Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in photoelectrochemical platforms based on porous materials for environmental pollutant detection
Shiben Liu
,
Jinhua Zhan
 * and
Bin Cai
* and
Bin Cai
 *
*
School of Chemistry and Chemical Engineering, Shandong University, 250100 Jinan, China. E-mail: bin.cai@sdu.edu.cn
First published on 6th March 2024
Abstract
Human health and ecology are seriously threatened by harmful environmental contaminants. It is essential to develop efficient and simple methods for their detection. Environmental pollutants can be detected using photoelectrochemical (PEC) detection technologies. The key ingredient in the PEC sensing system is the photoactive material. Due to the unique characteristics, such as a large surface area, enhanced exposure of active sites, and effective mass capture and diffusion, porous materials have been regarded as ideal sensing materials for the construction of PEC sensors. Extensive efforts have been devoted to the development and modification of PEC sensors based on porous materials. However, a review of the relationship between detection performance and the structure of porous materials is still lacking. In this work, we present an overview of PEC sensors based on porous materials. A number of typical porous materials are introduced separately, and their applications in PEC detection of different types of environmental pollutants are also discussed. More importantly, special attention has been paid to how the porous material's structure affects aspects like sensitivity, selectivity, and detection limits of the associated PEC sensor. In addition, future research perspectives in the area of PEC sensors based on porous materials are presented.
1. Introduction
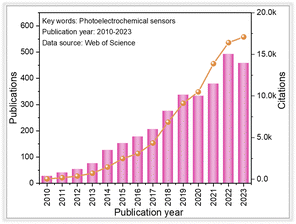
Over the past few decades, global population growth and industrial development have resulted in serious environmental pollution.1,2 Substantial amounts of harmful chemical compounds, such as heavy metal ions, antibiotics, pesticides, and phenolics, have been discharged into the environment. The self-purification capacity of natural water bodies cannot cope with these increasing environmental pollutants, posing a severe threat to both the ecological environment and public health.3,4 These contaminants can be identified using traditional detection techniques like high-performance liquid chromatography, atomic fluorescence spectroscopy, atomic absorption/emission spectrometry, and inductively coupled plasma mass spectrometry.5–9 However, the aforementioned detection techniques are limited by the large experimental instruments, specific operating conditions, and high training costs.10,11 Therefore, it is crucial to develop efficient techniques for the sensitive identification of these environmental contaminants.Recently, photoelectrochemical (PEC) detection methods, as a branch of electrochemical (EC) detection techniques, have gained much attention in the field of tracing environmental pollutants,12–15 which can be seen from the number of publications since 2010 (Fig. 1). Compared with conventional electrochemical detection techniques, the PEC method is based on the photo-induced electron–hole pair redox properties, which make it possible to generate signals (photocurrent).16–18 Since light and photocurrent are employed as the excitation source and identification signal, respectively, a PEC sensor handles relatively lower background noise and higher sensitivity than those of conventional electrochemical detection methods due to the difference in the energy form of the excitation source and the converted electrical signal. Moreover, the PEC sensor is not directly in contact with the measured object, resulting in less influence from the measurement condition.19 These advantages contribute to the simplicity of instrumentation, low cost, and ease of miniaturization associated with the PEC detection method.20–23
In a typical PEC sensing system, the incoming light is regarded as the excitation source, and the electrical signal is used as the detection signal.18,24,25 When exposed to light, the electrode containing the photoactive material is excited, resulting in the generation of photogenerated electron–hole pairs, which are subsequently transferred to the electrode surface. Cathodic photocurrent results from the electrons on the condition band (CB) being trapped by electron acceptors (A); otherwise, the holes on the valence band (VB) transfer to the surface of photoactive materials, where they react with the electron donors (D) to form anodic photocurrent (Fig. 2A).17 According to the different generation processes of the photocurrents at the interface between the electrode and electrolyte, the PEC detection sensors can be classified into two categories: (1) the analytes serve as electron donors or acceptors to directly react with photoactive materials;26,27 (2) the electrodes are first modified by recognition elements, after which the target concentration is determined through indirect physicochemical interactions between the targets and the recognition elements (Fig. 2B). For the commonly used recognition elements used in the PEC detection system, they can be divided into three categories: (1) aptamers refer to single-stranded synthetic nucleic acid molecules (DNA or RNA);28–30 (2) antibodies, which are mainly proteins, can be used as recognition elements to bind with target antigens such as proteins, toxins, and pathogens;31 (3) enzymes are biological catalysts that can bind to and act upon specific substrates.32
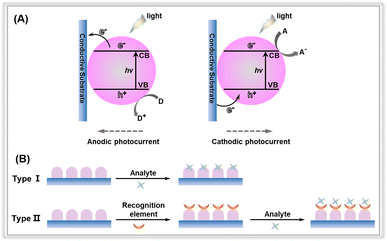 | ||
| Fig. 2 (A) Anodic and cathodic photocurrent generation mechanisms of photoactive material-based electrodes. (B) Different types of PEC sensors with or without the recognition elements. | ||
As can be seen from the inset above, whether the recognition element is contained or is free on photoelectrochemical sensing platforms, photoelectrochemical detection performance of a fabricated electrode is mainly determined by three factors, including light absorption capacity, photogenerated charge carrier separation and transportation within photoactive materials, and the transfer of surface charge carrier to the detection species.33 To achieve excellent detection activity with PEC sensors, researchers have focused on exploiting various sensing devices and detection modes, and the design engineering of photoactive materials. Much effort has been directed towards reducing costs and simplifying the device fabrication for versatile and portable PEC sensing platforms. For instance, in some detection devices, capacitors and digital multimeters are utilized to output electrical signals instead of electrochemical workstations.34–36 Another area of research involves exploring different detection patterns, such as split-type detection, self-power detection, visual detection, and high-throughput detection. Despite extensive efforts in designing detection devices and modifying detection modes, the primary focus in constructing PEC platforms has been on investigating photoelectrodes.19,37 To date, significant effort has been devoted to the development of photoactive materials as electrodes for PEC sensors. These materials include metal oxides, metal organic frameworks, graphene and carbon nitride.38,39
In recent years, porous materials have received considerable attention owing to their high surface area, relatively low density, large accessible space, and variable chemical composition.40–42 Research interests in the applications of porous material-based PEC sensors were rapidly increasing, particularly in the field of determining environmental pollutants.43 Due to their unique structure, porous materials are preferred over bulk materials for photoelectrochemical determination of environmental contaminants. As illustrated in Fig. 3, the porous material has a structural influence on the three factors governing the PEC detection activity of the sensor.
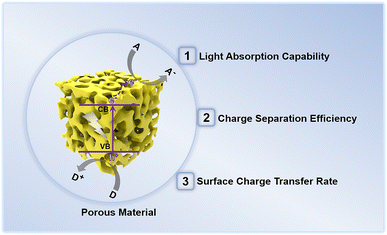 | ||
| Fig. 3 Structural effects of photoactive porous materials on the performance of photoelectrochemical detection of environmental pollutants. | ||
(1) Light absorption capability: porous materials with small pores enable the incident light to penetrate their pore walls and scatter intensely inside the pore channels, thereby greatly improving the light harvesting ability of the porous materials.44 Further, the feasible modifications of the band structure of the porous materials by altering the pore diameter also promote light absorption, particularly in the visible light region.
(2) Photogenerated charge carrier separation and transport efficiency: the photoelectric conversion efficiency of a photoelectrode depends on the separation and transport of photogenerated charge pairs within the photoactive material. In the case of bulk materials, photogenerated charge pair separation normally occurs in the space charge layer and the adjacent layers, leading to easy recombination. However, for porous materials, the charge separation and migration paths can be shortened due to their unique porous structures,45 enabling efficient photogenerated charge transfer within porous materials.
(3) Surface charge carrier transfer rate: porous materials possess a large specific surface area, which is advantageous for mass transfer during the photoelectrochemical detection. In addition, the exposed surface areas with abundant active sites provide sufficient functional groups conducive to interaction with recognition elements or direct trapping of the probe species. Hence, porous materials exhibit rapid surface charge carrier transfer rates and reaction kinetics.
Thus, photoelectrochemical sensing platforms based on porous materials have remarkable detection capabilities against environmental contaminants. This can be attributed to several factors, including enhanced light absorption, high photogenerated charge carrier separation and transport efficiency, and rapid surface charge carrier transfer rates during photoelectrochemical detection. As a result, these platforms offer a wide linear range, low detection limits, high sensitivity, excellent selectivity, and outstanding stability.
Recently, a significant number of reviews on PEC detection sensors for environmental pollutant determination have been published.11,16,46–50 However, these studies focused on strategies for fabricating PEC detection systems and identifying the multiple contaminants, neglecting to discuss the effect of the presence of porous materials on the detection activity of PEC sensors. For instance, in 2020, Shu et al. provided a comprehensive summary of current research in photoelectrochemical sensing, with a particular emphasis on material design and engineering to regulate photoelectrochemical sensing performance. They also discussed photoelectrochemical sensing devices and detection modes.33 In another review by Li and co-workers, the central theme was a comparative analysis of electrochemical detection and photoelectrochemical detection, specifically addressing the question of which analytical method is more effective for tracing environmental pollutants.11 Further, in 2021, Yan et al. provided a concise overview of the fundamental and research progress of functional materials (such as metals, metal oxides, inorganic 2D materials, and carbon nanomaterials) in electrochemical and photoelectrochemical technologies for monitoring environmental pollutants.51 Despite these efforts, few reviews have focused on the relationship between the structure of porous materials and the detection activity of associated PEC sensors. So, in this review, we seek to thoroughly investigate the recent advancements in porous materials for environmental contaminant detection. Indeed, porous materials encompass a wide range of different types. Here, we specifically focus on photoactive porous materials, including but not limited to metal oxides, metal–organic frameworks (MOFs), covalent organic frameworks (COFs), graphitic carbon nitride (g-C3N4), and MXene (Table 1). We extensively discuss the structural effects of porous materials on the performance of PEC sensors. Finally, we propose the main challenges and future prospects of porous materials in the realm of PEC detection sensors. However, due to the space limitations of this review and the widespread use of porous materials in the field of PEC detection sensors, many intriguing and significant studies may not have been addressed. We apologize for any inadvertent omissions and appreciate the valuable contributions of all researchers in this field.
| Photoactive porous materials | Common preparation method | Advantages |
|---|---|---|
| Metal oxides | Anodic oxidation, chemical bath deposition, hydrothermal | Suitable Eg, fast surface kinetics |
| Metal–organic frameworks | Hydrothermal, slow evaporation and diffusion, iono-thermal, microwave-assisted, mechanochemical, electrochemical, sonochemical, microemulsion | Unique porosity, adjustable light response range |
| Covalent organic frameworks | Hydrothermal, ionothermal, microwave-assisted, sonochemical, mechanochemical, light-induced processes | π-electron conjugation, low Eg |
| Graphitic carbon nitride | Chemical exfoliation, thermal oxidation | Metal-free nontoxic, visible light responsive |
| MXene | Etching method | Small diffusion barrier, high conductivity |
2. Photoactive porous materials
2.1. Metal oxides
Metal oxides play a crucial role in the field of photoelectrochemical sensing.52,53 Indeed, there is a large body of research focusing on porous metal oxides for PEC sensing, which is difficult to categorize in a simple way, so here we only present some representative porous metal oxides, in particular semiconductor metal oxides (Table 2). Among all the porous metal oxides applied in PEC sensing, TiO2 has always occupied a unique position.54–59 In addition, PEC detection sensors have been constructed using ZnO, SnO2, WO3, and Fe2O3 (ref. 60–65) (Table 3). For instance, in a study by Tavella and co-workers, a porous TiO2 array modified titanium electrode was constructed for PEC sensing of dopamine.66 The as-prepared electrode performed well for dopamine and has a wide response range (200∼1500 μM) and a low detection limit (20 μM). In addition to nanoarrays, nanotubes also have a large number of pores. For instance, Fan et al. prepared a BiOI nanoflowers/TiO2 nanotubes (BiOI/TiO2) composite through a hydrothermal method (Fig. 4A and B).67 Due to the presence of porous nanotube structure, the BiOI NFs/TiO2 NTs electrode provided more spaces for anchoring the aptamer, and thus the PEC platform demonstrated a significant activity for atrazine determination with a low detection limit of 0.5 pM (Fig. 4C and D).| Photoactive metal oxides | Band gap/eV | Preparation method |
|---|---|---|
| TiO2 | 3.0∼3.3 | Anodic oxidation, hydrothermal, sol–gel |
| ZnO | 3.7 | Electrodeposition, hydrothermal, chemical bath deposition |
| SnO2 | 3.6∼4.0 | Hydrothermal, electrospun |
| WO3 | 2.8 | Hydrothermal, solvothermal, co-precipitation |
| Fe2O3 | 2.2 | Electrodeposition, hydrothermal |
| Working electrode | Analyte | LOD | Linear concentration range | Ref. |
|---|---|---|---|---|
| a MIP: molecularly imprinted polymer; NTAs: nanotubes; NRs: nanorods; Vo-TNTAs: TiO2 nanotube arrays with oxygen vacancies; TiNTs: TiO2 nanotube arrays; GQDs: graphene quantum dots; P(33DT-co-3TPCA): poly(3,3′-dithiophene-co-3-thiophenecarboxylic acid); [BMIM]Cl: 1-butyl-3-methylimidazolium chloride. | ||||
| NiCo-LDHs/TiO2 TNAs | Cr(VI) | 0.12 μM | 0.5 μM∼1.8 mM | 55 |
| CdSe/TiO2 TNAs | Cd2+ | 0.35 nM | 1 nM∼10 mM | 72 |
| Fe3+/ZnO-Ag | Hg2+ | 0.1 nM | 0.5 nM∼100 nM | 60 |
| WO3/Au | Hg2+ | 2.0 pM | 4.2 pM∼840 pM | 61 |
| Fe2O3-CdS | Cu2+ | 0.5 nM | 50 nM∼600 μM | 73 |
| ZnO-CdS | Cu2+ | 3 nM | 0.01 μM∼1 mM | 69 |
| CdS/ZnO | Cd2+ | 3.3 μM | 0.01 mM∼5 mM | 74 |
| BiOI/ZnO NRs | Pb2+ | 7.5 μM | 10 μM∼100 μM | 75 |
| MIP@TiO2 NTAs | Perfluorooctane sulfonate | 86 ng mL−1 | 0.5 μM∼10 μM | 76 |
| CdS/MnO2 | Paraoxon | 0.017 ng mL−1 | 0.05 ng mL−1∼10 ng mL−1 | 77 |
| TiO2 TNAs/CdS | Bisphenol A | 0.5 pM | 1 pM∼100 pM | 78 |
| SnO2 | Bisphenol A | 1.2 nM | 2 nM∼500 nM | 79 |
| MoS2/ZnO | Propyl gallate | 12 nM | 0.125 μM∼1.47 μM | 80 |
| TiO2 array/Ti | Dopamine | 20 μM | 0.2 mM∼1.5 mM | 66 |
| CdS QDs/TiO2 TNAs | Asulam | 4.1 pg mL−1 | 0.02 ng mL−1∼2.0 ng mL−1 | 57 |
| BiOI/TiO2 NTAs | Atrazine | 0.5 pM | 1.0 pM∼600.0 pM | 67 |
| Bi2S3/Vo-TNTAs | Chlorpyrifos | 6 nM | 0.07 μM∼3.0 μM | 68 |
| GQDs/TiO2 TNAs | Chloramphenicol | 57.9 pM | 0.5 nM∼100 nM | 81 |
| WO3/CuMnO2 | Nitrofurazone | 1.19 nM | 0.015 μM∼32 μM | 70 |
| Bi2WO6/α-Fe2O3 | Tetracycline | 0.3 μM | 0.01 μM∼25 μM | 63 |
| P(33DT-co-3TPCA)/[BMIM]Cl-ZnO NRs | Aflatoxin B1 | 0.058 ng mL−1 | 0.10 ng mL−1∼10 ng mL−1 | 82 |
| rGO/TiO2 TNAs | Microcystin-LR | 0.5 fM | 1.0 fM∼500 fM | 83 |
| Cu2O/TiNTs | Sulfide | 0.6 μM | 1 μM∼300 μM | 84 |
| SnO2-AuNPs | Nitrite | 0.48 nM | 1 nM∼100 μM | 85 |
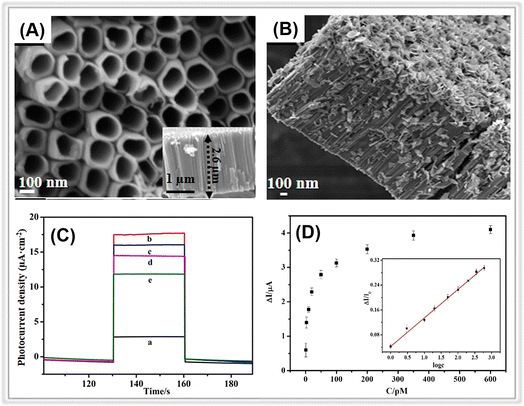 | ||
| Fig. 4 SEM images of (A) TiO2 NTs and (B) BiOI NFs/TiO2 NTs. (C) Photocurrent responses of (a) TiO2 NTs, (b) BiOI NFs/TiO2 NTs, (c) aptamer/BiOI NFs/TiO2 NTs, (d) BSA/aptamer/BiOI NFs/TiO2 NTs, and (e) atrazine/BSA/aptamer/BiOI NFs/TiO2 NTs. (D) Photocurrent change against atrazine concentrations. Adopted from ref. 67. Copyright 2021, with permission of Elsevier. | ||
Similarly, Wu et al. developed a PEC sensor for chlorpyrifos (CPF) detection based on bismuth sulfide nanoparticles decorating TiO2 nanotubes with oxygen vacancies (Bi2S3/Vo-TNTAs), which realized a rapid photocurrent response to CPF within a linear range of 0.07∼3.0 μM.68
In addition to the porous TiO2 nanomaterials, other photoactive porous metal oxides (ZnO, SnO2, WO3, and Fe2O3) have also exhibited excellent photoelectrochemical detection activity towards environmental pollutants. In a study by Wu and co-workers, CdS nanocrystals were decorated on porous one-dimensional (1D) ZnO nanorods through a pulsed electrodeposition technique.69 The resulting CdS/ZnO hybrid photoelectrode was employed for PEC detection of the heavy metal ion, Cu2+. The porous ZnO nanorods provided an enlarged surface area for the dispersion of nano-sized CdS nanocrystals, thereby enhancing the photogenerated electron transfer via the “1D electron highway”. The CdS/ZnO composite electrode demonstrated an ultrasensitive LOD of approximately 3 nM within a wide linear range of 0.01∼1000 μM. Meanwhile, Velmurugan et al. prepared a WO3/CuMnO2 p–n heterojunction composite and applied it for “signal-on” PEC sensing of nitrofurazone.70 Porous WO3 nano-tiles were synthesized via a hydrothermal method and coupled with CuMnO2 nanoparticles by an evaporative impregnation method. The porous WO3 provided abundant surface sites for interaction with CuMnO2 nanoparticles. Further, a p–n heterojunction was formed between the p-type CuMnO2 and the n-type WO3, which promoted photogenerated electron transfer due to the presence of a built-in electric field.71 As a result, the PEC nitrofurazone sensing performance of the WO3/CuMnO2 composite electrode demonstrated a wider detection range of 0.015∼32 μM with a lower LOD of 1.19 nM, compared to pure CuMnO2 nanoparticles.
α-Fe2O3 is a well-known visible light active semiconductor with a band gap of 2.2 eV, making it suitable for applications in PEC sensing. In 2019, Adhikari et al. prepared a Bi2WO6/α-Fe2O3 heterojunction photoelectrode by depositing porous α-Fe2O3 layers on the Bi2WO6 nanoflakes.63 The influence of the α-Fe2O3 layer thickness on the PEC detection activity of tetracycline was thoroughly investigated. After introducing porous α-Fe2O3 layers, the photocurrent of the hybrid composite-based photoelectrode increased (4.3 μA cm−2) compared to that of pristine Bi2WO6 (1.2 μA cm−2). Under optimized conditions, the photoelectrode demonstrated a LOD of around 0.3 μM in the range from 0.01 μM to 25 μM. Hence, PEC sensors based on porous metal oxides hold great potential for tracing environmental contaminants.
2.2. Metal–organic frameworks
Metal–organic frameworks (MOFs), which are potential porous materials composed of transition metal ions and organic ligands, have attracted much attention in the area of environmental pollutants determination.86,87 MOFs, particularly photosensitive MOFs, have many potential applications in PEC sensing due to their inherent characteristics, such as permanent porosity, chemical stability, and unique optical properties.39,88–91 The Zeolitic Imidazolate Framework (ZIF), Materials Institute Lavoisier frameworks (MILs), Universitetet i Oslo (UiO), copper(II) benzene-1,3,5-tricarboxylate (Cu-BTC), and porphyrin-based MOFs (PCN) series MOFs have been extensively investigated for PEC sensing applications against hazardous pollutants (Table 4).92| Working electrode | Analyte | LOD | Linear concentration range | Ref. |
|---|---|---|---|---|
| a Eu-MOF: europium(III)-based metal organic framework; Cu-MOF-NH2: copper(II) 2-aminoterephthalic acid; NiPc-Ni MOF: nickel phthalocyanine-based metal organic framework; Cu-BTC: copper(II) benzene-1,3,5-tricarboxylate; Ce-Por-MOFs: Ce-porphyrin-metal–organic frameworks; NH2-MIL-125(Ti): amino-functionalized titanium(IV) based MOFs; ZIF-8: zeolitic imidazolate framework-8; CdS QDs: CdS quantum dots; PCN-222, PCN-224: zirconium-porphyrin metal–organic framework; Ce-UiO-66: Ce doped zirconium-based MOF with terephthalic acid ligands; NH2-UiO-66: zirconium-based MOF with 2-aminoterephthalic acid ligands; Er-MOF: erbium(III)-based metal organic framework. | ||||
| Eu-MOF | Fe3+ | 0.0899 μM | 1 μM∼100 mM | 104 |
| Cu-MOF-NH2 | Kanamycin | 0.1 nM | 0.5 nM∼650 nM | 105 |
| NiPc-Ni MOF | Curcumin | 0.8 nM | 2.5 nM∼16 μM | 106 |
| Cu-BTC/g-C3N4 | Glyphosate | 0.13 pM | 1 pM∼10 nM | 100 |
| Cu-BTC@Cu2O | Dioctyl phthalate | 9.15 pM | 25.0 pM∼0.1 μM | 107 |
| PCN-224/rGO | p-arsanilic acid | 5.47 ng L−1 | 10 ng L−1∼10 mg L−1 | 108 |
| CdS QDs/PCN-224 | Doxorubicin hydrochloride | 3.57 nM | 10 nM∼1 μM | 102 |
| Gentamicin sulfate | 0.158 nM | 1 nM∼1 μM | ||
| PCN-222@g-C3N4 | Kanamycin sulfate | 0.127 nM | 1 nM∼100 nM | 101 |
| Ce-Por-MOFs/AgNWs | Ronidazole | 0.038 nM | 0.1 nM∼104 nM | 109 |
| ZIF-8@ZnIn2S4 | Tetracycline | 0.1 pM | 1 pM∼700 pM | 95 |
| MIP@NH2-MIL-125(Ti)/TiO2 | Oxytetracycline | 60 pM | 0.1 nM∼10 μM | 97 |
| CdS/Eu-MOF | Ampicillin | 0.093 nM | 0.1 nM∼0.2 μM | 110 |
| [Ru(bpy)3]2+@Ce-UiO-66/Mn:Bi2S3 | Ofloxacin | 6 pM | 0.01 nM∼100 nM | 98 |
| g-C3N4/Au/NH2-UiO-66 | D-penicillamine | 0.0046 μM | 10 nM∼400 μM | 99 |
| Er-MOF@AuNPs | Aflatoxin B1 | 19.6 fg mL−1 | 0.005 ng mL−1 ∼ 10.0 ng mL−1 | 111 |
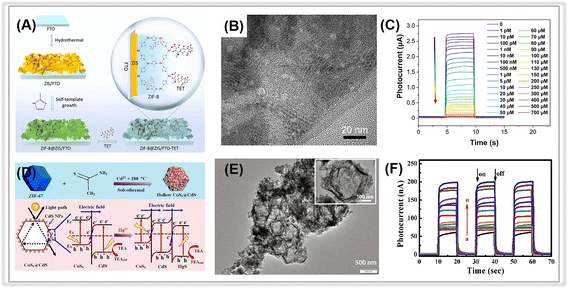 | ||
| Fig. 5 (A) The preparation of a ZIF-8@ZIS-based PEC sensor for tetracycline. (B) TEM image of ZIF-8@ZIS. (C) The corresponding calibration curve for the photocurrent signal responses toward various concentrations of tetracycline. Adopted from ref. 95. Copyright 2022, with permission of Elsevier. (D) The synthetic process of hollow CoSx@CdS composites and the band structures of CoSx@CdS/HgS composites and charge separation under visible light irradiation. (E) TEM image of CoSx@CdS composites. (F) Photocurrent responses of the CoSx@CdS-modified electrodes in the presence of Hg2+ of different concentrations. Adopted from ref. 103. Copyright 2020, with permission of Elsevier. | ||
| Working electrode | Template MOF | Analyte | LOD | Linear concentration range | Ref. |
|---|---|---|---|---|---|
| a ZIF-67: zeolitic imidazolate framework-67; MIPs: molecularly imprinted polymers; ZIF-8: zeolitic imidazolate framework-8; MIL-125(Ti): titanium(IV) terephthalic acid; Cu-BTC: Cu-BTC: copper(II) benzene-1,3,5-tricarboxylate; Zn-BTC: Zn(II) benzene-1,3,5-tricarboxylate; MIL-68(In): In(III) 1,4-benzenedicarboxylate; ZnCo-ZIF: bimetallic ZnCo-zeolitic imidazolate framework. | |||||
| CoSx@CdS | ZIF-67 | Hg2+ | 0.002 nM | 0.01 nM∼1 μM | 103 |
| Cu2O/CuO/TiO2 | NH2-MIL-125(Ti) | Pb2+ | 6.8 fM | 10 fM∼1 μM | 112 |
| CdCoS2 | ZIF-67-S | Chlorpyrifos | 0.57 ng mL−1 | 0.001 μg mL−1∼270 μg mL−1 | 113 |
| MIPs@TiO2-C | MIL-125(Ti) | Ofloxacin | 2.91 pg mL−1 | 0.01 ng mL−1∼3 μg mL−1 | 114 |
| CuO | Cu-BTC | Malathion | 0.086 nM | 0.1 nM∼104 nM | 115 |
| ZnO-Co3O4 | Zn-BTC | Sulfadiazine | 1.2 nM | 0.005 μM∼18.5 μM | 116 |
| ZnO/g-C3N4 | ZIF-8 | Oxytetracycline | 1.49 fM | 5 fM∼200 nM | 117 |
| ZnIn2S4@TiO2 | ZIF-8 | Lincomycin | 0.084 pM | 0.1 pM∼0.1 nM | 118 |
| ZnCdS@MoS2 | ZIF-8 | Lincomycin | 0.076 nM | 0.1 nM∼300 nM | 119 |
| AuNPs/In2O3@g-C3N4 | MIL-68(In) | Tetracycline | 3.3 pM | 0.01 nM∼0.5 μM | 120 |
| ZnxCo3−xO4/N-GQDs/AgBiS2 | ZnCo-ZIF | Ampicillin | 0.25 pM | 0.5 pM∼10 nM | 121 |
| In2O3–In2S3–Ti3C2 MXene | MIL-68(In) | Microcystin-LR | 0.169 pM | 0.5 pM∼400 nM | 122 |
Derivatives of the MIL family of MOFs have also demonstrated excellent PEC detection activities against environmental toxic species. In a study by Zhang and co-workers, COOH-functionalized TiO2 (TiO2-C) was achieved via one-step calcination of MIL-125(Ti).114 Due to the large specific surface area and abundant functional groups of TiO2-C, it demonstrated superior photochemical, electrochemical, and PEC detection performance compared to MIL-125(Ti). Furthermore, it was also useful for grafting molecularly imprinted polymers (MIPs). The MIPs@TiO2-C, with a large number of binding sites, provides precise electron transfer channels, resulting in improved sensitivity and selectivity for antibiotics such as ofloxacin. Under optimal conditions, the prepared sensor has a low detection limit (2.9 pg mL−1) and a wide linear concentration range (0.01 ng mL−1∼3 μg mL−1).
MIL-68(In) is also an ideal template for obtaining porous photoactive materials. For instance, Yan et al. synthesized an In2O3–In2S3–Ti3C2 MXene composite on the base of MIL-68(In)-derived In2O3 hollow tubular122 and then used it to construct a dual-mode (photoelectrochemical and photofuel cell) self-powered apta-sensing platform for detecting microcystin-LR (MC-LR). Porous In2O3 hollow tubulars with a large specific surface area provide abundant active sites, while the well-matched energy levels of In2O3 and In2S3 and the Ti3C2 MXene quantum dots acting as electron transfer mediators both accelerate the separation of photogenerated charge carriers. This sensing platform revealed excellent PEC detection activity in the range from 0.5 pM to 400 nM, with a LOD of 0.169 pM. In another research, Feng et al. used MIL-68(In) as the precursor for fabricating homogeneous In2O3 nanoparticles through high temperature calcination in an air atmosphere.120 The formation of a heterojunction between the porous In2O3 and g-C3N4 facilitated the separation and transfer of photogenerated charge pairs. The introduction of gold nanoparticles (Au NPs) with the localized surface plasmon resonance (LSPR) effect also improved visible light absorption and photoelectron transfer. The photocurrent of the Au NPs/In2O3@g-C3N4 composite reached 1.75 μA, much larger than that of pure g-C3N4. The Au NPs/In2O3@g-C3N4 was successfully applied to fabricate a label-free photoelectrochemical apta-sensing platform for tetracycline detection, which yielded a wide linear range from 0.01 nM to 500 nM with a LOD of 3.3 pM. Porous composites derived from Cu-BTC or Zn-BTC have also demonstrated great potential for PEC detection. In a study by Cao et al., Cu-BTC MOF (BTC: benzene-1,3,5-tricarboxylic acid) was calcinated to achieve porous hierarchical CuO.115 The CuO material has a large specific surface area, which is favorable for the capture of the target species, malathion. The facile PEC detection sensor achieved a LOD of 0.086 nM in the range of 0.1 nM to 104 nM.
2.3. Covalent organic frameworks
Like metal–organic frameworks, covalent organic frameworks (COFs) are a new class of porous materials that have attracted much interest for constructing the PEC analytic platforms.128,129 Generally, COFs are composed of covalently bonded light elements, such as C, N, H, O, B, and Si. The porous nature of COFs favors the trapping of probe species such as heavy metal ions. Moreover, the electronic interactions between the COF layers are realized by the π-stacked aromatic subunits, providing sufficient channels for charge transport.130–132 So far, several COFs have been employed in the field of PEC detection, such as D-TA COF,133,134 TAPP-COF,124 TTPA-COF,135 F-COF,125 p-bqy-COF,136 and PFA-130.137 However, the studies on PEC sensors based on covalent organic frameworks for tracing environmental pollutants are still in their early stages, as summarized in Table 6. In a study by Zhao and co-workers, a porphyrin-based covalent organic framework thin film (TAPP-COF) was synthesized via a liquid–liquid interfacial method and used as a photocathode material for photoelectrochemical “on-off-on” sensing of Pb2+.124 Due to the unique charge channels of porous COFs and the excellent photoelectric properties of porphyrin,138 the TAPP-COF-based PEC sensor displayed an improved “signal-on” photocathodic current response. The CdSe@SiO2 quantum dots, as a quenching agent, were introduced via a hybridization chain reaction to achieve a “signal off” photocurrent response, and then the PEC response switched to a “signal on” state after the addition of the detected species, Pb2+ (Fig. 6A). TAPP-COF is a p-type material that is beneficial to photogenerated electron–hole pair transfer.139 Hence, the as-prepared PEC detection sensor displayed a wide linear range from 0.05 nM to 100 nM with a LOD of 0.012 nM (Fig. 6C). Moreover, the flexibility of TAPP-COFs film was excellent, indicating its broad potential application in wearable PEC sensing devices (Fig. 6B).140–145| Working electrode | Analyte | LOD | Linear concentration range | Ref. |
|---|---|---|---|---|
| a TAPP-COF: porphyrin-based covalent organic framework; CoPc-PT-COF: tetra-amine cobalt phthalocyanine-2,9-bis[p-(formyl) phenyl]-1,10-phenanthroline-covalent organic framework; F-COF: fluoro-substituted covalent organic frameworks; TpPa-1-COF: p-phenylenediamine-covalent organic framework. | ||||
| TAPP-COF | Hg2+ | 0.012 nM | 0.05 nM∼1000 nM | 124 |
| CoPc-PT-COF@Cu-MOF | Cr(III) | 14.5 fM | 0.1 pM∼100 nM | 126 |
| F-COF/TiO2 NTAs | Dopamine | 0.032 μM | 0.1 μM∼300 μM | 125 |
| NH2-UiO-66/TpPa-1-COF | Dibutyl phthalate | 30 pM | 0.1 nM∼100 μM | 127 |
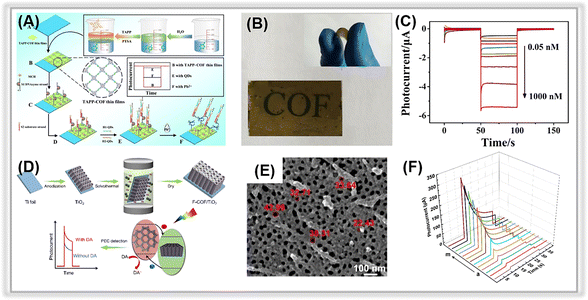 | ||
| Fig. 6 (A) Illustration of the TAPP-COF-based PEC sensor for tracing Pb2+. (B) The flexible photograph of TAPP-COF thin films. (C) Photocurrent response of the photoelectrochemical detection sensor to different concentrations of Pb2+. Adopted from ref. 124. Copyright 2021, with permission of American Chemical Society. (D) The construction of the F-COF/TiO2 NTA platform for PEC sensing for dopamine. (E) SEM image of F-COF/TiO2 NTA. (F) Photocurrent response of F-COF/TiO2 NTA to different concentrations of dopamine. Adopted from ref. 125. Copyright 2021, with permission of American Chemical Society. | ||
Alternatively, the combination of COFs with other materials could enhance their performance in PEC detection platforms by providing more reactive sites for the determination of target environmental contaminants. In a study by Wang et al., a heterostructure was formed between TiO2 NTAs and F-COFs through a simple hydrothermal method (Fig. 6D and E) and then used for constructing a PEC detection platform for dopamine.125 The introduction of F-COFs enhanced the visible light absorption and photogenerated electron–hole pairs separation efficiency. Meanwhile, the porous F-COFs processed a large specific surface area, and π–π interactions could be formed between aromatics of dopamine,146 which contributed to their ultra-sensitive detection activity. The resulting PEC sensor demonstrated excellent photocurrent response stability. More importantly, a LOD of 0.032 μM was obtained in an extended linear range (Fig. 6F).
Heterostructure COF/MOF hybrids have been fabricated as multifunctional materials for sensing applications.127,147 In 2021, Zhang et al. prepared a CoPc-PT-COF@Cu-MOF-based PEC-EC dual-mode biosensor for Cr(III) quantification.126 The 2D CoPc-PT-COF was in situ grown on a Cu-MOF by covalent binding between the carboxyl group in the Cu-MOF and the amino group in the CoPc-PT-COF. The DNA strands could be easily anchored on the composite via strong interaction owing to its large specific surface area and high porosity. Furthermore, due to the specific recognition between DNA and Cr(III), the composite-based biosensor could be used to trace Cr(III). The photocurrent of composite (419 nA) was about 14.5 times greater than that of Cu-MOF, indicating that the heterojunction between Cu-MOF and CoPc-PT-COF enhanced the photoelectric conversion efficiency. So, the obtained composite-based PEC biosensor displayed a LOD of 14.5 fM within the Cr(III) concentration range of 0.1 pM to 100 nM. Overall, porous COFs have significant potential for PEC sensing applications against environmental contaminants.
2.4. Graphitic carbon nitride
Graphitic carbon nitride (g-C3N4) is a well-known metal-free polymeric semiconductor that exhibits excellent visible light response and environmental friendliness.148–151 In recent years, g-C3N4 has been utilized in the area of PEC detection of environmental contaminants120,152–156 (Table 7). However, for bulk g-C3N4, the PEC detection performance is poor due to its relatively low photoelectric conversion efficiency. Activating pristine g-C3N4 with various treatments to achieve a porous structure is indeed an excellent way to speed up the separation of photogenerated charge pairs. Specifically, the methods employed to treat bulk g-C3N4 include chemical exfoliation and thermal oxidation.149,157,158| Working electrode | Analyte | LOD | Linear concentration range | Ref. |
|---|---|---|---|---|
| a A-CN: alkalized C3N4; 3DBC-C3N4: three-dimension branched crystalline carbon nitride; GA-C3N4: graphene-analogue carbon nitride; F-g-C3N4: formate anion-incorporated graphitic carbon nitride; Au/PCN-S: Au nanoparticle-decorated phosphorus-doped porous ultrathin C3N4 nanosheets; Au-doped 3D CN: Au nanoparticle doped three-dimensional graphitic carbon nitride; CNNS-Cu: copper cluster modified carbon nitride nanosheets; ZnPc/CN: zinc phthalocyanine/graphitic carbon nitride; utg-C3N4: ultrathin graphite-like carbon nitride; S-BN/Au/CN: sulfur doped hexagonal boron nitride/Au nanoparticles/graphitic carbon nitride; CB: carbon black; NS: nanosheets. | ||||
| A-CN | Cu2+ | 0.31 μM | 0.2 μM∼50 μM | 163 |
| 3DBC-C3N4 | Cu2+ | 0.38 nM | 1 nM∼100 nM | 43 |
| GA-C3N4 | Cu2+ | N/A | 0.4 μM∼7.6 μM | 164 |
| F-g-C3N4 | Cr(VI) | 0.006 μg L−1 | 0.01 μg L−1∼1000 μg L−1 | 165 |
| BiOI/CNx | Cr(VI) | 0.1 μM | 0.5 μM∼190 μM | 166 |
| g-C3N4@CdS QDs | Hg2+ | 12 nM | 20 nM∼550 nM | 167 |
| g-C3N4/CB | Cd2+ | 2.1 nM | 0∼700 nM | 168 |
| Pb2+ | 0.26 nM | 0∼300 nM | ||
| Hg2+ | 0.22 nM | 0∼500 nM | ||
| ND-g-CN | Ciprofloxacin | 20 ng L−1 | 60 ng L−1∼19.1 μg L−1 | 169 |
| CNNS-Cu | p-Nitrotoluene | 0.13 μM | 0.1 μM∼100 μM | 170 |
| Au/PCN-S | Oxytetracycline | 0.34 nM | 0.5 nM∼200 nM | 162 |
| Bi/BiVO4/g-C3N4 | Oxytetracycline | 0.0033 nM | 0.01 nM∼1000 nM | 171 |
| Bi/CV-PCN | Enrofloxacin | 3.3 fg mL−1 | 0.01 pg mL−1∼1 μg mL−1 | 172 |
| Au-doped 3D CN | Chloramphenicol | 0.1 pM | 0.5 pM∼300 nM | 173 |
| α-Fe2O3/d-C3N4 | Penbritin | 0.0125 pM | 0.5 pM∼50 nM | 62 |
| ZnPc/CN | Sulfadimethoxine | 0.03 nM | 0.1 nM∼300 nM | 174 |
| BiFeO3/utg-C3N4 | Ampicillin | 0.33 pM | 1 pM∼1 μM | 175 |
| ZnIn2S4/g-C3N4 | Bisphenol A | 0.016 μM | 0.05 μM∼30 μM | 156 |
| g-C3N4/BiOI | Bisphenol A | 26 ng mL−1 | 80 ng mL−1∼3.2 μg mL−1 | 176 |
| g-C3N4/CuO | Bisphenol A | 6.2 ng L−1 | 0.02 ng L−1∼10 ng L−1 | 177 |
| NiO-Ni-GCN | Octylphenol | 3.3 nM | 10 nM∼1 μM | 178 |
| g-C3N4/Bi24O31Cl10 | Enrofloxacin | 0.167 fM | 0.5 fM∼100 fM | 179 |
| Bi/CV-PCN | Enrofloxacin | 3.3 fg mL−1 | 0.01 pg mL−1∼1 μg mL−1 | 172 |
| S-BN/Au/CN | Diazinon | 6.8 pM | 0.01 nM∼100 nM | 180 |
| CoN/g-C3N4 | Atrazine | 3.3 × 10−5 fM | 1.0 × 10−4 fM∼10 fM | 181 |
| Ag2CrO4/g-C3N4/GO | Chloramphenicol | 0.29 pM | 0.5 pM∼50 nM | 182 |
| g-C3N4-AuNPs | Triclosan | 0.601 pM | 2 pM∼800 pM | 183 |
| C3N4-rGO | Rutin | 1.78 nM | 5 nM∼140 μM | 184 |
| CoO/Au/g-C3N4 | Microcystin-LR | 0.01 pM | 0.1 pM∼10 nM | 185 |
| BiVO4/2D-C3N4 | Microcystin-LR | 0.042 pg L−1 | 0.5 pg L−1∼10 μg L−1 | 186 |
For the chemical exfoliation process, the morphology and porosity of the porous carbon nitride are determined by the choice of etchant and the reaction conditions.159 Common exfoliation agents include K2Cr2O7 + H2SO4, KMnO4 + H2SO4, and HNO3 + H2SO4.160 However, these chemical agents pose environmental and safety concerns due to their hazardous nature. Thermal oxidation is another method for producing porous carbon nitride. This process disrupts the hydrogen bonds in carbon nitride framework, leading to the formation of porous carbon nitride nanosheets with a large specific surface area and a thin sheet structure.161 However, those above two aforementioned approaches can harm the environment. Further, it requires high-quality bulk g-C3N4, and the process is complicated and time-consuming.
For instance, in a study by Peng and co-workers, phosphorus-doped porous carbon nitride nanosheets (PCN-S) were synthesized using element doping and thermal oxidation method.162 The PCN-S demonstrated an ultrathin nanosheet structure, a large specific surface area, and numerous surface pores (Fig. 7D). Moreover, the Au NPs with the LSPR effect were in situ decorated on the porous surface to yield an Au/PCN-S composite via a photo-reduction method. The composite was used to construct a self-powered PEC apta-sensor for the oxytetracycline (OTC) assay. Under visible light irradiation, electrons were excited to the CB of PCN-S and subsequently transferred to the Au NPs. They then reacted with dissolvable oxygen in the aqueous solution to produce superoxide oxygen species, which react with analyte-OTC (Fig. 7F). Owing to its strong visible light absorption ability and enhanced photogenerated charge pair separation efficiency, the Au/PCN-S composite-based PEC sensor demonstrated excellent performance in terms of a wide linear detection range (0.5∼200 nM), a low detection limit (0.34 nM) (Fig. 7E).
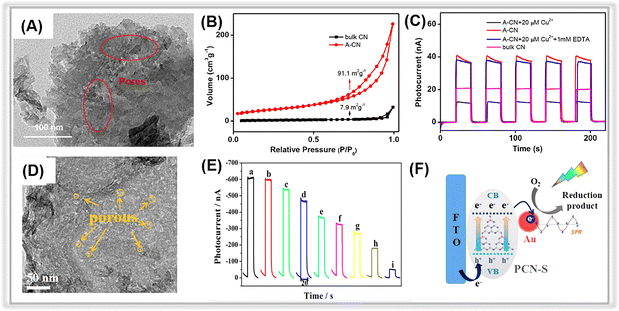 | ||
| Fig. 7 (A) TEM image of A-CN. (B) Nitrogen adsorption–desorption isotherm curves for bulk CN and A-CN. (C) Photocurrent responses of bulk CN and A-CN. Adopted from ref. 163. Copyright 2020, with permission of Elsevier. (D) TEM images of PCN-S. (E) Photocurrent responses of the Au/PCN-S at various oxytetracycline concentrations. (F) The photocurrent generation mechanism of Au/PCN-S under visible light irradiation. Adopted from ref. 162. Copyright 2018, with permission of Elsevier. | ||
Different from traditional methods, alkaline hydrothermal treatment is a new effective approach to achieving porous carbon nitride nanosheets.187,188 In 2020, Liang et al. prepared a porous carbon nitride via an alkaline hydrothermal method.163 The hydroxide effectively exfoliated the 2D framework of the pristine bulk graphitic carbon nitride to obtain a porous structure with a large number of functional groups on its surface. The TEM image of activated g-C3N4 (A-CN) clearly revealed the presence of porous structures on its surface (Fig. 7A). Moreover, the specific surface area (SSA) of A-CN was about 10 times larger than that of bulk g-C3N4 (Fig. 7B), further confirming the existence of porous structures in A-CN. In Fig. 7C, the photocurrent response of A-CN was much higher than that of bulk g-C3N4 due to the porous structure and the large number of functional groups on the surface, which promoted photogenerated charge separation and transfer. Thus, the A-CN-based PEC sensor displayed an outstanding performance for Cu2+ detection with a low detection limit of 0.31 μM in the range from 0.2 μM to 50 μM.
Aiming to overcome the inevitable restacking and agglomeration of the bulk g-C3N4 nanosheets, the fabrication of three-dimensional (3D) porous g-C3N4 is another simple method. It not only prevents the agglomeration of nanosheets but also improves the utilization of irradiation through multiple reflections in its open framework.189,190 For instance, Zhang et al. developed a dual-photoelectrode for detecting chloramphenicol, employing Au NPs doping porous three-dimensional g-C3N4 and porous N-doped Cu2O/C used as the photoanode and photocathode, respectively.173 The small-scale Au NPs were uniformly distributed across the 3D CN nanosheets. The photocurrent of Au-doped 3D CN was much higher than that of bulk g-C3N4, and it also had the smallest electron transfer resistance. The porous 3D g-C3N4 improved both the visible light absorption ability and photoelectric conversion efficiency. Additionally, the presence of Au NPs with the LSPR effect provided extra energy for photogenerated charge carrier generation with the aid of hot electron transfer. As a result, the prepared apta-sensor exhibited an ultra-low detection limit (0.1 pM) and a wide linear detection range (0.5 pM∼300 nM).
2.5. MXene
MXene, a 2D transition metal carbide or nitride, has been widely used in various applications, including electromagnetic interference shielding, energy storage batteries, supercapacitors, and sensors.38,191–194 Porous MXenes have demonstrated outstanding chemical reactivity and hydrophilicity owing to their superior electrical conductivity and significant number of functional groups like –F and –OH, enabling them to form heterojunctions with semiconductors.195 Additionally, exposed connector metal sites, such as titanium on MXenes, present a higher redox reactivity compared to conventional carbon materials, positioning MXene nanolayers as ideal optoelectronic platforms. Recent reports on MXene-based PEC sensors for environmental pollutant detection are summarized in Table 8. These reports illustrate that MXene can be combined with a variety of materials, including graphene oxide, graphitic carbon nitride, metal oxide, metal halide, and metal sulfide, to construct composite hybrid photoelectrochemical detection platforms.| Working electrode | Analyte | LOD | Linear concentration range | Ref. |
|---|---|---|---|---|
| a TiO2(001): active surface (001 facet) TiO2; AgI/Ti3C2 MXene/GO: AgI/3D porous Ti3C2 MXene/graphene aerogel. | ||||
| Ti3C2 MXene/SnS2 | Cr(VI) | 0.51 pM | 1.0 pM∼0.1 mM | 199 |
| Ti3C2 MXene/BiVO4 | Hg2+ | 1 pM | 1 pM∼2 nM | 200 |
| CdS/Ti3C2 MXene | Cu2+ | 0.05 nM | 0.1 nM∼10 μM | 201 |
| BiOBixI1−x/Ti3C2 MXene | Hg2+ | 42.1 pM | 0.1 nM∼1000 nM | 202 |
| BiOI/Ti3C2 MXene | L-cysteine | 0.005 nM | 0.01 nM∼10 μM | 203 |
| TiO2(001)/Ti3C2 MXene | Dopamine | 0.52 μM | 1.0 μM∼1000 μM | 204 |
| BiVO4/Ti3C2 MXene | Oxytetracycline | 0.03 nM | 0.1 nM∼100 nM | 205 |
| g-C3N4/Ti3C2 MXene | Ciprofloxacin | 0.13 nM | 0.4 nM∼1000 nM | 196 |
| Ti3C2 MXene/Bi4VO8Br/TiO2 | Ciprofloxacin | 0.3 nM | 1 nM∼1500 nM | 206 |
| AgBr/Ti3C2 MXene | Chlorpyrifos | 0.33 pg L−1 | 0.001 pg L−1∼1 ng L−1 | 207 |
| Bi4VO8Br/Ti3C2 MXene | Streptomycin | 0.3 nM | 1 nM∼1000 nM | 208 |
| Au@PtAg/TiO2-Ti3C2 MXene | Ochratoxin A | 1.73 fg mL−1 | 5 fg mL−1∼10 ng mL−1 | 209 |
| Anode: TiO2/S-Ti3C2 MXene | Microcystin-RR | 0.034 fM | 0.1 fM∼1 nM | 210 |
| Cathode: MoS2/S-Ti3C2 MXene | Aflatoxin B1 | 0.73 pg mL−1 | 0.01 pg mL−1∼1 μg mL−1 | 211 |
| MoS2-Ti3C2Tx MXene | ||||
| AgI/Ti3C2 MXene/GO | Sulfide | 1.54 nM | 5 nM∼200 μM | 198 |
Graphene oxide and graphitic carbon nitride can be effectively coupled with the porous MXenes due to the strong π–π interaction between them.196,197 For instance, in a study by Yuan and co-workers, the g-C3N4/Ti3C2 MXene composite was synthesized and proposed as a PEC sensing material for ciprofloxacin detection.196 In Fig. 8A, porous Ti3C2 MXene was observed on the surface of g-C3N4, suggesting a close connection between the two components facilitated by electrostatic self-assembly. Transient photocurrent measurements (Fig. 8B) revealed a photocurrent density of 1.36 μA cm−2 for the g-C3N4/Ti3C2 MXene, nearly twice that of g-C3N4. Meanwhile, the EIS of g-C3N4/Ti3C2 MXene has a smaller arc size compared to that of pure g-C3N4. These findings suggested that the introduction of Ti3C2 MXene promotes photogenerated charge transfer. The fabricated PEC sensor demonstrated an ultra-low detection limit of 0.13 nM over a broad linear range from 0.4 nM to 1000 nM.
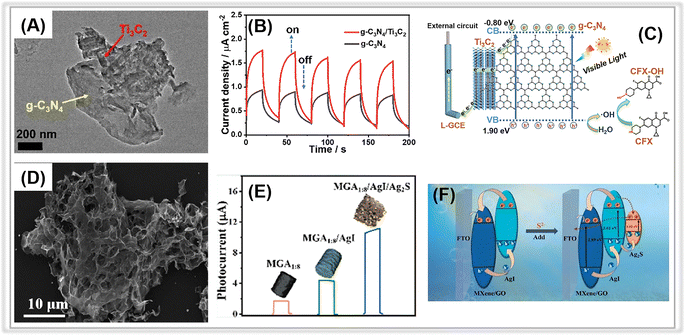 | ||
| Fig. 8 (A) TEM image of the g-C3N4/Ti3C2 MXene composite. (B) The transient photocurrents of g-C3N4 and g-C3N4/Ti3C2 MXene composite. (C) The proposed PEC mechanism at the g-C3N4/Ti3C2 MXene composite. Adopted from ref. 196. Copyright 2021, with permission of Elsevier. (D) SEM image of AgI/Ti3C2 MXene/GO composite. (E) The photocurrent responses of Ti3C2 MXene/GO, AgI/Ti3C2 MXene/GO, and Ag2S/AgI/Ti3C2 MXene/GO composites. (F) The possible electron-transfer mechanism of the AgI/Ti3C2 MXene/GO composite. Adopted from ref. 198. Copyright 2023, with permission of Elsevier. | ||
These results imply that the tight interaction between Ti3C2 MXene and g-C3N4 facilitates the smooth transfer of photogenerated electrons from g-C3N4 to Ti3C2 MXene, whose Fermi level (0.58 V vs. NHE) is much more positive than the conduction band energy of g-C3N4 (Fig. 8C).
Similarly, another typical carbon material, graphene oxide, has also been coupled with porous Ti3C2 MXene to construct smart PEC sensing platforms. In 2023, Zhang et al. reported a “signal-on” PEC sensor for sulfide detection based on in situ growth of AgI on a 3D porous Ti3C2 MXene/graphene aerogel (AgI/Ti3C2 MXene/GO).198 The porous Ti3C2 MXene/GO was synthesized through the solvothermal method, followed by in situ decoration of AgI NPs onto the Ti3C2 MXene/GO. Fig. 8D depicts the typical three-dimensional interconnected skeleton structure of aerogel. The porous structure of Ti3C2 MXene/GO aerogel with large specific surface area favored the anchoring of AgI NPs. Upon the addition of detection analyte-sulfide (S2−), Ag2S formed on the surface of AgI/Ti3C2 MXene/GO, resulting in an enhanced photocurrent response due to the newly created Ag2S/AgI heterojunction (Fig. 8F). As a result, the associated PEC sensor revealed an outstanding S2− detection activity, including a wide linear range (5 nM∼200 μM) and an ultra-low detection limit (1.54 nM).
Interestingly, MXene can serve as a co-catalyst to enhance the separation efficiency of photogenerated electron–hole pair.204,205,212,213 Recently, porous MXenes have been combined with various semiconductors (e.g., TiO2, BiVO4, MoS2, and BiOI) to fabricate composite-based PEC platforms for environmental contaminant assays. For example, Ling and co-workers reported a PEC sensing platform based on a BiVO4/Ti3C2 MXene composite for oxytetracycline (OTC) detection.205 Within the composite, the small porous MXene nanosheets acted as a co-catalyst to promote photo-generated charge pair separation. This was confirmed by the enhanced photocurrent responses, weaker PL intensity, and smaller arc resistance values of BiVO4/Ti3C2 MXene. The introduction of MXene created a Schottky barrier between BiVO4 and MXene, effectively suppressing electron reflux. During the PEC detection process, photogenerated electrons in the CB of BiVO4 were transferred to MXene via the Schottky junction and then injected into a conductive substrate (in this case, ITO) to generate photocurrent. Simultaneously, holes in the VB of BiVO4 contributed to the current generation by oxidizing OTC molecules on the composite surface. Thus, the improved photocurrent resulted in a wide detection range (0.1∼100 nM), an ultra-low detection limit (0.03 nM), and excellent sensitivity for the corresponding PEC sensing platform.
Schottky heterojunctions have also been observed in other Ti3C2 MXene-containing composites. For instance, Ye et al. reported the utilization of a flower-like BiOI/2D Ti3C2 MXene (BiOI/Ti3C2) heterostructure composite as a photocathode for L-cysteine (L-Cys) PEC biosensing.203 The Schottky interaction between the two components enhanced charge transfer, resulting in a superior cathodic photocurrent signal. Similarly, a Schottky junction was also found in CdS nanoparticles and Ti3C2 MXene in other studies.201 The CdS/Ti3C2 heterostructure-based PEC sensing platform showed a linear response for Cu2+ ranging from 0.1 nM to 10 μM with a LOD of 0.05 nM, attributed to significantly improved charge carrier transfer at the CdS/Ti3C2 interface. In another report, Du et al. employed a wet-chemical method to fabricate AgBr/Ti3C2 Schottky heterojunction composites for self-powered PEC sensing of chlorpyrifos (CPF).207 The synergistic effect of the Schottky heterojunction between AgBr and Ti3C2 MXene, combined with metal–ligand charge transfer (MLCT), promoted photogenerated charge carrier separation and transfer. The PEC sensor demonstrated excellent performance in CPF monitoring, with a linear detection range of 0.001∼1 ng L−1 and a LOD of 0.33 pg L−1. Recently, porous MBenes, which are respective transition metal borides,214–218 have been applied in the areas of energy storage and electrocatalysis. Until now, there have been few reports on the application of MBenes to photoelectrochemical sensors. However, we believe that MBenes have great potential in the fabrication of PEC detection platforms.
2.6. Perspectives
In summary, over the past decade, metal oxides, MOFs, COFs, graphitic carbon nitride, and MXene have made significant advances in the construction of PEC analytical platforms for environmental contaminants. Due to their porous structure, large specific surface area and abundant active sites, PEC sensors based on photoactive porous material exhibit superior optoelectronic response. (1) Among the photoactive metal oxides, TiO2 nanotubes or nanoarrays with unique porous channels can efficiently transmit photogenerated charge pairs. PEC sensors based on TiO2 nanotubes or nanoarrays can be applied to detect heavy metal ions and organic pollutants, such as Cr(VI) and atrazine. (2) MOFs and COFs both exhibit excellent photoelectrochemical detection performance against organic pollutants. Moreover, derivatives of MOFs have demonstrated robust PEC detection activities against environmental toxic species. (3) Bulk g-C3N4 can be treated with a variety of methods to obtain a porous structure, including chemical exfoliation, thermal oxidation, and alkaline hydrothermal treatment. Meanwhile, the construction of 3D frameworks is another approach to obtain porous structures. A number of research works have demonstrated that porous graphitic carbon nitride-based PEC sensors exhibit outstanding detection activity in terms of ultra-low detection limit and wide linear detection range. (4) Porous MXenes characterized by high redox reactivity, superior electrical conductivity, and rich functional groups, can effectively be coupled with other materials to construct composite hybrid PEC platforms for pollutant determination. Therefore, porous materials have great potential for the fabrication of PEC sensors.3. Applications for determining the environmental pollutants
3.1. Heavy metal ions
Heavy metal ions are highly detrimental environmental contaminants that can enter living organisms through the food chain and cause enzyme inhibition, poor antioxidant metabolism, DNA damage, and depletion of protein sulfhydryl groups.219–221 Copper (Cu), iron (Fe), manganese (Mn), cobalt (Co), and zinc (Zn) are required for biological functions at relatively low concentrations but become toxic in excessive amounts. Low amounts of lead (Pb), mercury (Hg), chromium (Cr), cadmium (Cd), and arsenic (As) are nevertheless dangerous.222,223 The World Health Organization (WHO) has established stringent restrictions regarding the concentration of heavy metal ions in drinking water. In recent years, many efforts have been dedicated to developing porous material-based PEC sensors for monitoring heavy metal ions.46,167,224,225 In this section, we present a selection of relevant works on PEC detection of heavy metal ions.The construction of heterojunctions was another strategy to improve the PEC detection activity for Cr(VI). For instance, in 2021, Cheng et al. devised a quick and highly sensitive PEC sensor for determining Cr(VI) in water samples.166 The photoactive electrode consisted of a p–n BiOI/CN heterojunction composite. The CN nanosheets were effectively coupled to the BiOI with a needle-like petal structure. The photocurrent density of BiOI/CN was significantly higher than that of pure CN and BiOI, suggesting that the introduction of porous CN enhanced the visible light absorption. Moreover, the BiOI/CN processed the smallest charge transfer resistance due to its unique structure, leading to increased active area and electric conductivity of the photoelectrode. Furthermore, the PEC detection performance of the composite-based electrode for Cr(VI) was studied at various Cr(VI) concentrations. It displayed a wide linear detection range of 0.5∼190 μM with a LOD of 0.1 μM. Under visible light irradiation, the electrons in the VB of CN and BiOI were excited to their CB. Due to the Fermi level of the p-type BiOI being close to the VB and that of the n-type CN being close to the CB, electrons in the CB of the CN tended to diffuse into the BiOI, while holes in the VB were transferred from the BiOI to the CN. The electrons in CB of CN could react with Cr(VI) to convert it to harmless Cr(III). As a result, an interfacial electric field was formed between BiOI and CN, facilitating the separation of photogenerated charge pairs.
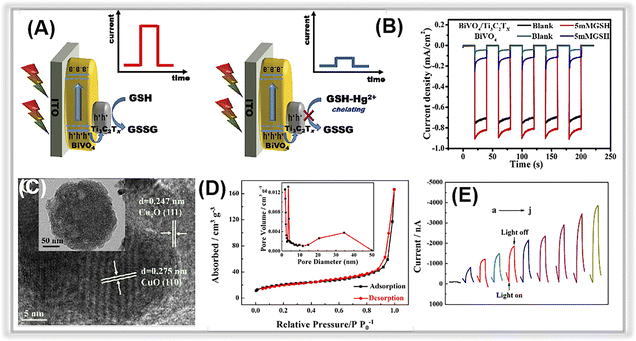 | ||
| Fig. 9 (A) The mechanism of sensing Hg2+ with the photoelectrochemical method. (B) The photocurrent responses of BiVO4 and Ti3C2 MXene/BiVO4. Adopted from ref. 200. Copyright 2020, with permission of Elsevier. (C) TEM and HR-TEM images of Cu2O–CuO–TiO2 composites. (D) Nitrogen adsorption–desorption isotherms of the Cu2O–CuO–TiO2. (E) Photocurrent responses with different Pb2+ concentrations. Adopted from.112 Copyright 2022, with permission of Elsevier. | ||
3.2. Organic pollutants
Organic pollutants found in wastewater encompass a wide range of compounds, including phenolics, antibiotics, pesticides, and toxins. These substances are extremely toxic and pose serious risks to human health. Thus, developing environmentally friendly methods for accurately determining these contaminants has become a focus of worldwide.236,237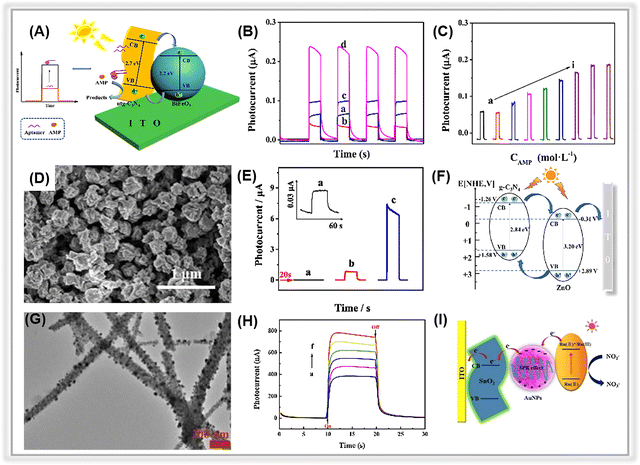 | ||
| Fig. 10 (A) The illustration of the PEC ampicillin apta-sensor based on the BiFeO3/utg-C3N4 composite. (B) The photocurrent signals of (a) utg-C3N4, (b) BiFeO3, (c) BiFeO3/bulk-C3N4, and (d) BiFeO3/utg-C3N4. (C) Photocurrent responses of the as-prepared apta-sensor at various ampicillin concentrations. Adopted from ref. 175. Copyright 2019, with permission of Elsevier. (D) SEM image of the ZnO/g-C3N4 composite. (E) The transient photocurrent responses of (a) g-C3N4, (b) ZnO, and (c) ZnO/g-C3N4. (F) Electron transfer mechanism for ZnO/g-C3N4. Adopted from ref. 117. Copyright 2023, with permission of Elsevier. (G) TEM images of SnO2-Au NPs. (H) The photocurrent responses of SnO2-Au NPs with different concentrations of nitrite. (I) The possible mechanism of PEC sensing nitrite. Adopted from ref. 85. Copyright 2020, with permission of Elsevier. | ||
Further, the porous graphitic carbon nitride can be combined with metal oxides derived from metal–organic frameworks to construct heterojunction composites. In 2023, Jiang et al. synthesized a MOF-derived ZnO nanopolyhedra/g-C3N4 composite via calcination of ZIF-8 and melamine.117 The g-C3N4 nanosheets were covered on the ZnO surface (Fig. 10D). In Fig. 10E, the remarkable photocurrent of the ZnO/g-C3N4 composite-based electrodes compared to pure ZnO was attributed to the presence of heterojunctions that facilitate the separation of photogenerated electron–hole pairs. A plausible electron transfer mechanism was proposed in Fig. 10F. Due to the suitable band energy levels of ZnO and g-C3N4, a type-II heterojunction is formed at the contact interface of the two components. As a result, the corresponding PEC apta-sensor demonstrated an ultra-low LOD (1.49 pM) in a wide linear range spanning from 5 pM to 200 nM. Furthermore, in addition to aptamer-based PEC sensors, recognition element-free sensors have been employed for the detection of antibiotics. For instance, Yan et al. presented a non-recognition element PEC sensor using a porous nitrogen-deficient graphitic carbon nitride nanosheet (ND-g-CN) for ciprofloxacin (CIP) detection.169 Notably, the presence of nitrogen vacancies serves as traps, effectively suppressing charge recombination, while the porous sheet structure promotes the separation and transfer of photogenerated charge carriers. Thus, the PEC sensor realized an ultra-sensitive determination of CIP (LOD: 20 ng L−1).
Different from the enzyme-based biosensor, a self-powered PEC apta-sensor was constructed for diazinon (DZN) detection based on porous graphitic carbon nitride (CN).180 In this PEC apta-sensor, sulfur-doped h-BN (S-BN) was combined with the porous graphitic carbon nitride (CN), and Au nanoparticles with localized surface plasmon resonance (LSPR) effect acted as bridges to create a Z-scheme heterojunction, thereby enhancing the separation efficiency of photogenerated charge pairs. The proposed PEC sensor exhibited the benefits of a low detection limit (6.8 pM), a broad linear detection range (0.01∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nM), and excellent selectivity for DZN detection.
000 nM), and excellent selectivity for DZN detection.
3.3. Non-metal ion pollutants
Apart from heavy metal ions and organic pollutants, there is growing concern regarding non-metal ion pollutants, including nitrites, sulfides, and cyanides.251 Recently, several works have focused on the PEC detection of these non-metal ion pollutants in the environment.252,253 In 2017, Su et al. described a novel PEC sensor for sulfide detection using Cu2O-decorated TiO2 nanotubes.84 The p–n heterojunction in the porous Cu2O–TiO2 heterostructure effectively suppressed the recombination of photogenerated charge pairs, leading to enhanced photocurrent response. The detection of sulfide by this sensor displayed a wide linear range from 1 μM to 300 μM, and the LOD was 0.6 μM. Furthermore, it has been successfully employed for accurate sulfide monitoring in tap and lake water, achieving outstanding recoveries ranging from 99.2% to 103%.In addition to the detection of toxic sulfides in water bodies, the PEC sensing platform has also been utilized for tracing nitrides. For instance, Luo et al. constructed a porous three-dimensional (3D) network of SnO2 nanofibers on an ITO substrate using an electrospinning technique, followed by the electrodeposition of gold nanoparticles (Au NPs).85 The presence of porous SnO2 nanofibers with a 3D network structure was beneficial for the photogenerated charge pair separation and transfer (as shown in Fig. 10G). Moreover, Au NPs with the LSPR effect suppressed recombination of electron–hole pairs. By utilizing the photosensitizer-Ru(bpy)32−, the photocurrent response was further increased. As illustrated in Fig. 10I, under the 473 nm light irradiation, the SnO2 could not be excited, whereas Au NPs and Ru(bpy)32− were both excited. Ru2+ was excited into unstable Ru2+*, and the electrons were transferred to the Au or SnO2 nanofibers. These electrons were then transferred into the conduction band of SnO2 with the aid of Au NPs in the surface plasma state. The Ru2+ ions were converted to Ru3+ ions, which then reacted with NO2− to form NO3− and Ru2+ ions. As a result, a linear relationship between photocurrent and NO2− concentration was established. Under optimized test conditions, the fabricated sensor demonstrated a wide linear range from 1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nM with a LOD of 0.48 nM (Fig. 10H).
000 nM with a LOD of 0.48 nM (Fig. 10H).
4. Conclusion and outlook
Photoelectrochemical detection has emerged as a promising analytical method for tracing environmental pollutants due to its distinct advantages, including cost-effectiveness, rapid response, minimal background noise, and high sensitivity. Crucially, photoactive materials, which significantly influence the monitoring activity of PEC sensors, are key components of these sensing systems. In particular, porous materials have garnered considerable interest in the design and fabrication of photoelectrochemical sensing platforms due to their unique properties, such as a high surface area, tunable pore sizes, and an abundance of functional groups. In this review, we summarize recent advances in photoactive porous material-based photoelectrochemical sensors and their applications in monitoring environmental contaminants in water bodies based on a substantial body of research. We introduce and categorize typical porous materials into five groups: metal oxides, metal–organic frameworks, covalent organic frameworks, graphitic carbon nitride, and MXene. Additionally, we separately discuss their applications in detecting heavy metal ions, organic pollutants, and non-metal ion pollutants.Most importantly, the structural effects of porous materials on the photoelectrochemical detection performance (sensitivity, detection limits, selectivity, and stability) of associated sensors have been discussed in detail in several representative works. Based on the comparison of numerous references, the structural effects of porous materials on PEC detection activity can be summarized into the following critical points: (1) enhancement of the light absorption ability through the multiple reflection of light in pores, improving the light harvesting capability; (2) acceleration of the photoinduced electron–hole pair separation and transfer through the unique porous structures with considerable small pores, shortening the separation and migration route and suppressing charge pair recombination; (3) improvement of the surface charge transfer rate, porous materials with the high surface area provide abundant active sites for chemical reactions and facilitate the detection of species capture. From the aforementioned advantages of porous materials for the construction of photoelectrochemical sensing platforms, we strongly confirm the remarkable potential of porous materials for environmental pollutant detection.
Despite excellent advances and rapid progress in the field of photoelectrochemical sensors based on porous materials for environmental toxic species assays, some fundamental issues and emerging challenges remain:
(1) Even though much effort has been devoted to the application of porous materials in PEC sensors for environmental hazard detection, only a small portion of the vast family of porous materials has been used to fabricate PEC sensing platforms. Meanwhile, some porous materials are only preliminary to PEC sensing applications for highly toxic species, such as photoactive COFs. Therefore, it still has huge potential for exploitation. Furthermore, relatively few porous materials have been used for heavy metal ion assays, only metal oxides and graphitic carbon nitride. Some toxic heavy metal ions, such as arsenic, have not been detected by porous material-based PEC sensors.
(2) Most studies of porous materials focus on the energy level structure and neglect the unique porous structures, e.g., pore diameter and surface area. In addition, the effect of the porous structure on the photoinduced charge transfer and separation efficiency needs to be explored more. More importantly, although the photoelectric coordination of the detection mechanism based on the porous materials has been explained in several works, they only prefer to discuss the photocurrent signal generation mechanism in terms of the classical semiconductor theory and the consideration of the structural effects on the detection mechanism are somewhat insufficient. More attention should be paid to it for future studies, as porous structures favor trapping of probe species and an abundance of functional groups provides more active sites for the occurrence of relevant redox reactions. Moreover, the stability of PEC sensors based on porous materials requires more attention due to the fragile nature of porous structures and multiple assembly processes.
(3) The application of porous materials to PEC sensing is primarily at the laboratory stage. For practical applications, it still suffers from several shortcomings, such as relatively low reliability and reproducibility. Moreover, real samples may contain multiple environmental hazards, thus requiring multiplexing sensing capabilities for PEC sensors based on porous materials. In the field of environmental sample detection, integrating PEC detection methods with flexible electrodes, such as conductive polymers and carbon paper, is highly recommended. Flexible electrodes support the miniaturization and development of wearable and smart devices. PEC detection strategies should be combined with intelligent technologies to enable real-time and in situ detection, while still emphasizing the stability, convenience, and operability of integrated detection devices. Additionally, there are currently few commercial PEC sensing devices on the market, which hinders the development of PEC sensing methods. Therefore, future research should focus more on the fabrication of PEC-related equipment. With the help of smart technologies and the potential for commercial benefits, the current limitations of PEC sensing can be resolved.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Shiben Liu, conceptualization, paper writing; Jinhua Zhan, analysis of article structure and error proofreading; Bin Cai, collecting data and providing financial support. Shiben Liu's writing, first draft preparation. Jinhua Zhan and Bin Cai's writing, review and proofreading.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This project was supported by the National Natural Science Foundation of China (52201262), Guangdong Basic and Applied Basic Research Foundation (2021A1515110920), Natural Science Foundation of Shandong Province (ZR2022QE001), Taishan Scholars Program of Shandong Province (tsqn202211042), and Shandong Postdoctoral Science Foundation (SDCX-ZG-202303013).References
- H. Xu, Y. Jia, Z. Sun, J. Su, Q. S. Liu, Q. Zhou and G. Jiang, Eco-Environ. Health, 2022, 1, 31–45 CrossRef PubMed.
- B. Wang and Y. Wang, Sci. Total Environ., 2022, 831, 154906 CrossRef CAS PubMed.
- A. Saravanan, P. S. Kumar, R. V. Hemavathy, S. Jeevanantham, P. Harikumar, G. Priyanka and D. R. A. Devakirubai, Sci. Total Environ., 2022, 812, 152456 CrossRef CAS PubMed.
- P. Chowdhary, A. Raj and R. N. Bharagava, Chemosphere, 2018, 194, 229–246 CrossRef CAS PubMed.
- O. Surucu, Mater. Today Chem., 2022, 23, 100639 CrossRef CAS.
- C. Xu, M. He, B. Chen and B. Hu, Talanta, 2022, 245, 123470 CrossRef CAS PubMed.
- J. Borrull, A. Colom, J. Fabregas, F. Borrull and E. Pocurull, J. Chromatogr. A, 2020, 1621, 461090 CrossRef CAS PubMed.
- H. Zheng, J. Hong, X. Luo, S. Li, M. Wang, B. Yang and M. Wang, Microchem. J., 2019, 145, 806–812 CrossRef CAS.
- M. Soylak, M. Alasaad and Ö. Özalp, Microchem. J., 2022, 178, 107329 CrossRef CAS.
- V. Vogiazi, A. de la Cruz, S. Mishra, V. Shanov, W. R. Heineman and D. D. Dionysiou, ACS Sens., 2019, 4, 1151–1173 CrossRef CAS PubMed.
- Z. Li and M. Zhu, Chem. Commun., 2020, 56, 14541–14552 RSC.
- C. Zhu, D. Liu, Y. Li, X. Shen, L. Li, Y. Liu and T. You, Curr. Opin. Electrochem., 2019, 17, 47–55 CrossRef CAS.
- L. Ge, Q. Liu, N. Hao and W. Kun, J. Mater. Chem. B, 2019, 7, 7283–7300 RSC.
- Q. Liu, H. Zhang, H. Jiang, P. Yang, L. Luo, Q. Niu and T. You, Biosens. Bioelectron., 2022, 216, 114634 CrossRef CAS PubMed.
- Y. Zhou, H. Yin, W. W. Zhao and S. Ai, Coord. Chem. Rev., 2020, 424, 213519 CrossRef CAS.
- W. W. Zhao, J. J. Xu and H. Y. Chen, Chem. Soc. Rev., 2015, 44, 729–741 RSC.
- L. Yang, S. Zhang, X. Liu, Y. Tang, Y. Zhou and D. K. Y. Wong, J. Mater. Chem. B, 2020, 8, 7880–7893 RSC.
- Z. Qiu and D. Tang, J. Mater. Chem. B, 2020, 8, 2541–2561 RSC.
- Y. Zang, J. Lei and H. Ju, Biosens. Bioelectron., 2017, 96, 8–16 CrossRef CAS PubMed.
- M. Azriouil, M. Matrouf, F. E. Ettadili, F. Laghrib, A. Farahi, S. Saqrane, M. Bakasse, S. Lahrich and M. A. El Mhammedi, Food Chem. Toxicol., 2022, 168, 113378 CrossRef CAS PubMed.
- C. Huang, L. Zhang, Y. Zhu, Z. Zhang, Y. Liu, C. Liu, S. Ge and J. Yu, Anal. Chem., 2022, 94, 8075–8084 CrossRef CAS PubMed.
- J. Zhao, C. Fu, C. Huang, S. Zhang, F. Wang, Y. Zhang, L. Zhang, S. Ge and J. Yu, Chem. Eng. J., 2021, 406, 126892 CrossRef CAS.
- S. Wang, F. Wang, C. Fu, Y. Sun, J. Zhao, N. Li, Y. Liu, S. Ge and J. Yu, Anal. Chem., 2020, 92, 7604–7611 CrossRef CAS PubMed.
- I. Ibrahim, H. N. Lim, R. Mohd Zawawi, A. Ahmad Tajudin, Y. H. Ng, H. Guo and N. M. Huang, J. Mater. Chem. B, 2018, 6, 4551–4568 RSC.
- H. Wang, B. Zhang, Y. Tang, C. Wang, F. Zhao and B. Zeng, TrAC, Trends Anal. Chem., 2020, 131, 116020 CrossRef CAS.
- W. Ma, D. Han, M. Zhou, H. Sun, L. Wang, X. Dong and L. Niu, Chem. Sci., 2014, 5, 3946–3951 RSC.
- M. Song, H. Sun, J. Yu, Y. Wang, M. Li, M. Liu and G. Zhao, ACS Appl. Mater. Interfaces, 2021, 13, 37212–37222 CrossRef CAS PubMed.
- M. Lv, W. Zhou, H. Tavakoli, C. Bautista, J. Xia, Z. Wang and X. Li, Biosens. Bioelectron., 2021, 176, 112947 CrossRef CAS PubMed.
- B. Peng, L. Tang, G. Zeng, Y. Zhou, Y. Zhang, B. Long, S. Fang, S. Chen and J. Yu, Curr. Anal. Chem., 2018, 14, 4–12 CrossRef CAS.
- W. W. Zhao, J. J. Xu and H. Y. Chen, TrAC, Trends Anal. Chem., 2016, 82, 307–315 CrossRef CAS.
- Y. Zheng, Y. Zhou, X. Cui, H. Yin and S. Ai, Mater. Today Chem., 2022, 24, 100878 CrossRef CAS.
- J. H. Zhu, M. Wang, L. H. Tu, A. J. Wang, X. Luo, L. P. Mei, T. Zhao and J. J. Feng, Sens. Actuators, B, 2021, 347, 130553 CrossRef CAS.
- J. Shu and D. Tang, Anal. Chem., 2020, 92, 363–377 CrossRef CAS PubMed.
- Z. Qiu, J. Shu, J. Liu and D. Tang, Anal. Chem., 2019, 91, 1260–1268 CrossRef CAS PubMed.
- K. Zhang, S. Lv and D. Tang, Analyst, 2019, 144, 5389–5393 RSC.
- J. Shu, Z. Qiu, Q. Zhou, Y. Lin, M. Lu and D. Tang, Anal. Chem., 2016, 88, 2958–2966 CrossRef CAS PubMed.
- Y. Zhou, H. Yin and S. Ai, Coord. Chem. Rev., 2021, 447, 214156 CrossRef CAS.
- A. Y. S. Tan, H. T. A. Awan, F. Cheng, M. Zhang, M. T. T. Tan, S. Manickam, M. Khalid and K. Muthoosamy, Chem. Eng. J., 2024, 482, 148774 CrossRef CAS.
- X. Ma, J. Kang, Y. Wu, C. Pang, S. Li, J. Li, Y. Xiong, J. Luo, M. Wang and Z. Xu, TrAC, Trends Anal. Chem., 2022, 157, 116793 CrossRef CAS.
- R. Yadav, T. Baskaran, A. Kaiprathu, M. Ahmed, S. V. Bhosale, S. Joseph, A. H. Al-Muhtaseb, G. Singh, A. Sakthivel and A. Vinu, Chem.–Asian J., 2020, 15, 2588–2621 CrossRef CAS PubMed.
- B. R. Thompson, T. S. Horozov, S. D. Stoyanov and V. N. Paunov, J. Mater. Chem. A, 2019, 7, 8030–8049 RSC.
- Z. Li, Y. Li, S. Chen, Q. Zha and M. Zhu, Chem. Eng. J., 2023, 460, 141657 CrossRef CAS.
- J. Du, Y. Fan, X. Gan, X. Dang and H. Zhao, Electrochim. Acta, 2020, 330, 135336 CrossRef CAS.
- H. Xiong, Y. Dong, D. Liu, R. Long, T. Kong and Y. Xiong, J. Phys. Chem. Lett., 2022, 13, 1272–1282 CrossRef CAS PubMed.
- Y. Chen, L. Li, Q. Xu, W. Chen, Y. Dong, J. Fan and D. Ma, Sol. RRL, 2021, 5, 2000541 CrossRef CAS.
- X. Dong, D. Liu, X. Meng and T. You, Anal. Sci., 2022, 38, 459–481 CrossRef CAS PubMed.
- L. Li, J. Chen, C. Xiao, Y. Luo, N. Zhong, Q. Xie, H. Chang, D. Zhong, Y. Xu, M. Zhao and Q. Liao, Chin. Chem. Lett., 2022, 34, 107904 CrossRef.
- Q. Zhou and D. Tang, TrAC, Trends Anal. Chem., 2020, 124, 115814 CrossRef CAS.
- Y. T. Xu, S. Y. Yu, Y. C. Zhu, G. C. Fan, D. M. Han, P. Qu and W. W. Zhao, TrAC, Trends Anal. Chem., 2019, 114, 81–88 CrossRef CAS.
- B. Wang, J. T. Cao and Y. M. Liu, Analyst, 2020, 145, 1121–1128 RSC.
- K. Yan, P. Karthick Kannan, D. Doonyapisut, K. Wu, C. H. Chung and J. J. A. F. M. Zhang, Adv. Funct. Mater., 2021, 31, 2008227 CrossRef CAS.
- A. Huang, Y. He, Y. Zhou, Y. Zhou, Y. Yang, J. Zhang, L. Luo, Q. Mao, D. Hou and J. Yang, J. Mater. Sci., 2018, 54, 949–973 CrossRef.
- H. Peng, W. Xiong, Z. Yang, J. Tong, M. Jia, Y. Xiang, S. Sun and Z. Xu, Chem. Eng. J., 2023, 457, 141317 CrossRef CAS.
- Y. Wang, M. Zu, X. Zhou, H. Lin, F. Peng and S. Zhang, Chem. Eng. J., 2020, 381, 122605 CrossRef CAS.
- J. Qiao, Y. Wang, Q. Liang, S. Dong, Z. Zeng and S. Shao, ACS Appl. Nano Mater., 2022, 5, 5535–5543 CrossRef CAS.
- X. Sun, C. Gao, L. Zhang, M. Yan, J. Yu and S. Ge, Sens. Actuators, B, 2017, 251, 1–8 CrossRef CAS.
- J. Tian, Y. Li, J. Dong, M. Huang and J. Lu, Biosens. Bioelectron., 2018, 110, 1–7 CrossRef CAS PubMed.
- M. Jia, Z. Yang, H. Xu, P. Song, W. Xiong, J. Cao, Y. Zhang, Y. Xiang, J. Hu, C. Zhou, Y. Yang and W. Wang, Chem. Eng. J., 2020, 388, 124388 CrossRef CAS.
- W. Dong, B. Wang, Y. Xue and G. Jie, Sens. Actuators, B, 2023, 393, 134293 CrossRef CAS.
- B. Zhang, H. Meng, X. Wang, J. Li, H. Chang and W. Wei, Sens. Actuators, B, 2018, 255, 2531–2537 CrossRef CAS.
- B. Zhang, H. Wang, H. Ye, B. Xu, F. Zhao and B. Zeng, Sens. Actuators, B, 2018, 273, 1435–1441 CrossRef CAS.
- X. Ouyang, C. Feng, L. Tang, X. Zhu, B. Peng, X. Fan, Y. Liao, Z. Zhou and Z. Zhang, Biosens. Bioelectron., 2022, 197, 113734 CrossRef CAS PubMed.
- S. Adhikari, S. Selvaraj and D. H. Kim, Appl. Catal., B, 2019, 244, 11–24 CrossRef CAS.
- Q. Cai, T. Yin, Y. Ye, G. Jie and H. Zhou, Anal. Chem., 2022, 94, 5814–5822 CrossRef CAS PubMed.
- N. Zhang, Y. Li, G. Zhao, J. Feng, Y. Li, Y. Wang, D. Zhang and Q. Wei, Talanta, 2023, 253, 124048 CrossRef CAS.
- F. Tavella, C. Ampelli, S. G. Leonardi and G. Neri, Sensors, 2018, 18, 3566 CrossRef PubMed.
- L. Fan, G. Liang, C. Zhang, L. Fan, W. Yan, Y. Guo, S. Shuang, Y. Bi, F. Li and C. Dong, J. Hazard. Mater., 2021, 409, 124894 CrossRef CAS PubMed.
- J. Wu, P. Ou, Y. Lin, X. Tan, F. Wei, Y. Mi, S. Liu and K. Huang, J. Electroanal. Chem., 2022, 911, 116220 CrossRef CAS.
- H. Wu, Z. Zheng, Y. Tang, N. M. Huang, R. Amal, H. N. Lim and Y. H. Ng, Sustainable Mater. Technol., 2018, 18, e00075 CrossRef CAS.
- S. Velmurugan, C. K. Y. T, J. Ching Juan and J. N. Chen, J. Colloid Interface Sci., 2021, 596, 108–118 CrossRef CAS PubMed.
- J. Jana, T. S. K. Sharma, J. S. Chung, W. M. Choi and S. H. Hur, J. Alloys Compd., 2023, 946, 169395 CrossRef CAS.
- Y. Liang, B. Kong, A. Zhu, Z. Wang and Y. Tian, Chem. Commun., 2012, 48, 245–247 RSC.
- J. Tang, J. Li, Y. Zhang, B. Kong, Yiliguma, Y. Wang, Y. Quan, H. Cheng, A. M. Al-Enizi, X. Gong and G. Zheng, Anal. Chem., 2015, 87, 6703–6708 CrossRef CAS PubMed.
- R. Wang, X. Pang, H. Zhang, P. Gao, B. Du, H. Ma and Q. Wei, Anal. Methods, 2015, 7, 5406–5411 RSC.
- K. Wang, N. Sun, X. Li, R. Zhang, G. Zu, J. Wang and R. Pei, Anal. Methods, 2015, 7, 9340–9346 RSC.
- T. Tran.T., J. Li, H. Feng, J. Cai, L. Yuan, N. Wang and Q. Cai, Sens. Actuators, B, 2014, 190, 745–751 CrossRef CAS.
- Y. Qin, Y. Wu, G. Chen, L. Jiao, L. Hu, W. Gu and C. Zhu, Anal. Chim. Acta, 2020, 1130, 100–106 CrossRef CAS PubMed.
- L. Wang, H. Zhang, H. Shi, B. Jin, X. Qin, G. Wang, K. Li, T. Zhang and H. Zhang, Anal. Methods, 2021, 13, 2857–2864 RSC.
- B. Zhang, L. Lu, F. Huang and Z. Lin, Anal. Chim. Acta, 2015, 887, 59–66 CrossRef CAS PubMed.
- F. Han, Z. Song, M. H. Nawaz, M. Dai, D. Han, L. Han, Y. Fan, J. Xu, D. Han and L. Niu, Anal. Chem., 2019, 91, 10657–10662 CrossRef CAS PubMed.
- X. Qin, Q. Wang, L. Geng, X. Shu and Y. Wang, Talanta, 2019, 197, 28–35 CrossRef CAS PubMed.
- C. Wang, H. Wang, M. Zhang, B. Zeng and F. Zhao, Sens. Actuators, B, 2021, 339, 129900 CrossRef CAS.
- M. Liu, J. Yu, X. Ding and G. Zhao, Electroanalysis, 2016, 28, 161–168 CrossRef CAS.
- Y. Su, S. Yang, W. Liu, L. Qiao, J. Yan, Y. Liu, S. Zhang and Y. Fang, Microchim. Acta, 2017, 184, 4065–4072 CrossRef CAS.
- J. Luo, Y. Jiang, X. Guo, Y. Ying, Y. Wen, P. Lin, Y. Sun, H. Yang and Y. Wu, Sens. Actuators, B, 2020, 309 Search PubMed.
- S. Hu, Y. Wei, J. Wang and Y. Yu, Anal. Chim. Acta, 2021, 1178, 338793 CrossRef CAS PubMed.
- Y. Shi, Y. Zou, M. S. Khan, M. Zhang, J. Yan, X. Zheng, W. Wang and Z. Xie, J. Mater. Chem. C, 2023, 11, 3692–3709 RSC.
- P. Samanta, A. V. Desai, S. Let and S. K. Ghosh, ACS Sustain. Chem. Eng., 2019, 7, 7456–7478 CrossRef CAS.
- Y. Hua, D. Kukkar, R. J. C. Brown and K. H. Kim, Crit. Rev. Environ. Sci. Technol., 2022, 53, 258–289 CrossRef.
- R. Gupta, F. Rahi Alhachami, I. Khalid, H. S. Majdi, N. Nisar, Y. Mohamed Hasan, R. Sivaraman, R. M. Romero Parra, Z. I. Al Mashhadani and Y. Fakri Mustafa, Crit. Rev. Anal. Chem., 2022, 1–22 CrossRef PubMed.
- H. Du, T. Yin, J. Wang and G. Jie, Anal. Chem., 2023, 95, 7053–7061 CrossRef CAS PubMed.
- X. Zhang, T. Yan, T. Wu, Y. Feng, M. Sun, L. Yan, B. Du and Q. Wei, Biosens. Bioelectron., 2019, 135, 88–94 CrossRef CAS PubMed.
- H. Dai, X. Yuan, L. Jiang, H. Wang, J. Zhang, J. Zhang and T. Xiong, Coord. Chem. Rev., 2021, 441, 213985 CrossRef CAS.
- Z. Mo, D. Tai, H. Zhang and A. Shahab, Chem. Eng. J., 2022, 443, 136320 CrossRef CAS.
- F. Z. Chen, Y. Gao, Y.-J. Li, W. Li, X. Y. Wu, D. M. Han and W. W. Zhao, Sens. Actuators, B, 2022, 361, 136651 Search PubMed.
- L. Hanna, C. L. Long, X. Zhang and J. V. Lockard, Chem. Commun., 2020, 56, 11597–11600 RSC.
- Y. Yang, W. Yan, X. Wang, L. Yu, J. Zhang, B. Bai, C. Guo and S. Fan, Biosens. Bioelectron., 2021, 177, 113000 CrossRef CAS PubMed.
- R. Feng, X. Zhang, X. Xue, Y. Xu, H. Ding, T. Yan, L. Yan and Q. Wei, ACS Appl. Bio Mater., 2021, 4, 7186–7194 CrossRef CAS PubMed.
- Y. Wu, K. Lu, F. Pei, Y. Yan, S. Feng, Q. Hao, M. Xia and W. Lei, Talanta, 2022, 248, 123617 CrossRef CAS PubMed.
- Y. Cao, L. Wang, C. Wang, X. Hu, Y. Liu and G. Wang, Electrochim. Acta, 2019, 317, 341–347 CrossRef CAS.
- W. Dong, Z. Li, W. Wen, S. Feng, Y. Zhang and G. Wen, RSC Adv., 2021, 11, 28320–28325 RSC.
- W. Dong, Z. Li, W. Wen, B. Liu and G. Wen, ACS Appl. Mater. Interfaces, 2021, 13, 57497–57504 CrossRef CAS PubMed.
- L. Zhang, L. Feng, P. Li, X. Chen, J. Jiang, S. Zhang, C. Zhang, A. Zhang, G. Chen and H. Wang, Chem. Eng. J., 2020, 395, 125702 Search PubMed.
- J. Liu, X. Yu, Y. Xu, Y. Zhao and D. Li, Inorg. Chem., 2021, 60, 19044–19052 CrossRef CAS PubMed.
- Y. Wang, D. Jin, X. Zhang, J. Zhao and X. Hun, Mikrochim. Acta, 2022, 189, 193 CrossRef CAS PubMed.
- J. Xiong, W. Tan, J. Cai, K. Lu and X. Mo, Int. J. Electrochem. Sci., 2022, 17, 22048 CrossRef CAS.
- P. W. Gao, Y. Z. Shen, C. Ma, Q. Xu and X. Y. Hu, Analyst, 2021, 146, 6178–6186 RSC.
- M. Peng, G. Guan, H. Deng, B. Han, C. Tian, J. Zhuang, Y. Xu, W. Liu and Z. Lin, Environ. Sci.: Nano, 2019, 6, 207–215 RSC.
- J. Kang, X. Ma, Y. Wu, C. Pang, S. Li, J. Li, Y. Xiong, J. Luo, M. Wang and Z. Xu, Mikrochim. Acta, 2022, 189, 383 CrossRef CAS PubMed.
- J. Gao, Y. Chen, W. Ji, Z. Gao and J. Zhang, Analyst, 2019, 144, 6617–6624 RSC.
- W. Li, L. Xu, X. Zhang, Z. Ding, X. Xu, X. Cai, Y. Wang, C. Li and D. Sun, Sens. Actuators, B, 2023, 378, 133153 CrossRef CAS.
- Q. Yu, Y. Fu, K. Xiao, X. Zhang, C. Du and J. Chen, Anal. Chim. Acta, 2022, 1195, 339456 CrossRef CAS PubMed.
- D. Zheng, M. Chen, Y. Chen and W. Gao, Anal. Chim. Acta, 2022, 1212, 339913 CrossRef CAS PubMed.
- Z. Zhang, L. Huang, S. Sheng, C. Jiang and Y. Wang, Sens. Actuators, B, 2021, 343 Search PubMed.
- Y. Cao, L. Wang, C. Wang, D. Su, Y. Liu and X. Hu, Mikrochim. Acta, 2019, 186, 481 CrossRef PubMed.
- S. Velmurugan, T. C. K. Yang, S. W. Chen and J.-N. Chen, J. Environ. Chem. Eng., 2021, 9, 106169 CrossRef CAS.
- H. Jiang, Q. Liu, H. Zhang, P. Yang and T. You, J. Colloid Interface Sci., 2023, 632, 35–43 CrossRef CAS PubMed.
- J. Peng, J. Yang, B. Chen, S. Zeng, D. Zheng, Y. Chen and W. Gao, Biosens. Bioelectron., 2021, 175, 112873 CrossRef CAS PubMed.
- X. Zhang, J. Peng, Y. Ding, D. Zheng, Y. Lin, Y. Chen and W. Gao, Sens. Actuators, B, 2020, 306, 127552 CrossRef CAS.
- Y. Feng, T. Yan, T. Wu, N. Zhang, Q. Yang, M. Sun, L. Yan, B. Du and Q. Wei, Sens. Actuators, B, 2019, 298, 126817 CrossRef CAS.
- T. Yan, X. Zhang, X. Ren, Y. Lu, J. Li, M. Sun, L. Yan, Q. Wei and H. Ju, Sens. Actuators, B, 2020, 320, 128387 CrossRef CAS.
- T. Yan, H. Ding, R. Feng, R. Yuan, Y. Zhao, M. Sun, L. Yan and Q. Wei, ACS Appl. Mater. Interfaces, 2022, 14, 25308–25316 CrossRef CAS PubMed.
- J. Tan, B. Peng, L. Tang, G. Zeng, Y. Lu, J. Wang, X. Ouyang, X. Zhu, Y. Chen and H. Feng, Anal. Chem., 2020, 92, 13073–13083 CrossRef CAS PubMed.
- C. Zhao, L. Zhang, Q. Wang, L. Zhang, P. Zhu, J. Yu and Y. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 20397–20404 CrossRef CAS PubMed.
- C. Wang, N. Liu, X. Liu, Y. Tian, X. Zhai, X. Chen and B. Hou, ACS Appl. Nano Mater., 2021, 4, 8801–8812 CrossRef CAS.
- S. Zhang, K. Chen, L. Zhu, M. Xu, Y. Song, Z. Zhang and M. Du, Dalton Trans., 2021, 50, 14285–14295 RSC.
- Y. Yang, H. Wei, X. Wang, D. Sun, L. Yu, B. Bai, X. Jing, S. Qin and H. Qian, Biosens. Bioelectron., 2023, 223, 115017 CrossRef CAS PubMed.
- X. Liu, D. Huang, C. Lai, G. Zeng, L. Qin, H. Wang, H. Yi, B. Li, S. Liu, M. Zhang, R. Deng, Y. Fu, L. Li, W. Xue and S. Chen, Chem. Soc. Rev., 2019, 48, 5266–5302 RSC.
- J. Liu, J. Wang, Y. Wang and Y. Wang, J. Polym. Sci., 2022, 1–24 Search PubMed.
- S. Kandambeth, K. Dey and R. Banerjee, J. Am. Chem. Soc., 2019, 141, 1807–1822 CrossRef CAS PubMed.
- K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Chem. Rev., 2020, 120, 8814–8933 CrossRef CAS PubMed.
- X. Yan, H. Li, T. Yin, G. Jie and H. Zhou, Biosens. Bioelectron., 2022, 217, 114694 CrossRef CAS PubMed.
- T. Liu, L. Cui, H. Zhao and X. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 47090–47098 CrossRef CAS PubMed.
- K. Xiao, R. Zhu, C. Du, X. Zhang and J. Chen, Sens. Actuators, B, 2023, 380, 133403 CrossRef CAS.
- N. Li, C. Wang, L. Chen, C. Ye and Y. Peng, Molecules, 2022, 27, 6732 CrossRef CAS PubMed.
- M. Wang, G. Liang, M. Wang, M. Hu, L. Zhu, Z. Li, Z. Zhang, L. He and M. Du, Chem. Eng. J., 2022, 448, 137779 CrossRef CAS.
- Y. Gao, J. Zhang, X. Zhang, J. Li, R. Zhang and W. Song, Anal. Chem., 2021, 93, 8647–8655 CrossRef CAS PubMed.
- S. Wang, X. Xu, Y. Yue, K. Yu, Q. Shui, N. Huang and H. Chen, Small Struct., 2020, 1, 2000021 CrossRef.
- Z. Xu, X. Cui, Y. Li, Y. Li, Z. Si and Q. Duan, Appl. Surf. Sci., 2023, 613, 155966 CrossRef CAS.
- T. Yan, G. Zhang, K. Yu, H. Chai, M. Tian, L. Qu, H. Dong and X. Zhang, Chem. Eng. J., 2023, 455, 104779 Search PubMed.
- D. Feng, P. Huang, Y. Miao, A. Liang, X. Wang, B. Tang, H. Hou, M. Ren, S. Gao, L. Geng and A. Luo, Sens. Actuators, B, 2022, 368, 132121 CrossRef CAS.
- Y. Liu, L. Zhong, S. Zhang, J. Wang and Z. Liu, Sens. Actuators, B, 2022, 354, 131204 CrossRef CAS.
- B. Dong, X. Zhang, L. Cao, X. Jiang and F. Wang, J. Mater. Chem. C, 2023, 11, 2074–2081 RSC.
- H. Zhao, R. Su, L. Teng, Q. Tian, F. Han, H. Li, Z. Cao, R. Xie, G. Li, X. Liu and Z. Liu, Nanoscale, 2022, 14, 1653–1669 RSC.
- Q. Wang, X. Sun, C. Liu, C. Wang, W. Zhao, Z. Zhu, S. Ma and S. Zhang, Front. Bioeng. Biotechnol., 2023, 11, 1164805 CrossRef PubMed.
- W. Y. Geng, H. Zhang, Y. H. Luo, X. G. Zhu, A. D. Xie, J. Wang and D. E. Zhang, Microporous Mesoporous Mater., 2021, 323, 111186 CrossRef CAS.
- M. Xu, K. Chen, L. Zhu, S. Zhang, M. Wang, L. He, Z. Zhang and M. Du, Langmuir, 2021, 37, 13479–13492 CrossRef CAS PubMed.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- X. Huo, H. Yi, Y. Fu, Z. An, L. Qin, X. Liu, B. Li, S. Liu, L. Li, M. Zhang, F. Xu, G. Zeng and C. Lai, Energy Environ. Sci., 2021, 8, 1835–1862 CAS.
- H. Li, Q. Cai, Y. Xue, G. Jie and H. Zhou, Sens. Actuators, B, 2023, 391, 134062 CrossRef CAS.
- T. S. K. Sharma, A. Ganguly, A. Santhan and K. Y. Hwa, ACS Appl. Nano Mater., 2022, 5, 5208–5222 CrossRef CAS.
- L. Xu, H. Li, P. Yan, J. Xia, J. Qiu, Q. Xu, S. Zhang, H. Li and S. Yuan, J. Colloid Interface Sci., 2016, 483, 241–248 CrossRef CAS PubMed.
- Y. Liu, K. Yan and J. Zhang, ACS Appl. Mater. Interfaces, 2015, 8, 28255–28264 CrossRef PubMed.
- L. Xu, S. Ling, H. Li, P. Yan, J. Xia, J. Qiu, K. Wang, H. Li and S. Yuan, Sens. Actuators, B, 2017, 240, 308–314 CrossRef CAS.
- S. S. Low, Z. Chen, Y. Li, Y. Lu and Q. Liu, TrAC, Trends Anal. Chem., 2021, 145, 116454 CrossRef CAS.
- Q. W. Chen, C. Yuan and C. Y. Zhai, Chin. Chem. Lett., 2022, 33, 983–986 CrossRef CAS.
- F. K. Kessler, Y. Zheng, D. Schwarz, C. Merschjann, W. Schnick, X. Wang and M. J. Bojdys, Nat. Rev. Mater., 2017, 2, 17030 CrossRef CAS.
- Y. Li, X. Li, H. Zhang and Q. Xiang, Nanoscale Horiz., 2020, 5, 765–786 RSC.
- X. Gao, J. Feng, D. Su, Y. Ma, G. Wang, H. Ma and J. Zhang, Nano Energy, 2019, 59, 598–609 CrossRef CAS.
- H. J. Li, B. W. Sun, L. Sui, D. J. Qian and M. Chen, Phys. Chem. Chem. Phys., 2015, 17, 3309–3315 RSC.
- J. Zhang, Y. Chen and X. Wang, Energy Environ. Sci., 2015, 8, 3092–3108 RSC.
- B. Peng, L. Tang, G. Zeng, S. Fang, X. Ouyang, B. Long, Y. Zhou, Y. Deng, Y. Liu and J. Wang, Biosens. Bioelectron., 2018, 121, 19–26 CrossRef CAS PubMed.
- D. Liang, X. Liang, Z. Zhang, H. Wang, N. Zhang, J. Wang and X. Qiu, Microchem. J., 2020, 156, 104922 CrossRef CAS.
- H. Xu, J. Yan, X. She, L. Xu, J. Xia, Y. Xu, Y. Song, L. Huang and H. Li, Nanoscale, 2014, 6, 1406–1415 RSC.
- T. Fang, X. Yang, L. Zhang and J. Gong, J. Hazard. Mater., 2016, 312, 106–113 CrossRef CAS PubMed.
- D. Cheng, H. Wu, C. Feng, Y. Zhang, Y. Ding and H. Mei, J. Alloys Compd., 2021, 882, 160690 CrossRef CAS.
- Z. Li, W. Dong, X. Du, G. Wen and X. Fan, Microchem. J., 2020, 152, 104259 CrossRef CAS.
- J. Hu, Z. Li, C. Zhai, L. Zeng and M. Zhu, Anal. Chim. Acta, 2021, 1183, 338951 CrossRef CAS PubMed.
- P. Yan, J. Dong, Z. Mo, L. Xu, J. Qian, J. Xia, J. Zhang and H. Li, Biosens. Bioelectron., 2020, 148, 111802 CrossRef CAS PubMed.
- L. Chen, Z. Li, Q. Xiao, M. Li, Y. Xu and X. Qiu, Appl. Catal., A, 2023, 649, 118964 CrossRef CAS.
- Y. Xu, Z. Wen, T. Wang, M. Zhang, C. Ding, Y. Guo, D. Jiang and K. Wang, Biosens. Bioelectron., 2020, 166, 112453 CrossRef CAS PubMed.
- Y. Xu, D. Jiang, M. Zhang, Z. Zhang, J. Qian, N. Hao, C. Ding and K. Wang, Sens. Actuators, B, 2020, 316, 128142 CrossRef CAS.
- Z. Zhang, B. Peng, X. Ouyang, X. Zhu, L. Chen, X. Fan, Z. Zhou, J. Wang and L. Tang, Sens. Actuators, B, 2022, 368, 132144 CrossRef CAS.
- Q. Liu, T. Shi, Y. Cheng, Z. Wen, C. Ding, Y. Li and K. Wang, J. Hazard. Mater., 2021, 406, 124749 CrossRef CAS PubMed.
- L. Ge, Y. Xu, L. Ding, F. You, Q. Liu and K. Wang, Biosens. Bioelectron., 2019, 124–125, 33–39 CrossRef CAS PubMed.
- L. Xu, P. Yan, H. Li, S. Ling, J. Xia, Q. Xu, J. Qiu and H. Li, RSC Adv., 2017, 7, 7929–7935 RSC.
- L. Yang, Z. Zhao, J. Hu, H. Wang, J. Dong, X. Wan, Z. Cai and M. Li, Electroanalysis, 2020, 32, 1651–1658 CrossRef CAS.
- W. Gong, J. Zou, S. Zhang, X. Zhou and J. Jiang, Electroanalysis, 2016, 28, 227–234 CrossRef CAS.
- M. Yang, Y. Jia, Y. Chen, P. Yan, L. Xu, J. Qian, F. Chen and H. Li, J. Environ. Chem. Eng., 2022, 10, 107208 CrossRef CAS.
- J. Tan, B. Peng, L. Tang, C. Feng, J. Wang, J. Yu, X. Ouyang and X. Zhu, Biosens. Bioelectron., 2019, 142, 111546 CrossRef CAS PubMed.
- Z. Qi, P. Yan, J. Qian, L. Zhu, H. Li and L. Xu, Sens. Actuators, B, 2023, 387, 133792 CrossRef CAS.
- B. Peng, Y. Lu, J. Luo, Z. Zhang, X. Zhu, L. Tang, L. Wang, Y. Deng, X. Ouyang, J. Tan and J. Wang, J. Hazard. Mater., 2021, 401, 123395 CrossRef CAS PubMed.
- S. Feng, X. Wei, L. Zhong and J. Li, Electroanalysis, 2018, 30, 320–327 CrossRef CAS.
- J. Wang, B. Yang, S. Li, B. Yan, H. Xu, K. Zhang, Y. Shi, C. Zhai and Y. Du, J. Colloid Interface Sci., 2017, 506, 329–337 CrossRef CAS PubMed.
- L. Tang, X. Ouyang, B. Peng, G. Zeng, Y. Zhu, J. Yu, C. Feng, S. Fang, X. Zhu and J. Tan, Nanoscale, 2019, 11, 12198–12209 RSC.
- Y. Li, Y. Bu, F. Jiang, X. Dai and J. P. Ao, Biosens. Bioelectron., 2020, 150, 111903 CrossRef CAS PubMed.
- H. Nie, M. Ou, Q. Zhong, S. Zhang and L. Yu, J. Hazard. Mater., 2015, 300, 598–606 CrossRef CAS PubMed.
- H. Yu, R. Shi, Y. Zhao, T. Bian, Y. Zhao, C. Zhou, G. I. N. Waterhouse, L. Z. Wu, C. H. Tung and T. Zhang, Adv. Mater., 2017, 29, 1605148 CrossRef PubMed.
- R. Cao, H. Yang, S. Zhang and X. Xu, Appl. Catal. B: Environ., 2019, 258, 117997 CrossRef CAS.
- X. Chen, R. Shi, Q. Chen, Z. Zhang, W. Jiang, Y. Zhu and T. Zhang, Nano Energy, 2019, 59, 644–650 CrossRef CAS.
- Z. Chen, M. Asif, R. Wang, Y. Li, X. Zeng, W. Yao, Y. Sun and K. Liao, Chem. Rec., 2022, 22, e202100261 CrossRef CAS PubMed.
- X. Wu, P. Ma, Y. Sun, F. Du, D. Song and G. Xu, Electroanalysis, 2021, 33, 1827–1851 CrossRef CAS.
- Y. Pei, X. Zhang, Z. Hui, J. Zhou, X. Huang, G. Sun and W. Huang, ACS Nano, 2021, 15, 3996–4017 CrossRef CAS PubMed.
- D. Jiang, Y. Zhang, X. Du, L. Zhang, X. Shan, W. Wang, H. Shiigi and Z. Chen, Chem. Commun., 2023, 59, 1185–1188 RSC.
- N. H. Solangi, N. M. Mubarak, R. R. Karri, S. A. Mazari and A. S. Jatoi, Environ. Res., 2023, 222, 115279 CrossRef CAS PubMed.
- C. Yuan, Z. He, Q. Chen, X. Wang, C. Zhai and M. Zhu, Appl. Surf. Sci., 2021, 539, 148241 CrossRef CAS.
- Y. Zhou, K. Maleski, B. Anasori, J. O. Thostenson, Y. Pang, Y. Feng, K. Zeng, C. B. Parker, S. Zauscher, Y. Gogotsi, J. T. Glass and C. Cao, ACS Nano, 2020, 14, 3576–3586 CrossRef CAS PubMed.
- Q. Zhang, C. Wang, Y. Tian, Y. Liu, F. You, K. Wang, J. Wei, L. Long and J. Qian, Anal. Chim. Acta, 2023, 1245, 340845 CrossRef CAS PubMed.
- Y. Tian, C. Wang, Q. Zhang, H. Cui, Y. Liu, K. Wang, J. Wei, L. Long and J. Qian, Sens. Actuators, B, 2023, 382, 133496 CrossRef CAS.
- Q. Jiang, H. Wang, X. Wei, Y. Wu, W. Gu, L. Hu and C. Zhu, Anal. Chim. Acta, 2020, 1119, 11–17 CrossRef CAS PubMed.
- C. Ye, F. Xu, F. Ullah and M. Wang, Anal. Bioanal. Chem., 2022, 414, 3571–3580 CrossRef CAS PubMed.
- W. Xiao, W. Xu, W. Huang, Y. Zhou, Z. Jin, X. Wei and J. Li, ACS Appl. Nano Mater., 2022, 5, 18168–18177 CrossRef CAS.
- C. Ye, Z. Wu, K. Ma, Z. Xia, J. Pan, M. Wang and C. Ye, J. Alloys Compd., 2021, 859, 157787 CrossRef CAS.
- Y. Chen, X. Li, G. Cai, M. Li and D. Tang, Electrochem. Commun., 2021, 125, 106897 Search PubMed.
- L. Ling, C. Yuan, Q. Xu, T. Li, M. Zhu and C. Zhai, Surf. Interfaces, 2023, 36, 102483 CrossRef CAS.
- F. You, Z. Wen, R. Yuan, L. Ding, J. Wei, J. Qian, L. Long and K. Wang, J. Electroanal. Chem., 2022, 914, 116285 CrossRef CAS.
- X. Du, W. Du, J. Sun and D. Jiang, Food Chem., 2022, 385, 132731 CrossRef CAS PubMed.
- F. You, J. Wei, Y. Cheng, Z. Wen, C. Ding, N. Hao and K. Wang, J. Hazard. Mater., 2021, 414, 125539 CrossRef CAS PubMed.
- C. Chen, X. Zhou, Z. Wang, J. Han and S. Chen, Anal. Chim. Acta, 2022, 1216, 339943 CrossRef CAS PubMed.
- Y. Zhang, X. Du, M. Wei, X. Shan, W. Wang, D. Jiang, H. Shiigi and Z. Chen, Anal. Chim. Acta, 2023, 1238, 340645 CrossRef CAS PubMed.
- Y. Jin, Y. Luan, Z. Wu, W. Wen, X. Zhang and S. Wang, Anal. Chem., 2021, 93, 13204–13211 CrossRef CAS PubMed.
- Y. Sun, X. Meng, Y. Dall'Agnese, C. Dall'Agnese, S. Duan, Y. Gao, G. Chen and X. F. Wang, Nano-Micro Lett., 2019, 11, 79 CrossRef CAS PubMed.
- M. Tahir, A. Ali Khan, S. Tasleem, R. Mansoor and W. K. Fan, Energy Fuels, 2021, 35, 10374–10404 CrossRef CAS.
- V. G. Nair, M. Birowska, D. Bury, M. Jakubczak, A. Rosenkranz and A. M. Jastrzebska, Adv. Mater., 2022, 34, e2108840 CrossRef PubMed.
- M. Jakubczak, A. Szuplewska, A. Rozmysłowska Wojciechowska, A. Rosenkranz and A. M. Jastrzębska, Adv. Funct. Mater., 2021, 31, 2103048 CrossRef CAS.
- M. Khazaei, J. Wang, M. Estili, A. Ranjbar, S. Suehara, M. Arai, K. Esfarjani and S. Yunoki, Nanoscale, 2019, 11, 11305–11314 RSC.
- B. Zhang, J. Zhou and Z. Sun, J. Mater. Chem. A, 2022, 10, 15865–15880 RSC.
- A. Sharma, V. S. Rangra and A. Thakur, J. Mater. Sci., 2022, 57, 12738–12751 CrossRef CAS.
- C. Venkateswara Raju, C. Hwan Cho, G. Mohana Rani, V. Manju, R. Umapathi, Y. Suk Huh and J. Pil Park, Coord. Chem. Rev., 2023, 476, 214920 CrossRef CAS.
- R. Sharma, N. F. Suhendra, S. H. Jung and H. i. Lee, Chem. Eng. J., 2023, 451, 138506 CrossRef CAS.
- R. Sharma, S. N. Chavan and H. i. Lee, Sens. Actuators, B, 2023, 396, 134625 CrossRef CAS.
- W. W. Zhao, J. J. Xu and H. Y. Chen, Analyst, 2016, 141, 4262–4271 RSC.
- X. Gan, H. Zhao, R. Schirhagl and X. Quan, Mikrochim. Acta, 2018, 185, 478 CrossRef PubMed.
- R. Ahmad, N. Tripathy, A. Khosla, M. Khan, P. Mishra, W. A. Ansari, M. A. Syed and Y. B. Hahn, J. Electrochem. Soc., 2020, 167, 037519 CrossRef CAS.
- M. Xu, X. Wang and X. Liu, J. Agric. Food Chem., 2022, 70, 11468–11480 CrossRef CAS PubMed.
- S. Prasad, K. K. Yadav, S. Kumar, N. Gupta, M. M. S. Cabral Pinto, S. Rezania, N. Radwan and J. Alam, J. Environ. Manage., 2021, 285, 112174 CrossRef CAS PubMed.
- K. Dashtian, M. Ghaedi and S. Hajati, Biosens. Bioelectron., 2019, 132, 105–114 CrossRef CAS PubMed.
- W. L. Cui, Z. H. Zhang, L. Wang, J. Qu and J. Y. Wang, Spectrochim. Acta, Part A, 2022, 267, 120516 CrossRef CAS PubMed.
- Y. Wang, Y. Wang, F. Wang, H. Chi, G. Zhao, Y. Zhang, T. Li and Q. Wei, J. Colloid Interface Sci., 2022, 606, 510–517 CrossRef CAS PubMed.
- J. Wang, Y. Pan, L. Jiang, M. Liu, F. Liu, M. Jia, J. Li and Y. Lai, ACS Appl. Mater. Interfaces, 2019, 11, 37541–37549 CrossRef CAS PubMed.
- K. Wang, J. Yang, X. Yang, Q. Guo and G. Nie, Microchem. J., 2022, 182, 107952 CrossRef CAS.
- EPA, Office of Water, Washington, DC, USA, 2009 Search PubMed.
- Y. Niu, G. Luo, H. Xie, Y. Zhuang, X. Wu, G. Li and W. Sun, Mikrochim. Acta, 2019, 186, 826 CrossRef CAS PubMed.
- L. Meng, M. Liu, K. Xiao, X. Zhang, C. Du and J. Chen, Chem. Commun., 2020, 56, 8261–8264 RSC.
- Y. Wang, G. Zhao, G. Zhang, Y. Zhang, H. Wang, W. Cao, T. Li and Q. Wei, Sens. Actuators, B, 2020, 319, 128313 CrossRef CAS.
- B. M. da Costa Filho, A. C. Duarte and T. A. P. Rocha-Santos, Trends Environ. Anal. Chem., 2022, 33, e00154 CrossRef CAS.
- Z. Li, H. Zhang, Q. Zha, J. Li and M. Zhu, Catalysts, 2023, 13, 91 CrossRef CAS.
- R. Gusain, K. Gupta, P. Joshi and O. P. Khatri, Adv. Colloid Interface Sci., 2019, 272, 102009 CrossRef CAS PubMed.
- C. Liu, B. Li, M. Liu and S. Mao, Sens. Actuators, B, 2022, 369, 132383 CrossRef CAS.
- J. Wang and R. Zhuan, Sci. Total Environ., 2020, 701, 135023 CrossRef CAS PubMed.
- P. Yan, D. Jiang, Y. Tian, L. Xu, J. Qian, H. Li, J. Xia and H. Li, Biosens. Bioelectron., 2018, 111, 74–81 CrossRef CAS PubMed.
- T. S. K. Sharma and K. Y. Hwa, Chem. Eng. J., 2022, 439, 135591 CrossRef CAS.
- X. Liu, P. Liu, Y. Tang, L. Yang, L. Li, Z. Qi, D. Li and D. K. Y. Wong, Biosens. Bioelectron., 2018, 112, 193–201 CrossRef CAS PubMed.
- X. Qin, L. Geng, Q. Wang and Y. Wang, Mikrochim. Acta, 2019, 186, 430 CrossRef PubMed.
- Y. Wang, F. Bian, X. Qin and Q. Wang, Mikrochim. Acta, 2018, 185, 161 CrossRef PubMed.
- Y. Chen, Y. Wang, P. Yan, Q. Ouyang, J. Dong, J. Qian, J. Chen, L. Xu and H. Li, Anal. Chim. Acta, 2020, 1125, 299–307 CrossRef CAS PubMed.
- D. Vaya and P. K. Surolia, Environ. Technol. Innovation, 2020, 20, 101128 CrossRef CAS.
- H. Wang, D. Liang, Y. Xu, X. Liang, X. Qiu and Z. Lin, Environ. Sci.: Nano, 2021, 8, 773–783 RSC.
- Q. Liu, Y. Yin, N. Hao, J. Qian, L. Li, T. You, H. Mao and K. Wang, Sens. Actuators, B, 2018, 260, 1034–1042 CrossRef CAS.
- Z. Lei, P. Lei, J. Guo and Z. Wang, Talanta, 2022, 248, 123607 CrossRef CAS PubMed.
- R. Sharma, N. F. Suhendra and H. i. Lee, Sens. Actuators, B, 2023, 382, 133570 CrossRef CAS.
- Q. Li, J. Yao, Y. Jiang, X. Guo, Y. Ying, Y. Wen, X. Liu, Y. Wu, H. Yang and C. Le, J. Electroanal. Chem., 2022, 917, 116412 CrossRef CAS.
- P. Teng, X. Wen, Z. Liu, J. Zhang, Y. Zhang, N. Copner, J. Yang, K. Li, M. Bowkett, D. Gao, L. Yuan and X. Zhu, Opt. Commun., 2022, 502, 127436 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |