Open Access Article
Open Access ArticlepH-responsive niosome-based nanocarriers of antineoplastic agents†
Viliana Guglevaa,
Rositsa Mihaylovab,
Georgi Momekovb,
Katya Kamenovac,
Aleksander Forysd,
Barbara Trzebickad,
Maria Petrovae,
Iva Ugrinovae,
Denitsa Momekova*f and
Petar D. Petrov *c
*c
aDepartment of Pharmaceutical Technologies, Faculty of Pharmacy, Medical University of Varna “Prof. Dr Paraskev Stoyanov”, 84 Tsar Osvoboditel Str., 9000 Varna, Bulgaria
bDepartment of Pharmacology, Pharmacotherapy and Toxicology, Faculty of Pharmacy, Medical University of Sofia, 2 Dunav Str., 1000 Sofia, Bulgaria
cInstitute of Polymers, Bulgarian Academy of Sciences, bl.103 Akad. G. Bonchev Str.,1113, Sofia, Bulgaria. E-mail: ppetrov@polymer.bas.bg
dCentre of Polymer and Carbon Materials, Polish Academy of Sciences, ul. M. Curie-Skłodowskiej 34, Zabrze, Poland
eInstitute of Molecular Biology “Akad. Roumen Tsanev”, Bulgarian Academy of Sciences, Acad. G. Bonchev str., bl 21, Sofia 1113, Bulgaria
fDepartment of Pharmaceutical Technology and Biopharmaceutics, Faculty of Pharmacy, Medical University of Sofia, 2 Dunav Str., 1000 Sofia, Bulgaria. E-mail: dmomekova@pharmfac.mu-sofia.bg
First published on 11th April 2024
Abstract
Differences in pH between the tumour interstitium and healthy tissues can be used to induce conformational changes in the nanocarrier structure, thereby triggering drug release at the desired site. In the present study, novel pH-responsive nanocarriers were developed by modifying conventional niosomes with hexadecyl-poly(acrylic acid)n copolymers (HD-PAAn). Niosomal vesicles were prepared by the thin film hydration method using Span 60, Span 60/Tween 60 and cholesterol as main constituents, and HD-PAA modifiers of different concentrations (0.5, 1, 2.5, 5 mol%). Next, two model substances, a water-soluble fluorescent dye (calcein) and a hydrophobic agent with pronounced antineoplastic activity (curcumin), were loaded in the aqueous core and hydrophobic membrane of the elaborated niosomes, respectively. Physicochemical properties of blank and loaded nanocarriers such as hydrodynamic diameter (Dh), size distribution, zeta potential, morphology and pH-responsiveness were investigated in detail. The cytotoxicity of niosomal curcumin was evaluated against human malignant cell lines of different origins (MJ, T-24, HUT-78), and the mechanistic aspects of proapoptotic effects were elucidated. The formulation composed of Span 60/Tween 60/cholesterol/2.5% HD-PAA17 exhibited optimal physicochemical characteristics (Dh 302 nm; ζ potential −22.1 mV; high curcumin entrapment 83%), pH-dependent drug release and improved cytotoxic and apoptogenic activity compared to free curcumin.
Introduction
The key advantages of nanoparticulate drug delivery systems such as small size, functionality, capacity to encapsulate wide variety of bioactive substances and thus to improve their physicochemical characteristics and bioavailability have attracted enormous research interest, leading to constant improvement and evolution of nanomedicine.1,2 The site-specific drug delivery is especially beneficial in cancer therapeutics, as it contributes to diminished systemic toxicity, reduced side effects and enhanced therapeutic efficacy.3,4 In particular, the elaboration of stimuli-responsive nanocarriers, which are able to release their cargo in response to specific triggers (e.g., temperature, pH, redox potential, ionic composition, etc.) is a very promising concept for development of advanced nanocarriers.5,6 Niosomes are nanoscale drug delivery systems possessing good biocompatibility, biodegradability, and low toxicity.7,8 They are vesicular aggregates, identical to liposomes, but their bilayer membrane is composed of non-ionic surfactants (along with cholesterol) instead of phospholipids.9 This fact imparts to niosomes superior chemical stability, reduced cost of production, and facilitated scale-up.10,11 Niosomes have been exploited as feasible nanocarriers for delivery of hydrophobic and hydrophilic drugs, biologically active compounds, genetic material, as well as imaging agents in diagnostics/theranostics.12,13 Drug delivery of natural phytochemicals has been a challenging task due to their inherent disadvantageous properties such as high molar mass, limited aqueous solubility, low chemical stability, predisposition to photodegradation.14 In terms of topical application, these limitations may be overcome by developing advanced vesicular carriers, e.g., aspasomes (ascorbyl palmitate-based vesicles),15 bilosomes (biles salts containing vesicular systems),16 elastic liposomes,17,18 or ultradeformable vesicles.19–21 The last two types of nanocarriers contain in their structure an edge activator like sodium cholate or Polysorbate 80 to impart enhanced elasticity/deformability (vs. the conventional liposomes) and to increase thereby drug permeation and bioavailability. In terms of systemic delivery of natural chemotherapeutics, improved therapeutic effectiveness may be obtained by modifying the vesicular structure via inclusion of different ligands (aptamers, receptor ligands, antibodies, etc.) intended to achieve target selectivity, or functional polymers for imparting stimuli-responsive properties.22,23 For instance, elaborating polymer-modified pH-responsive niosomes, has been exploited as cancer targeting strategy as the altered microenvironment in the tumour affected tissues (mild hyperthermia, acidic pH, increased glutathione concentration), may serve as feasible and predictable indicator to utilize, in comparison to the uneven and diverse expression of target receptors in the affected areas.24,25 The increased anaerobic glycolysis in cancer cells determines an extracellular pH ranging from 6.4 to 7.0 (vs. 7.2–7.4 in normal tissues), whereas the ATP-driven H+ pump further lowers the pH in endosomes to 4.5–6.5, creating thereby a pH gradient, that allows a selective cargo release from the pH-responsive nanocarriers.26,27 By analogy with liposomes, this process is facilitated by a pH-dependent destabilization of the niosomal membrane,5,28 or by the fusogenic activity of the vesicles.29 A series of pH-responsive niosomes have already been reported in the literature. In some of the formulations the stimuli responsiveness is acquired by altering the constituents, for example using cholesteryl hemisuccinate instead of cholesterol,25,30 or by derivatizing the non-ionic surfactants, as reported by Marzoli et al., which developed Tween 20-glycine based niosomes.31 Other interesting approaches include coating the vesicles with pH (low) insertion peptide,32 or pH-sensitive polymers. Leroux et al. synthesized randomly- and terminally-alkylated copolymers of N-isopropylacrylamide and glycine acrylamide as the pH-sensitive moiety.28,33 The binding of copolymers to niosomes was achieved by hydrogen bonding, but the complexes formed were unstable in serum and the systems lost their pH-sensitivity. Hyaluronic acid (HA)-decorated niosomes have been developed for tacrolimus ocular delivery34 and targeted delivery of epirubicin to breast cancer cells.35 The authors emphasized the positive effect of modifying niosomal surface properties regarding the interaction of nanocarriers with cells (improved mucoadhesion and cell internalization as compared to the non-modified niosomes), but they did not unambiguously discuss whether the presence of pH sensitive moiety is beneficial in terms of release of the active substance.Our research group adopted the polymer-based approach to impart pH-responsiveness of the vesicles by newly synthesized amphiphilic copolymers based on hexadecyl- and poly(acrylic acid) segments. The pH-sensitivity of the nanocarriers was demonstrated via the release profile of two model substances: the hydrophilic dye (calcein) and the hydrophobic drug (curcumin) as a function of pH.
Curcumin, a polyphenol extracted from the roots of Curcuma longa, is well-known for its antineoplastic properties.36 The latter are attributed to a variety of complex mechanisms, including downregulation of STAT 3 (signal transducer and activator of transcription 3) and NF-κB (nuclear factor kappa B) signalling pathways, stimulation of apoptosis, as well suppression of tumour proliferation.37,38 However, curcumin is characterized by low solubility in water, chemical and photoinstability.39,40 Additionally, it possesses insufficient oral bioavailability, due to its low absorption and rapid biotransformation, which further favours its inclusion into nanocarriers such as niosomes.41–44
The present study describes the development of novel pH-responsive niosome-based carriers for delivery of active agents with potential anticancer application. Polymers comprising a lipophilic hexadecyl and a pH-sensitive poly(acrylic acid) segments were synthesized and incorporated into the niosomal aggregates, fabricated from Tween 60, Span 60, and cholesterol. The effect of polymer concentration on the structural stability of niosomes, as well as the pH-triggered release of curcumin at conditions resembling tumour cells were evaluated. Finally, comprehensive in vitro assays were applied to evaluate the anticancer activity and the mechanistic aspects behind it of the developed pH-responsive niosomal formulations in comparative way vs. free drug or loaded into plain niosomes curcumin.
Results and discussion
Synthesis of copolymers
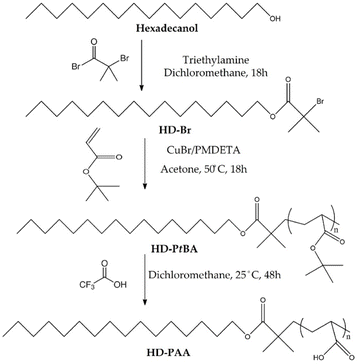
Polymers comprising a short lipophilic hexadecyl and a pH-sensitive poly(acrylic acid) segments were synthesized by a three step procedure involving preparation of: (i) hexadecyl-based ATRP initiator, (ii) hexadecyl-poly(t-butyl acrylate) precursors, and (iii) hexadecyl-poly(acrylic acid) final products (Fig. 1). We aimed at obtaining polymers with an aliphatic hexadecyl (C16) segment, which is compatible with the constituents of the lipid bilayer of niosomes, and a PAA segment which is soluble in aqueous media at neutral pH and tends to be insoluble in acidic media, due to the protonation/deprotonation of the pendant carboxyl groups. The synthesized compounds were characterized by 1H-NMR (Fig. 2) analysis and the calculated composition and number-average molar mass (Mn) are listed in Table 1. The number-average degree of polymerization of tBA was determined taking into account the relative intensity of integrals assigned to the methyl protons of hexadecyl chain-end (–CH3, δ = 0.88 ppm, a) and methine protons of tBA main chain (–CH–, δ = 2.21 ppm, f). 1H-NMR provided also direct evidence for the efficient reactions of obtaining an ATRP initiator from hexadecanol and converting PtBA to PAA via hydrolysis. In the last case, the characteristic peak of the tert-butyl protons at 1.45 ppm (h, Fig. 2) was not detected in the spectra of HD-PAA polymers (Fig. S1†).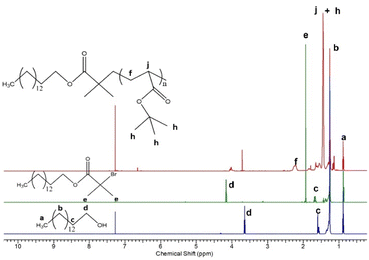 | ||
| Fig. 2 1H-NMR spectra of hexadecanol (bottom), hexadecyl-based ATRP initiator (middle), and hexadecyl-poly(t-butyl acrylate)12 precursor (top) in CDCl3. | ||
| Sample code | MNMRn (g mol−1) | MGPCn (g mol−1) | Mw/Mn |
|---|---|---|---|
| HD-Br | 390 | 510 | 1.01 |
| HD-PtBA8 | 1415 | 1850 | 1.10 |
| HD-PAA8 | 820 | — | — |
| HD-PtBA12 | 1930 | 3100 | 1.17 |
| HD-PAA12 | 1100 | — | — |
| HD-PtBA17 | 2600 | 3200 | 1.20 |
| HD-PAA17 | 1470 | — | — |
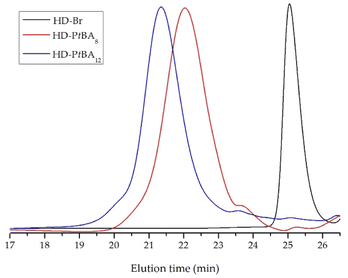
GPC chromatograms of HD-PtBA precursors were monomodal with narrow molar mass distribution, which indicate the high efficiency of HD-Br initiator and good control over the ATRP process (Fig. 3). The number-average molar masses and dispersity indices (Mw/Mn) of the precursors, calculated from the GPC analysis are given in Table 1.
Preparation and characterization of pH-responsive niosomes
A series of conventional and polymer-modified niosomes based on Span 60, Span 60/Tween 60 and cholesterol were prepared by the thin film hydration method. The niosomal compositions (type of surfactants as well surfactant/cholesterol ratio) were selected based on our previous study.45 The design of the current study was chosen following the consideration to investigate the influence of copolymers type and concentration on the physicochemical characteristics on two types of vesicles: the one exhibiting tight and rigid bilayer membrane (Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol 1
Chol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) and second characterized with leakier vesicular structure, but high curcumin entrapment (Tw60
1 molar ratio) and second characterized with leakier vesicular structure, but high curcumin entrapment (Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60
Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol 3.5
Chol 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5
3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The results from the conducted experiments are summarized in Table 2. The expected pH-induced change in membrane permeability, allowing release of the active substance, for HD-PAA8-modified niosomes was not observed (see section pH-induced calcein leakage). Therefore, data related to these compositions are not described. As evident from the obtained results, copolymers type and concentration, as well as curcumin's inclusion exhibit a pronounced effect on nanocarriers' size. Blanc niosomes are characterized by larger sizes compared to drug-loaded, which may be rated to curcumin's interaction with the lipophilic region of the surfactant molecules and its accommodation in the hydrophobic membrane. The latter determines the formation of more compact structure, resulting in vesicles size reduction. Regarding copolymers inclusion, an inverse relationship was estimated between their concentration and vesicles size – a higher molar fraction of HD-PAA12 or HD-PAA17 leads to a decrease in niosomal diameter. The latter may be a result of the enhanced steric repulsion between the hydrophilic blocks of the copolymer, determining an increase in niosomes' curvature and, respectively a decrease in their size. Additionally, the incorporated in the niosomal membrane hydrophobic block of the copolymer may interact with the molecules of non-ionic surfactants and cholesterol, which increases the integrity of the niosomal membrane. Polymer content also affected the size distribution pattern of the vesicles – the higher the molar ratio of HD-PAA12 and HD-PAA17 the higher the polydispersity index. A possible explanation for such behavior may be the reaching and exceeding of the saturation limit of membranes with respect to investigated polymers, which leads to a subsequent destabilizing effect onto niosomal integrity, respectively disruption of the lamellar structure and formation of transient structures – discs or micelles. Zeta potential also increased with the inclusion of higher molar fraction of modifying copolymers. The obtained high negative values are a prerequisite for optimal colloidal stability of niosomal dispersions due to the electrostatic repulsion between the vesicles, preventing their aggregation (Table 2). Regarding the entrapment efficiency values, no significant changes in curcumin encapsulation were observed within the concentration range (0.5–2.5 mol%) for both studied polymers. However, in the formulations based on HD-PAA12 the increase of copolymer concentration above 2.5 mol% (S8 and S12) was accompanied by more than 10% decrease in curcumin entrapment efficiency, which was probably due to the competitive interactions between the plain counterparts are very small to claim a beneficial effect of the membrane modification of niosomes on the solubilization and entrapment of curcumin. Based on the obtained results, it can be concluded that the optimal concentration of modifying copolymer for the elaboration of curcumin loaded niosomes was 2.5 mol%. This conclusion was also confirmed by the microscopic evaluation of the structure of elaborated vesicles. Representative micrographs of conventional and copolymer modified niosomes are depicted in Fig. 4. All formulated vesicles exhibited spherical shape with a smooth surface. As evident from the presented data, the inclusion of the pH-sensitive copolymers in a concentration of up to 2.5 mol% doesn't disrupt the unilamellar vesicular structure and copolymer molecules and the hydrophobic curcumin. In the formulations modified with HD-PAA17 the tendency was preserved, however in a less pronounced manner. A similar trend is observed also in regard of drug loading capacity. Despite the observed slight increase in the curcumin loading with the increase of the mole fraction of copolymers (up to 2.5 mol%), the differences in the loading capacity of the modified vesicles compared to their integrity of niosomes. However, at 5 mol%, a concomitant small fraction of discs and micelles was observed (Fig. S2†).
3). The results from the conducted experiments are summarized in Table 2. The expected pH-induced change in membrane permeability, allowing release of the active substance, for HD-PAA8-modified niosomes was not observed (see section pH-induced calcein leakage). Therefore, data related to these compositions are not described. As evident from the obtained results, copolymers type and concentration, as well as curcumin's inclusion exhibit a pronounced effect on nanocarriers' size. Blanc niosomes are characterized by larger sizes compared to drug-loaded, which may be rated to curcumin's interaction with the lipophilic region of the surfactant molecules and its accommodation in the hydrophobic membrane. The latter determines the formation of more compact structure, resulting in vesicles size reduction. Regarding copolymers inclusion, an inverse relationship was estimated between their concentration and vesicles size – a higher molar fraction of HD-PAA12 or HD-PAA17 leads to a decrease in niosomal diameter. The latter may be a result of the enhanced steric repulsion between the hydrophilic blocks of the copolymer, determining an increase in niosomes' curvature and, respectively a decrease in their size. Additionally, the incorporated in the niosomal membrane hydrophobic block of the copolymer may interact with the molecules of non-ionic surfactants and cholesterol, which increases the integrity of the niosomal membrane. Polymer content also affected the size distribution pattern of the vesicles – the higher the molar ratio of HD-PAA12 and HD-PAA17 the higher the polydispersity index. A possible explanation for such behavior may be the reaching and exceeding of the saturation limit of membranes with respect to investigated polymers, which leads to a subsequent destabilizing effect onto niosomal integrity, respectively disruption of the lamellar structure and formation of transient structures – discs or micelles. Zeta potential also increased with the inclusion of higher molar fraction of modifying copolymers. The obtained high negative values are a prerequisite for optimal colloidal stability of niosomal dispersions due to the electrostatic repulsion between the vesicles, preventing their aggregation (Table 2). Regarding the entrapment efficiency values, no significant changes in curcumin encapsulation were observed within the concentration range (0.5–2.5 mol%) for both studied polymers. However, in the formulations based on HD-PAA12 the increase of copolymer concentration above 2.5 mol% (S8 and S12) was accompanied by more than 10% decrease in curcumin entrapment efficiency, which was probably due to the competitive interactions between the plain counterparts are very small to claim a beneficial effect of the membrane modification of niosomes on the solubilization and entrapment of curcumin. Based on the obtained results, it can be concluded that the optimal concentration of modifying copolymer for the elaboration of curcumin loaded niosomes was 2.5 mol%. This conclusion was also confirmed by the microscopic evaluation of the structure of elaborated vesicles. Representative micrographs of conventional and copolymer modified niosomes are depicted in Fig. 4. All formulated vesicles exhibited spherical shape with a smooth surface. As evident from the presented data, the inclusion of the pH-sensitive copolymers in a concentration of up to 2.5 mol% doesn't disrupt the unilamellar vesicular structure and copolymer molecules and the hydrophobic curcumin. In the formulations modified with HD-PAA17 the tendency was preserved, however in a less pronounced manner. A similar trend is observed also in regard of drug loading capacity. Despite the observed slight increase in the curcumin loading with the increase of the mole fraction of copolymers (up to 2.5 mol%), the differences in the loading capacity of the modified vesicles compared to their integrity of niosomes. However, at 5 mol%, a concomitant small fraction of discs and micelles was observed (Fig. S2†).
| Sample | SF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (mol Chol (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) mol) |
HD-PAA12 (mol%) | HD-PAA17 (mol%) | Dh (nm) | PDI | ζ-Potential (mV) | EE (%) | DL (%) |
|---|---|---|---|---|---|---|---|---|
| S1 | Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (1 Chol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (blank) 1) (blank) |
— | — | 573 ± 5.2 | 0.45 ± 0.03 | −8.7 ± 0.4 | — | — |
| S2 | Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (1 Chol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | — | 389 ± 5.2 | 0.31 ± 0.02 | −5.7 ± 0.4 | 27 ± 1.6 | 1.23 |
| S3 | Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (3.5 Chol (3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (blank) 3) (blank) |
— | — | 489 ± 4.8 | 0.34 ± 0.06 | −12.3 ± 2.3 | — | — |
| S4 | Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (3.5 Chol (3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
— | — | 379 ± 3.3 | 0.32 ± 0.02 | −12.2 ± 2.1 | 80 ± 1.8 | 2.03 |
| S5 | Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol ((1 Chol ((1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.5 | — | 464 ± 4.2 | 0.27 ± 0.04 | −17.7 ± 0.7 | 28 ± 1.6 | 1.28 |
| S6 | Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (1 Chol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1 | — | 478 ± 5.9 | 0.31 ± 0.02 | −20.2 ± 1.4 | 29 ± 1.6 | 1.33 |
| S7 | Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (1 Chol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
2.5 | — | 330 ± 4.2 | 0.25 ± 0.05 | −21.2 ± 1.1 | 32 ± 1.6 | 1.47 |
| S8 | Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (1 Chol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
5 | — | 237 ± 8.4 | 0.5 ± 0.02 | −23.9 ± 2.5 | 21 ± 1.6 | 0.96 |
| S9 | Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (3.5 Chol (3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
0.5 | — | 293 ± 5.5 | 0.37 ± 0.02 | −19.2 ± 1.8 | 81 ± 0.9 | 2.06 |
| S10 | Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (3.5 Chol (3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
1 | — | 391 ± 5.9 | 0.41 ± 0.06 | −21.7 ± 0.6 | 80 ± 2.8 | 2.04 |
| S11 | Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (3.5 Chol (3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
2.5 | 324 ± 3.3 | 0.38 ± 0.03 | −22.1 ± 1.4 | 83 ± 1.3 | 2.12 | |
| S12 | Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (3.5 Chol (3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
5 | — | 332 ± 8.1 | 0.55 ± 0.06 | −24.8 ± 1.3 | 71 ± 1.8 | 1.81 |
| S13 | Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (1 Chol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | 0.5 | 602 ± 3.3 | 0.38 ± 0.06 | −21.3 ± 0.7 | 29 ± 2.8 | 1.33 |
| S14 | Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (1 Chol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | 1 | 654 ± 7.5 | 0.4 ± 0.01 | −22.5 ± 1.1 | 30 ± 1.1 | 1.38 |
| S15 | Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (1 Chol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | 2.5 | 563 ± 4.6 | 0.36 ± 0.04 | −23.8 ± 1.2 | 30 ± 3.4 | 1.38 |
| S16 | Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (1 Chol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | 5 | 519 ± 5.8 | 0.48 ± 0.05 | −23.1 ± 0.9 | 28 ± 2.5 | 1.28 |
| S17 | Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (3.5 Chol (3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
— | 0.5 | 324 ± 9.5 | 0.32 ± 0.04 | −20.4 ± 0.4 | 81 ± 1.8 | 2.06 |
| S18 | Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (3.5 Chol (3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
— | 1 | 363 ± 6.7 | 0.42 ± 0.07 | −24.3 ± 1.5 | 80 ± 3.4 | 2.04 |
| S19 | Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (3.5 Chol (3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
— | 2.5 | 302 ± 6.6 | 0.34 ± 0.02 | −21.1 ± 1.7 | 83 ± 2.8 | 2.12 |
| S20 | Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (3.5 Chol (3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
— | 5 | 299 ± 9.5 | 0.45 ± 0.03 | −23.5 ± 0.8 | 76 ± 2.2 | 1.94 |
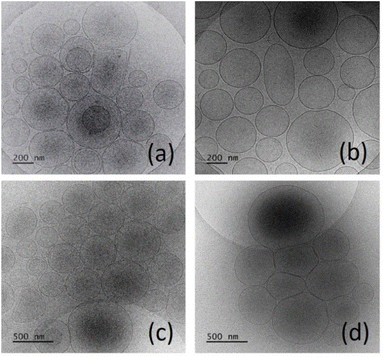 | ||
Fig. 4 Cryo-TEM images of niosomes: (a) Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol, (b) Tw60 Chol, (b) Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol; (c) Sp60 Chol; (c) Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol Chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HD-PAA12 (2.5 mol%), (d) Tw60 HD-PAA12 (2.5 mol%), (d) Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol Chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HD-PAA12 (2.5 mol%). HD-PAA12 (2.5 mol%). | ||
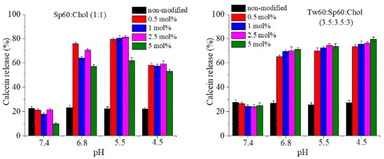
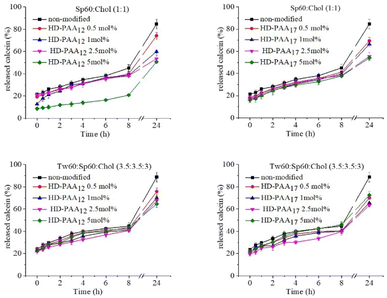
pH-induced calcein leakage
An integral part of the detailed characterization of modified niosomes was the evaluation of their pH-responsiveness. For this purpose, a series of calcein-loaded niosomes, modified with HD-PAA12 and HD-PAA17 were prepared. Calcein is a water-soluble, polar compound, which fluorescence at concentration of 80 mM is self-quenched and is not dependent on variations in pH. The polar nature of calcein limits its passage through intact niosomal membranes. However, when the membrane is disrupted as result of changes in the pH of the medium, calcein will be released. Consequently, its concentration decreases due to dilution in the release medium, leading to an increase in the fluorescence of the dye. The results of the spectrofluorometric study of niosomes as a function of pH are illustrated in Fig. 5. As evident from the obtained results, the inclusion of studied copolymers into niosomal membranes leads to pH-triggered destabilization of the niosomal membranes. Thus, a pH-induced release of more than 70% of the encapsulated fluorescent dye was achieved only after 10 minutes of incubation in a slightly acidic media. At the same time, calcein release from modified niosomes after 24 h incubation in PBS pH 7.4 at 37 °C exhibited lower release rate compared to non-modified vesicles (Fig. 6). To elucidate the mechanism of the observed pH-dependent calcein release profile, we performed a comparative DLS analysis of HD-PAA17 modified niosomes before and after 10 min incubation at 37 °C in isotonic media with pH 7.4 and 5.5, respectively. While at the initial moment there is no difference in the size and size distribution between the vesicles at pH 7.4 and 5.5, after 10 minutes of incubation at 37 °C, a decrease by about 10% of size of niosomes in the acidic medium, as well as a slight increase in PDI compared to vesicles at pH 7.4 was clearly observed (Fig S3†). This is probably due to changes in the lamellar organization of the bilayers, in response to conformational changes of PAA moieties upon their protonation in the acidic environment.Usually, upon gradual decrease in pH the polyanion chains undergo a collapse from an expanded coil-like conformation to a contracted, globule-like conformation. This is due to the protonation of the carboxylic groups, which makes the polymer more hydrophobic. In the case of polyanion-modified vesicles, at a certain point the deprotonated polymer becomes membrane active, i.e., it leads to an increased lateral compression of the bilayer and disruption of the membrane.46 The pH at which this happens is dependent on the hydrophilic/hydrophobic balance of the modifying molecules. Philippova et al. reported that the addition of hydrophobic alkyl residue to the poly(acrylic acid) backbone shifts the transition to a higher value as compare to the pure PAA.47 In addition, hydrogen bonding between the polyanion molecules and the vesicle surface also contributes to the shift of the coil-to-globule transition to higher pH values.46 The results from calcein leakage experiments undoubtedly demonstrate the potential of the elaborated pH-responsive niosomes to serve as effective platform for targeted drug delivery and pH-triggered release of water-soluble drugs in pathologically altered tissues (e.g., in tumour interstitium, or in specific subcellular compartments, characterized with lower pH values than physiological), while simultaneously preserve their stability and integrity in systemic circulation environment. A subsequent stage in our study was to evaluate curcumin release from copolymer modified niosomes in dissolution media with different pH values. As all copolymer modified vesicles exhibited pH-responsive properties, the in vitro release studies were conducted with the formulation characterized with the smallest size and highest curcumin entrapment efficiency – Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tw60
Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol
Chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HD-PAA17 (S19).
HD-PAA17 (S19).
In vitro release of curcumin from optimal niosomal formulation
The release profile of curcumin from HD-PAA17 modified niosomes was investigated as a function of pH for 24 hours (Fig. 7a). Curcumin exhibited a pH-dependent release pattern inversely proportional to the corresponding pH values. The highest release rate was estimated at pH 5.5, with approximately 1.6 and 1.5-fold increased cumulative drug release compared to those at pH 7.4 and pH 7.4 + 10% albumin. A possible explanation may lie in the pH-dependent ionization of polyelectrolytes. At neutral or alkaline conditions, PAA is deprotonated, which contributes to its good solubility and swelling in aqueous medium. In an acidic environment, on the contrary, PAA is prone to protonation, which leads to diminished solubility, dissociation48 and ultimately determines the higher release of encapsulated curcumin. A schematic illustration of the process is shown on Fig. 7b. Similar tendency in the drug release pattern was reported also by Saroj and Rajput, which elaborated poly(acrylic acid)-modified mesoporous silica nanoparticles.49 The included albumin in the release medium (PBS, pH 7.4) determined the slightly higher curcumin release compared to plain PBS. The latter may be due to the absorption of albumin onto niosomal surface, which may result into a transient void in their structure, leading to increased membrane permeability. Similar findings were reported by Hioki et al., which investigated doxorubicin release from conventional and PEGylated liposomes.50 This high retention of curcumin in niosomes at pH 7.4 is a precondition for improved chemical stability of the drug in the circulation and is also essential for the aptitude of the carrier to deliver the cargo to the desired tissue or cell compartment at optimal concentration.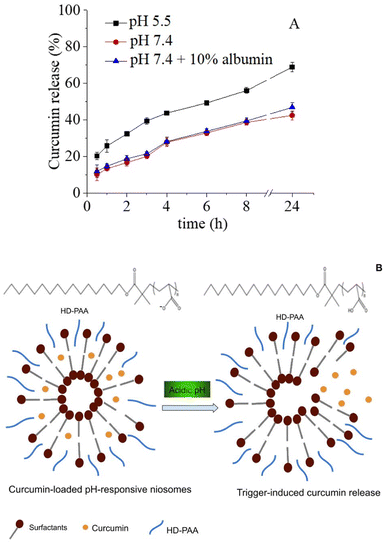 | ||
Fig. 7 Curcumin cumulative release from HD-PAA17 modified niosomes based on Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tw60 Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol as a function of pH and time (A) and schematic presentation of membrane rearrangements at acid pH (B). Chol as a function of pH and time (A) and schematic presentation of membrane rearrangements at acid pH (B). | ||
As the elaborated systems are designed as potential parenteral preparations, following systemic administration the tissue distribution is expected to occur within several hours and at such stage the pH-sensitive properties would condition beneficial behavior in tissues and compartments with lower pH, incl. tumor interstitium and subcellulary inside endosomes, long before the first 24 h.
Stability of niosomes
The stability is one of the main requirements for niosomes as drug carriers. In other words, they have to maintain their key physicochemical characteristics and the amount of the encapsulated drug unchanged for a certain period of time. In this regard, the stability of selected niosomal compositions was monitored for a period of one month when stored in a refrigerator at a temperature of 4 °C. The data on the main parameters of the vesicles immediately after their preparation and one month later are presented in Table 3. The results obtained demonstrated that both plain and polymer modified niosomal formulations are stable upon storage at low temperature for at least one month as there is no evidence for deterioration of the main parameters of niosomes: size, PDI and encapsulation efficacy of curcumin. This can be due to the presence of cholesterol and modifying copolymer in niosomal membranes, which in combination with low temperature of storage hampers the molecular movements of membrane constituents and thus, preventing the merging of niosomes.| Sample | Time (days) | Dh (nm) | PDI | ζ-Potential (mV) | EE (%) |
|---|---|---|---|---|---|
Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ch (S4) Ch (S4) |
0 | 379 ± 3.3 | 0.32 ± 0.02 | −12.2 ± 2.1 | 80 ± 2 |
| 30 | 382 ± 4.2 | 0.35 ± 0.01 | −11.5 ± 1.8 | 79 ± 2 | |
Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ch Ch![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HD-PAA17 (S19) HD-PAA17 (S19) |
0 | 302 ± 6.6 | 0.34 ± 0.01 | −21.1 ± 1.7 | 83 ± 3 |
| 30 | 298 ± 2.4 | 0.34 ± 0.03 | −22.6 ± 2.1 | 83 ± 2 |
Cellular internalization of niosomes
Habitually, the cellular internalization of nanocarriers involves a near contact between cell and niosomal membranes. As the PAA segments of the copolymers used in this study are deprotonated at physiological environment (pH 7.4), this fact determines the negative zeta potential of the developed HD-PAA modified niosomes (Table 2). Although the influence of the ζ-potential on the in vivo behavior of niosomes is complex, and the effects described in the literature are not unambiguous, there are many data proving that the presence of a negative zeta-potential favors the cellular internalization of the vesicles.51 On this ground it was worthy to investigate whether the presence of hydrophilic PAA layer on the niosomal surface could affect their ability to interact with target cells. Thus, in vitro cellular uptake of curcumin by H1299 and L929 (data not shown) cells, treaded with subequieffective concentrations of free or niosomal curcumin encapsulated into plain Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60
Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
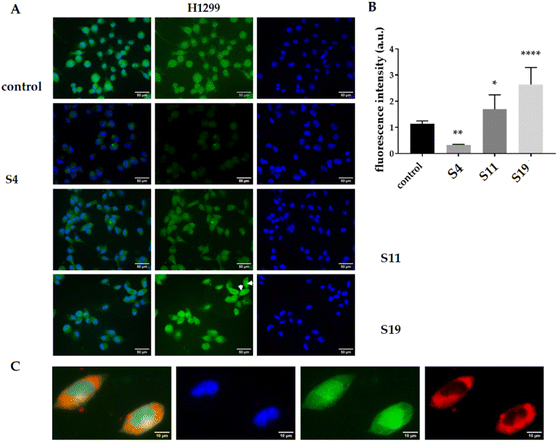
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol niosomes (S4) or into their HD-PAA12 or HD-PAA17 modified counterparts (S11, S19) was studied by fluorescence microscopy. The tested cell lines H1299 and L929 were selected as models of sensitive adherent malignant and nonmalignant cells. As shown in Fig. 8 A and B, H1299 cells treated with the niosomal curcumin showed greater green fluorescence intensity than those treated with the free drug. Presumably, the encapsulated curcumin in niosomes penetrates the cell by endocytosis, which is the predominantly mechanism of internalization of nanosized carriers and is much more effective than the simple diffusion of free drug.52 The encapsulation into the niosomes increased its accumulation in the cells, which is evident from the bar graph representing the quantification of the normalized integrated density. In the case of plain niosomes, an increase of 60% was observed, while polymer-modified niosomes showed approximately 2.6-fold higher fluorescence intensity as compared to the free curcumin (Fig. 8B). Normally, after internalization in the cells, curcumin accumulates partially on endoplasmic membranes, clathrin vesicles, and lysosomes.53 Such localization was observed for the free and niosomal curcumin encapsulated into the HD-PAA12 modified niosomes. Interestingly, HD-PAA17 modified vesicles, which contains a more extended PAA component, shows colocalization of curcumin with the nuclear-specific marker DAPI (Fig. 8C and arrows in panel A), demonstrating the ability of the engineered vesicles to overcome lysosomal retention and deliver cargo to target subcellular compartments. The observed effective targeting of the nucleus was not a primary consideration in our formulation design strategy, but it is a noteworthy discovery.
Chol niosomes (S4) or into their HD-PAA12 or HD-PAA17 modified counterparts (S11, S19) was studied by fluorescence microscopy. The tested cell lines H1299 and L929 were selected as models of sensitive adherent malignant and nonmalignant cells. As shown in Fig. 8 A and B, H1299 cells treated with the niosomal curcumin showed greater green fluorescence intensity than those treated with the free drug. Presumably, the encapsulated curcumin in niosomes penetrates the cell by endocytosis, which is the predominantly mechanism of internalization of nanosized carriers and is much more effective than the simple diffusion of free drug.52 The encapsulation into the niosomes increased its accumulation in the cells, which is evident from the bar graph representing the quantification of the normalized integrated density. In the case of plain niosomes, an increase of 60% was observed, while polymer-modified niosomes showed approximately 2.6-fold higher fluorescence intensity as compared to the free curcumin (Fig. 8B). Normally, after internalization in the cells, curcumin accumulates partially on endoplasmic membranes, clathrin vesicles, and lysosomes.53 Such localization was observed for the free and niosomal curcumin encapsulated into the HD-PAA12 modified niosomes. Interestingly, HD-PAA17 modified vesicles, which contains a more extended PAA component, shows colocalization of curcumin with the nuclear-specific marker DAPI (Fig. 8C and arrows in panel A), demonstrating the ability of the engineered vesicles to overcome lysosomal retention and deliver cargo to target subcellular compartments. The observed effective targeting of the nucleus was not a primary consideration in our formulation design strategy, but it is a noteworthy discovery.
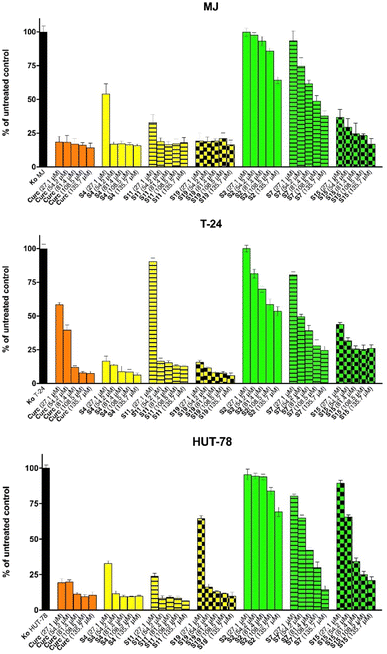
Cytotoxicity assessment of free and niosomal curcumin
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60
Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol, equivalent (in the MJ cell line) and nearly seven times exceeding (in the T-24 malignant culture) the cytotoxicity of the reference substance curcumin. The alternative HD-PAA12-modified niosomal formulation (S11) demonstrated a slightly lower antiproliferative potential, however, still matching the activity of the reference free drug in the other screened T-cell lymphoma model (HUT-78). Satisfactory results were also obtained with the unmodified variant of curcumin-loaded Tw60
Chol, equivalent (in the MJ cell line) and nearly seven times exceeding (in the T-24 malignant culture) the cytotoxicity of the reference substance curcumin. The alternative HD-PAA12-modified niosomal formulation (S11) demonstrated a slightly lower antiproliferative potential, however, still matching the activity of the reference free drug in the other screened T-cell lymphoma model (HUT-78). Satisfactory results were also obtained with the unmodified variant of curcumin-loaded Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60
Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol niosomes, which inhibited T-24 cells' growth nearly seven times more effectively than the reference substance (IC50 5.5 μM vs. 34.9 μM, respectively). The least favourable cytotoxicity profile showed plain Sp60
Chol niosomes, which inhibited T-24 cells' growth nearly seven times more effectively than the reference substance (IC50 5.5 μM vs. 34.9 μM, respectively). The least favourable cytotoxicity profile showed plain Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol niosomes and its stimuli-responsive counterparts modified with HD-PAA12 (S7) in all of the screened malignant cell lines.
Chol niosomes and its stimuli-responsive counterparts modified with HD-PAA12 (S7) in all of the screened malignant cell lines.
| IC50 (μM ± SD) in terms of curcumin concentration | |||
|---|---|---|---|
| Sample/cell line | MJ | T-24 | HUT-78 |
| Curc | 3.3 ± 1.2 | 34.9 ± 2.8 | 6.4 ± 1.8 |
Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ch (S4) Ch (S4) |
26.4 ± 2.3 | 5.5 ± 1.7 | 14.2 ± 2.4 |
Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ch Ch![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HD-PAA12 (S11) HD-PAA12 (S11) |
18.2 ± 3.4 | 41.2 ± 4.3 | 8.7 ± 1.7 |
Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60 Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ch Ch![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HD-PAA17 (S19) HD-PAA17 (S19) |
2.3 ± 1.1 | 5.2 ± 1.5 | 33.0 ± 2.5 |
Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ch (S2) Ch (S2) |
140.2 ± 8.3 | 142.9 ± 7.6 | 154.8 ± 9.5 |
Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ch Ch![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HD-PAA12 (S7) HD-PAA12 (S7) |
101.8 ± 10.4 | 59.7 ± 3.6 | 67.5 ± 4.4 |
Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ch Ch![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HD-PAA17 (S15) HD-PAA17 (S15) |
10.39 ± 2.1 | 18.7 ± 3.1 | 67.6 ± 3.2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60
Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ch
Ch![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
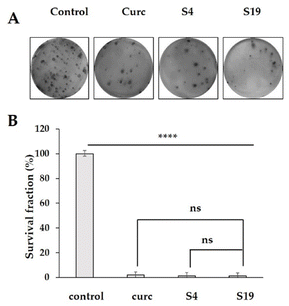
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HD-PAA17 were further assessed in a comparative way vs. free agent or loaded into plain niosomes curcumin by using clonogenic assay in H1299 cancer cells after 48 h exposure to their equieffective levels. The clonogenic assay is a valuable tool for assessing the fraction of cells that retain their proliferative ability and form colonies after exposure to a cytotoxic agent. We treated H1299 cells with calculated IC50 values (see ESI, Table S1 and Fig. S4†) of free curcumin, loaded in plain niosomes curcumin (S4), and their HD-PAA17 modified counterparts (S19) for 48 hours at two different cell concentrations (500 and 1000 cells per well). Then the cells were allowed to grow for 10 days in drug-free medium. Curcumin has demonstrated potential anti-cancer effects, including inhibition of cancer stem cells and induction of senescence and clonogenic death in breast cancer cells.54 We sought to determine whether curcumin loaded in pH-sensitive niosomes retained the cytotoxic capacity of the free drug. Our results revealed that although there are no statistically significant differences in the survival fractions of H1299 cells treated with either free curcumin or niosomal curcumin (Fig. 10) the less colonies were count in cells treated with HD-PAA-modified systems. These findings suggest that curcumin-loaded pH-sensitive niosomes could serve as a promising drug delivery system for cancer treatment. Encapsulation of the drug in niosomes may enhance its effectiveness and minimize potential side effects. However, further investigation is necessary to explore the full potential of this approach and its clinical applications.
HD-PAA17 were further assessed in a comparative way vs. free agent or loaded into plain niosomes curcumin by using clonogenic assay in H1299 cancer cells after 48 h exposure to their equieffective levels. The clonogenic assay is a valuable tool for assessing the fraction of cells that retain their proliferative ability and form colonies after exposure to a cytotoxic agent. We treated H1299 cells with calculated IC50 values (see ESI, Table S1 and Fig. S4†) of free curcumin, loaded in plain niosomes curcumin (S4), and their HD-PAA17 modified counterparts (S19) for 48 hours at two different cell concentrations (500 and 1000 cells per well). Then the cells were allowed to grow for 10 days in drug-free medium. Curcumin has demonstrated potential anti-cancer effects, including inhibition of cancer stem cells and induction of senescence and clonogenic death in breast cancer cells.54 We sought to determine whether curcumin loaded in pH-sensitive niosomes retained the cytotoxic capacity of the free drug. Our results revealed that although there are no statistically significant differences in the survival fractions of H1299 cells treated with either free curcumin or niosomal curcumin (Fig. 10) the less colonies were count in cells treated with HD-PAA-modified systems. These findings suggest that curcumin-loaded pH-sensitive niosomes could serve as a promising drug delivery system for cancer treatment. Encapsulation of the drug in niosomes may enhance its effectiveness and minimize potential side effects. However, further investigation is necessary to explore the full potential of this approach and its clinical applications.
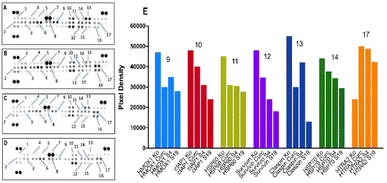
As indicated by the proteomic data for T-24 cells (Fig. 12), both curcumin and its niosomal formulations failed to evoke cleavage of the procaspase 3 zymogen (7), that acts as a central executor caspase in the final stages of the apoptotic cascade. Changes in the activity of the bcl-2 cell death regulators bad (1, proapoptotic), bcl-2 and bcl-x (antiapoptotic, 2 and 3, respectively) were also minor (Fig. 12 A–D).
Nevertheless, distinguishable changes in spot signals were registered for a number of survival factors that inhibit apoptosis at an earlier stage, including cIAP (10), survivin (12) and the chaperons HSP60, HSP70 (11 and 14, respectively). The expression profiles of these proteins follow a repetitive pattern where the strongest down-regulation (ca. 50% in cIAP1 and survivin levels) was induced by the HD-PAA17-modified niosomes (S19) followed by the plain composition (S4) and the free curcumin. A similar trend with a slightly better performance of curcumin shared the proteomic imprints of HMOX1 (9) and claspin (13), although the strongest proapoptotic stimuli was invariably induced by the modified with HD-PAA17 niosomal variant. On the contrary, the proapoptotic serine protease HTRA/Omi that is released from mitochondria in response to cellular stress was significantly up-regulated in the treated samples regardless of curcumin formulation and slightly decreased in the following order: curcumin > plain niosomes > HD-PAA17-modified niosomes (Fig. 12E).
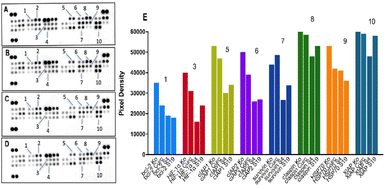
Comparative analysis of the apoptotic response in CTCL cells (Fig. 13) also showed consistent reduction in the expression of the aforementioned survival factors cIAP1 (5), cIAP2 (6), survivin (7) and XIAP (10). However, the strongest inhibition of these factors was achieved by the plain niosomal formulation (S4) leading to a nearly 2-fold weakening in the signals for (5), (6) and (7) and a modest decline in (10). Unlike bladder carcinoma T-24 cell line, the proapoptotic activity of curcumin formulations against MJ cells followed a different trend. The most prominent changes in protein expression across all samples were induced by the unmodified niosomes, followed by its HD-PAA17-modified variant and free curcumin (Fig. 13E). A slightly better performance of the pH-sensitive system was only observed in the chaperone HSP70 (9) expression profile. The two niosomal compositions yielded a decent 50% decrease of the major bcl-2 family member (1) that was less influenced by the free curcumin formulation. The found predominant modulation of early regulatory factors of cell cycle and the lack of capacity to initiate core components of the apoptotic machinery (i.e. procaspase 3) in curcumin and niosome samples may be a time-dependent phenomenon since protein expression was measured only 24 hours following treatment.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60
Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
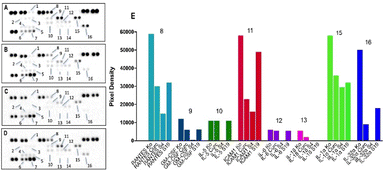
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (S4). An absolute inhibition was also reached in IL-32 expression (16), which was initially high in the control group and plays a major role in cancer-induced chronic inflammation. The same formulation induced substantial reduction in the chemotactic RANTES protein (8) and its downstream target – the intercellular adhesion molecule 1 (ICAM-1, 11). Both mediators have been recognized as major triggers in skin inflammation that is a characteristic trait of the CTCL lesions. According to the presented data from the conducted pharmacodynamic study, both types of niosome formulations exerted an augmenting effect on curcumin performance in either of the investigated mechanistic aspects. The strongest proapoptotic stimuli in bladder carcinoma T-24 cells exerted the pH-responsive Tw60
Chol (S4). An absolute inhibition was also reached in IL-32 expression (16), which was initially high in the control group and plays a major role in cancer-induced chronic inflammation. The same formulation induced substantial reduction in the chemotactic RANTES protein (8) and its downstream target – the intercellular adhesion molecule 1 (ICAM-1, 11). Both mediators have been recognized as major triggers in skin inflammation that is a characteristic trait of the CTCL lesions. According to the presented data from the conducted pharmacodynamic study, both types of niosome formulations exerted an augmenting effect on curcumin performance in either of the investigated mechanistic aspects. The strongest proapoptotic stimuli in bladder carcinoma T-24 cells exerted the pH-responsive Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60
Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HD-PAA17 niosomes, whereas plain Tw60
HD-PAA17 niosomes, whereas plain Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60
Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol vesicles had a more pronounced effect in the CTC lymphoma cells. In terms of inflammation, prevalent inhibitory effect evoked the polymer-free Tw60
Chol vesicles had a more pronounced effect in the CTC lymphoma cells. In terms of inflammation, prevalent inhibitory effect evoked the polymer-free Tw60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60
Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol formulation in both screened cell lines.
Chol formulation in both screened cell lines.Experimental
Materials
Hexadecanol, α-bromoisobutyryl bromide, triethylamine, tert-butyl acrylate (tBA), Na2CO3, MgSO4 Cu(I)Br, N,N,N′,N′′,N′′-pentamethyldiethylenetriamine (PMDETA), and trifluoroacetic acid (TFA) were purchased from Sigma-Aldrich (FOT, Bulgaria) and used as received. Dichloromethane (DCM), acetone, methanol, 1,4-dioxane and tetrahydrofuran (THF) were obtained from Fisher Chemical (Labimex, Bulgaria) and distilled prior use. Curcumin (Curc), Span 60, Tween 60 and cholesterol were provided by Sigma-Aldrich (FOT, Bulgaria). Chloroform, methanol and other solvents were of analytical grade.Methods
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O), 1.68 (m, 2H, –CH2–C
2–O), 1.68 (m, 2H, –CH2–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–CH2–O–), 1.93 (s, 6H, –C–C
2–CH2–O–), 1.93 (s, 6H, –C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.26 (m, 26H, –CH2–C
3), 1.26 (m, 26H, –CH2–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–CH2–), 0.88 (m, 3H, CH2–C
2–CH2–), 0.88 (m, 3H, CH2–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3).
3).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) at 4 °C. Finally, the polymer was recovered by freeze drying. Yield 76%. 1H-NMR (CDCl3, δ ppm): 4.17 (m, 2H, CH2–C
30) at 4 °C. Finally, the polymer was recovered by freeze drying. Yield 76%. 1H-NMR (CDCl3, δ ppm): 4.17 (m, 2H, CH2–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O), 1.68 (m, 2H, –CH2–C
2–O), 1.68 (m, 2H, –CH2–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–CH2–O–), 1.93 (s, 6H, –C–C
2–CH2–O–), 1.93 (s, 6H, –C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.26 (m, 26H, –CH2–C
3), 1.26 (m, 26H, –CH2–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–CH2–), 0.88 (m, 3H, CH2–C
2–CH2–), 0.88 (m, 3H, CH2–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); tBA protons: 2.21 (s, H, –CH2–C(C
3); tBA protons: 2.21 (s, H, –CH2–C(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)
O)![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –), 1.53 (m, 2H, –C
–), 1.53 (m, 2H, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–C(C
2–C(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)H–), and 1.45 (m, 9H, O–C(C
O)H–), and 1.45 (m, 9H, O–C(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)3).
3)3).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) mixture, transferred into dialysis tube (3500 MWCO), and dialyzed against deionized water for 3 days. Finally, HDPAA was recovered by freeze drying.
20) mixture, transferred into dialysis tube (3500 MWCO), and dialyzed against deionized water for 3 days. Finally, HDPAA was recovered by freeze drying.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tween 60) and cholesterol (1
Tween 60) and cholesterol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3.5
1 and 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5
3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio, total lipid content 30 μmoL ml−1) and curcumin (1.5 μmol mL−1) were dissolved in 10 mL chloroform. For the preparation of polymer modified niosomes 0.5, 1, 2.5 and 5 mol% HD-PAA12 or HD-PAA17 were dissolved in chloroform
3 molar ratio, total lipid content 30 μmoL ml−1) and curcumin (1.5 μmol mL−1) were dissolved in 10 mL chloroform. For the preparation of polymer modified niosomes 0.5, 1, 2.5 and 5 mol% HD-PAA12 or HD-PAA17 were dissolved in chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol mixture (2
methanol mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) and added to the niosomal constituents. The organic solvents were evaporated at 60 °C using a rotary evaporator (Buchi, Germany) (150 rpm), resulting into a dried lipid film on the interior of the flask. Next, the lipid film was subsequently hydrated for 1 hour at 60 °C (100 rpm) with 10 mL PBS (pH 7.4, composition: NaCl 8.0 g L−1, KCl 0.2 g L−1, Na2HPO4 1.44 g L−1 and KH2PO4 0.245 g L−1). Afterwards niosomal dispersions were sonicated for 2 min using probe sonicator (Bandelin). The elaborated curcumin-loaded niosomes were stored at 4 ± 2 °C for further evaluation.
1 v/v) and added to the niosomal constituents. The organic solvents were evaporated at 60 °C using a rotary evaporator (Buchi, Germany) (150 rpm), resulting into a dried lipid film on the interior of the flask. Next, the lipid film was subsequently hydrated for 1 hour at 60 °C (100 rpm) with 10 mL PBS (pH 7.4, composition: NaCl 8.0 g L−1, KCl 0.2 g L−1, Na2HPO4 1.44 g L−1 and KH2PO4 0.245 g L−1). Afterwards niosomal dispersions were sonicated for 2 min using probe sonicator (Bandelin). The elaborated curcumin-loaded niosomes were stored at 4 ± 2 °C for further evaluation.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt/wt). Afterwards aliquots of phosphate buffered saline (pH 7.4) were added and the resultant mixture were vortexed for 10 s and centrifuged for 5 min at 6000 rpm. Curcumin's concentration in the organic layer was evaluated spectrophotometrically at 427 nm, using a UV-VIS spectrophotometer (Shimadzu UV-1800). The entrapment efficiency (EE%) and drug loading capacity (DL%) were calculated using the following equations:
1 wt/wt). Afterwards aliquots of phosphate buffered saline (pH 7.4) were added and the resultant mixture were vortexed for 10 s and centrifuged for 5 min at 6000 rpm. Curcumin's concentration in the organic layer was evaluated spectrophotometrically at 427 nm, using a UV-VIS spectrophotometer (Shimadzu UV-1800). The entrapment efficiency (EE%) and drug loading capacity (DL%) were calculated using the following equations:
 | (1) |
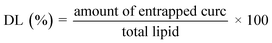 | (2) |
The experiments were carried out three times and the average values were used.
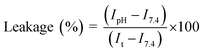 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Sigma-Aldrich, Steinheim, Germany) and placed in a 100 mL dissolution medium – phosphate-buffered saline (PBS) pH 5.5, PBS pH 7.4 and PBS pH 7.4 + 10% albumin. To all acceptor media 10% ethanol was added to maintain the solubility of the released curcumin. The acceptor medium was in constant motion (200 rpm) and the circulating water jacket (Huber, Germany) maintained the temperature at 37 ± 0.5 °C during the study. At predetermined time points 1 mL samples from the released medium were withdrawn and analyzed for curcumin by UV-VIS spectroscopy at 427 nm. The aliquots were replaced with equal volume fresh medium. The drug release studies were carried out threefold.
000, Sigma-Aldrich, Steinheim, Germany) and placed in a 100 mL dissolution medium – phosphate-buffered saline (PBS) pH 5.5, PBS pH 7.4 and PBS pH 7.4 + 10% albumin. To all acceptor media 10% ethanol was added to maintain the solubility of the released curcumin. The acceptor medium was in constant motion (200 rpm) and the circulating water jacket (Huber, Germany) maintained the temperature at 37 ± 0.5 °C during the study. At predetermined time points 1 mL samples from the released medium were withdrawn and analyzed for curcumin by UV-VIS spectroscopy at 427 nm. The aliquots were replaced with equal volume fresh medium. The drug release studies were carried out threefold.Proteome Profiler Human Apoptosis Array Kit, R&D Systems and a Proteome Profiler Human Cytokine Array Kit, R&D Systems were used for the parallel determination of multiple apoptosis- and inflammation-related protein endpoints.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60
Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ch niosomes for 48 hours. After incubation the medium was replaced with fresh one and the cells were allowed to grow for 10 days. Then the cells were washed 2 times for 5 min with 1× PBS. After that the cells were fixated with 4% PFA (paraformaldehyde) for 20 min at gentle shaking. After the fixation the cells were washed with 1× PBS for 5 min and stained with 0.2% Crystal-violet (Sigma-Aldrich) for 20 min at gentle shaking and room temperature. The last step was washing the cells 3 times for 5 min with dH2O and dried at room temperature. Then the colonies were counted on ImageJ software and plating efficiency (PE) and survival fraction (SF) were calculated. Photoshop software was used for image processing. Statistical analysis was performed on GraphPad Prism v. 8.0 (Dotmatics, San Diego, CA, USA) and One-way ANOVA Tukey's multiple comparisons test was used.
Ch niosomes for 48 hours. After incubation the medium was replaced with fresh one and the cells were allowed to grow for 10 days. Then the cells were washed 2 times for 5 min with 1× PBS. After that the cells were fixated with 4% PFA (paraformaldehyde) for 20 min at gentle shaking. After the fixation the cells were washed with 1× PBS for 5 min and stained with 0.2% Crystal-violet (Sigma-Aldrich) for 20 min at gentle shaking and room temperature. The last step was washing the cells 3 times for 5 min with dH2O and dried at room temperature. Then the colonies were counted on ImageJ software and plating efficiency (PE) and survival fraction (SF) were calculated. Photoshop software was used for image processing. Statistical analysis was performed on GraphPad Prism v. 8.0 (Dotmatics, San Diego, CA, USA) and One-way ANOVA Tukey's multiple comparisons test was used.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sp60
Sp60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
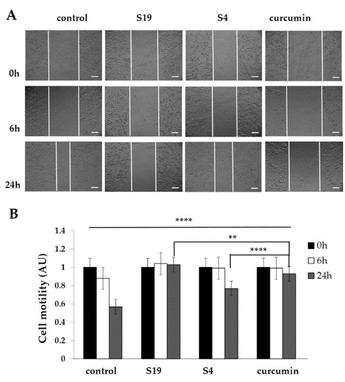
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol and pH-sensitive HD-PAA17 vesicles. Images of the formed “wounded” area were taken immediately (0 hours) on Zeiss AxioVert 200M microscope using a 5× objective lens, equipped with a CCD camera Axio Cam MRm. Additional images were taken 6 and 24 hours after treatment. The length of the scratch for every time interval was measured using the Carl Zeiss™ AxioVision Rel. version 4.8 software (Fisher Scientific, Waltham, MA, USA). The cell migration at every tested condition was quantified and processed on GraphPad Prism v. 8.0 (Dotmatics, San Diego, CA, USA).
Chol and pH-sensitive HD-PAA17 vesicles. Images of the formed “wounded” area were taken immediately (0 hours) on Zeiss AxioVert 200M microscope using a 5× objective lens, equipped with a CCD camera Axio Cam MRm. Additional images were taken 6 and 24 hours after treatment. The length of the scratch for every time interval was measured using the Carl Zeiss™ AxioVision Rel. version 4.8 software (Fisher Scientific, Waltham, MA, USA). The cell migration at every tested condition was quantified and processed on GraphPad Prism v. 8.0 (Dotmatics, San Diego, CA, USA).Conclusions
Original pH-responsive nanocarriers for delivery of bioactive agents were successfully elaborated by membrane modification of conventional niosomes with newly synthesized hexadecyl-poly(acrylic acid) copolymers (HD-PAA12 and HD-PAA17). All copolymer modified niosomes exhibited pH-dependent release of the encapsulated cargo (a water soluble and a hydrophobic model drug), with higher release rate in acidic media, which may be exploited as a feasible targeting approach in cancer therapeutics. The formulation composed of Span60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tween60
Tween60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cholesterol
cholesterol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HD-PAA17 (2.5 mol%) were characterized by small size (302 nm), high curcumin entrapment efficiency (83%) and increased cellular internalization. In addition, the curcumin-loaded pH-responsive niosomes showed a higher inhibition on colony formation and enhanced antineoplastic and anti-inflammatory activity compared to free drug. Furthermore, the results from the conducted pharmacodynamic study revealed the strongest proapoptotic stimuli in bladder carcinoma T-24 cells for the optimal Span60
HD-PAA17 (2.5 mol%) were characterized by small size (302 nm), high curcumin entrapment efficiency (83%) and increased cellular internalization. In addition, the curcumin-loaded pH-responsive niosomes showed a higher inhibition on colony formation and enhanced antineoplastic and anti-inflammatory activity compared to free drug. Furthermore, the results from the conducted pharmacodynamic study revealed the strongest proapoptotic stimuli in bladder carcinoma T-24 cells for the optimal Span60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tween60
Tween60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cholesterol
cholesterol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HD-PAA17 formulation. Therefore, the elaborated pH-responsive niosomes may be explored as a feasible platform for targeting delivery of curcumin.
HD-PAA17 formulation. Therefore, the elaborated pH-responsive niosomes may be explored as a feasible platform for targeting delivery of curcumin.
Author contributions
Denitsa Momekova: conceptualization, formal analysis, investigation, writing—original draft preparation, writing – review and editing, funding acquisition. Petar D. Petrov: conceptualization, formal analysis, investigation, writing – sections, writing – review and editing. Georgi Momekov: formal analysis, investigation, writing – review and editing. Iva Ugrinova: formal analysis, investigation, writing – sections, writing – review and editing. Viliana Gugleva: investigation, writing – original draft preparation, writing – sections, review, and editing. Rositsa Mihaylova: investigation, writing – sections, review, and editing. Katya Kamenova: investigation, writing – sections. Aleksander Forys: investigation, writing – sections. Barbara Trzebicka: writing – sections, review, and editing. Maria Petrova: investigation, writing – sections.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Bulgarian National Science Fund (grant KΠ-06-43/3-2020). P. P. and B. T. acknowledge the scientific cooperation between the Bulgarian Academy of Sciences and the Polish Academy of Sciences (project IC-PL/12/2024-2025).Notes and references
- S. A. A. Rizvi and A. M. Saleh, Applications of nanoparticle systems in drug delivery technology, Saudi Pharm. J., 2018, 26(1), 64–70 CrossRef.
- O. Afzal, A. S. A. Altamimi, M. S. Nadeem, S. I. Alzarea, W. H. Almalki, A. Tariq, B. Mubeen, B. N. Murtaza, S. Iftikhar, N. Riaz and I. Kazmi, Nanoparticles in drug delivery: from history to therapeutic applications, Nanomaterials, 2022, 12(24), 4494 CrossRef CAS.
- Y. Yao, Y. Zhou, L. Liu, Y. Xu, Q. Chen, Y. Wang, S. Wu, Y. Deng, J. Zhang and A. Shao, Nanoparticle-based drug de-livery in cancer therapy and its role in overcoming drug resistance, Front. Mol. Biosci., 2020, 7, 193 CrossRef CAS.
- D. Mundekkad and W. C. Cho, Nanoparticles in clinical translation for cancer therapy, Int. J. Mol. Sci., 2022, 23(3), 1685 CrossRef CAS.
- S. Saraf, A. Jain, A. Tiwari, A. Verma, P. K. Panda and S. K. Jain, Advances in liposomal drug delivery to cancer: An overview, J. Drug Delivery Sci. Technol., 2020, 56, 101549 CrossRef CAS.
- N. M. AlSawaftah, N. S. Awad, W. G. Pitt and G. A. Husseini, pH-Responsive nanocarriers in cancer therapy, Polymers, 2022, 14(5), 936 CrossRef CAS.
- D. G. Bhavani and V. L. Lakshmi, Recent advances of non-ionic surfactant-based nano-vesicles (niosomes and proniosomes): a brief review of these in enhancing transdermal delivery of drug, Futur. J. Pharm. Sci., 2020, 6, 100 CrossRef.
- B. A. Witika, K. E. Bassey, P. H. Demana, X. Siwe-Noundou and M. S. Poka, Current advances in specialised niosomal drug delivery: manufacture, characterization and drug delivery applications, Int. J. Mol. Sci., 2022, 23(17), 9668 CrossRef CAS.
- M. Masjedi and T. Montahaei, An illustrated review on nonionic surfactant vesicles (niosomes) as an approach in modern drug delivery: Fabrication, characterization, pharmaceutical, and cosmetic applications, J. Drug Delivery Sci. Technol., 2021, 61, 102234 CrossRef CAS.
- X. Ge, M. Wei, S. He and W. E. Yuan, Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery, Pharmaceutics, 2019, 11(2), 55 CrossRef CAS.
- S. Grijalvo, G. Puras, J. Zárate, M. Sainz-Ramos, N. A. L. Qtaish, T. López, M. Mashal, N. Attia, D. Díaz Díaz, R. Pons, E. Fernández, J. L. Pedraz and R. Eritja, Cationic niosomes as non-viral vehicles for nucleic acids: challenges and opportunities in gene delivery, Pharmaceutics, 2019, 11(2), 50 CrossRef CAS.
- C. Marianecci, L. Di Marzio, F. Rinaldi, C. Celia, D. Paolino, F. Alhaique, S. Esposito and M. Carafa, Niosomes from 80s to present: the state of the art, Adv. Colloid Interface Sci., 2014, 205, 187–206 CrossRef CAS.
- M. Schlich, F. Lai, R. Pireddu, E. Pini, G. Ailuno, A. M. Fadda, D. Valenti and C. Sinico, Resveratrol proniosomes as a convenient nanoingredient for functional food, Food Chem., 2020, 310, 125950 CrossRef CAS.
- R. Ahmad, S. Srivastava, S. Ghosh and S. K. Khare, Phytochemical delivery through nanocarriers: a review, Colloids Surf., B, 2021, 197, 111389 CrossRef CAS.
- N. d’Avanzo, M. C. Cristiano, L. Di Marzio, M. C. Bruno, D. Paolino, C. Celia and M. Fresta, Multidrug Idebenone/Naproxen Co-loaded Aspasomes for Significant in vivo Anti-inflammatory Activity, ChemMedChem, 2022, 17(9), e202200067 CrossRef.
- M. A. Abdelbari, A. A. El-Gazar, A. A. Abdelbary, A. H. Elshafeey and S. Mosallam, Brij integrated bilosomes for improving the transdermal delivery of niflumic acid for effective treatment of osteoarthritis: In vitro characterization, ex vivo permeability assessment, and in vivo study, Int. J. Pharm., 2023, 640, 123024 CrossRef CAS.
- A. C. Caritá, J. Resende de Azevedo, Y. Chevalier, D. Arquier, M. V. Buri, K. A. Riske, G. Ricci Leonardi and M. A. Bolzinger, Elastic cationic liposomes for vitamin C delivery: Development, characterization and skin absorption study, Int. J. Pharm., 2023, 638, 122897 CrossRef.
- M. A. Altamimi, A. Hussain, W. A. Mahdi, S. S. Imam, M. A. Alshammari, S. Alshehri and M. R. Khan, Mechanistic Insights into Luteolin-Loaded Elastic Liposomes for Transdermal Delivery: HSPiP Predictive Parameters and Instrument-Based Evidence, ACS Omega, 2022, 7(51), 48202–48214 CrossRef CAS.
- M. B. Marzola Coronel, C. C. Fraenza and E. Anoardo, On the deformability of additivated phosphatidylcholine liposomes: Molecular dynamic regimes and membrane elasticity, Chem. Phys. Lipids, 2023, 252, 105290 CrossRef CAS PubMed.
- D. Paolino, D. Cosco, F. Cilurzo, E. Trapasso, V. M. Morittu, C. Celia and M. Fresta, Improved in vitro and in vivo collagen biosynthesis by asiaticoside-loaded ultradeformable vesicles, J. Controlled Release, 2012, 162(1), 143–151 CrossRef CAS PubMed.
- A. Barone, M. C. Cristiano, F. Cilurzo, M. Locatelli, D. Iannotta, L. Di Marzio, C. Celia and D. Paolino, Ammonium glycyrrhizate skin delivery from ultradeformable liposomes: A novel use as an anti-inflammatory agent in topical drug delivery, Colloids Surf., B, 2020, 193, 111152 CrossRef CAS PubMed.
- R. Muzzalupo and E. Mazzotta, Do niosomes have a place in the field of drug delivery?, Expert Opin. Drug Delivery, 2019, 16(11), 1145–1147 CrossRef CAS PubMed.
- X. Hu, J. Zhang, L. Deng, H. Hu, J. Hu and G. Zheng, Galactose-modified pH-sensitive niosomes for controlled release and hepatocellular carcinoma target delivery of tanshinone IIA, AAPS PharmSciTech, 2021, 22(3), 96 CrossRef CAS PubMed.
- P. Aparajay and A. Dev, Functionalized niosomes as a smart delivery device in cancer and fungal infection, Eur. J. Pharm. Sci., 2022, 168, 106052 CrossRef CAS PubMed.
- D. Tila, S. N. Yazdani-Arazi, S. Ghanbarzadeh, S. Arami and Z. Pourmoazzen, pH-sensitive, polymer modified, plasma stable niosomes: promising carriers for anti-cancer drugs, EXCLI J., 2015, 14, 21–32 Search PubMed.
- G. Hao, Z. P. Xu and L. Li, Manipulating extracellular tumour pH: an effective target for cancer therapy, RSC Adv., 2018, 8, 22182–22192 RSC.
- Y. B. Hu, E. B. Dammer, R. J. Ren and G. Wang, The endosomal-lysosomal system: from acidification and cargo sorting to neurodegeneration, Transl. Neurodegener., 2015, 4, 18 CrossRef PubMed.
- E. Roux, M. Francis, F. M. Winnik and J. C. Leroux, Polymer based pH-sensitive carriers as a means to improve the cytoplasmic delivery of drugs, Int. J. Pharm., 2002, 242, 25–36 CrossRef CAS PubMed.
- M. Di Francesco, C. Celia, R. Primavera, N. D'Avanzo, M. Locatelli, M. Fresta, F. Cilurzo, C. A. Ventura, D. Paolino and L. Di Marzio, Physicochemical characterization of pH-responsive and fusogenic self-assembled non-phospholipid vesicles for a potential multiple targeting therapy, Int. J. Pharm., 2017, 528, 18–32 CrossRef CAS PubMed.
- S. Sargazi, S. M. Hosseinikhah, F. Zargari, N. P. S. Chauhana, M. Hassanisaadi and S. Amani, pH-responsive cisplatin-loaded niosomes: synthesis, characterization, cytotoxicity study and interaction analyses by simulation methodology, Nanofabrication, 2021, 6, 1–15 CrossRef.
- F. Marzoli, C. Marianecci, F. Rinaldi, D. Passeri, M. Rossi, P. Minosi, M. Carafa and S. Pieretti, Long-lasting, antinociceptive effects of pH-sensitive niosomes loaded with ibuprofen in acute and chronic models of pain, Pharmaceutics, 2019, 11(2), 62 CrossRef CAS PubMed.
- M. C. Pereira, M. Pianella, D. Wei, A. Moshnikova, C. Marianecci, M. Carafa, O. A. Andreev and Y. K. Reshetnyak, pH-sensitive pHLIP coated niosomes, Mol. Membr. Biol., 2016, 33, 51–63 CrossRef CAS PubMed.
- M. F. Francis, G. Dhara, F. M. Winnik and J. C. Leroux, In vitro evaluation of pH-sensitive polymer/niosome complexes, Biomacromolecules, 2001, 2, 741–749 CrossRef CAS PubMed.
- W. Zeng, Q. Li, T. Wan, C. Liu, W. Pan, Z. Wu, G. Zhang, J. Pan, M. Qin, Y. Lin, C. Wu and Y. Xu, Hyaluronic acid-coated niosomes facilitate tacrolimus ocular delivery: mucoadhesion, precorneal retention, aqueous humor pharmacokinetics, and transcorneal permeability, Colloids Surf., B, 2016, 141, 28–35 CrossRef CAS PubMed.
- A. Mansoori-Kermani, S. Khalighi, I. Akbarzadeh, F. R. Niavol, H. Motasadizadeh, A. Mahdieh, V. Jahed, M. Abdinezhad, N. Rahbariasr, M. Hosseini, N. Ahmadkhani, B. Panahi, Y. Fatahi, M. Mozafari, A. P. Kumar and E. Mostafavi, Engineered hyaluronic acid-decorated niosomal nanoparticles for controlled and targeted delivery of epirubicin to treat breast cancer, Mater. Today Bio, 2022, 16, 100349 CrossRef CAS PubMed.
- K. Mansouri, S. Rasoulpoor, A. Daneshkhah, S. Abolfathi, N. Salari, M. Mohammadi, S. Rasoulpoor and S. Shabani, Clinical effects of curcumin in enhancing cancer therapy: a systematic review, BMC Cancer, 2020, 20(1), 791 CrossRef CAS PubMed.
- N. G. Vallianou, A. Evangelopoulos, N. Schizas and C. Kazazis, Potential anticancer properties and mechanisms of action of curcumin, Anticancer Res., 2015, 35(2), 645–651 CAS.
- M. A. Tomeh, R. Hadianamrei and X. Zhao, A review of curcumin and its derivatives as anticancer agents, Int. J. Mol. Sci., 2019, 20(5), 1033 CrossRef CAS PubMed.
- M. Kharat, G. Zhang and D. J. McClements, Stability of curcumin in oil-in-water emulsions: Impact of emulsifier type and concentration on chemical degradation, Food Res. Int., 2018, 111, 178–186 CrossRef CAS PubMed.
- M. Urošević, L. Nikolić, I. Gajić, V. Nikolić, A. Dinić and V. Miljković, Curcumin: biological Activities and Modern Pharmaceutical Forms, Antibiotics, 2022, 11(2), 135 CrossRef PubMed.
- R. Tabanelli, S. Brogi and V. Calderone, Improving curcumin bioavailability: current strategies and future perspectives, Pharmaceutics, 2021, 13(10), 1715 CrossRef CAS PubMed.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Bioavailability of curcumin: problems and promises, Mol. Pharm., 2007, 4(6), 807–818 CrossRef CAS PubMed.
- A. L. Lopresti, The problem of curcumin and its bioavailability: could its gastrointestinal influence contribute to its overall health-enhancing effects?, Adv. Nutr., 2018, 9(1), 41–50 CrossRef PubMed.
- L. Casula, F. Lai, E. Pini, D. Valenti, C. Sinico, M. C. Cardia, S. Marceddu, G. Ailuno and A. M. Fadda, Pulmonary delivery of curcumin and beclomethasone dipropionate in a multicomponent nanosuspension for the treatment of bronchial asthma, Pharmaceutics, 2021, 13(8), 1300 CrossRef CAS PubMed.
- V. Gugleva, V. Michailova, R. Mihaylova, G. Momekov, M. M. Zaharieva, H. Najdenski, P. Petrov, S. Rangelov, A. Forys, B. Trzebicka and D. Momekova, Formulation and evaluation of hybrid niosomal in situ gel for intravesical co-delivery of curcumin and gentamicin sulfate, Pharmaceutics, 2022, 14(4), 747 CrossRef CAS PubMed.
- D. C. Drummond, M. Zignani and J.-C. Leroux, Current status of pH-sensitive liposomes in drug delivery, Prog. Lipid Res., 2000, 39(5), 406–460 CrossRef PubMed.
- O. E. Philippova, D. Hourdet, R. Audebert and A. Khokhlov, pH-Responsive Gels of Hydrophobically Modified Poly(acrylic acid), Macromolecules, 1997, 30(26), 8278–8285 CrossRef CAS.
- X. Xiao, Y. Liu, M. Guo, W. Fei, H. Zheng, R. Zhang, Y. Zhang, Y. Wei, G. Zheng and F. Li, pH-triggered sustained re-lease of arsenic trioxide by polyacrylic acid capped mesoporous silica nanoparticles for solid tumor treatment in vitro and in vivo, J. Biomater. Appl., 2016, 31, 23–35 CrossRef CAS PubMed.
- S. Saroj and S. J. Rajput, Tailor-made pH-sensitive polyacrylic acid functionalized mesoporous silica nanoparticles for efficient and controlled delivery of anticancer drug etoposide, Drug Dev. Ind. Pharm., 2018, 44, 1198–1211 CrossRef CAS PubMed.
- A. Hioki, A. Wakasugi, K. Kawano, Y. Hattori and Y. Maitani, Development of an in vitro drug release assay of PEGylated liposome using bovine serum albumin and high temperature, Biol. Pharm. Bull., 2010, 33(9), 1466–1470 CrossRef CAS PubMed.
- R. Bajoria and S. F. Contractor, Effect of surface charge of small unilamellar liposomes on uptake and transfer of carboxyfluorescein across the perfused human term placenta, Pediatr. Res., 1997, 42, 520–527 CrossRef CAS PubMed.
- S. Mazumdar, D. Chitkara and A. Mittal, Exploration and insights into the cellular internalization and intracellular fate of amphiphilic polymeric nanocarriers, Acta Pharm. Sin. B, 2021, 11(4), 903–924 CrossRef CAS PubMed.
- M. Nazıroğlu, B. Çiğ, Y. Yazğan, G. K. Schwaerzer, F. Theilig and L. Pecze, Albumin evokes Ca2+-induced cell oxidative stress and apoptosis through TRPM2 channel in renal collecting duct cells reduced by curcumin, Sci. Rep., 2019, 9, 12403 CrossRef PubMed.
- N. Güllü, J. Smith, P. Herrmann and U. Stein, MACC1-dependent antitumor effect of curcumin in colorectal cancer, Nutrients, 2022, 14(22), 4792 CrossRef PubMed.
- G. Mudduluru, J. N. George-William, S. Muppala, I. A. Asangani, R. Kumarswamy, L. D. Nelson and H. Allgayer, Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer, Biosci. Rep., 2011, 31(3), 185–197 CrossRef CAS PubMed.
- L. Zhang, X. Cheng, Y. Gao, C. Zhang, J. Bao, H. Guan, H. Yu, R. Lu, Q. Xu and Y. Sun, Curcumin inhibits metastasis in human papillary thyroid carcinoma BCPAP cells via down-regulation of the TGF-β/Smad2/3 signaling pathway, Exp. Cell Res., 2016, 341(2), 157–165 CrossRef CAS PubMed.
- Y. Thabet, M. Elsabahy and N. G. Eissa, Methods for preparation of niosomes: a focus on thin-film hydration method, Methods, 2022, 199, 9–15 CrossRef CAS PubMed.
- S. S. D'Souza and P. P. DeLuca, Methods to assess in vitro drug release from injectable polymeric particulate systems, Pharm. Res., 2006, 23, 460–474 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra01334d |
| This journal is © The Royal Society of Chemistry 2024 |