Open Access Article
Open Access ArticleSynergistic effects of a copper–cobalt–nitroisophthalic acid/neodymium oxide composite on the electrochemical performance of hybrid supercapacitors
Asma Khizara,
Muhammad Zahir Iqbal*a,
Ayesha Zakira,
Misbah Shaheena,
Saikh Mohammad Wabaidurb and
Mian Muhammad Faisalc
aRenewable Energy Research Laboratory, Faculty of Engineering Sciences, Ghulam Ishaq Khan Institute of Engineering Sciences and Technology, Topi, 23640, Khyber Pakhtunkhwa, Pakistan. E-mail: zahir@giki.edu.pk
bDepartment of Chemistry, College of Science, King Saud University, Riyadh 11451, Saudi Arabia
cDepartment of Physics, Durham University, DH1 3LE, UK
First published on 2nd April 2024
Abstract
Hybrid supercapacitors can produce extraordinary advances in specific power and energy to display better electrochemical performance and better cyclic stability. Amalgamating metal oxides with metal–organic frameworks endows the prepared composites with unique properties and advantageous possibilities for enhancing the electrochemical capabilities. The present study focused on the synergistic effects of the CuCo(5-NIPA)–Nd2O3 composite. Employing a half-cell configuration, we conducted a comprehensive electrochemical analysis of CuCo(5-NIPA), Nd2O3, and their composite. Owing to the best performance of the composite, the hybrid device prepared from CuCo(5-NIPA)–Nd2O3 and activated carbon demonstrated a specific capacity of 467.5 C g−1 at a scan rate of 3 mV s−1, as well as a phenomenal energy and power density of 109.68 W h kg−1 and 4507 W kg−1, respectively. Afterwards, semi-empirical techniques and models were used to investigate the capacitive and diffusive mechanisms, providing important insights into the unique properties of battery–supercapacitor hybrids. These findings highlight the enhanced performance of the CuCo(5-NIPA)–Nd2O3 composite, establishing it as a unique and intriguing candidate for applications requiring the merging of battery and supercapacitor technologies.
1. Introduction
The rapid development of electronic technology has spurred an increasing demand for energy-storage devices. This spike in demand coincides with the depletion of the fossil fuel resources, prompting the need for the development of renewable energy-storage systems.1 Due to their extended cycle life, and high power and energy density, researchers have focused on second-generation lithium-ion batteries, fuel cells, and supercapacitors as viable and sustainable energy-storage options.2,3 In this regard, batteries and supercapacitors (SCs) are both essential for storing renewable energy. Batteries have a high energy density due to redox processes, but their power density is restricted compared to supercapacitors.4,5 Considering this, an extensive amount of research is being done to improve the energy density of SCs while maintaining their high-power properties. A major endeavor in this area is the development of hybrid energy-storage devices, with an aim to bridge the energy capacity gap between standard batteries and the power output of SCs. The hybrid supercapacitor (HSC) is one such invention that combines extraordinarily efficient battery-type electrode materials with capacitive grade materials.6,7 In an HSC, faradaic reaction processes are enabled by the high energy potential of redox-active materials, which are generally used as positive electrodes (with high energy density), paired with carbonaceous materials that serve as negative electrodes (with high power density).8,9 Redox-active metal oxides, owing to their high energy density and remarkable rate capabilities, are suitable choices for positive electrode materials.5 In contrast, carbonaceous materials are frequently employed as electrodes in electric double-layer capacitors (EDLCs) due to their cost-effectiveness, resistance to high temperatures, high electrical conductivity, and excellent power density.10,11In this regard metal–organic frameworks (MOFs) have emerged as a new class of crystalline materials, characterized by the coordination interactions between organic ligands and inorganic nodes, which are often metal ions or clusters with a high ability to store charge.12 MOFs have a wide specific surface area, fully exposed active sites, and changeable structure due to their unusual inorganic and organic hybrid structure, and can be employed directly as electrode materials.13 However, MOFs have several restrictions, such as low chemical stability and electrical conductivity,14,15 that prevent them from reaching their full potential. However, their combination with various functional materials, like metal oxides, can produce composites with the benefits of both parent materials while limiting their drawbacks.16,17 Metal oxides with their unique properties have proven themselves as chemically appropriate and commercially feasible materials for energy technologies, such as batteries, fuel cells, water electrolysis, and small-molecules (e.g., H2O, CO2, N2) activation.18–20 Lanthanides, having an electron configuration of [Xe]4fN (N = 0–14), are widely used in various applications due to the unique properties derived from their 4f electron configurations.21–24 The majority of their chemical and physical properties are determined by the structure of their outer electronic layer.25 Due to their redox activity, substantial bulk density, and high conductivity, lanthanides, notably rare earth metal oxides, are a major focus of research as an active electrode material for supercapacitors.26,27
In this regard, neodymium oxide (Nd2O3) is an important, highly redox active, and naturally abundant element.28 Because of its strong structural qualities, resistance to corrosion, and high chemical stability it helps to improve the specific capacity and performance of the overall system by offering a long-lasting effectiveness and reliability in energy-storage devices by preventing deterioration and structural changes. Moreover, with a large surface area, copper cobalt–5-nitroisophthalic acid (CuCo(5-NIPA)) is ideal for cation intercalation/de-intercalation and electrochemical reactions during energy-storage activities, whereas the addition of metal oxides effectively increases the redox-active sites.29,30 5-Nitroisophthalic acid (5-NIPA) is a multifunctional ligand with a nitro group that has beneficial thermal as well as chemical stability and can also act as a hydrogen-bond acceptor to form hydrogen bonds. It also has a large number of coordination modes and the capacity to form clusters, which can serve as secondary building blocks in the formation of novel structural frameworks.31 Porous coordination frameworks of 5-NIPA are observed due to their high coordination numbers as well as their unexpected and adaptive coordination behavior.32
Our study was aimed at merging the benefits of CuCo(5-NIPA) and Nd2O3 by making their composite with high surface areas, in which each component would preserve its uniqueness while imparting outstanding properties to the whole system.33 The electron-withdrawing nitro group within 5-NIPA gave CuCo(5-NIPA) unique electrical characteristics. To further strengthen the energy-storage capacity of CuCo(5-NIPA), we utilized Nd2O3 to improve the stability and conductivity. The novelty of our work lies in the enhancement of the electrochemical properties of CuCo-based MOFs with a functionalized linker (5-NIPA) by integrating a rare earth metal oxide (Nd2O3). By utilizing the distinct advantages of each component, this study leveraged the synergistic interaction between Nd2O3 and the porous structure of CuCo(5-NIPA)–Nd2O3 to obtain higher electrochemical performance. Further, ample active sites for charge preservation and transport were provided by the MOF structure, while Nd2O3 improved the overall conductivity, which raised the redox activity and improved the energy-storage capacity. By working together, the potential of both materials was fully utilized, which could lead to further improvements in the functioning of energy-storage devices, such as batteries and supercapacitors. Complete electrochemical investigations were performed in a three-electrode assembly to compare the characteristics of CuCo(5-NIPA), Nd2O3, and CuCo(5-NIPA)–Nd2O3. Furthermore, CuCo(5-NIPA)–Nd2O3 was examined in a two-cell configuration to reveal the device performance. Following this, a semi-empirical approach was further employed to evaluate the capacitive as well as diffusive contributions using linear and quadratic models.
2. Experimental
2.1 Materials
CuCo(5-NIPA), Nd2O3, and their composite were examined via electrochemical analysis. N-Methyl-2-pyrrolidone (NMP), neodymium oxide, potassium hydroxide (KOH) pellets, activated carbon, acetylene black, DI water, and polyvinylidene fluoride (PVDF) were used in the electrochemical tests and were brought from Sigma Aldrich.2.2 Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
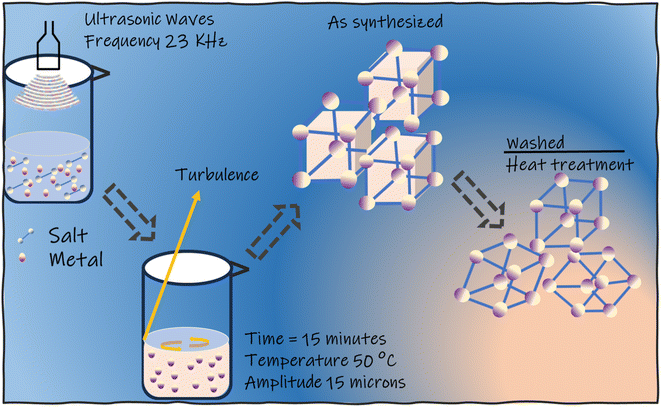
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v) in a beaker. Then, in 3 mL of the previously mentioned mixture, an equal quantity of copper acetate monohydrate was dissolved. Furthermore, HCl solution was added dropwise for combining the ligand and metal salt, which was then sonicated at an amplitude and frequency of 15 micron for 15 min, respectively, followed by temperature maintenance at 50 °C and slow evaporation. Resulting dark-blue needle-like crystals were obtained and were washed three times with DMF, DI water, acetone, and methanol before drying in the air.
70 v/v) in a beaker. Then, in 3 mL of the previously mentioned mixture, an equal quantity of copper acetate monohydrate was dissolved. Furthermore, HCl solution was added dropwise for combining the ligand and metal salt, which was then sonicated at an amplitude and frequency of 15 micron for 15 min, respectively, followed by temperature maintenance at 50 °C and slow evaporation. Resulting dark-blue needle-like crystals were obtained and were washed three times with DMF, DI water, acetone, and methanol before drying in the air.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v) 0.25 mmol of NIPA was dissolved. Following that, an equivalent amount of cobalt acetate monohydrate was mixed in 3 mL of the previously described mixture. The purification and synthesis processes for the cobalt-containing MOF were similar to those for the Cu(5-NIPA) MOF described above.
70 v/v) 0.25 mmol of NIPA was dissolved. Following that, an equivalent amount of cobalt acetate monohydrate was mixed in 3 mL of the previously described mixture. The purification and synthesis processes for the cobalt-containing MOF were similar to those for the Cu(5-NIPA) MOF described above.The mass loading on the electrode was calculated by mass balance according to eqn (1) below:
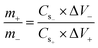 | (1) |
2.3 Characterization
The structural and electrochemical characteristics of the synthesized CuCo(5-NIPA), Nd2O3, and CuCo(5-NIPA)–Nd2O3 electrodes were assessed through several characterizations. For examining the surface, morphological, and elemental features of the electrodes, X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy dispersive X-ray analysis (EDX) were utilized. By utilizing a GAMRY Reference 3000 Potentiostat/Galvanostat, several techniques, including cyclic voltammetry (CV), galvanostatic charging and discharging (GCD), electrochemical impedance spectroscopy (EIS), and theoretical model fitting, were employed to assess the electrochemical performances of the electrodes. The synthesized electrode's performance was first evaluated in a three-cell configuration. Based on the results from the three-cell assembly, the top-performing sample was then assessed in a two-cell configuration for the battery–supercapacitor hybrid after being combined with activated carbon.3. Results and discussion
3.1 Structural characterization
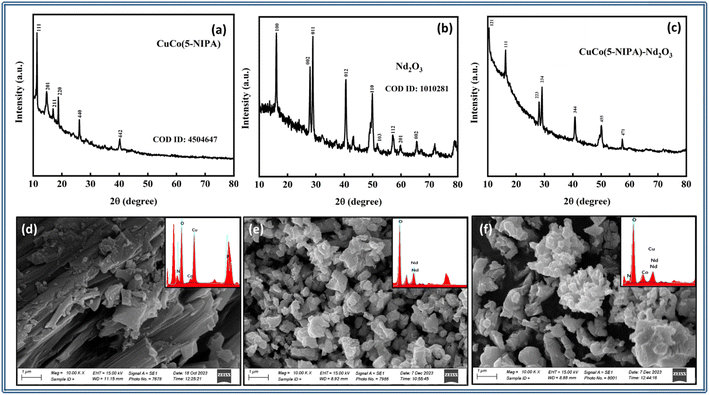
For analyzing the structural and morphological properties of CuCo(5-NIPA), Nd2O3, and their composite (CuCo(5-NIPA)–Nd2O3), XRD, EDX, and SEM were performed. Fig. 2(a)–(c) depict the XRD results, which revealed distinct peaks for CuCo(5-NIPA), Nd2O3, and their composite. The XRD patterns highlight the structural properties with the crystalline phases of the three samples. Moreover, the presence of peaks in the composite XRD pattern verified the existence of CuCo(5-NIPA) and Nd2O3.Following this, for further assessing the elemental analysis and morphological features of the synthesized electrodes, EDX and SEM were utilized. The SEM micrographs along with the respective elemental compositions (EDX) for CuCo(5-NIPA), Nd2O3, and CuCo(5-NIPA)–Nd2O3 are displayed in Fig. 2(d)–(f). From the micrographs, a flake-like morphology was noticed for CuCo(5-NIPA), which was attributed to their layered structure (a vital feature relevant to supercapacitor applications). A layered arrangement facilitates fast charge and discharge kinetics by accelerating the fundamental motion of ions in the electrode matrix, leading to an increase in ionic conductivity and rate capability for a material. Moreover, Nd2O3's crystalline nature could contribute to reducing the internal resistance and an increase in the energy-storage capacity through the effective conveyance, carrying, and conducting of charge. Furthermore, it could reduce structural degradation while promoting continuous ion adsorption and desorption, either of which could improve the supercapacitor's long-term robustness. Subsequently the composite CuCo(5-NIPA)–Nd2O3 showed that both Nd2O3 and CuCo(5-NIPA) coexisted. This offers more surface area and active sites by combining both their flaky and crystalline traits. These observations were further supported by electrochemical measurements in the half- and full-cells.
3.2 Half-cell assembly electrochemical characterization
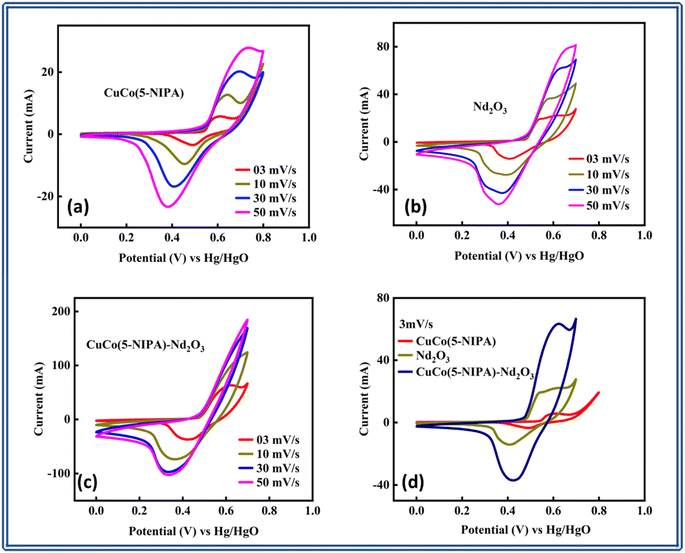 | ||
| Fig. 3 Cyclic voltammograms of (a) CuCo(5-NIPA), (b) Nd2O3, and (c) CuCo(5-NIPA)–Nd2O3, (d) comparison of all three samples. | ||
Moreover, the morphologies of the CV curves of CuCo(5-NIPA) remained unaltered even at a high scan rate of 50 mV s−1, demonstrating its exceptional stability, while there was minimal change in the CV shape of Nd2O3, with one notable exception of an alteration in some peak positions. These voltammogram patterns show that the reactions were diffusion regulated, with the mass transport rate acting as the governing factor. This shows that the synthesized electrodes are capable of rapid and reversible faradaic reactions, highlighting their potential value in electrochemical applications. On the other hand, the CuCo(5-NIPA) and Nd2O3 composite's CV shape showed notable alterations, revealing the dominancy of electrochemical kinetics. This indicates that by adding one element to the other's matrix the dynamics of the electrochemical process will change by providing new reaction avenues. Despite these modifications, the composite showed a larger integral area and peak current than the individual components, as shown by the comparison of the CV profiles of all electrodes at the lowest scan rate of 3 mV s−1 as illustrated in Fig. 3(d). CuCo(5-NIPA) most likely contributed to this improvement because of its large surface area and its abundance of redox-active sites derived from the cobalt and copper ions. Moreover, adding 5-NIPA could result in the emergence of novel functional groups or an upsurge in the quantity of charge-storage active sites that are easily accessible. The electrochemical performance of these sites was enhanced by the improved electron-transport mechanisms during charge/discharge cycles. Meanwhile, the electrochemically active Nd2O3 increased the number of redox-active centers in the composite. The presence of Nd2O3 facilitated the transition between Nd3+ and Nd4+ ions at the topmost layer, hence improving the total capacitance of the electrode material.
 | ||
| Fig. 4 Galvanostatic charge–discharge curves of (a) CuCo(5-NIPA), (b) Nd2O3, and (c) CuCo(5-NIPA)–Nd2O3, (d) comparison of the GCD curves of all three samples. | ||
An electrode's charge-storage capacity may be evaluated by examining its CV curves, which are represented in terms of the specific capacity (C g−1).
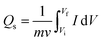 | (2) |
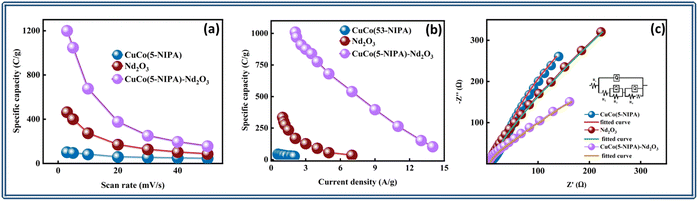
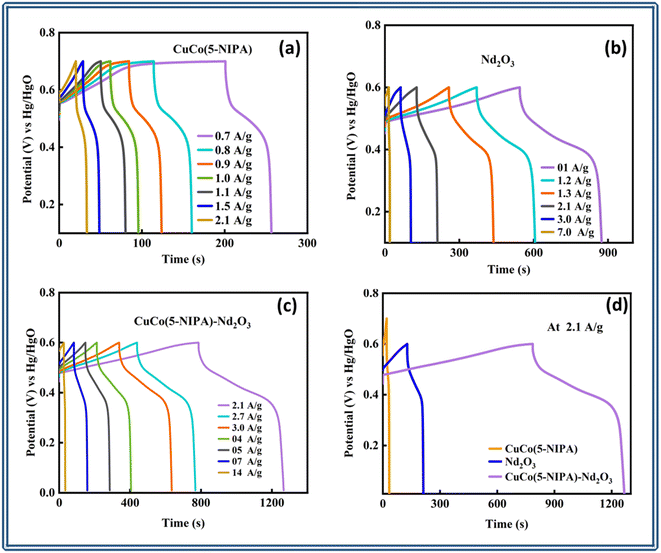
The area under the CV curve is defined by the integral component, which determines the specific capacity, and is designated as Qs in the equation above and represented in C g−1. The scan rate and mass of the active material is denoted by “ν” and “m”, respectively. According to the prior correlation, a cyclic voltammetry (CV) curve with a larger active area is associated with a greater specific capacity at any given scan rate. Fig. 5(a) depicts the Qs trend for all three samples in the half-cell assembly, where CuCo(5-NIPA), Nd2O3, and CuCo(5-NIPA)–Nd2O3 achieved Qs values of 105, 467, and 1202 C g−1, respectively, at a scan rate of 3 mV s−1. It is critical to note that when the scan rates increase, the specific capacity falls, because the swift kinetics of the electrolyte ions restrict their contact time with the conducting electrode material. Additionally, eqn (3) was used to calculate the specific capacities (Qs) of CuCo(5-NIPA), Nd2O3, and CuCo(5-NIPA)–Nd2O3 from the GCD analysis for all current densities.
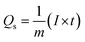 | (3) |
Fig. 5(b) illustrates the Qs for all samples determined by using the aforementioned relation at various current densities. The maximum Qs values for CuCo(5-NIPA), Nd2O3, and CuCo(5-NIPA)–Nd2O3 were 45.6, 355.7, and 1012.2 C g−1, respectively. At high current density values, Qs had a diminishing tendency because of the quick kinetics of the electrolyte ions, which prevented them from successfully interacting with the active electrode material. The data demonstrate that the CuCo(5-NIPA)–Nd2O3 composite had exceptional rate capacity, which supports the electrode material's greater performance.
3.3 Electrochemical characterizations of a hybrid supercapacitor
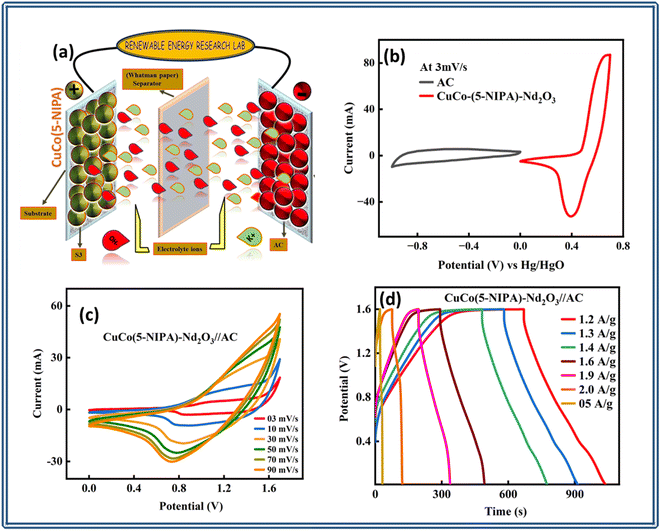
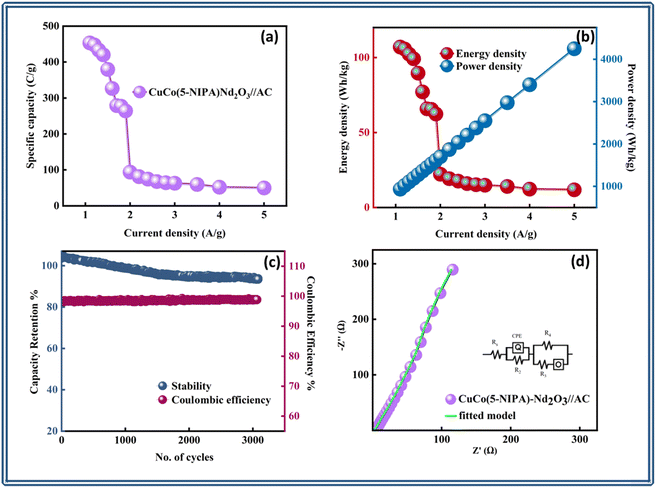
It is evident from the electrochemical evaluation of all the samples in the three-cell configuration that the composite of MOF and the oxide performed better. Therefore, in an asymmetric design, the device was fabricated with the best-performing sample and activated carbon (AC) as positive and negative electrode materials, respectively. Fig. 6(a) displays a schematic representation of the device (CuCo–(5-NIPA)–Nd2O3//AC). The CV results for the CuCo(5-NIPA)–Nd2O3 and activated carbon electrodes are shown in Fig. 6(b), where the presence and absence of redox peaks, respectively, validate the capacitive and the battery-grade nature of the AC and CuCo(5-NIPA)/Nd2O3, respectively. The redox peaks made it easy to observe that the sample was capacitive and diffusion controlled. The device's CV curves for CuCo–(5-NIPA)–Nd2O3//AC at various scan rates ranging from 3 to 90 mV s−1 are depicted in Fig. 6(c), which highlights the device's stability and high capacity due to its ability to maintain its shape even at high scan rates. Additionally, the rectangular zone and spikes in the CV profiles confirmed the existence of both faradaic and non-faradaic processes.The voltage window used in the CV measurements was repeated, and the GCD characterization was performed at different current density levels. The resulting charge–discharge curves are displayed in Fig. 6(d), where the non-linearity and humps in the GCD curves indicate both faradaic and non-faradaic charge-storage processes. The reversible electrochemical nature of the real device was illustrated by the regular symmetry of the curves. Notably, even at high scan rates, the GCD profiles of CuCo–(5-NIPA)–Nd2O3//AC remained unchanged, which emphasizes the device's exceptional rate capabilities. Furthermore, based on the GCD results, the specific capacities (Qs) were estimated, and their trends are shown in Fig. 7(a). CuCo–(5-NIPA)–Nd2O3//AC showed a specific capacity (Qs) of 467.5 C g−1, which demonstrates the device's amazing rate capacity.
The actual device's energy density and power density were estimated by using the following equations:
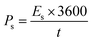 | (4) |
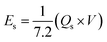 | (5) |
Furthermore, EIS tests were performed to evaluate the conductive properties of the CuCo–(5-NIPA)–Nd2O3//AC device. Fig. 7(d) depicts the Nyquist plot along with the fitted curve with an inset of the fitted circuit model R(Q(R))–(R(RO)). The device's exceptional conductivity could be attributed to its low ESR value of 0.13 Ω.
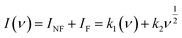 | (6) |
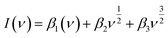 | (7) |
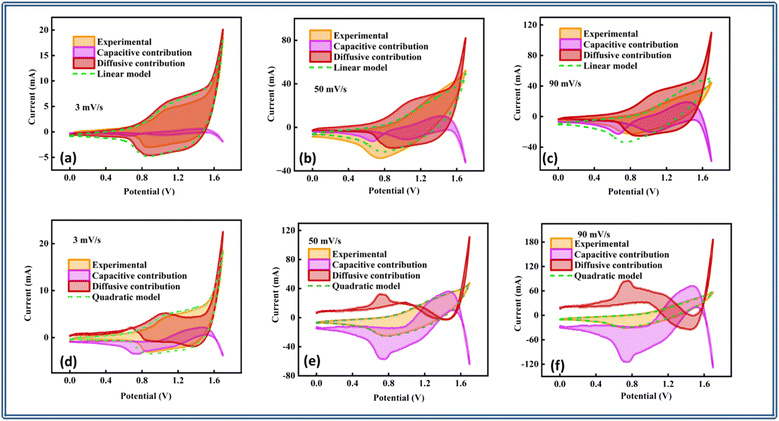 | ||
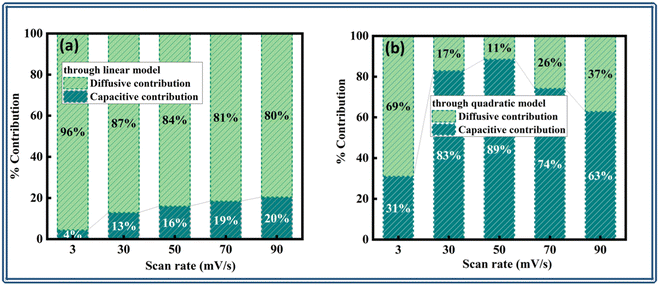
| Fig. 8 Capacitive diffusive contributions at 3, 50, and 90 mV s−1 obtained through the (a)–(c) linear model and (d)–(f) quadratic model. | ||
Furthermore, a bar plot was done that clearly illustrated the percentage fluctuation in the diffusive and capacitive components versus the matching scan speeds from both models, as shown in Fig. 9(a) and (b). The hybrid device's charge-storage chemistry demonstrated the involvement of both non-faradaic and faradaic (redox) processes due to the synergistic impact of the controlled diffusion and capacitive materials.
4. Conclusion
Concisely, for developing energy-storage devices, Nd2O3, CuCo(5-NIPA), and their composite, CuCo(5-NIPA)–Nd2O3, were electrochemically analyzed in half- and full-cell configurations. An in-depth study revealed the outstanding capabilities of the CuCo(5-NIPA)–Nd2O3 composite, with a specific capacity value of 1012.2 C g−1 at 2.1 A g−1. This promising outcome prompted us to apply it in a hybrid supercapacitor device as the positive electrode material. This combination produced a specific capacity of 467.5 C g−1. Furthermore, the real device exhibited exceptional energy-storage capacities, with a specific energy of 109.68 W h kg−1 and a specific power of 4507 W kg−1. Afterwards, the capacitive and diffusive contributions were deconvoluted through linear and quadratic models. These findings highlight the enormous potential and step forward toward the development of self-supported electrode materials for innovative energy-storage applications that require high-performance solutions. This shows the prospects for developing enhanced energy-storage technologies utilizing these novel electrode materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by Researchers Supporting Project number (RSP2024R448), King Saud University, Riyadh, Saudi Arabia.References
- M. Mamizadeh, S. M. Masoudpanah, M. S. Bafghi and M. P. Dabir, J. Energy Storage, 2023, 63, 106989 CrossRef.
- Y. Lin, S. Su, Y. Cui, H. Dai, L. Lai and X. Zhu, J. Mater. Sci.: Mater. Electron., 2023, 34, 1308 CrossRef CAS.
- S. Yao and Y. Zhu, Adv. Mater., 2015, 27, 1480–1511 CrossRef CAS PubMed.
- Z. Wu, L. Li, J. m. Yan and X. b. Zhang, Adv. Sci., 2017, 4, 1600382 CrossRef PubMed.
- T. Meng, Q.-Q. Xu, Z.-H. Wang, Y.-T. Li, Z.-M. Gao, X.-Y. Xing and T.-Z. Ren, Electrochim. Acta, 2015, 180, 104–111 CrossRef CAS.
- F. Yu, C. Zhang, F. Wang, Y. Gu, P. Zhang, E. R. Waclawik, A. Du, K. K. Ostrikov and H. Wang, Mater. Horiz., 2020, 7, 495–503 RSC.
- H. Shao, N. Padmanathan, D. McNulty, C. O'Dwyer and K. M. Razeeb, ACS Appl. Energy Mater., 2018, 2, 569–578 CrossRef.
- J. Iqbal, A. Numan, S. Rafique, R. Jafer, S. Mohamad, K. Ramesh and S. Ramesh, Electrochim. Acta, 2018, 278, 72–82 CrossRef CAS.
- J. Iqbal, A. Numan, R. Jafer, S. Bashir, A. Jilani, S. Mohammad, M. Khalid, K. Ramesh and S. Ramesh, J. Alloys Compd., 2020, 821, 153452 CrossRef CAS.
- S. Najib and E. Erdem, Nanoscale Adv., 2019, 1, 2817–2827 RSC.
- R. S. Kate, S. A. Khalate and R. J. Deokate, J. Alloys Compd., 2018, 734, 89–111 CrossRef CAS.
- Y. Cui, B. Li, H. He, W. Zhou, B. Chen and G. Qian, Accounts Chem. Res, 2016, 49, 483–493 CrossRef CAS PubMed.
- Z. Liu, F. Zheng, W. Xiong, X. Li, A. Yuan and H. Pang, SmartMat, 2021, 2, 488–518 CrossRef CAS.
- L. Wang, Y. Han, X. Feng, J. Zhou, P. Qi and B. Wang, Coord. Chem. Rev., 2016, 307, 361–381 CrossRef CAS.
- H. Wang, Q.-L. Zhu, R. Zou and Q. Xu, Chem, 2017, 2, 52–80 CAS.
- S. Zheng, H. Xue and H. Pang, Coord. Chem. Rev., 2018, 373, 2–21 CrossRef CAS.
- F. Y. Yi, R. Zhang, H. Wang, L. F. Chen, L. Han, H. L. Jiang and Q. Xu, Small Methods, 2017, 1, 1700187 CrossRef.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS PubMed.
- D. G. Nocera, Accounts Chem. Res, 2012, 45, 767–776 CrossRef CAS PubMed.
- R. Schlögl, Angew. Chem., Int. Ed., 2011, 50, 6424–6426 CrossRef PubMed.
- M. A. Farrukh, K. M. Butt, A. Altaf and S. Khadim, Silicon, 2019, 11, 2591–2598 CrossRef CAS.
- X. Li, F. Zhang and D. Zhao, Nano Today, 2013, 8, 643–676 CrossRef CAS.
- Y. Zhang, Z. Song and F. Dong, J. Lumin., 2012, 132, 2462–2467 CrossRef CAS.
- J. Feng, R. Tang, G. Liu and T. Meng, Chem. Eng. J., 2023, 452, 139131 CrossRef CAS.
- J. Xu, H. Zou, H. Li, G. Li, S. Gan and G. Hong, J. Alloys Compd., 2010, 490, 552–556 CrossRef CAS.
- H. R. Naderi, M. R. Ganjali, A. S. Dezfuli and P. Norouzi, RSC Adv., 2016, 6, 51211–51220 RSC.
- H. Li, S. X. Wang, Z. Huang, S. Zhang, Y. Li, L. Han and Z. Tan, Polym. Adv. Technol., 2014, 25, 1163–1168 CrossRef CAS.
- K. T. Kubra, R. Sharif, B. Patil, A. Javaid, S. Shahzadi, A. Salman, S. Siddique and G. Ali, J. Alloys Compd., 2020, 815, 152104 CrossRef CAS.
- J. Yang, F. Zhang, H. Lu, X. Hong, H. Jiang, Y. Wu and Y. Li, Angew. Chem., 2015, 127, 11039–11043 CrossRef.
- J. Yu, C. Mu, B. Yan, X. Qin, C. Shen, H. Xue and H. Pang, Mater. Horiz., 2017, 4, 557–569 RSC.
- S.-P. Chen, Y.-X. Ren, W.-T. Wang and S.-L. Gao, Dalton Trans., 2010, 39, 1552–1557 RSC.
- M.Z. Iqbal, J. Electroanal. Chem., 2020, 879, 114812 CrossRef CAS.
- W.-w. Zhan, Q. Kuang, J.-z. Zhou, X.-j. Kong, Z.-x. Xie and L.-s. Zheng, J. Am. Chem. Soc., 2013, 135, 1926–1933 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |