Open Access Article
Open Access ArticlePreparation of an injectable zinc-containing hydrogel with double dynamic bond and its potential application in the treatment of periodontitis
Mei Yang,
Dejiang Du,
Yuanping Hao ,
Zhaojian Meng,
Haiyu Zhang and
Yuhan Liu
,
Zhaojian Meng,
Haiyu Zhang and
Yuhan Liu *
*
Qingdao Stomatological Hospital Affiliated to Qingdao University, Qingdao 266000, Shandong, China
First published on 17th June 2024
Abstract
Periodontal tissue regeneration continues to face significant clinical challenges. Periodontitis leads to alveolar bone resorption and even tooth loss due to persistent microbial infection and persistent inflammatory response. As a promising topical drug delivery system, the application of hydrogels in the controlled release of periodontal bioactive drugs has aroused great interest. Therefore, the design and preparation of an injectable hydrogel with self-repairing properties for periodontitis treatment is still in great demand. In this study, polysaccharide-based self-healing hydrogels with antimicrobial osteogenic properties were developed. Zinc ions are introduced into a dynamic cross-linking network formed by dynamic Schiff bases between carboxymethyl chitosan and oxidized hyaluronic acid via coordination bonds. The OC–Zn hydrogels exhibited good tissue adhesion, good fatigue resistance, excellent self-healing ability, low cytotoxicity, good broad-spectrum antimicrobial activity, and osteogenic activity. Therefore, the designed hydrogels allow the development of drug delivery systems as a potential treatment for periodontitis.
Introduction
Periodontitis is an inflammatory disease caused by a biofilm that leads to loss of gingival attachment, periodontal pocket formation, and alveolar bone resorption, which may eventually progress to tooth loss if left untreated.1 Periodontitis is one of the most common dental diseases, affecting 90% of the global population, and is strongly associated with chronic non-communicable diseases such as cerebrovascular disease, coronary heart disease, cardiovascular disease, and cancer.2,3 Long-term, effective control of infection and inflammation is the key to the treatment of periodontitis. Traditionally, mechanical debridement combined with adjunctive antibiotic therapy has been considered the mainstay of treatment for periodontal disease and imposes a heavy financial burden on patients.4,5 However, due to the complex anatomy of periodontal pockets and antibiotic resistance, it is almost difficult to completely remove microorganisms and pathogens deep inside the periodontal pockets using conventional treatments.6 It has been reported that 74.2% of patients with chronic periodontitis show subgingival pathogens resistant to one or more of the experimental antibiotics, and these challenges will limit the therapeutic outcomes and the future application of antibiotic-based topical drug delivery systems.7 To overcome these challenges, extensive research has been devoted to the development of novel topical antimicrobial systems to improve the therapeutic outcome of periodontitis.8,9Hydrogels, with good hydrophilicity and three-dimensional porous structure, are an ideal platform for periodontal drug delivery.10–13 Compared to pre-formed scaffolds, injectable hydrogels are administered in a minimally invasive manner, which is ideal for irregularly shaped areas and reduces the risk of infection.14,15 The hydrogel is injected into the periodontal pocket and then cured in situ. Self-healing hydrogels are triggered by the inherent regenerative capacity of natural tissues that develop through dynamic covalent bonding and non-covalent interactions.16,17 Self-healing hydrogels can self-repair after minor injuries and maintain structural integrity in a timely manner, facilitating drug delivery in dynamic environments.18–20
Chitosan (CS) is a biocompatible and cationic polysaccharide with intrinsic antimicrobial activity by binding to negatively charged bacterial cell membranes or biomolecules, disrupting cell membranes and metabolism.21 Carboxymethyl chitosan (CMCS), as a water-soluble derivative of CS, is a promising candidate for treating periodontal diseases due to its excellent antimicrobial properties, biodegradability, and biocompatibility.22 In addition, hyaluronic acid (HA), another polysaccharide commonly found in the extracellular matrix, accumulates at the wound site during the healing process.23 It regulates cellular function by interacting directly with receptors on the cell membrane or indirectly with cytokines in the extracellular matrix.24 Metal ions have been used as a therapeutic agent for various biomedical applications due to their availability, low cost, stability, and enhanced safety.25 Among metal ions, zinc is a micronutrient that aids in wound healing and inhibits bacterial growth during the healing process.26 To the best of our knowledge, there are no studies on the synthesis and characterization of self-healing hydrogels based on CMCS, aldehyde–hyaluronic acid, and zinc.
This study aimed to develop and evaluate self-healing hydrogels for periodontal disease. We constructed a drug-free polysaccharide-based injectable hydrogel (OC–Zn) through dynamic imine bonding and electrostatic interactions between CMCS and OHA, as well as ligand bonding between zinc ions, as shown in Scheme 1. The synthesized OC–Zn hydrogel showed synergistic therapeutic potential to promote periodontal tissue regeneration by killing pathogenic bacteria and modulating the osteogenic microenvironment. The structural and physicochemical properties of OC–Zn hydrogels were systematically characterized in terms of microstructure, gelation time, swelling behavior, rheological and self-healing behavior, mechanical properties, and tissue adhesion. In addition, the hemocompatibility and cytotoxicity, in vitro antimicrobial activity, and osteogenesis-promoting performance of OC–Zn hydrogels were also evaluated.
 | ||
| Scheme 1 Schematic of the fabrication route of multifunctional OC–Zn hydrogels with the potential for periodontitis. | ||
Results and discussion
Preparation and characterization of the OC–Zn hydrogels
To address the multiple needs of periodontal treatment, a self-healing, antibacterial, and osteogenic hydrogel was designed herein. The preparation diagram of the injectable hydrogel comprised of OHA, CMCS, and ZnCl2 is displayed in Scheme 1. Aldehyde-decorated hyaluronate was synthesized through the oxidation of hyaluronate in the presence of sodium periodate and the structure was confirmed by 1H NMR and FTIR spectrum (Fig. 1).13,27 The 1H NMR spectrum of OHA displayed a triplet peak near 4.93, 5.02 and 5.15 ppm corresponding to the ATR-FTIR absorption region of 1732 cm−1, which indicates the format of hydrogen on the skeleton of the open-loop D-glucuronic acid unit in HA due to the stretching vibration of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in the aldehyde. These results confirm the successful synthesis of OHA. Hydrogels can be quickly formed at 37 °C via multiple crosslinking, including the electrostatic interactions between macromolecular chains, Zn–O, Zn–N coordination, and a Schiff's base reaction between the amino group from CMCS and the aldehyde group from OHA.22,28 Fig. 1B shows the sol–gel process of OC–Zn hydrogel where the OC–Zn hydrogel was successfully formed. Besides, the OC–Zn hydrogel can instantly recover to its original state without any damage after compression. Additionally, the OC–Zn series hydrogels were further characterized by Fourier-transform infrared (FTIR), and the results a new characteristic peak of 1607 cm−1 for the dynamic Schiff base (imine) C
O bond in the aldehyde. These results confirm the successful synthesis of OHA. Hydrogels can be quickly formed at 37 °C via multiple crosslinking, including the electrostatic interactions between macromolecular chains, Zn–O, Zn–N coordination, and a Schiff's base reaction between the amino group from CMCS and the aldehyde group from OHA.22,28 Fig. 1B shows the sol–gel process of OC–Zn hydrogel where the OC–Zn hydrogel was successfully formed. Besides, the OC–Zn hydrogel can instantly recover to its original state without any damage after compression. Additionally, the OC–Zn series hydrogels were further characterized by Fourier-transform infrared (FTIR), and the results a new characteristic peak of 1607 cm−1 for the dynamic Schiff base (imine) C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond with the disappearance of the aldehyde groups in OHA, which confirmed the successful preparation of OC–Zn hydrogel. Besides, scanning electron microscope (SEM) exhibited OC–Zn hydrogels have a homogeneous microporous structure and the internal pore size of the hydrogel decreased after increasing Zn2+ concentrations, which may be attributable to the good crosslinking between OHA, CMCS via Schiff-based bond and Zn–O, Zn–N coordination and simultaneous interactions.
N bond with the disappearance of the aldehyde groups in OHA, which confirmed the successful preparation of OC–Zn hydrogel. Besides, scanning electron microscope (SEM) exhibited OC–Zn hydrogels have a homogeneous microporous structure and the internal pore size of the hydrogel decreased after increasing Zn2+ concentrations, which may be attributable to the good crosslinking between OHA, CMCS via Schiff-based bond and Zn–O, Zn–N coordination and simultaneous interactions.
Evaluation of the injectable self-healing behavior, rheology, swelling, adhesiveness performance of the OC–Zn hydrogels
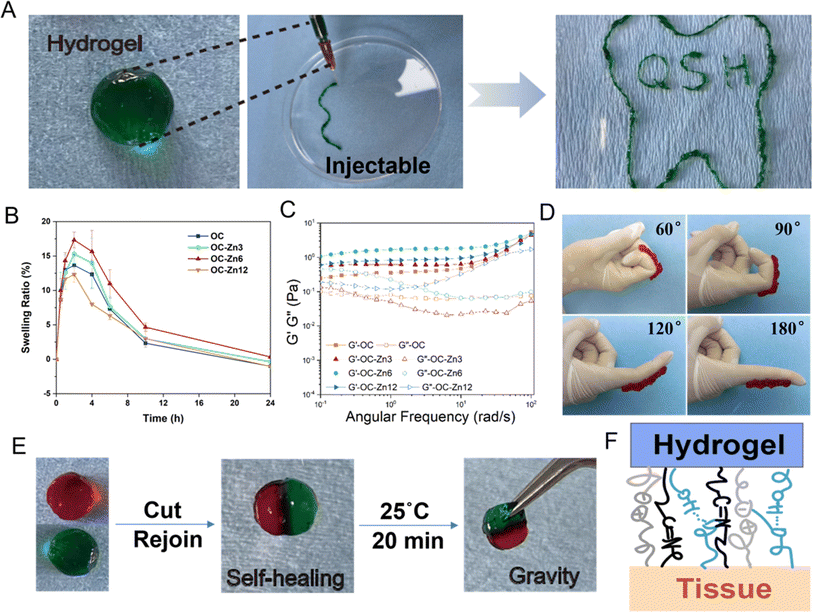
To demonstrate that OC–Zn hydrogels can maintain the periodontal microenvironment, it is vital that we confirm their injectable self-healing behavior, rheology, adhesiveness, and swelling performance. First, the injectable self-healing properties of the hydrogel are proven. As shown in Fig. 2A, OC–Zn hydrogel could be injected into the shape of teeth and “QSH” through commonly used-gauge needles, and subsequently re-obtained their original shape integrity post-injection. The excellent injectable properties of OC–Zn hydrogels will benefit greatly from later in situ injection filling of periodontal pockets, where they can be applied to irregular shapes.As hydrogel is administered for periodontal tissues, their great architecture stability after the swelling is crucial. The swelling ratio of freeze-dried samples in PBS as the concentration of Zn increased. Meanwhile, the degradation behavior was also observed, and the hydrogels could maintain the original integral shape within 24 h (Fig. 2B). Due to external forces such as oral chewing and the semi-enclosed anatomical position of the periodontal tissue, hydrogels' mechanical properties (e.g., recoverability, deformability, etc.) are particularly important.10,29 As a promising candidate for periodontal use, OC–Zn hydrogels formed by multiple dynamic cross-linking had satisfactory mechanical properties, including excellent recoverability and deformability.30 Rheological measurements were performed on the OC–Zn hydrogels to explore their mechanical properties. Here, the elasticity and flowability were evaluated in terms of storage modulus and loss modulus, termed G′ and G′′, respectively. As shown in Fig. 2C, all the tested groups of hydrogels exhibited elastic behavior, with G′ being greater than G′′. To evaluate the self-healing ability of OC–Zn hydrogel, the samples that were stained with red and green pigments were split in half and then recombined into the differently stained parts. After 20 min, the integrated hydrogels could lift under self-weight (Fig. 2E). The results indicated that the OC–Zn hydrogels could rapidly self-heal even after damage, prevent mechanical damage, and maintain the stability of periodontal tissue delivery under oral movement. We proposed that the Schiff base bonds between the –NH2 of CMCS and –CHO of OHA and the coordination bond between zinc ion and O, N undergo dynamic cross-linking to achieve self-healing.28 As exhibited in Fig. 2D, OC–Zn hydrogel can be rotated arbitrarily on the model's finger without shedding and shows good fatigue resistance in 10 bending cycles. The repeated bending and extension of the finger did not affect its adhesion ability. This hydrogel could produce instant tissue-material interfacial interactions with the tissue through multiple forces, including electrostatic interactions, hydrogen bonding, and Schiff base bonding (Fig. 2F).
Evaluation of the biocompatibility of the OC–Zn hydrogels
Good biocompatibility is an essential factor for biomaterials.27,31 Here, the in vitro hemolysis test was performed to estimate the hemocompatibility of the OC–Zn hydrogels. Fig. 3A shows the distinct differences in color between the four hydrogel groups, the negative control (NS) and the positive control (Triton X-100). All four hydrogel groups were colorless and transparent, similar to the negative control, while the deionized water group was bright red. From the quantitative data in Fig. 3A, the hemolysis rate of the OC–Zn hydrogel was extremely low compared to the OC hydrogel (∼1.3%), indicating that the hemolysis rate was significantly reduced by the addition of zinc ions. Furthermore, hPDLSCs and MC3T3 cells were used to evaluate the cytocompatibility through the cytoskeleton/nuclear and cell counting kit-8 (CCK8) assays. As shown in Fig. 3C, the morphology of the cytoskeleton and nucleus in the hydrogel groups presented normal structural characteristics like those of the control group. The CCK8 assay examined the cell proliferation rate in the hydrogel extract. The cell vitality in OC–Zn12 group was significantly lower than that of the other groups at 1 day and 3 days. Nonetheless, no significant difference was observed in the proliferation rate of each hydrogel group compared to the control at 5 days. Overall, all experiments confirmed that the OC–Zn hydrogels had excellent biocompatibility.Evaluation of the anti-bacterial activity of the OC–Zn hydrogels
Microbial infections are an extremely distressing problem that can accelerate alveolar bone degeneration and aggravate periodontal disease.32,33 Two common pathogens – Fusobacterium nucleatum (F. nucleatum) and Staphylococcus aureus (S. aureus) in periodontal pockets were used as representative pathogenic bacteria to evaluate the antibacterial activity of these hydrogels. The microbial proliferation was assessed by colony assay.34 The images of the spread plates of F. nucleatum and S. aureus disclosed a lower bacterial count in the hydrogel groups compared to the control group, and the bactericidal effect was significantly enhanced with increasing zinc ion concentration (p < 0.05). In the time kill assay (Fig. 4C, p < 0.05), it can be seen that the antibacterial ability of hydrogel increases with the increase of time, which may be due to the slow release of components in hydrogel. CMCS is one of the main antibacterial active ingredients of hydrogels. The pristine OC hydrogel (without ZnCl2) exhibits good antimicrobial capacity by continuously releasing cations to disrupt bacterial cell walls and boost cell membrane permeability, leading to the lysis of the bacteria and the exudation of cell contents.35,36 On the other hand, zinc ions might generate reactive oxygen species (ROS) in cells, induce gene expression related to oxidative stress, inhibit the synthesis of the cell wall, and thus result in microbial growth inhibition and death.37,38 The integrity of the bacterial wall was further detected using TEM. As depicted in Fig. 4D, the bacteria in the control group showed a typical shaped morphology with integrated cell walls. As we suspected, after OC–Zn12 treatment, the bacterial surface disintegrated into the fuzzy boundary. Thus, these results imply that cellular leakage caused by bacterial wall disruption may be one of the antimicrobial mechanisms of the prepared hydrogel. Therefore, the high antibacterial efficiency of OC–Zn hydrogels may be due to the synergistic attributes of both CMCS and zinc ions.Evaluation of the anti-biofilm activity of the OC–Zn hydrogels
Biofilms composed of microorganisms and extracellular polymeric substances (EPS) can induce persistent bacterial infections.39,40 First, the biomass of biofilm after different treatments was quantitatively determined by crystal violet staining. As shown in Fig. 5A/B, all hydrogel groups had different degrees of anti-biofilm effect, but there was no significant difference between groups. To investigate the effect of hydrogels on bacterial biofilms, biofilms cultured for 48 h were treated with the prepared hydrogels for 12 h. The biofilms were stained with live/dead fluorescence kits, and the disruptive effect was observed with CLSM.41,42 As shown in Fig. 5C, the CLSM image showed that the blank group was covered by biofilm. Importantly, with the increase of zinc ion concentration, the number of dead red bacteria embedded in the biofilm increased, demonstrating the good biofilm eradication capacity of the hydrogel.Evaluation of the OC–Zn hydrogels
ALP staining and alizarin red staining (ARS) were used to detect the osteogenic properties of OC and OC–Zn hydrogels and their effects on the osteogenic differentiation of hPDLSCs. As shown in Fig. 6A, zinc-based hydrogels significantly increased the expression level of ALP compared with the blank control group. However, with the increase in zinc ion concentration, the cell viability of hPDLSCs decreased. Among them, OC–Zn3 has been shown to maintain the survival rate and osteogenic differentiation of hPDLSCs. As can be seen from ARS images, OC–Zn3 significantly promoted the mineralization of osteogenic differentiated hPDLSCs, which further confirms the osteogenic efficacy of zinc-containing hydrogel.43,44 | ||
| Fig. 6 (A) ALP staining of hPDLSCs on day 7 (scale bar: 100 μm). (B) ARS staining on day 21 (scale bar: 100 μm). | ||
Materials and methods
Materials
Sodium hyaluronate (HA, molecular weight 40–80 kDa), sodium (meta) periodate and glycol were purchased from Aladdin Biochemical Technology Co. Ltd (Shanghai, China). Carboxymethyl chitosan (CMCS) was purchased from Solarbio® Life Sciences. All the chemicals were used without further purification.Synthesis and characterization of oxidized hyaluronate (OHA)
HA (1 g) was first dissolved in deionized water to obtain 1.0% w/v solution. Then, 10 mL of sodium (meta) periodate aqueous solution (0.5 M) was added dropwise to the solution. The reaction was stirred vigorously in the dark at room temperature for 24 h (as the sodium meta periodate is light sensitive).13,45 The reaction was terminated by adding glycol (0.05% v/v) and kept for 1 h. Afterward, the oxidized HA solution was dialyzed against deionized water for 72 h (molecular weight cutoff 3500 D, Solarbio® Life Sciences). Samples were frozen and lyophilized in a freezer and stored at 4 °C until further processing. The formation of aldehyde functional groups was confirmed by using Attenuated Total Reflectance Fourier transformed Infrared Spectroscopy (ATR-FTIR, Nicolet iN10 FTIR spectrometer in the 400–4000 cm−1, Thermo Fisher Scientific, Waltham, MA, USA). Proton nuclear magnetic resonance (1H NMR, Bruker Advance III, Germany) measurement was performed to further validate the successful OHA and the degree of modification.Preparation of oxidized hyaluronate (OHA)/carboxymethyl chitosan (OC) and ZnCl2 cross-linked OC–Zn hydrogel
OHA was dissolved in deionized water to a final concentration of 5% (w/v). CMCS was dissolved in deionized water to obtain the final concentration of 3% (w/v). The above solutions were blended in equal volumes to prepare the OC hydrogel. To endow the self-healing hydrogels with more antibacterial and osteogenic functions, a certain amount of ZnCl2 was dissolved in the OHA solution. The final concentration of ZnCl2 in the hydrogel was 0.03, 0.06, and 0.12% (w/v), respectively. These three hydrogels are named OC–Zn3, OC–Zn6, and OC–Zn12.Characterization of the hydrogels
 | (1) |
 | (2) |
 | (3) |
 | (4) |
Conclusion
In summary, OC–Zn hydrogels were successfully designed and prepared in this study. The formation of dynamic Schiff base (imine) bonds between –CHO in OHA and –NH2 in CMCS, and dynamic coordination bonds between COO-, –NH2, and –OH in polysaccharides and Zn2+ constitute a dynamic network that forms injectable and self-healing hydrogels. The hydrogels exhibited rapid gelation and shear thinning, which contributed to their injectability, as well as rapid and reproducible self-healing properties. In addition, tunable mechanical and swelling degradation was achieved by adding different concentrations of Zn2+ to form different ratios of bi-dynamic bonds. The addition of zinc ions enhanced the antimicrobial properties of the hydrogel while conferring satisfactory osteogenic activity to the hydrogel (OC–Zn3). The developed hydrogel has great potential as a drug delivery system for periodontitis.Author contributions
Yuhan Liu: supervision, methodology, writing – review & editing; Mei Yang: conceptualization, methodology, investigation, software, writing – original draft; Yuanping Hao: software; Dejiang Du: methodology. Haiyu Zhang: investigation, methodology. All authors have read and approved the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This research was financially supported by Qingdao South District Science and Technology Planning Project (Grant No. 2022-2-019-YY), the Qingdao Key Health Discipline Development Fund (2022–2024), Qingdao Clinical Research Center for Oral Diseases (Grant No. 22-3-7-lczx-7-nsh), Shandong Provincial Key Medical and Health Discipline of Oral Medicine (Qingdao University Affiliated Qingdao Stomatological Hospital) (2024–2026), and the Doctor Fund Project of Qingdao Stomatological Hospital (2023QN07).References
- G. C. Armitage, Periodontology 2000, 2004, 34, 9–21 CrossRef PubMed.
- G. Hajishengallis and T. Chavakis, Nat. Rev. Immunol., 2021, 21, 426–440 CrossRef CAS PubMed.
- R. J. Genco and M. Sanz, Periodontology 2000, 2020, 83, 7–13 CrossRef PubMed.
- D. Herrera, P. Matesanz, C. Martín, V. Oud, M. Feres and W. Teughels, J. Clin. Periodontol., 2020, 47, 239–256 CrossRef PubMed.
- F. Graziani, D. Karapetsa, B. Alonso and D. Herrera, Periodontology 2000, 2017, 75, 152–188 CrossRef PubMed.
- R. G. Caffesse, P. L. Sweeney and B. A. Smith, J. Clin. Periodontol., 1986, 13, 205–210 CrossRef CAS PubMed.
- R. S. Wilder and K. S. Bray, Periodontology 2000, 2016, 71, 65–81 CrossRef PubMed.
- X. Tan, S. Liu, X. Hu, R. Zhang, X. Su, R. Qian, Y. Mai, Z. Xu, W. Jing, W. Tian and L. Xie, ACS Appl. Mater. Interfaces, 2023, 15, 391–406 CrossRef CAS PubMed.
- Y. Tang, Q.-X. Huang, D.-W. Zheng, Y. Chen, L. Ma, C. Huang and X.-Z. Zhang, Mater. Today, 2022, 53, 71–83 CrossRef CAS.
- Y. Yin, S. Yang, D. Ai, H. Qin, Y. Sun, X. Xia, X. Xu, W. Ji and J. Song, Adv. Funct. Mater., 2023, 33, 2301062 CrossRef CAS.
- X. Zhao, Y. Yang, J. Yu, R. Ding, D. Pei, Y. Zhang, G. He, Y. Cheng and A. Li, Biomaterials, 2022, 282, 121387 CrossRef CAS PubMed.
- S. Liu, Y.-N. Wang, L. Yu, J. Li and S. Ge, Chem. Eng. J., 2022, 432, 134308 CrossRef CAS.
- H. Guo, S. Huang, X. Yang, J. Wu, T. B. Kirk, J. Xu, A. Xu and W. Xue, ACS Appl. Mater. Interfaces, 2021, 13, 61638–61652 CrossRef CAS PubMed.
- Z. Yu, Q. Li, X. He, X. Wang, Y. Wen, L. Zeng, W. Yu, P. Hu and H. Chen, Eur. Polym. J., 2023, 197, 112330 CrossRef CAS.
- S. Li, M. Pei, T. Wan, H. Yang, S. Gu, Y. Tao, X. Liu, Y. Zhou, W. Xu and P. Xiao, Carbohydr. Polym., 2020, 250, 116922 CrossRef CAS PubMed.
- P. Bertsch, M. Diba, D. J. Mooney and S. C. G. Leeuwenburgh, Chem. Rev., 2023, 123, 834–873 CrossRef CAS PubMed.
- B. D. Zheng, J. Ye, Y. C. Yang, Y. Y. Huang and M. T. Xiao, Carbohydr. Polym., 2022, 275, 118770 CrossRef CAS PubMed.
- Z. Zou, Z. Zhang, H. Ren, X. Cheng, X. Chen and C. He, Biomaterials, 2023, 301, 122251 CrossRef CAS PubMed.
- Y. Pang, H. Wang, Y. Yao, D. Chen, R. Yang, Z. Wang, J. Yang, Y. Li and W. Liu, Adv. Funct. Mater., 2023, 33, 2303095 CrossRef CAS.
- S. H. Lin, A. P. H. Huang and S. h. Hsu, Adv. Funct. Mater., 2023, 33, 2303853 CrossRef CAS.
- A. Moeini, P. Pedram, P. Makvandi, M. Malinconico and G. Gomez d'Ayala, Carbohydr. Polym., 2020, 233, 115839 CrossRef CAS PubMed.
- Y. Hao, W. Zhao, H. Zhang, W. Zheng and Q. Zhou, Carbohydr. Polym., 2022, 287, 119336 CrossRef CAS PubMed.
- H. Weng, W. Jia, M. Li and Z. Chen, Carbohydr. Polym., 2022, 294, 119767 CrossRef CAS PubMed.
- Y. Lin, J. Xu, Y. Dong, Y. Wang, C. Yu, Y. Li, C. Zhang, Q. Chen, S. Chen and Q. Peng, Carbohydr. Polym., 2023, 314, 120962 CrossRef CAS PubMed.
- N. Mutlu, L. Liverani, F. Kurtuldu, D. Galusek and A. R. Boccaccini, Int. J. Biol. Macromol., 2022, 213, 845–857 CrossRef CAS PubMed.
- T. Khodaei, J. Nourmohammadi, A. Ghaee and Z. Khodaii, Carbohydr. Polym., 2023, 302, 120371 CrossRef CAS PubMed.
- C. Zhou, Y. Zou, R. Xu, X. Han, Z. Xiang, H. Guo, X. Li, J. Liang, X. Zhang, Y. Fan and Y. Sun, Mater. Horiz., 2023, 10, 3114–3123 RSC.
- X. Yin, Y. Hao, Y. Lu, D. Zhang, Y. Zhao, L. Mei, K. Sui, Q. Zhou and J. Hu, Adv. Funct. Mater., 2021, 31, 2105614 CrossRef CAS.
- J. Xing, Y. Ding, X. Zheng, P. Yu, M. Qin, R. Qiu, Y. Li, S. Shang, J. Xie and J. Li, Chem. Eng. J., 2022, 444, 136580 CrossRef CAS.
- K. Yang, X. Zhou, Z. Li, Z. Wang, Y. Luo, L. Deng and D. He, ACS Appl. Mater. Interfaces, 2022, 14, 43010–43025 CrossRef CAS PubMed.
- J. Wang, H. Cheng, W. Chen, P. Han, X. Yao, B. Tang, W. Duan, P. Li, X. Wei, P. K. Chu and X. Zhang, Chem. Eng. J., 2023, 452, 139474 CrossRef CAS.
- Y. Xin, Z. Guo, A. Ma, E. Shi, Z. Li, Z. Liang, Z. Qian, L. Yang, Y. Wang, M. Cao and X. Yang, Chem. Eng. J., 2023, 451, 138782 CrossRef CAS.
- Y. Tian, Y. Li, J. Liu, Y. Lin, J. Jiao, B. Chen, W. Wang, S. Wu and C. Li, Bioact. Mater., 2022, 9, 428–445 CAS.
- N. Mukherjee, S. Ghosh, J. Sarkar, R. Roy, D. Nandi and S. Ghosh, ACS Appl. Mater. Interfaces, 2023, 15, 33457–33479 CrossRef CAS PubMed.
- L. Yang, Z. Han, C. Chen, Z. Li, S. Yu, Y. Qu and R. Zeng, Mater. Sci. Eng., C, 2020, 117, 111265 CrossRef CAS PubMed.
- W. Tan, T. Long, Y. Wan, B. Li, Z. Xu, L. Zhao, C. Mu, L. Ge and D. Li, Carbohydr. Polym., 2023, 312, 120824 CrossRef CAS PubMed.
- A. Sirelkhatim, S. Mahmud, A. Seeni, N. H. M. Kaus, L. C. Ann, S. K. M. Bakhori, H. Hasan and D. Mohamad, Nano-Micro Lett., 2015, 7, 219–242 CrossRef CAS PubMed.
- L. E. Shi, Z. H. Li, W. Zheng, Y. F. Zhao, Y. F. Jin and Z. X. Tang, Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess., 2014, 31, 173–186 CrossRef CAS PubMed.
- X. Zhu, C. Liang, J. Chen, J. Gao, W. Chen, Q. Ouyang, L. Luo, Z. Huang, H. Luo, L. Chen and J. Chen, Chem. Eng. J., 2024, 481, 148136 CrossRef CAS.
- Y. Sheng, Z. Chen, W. Wu and Y. Lu, Drug Discovery Today, 2023, 28, 103455 CrossRef CAS PubMed.
- B. P. Lima, L. I. Hu, G. W. Vreeman, D. B. Weibel and R. Lux, Microb. Ecol., 2019, 78, 336–347 CrossRef CAS PubMed.
- A. Adak, S. Ghosh, V. Gupta and S. Ghosh, Biomacromolecules, 2019, 20, 1889–1898 CrossRef CAS PubMed.
- T. Lu, J. Zhang, X. Yuan, C. Tang, X. Wang, Y. Zhang, K. Xiong and J. Ye, Mater. Sci. Eng. C., 2021, 131, 112490 CrossRef CAS PubMed.
- Y. Zhao, J. Li, L. Liu, Y. Wang, Y. Ju, C. Zeng, Z. Lu, D. Xie and J. Guo, Adv. Healthcare Mater., 2023, 12, 2300303 CrossRef CAS PubMed.
- Y. Xu, R. Rothe, D. Voigt, S. Hauser, M. Cui, T. Miyagawa, M. Patino Gaillez, T. Kurth, M. Bornhauser, J. Pietzsch and Y. Zhang, Nat. Commun., 2021, 12, 2407 CrossRef CAS PubMed.
- M. Wang, S. Lin, M. Liu, J. Jiao, H. Mi, J. Sun, Y. Liu, R. Guo, S. Liu, H. Fu, Y. Yang and R. Li, Chem. Eng. J., 2023, 463, 142283 CrossRef CAS.
- Y. Hao, C. Yuan, J. Deng, W. Zheng, Y. Ji and Q. Zhou, ACS Appl. Mater. Interfaces, 2022, 14, 16006–16017 CrossRef CAS PubMed.
- Z. Zhou, J. Xiao, S. Guan, Z. Geng, R. Zhao and B. Gao, Carbohydr. Polym., 2022, 285, 119235 CrossRef CAS PubMed.
- Z. Ahmadian, A. Correia, M. Hasany, P. Figueiredo, F. Dobakhti, M. R. Eskandari, S. H. Hosseini, R. Abiri, S. Khorshid, J. Hirvonen, H. A. Santos and M. A. Shahbazi, Adv. Healthcare Mater., 2021, 10, e2001122 CrossRef PubMed.
- J. Yu, X. Wu, W. Zhang, F. Chu, Q. Zhang, M. Gao, Y. Xu and Y. Wu, Hum. Cell, 2023, 36, 1389–1402 CrossRef CAS PubMed.
- M. Liu, L. Huang, X. Xu, X. Wei, X. Yang, X. Li, B. Wang, Y. Xu, L. Li and Z. Yang, ACS Nano, 2022, 16, 9479–9497 CrossRef CAS PubMed.
- A. Adak, V. Castelletto, A. de Sousa, K.-A. Karatzas, C. Wilkinson, N. Khunti, J. Seitsonen and I. W. Hamley, Biomacromolecules, 2024, 25, 1205–1213 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |