Open Access Article
Open Access ArticleDesign, synthesis, and anti-inflammatory activity of indole-2-formamide benzimidazole[2,1-b]thiazole derivatives†
Hai-Feng Lin a,
Yu-Cai Jiang
a,
Yu-Cai Jiang *b,
Zhi-Wei Chen
*b,
Zhi-Wei Chen c and
Lin-Lin Zhengd
c and
Lin-Lin Zhengd
aDepartment of Gastroenterology, Affiliated Hospital of Putian University, Putian, China
bDepartment of Pharmacy, Affiliated Hospital of Putian University, Putian, China. E-mail: jiangyucai2030@ptu.edu.cn
cDepartment of Pathology, Affiliated Hospital of Putian University, Putian, China
dDepartment of Oncology, Affiliated Hospital of Putian University, Putian, China
First published on 29th May 2024
Abstract
Molecular hybridization is a widely employed technique in medicinal chemistry for drug modification, aiming to enhance pharmacological activity and minimize side effects. The combination of an indole ring and imidazole[2,1-b]thiazole has shown promising potential as a group that exhibits potent anti-inflammatory effects. In this study, we designed and synthesized a series of derivatives comprising indole-2-formamide benzimidazole[2,1-b]thiazole to evaluate their impact on LPS-induced production of pro-inflammatory cytokines NO, IL-6, and TNF-α release, as well as iron death in RAW264.7 cells. The findings revealed that most compounds effectively inhibited LPS-induced production of pro-inflammatory cytokines NO, IL-6, and TNF-α release in RAW264.7 cells. Compound 13b exhibited the most potent anti-inflammatory activity among the tested compounds. The results of the cytotoxicity assay indicated that compound 13b was nontoxic. Additionally, compound 13b was found to elevate the levels of ROS, MDA, and Fe2+, while reducing GSH content, thereby facilitating the iron death process. Consequently, compound 13b showed promise for future development as an anti-inflammatory drug.
Introduction
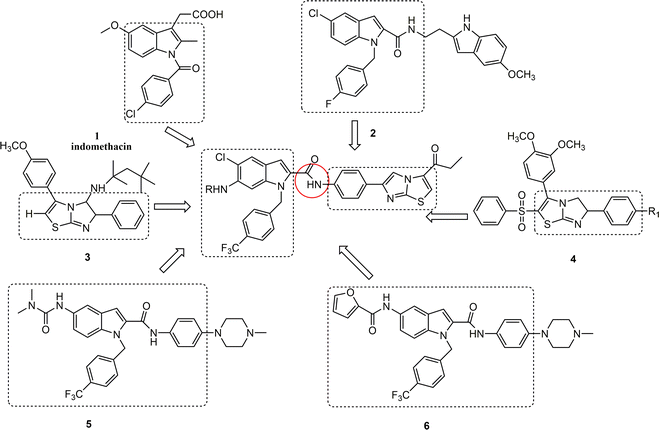
Inflammation is the natural immune response of the body to pathogens, physical injuries, and irritants, and is characterized by localized symptoms such as redness, swelling, heat, and pain, as well as systemic effects including fever, chills, and organ dysfunction.1 Clinical manifestations vary among individuals, with inflammation typically serving as a protective response to dilute toxins, remove inflammatory agents, and prevent the spread of microorganisms throughout various bodily systems. Additionally, inflammation aids in the regeneration of tissues and organs.2 Pro-inflammatory mediators, including nitrogen monoxide (NO), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-1β (IL-1β), are essential for facilitating tissue and cell healing as well as for the prevention and management of a number of illnesses.3–5 However, excessive production of pro-inflammatory mediators or their inadequate control may lead to acute lung damage, rheumatoid arthritis, and chronic inflammatory conditions in the gut.6,7 Therefore, it is crucial to identify highly effective medications and treatments that efficiently inhibit the synthesis and release of these cytokines.The fusion of numerous heterocyclic compounds, including nitrogen and sulfur atoms, plays an essential role in the field of medicinal chemistry.8,9 These chemicals are extensively used to treat a broad range of conditions. Indole and imidazole[2,1-b]thiazole derivatives have garnered considerable interest, probably owing to their extensive pharmacological activities. These activities include anti-tumor effects,10,11 antibacterial properties,12,13 anti-inflammatory and analgesic effects,14,15 as well as antioxidant capabilities.16,17 Importantly, indomethacin 1, compound 2,18 compound 3,19 compound 4,15 compound 5,20 and compound 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 have shown favorable anti-inflammatory activities and safety (Fig. 1).
20 have shown favorable anti-inflammatory activities and safety (Fig. 1).
Molecular hybridization is widely used in pharmaceutical chemistry, involving the combination of two drug molecules or their pharmacodynamic groups into a single entity.21 This strategy leads to produce new chemicals with combined features of the original medications that demonstrate synergistic therapy benefits or reduce adverse effects.22 The non-steroidal anti-inflammatory medicine benorylate is a notable example of a hybrid molecule, produced by combining the carboxyl group of aspirin with the phenolic hydroxyl group of acetaminophen. This combination results in the formation of a hybrid molecule adjusted to reduce the incidence of gastrointestinal discomfort.
Based on results of previous studies, this research group devised and synthesized a series of indole-2-formamide benzimidazole[2,1-b]thiazole compounds as the core skeletons (Fig. 1). An in vitro investigation assessed the inhibitory effect these compounds on the production of NO, IL-6 and TNF-α from RAW264.7 cells that were triggered by lipopolysaccharide (LPS). Structure–activity analysis was conducted, and the compounds were tested to determine their ability to induce ferroptosis. The aim is to develop medications that are both highly effective and non-toxic.
Results and discussion
Chemistry
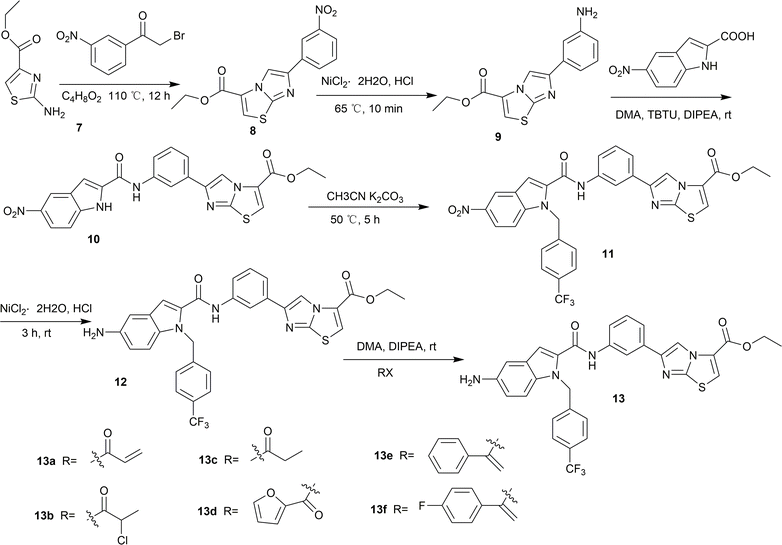
It is possible to synthesize indole-2-formamide benzimidazole[2,1-b]thiazole derivatives as shown in Scheme 1. Under the conditions of a reflux reaction carried out at 110 °C in a solution of 1,4-dioxane, compound 8 was produced in 84% yield. To complete the nitro reduction of compound 8, tin(II) chloride dihydrate was used as a catalyst. The reaction was carried out by extracting, filtering, and removing the solvent while the pH was adjusted to an alkaline state. Subsequently, compound 9 was esterified with 5-nitro-1H-indole-2-carboxylic acid in dimethyl adipate (DMA) under the suitable conditions using tributylthiourea (TBTU) and N,N-diisopropylethylamine (DIPEA), which resulted in the production of compound 10 in 74.2% yield. Subsequently, heat and stirring were used to condense compound 10 with 4-trifluoromethyl benzyl bromide in anhydrous acetonitrile. The conditions surrounding the condensation process were favorable for potassium carbonate (K2CO3). Silica gel column chromatography was used to purify the product, that is, compound 11. Finally, to convert the target molecule 11 into a new derivative 12, it was subjected to nitro reduction and halogen introduction in anhydrous ethanol. This was accomplished by using tin(II) chloride dihydrate and strong hydrochloric acid. Additionally, the use of DIPEA as a catalyst allowed for effective completion of reactions between nucleophile 12 and various acyl halides in DMA. These reactions led to the synthesis of compounds 13a–13f of the compound series. Proton nuclear magnetic resonance (1H NMR), carbon nuclear magnetic resonance (13C NMR), and high-resolution electrospray ionization mass spectrometry (HR-ESI-MS) were used to comprehensively characterize the newly synthesized indole-2-formamide benzimidazole[2,1-b]thiazole derivatives.Initial evaluation against LPS-induced NO, IL-6, and TNF-α release by the derivatives (13a–13f)
In terms of the progression of the systemic inflammatory response, LPS has a significant role, notably in the synthesis of proinflammatory cytokines such as NO, IL-6, and TNF-α. To determine whether the compounds possessed anti-inflammatory effects (Fig. 2), an evaluation was conducted using the Griess reagent test to determine the inhibitory effects of the synthesized compounds on the production of NO by LPS in RAW264.7 cells. Additionally, the enzyme-linked immunosorbent assay (ELISA) was utilized to assess the inhibitory effects of these compounds on the production of IL-6 and TNF-α by LPS in RAW264.7 cells. A demonstration of the inhibitory effects of the derivatives on the release of NO, IL-6, and TNF-α from RAW264.7 cells that have been stimulated by LPS can be discovered in Fig. 2. The vast majority of the compounds exhibited varying degrees of inhibition in LPS-activated RAW264.7 cells. Furthermore, these effects increased as the dose the drug increased, particularly with respect to NO and IL-6 levels.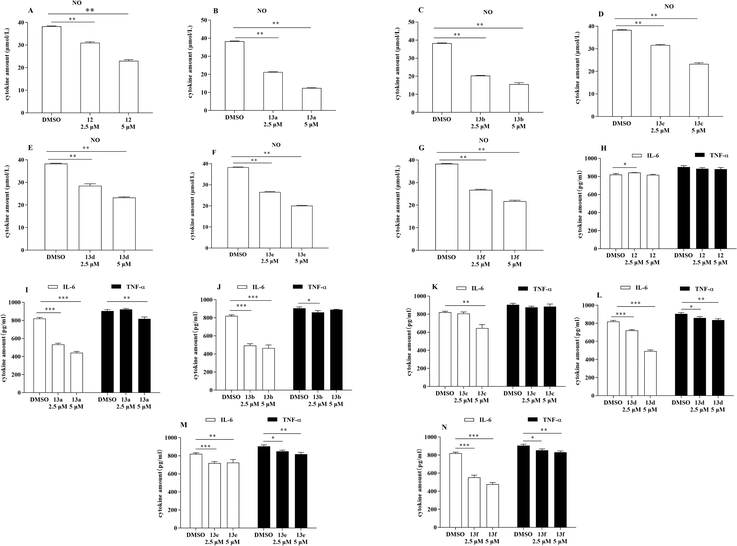 | ||
| Fig. 2 Anti-inflammatory screening of compounds 12 and 13a–13f (*p < 0.05, **p < 0.01, ***p < 0.001 vs. DMSO). | ||
Dose-dependent inhibition of NO, IL-6, and TNF-α release by the derivatives (13a, 13b, and 13d–13f)
The compounds capable of inhibiting the release of NO, IL-6, and TNF-α were selected based on their anti-inflammatory activity, and subsequent the half maximal inhibitory concentration (IC50) assays were conducted with dose–response experiments. According to the anti-inflammatory activity, compounds that can inhibit the release of NO, IL-6, and TNF-α were screened. Compounds 13a, 13b, and 13d–13f were selected for subsequent experiments. As shown in Table 1, 13b exhibited the strongest inhibition of NO, IL-6, and TNF-α with IC50 values of 10.992 μM, 2.294 μM, and 12.901 μM, respectively, followed by compound 13d with IC50 values of 19.969 μM, 4.715 μM, and 22.044 μM, respectively.| Compound | IC50 (μM) | ||
|---|---|---|---|
| NO | IL-6 | TNF-α | |
| DEX | 21.197 ± 0.517 | 0.247 ± 0.073 | 27.796 ± 0.643 |
| 13a | 61.494 ± 0.560 | 1.437 ± 0.351 | 62.835 ± 1.198 |
| 13b | 10.992 ± 0.328 | 2.294 ± 0.791 | 12.901 ± 1.591 |
| 13d | 19.969 ± 0.674 | 4.715 ± 1.995 | 22.044 ± 0.449 |
| 13e | 22.143 ± 1.019 | 4.879 ± 0.112 | 21.795 ± 1.143 |
| 13f | 36.041 ± 1.375 | 1.539 ± 0.515 | 46.777 ± 0.197 |
Cytotoxicity of the derivatives (13b and 13d)
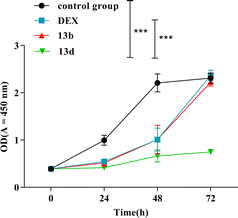
Subsequently, the cytotoxicity experiments of dexamethasone (DEX), 13b, and 13d were performed in RAW264.7 cells by using Cell Counting Kit-8 (CCK-8) assays. The results are shown in Fig. 3. During the 72 hour period, there was no discernible change in cell activity between the compound 13b group, the control group, and the DEX group. The cell proliferation rate of compound 13d was noticeably reduced when compared with the proliferation rates of the control group and the DEX group. These experimental results indicate that compound 13b does not exhibit any toxic effects on RAW264.7 cells, thus demonstrating its safety.Structure–activity relationship of the derivatives
Based on the anti-inflammatory activity results, we summarized the structure–activity relationships of the compounds. Most of these compounds inhibited LPS-induced release of inflammatory factors from RAW264.7 cells. Notably, the compounds strongly inhibited the release of NO and IL-6, while the inhibition of TNF-α release was relatively weak. The relatively weak inhibitory effect of lead compound 12 suggests that introducing of a hydrophobic group at the amino site may enhance the activity. Branching at the α-position to the nitrogen of compound 12, such as with a propenylcarbonyl group (13a), diminished IL-6 activity (IC50 = 1.437 μM) compared with compound 13b, however, the opposite trend was observed for the TNF-α inhibition capabilities of 13a and 13b. Compared with 13a, 13b has an approximately 6-fold higher IC50 for NO inhibition and an approximately 5-fold higher IC50 for TNF-α. When compared with compounds 13e and 13f, compound 13d, which included α-furanocarbonyl at the 5-position, demonstrated a considerable overall reduction of NO, IL-6, and TNF-α. The IC50 values of compound 13d were 19.969 μM, 4.715 μM, and 22.044 μM, respectively. Among them, compound 13f showed the most significant inhibition of IL-6 (IC50 = 1.539 μM), with poorer inhibitory vigor against NO and TNF-α. Taken together, the structure–activity relationships in this series are complex. Among them, compound 13b substituted with 2-chloropropylcarbonyl was the most potent in the series with an IC50 (NO) of 10.992 μM, an IC50 (IL-6) of 2.294 μM and an IC50 (TNF-α) of 12.901 μM. All of these findings provide new evidence for the anti-inflammatory effects of these indole-2-formamide benzimidazole[2,1-b]thiazole derivatives.The promotion of ferroptosis was observed in RAW264.7 cells by compounds 13b and 13d
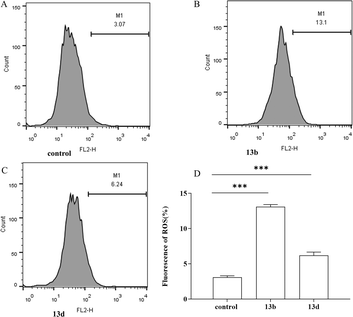 | ||
| Fig. 4 The effects of 13b and 13d on LPS-induced intracellular ROS production in RAW264.7 cells (***p < 0.001 vs. control). | ||
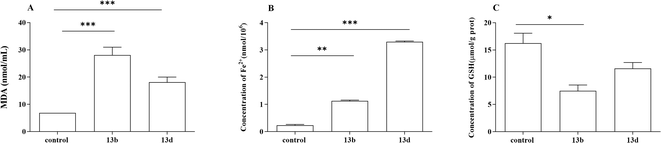
Ferroptosis is a form of programmed cell death triggered by iron-dependent lipid peroxidation, and is characterized by the excessive accumulation of iron ions and lipid peroxides within cells.23,24 Previous studies have shown that ROS are essential for ferroptosis and that increasing the activity of superoxide dismutase (SOD) may efficiently reduce ROS levels in vivo. Intracellular iron build-up aggravates oxidative damage and promotes ferroptosis by suppressing the antioxidant system and causing the Fenton reaction, which directly produces excess ROS.25,26 The degree of cellular oxidative damage can be measured using MDA.27,28 GSH, catalyzed by glutathione peroxidase 4 (GPX4), is the most abundant intracellular antioxidant capable of scavenging ROS and inhibiting ferroptosis.29,30 Based on our experimental results, we observed a significant decrease in intracellular GSH content in the treatment group. Additionally, increased levels of ROS and MDA were detected, along with intracellular Fe2+ accumulation, suggesting that compounds 13b and 13d may exert therapeutic effects through their involvement in the iron death pathway. Overall, compounds 13b and 13d exhibited anti-inflammatory effects by suppressing the release of inflammatory cytokines and promoting ferroptosis.
A common mechanism underlying the sepsis caused by various diseases is the entry of harmful bacteria into the body, which initiates an innate immune reaction. Macrophages are equipped with pattern-recognition receptors that recognize chemical patterns linked to infections and trigger the production of pro-inflammatory substances by immune cells. Cells respond to this stimulation by becoming inflamed, and prolonged inflammation eventually suppresses the immune system.31–33 Iron death, a type of regulatory cell death linked to inflammation, releases harmful molecules including high mobility group box-1 (HMGB1). Due to its immunogenic qualities, HMGB1 may intensify excessive sepsis-related inflammatory response.34 Neutralizing anti-HMGB1 antibodies has been shown to be an effective way to reduce the aberrant immunological response of macrophages to systemic or local infections brought on by iron death. This suggests that HMGB1 targeting may be a therapeutic strategy for mitigating the abnormal immune response linked to iron death.35 However, some studies have indicated the potential of iron-induced cell death in suppressing inflammatory responses. The steroid dexamethasone has been found to have anti-inflammatory properties and cause ferroptosis. This was accomplished by increasing the production of dipeptidase-1 by activating the glucocorticoid receptors and decreasing glutathione levels. Ferroptosis can be induced to a significant degree by dexamethasone.36 Oh et al.37 found that by blocking its effect on the NF-κB signaling pathway in bone marrow-derived macrophages, erastin may improve the prognosis of animal models of cecal ligation and perforation (CLP) or LPS-induced septic shock. This reduced the production of inflammatory mediators. Arbiser et al.38 utilized the ceramide analogue solenopsin to facilitate skin cell uptake and induce iron-mediated cell death for treating psoriasis, yielding remarkable outcomes and suggesting a potential positive role of iron-induced cell death in inflammation reduction. These disparities imply that the mechanism underlying iron-induced cell death may yield two distinct outcomes under different scenarios: anti-inflammatory and pro-inflammatory. Future research on septic infections should explore the conditions under which these disparities arise and how they can be harnessed to exert beneficial effects on the body.
Conclusions
The structures of a series of indole-2-formamide benzimidazole[2,1-b]thiazole derivatives were synthesized and characterized using hydrogen, carbon, and mass spectrometry. According to the in vitro cell activity results, most of the compounds demonstrated effective inhibition of the release of the inflammatory cytokines NO, IL-6, and TNF-α. Furthermore, a structure–activity relationship analysis revealed that the introduction of electron-withdrawing groups at the amino position enhanced their activity. The cytotoxicity assay showed that compound 13b was non-toxic. Additionally, in iron death experiments, it was observed that these compounds significantly increased ROS content as well as the levels of MDA and Fe2+, while decreasing GSH content. This suggests their potential role in promoting the occurrence of the iron death process. In conclusion, compound 13b not only exhibited the most efficient inhibitory effects on inflammatory factor release but also had the potential to promote iron death. Therefore, further investigation is warranted.Experimental
Materials and characterization
All commercial chemicals and solvents were of reagent grade and were used without further purification (Changsha Junshu Biotechnology Co., Ltd, Hunan, China). TLC was performed on 0.20 mm silica gel 60 F254 plates (Qingdao Ocean Chemical Factory, Shangdong, China). Melting points were obtained using a WRS-1B melting apparatus and were uncorrected. 1H NMR and 13C NMR spectra were recorded in DMSO-d6 solution with a Bruker Instrument at 400 or 100 MHz, and peak positions are given in parts per million (δ) downfield from tetramethylsilane as the internal standard. J values are given in Hz. Mass spectra were acquired using an Agilent 6210 ESI/TOF or Agilent 6545 LC/Q-TOF mass spectrometer. All chemicals were of the highest available grade, and the purity of all synthesized compounds was >95% as determined by high performance liquid chromatography (HPLC) analysis.General procedure for synthesis of compounds
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. This resulted in the production of 222.2 mg (11), corresponding to a yield of 70.2%.
5. This resulted in the production of 222.2 mg (11), corresponding to a yield of 70.2%.General procedure A for the preparation of indole-2-formamide benzimidazol[2,1-b]thiazole derivatives (13a–13f)
Following the addition of 6 mL of DMA and 0.75 mmol of an acyl chloride derivative, compound 12 was mixed into a flask with a round bottom while the temperature was kept at room temperature. Twenty-four hours were spent stirring the reaction mixture at room temperature, while TLC was used to check its progress. The reaction was stopped by adding 20 mL of a saturated sodium bicarbonate solution after the starting ingredient had completely passed away. The final product, 13a–13f, using ethyl acetate (20 mL × 3), combined it with the organic layer, dried it with anhydrous magnesium sulfate, and then separating it using column chromatography. The yield of the product ranged from 56 to 82%.The characterization 1H NMR, 13C NMR, HR-ESI-MS data of target compounds are shown in the ESI.†
Biological evaluation
The RAW264.7 cells were cultured in a humidified incubator at 37 °C with 5% CO2. The culture medium used was Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% FBS (Hyclone, Logan, UT, USA), 100 U mL−1 of penicillin, and 100 mg mL−1 of streptomycin.
The organization and intervention of cells
The cells were at a density of 4 × 105 cells in milliliter for RAW264.7 cells that were in the logarithmic growth phase in 96-well plates and incubated for 24 h prior to drug administration. In the indomethacin-treated and experimental groups, macrophages were incubated for 30 min, followed by stimulation with LPS (0.5 mg L−1), and then for an additional 24 h.The impact of target compounds on the level of NO released from LPS-induced RAW264.7 cells
The Griess technique was used in order to determine the amount of nitric oxide (NO) that was present in the supernatant of RAW264.7 cells that had been stimulated by LPS. The NO content of each sample was determined by referring to an NaNO2 standard curve.The impact of target compounds on the production of IL-6 and TNF-α cytokines LPS-induced RAW264.7 cells
After treatment of RAW264.7 cells, IL-6 and TNF-α concentration in culture medium was determined with commercial ELISA assay kits according to its manufacturer's instructions. The absorbance was measured using a microplate reader at 450 nm, and the concentrations were calculated using a standard curve.Cytotoxicity experiments of 13b and 13d
The cytotoxicity of compounds 13b and 13d were evaluated in RAW264.7 cells. Cell viability was determined using the CCK-8 assay. Cells (1 ×104 cells per well) in 96-well plates were treated with different concentrations of molecule for 24, 48 and 72 h. Then, removed the culture medium from the wells and added 100 μL of the CCK-8 working solution (10% CCK-8 reagent in the culture medium), followed by incubation at 37 °C in a 5% CO2 humidified incubator. The absorbance of each well was measured at 450 nm using a microplate reader at the following concentrations: (1) control group: normal medium; (2) dexamethasone (DEX): 27.964 μM; (3) compound 13b: 12.326 μM (4) compound 13d: 23.684 μM.Measurement of indicators related to iron death
After treatment of RAW264.7 cells, ROS, MDA, GSH and Fe2+ concentrations in the culture medium were determined using commercial ELISA assay kits according to the manufacturer's instructions (compounds 13a and 13b: 10 μM).Statistical analysis
Statistical analyses were analyzed by GraphPad Prism 9.5.1, and expressed as mean ± standard (SD). Statistical data were considered to be statistically significant if p-values less than 0.05.Author contributions
Yu-Cai Jiang and Hai-Feng Lin conceived the study and supervised the study. Hai-Feng Lin, Yu-Cai Jiang, Zhi-Wei Chen and Lin-Lin Zheng performed the experiments. Hai-Feng Lin, Yu-Cai Jiang and Zhi-Wei Chen analyzed data. Yu-Cai Jiang wrote the manuscript. Hai-Feng Lin, Yu-Cai Jiang, Zhi-Wei Chen and Lin-Lin Zheng review the manuscript and discussion of results. All the authors have read and approved the final version of the manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this study.Acknowledgements
This study was supported by the Natural Science Foundation of Fujian Province of China (2021J011376), Educational Commission of Fujian Province of China (JAT200512), Educational Commission of Fujian Province of China (JAT210398), and the Scientific Research Project of Putian University (2021031).Notes and references
- Z. Ju, Z. Shang, T. Mahmud, J. Fang, Y. Liu, Q. Pan, X. Lin and F. Chen, J. Nat. Prod., 2023, 86, 958–965 CrossRef CAS PubMed.
- L. Z. Chen, L. Yao, M. M. Jiao, J. B. Shi, Y. Tan, B. F. Ruan and X. H. Liu, Eur. J. Med. Chem., 2019, 175, 114–128 CrossRef CAS PubMed.
- S. Lv, M. Han, R. Yi, S. Kwon, C. Dai and R. Wang, Int. J. Clin. Pract., 2014, 68, 520–528 CrossRef CAS PubMed.
- L. Song, G. Li, W. Guan, Z. Zeng, Y. Ou, T. Zhao, J. Li, D. He, X. Fang, Y. Zhang, J. Q. Wu, R. Tong and H. Yao, Biomed. Pharmacother., 2023, 166, 115412 CrossRef CAS PubMed.
- M. Naffaa, B. F. Makhoul, A. Tobia, M. Kaplan, D. Aronson, W. Saliba and Z. S. Azzam, Am. J. Emerg. Med., 2013, 31, 1361–1364 CrossRef.
- F. Imam, N. O. Al-Harbi, M. M. Al-Harbi, M. A. Ansari, K. M. Zoheir, M. Iqbal, M. K. Anwer, A. R. Al Hoshani, S. M. Attia and S. F. Ahmad, Pharmacol. Res., 2015, 102, 1–11 CrossRef CAS PubMed.
- W. Albrecht, A. Unger, S. M. Bauer and S. A. Laufer, J. Med. Chem., 2017, 60, 5290–5305 CrossRef CAS PubMed.
- S. S. Zhang, Q. W. Tan and L. P. Guan, Mini-Rev. Med. Chem., 2021, 21, 2261–2275 CrossRef CAS PubMed.
- O. Soyer Can, S. Ünlü, H. Ocak, E. Ç. Çöldür, H. Sipahi and B. Bilgin Eran, J. Iran. Chem. Soc., 2021, 19, 579–587 CrossRef.
- B. Karaman and N. Ulusoy Güzeldemirci, Med. Chem. Res., 2016, 25, 2471–2484 CrossRef CAS.
- F. Centofanti, A. Buono, M. Verboni, C. Tomino, S. Lucarini, A. Duranti, P. P. Pandolfi and G. Novelli, Pharmaceuticals, 2023, 16, 240 CrossRef CAS PubMed.
- M. A. Salem, A. Ragab, A. A. Askar, A. El-Khalafawy and A. H. Makhlouf, Eur. J. Med. Chem., 2020, 188, 111977 CrossRef CAS.
- M. A. Syed, P. R. Yiragamreddy and K. B. Chandrasekhar, Immunobiology, 2017, 27(4), 329–338 CAS.
- S. Hou, X. Yang, Y. Tong, Y. Yang, Q. Chen, B. Wan, R. Wei, Y. Wang, Y. Zhang, B. Kong, J. Huang, Y. Chen, T. Lu, Q. Hu and D. Du, Eur. J. Med. Chem., 2021, 211, 113114 CrossRef CAS PubMed.
- N. S. Shetty, I. A. M. Khazi and C.-J. Ahn, Bull. Korean Chem. Soc., 2010, 31, 2337–2340 CrossRef CAS.
- E. D. Dincel, E. Gursoy, T. Yilmaz-Ozden and N. Ulusoy-Guzeldemirci, Bioorg. Chem., 2020, 103, 104220 CrossRef CAS PubMed.
- P. V. Thanikachalam, R. K. Maurya, V. Garg and V. Monga, Eur. J. Med. Chem., 2019, 183, 111680 CrossRef CAS PubMed.
- Y. Huang, B. Zhang, J. Li, H. Liu, Y. Zhang, Z. Yang and W. Liu, Eur. J. Med. Chem., 2019, 180, 41–50 CrossRef CAS PubMed.
- M. B. Tehrani, S. Emami, M. Asadi, M. Saeedi, M. Mirzahekmati, S. M. Ebrahimi, M. Mahdavi, H. Nadri, A. Moradi, F. H. Moghadam, S. Farzipour, M. Vosooghi, A. Foroumadi and A. Shafiee, Eur. J. Med. Chem., 2014, 87, 759–764 CrossRef CAS PubMed.
- Z. Liu, L. Tang, H. Zhu, T. Xu, C. Qiu, S. Zheng, Y. Gu, J. Feng, Y. Zhang and G. Liang, J. Med. Chem., 2016, 59, 4637–4650 CrossRef CAS PubMed.
- M. H. Guo, P. Wen, Y. Xiao, W. S. Ji, X. L. Zhou, F. Gao and L. H. Shan, Fitoterapia, 2023, 168, 105536 CrossRef CAS PubMed.
- S. Wang, G. Dong and C. Sheng, Chem. Rev., 2019, 119, 4180–4220 CrossRef CAS PubMed.
- Q. Song, S. Peng, F. Che and X. Zhu, J. Pharmacol. Sci., 2022, 148, 300–306 CrossRef CAS PubMed.
- X. Jiang, B. R. Stockwell and M. Conrad, Nat. Rev. Mol. Cell Biol., 2021, 22, 266–282 CrossRef PubMed.
- X. Chen, R. Kang, G. Kroemer and D. Tang, Nat. Rev. Clin. Oncol., 2021, 18, 280–296 CrossRef CAS PubMed.
- H. Wang, P. An, E. Xie, Q. Wu, X. Fang, H. Gao, Z. Zhang, Y. Li, X. Wang, J. Zhang, G. Li, L. Yang, W. Liu, J. Min and F. Wang, Hepatology, 2017, 66, 449–465 CrossRef CAS PubMed.
- J. Du, Y. Zhou, Y. Li, J. Xia, Y. Chen, S. Chen, X. Wang, W. Sun, T. Wang, X. Ren, X. Wang, Y. An, K. Lu, W. Hu, S. Huang, J. Li, X. Tong and Y. Wang, Redox Biol., 2020, 32, 101483 CrossRef CAS PubMed.
- L. Li, S. Sun, L. Tan, Y. Wang, L. Wang, Z. Zhang and L. Zhang, Nano Lett., 2019, 19, 7781–7792 CrossRef CAS PubMed.
- J. Cao, X. Chen, L. Jiang, B. Lu, M. Yuan, D. Zhu, H. Zhu, Q. He, B. Yang and M. Ying, Nat. Commun., 2020, 11, 1251 CrossRef CAS.
- Z. Tang, Y. Ju, X. Dai, N. Ni, Y. Liu, D. Zhang, H. Gao, H. Sun, J. Zhang and P. Gu, Redox Biol., 2021, 43, 101971 CrossRef CAS.
- S. L. Raymond, D. C. Holden, J. C. Mira, J. A. Stortz, T. J. Loftus, A. M. Mohr, L. L. Moldawer, F. A. Moore, S. D. Larson and P. A. Efron, Biochim. Biophys. Acta, Mol. Basis Dis., 2017, 1863, 2564–2573 CrossRef CAS.
- O. Takeuchi and S. Akira, Cell, 2010, 140, 805–820 CrossRef CAS PubMed.
- R. V. D'Elia, K. Harrison, P. C. Oyston, R. A. Lukaszewski and G. C. Clark, Clin. Vaccine Immunol., 2013, 20, 319–327 CrossRef PubMed.
- G. H. Son, Y. Kim, J. J. Lee, K. Y. Lee, H. Ham, J. E. Song, S. T. Park and Y. H. Kim, Sci. Rep., 2019, 9, 19746 CrossRef CAS PubMed.
- Q. Wen, J. Liu, R. Kang, B. Zhou and D. Tang, Biochem. Biophys. Res. Commun., 2019, 510, 278–283 CrossRef CAS.
- A. von Mässenhausen, N. Zamora Gonzalez, F. Maremonti, A. Belavgeni, W. Tonnus, C. Meyer, K. Beer, M. T. Hannani, A. Lau, M. Peitzsch, P. Hoppenz, S. Locke, T. Chavakis, R. Kramann, D. A. Muruve, C. Hugo, S. R. Bornstein and A. Linkermann, Sci. Adv., 2022, 8, eabl8920 CrossRef.
- B. M. Oh, S. J. Lee, G. L. Park, Y. S. Hwang, J. Lim, E. S. Park, K. H. Lee, B. Y. Kim, Y. T. Kwon, H. J. Cho and H. G. Lee, J. Clin. Med., 2019, 8, 2210 CrossRef CAS.
- J. L. Arbiser, M. Y. Bonner, N. Ward, J. Elsey and S. Rao, Biochim. Biophys. Acta, Gen. Subj., 2018, 1862, 2518–2527 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedure and its characterization 1H NMR, 13C NMR, HR-MS data of target compounds. See DOI: https://doi.org/10.1039/d4ra00557k |
| This journal is © The Royal Society of Chemistry 2024 |