2.04 | 58.89 ± 2.73 | 40.81 ± 2.16 | 12.10 ± 2.13 | 0 |
| 6f | 6.38 ± 1.25 | 1.47 ± 1.71 | 41.58 ± 3.88 | 20.16 ± 1.35 | 15.95 ± 3.49 | 7.91 ± 1.71 |
| 6g | 17.89 ± 3.43 | 8.49 ± 2.70 | 40.46 ± 1.25 | 26.64 ± 4.15 | 19.88 ± 1.94 | 11.05 ± 5.93 |
| 6h | 47.89 ± 0.53 | 46.16 ± 4.69 | 71.93 ± 3.26 | 33.32 ± 3.03 | 39.28 ± 0.66 | 8.70 ± 2.91 |
| 6i | 38.40 ± 2.05 | 17.98 ± 1.21 | 45.84 ± 3.28 | 22.13 ± 1.35 | 21.05 ± 2.49 | 10.83 ± 1.91 |
| 6j | 16.03 ± 2.72 | 1.60 ± 0.19 | 14.45 ± 4.05 | 10.59 ± 1.63 | 23.10 ± 3.44 | 0 |
| 6k | 54.27 ± 3.52 | 29.31 ± 1.11 | 18.46 ± 1.28 | 10.54 ± 0.95 | 28.94 ± 1.59 | 19.32 ± 1.71 |
| 6l | 56.43 ± 0.65 | 30.25 ± 0.81 | 40.58 ± 2.28 | 20.06 ± 1.35 | 45.73 ± 3.47 | 19.85 ± 0.97 |
| 6m | 36.64 ± 2.57 | 13.95 ± 4.42 | 51.85 ± 2.81 | 42.63 ± 3.03 | 36.34 ± 3.24 | 0 |
| 6n | 30.66 ± 3.01 | 11.95 ± 4.47 | 68.91 ± 0.27 | 50.46 ± 1.31 | 35.89 ± 3.79 | 0 |
| 6o | 15.48 ± 1.35 | 7.14 ± 3.41 | 52.99 ± 1.28 | 35.53 ± 1.38 | 17.08 ± 5.49 | 8.81 ± 1.15 |
| 6p | 37.46 ± 1.07 | 10.34 ± 3.41 | 85.76 ± 0.09 | 57.85 ± 2.41 | 28.59 ± 1.43 | 0 |
| 6q | 11.38 ± 0.35 | 7.73 ± 0.61 | 58.15 ± 2.18 | 34.73 ± 2.31 | 54.17 ± 3.49 | 24.91 ± 1.08 |
| 6r | 5.60 ± 1.75 | 0.85 ± 1.91 | 41.38 ± 1.28 | 22.13 ± 1.37 | 23.58 ± 1.09 | 10.16 ± 3.11 |
| 6s | 35.86 ± 1.25 | 17.34 ± 0.81 | 55.35 ± 1.18 | 36.13 ± 0.35 | 39.84 ± 2.38 | 19.91 ± 1.01 |
| 6t | 40.28 ± 3.10 | 19.18 ± 0.92 | 46.22 ± 1.71 | 42.77 ± 1.90 | 35.32 ± 3.91 | 5.05 ± 1.16 |
| 6u | 19.00 ± 4.35 | 13.73 ± 6.91 | 39.01 ± 8.98 | 37.03 ± 2.35 | 19.18 ± 5.49 | 4.41 ± 1.71 |
| 6v | 24.50 ± 1.04 | 19.72 ± 6.78 | 58.31 ± 1.72 | 43.93 ± 3.44 | 29.59 ± 1.32 | 10.61 ± 1.55 |
| 6w | 31.16 ± 0.33 | 15.63 ± 1.39 | 56.58 ± 1.26 | 32.55 ± 0.93 | 44.21 ± 1.56 | 20.22 ± 1.74 |
| 6x | 44.38 ± 1.23 | 31.09 ± 2.17 | 18.33 ± 1.06 | 11.54 ± 1.03 | 45.38 ± 2.06 | 22.56 ± 1.21 |
| Thiadiazole copper | 61.36 ± 3.17 | 50.36 ± 3.72 | 76.59 ± 3.10 | 48.01 ± 2.33 | 92.67 ± 5.22 | 45.29 ± 3.25 |
3.4. Docking analysis
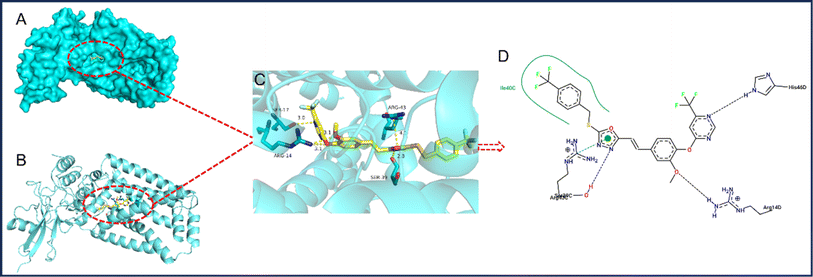
The interaction between a potent compound and the SDH enzyme was investigated using docking techniques, with results shown in Fig. 3. The observed lowest binding energy was −9.8 kcal mol−1. In compound 6f, the oxygen atoms belonging to the pyrimidinyloxy ether and phenyloxy ether groups engage in a dual hydrogen bonding interaction with the ARG-14 residue, maintaining a consistent distance of 3.1 Å for both bonds. Otherwise, compound 6f utilizes nitrogen atoms on its pyrimidine and 1,3,4-oxadiazole rings to establish hydrogen bonds with SER-17 and SER-39 residues at distances of 3.0 Å and 2.8 Å, respectively. Additionally, the oxadiazole ring engages in a pi–cation interaction with ARG-43, involving electrostatic attraction. These findings enhance our understanding of the compound's interaction with SDH, revealing potential mechanisms of action and informing future therapeutic designs.4. Conclusion
In this investigation, 24 innovative ferulic acid derivatives, containing 1,3,4-oxadiazole thioether and trifluoromethyl pyrimidine moieties, were devised and synthesized. Remarkably, compound 6f exhibited pronounced antifungal efficacy against Phomopsis sp., surpassing that of pyrimethanil and hymexazol in laboratory tests. This research provides a valuable framework for developing novel ferulic acid derivatives tailored for managing fungal and bacterial infections in plants. Furthermore, molecular docking simulations elucidated that compound 6f engages in hydrogen bonding with the SDH enzyme at SER-17, SER-39, ARG-14 and ARG-43 sites, clarifying its mode of action. Consequently, these ferulic acid derivatives arise as promising agents for tackling fungal and bacterial threats in plant health.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by Science and Technology Fund Project of Guizhou (No. [2020]1Z023), Science and Technology Fund Project of Guizhou (Qian Ke He Pingtai Rencai-CXTD [2022]002), Education Department of Guizhou Province-Natural Science Research Project (QJJ[2023]042), and Key Laboratory of Green Pesticide Agricultural Engineering Ministry of Education, Guizhou University (QJJ[2022]434).References
- C. L. Price, J. E. Parker, A. G. Warrilow, D. E. Kelly and S. L. Kelly, Pest Manage. Sci., 2015, 7, 1054 CrossRef PubMed.
- D. A. Pereira, B. A. McDonald and P. C. Brunner, Pest Manage. Sci., 2017, 73, 1503 CrossRef CAS PubMed.
- J. Y. Dong, W. Gao, K. Li, Z. Y. Hong, L. F. Tang, L. J. Han, Z. H. Wang and Z. J. Fan, J. Agric. Food Chem., 2022, 70, 3435 CrossRef CAS PubMed.
- C. Wang, Z. Yang, L. Tang, X. Wang and Q. Zhang, Environ. Sci. Technol. Lett., 2018, 5, 635 CrossRef CAS.
- L. G. Copping and S. O. Duke, Pest Manage. Sci., 2007, 63, 524 CrossRef CAS PubMed.
- X. Qian, P. W. Lee and S. Cao, J. Agric. Food Chem., 2010, 58, 2613 CrossRef CAS PubMed.
- J. N. Seiber, J. Agric. Food Chem., 2011, 59, 1 CrossRef CAS PubMed.
- L. G. Copping and S. O. Duke, Pest Manage. Sci., 2007, 63, 524 CrossRef CAS PubMed.
- X. L. Ren, X. Y. Li, L. M. Yin, D. H. Jiang and D. Y. Hu, ACS Omega, 2020, 5, 19721 CrossRef CAS PubMed.
- S. J. Ma, R. J. Xin, L. W. Wang, L. Y. Jin, X. X. Zhang, H. L. Yu, J. Yang, L. L. Dong, L. H. Zhang and J. A. Dong, J. Agric. Food Chem., 2023, 71, 276 CrossRef CAS PubMed.
- L. H. Zhang, J. L. Zhang, Y. C. Liu, Z. Y. Cao, J. M. Han, J. Yang and J. G. Dong, J. Integr. Agric., 2013, 12, 1026 CrossRef.
- H. Patzke and A. Schieber, Food Res. Int., 2018, 113, 18 CrossRef CAS PubMed.
- Y. Han, X. Y. Meng, X. F. Lin, N. Duan, Z. P. Wang and S. J. Wu, Food Biosci., 2023, 52, 102414 CrossRef.
- X. H. Gan, W. Zhang, S. C. Lan and D. Y. Hu, J. Agric. Food Chem., 2023, 71, 1369 CrossRef CAS PubMed.
- T. Yuan, Z. X Wang, D. Liu, H. Zeng, J. Liang, D. Hu and X. Gan, Pest Manage. Sci., 2022, 78, 1749 CrossRef CAS PubMed.
- A. Dai, Y. Huang, L. Yu, Z. Zheng and J. Wu, BMC Chem., 2022, 16, 34 CrossRef CAS PubMed.
- R. Zhang, P. Deng, A. Dai, S. Guo, Y. Wang, P. Wei and J. Wu, ACS Omega, 2021, 6, 27561 CrossRef CAS PubMed.
- S. Wang, J. Chen, J. Shi, Z. Wang, D. Hu and B. Song, J. Agric. Food Chem., 2021, 69, 11804 CrossRef CAS PubMed.
- J. X. Chen, Y. Z. Chen, X. H. Gan, B. J. Song, D. Y. Hu and B. A. Song, J. Agric. Food Chem., 2018, 66, 9616 CrossRef CAS PubMed.
- Z. B. Yang, P. Li, Y. J. He, J. Luo, J. Zhou, Y. H. Wu and L. T. Chen, J. Heterocycl. Chem., 2020, 57, 81 CrossRef CAS.
- J. Shi, N. Luo, M. Ding and X. Bao, Chin. Chem. Lett., 2020, 31, 434 CrossRef CAS.
- N. J. Pan, R. R. Wu, C. Yan, M. Zhou, Q. Fei, P. Li and W. N. Wu, J. Heterocycl. Chem., 2023, 60, 1768 CrossRef CAS.
- Y. E. Wang, D. Yang, L. Dai, J. Huo, L. Chen, Z. Kang, J. Mao and J. Zhang, J. Agric. Food Chem., 2022, 70, 2510 CrossRef CAS PubMed.
- F. Peng, T. Liu, Q. Wang, F. Liu, X. Cao, J. Yang, L. Liu, C. Xie and W. Xue, J. Agric. Food Chem., 2021, 69, 11085 CrossRef CAS PubMed.
- J. X. Chen, Y. Q. Luo, C. Q. Wei, S. K. Wu, R. Wu, S. B. Wang, D. Y. Hu and B. A. Song, Pest Manage. Sci., 2020, 76, 3188 CrossRef CAS PubMed.
- W. N. Wu, Q. Chen, A. Q. Tai, G. Q. Jiang and G. P. Ouyang, Bioorg. Med. Chem. Lett., 2015, 25, 2243 CrossRef CAS PubMed.
- X. Zhang, Z. Yang, H. Xu, Y. Liu, X. Yang, T. Sun, X. Lu, F. Shi, Q. Yang, W. Chen, H. Duan and Y. Ling, J. Agric. Food Chem., 2022, 70, 9262 CrossRef CAS PubMed.
- R. Wu, T. Liu, S. K. Wu, H. D. Li, R. J. Song and B. A. Song, J. Agric. Food Chem., 2022, 70, 9305 CrossRef CAS PubMed.
- S. Lan, T. Xiao, S. Guan, A. Liu, C. Long, F. Zhong, Z. Huang, X. Liu, Z. Zhang and W. Liu, J. Heterocycl. Chem., 2023, 60, 1058 CrossRef CAS.
- J. Li, Y. Wang, Y. Wu, R. Li, S. Liang, J. Zhang, Y. Zhu and B. Xie, Pestic. Biochem. Physiol., 2021, 172, 104766 CrossRef CAS PubMed.
- M. Yu, H. Liu, L. Guo, T. Zhou, Y. Shan, Z. Xia, X. Li, M. An and Y. Wu, Pest Manage. Sci., 2022, 78, 5259 CrossRef CAS PubMed.
- X. M. Tang, W. Zhan, S. Chen, R. Zhou, D. Hu, N. Sun, Q. Fei, W. Wu and W. Xue, Arabian J. Chem., 2022, 15, 104110 CrossRef CAS.
- X. T. Yu, W. J. Lan, M. H. Chen, S. Xu, X. X. Luo, S. He, H. J. Chen, Q. Fei and W. N. Wu, J. Chem., 2021, 8370407 CAS.
- W. N. Wu, W. J. Lan, C. Y. Wu and Q. Fei, Front. Chem., 2021, 695628 CrossRef CAS PubMed.
- W. N. Wu, M. Chen, R. Wang, H. Tu, M. Yang and G. Ouyang, Chem. Pap., 2019, 73, 719 CrossRef.
- N. J. Pan, C. Y. Liu, R. R. Wu, Q. Fei and W. N. Wu, Front. Chem., 2022, 922813 CrossRef CAS PubMed.
- N. J. Pan, H. Wang, J. S. An, C. Y. Liu, H. J. Chen, Q. Fei, P. Li and W. N. Wu, ACS Omega, 2024, 9, 1424 CrossRef CAS PubMed.
- N. J. Pan, R. R. Wu, C. Yan, M. Zhou, Q. Fei, P. Li and W. N. Wu, J. Heterocycl. Chem., 2023, 60, 17678 CrossRef.
- S. B. Wang, J. X. Chen, J. Shi, Z. J. Wang, D. Y. Hu and B. A. Song, J. Agric. Food Chem., 2021, 69, 11804 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra01765j |
| This journal is © The Royal Society of Chemistry 2024 |