Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The influence of hydrogen bonding on the structure of organic–inorganic hybrid catalysts and its application in the solvent-free epoxidation of α-olefins†
Hong-Bin Ju‡
abde,
Li-Zhi Zhang‡ce,
De-Bao Li*a,
Tao Geng *be,
Ya-Jie Jiangbe and
Ya-Kui Wangbe
*be,
Ya-Jie Jiangbe and
Ya-Kui Wangbe
aInstitute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, Shanxi, China. E-mail: dbli@sxicc.ac.cn
bChina Research Institute of Daily Chemistry Co., Ltd, Taiyuan 030001, Shanxi, China. E-mail: anhuayanjiushi@163.com
cChina Research Institute of Daily Chemical Industry, Taiyuan 030001, Shanxi, China
dUniversity of Chinese Academy of Sciences, Beijing 100049, China
eShanxi Key Laboratory of Functional Surfactants, Taiyuan 030001, Shanxi, China
First published on 22nd April 2024
Abstract
In this study, two types of catalysts were prepared by the combination of gemini quaternary ammonium salt with two distinct species of phosphotungstic acid. Catalysts prepared by the Wells–Dawson type of phosphotungstic acid and Keggin-type phosphotungstic acid both exhibited dual-phase catalytic behavior, demonstrating both heterogeneous and homogeneous catalytic activities. In comparison to the catalyst prepared by the Keggin-type phosphotungstic acid, due to the higher size of Wells–Dawson type of phosphotungstic acid, hydrogen bonding could not effectively affect the catalyst prepared by H6P2W18O62. Subsequently, the influential factors on the catalytic reaction were investigated. Through the utilization of techniques such as XPS, FT-IR, Raman spectra and other characterization methods, two distinct structure and reaction mechanisms for these catalysts were elucidated under the influence of hydrogen bonding.
1. Introduction
Epoxides represent crucial raw materials for the synthesis of fine chemicals, finding extensive applications in nanomaterials, surfactants, food additives, and cosmetics.1,2 Industrially, olefin epoxidation has consistently remained the primary method for producing epoxides. The 1,2-epoxy alkanes synthesized by α-olefins serve as specific intermediates for synthesizing high-value chemicals,3,4 presenting a distinct application potential, particularly in the realm of surfactants.5,6In the context of olefin epoxidation, catalysts have played a pivotal role.7,8 Broadly, these catalysts can be categorized as either metal-based or inorganic catalysts.9,10 Among metal-based catalysts, transition metal-based catalysts have garnered extensive attention owing to their efficient atomic utilization of oxidants.11 Typically, these catalysts are prepared into metal–organic frameworks,6 polymers,12,13 phase-transfer catalysts (PTCs),14 and microemulsions15 to enhance their catalytic efficiency. The majority of these catalysts are heterogeneous in nature, they may pose certain risks due to the rigorous reaction conditions required for achieving high yields.16 Furthermore, the limited solubility of certain types of homogeneous catalysts under catalytic conditions, along with challenges in separation processes, present significant hurdles for researchers.17
As a kind of widely used phase-transfer catalysts (PTCs), Ishii–Venturello catalysts18 have gained widespread use in epoxidation, owing to their mild catalytic conditions and recyclable characteristics.19 Prepared by heteropoly acid and ammonium salt,20 these catalysts offer a blend of advantages associated with both heterogeneous and homogeneous catalysts. However, the addition of solvents in the catalytic process for catalyst protection contradicts the principles of green chemistry.21,22 Catalysts developed for olefin epoxidation under solvent-free conditions were generally only used to oxidize cycloolefins23 or require more stringent reaction conditions.24 Consequently, the present research goals lie in enabling catalysts to function without the addition of solvents.
PTCs prepared by polyoxometalates and Gemini quaternary ammonium salts exhibited an enhanced ability to penetrate the oil–water interface to the oil phase under solvent-free conditions. Not only did they demonstrate exceptional catalytic performance, but also facilitated easier recovery from the reaction system.25,26 Among these catalysts, the utilization of Keggin-type polyoxometalates (POMs) was prevalent due to their outstanding oxidation catalytic performance.27,28 In theory, by the formation of hydrogen bonds in POM, on the one hand, suggesting strong host–guest interactions in two substances could be enhanced,29 on the other hand, such strong interactions tightened interface contact and accelerated interface electron transfer of catalyst,30 making the catalytic performance more effective. Wells–Dawson-type phosphotungstic acid, characterized by its nanoscale dimensions of approximately 1 nm, can be viewed as an enhanced Keggin-type cluster, offering a greater number of organic ligands.31,32 Additionally, it provides more redox active sites. Despite these advantages, this species has not been as extensively employed in epoxidation reactions.33,34
In this study, two different types of organic–inorganic composite catalyst was prepared, incorporating Wells–Dawson-type phosphotungstic acid and Keggin-type phosphotungstic acid in combination with a gemini quaternary ammonium salt. Compared with our previous study,35 due to the addition of hydroxyl groups in the quaternary ammonium salt, the new catalyst prepared by Keggin-type phosphotungstic acid showed distinct microstructure and reaction mechanism. Subsequently, a comparative analysis was performed between catalysts prepared by Keggin type and Wells–Dawson type phosphotungstic acid, leading to the deduction of distinct reaction mechanisms underlying these catalysts.
2. Material and methods
2.1 Materials
1,3-Dichloro-2-propanol, propanol, sodium carbonate, 1-dodecene, 1-octene, 1-tetradecene, 1-hexadecene, hydrogen peroxide, phosphotungstic acid, phosphoric acid ethyl alcohol, and methyl alcohol were purchased from Aladdin. Diethyl ether, dodecyl-tetradecyl-dimethylamine, and sodium tungstate were purchased from Wilmar. The above reagents were analytically pure samples. The standard samples used for gas chromatography (1-dodecene, 1-octene, dodecane epoxide and epoxyoctane) were chromatographically pure and purchased from Aladdin.2.2 Synthesis of surfactant DDC
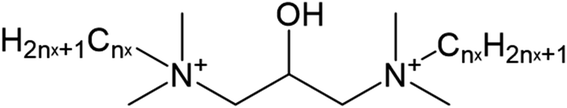
The chemical structure of N1,N3-didodecyl,tetradecyl-2-hydroxy-N1,N1,N3,N3-tetramethyl-1,3-propane-diaminium chloride (DDC) was depicted in Scheme 1, which was a type of mixture, with the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of dodecylamine to tetradecylamine. The synthesis was conducted under oxygen-free conditions. Specifically, 2 mol of 1,3-dichloro-2-propanol were added into a reactor containing 0.4 mol of dodecyl, tetradecyl-dimethylamine in 70 mL of propanol, with a total mass of 0.3 wt% sodium carbonate acting as the catalyst. The reaction mixture was then refluxed at 60 °C for a period of 4–6 hours. After the reaction, a white precipitate was obtained through vacuum distillation for solvent removal, followed by 3 times recrystallization using a solvent mixture of ethyl acetate and ethanol (20
1 molar ratio of dodecylamine to tetradecylamine. The synthesis was conducted under oxygen-free conditions. Specifically, 2 mol of 1,3-dichloro-2-propanol were added into a reactor containing 0.4 mol of dodecyl, tetradecyl-dimethylamine in 70 mL of propanol, with a total mass of 0.3 wt% sodium carbonate acting as the catalyst. The reaction mixture was then refluxed at 60 °C for a period of 4–6 hours. After the reaction, a white precipitate was obtained through vacuum distillation for solvent removal, followed by 3 times recrystallization using a solvent mixture of ethyl acetate and ethanol (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
2.3 Synthesis of Wells–Dawson type phosphotungstic acid
Wells–Dawson phosphotungstic acid was synthesized by the documented method:36 50 g NaWO4·2H2O was dissolved in 60 mL distilled water, then poured 35.0 mL of concentrated phosphoric acid. The mixture was heated for 20 minutes until it turned milky white, then further heated to 120 °C for 8 hours. After the reaction, the mixture was cooled down to room temperature, and a specific quantity of concentrated hydrochloric acid was added to acidify the mixture. Diethyl ether was added to extract the phosphotungstic acid. Upon phase separation, the lower diethyl ether phase, consisted with diethyl ether, water and phosphotungstic acid were selected, and diethyl ether was evaporated under 40 °C conditions. The resulting residue was subjected to drying until a constant weight was achieved, yielding the final light yellow solid H6P2W18O62·14H2O.2.4 Catalyst preparation
1 mmol H6P2W18O62·14H2O was dissolved in the 20 mL H2O2 (30wt%), and solution of 3 mmol of the surfactant DDC in 20 mL of anhydrous ethanol was also prepared. The anhydrous ethanol–DDC mixture was then added to the H6P2W18O62–H2O2 mixture and stirred for 1 hour. The resulting suspension was washed with anhydrous ethanol and repeated 3 or 4 times. Following filtration, the obtained precipitate was subjected to vacuum drying for a minimum of 12 hours, yielding the catalyst (P2W18-DDC).1 mmol H3PW12O40·xH2O was dissolved in the 20 mL H2O2 (30wt%), and solution of 3 mmol of the surfactant DDC in 20 mL of anhydrous ethanol was also prepared. The anhydrous ethanol–DDC mixture was then added to the H3PW12O40–H2O2 mixture and stirred for 1 hour. The resulting suspension was washed with anhydrous ethanol and repeated 3 or 4 times. Following filtration, the obtained precipitate was subjected to vacuum drying for a minimum of 12 hours, yielding the catalyst (PW12-DDC).
2.5 Catalyst testing
0.1 mol 1-dodecene, 0.1 mol H2O2 (30% (mass) solution in water), and 5wt% catalysts were added into the flask, the mixture was heated by the heating jacket and stirred by a polytetrafluoroethylene stirring paddle, besides, a mercurial thermometer was immersed into the solution to make accurate temperature measurements. Due to the different reaction conditions of these 2 catalysts, the specific reaction conditions would be provided in results and discussion.
Analysis of reaction products were used by external standard method. The conversion of α-olefin and selectivity of epoxyalkane were calculated according to the formula below: Conversion X = (C0 − Ct)/C0, C0, Ct represents the initial concentration of the reactant and the concentration at time t respectively. Yield γ = Cpt/Cp0, Cpt, Cp0 represent the concentration at time t and the theoretical concentration of the product respectively. Selectivity S = γ/X. The curves of standard samples were given in ESI (Fig. S1†).
3. Results and discussion
3.1 Catalytic testing
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of H2O2 to 1-dodecene, dosage of substrate: 0.1 mol. As depicted in Table 1, after 6 hours reaction, P2W18-DDC exhibited higher selectivity towards dodecane epoxide but lower conversion of 1-dodecene compared to PW12-DDC. In contrast to PW12-DDC, almost all of the P2W18-DDC catalyst could be recovered after the reaction.
1 ratio of H2O2 to 1-dodecene, dosage of substrate: 0.1 mol. As depicted in Table 1, after 6 hours reaction, P2W18-DDC exhibited higher selectivity towards dodecane epoxide but lower conversion of 1-dodecene compared to PW12-DDC. In contrast to PW12-DDC, almost all of the P2W18-DDC catalyst could be recovered after the reaction.
| No. | Catalyst | Conversion/% | Selectivity/% | Catalyst state |
|---|---|---|---|---|
| 1 | PW12-DDC | 49 | 85 | Partial soluble |
| 2 | P2W18-DDC | 35 | 90 | Insoluble |
Subsequently, the catalytic performance of different olefins under identical conditions was investigated (Table S1†). P2W18-DDC exhibited higher selectivity towards epoxides and lower conversion to olefins, especially notable in the case of 1-tetradecene, which showed 93% selectivity to tetradecane epoxide and 36% conversion. In comparison with other catalysts,37,38 P2W18-DDC demonstrated superior selectivity in the epoxidation of long-chain olefins within a relatively short reaction time (only 6 hours), although with the lower olefin conversion. Additionally, a notable advantage of this catalyst was its ability to maintain high catalytic activity under solvent-free conditions and be readily reused through a straightforward filtration process by anhydrous ethanol, thus reducing production costs and aligning with the concept of green chemistry.
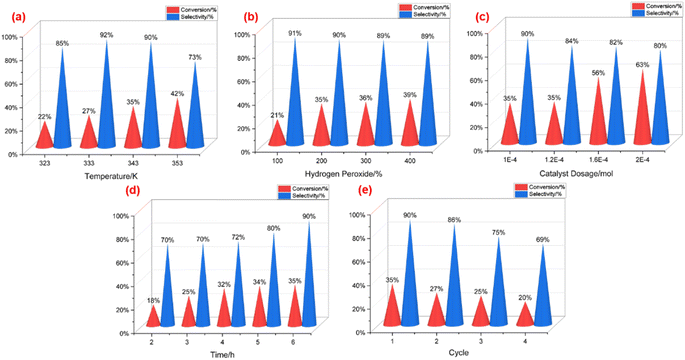
Initially, the impact of temperature was examined under other invariant reaction conditions (Fig. 1a), with all reaction times kept six hours. As the temperature surpassed 323 K, the conversion of 1-dodecene exhibited a steady increase, while the selectivity of dodecane epoxide initially rose before subsequently declining. At 333 K, P2W18-DDC demonstrated an optimal selectivity of 92% towards dodecane epoxide. Moreover, the yield of epoxy dodecane continued to rise as the temperature elevated from 323 K to 353 K. Considering both selectivity and product yield, 343 K was selected as the most suitable temperature for the reaction.
Subsequently, the impact of hydrogen peroxide was examined under 343 K with other invariant reaction conditions. The initial ratio of H2O2 to 1-dodecene was varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. As depicted in Fig. 1b, elevated volumes of hydrogen peroxide led to greater conversion of 1-dodecene but lower selectivity of dodecane epoxide. Notably, the selectivity of dodecane epoxide dropped below 90% when the ratio of hydrogen peroxide to 1-dodecene was 2
1. As depicted in Fig. 1b, elevated volumes of hydrogen peroxide led to greater conversion of 1-dodecene but lower selectivity of dodecane epoxide. Notably, the selectivity of dodecane epoxide dropped below 90% when the ratio of hydrogen peroxide to 1-dodecene was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Consequently, an initial ratio of 2
1. Consequently, an initial ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for H2O2 and 1-dodecene was deemed the relatively suitable condition.
1 for H2O2 and 1-dodecene was deemed the relatively suitable condition.
Next, the influence of catalyst dosage was investigated using four different dosage levels, while keeping other conditions constant. As illustrated in Fig. 1c, P2W18-DDC exhibited the lowest conversion of 1-dodecene, along with the highest selectivity towards epoxy dodecane, at 1 × 10−4 mol catalyst dosage. With an increase in catalyst dosage, the interfacial tension between oil and water decreased due to the presence of long-chain quaternary ammonium salt. This facilitated the formation of smaller droplets and increased the interface area between water phase and oil phase, thereby enhancing the reaction rate. The conversion of 1-dodecene continued to rise, and the selectivity of epoxy dodecane decreased from 90% to 80% as the catalyst dosage increased from 1 × 10−4 mol to 2 × 10−4 mol. This trend indicated that an elevated amount of catalyst also resulted in the formation of by-products.
Under the determined appropriate conditions, the influence of reaction time was investigated for 2 to 6 hours. As depicted in Fig. 1d, the selectivity of epoxy dodecane exhibited a rapid increase as the reaction progressed, reaching its maximum at 6 hours, while the conversion of 1-dodecene increased at a slower rate.
Finally, cycle experiments were conducted under the specified conditions: Catalyst dosage at 1 × 10−4 mol; rotational speed at 250 rpm; temperature at 343 K; and an initial ratio of H2O2 to 1-dodecene at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The catalyst was subsequently filtered for recycling and washed with anhydrous ethanol at least three times after each reaction cycle. The outcomes were presented in Fig. 1e. As the P2W18-DDC catalyst was reused, the conversion of 1-dodecene experienced a gradual reduction, while the selectivity of epoxy dodecane dropped steeply after three cycles of recycling. These findings suggested that the catalyst possesses a limited recycling capacity.
1. The catalyst was subsequently filtered for recycling and washed with anhydrous ethanol at least three times after each reaction cycle. The outcomes were presented in Fig. 1e. As the P2W18-DDC catalyst was reused, the conversion of 1-dodecene experienced a gradual reduction, while the selectivity of epoxy dodecane dropped steeply after three cycles of recycling. These findings suggested that the catalyst possesses a limited recycling capacity.
3.2 Catalyst characterization
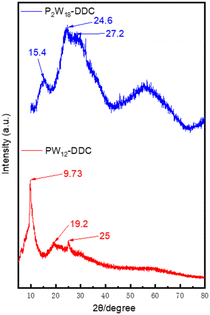
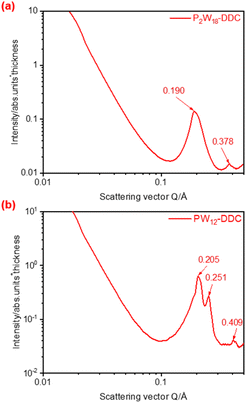
To further determine the microstructure of the catalyst, SAXS analysis was used to characterize P2W18-DDC and PW12-DDC. In the SAXS pattern presented in Fig. 3a, P2W18-DDC exhibited two scattering peaks arising from ordered structures, characterized by q values of 0.190 nm−1 and 0.378 nm−1, respectively. The nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of the q values was indicative of lamellar phases. Conversely, in the SAXS pattern of PW12-DDC (Fig. 3b), the q-value ratio was approximately 1
2 ratio of the q values was indicative of lamellar phases. Conversely, in the SAXS pattern of PW12-DDC (Fig. 3b), the q-value ratio was approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √1.5
√1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √4, indicating an irregular layered arrangement. The interlayer spacing (d-spacing) was determined from the q1 value of the first scattering peak using the formula d1 = 2π/q1. For P2W18-DDC, the calculated d value was 3.3 nm, while for PW12-DDC it was 3.06 nm. This observation suggested that P2W18-DDC possessed a greater layer spacing, which could be attributed to the larger peroxy structure of Wells–Dawson-type phosphotungstic acid. Furthermore, compared with the previously prepared catalys,35 quaternary ammonium salts with shorter chain lengths in PW12-DDC had performed longer interlayer spacing and more irregular lamellar phases, indicated that with the participation of hydrogen bonding, C–OH in DDC could combine a certain quantity of peroxy phosphotungstic acid, facilitated the binding of a certain quantity of peroxy phosphotungstic acid, resulting in the irregular arrangement between phosphotungstic acid molecules to form PW12-DDC, while the larger peroxy structure of Wells–Dawson-type phosphotungstic acid could not accommodate more phosphotungstic acid unit, made the hydrogen bonding ineffective. Due to the enhanced capacity of Wells–Dawson-type phosphotungstic acid to bind quaternary ammonium cations, scanning electron microscopy (SEM) was used to provide detailed micromorphological information. The micrographs of P2W18-DDC and PW12-DDC were presented in Fig. S3 and S4,† respectively. The layered structures of both catalysts were observed, P2W18-DDC demonstrated a more obvious and denser layered arrangement in comparison to PW12-DDC.
√4, indicating an irregular layered arrangement. The interlayer spacing (d-spacing) was determined from the q1 value of the first scattering peak using the formula d1 = 2π/q1. For P2W18-DDC, the calculated d value was 3.3 nm, while for PW12-DDC it was 3.06 nm. This observation suggested that P2W18-DDC possessed a greater layer spacing, which could be attributed to the larger peroxy structure of Wells–Dawson-type phosphotungstic acid. Furthermore, compared with the previously prepared catalys,35 quaternary ammonium salts with shorter chain lengths in PW12-DDC had performed longer interlayer spacing and more irregular lamellar phases, indicated that with the participation of hydrogen bonding, C–OH in DDC could combine a certain quantity of peroxy phosphotungstic acid, facilitated the binding of a certain quantity of peroxy phosphotungstic acid, resulting in the irregular arrangement between phosphotungstic acid molecules to form PW12-DDC, while the larger peroxy structure of Wells–Dawson-type phosphotungstic acid could not accommodate more phosphotungstic acid unit, made the hydrogen bonding ineffective. Due to the enhanced capacity of Wells–Dawson-type phosphotungstic acid to bind quaternary ammonium cations, scanning electron microscopy (SEM) was used to provide detailed micromorphological information. The micrographs of P2W18-DDC and PW12-DDC were presented in Fig. S3 and S4,† respectively. The layered structures of both catalysts were observed, P2W18-DDC demonstrated a more obvious and denser layered arrangement in comparison to PW12-DDC.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, and the molar ratio of P to O was nearly 2
7, and the molar ratio of P to O was nearly 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39. The molar ratio of P to W was 2
39. The molar ratio of P to W was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11. Moreover, the molar ratio of P to N was nearly 4
11. Moreover, the molar ratio of P to N was nearly 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, indicating that 1 mol Wells–Dawson type phosphotungstic acid molecule was capable of combining with a maximum of 2.25 mol surfactant DDC molecules. This suggested that the charges carried by Dawson type peroxy phosphotungstic acid was lower than the theoretical calculations. Furthermore, in theory, the molar ratio of W to P in PW12-DDC was 4
9, indicating that 1 mol Wells–Dawson type phosphotungstic acid molecule was capable of combining with a maximum of 2.25 mol surfactant DDC molecules. This suggested that the charges carried by Dawson type peroxy phosphotungstic acid was lower than the theoretical calculations. Furthermore, in theory, the molar ratio of W to P in PW12-DDC was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, N to P was nearly 3
1, N to P was nearly 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, but in fact W to P in PW12-DDC was approximately 10
1, but in fact W to P in PW12-DDC was approximately 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, N to P was nearly 8
1, N to P was nearly 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, far greater than the ideal structure reported previously.37 Although it is reported that the peroxy phosphotungstic acid anion would be converted to a more stable structure of PW11/PW12 during the immobilization process, more quaternary ammonium salts combined with Keggin type peroxy phosphotungstic acid in PW12-DDC might be attributed to the hydrogen bonding between peroxy phosphotungstic acid and surfactant DDC.
1, far greater than the ideal structure reported previously.37 Although it is reported that the peroxy phosphotungstic acid anion would be converted to a more stable structure of PW11/PW12 during the immobilization process, more quaternary ammonium salts combined with Keggin type peroxy phosphotungstic acid in PW12-DDC might be attributed to the hydrogen bonding between peroxy phosphotungstic acid and surfactant DDC.
| C/% | H/% | O/% | N/% | P/% | W/% | |
|---|---|---|---|---|---|---|
| P2W18-DDC | 24.11 | 55.67 | 14.06 | 1.56 | 0.68 | 3.92 |
| PW12-DDC | 25.88 | 58.94 | 11.45 | 1.59 | 0.19 | 1.95 |
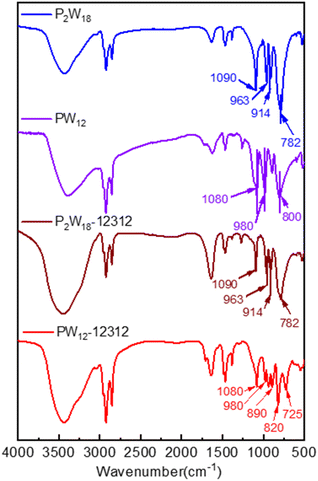
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 890 cm−1 (W–Ob–W), and 725 cm−1 (W–Oc–W), compared with the Keggin-type phosphotungstic acid at 1080 cm−1 (P–O), 980 cm−1 (W
O), 890 cm−1 (W–Ob–W), and 725 cm−1 (W–Oc–W), compared with the Keggin-type phosphotungstic acid at 1080 cm−1 (P–O), 980 cm−1 (W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 890 cm−1 (W–Ob–W), and 800 cm−1 (W–Oc–W), the peaks at 820 cm−1 was attributed to the –O–O–, while the peaks of W–Oc–W performed a red shift from 800 cm−1 to 725 cm−1, which could not be observed at hydroxy-free quaternary ammonium salt and phosphotungstate acid system, leading to the formation of hydrogen bonding between C–OH and W–Oc–W, moreover, the new band at 938 cm−1 originally branched from 980 cm−1 for W
O), 890 cm−1 (W–Ob–W), and 800 cm−1 (W–Oc–W), the peaks at 820 cm−1 was attributed to the –O–O–, while the peaks of W–Oc–W performed a red shift from 800 cm−1 to 725 cm−1, which could not be observed at hydroxy-free quaternary ammonium salt and phosphotungstate acid system, leading to the formation of hydrogen bonding between C–OH and W–Oc–W, moreover, the new band at 938 cm−1 originally branched from 980 cm−1 for W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O was assignable to the reduced W5+ species due to the intramolecular charge transfer from quaternary ammonium salt to terminal oxygen of PW.41 Conversely, the peaks at 914, 963, and 1090 cm−1 showed the successful synthesis of Wells–Dawson type of phosphotungstic acid, with the similar peaks in catalyst P2W18-DDC, they exhibited common corresponding to the vibration of W–Ob–W, W
O was assignable to the reduced W5+ species due to the intramolecular charge transfer from quaternary ammonium salt to terminal oxygen of PW.41 Conversely, the peaks at 914, 963, and 1090 cm−1 showed the successful synthesis of Wells–Dawson type of phosphotungstic acid, with the similar peaks in catalyst P2W18-DDC, they exhibited common corresponding to the vibration of W–Ob–W, W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and P–O, respectively. In conjunction with the elemental analysis results, treatment with H2O2 resulted in the conversion of partial H6P2W18O62 into a peroxy structure. No significant shift was observed in P2W18-DDC, indicating that the combination of Dawson type phosphotungstic acid and quaternary ammonium salts occurred solely through ionic bonding. Furthermore, both catalysts retained the characteristic peak of W–Oc–W, confirming the preservation of their Keggin and Wells–Dawson type phosphotungstic acid structures.
O, and P–O, respectively. In conjunction with the elemental analysis results, treatment with H2O2 resulted in the conversion of partial H6P2W18O62 into a peroxy structure. No significant shift was observed in P2W18-DDC, indicating that the combination of Dawson type phosphotungstic acid and quaternary ammonium salts occurred solely through ionic bonding. Furthermore, both catalysts retained the characteristic peak of W–Oc–W, confirming the preservation of their Keggin and Wells–Dawson type phosphotungstic acid structures.
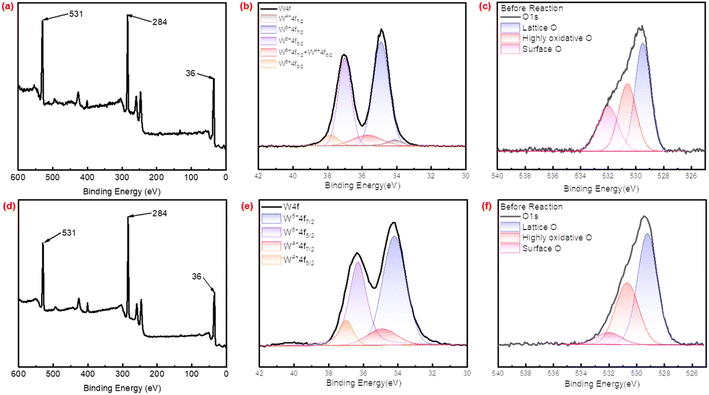
In the O 1s spectra in Fig. 5c and f, the peak fitting revealed three distinct peaks, the peak at 529.5 eV was attributed to lattice oxygen, while the peak at 530.5 eV corresponded to highly oxidative oxygen species, the peak at 531.5 eV was attributed to surface oxygen species. The surface O in catalyst could be classified as reaction intermediates and other active O species, such as W(O)2 and less C–OH of quaternary ammonium salt, while W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O could be attributed to highly oxidative O, which was reported to be closely correlated to surface O, could participate in the reaction by combining O to become an active substance, W–O–W could be attributed to lattice O.43,44 During the catalytic reaction, only surface oxygen, along with highly oxidative oxygen participate in the reaction process. It was worthy noting that although the relative content of quaternary ammonium salts in PW12-DDC was relatively higher than P2W18-DDC, the content of surface O specie was just 9.36%, lower than 24.87% in P2W18-DDC, but the content of highly oxidative O specie and FWHM were higher than P2W18-DDC, which could be attributed to the formation of hydrogen bonding between phosphotungstic acid and surfactant DDC during the preparation of PW12-DDC.
O could be attributed to highly oxidative O, which was reported to be closely correlated to surface O, could participate in the reaction by combining O to become an active substance, W–O–W could be attributed to lattice O.43,44 During the catalytic reaction, only surface oxygen, along with highly oxidative oxygen participate in the reaction process. It was worthy noting that although the relative content of quaternary ammonium salts in PW12-DDC was relatively higher than P2W18-DDC, the content of surface O specie was just 9.36%, lower than 24.87% in P2W18-DDC, but the content of highly oxidative O specie and FWHM were higher than P2W18-DDC, which could be attributed to the formation of hydrogen bonding between phosphotungstic acid and surfactant DDC during the preparation of PW12-DDC.
3.3 Mechanism analysis
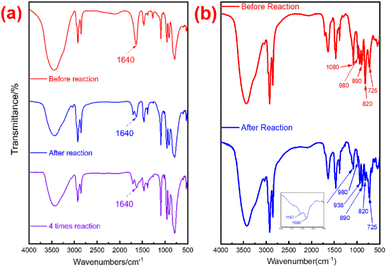
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and P–O, respectively, were still prominently present. Interestingly, in comparison to after reaction P2W18-DDC, the majority of the W
O, and P–O, respectively, were still prominently present. Interestingly, in comparison to after reaction P2W18-DDC, the majority of the W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks and –O–O– peaks in PW12-DDC nearly vanished (at 980 cm−1 and 820 cm−1), leaving only very faint residual peaks, the peak of W–Oc–W at 725 cm−1 increased noticeably, which was not observed before.35 The reason for these significant conversion among W
O peaks and –O–O– peaks in PW12-DDC nearly vanished (at 980 cm−1 and 820 cm−1), leaving only very faint residual peaks, the peak of W–Oc–W at 725 cm−1 increased noticeably, which was not observed before.35 The reason for these significant conversion among W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, W(O2) and W–Oc–W might not only be due to the transformation from W
O, W(O2) and W–Oc–W might not only be due to the transformation from W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O to W(O2), the peroxidation from W–Oc–W to W(O2) induced by hydrogen bonding also involved in the reaction during the catalytic reaction. Furthermore, the peaks at 1080 cm−1 and 1107 cm−1 also confirmed the formation of hydrogen bonding among the terminal oxygen atoms of PW12 anions and the quaternary ammonium salt,45 leading to the redox properties of W species tuned by W–O⋯H.
O to W(O2), the peroxidation from W–Oc–W to W(O2) induced by hydrogen bonding also involved in the reaction during the catalytic reaction. Furthermore, the peaks at 1080 cm−1 and 1107 cm−1 also confirmed the formation of hydrogen bonding among the terminal oxygen atoms of PW12 anions and the quaternary ammonium salt,45 leading to the redox properties of W species tuned by W–O⋯H.
The XPS spectra of O 1s and W 4f of utilized P2W18-DDC and PW12-DDC were demonstrated in Fig. S9.† After reaction, the ratio of surface O (C–OH and W(O2)) to lattice O (W–O–W) for the catalyst was lower than before the reaction in both catalysts. This reduction in ratio likely contributed to the decrease in catalytic reactivity of catalysts, but the content of highly oxidative O nearly unchanged, indicating the transformation between W(O2) and W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Notably, after the reaction, the content of W6+ in P2W18-DDC have been increased with the decreased content of W4+(Fig. S9d†), with a narrower FWHM of W5+ peaks in 34.9 eV. This observation suggested that during the reaction, some of the W5+ and W4+ species were oxidized back to higher valence states. Conversely, in the W 4f spectra of PW12-DDC (Fig. S9b†), the surface tungsten (W) species of PW12-DDC predominantly existed in the +5 valence state or lower. After the reaction, a decrease in W5+ contents have been observed, while the content of highly oxidative O and lattice O increased obviously, indicating the formation of WOX groups instead of the original structure of phosphotungstic acid,44 leading to the weakening of layered structure observed on the SEM image.
O. Notably, after the reaction, the content of W6+ in P2W18-DDC have been increased with the decreased content of W4+(Fig. S9d†), with a narrower FWHM of W5+ peaks in 34.9 eV. This observation suggested that during the reaction, some of the W5+ and W4+ species were oxidized back to higher valence states. Conversely, in the W 4f spectra of PW12-DDC (Fig. S9b†), the surface tungsten (W) species of PW12-DDC predominantly existed in the +5 valence state or lower. After the reaction, a decrease in W5+ contents have been observed, while the content of highly oxidative O and lattice O increased obviously, indicating the formation of WOX groups instead of the original structure of phosphotungstic acid,44 leading to the weakening of layered structure observed on the SEM image.
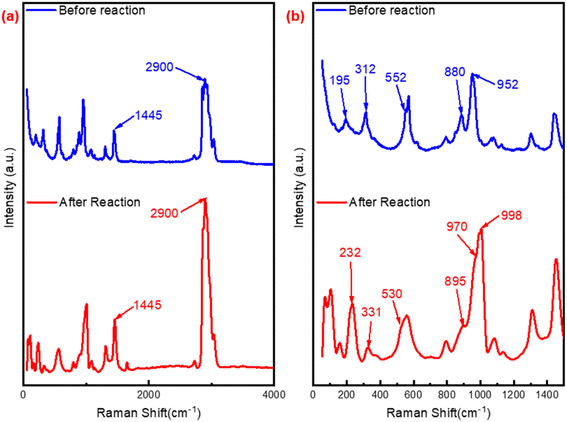
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O,47 the blue shift from 552 to 530 cm−1 revealed the transform between W–Oc–W and W(O)2, while the red shift from 195 to 232 cm−1, 312 to 331 cm−1 could also confirm this conversion. Furthermore, compared with P2W18-DDC, two new peaks at 100 cm−1 and 998 cm−1 were observed in the PW12-DDC, indicated that during the catalytic reaction, the peroxy structure of catalyst underewent a process of forming WOX groups influenced by hydrogen bonding between DDC and phosphotungstic acid,43 while the P2W18-DDC tended to form the peroxy structure. Combined with the results of FT-IR, XPS spectra and Raman spectra, after the reaction, a portion of the W–O–W/W
O,47 the blue shift from 552 to 530 cm−1 revealed the transform between W–Oc–W and W(O)2, while the red shift from 195 to 232 cm−1, 312 to 331 cm−1 could also confirm this conversion. Furthermore, compared with P2W18-DDC, two new peaks at 100 cm−1 and 998 cm−1 were observed in the PW12-DDC, indicated that during the catalytic reaction, the peroxy structure of catalyst underewent a process of forming WOX groups influenced by hydrogen bonding between DDC and phosphotungstic acid,43 while the P2W18-DDC tended to form the peroxy structure. Combined with the results of FT-IR, XPS spectra and Raman spectra, after the reaction, a portion of the W–O–W/W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species involved in the reaction might not return to their original structure, but rather combine with the remaining C–OH in quaternary ammonium salts through hydrogen bonding to form groups like WO3/4.
O species involved in the reaction might not return to their original structure, but rather combine with the remaining C–OH in quaternary ammonium salts through hydrogen bonding to form groups like WO3/4.
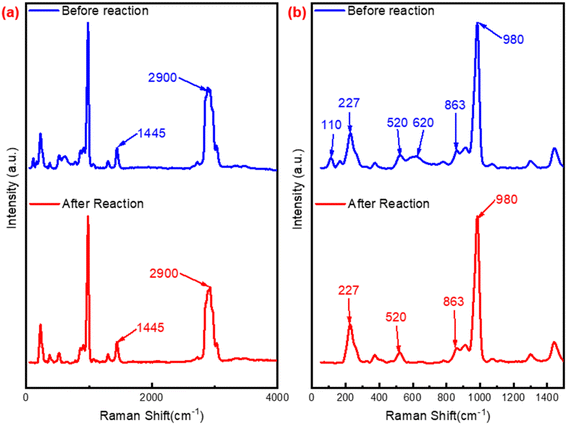 | ||
| Fig. 7 Raman spectra of P2W18-DDC and used P2W18-DDC (a) range from 50 to 4000 cm−1; (b) range from 50 to 1500 cm−1. | ||
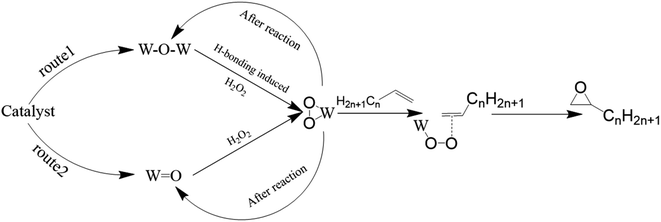
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (phosphotungstic acid) participates in the catalytic reaction. After the reaction, the W(O2) species transform into W–O–W to next catalytic reaction. Conversely, in the epoxidation reaction catalyzed by W
O (phosphotungstic acid) participates in the catalytic reaction. After the reaction, the W(O2) species transform into W–O–W to next catalytic reaction. Conversely, in the epoxidation reaction catalyzed by W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species, the reaction pathways were the same as previous study,37 the W
O species, the reaction pathways were the same as previous study,37 the W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species combine with oxygen to form W(O2). After the reaction, a portion of the W(O2) species revert to W
O species combine with oxygen to form W(O2). After the reaction, a portion of the W(O2) species revert to W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, while the remaining W(O2) species retain their hypervalent state structure. By using various characterization methods, it is proved that the catalyst prepared by Keggin type phosphotungstic acid would work through the above two routes in the catalytic reaction. While there was only one route (W(O2) formed from W
O, while the remaining W(O2) species retain their hypervalent state structure. By using various characterization methods, it is proved that the catalyst prepared by Keggin type phosphotungstic acid would work through the above two routes in the catalytic reaction. While there was only one route (W(O2) formed from W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) worked in catalytic reaction of the catalyst prepared by Wells–Dawson type phosphotungstic acid.
O) worked in catalytic reaction of the catalyst prepared by Wells–Dawson type phosphotungstic acid.
4. Conclusions
In this study, two new types of catalyst was prepared to utilize Wells–Dawson-type phosphotungstic acid and Keggin type phosphotungstic acid combined with a biquaternary ammonium cation. Various characterizations were used to elucidate the fundamental structure of the catalysts. Compared with a catalyst synthesized with Keggin-type phosphotungstic acid, the Wells–Dawson-type phosphotungstic acid catalyst exhibited superior selectivity and recyclability, but lower conversion in the epoxidation of 1-dodecene. Subsequently, the impact of various reaction conditions on catalyst performance was investigated, leading to the determination of optimal reaction conditions under solvent-free circumstances, thereby highlighting its potential industrial applicability. Throughout the course of the reaction, significant structural alterations in the catalyst were observed. Consequently, a comparative analysis was conducted between the pre- and after-reaction states, enabling the deduction of the catalytic mechanism influenced by hydrogen bonds for the catalyst synthesized with Keggin type phosphotungstic acid. Notably, this mechanism revealed a distinct reaction pathway in contrast to catalysts synthesized with Wells–Dawson type phosphotungstic acid.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the Basic research project of POLY sinolight (ZQ2021JC01) and the Project of JALA Research Funds (JALA2021).References
- J. Liu, G. Yang, Y. Liu, Z. Zhou, Z. Zhang and X. Hu, Selective Oxidation of Cyclohexene with H2O2 Catalyzed by Resin Supported Peroxo Phosphotungstic Acid Under Mild Conditions, Catal. Lett., 2021, 151, 147–152 CrossRef CAS.
- J. W. Bae and K.-Y. Lee, Special issue of the 15th Korea–Japan Symposium on Catalysis (15th KJSC), Res. Chem. Intermed., 2016, 42, 1–2 CrossRef CAS.
- B. Bahramian, V. Mirkhani, M. Moghadam and S. Tangestaninejad, Selective alkene epoxidation and alkane hydroxylation with sodium periodate catalyzed by cationic Mn(III)-salen supported on Dowex MSC1, Appl. Catal., A, 2006, 301, 169–175 CrossRef CAS.
- M. A. Nasseri, A. Allahresani and H. Raissi, Grafting of a chiral Mn(III) complex on graphene oxide nanosheets and its catalytic activity for alkene epoxidation, RSC Adv., 2014, 4, 26087–26093 RSC.
- Y.-L. Hu, Y.-W. Liu and D.-J. Li, Efficient and convenient epoxidation of alkenes to epoxides with H2O2 catalyzed by Co(OAc)2 in ionic liquid [C12py][PF6], J. Iran. Chem. Soc., 2015, 12, 2179–2184 CrossRef CAS.
- A. Bafti, M. Razum, E. Topić, D. Agustin, J. Pisk and V. Vrdoljak, Implication of oxidant activation on olefin epoxidation catalysed by Molybdenum catalysts with aroylhydrazonato ligands: Experimental and theoretical studies, Mol. Catal., 2021, 512, 111764 CrossRef CAS.
- M. Jafarpour, H. Kargar and A. Rezaeifard, A synergistic effect of a cobalt Schiff base complex and TiO2 nanoparticles on aerobic olefin epoxidation, RSC Adv., 2016, 6, 79085–79089 RSC.
- R. Dileep and B. J. Rudresha, An ionic liquid immobilized copper complex for catalytic epoxidation, RSC Adv., 2015, 5, 65870–65873 RSC.
- A. Freites Aguilera, J. Rahkila, J. Hemming, M. Nurmi, G. Torres, T. Razat, P. Tolvanen, K. Eränen, S. Leveneur and T. Salmi, Epoxidation of Tall Oil Catalyzed by an Ion Exchange Resin under Conventional Heating and Microwave Irradiation, Ind. Eng. Chem. Res., 2020, 59, 10397–10406 CrossRef CAS.
- J. Rakhtshah, A comprehensive review on the synthesis, characterization, and catalytic application of transition-metal Schiff-base complexes immobilized on magnetic Fe3O4 nanoparticles, Coord. Chem. Rev., 2022, 467, 214614 CrossRef CAS.
- M. Mitra, O. Cusso, S. S. Bhat, M. Sun, M. Cianfanelli, M. Costas and E. Nordlander, Highly enantioselective epoxidation of olefins by H2O2 catalyzed by a non-heme Fe(ii) catalyst of a chiral tetradentate ligand, Dalton Trans., 2019, 48, 6123–6131 RSC.
- X.-Y. Shi, P.-M. Wang, K.-Y. Liu, X.-F. Dong, X.-Y. Han and J.-F. Wei, Peroxophosphotungstate held in an ionic liquid brush: an efficient and reusable catalyst for the dihydroxylation of olefins with H2O2 in neat water, Appl. Organomet. Chem., 2014, 28, 760–763 CrossRef CAS.
- Y. Li, S. Pan, R. Han, J. Gao, D. Liu and J. Gui, Rational Design of Phosphotungstate Hybrids with Dual Active Sites for Efficient Catalytic Oxidation, Catal. Lett., 2023, 153, 3092–3102 CrossRef CAS.
- V. Jallet, G. Guillemot, J. Lai, P. Bauduin, V. Nardello-Rataj and A. Proust, Covalent amphiphilic polyoxometalates for the design of biphasic microemulsion systems, Chem. Commun., 2014, 50, 6610–6612 RSC.
- F. Zhang, Y. Dong, S. Lin, X. Gui and J. Hu, A novel amphiphilic phase transfer catalyst for the green epoxidation of soybean oil with hydrogen peroxide, Mol. Catal., 2023, 547, 113384 CrossRef CAS.
- P. Mekrattanachai, C. Cao, Z. Li, H. Li and W. Song, Cobalt immobilized on hydroxyapatite as a low-cost and highly effective heterogeneous catalyst for alkenes epoxidation under mild conditions, RSC Adv., 2018, 8, 37303–37306 RSC.
- J. Tong, W. Wang, L. Su, Q. Li, F. Liu, W. Ma, Z. Lei and L. Bo, Highly selective oxidation of cyclohexene to 2-cyclohexene-1-one over polyoxometalate/metal–organic framework hybrids with greatly improved performances, Catal. Sci. Technol., 2017, 7, 222–230 RSC.
- C. Venturello, E. Alneri and M. Ricci, A new, effective catalytic system for epoxidation of olefins by hydrogen peroxide under phase-transfer conditions, J. Org. Chem., 1983, 48, 3831–3833 CrossRef CAS.
- M.-L. Wang and T.-H. Huang, Kinetic Study of the Epoxidation of 1,7-Octadiene under Phase-Transfer-Catalysis Conditions, Ind. Eng. Chem. Res., 2004, 43, 675–681 CrossRef CAS.
- Y. Ishii, K. Yamawaki, T. Ura, H. Yamada, T. Yoshida and M. Ogawa, Hydrogen peroxide oxidation catalyzed by heteropoly acids combined with cetylpyridinium chloride. Epoxidation of olefins and allylic alcohols, ketonization of alcohols and diols, and oxidative cleavage of 1,2-diols and olefins, J. Org. Chem., 1988, 53, 3587–3593 CrossRef CAS.
- A. Patel and R. Sadasivan, Hybrid Catalyst Based on Cu Substituted Phosphotungstate and Imidazole: Synthesis, Spectroscopic Characterization, Solvent Free Oxidation of Styrene with TBHP and Kinetics, Catal. Lett., 2020, 150, 353–364 CrossRef CAS.
- R. Sadasivan and A. Patel, Flexible oxidation of styrene using TBHP over zirconia supported mono-copper substituted phosphotungstate, RSC Adv., 2019, 9, 27755–27767 RSC.
- U. Jameel, M. Zhu, X. Chen, Y. Liu and Z. Tong, Green epoxidation of cyclooctene with molecular oxygen over an ecofriendly heterogeneous polyoxometalate-gold catalyst Au/BW11/Al2O3, J. Zhejiang Univ., Sci., A, 2016, 17, 1000–1012 CrossRef CAS.
- F. Chen, X. Li, B. Wang, T. Xu, S.-L. Chen, P. Liu and C. Hu, Mechanism of the Cycloaddition of Carbon Dioxide and Epoxides Catalyzed by Cobalt-Substituted 12-Tungstenphosphate, Chem.–Eur. J., 2012, 18, 9870–9876 CrossRef CAS PubMed.
- X. Zuwei, Z. Ning, S. Yu and L. Kunlan, Reaction-Controlled Phase-Transfer Catalysis for Propylene Epoxidation to Propylene Oxide, Science, 2001, 292, 1139–1141 CrossRef CAS PubMed.
- H. Gao, J. Yu, J. Du, H. Niu, J. Wang, X. Song, W. Zhang and M. Jia, Supramolecular Assembly Based on Octamolybdate and Triazole Derivative: Crystal Structure and Catalytic Application in Olefin Epoxidation, J. Cluster Sci., 2014, 25, 1263–1272 CrossRef CAS.
- Y.-F. Song and R. Tsunashima, Recent advances on polyoxometalate-based molecular and composite materials, Chem. Soc. Rev., 2012, 41, 7384–7402 RSC.
- X. Sun, J. Zhang, X. Yuan and Z. Fu, A silicotungstate-based copper–viologen hybrid photocatalytic compound for efficient degradation of organic dyes under visible light, CrystEngComm, 2019, 21, 5563–5567 RSC.
- P. Zhang, T. Wang, H. Ma, R. Ma, Z. Xia, Q. Yang, X. Yang, G. Xie and S. Chen, Heterointerface Connection with Multiple Hydrogen-Bonding in Z-Scheme Heterojunction SiW9Co3@UiO-67-NH2 Deciding High Stability and Photocatalytic CO2 Reduction Performance, Inorg. Chem., 2023, 62, 20401–20411 CrossRef CAS.
- S.-M. Wang, Y.-H. Jin, L. Zhou, K.-H. Wang, H. J. Kim, L. Liu, E. Kim and Z. Han, Hydrogen-Bonded Organic Framework-Polyoxometalate-Based System for Electrochromic Devices, ACS Appl. Mater. Interfaces, 2023, 15, 56242–56252 CrossRef CAS PubMed.
- X. Ma, S. Li, J. Hua, P. Ma, J. Wang and J. Niu, Designing a new organic–inorganic arsenomolybdate by modification of traditional Keggin-type building fragment, J. Coord. Chem., 2013, 66, 725–736 CrossRef CAS.
- S.-F. Jia, X.-L. Hao, Y.-Z. Wen and Y. Zhang, Synthesis, cytotoxicity, apoptosis and cell cycle arrest of a monoruthenium(II)-substituted Dawson polyoxotungstate, J. Coord. Chem., 2019, 72, 633–644 CrossRef CAS.
- T. Ma, J. Ding, R. Shao, W. Xu and Z. Yun, Dehydration of glycerol to acrolein over Wells–Dawson and Keggin type phosphotungstic acids supported on MCM-41 catalysts, Chem. Eng. J., 2017, 316, 797–806 CrossRef CAS.
- L. E. Briand, G. T. Baronetti and H. J. Thomas, The state of the art on Wells–Dawson heteropoly-compounds: A review of their properties and applications, Appl. Catal., A, 2003, 256, 37–50 CrossRef CAS.
- L.-Z. Zhang, H.-B. Ju, T. Geng, D.-B. Li, Y.-J. Jiang and Y.-K. Wang, Study on the epoxidation of chain olefins using biquaternary ammonium phosphotungstic acid phase transfer catalysts under no-solvent condition, Chem.–Eur. J., 2024, 30, e202303559 CrossRef CAS PubMed.
- L. E. Briand, G. M. Valle and H. J. Thomas, Stability of the phospho-molybdic Dawson-type ion P2Mo18O626− in aqueous media, J. Mater. Chem., 2002, 12, 299–304 RSC.
- Z. Zhang, Q. Zha, Y. Liu, Z. Zhang, J. Liu and Z. Zhou, Study on the epoxidation of olefins with H2O2 catalyzed by biquaternary ammonium phosphotungstic acid, Chin. J. Chem. Eng., 2023, 58, 146–154 CrossRef.
- S. Zhao, Y. Jia and Y.-F. Song, Highly efficient and selective oxidation of various substrates under mild conditions using a lanthanum-containing polyoxometalate as catalyst, Appl. Catal., A, 2013, 453, 188–194 CrossRef CAS.
- Y. Wang, W. Li and L. Wu, Organic–Inorganic Hybrid Supramolecular Gels of Surfactant-Encapsulated Polyoxometalates, Langmuir, 2009, 25, 13194–13200 CrossRef CAS PubMed.
- Y. Shen, X.-H. Lu, C.-C. Wei, X.-T. Ma, C. Peng, J. He, D. Zhou and Q.-H. Xia, Highly selective mono-epoxidation of dicyclopentadiene with aqueous H2O2 over heterogeneous peroxo-phosphotungstic catalysts, Mol. Catal., 2017, 433, 185–192 CrossRef CAS.
- M. Zhang, P. Zhao, Y. Leng, G. Chen, J. Wang and J. Huang, Schiff Base Structured Acid–Base Cooperative Dual Sites in an Ionic Solid Catalyst Lead to Efficient Heterogeneous Knoevenagel Condensations, Chem.–Eur. J., 2012, 18, 12773–12782 CrossRef CAS PubMed.
- J. Zhao, B. Hou, H. Guo, L. Jia, P. Niu, C. Chen, H. Xi, D. Li and J. Zhang, Insight into the Influence of WOx-Support Interaction over Pt/W/SiZr Catalysts on 1,3-Propanediol Synthesis from Glycerol, ChemCatChem, 2022, 14, e202200341 CrossRef CAS.
- Y. Wang, Z. Li, Z. Liu and X. Shi, Heterogenous carboxyl-functionalized bilayer ionic liquids/polyoxometalate catalysts for extractant-free oxidative desulfurization, J. Mol. Liq., 2023, 373, 121245 CrossRef CAS.
- H. Chen, C. Lim, M. Zhou, Z. He, X. Sun, X. Li, Y. Ye, T. Tan, H. Zhang, C. Yang, J. W. Han and Y. Chen, Activating Lattice Oxygen in Perovskite Oxide by B-Site Cation Doping for Modulated Stability and Activity at Elevated Temperatures, Adv. Sci., 2021, 8, 2102713 CrossRef CAS PubMed.
- Y. Chen, R. Tan, W. Zheng, Y. Zhang, G. Zhao and D. Yin, Dendritic phosphotungstate hybrids efficiently catalyze the selective oxidation of alcohols with H2O2, Catal. Sci. Technol., 2014, 4, 4084–4092 RSC.
- Y. Zhang, G. Ji and A. Li, Production of limonene epoxides from tire pyrolysis oil by polyoxometalate immobilized on SBA-15, Process Saf. Environ. Prot., 2023, 178, 287–295 CrossRef CAS.
- M. A. Rezvani and O. F. Miri, Synthesis and characterization of PWMn/NiO/PAN nanosphere composite with superior catalytic activity for oxidative desulfurization of real fuel, Chem. Eng. J., 2019, 369, 775–783 CrossRef CAS.
- X. Lu, W.-J. Zhou, Y. Guan, A. Liebens and P. Wu, Enhancing ethylene epoxidation of a MWW-type titanosilicate/H2O2 catalytic system by fluorine implanting, Catal. Sci. Technol., 2017, 7, 2624–2631 RSC.
- M. Rocha, S. L. H. Rebelo and C. Freire, Enantioselective arene epoxidation under mild conditions by Jacobsen catalyst: The role of protic solvent and co-catalyst in the activation of hydrogen peroxide, Appl. Catal., A, 2013, 460–461, 116–123 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra01399a |
| ‡ These authors contributed to the work equally and should be regarded as co-first authors. |
| This journal is © The Royal Society of Chemistry 2024 |