Open Access Article
Open Access ArticleRecent progress on CO2 separation membranes†
Yuheng Fan a,
Weichu Yu
a,
Weichu Yu *ab,
Aibin Wu
*ab,
Aibin Wu a,
Wenming Shu
a,
Wenming Shu a and
Ying Zhanga
a and
Ying Zhanga
aCollege of Chemistry & Environmental Engineering, Yangtze University, Jingzhou, Hubei 434023, P. R. China. E-mail: yuweichu@126.com
bHubei Engineering Research Centers for Clean Production and Pollution Control of Oil and Gas Fields, Jingzhou, Hubei 434023, P. R. China
First published on 1st July 2024
Abstract
Presently, excessive carbon dioxide emissions represent a critical environmental challenge. Thus, urgent efforts are required to develop environmentally friendly and low-energy technologies for carbon dioxide treatment. In this case, membrane separation technology stands out as a promising avenue for CO2 separation, with selective membrane materials of high permeability playing a pivotal role in this process. Herein, we categorize CO2 separation membranes into three groups: inorganic membranes, organic membranes, and emerging membranes. Moreover, representative high-performance membranes are introduced and their synthesis methods, gas separation performances, and applications are examined. Furthermore, a brief analysis of the challenges encountered by carbon dioxide separation membrane materials is provided together with a discussion on the future research direction. It is expected that this review will provide some potential insights and guidance for the future development of CO2 separation membranes, which can promote their development.
1. Introduction
CO2 capture, utilization, and storage (CCUS) technology stands out as the most effective approach to mitigate greenhouse gas emissions, attracting considerable attention worldwide.1,2 CCUS technology is based on the capture and separation of carbon dioxide.3 To realize the objective of capturing and isolating carbon dioxide, membrane separation has emerged as the prevalent method. This technique allows for the selective permeation of carbon dioxide through physical or chemical interactions between carbon dioxide and the membrane.Research on carbon dioxide membrane separation methods centered around the preparation and acquisition of high-efficiency membranes. Currently, extensively studied CO2 separation membranes include inorganic, organic, and emerging membranes. Inorganic membranes primarily consist of silica, zeolite, and graphene membranes. Organic membranes include cellulose, polyamide, polysulfone, and polyether membranes. Emerging membranes include composite, metal–organic framework (MOF), zeolitic imidazolate framework (ZIF), carbon molecular sieve (CMS), polymers of intrinsic microporosity (PIM), and facilitated transport membranes. With its notable advantages of low energy consumption and high separation efficiency, the membrane separation method is rapidly emerging as globally advancing technology for carbon dioxide capture and separation.4
In the case of concentration difference or pressure difference on both sides of a gas film, the mixed gas to be selectively separated passes through the gas separation film, and gas separation is realized on the basis of the difference in the rate of different components in the mixed gas passing through the gas film. The selectivity of gas separation membranes represents the degree of separation of the required gas molecules from other molecules, and the separation factor represents the efficiency of gas separation.5 The amount of gas passing through a membrane with a certain area and thickness per unit time and differential pressure can be determined. Robeson upper limit represents the limit of the separation performance of homopolymer membranes for a specific gas pair (CO2/CH4, CO2/N2, etc.) and is useful for guiding breakthroughs in optimizing the structure/performance of polymer membranes. The upper limit reflects the trade-off effect, whereby an increase in permeability leads to a decrease in selectivity and vice versa. Fitting parameters for Robeson upper bounds (2008) and proposed CO2/N2 upper bounds are determined using the formula Px = 30.967 × 106αxy−2.888 and CO2/CH4 upper bounds using the formula Px = 5.369 × 106αxy−2.636 (where Px is the permeability (barrer) of the most permeable x-gas and αxy is the selectivity for the x/y gas pair).6
Concerning CO2 separation membranes, according to the literature, their key drawback is the trade-off between high gas selectivity and separation efficiency. Building on the current research landscape, the latest advancements in CO2 separation membranes are meticulously presented herein. Also, the challenges and future developmental trends in CO2 separation membranes are explored in detail, presenting valuable reference and guidance for subsequent endeavors in the creation of novel CO2 separation membranes.
2. Mechanism of carbon dioxide membrane separation
With the development of technologies linked to polymer materials, technology known as the multi-component mixed gas membrane separation method has been gradually developed. When there is a concentration or pressure difference between two sides of the gas membrane as the driving force, the mixed gas can preferentially pass through it based on the difference in the rate of various gas components passing through the membrane. The fundamental membrane separation mechanisms are primarily split into the following three separation mechanisms because of the various membrane materials and properties.2.1 Microporous diffusion
When a gas passes through a porous membrane, the gas molecules with different mixed components have different diameters, and thus the speed of passing through the membrane driven by the pressure discrepancy or concentration discrepancy is different. Small gas molecules pass through the membrane preferentially, while large gas molecules are difficult to pass through. The gas separation effect of porous membranes is mainly affected by the gas properties and membrane pore size.7 Specifically, according to the membrane pore diameter, r, and the average diameter of the gas molecules, λ, the transfer mechanism of mixed gas in porous membranes can be classified into Knudsen diffusion, surface diffusion, capillary condensation, and molecular sieve diffusion,8 as shown in Fig. 1.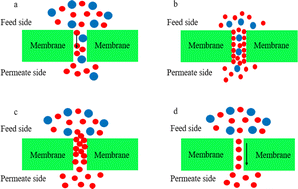 | ||
| Fig. 1 Schematic diagram of the porous membrane transfer mechanism ((a) Knudsen diffusion, (b) surface diffusion, (c) capillary condensation, and (d) molecular sieve diffusion). | ||
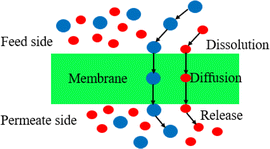
2.2 Dissolution permeation diffusion
Different from the microporous diffusion mechanism of porous membranes, the process of gas passing through a dense membrane generally occurs as the following steps: firstly, the gas is adsorbed on the upstream side of the membrane and dissolved on the membrane surface; secondly, the gas diffuses within the membrane towards the downstream side due to concentration or pressure differences; and finally, the gas is released and desorbed on the downstream surface of the membrane (Fig. 2).9 The dissolution diffusion mechanism, which realizes separation according to the difference between the amount of various gases dissolved in the membrane and their diffusion rate through the membrane, is the name given to this interpretation of gas permeation.In the actual membrane separation procedure, the gas mixture passes through the membrane, but there is a difference in the speed at which the different gases pass through. After a multi-stage membrane separation process, the gas purity can be effectively increased through advancements in membrane separation technology, but this will also increase the energy consumption, limiting its potential for further commercial use.
2.3 Facilitated transport
The mechanism of facilitated transport membranes was inspired by biofilms and has been extensively studied. Unlike the dissolution diffusion mechanism, there is a specific interaction between CO2 and the functional groups in the facilitated transport membrane. Due to the reversible chemical reaction between the functional groups and CO2, specific functional groups enhance the transport of CO2 within the membrane.The process of gas passing through facilitated transport membranes generally occurs as the following steps: at the feed-side interface of the membrane, CO2 reacts with the carrier and forms a CO2-carrier reaction product, which diffuses along its concentration gradient to the permeate side of the membrane. Due to the lower CO2 partial pressure on the permeate side, CO2 is released from the CO2-carrier reaction product to the permeate side, while regenerating the carrier, which can then react with another CO2 molecule on the feed side (Fig. 3). Therefore, other gases only undergo solution-diffusion, such as N2, H2, and CH4.
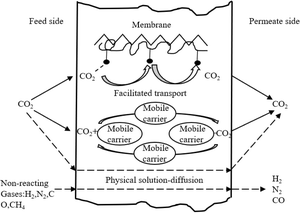 | ||
| Fig. 3 Schematic diagram of the facilitated transport mechanism.10 | ||
The carriers of facilitated transport membranes can be small and mobile molecules, which are referred to as mobile carriers, or functional groups anchored on polymer backbones, the so-called fixed-site carriers. The fixed-site carrier can increase the membrane stability due to its polymeric nature and is inherently stable in the membrane. In addition, the mobile carrier may enhance the CO2 flux due to its high mobility compared to the fixed carrier. Thus, a combination of fixed-site and mobile carriers should provide good membrane stability together with good CO2 facilitation.10
3. Membranes for carbon dioxide separation
Membrane materials serve as the foundation of membrane separation technology, given that their performance directly impacts the potential applications of this technology. Gas separation membranes have a Robeson upper limit for permeability and selectivity, indicating that higher permeability results in lower selectivity, and vice versa. Therefore, in practical applications, membrane materials are selected based on the separation requirements, operating conditions and other relevant factors. Gas separation membranes can be classified into three groups including inorganic membranes, organic membranes, and emerging membranes.3.1 Inorganic membranes
Membranes for gas separation that use inorganic materials as the separation medium are known as inorganic separation membranes. Inorganic membranes exhibit the benefits of superior chemical stability, controllable pore size distribution, etc. due to the characteristics of inorganic materials. Furthermore, the gas separation performance of inorganic membranes can be promoted by improving their pore size and structure. Compared with polymer organic membranes, some inorganic membranes such as graphene membranes have higher diffusion and selectivity. Some commonly employed CO2 inorganic separation membranes include silica, zeolite, and graphene membranes.Starting from the introduction of transition metals, Yan et al.13 constructed cobalt-doped silica membranes by the sol–gel method, and explored the existing form of cobalt and the effect of the addition of cobalt on the pore structure and membrane separation performance. The findings demonstrated that the microporous SiO2 membranes with 10% Co doping had a typical microporous structure, and the permeability of the Co-doped SiO2 membranes to H2 at 300 °C was 64.1 barrer and the H2/CO2 separation coefficient reached 6.61. Li et al.14 selected several precursors and nickel sources to prepare nickel-doped silica membranes, and the CH4 and CO2 permeation fluxes of the Ni-doped SiO2 membranes were 15.6 barrer and 6.4 barrer, respectively, and the CH4/CO2 separation factor was 2.43. Gu et al.15 prepared aluminum-doped microporous SiO2 membranes with tetraethyl orthosilicate and sec-butanol aluminum. After aging treatment for 500 h at 873 K and 16% water vapor, the H2 passing rate of the aluminum-doped SiO2 membranes changed slightly, while the hydrothermal stability was much higher than that of pure SiO2 membranes.
There have been numerous investigations on enhancing the hydrothermal stability of SiO2 membranes by adding hydrophobic groups. To obtain a hydrophobic SiO2 membrane, Zhang et al.16 used CTAB (cetyltrimethylammonium bromide), vinyl triethoxysilane and ethyl orthosilicate. The SiO2 membrane had good permeability to CH4 and CO2, and the CH4/CO2 gas separation factor was 2.27 at a pressure difference of 20 kPa. Hong et al.17 obtained a trifluoropropyl-modified SiO2 membrane material and hydrophobically modified the ordinary SiO2 membrane using trifluoropropyltrimethoxysilane. At 300 °C, the permeability of the membrane to H2 was 47.7 barrer and the H2/CO2 separation coefficient reached 6.99.
Ding et al.18 successfully prepared a heptadecafluorodecyl-modified SiO2 membrane, and at 300 °C, the permeability of the membrane to H2 was 100 barrer and the H2/CO2 separation coefficient reached 13.30. Compared with methyl, trifluoropropyl and other hydrophobic groups with relatively simple structures, the modification effect of heptadecafluorodecyl on the SiO2 membrane was more obvious. The CO2 separation performances of different silica membranes are summarized in Table 1. These are the best-performing membranes reported in the literature, and thus the selected values were chosen for inclusion in this table and the value in the subsequent tables are based on the same principle.
| Membrane | T (°C) | P (atm) | Permeability (barrer) | Selectivity |
|---|---|---|---|---|
| 10% Co–SiO2 membrane13 | 300 | 0.99 | H2: 64.1 | CO2/H2: 6.61 |
| 10% Ni–SiO2 membrane14 | 50 | 1.00 | CO2: 6.4 | CO2/CH4: 2.43 |
| 0.04C-(0.9A-151) SiO2 membrane16 | 75 | 0.20 | CO2: 13.4 | CO2/CH4: 2.27 |
| Trifluoropropyl–SiO2 membrane17 | 300 | 0.99 | H2: 47.7 | CO2/H2: 6.93 |
| Heptadecafluorodecyl-modified SiO2 membrane18 | 300 | 1.00 | H2: 100 | CO2/H2: 13.30 |
As can be seen in Table 1, most silica membranes were enhanced through the doping of transition metal ions and the introduction of hydrophobic groups. The efficacy of various transition metal ions in improving the gas separation performance of silica membranes varies. Notably, doping Co ions demonstrated superior effects compared to doping Ni ions. This discrepancy can be attributed to the presence of Co elements in Co-doped silica membranes, which existed in the form of Si–O–Co within the SiO2 framework. In contrast, Ni-doped silica membranes incorporated Ni elements not only in the Si–O–Ni form in the SiO2 framework but also filled some Ni and NiO crystals in the SiO2 pores. This alteration in pore size compromised the gas separation performance of Ni-doped silica membranes compared to Co-doped silica membranes.19
The structural stability, pore size controllability, gas permeability and separation performance of SiO2 membranes can be effectively improved by doping transition metal ions and introducing hydrophobic groups for modification. Nevertheless, these two methods have certain drawbacks, where the pore size of the membrane material is easily changed by the metal ions by doping into the skeleton of the SiO2 membrane, making it difficult to meet the requirements for gas separation. Furthermore, to date, the mechanism of the effect of hydrophobic group structure on the performance of SiO2 membranes is unclear, which are still far from practical application.
Himeno et al.24 prepared a DDR (deca-dodecasil R) membrane for CO2 separation based on a zeolite membrane. At 25 °C, the permeability of the membrane to the CO2 single component reached 100 barrer, and the selectivity of CO2/CH4 and CO2/N2 mixed gas with a 50/50 feed ratio was 200. Cui et al.25 prepared a T-type zeolite membrane with strong CO2 separation capability. At 70 °C, the optimal selectivity of the membrane for CO2/CH4 and CO2/N2 mixtures was 266 and 31, respectively. The 50/50 selectivity for CO2/CH4 and CO2/N2 at 35 °C was 400 and 107, respectively, indicating that the zeolite membrane has an excellent CO2 separation performance. Gong26 prepared a rhombic zeolite membrane via the secondary growth synthesis method. The ideal separation coefficient for the CO2/N2 single component and the separation coefficient of the two components reached 3.5 and 4.05, respectively. With an increase in temperature, the separation performance only decreased slightly and had good thermal stability.
Jang et al.27 used the secondary growth method to synthesize OSDA (organic structure directing agent)-free CHA (chabazite) zeolite membrane. The maximum selectivity coefficient of the obtained CHA zeolite membrane for CO2/N2 was 12.5, indicating that the zeolite membrane has a good CO2 separation performance. Kida et al.28 synthesized a pure silica CHA zeolite (Si-CHA) membrane by using a porous α-alumina support and the results revealed that the Si-CHA membrane had ultra-high permeability, where at 25 °C and 0.1 MPa, the selectivity of the Si-CHA membrane for CO2/CH4 was as high as 130. Analogously, high-quality Si-CHA zeolite membranes were prepared using a green and fluorite-free synthesis route by Zhou et al.,29 where the best membrane displayed a CO2 permeability of 120 barrer and CO2/CH4 selectivity as high as 480 for an equimolar CO2/CH4 gas mixture at 25 °C and 0.2 MPa pressure drop.
Yu et al.30 successfully prepared a CHA nanocrystalline membrane by adding fluoride. The addition of fluoride increased the permeability and separation performance of the CHA nanocrystalline membrane and increased the selectivity for CO2/CH4 to 32. The Y-type zeolite membrane with CO2/N2 selectivity of up to 500 prepared by Jeremy et al.31 had high CO2 selectivity, but low CO2 permeability. The CO2 separation performances of different zeolite membranes are reported in Table 2.
| Membrane | T (°C) | P (atm) | Permeability (barrer) | Selectivity |
|---|---|---|---|---|
| DDR-type membrane24 | 25 | 1.97 | CO2: 30 | CO2/CH4: 200 |
| Zeolite T membrane25 | 35 | 0.99 | CO2: 46 | CO2/CH4: 400 |
| Chabazite membrane26 | 20 | 0.19 | CO2: 27 | CO2/N2: 4.05 |
| OSDA-free CHA membrane27 | 75 | 1.00 | CO2: 100 | CO2/N2: 12.5 |
| Si-CHA membrane28 | 25 | 0.99 | CO2: 400 | CO2/CH4: 130 |
| Si-CHA membrane29 | 25 | 1.97 | CO2: 120 | CO2/CH4: 480 |
| All-silica CHA nanocrystals membrane30 | 30 | 8.88 | CO2: 780 | CO2/CH4: 32 |
| Zeolite Y membrane31 | 30 | 1.36 | CO2: 9.6 | CO2/N2: 503 |
Based on the information in Table 2, it is evident that the selectivity and permeability of Si-CHA zeolite membranes surpass that of ordinary zeolite membranes (T/Y type). This superiority can be attributed to the unique and regular crystalline pores of Si-CHA zeolite membranes and their adjustable skeleton silicon–aluminum ratio (molar ratio of membrane skeleton silicon element to aluminum element). The pore size of Si-CHA zeolite membranes is larger than that of water molecules, H2 molecules, and CO2 molecules, but smaller than that of most gas molecules. This characteristic endows Si-CHA zeolite membranes with the potential for high molecular sieving selectivity, enabling them to achieve elevated permeability and selectivity.32
CHA-type zeolite membranes demonstrated an attractive separation performance for most studied applications. The two major contributing factors are as follows: CHA-type zeolite membranes (1) are more mature and (2) have three-dimensional channels and a suitable pore size. Preventing zeolite growth inside porous substrates and reducing the membrane thickness are effective strategies for improving the membrane permeance. In addition, it is important to conduct more membrane tests under close-to-realistic operating conditions, such as complex/real feed mixture and high operating pressure, to reveal the real-world performance for better assessment of the membrane potential.33
Zeolite membranes are beneficial for the adsorption and separation of CO2 due to their small pore structure and strong electrostatic field. Nonetheless, adequate control of their microstructure and research into the mechanism for their generation remain significant barriers to their wider deployment. Therefore, high selectivity and high permeability remain key areas for zeolite membrane research.
Jiang et al.35 demonstrated that porous graphene can be used in gas separation from the perspective of first principles, and then designed and synthesized two types of porous graphene membranes and proved that atoms modified the porous edges had a great impact on the separation performance of the membranes. Wang et al.36 designed and prepared a double-layer porous graphene membrane with a single-layer pore size of 2.5 nm, and by only shifting the relative position of the graphene layer, the effective pore size of the graphene membrane could reach 0.36 nm, which satisfies the technical specifications for the separation of CO2, N2 and CH4. Celebi et al.37 introduced nanopores in a CVD bilayer graphene structure by focused ion beam, with a size in the range of 10 nm to 1 μm. Among them, the permeability coefficient of the porous graphene membrane with a pore size of 7.6 nm for H2 was much higher than that of the reported gas separation membranes and it had similar selectivity to H2/CO2.
Currently, porous graphene membranes are difficult to manufacture and puncture precisely, and their real gas selectivity is substantially lower than their theoretical selectivity.38 The theoretical selectivity of graphene films surpasses their actual selectivity primarily due to the challenges in precisely controlling the interlayer spacing of graphene oxide films on the sub-nanometer scale using current technical methods. Consequently, the resulting graphene oxide films exhibit a non-uniform interlayer spacing, leading to an actual selectivity lower than the theoretical selectivity.
Therefore, researchers found that the nanoscale-thickness GO (graphene oxide) laminate films had excellent gas separation capabilities.
Kim et al.39 created GO layered membranes via two distinct methods, and then studied how the micro-structure of the GO laminar membrane affected its ability to separate gases. It was found that GO membranes with a tightly packed microstructure had more complicated airflow channels and higher selective separation effects for CO2/N2 mixed gas. Li et al.40 obtained an ultra-thin GO membrane with a thickness of 1.8 nm by vacuum filtration. The membrane exhibited high selectivity for H2/CO2 with a 3400 selectivity level due to the size sieving effect. Chi et al.41 prepared GO nanosheets with a size of 13 μm and a complete structure. Based on this substance, GO membranes were assembled by various techniques. The selectivity factor for H2/CO2 was found to reach 240 in the GO membranes with more regular stacking structures, which had superior gas selectivity.
The preparation of large-scale, high-quality membranes remains a major challenge although GO layered membranes have significant advantages in the preparation of selective interlayer channels and high gas selectivity.42 This is because different preparation methods have a significant impact on the gas selectivity of membranes. Therefore, researchers combined GO sheets with organic matter to improve the processability of the membrane.43 The composite forms included GO sheets added to the membrane basal planes and filling organic matter or ionic liquid into the GO sheets.44
Xin et al.45 modified GO nanosheets with sulfonated polymer molecular brushes and introduced GO sheets in a sulfonated polyetheretherketone (SPEEK) matrix to obtain graphene mixed matrix membranes. Further studies showed that the addition of sulfonated polymer-modified GO was beneficial to improve the CO2 adsorption selectivity and diffusion selectivity of the membranes. Shen et al.46 developed GO/PEBA (polyether block amide) mixed matrix membranes with various GO oxygen contents and studied the effect of oxygen content on the membrane separation performance. It was found that when the O/C ratio of GO was 0.55, the screening effect was strong and the gas separation performance was the best. The CO2 permeability coefficient reached 97 barrer and the CO2/N2 selectivity reached 86. Shen et al.47 used a vacuum spin coating method to fill polyethyleneimine (PEI) layer by layer between the GO film layers using the combined action of mechanical external forces and intermolecular forces to make the GO film microstructure more regular and orderly, and the H2 permeability coefficient reached 840 barrer, with an H2/CO2 selectivity of 33. The CO2 separation performances of different graphene membranes are summarized in Table 3.
| Membrane | T (°C) | P (atm) | Permeability (barrer) | Selectivity |
|---|---|---|---|---|
| GO layered membrane39 | 25 | 0.99 | CO2: 8500 | CO2/N2: 20 |
| Ultra-thin GO membrane40 | 20 | 1 | H2: 10 | H2/CO2: 3400 |
| GO nanosheets membrane41 | 25 | 0.99 | H2: 3400 | H2/CO2: 240 |
| Graphene oxide-doped ionic liquid membrane44 | 25 | 1 | CO2: 37 | CO2/N2: 130 |
| Graphene mixed matrix membrane45 | 25 | 0.99 | CO2: 1327 | CO2/N2: 86.4 |
| GO/PEBA membrane46 | 25 | 2.96 | CO2: 97 | CO2/N2: 86 |
| GO mixed matrix membrane47 | 25 | 1.97 | H2: 840 | H2/CO2: 33 |
Referring to the data presented in Table 3, it is noticeable that the higher permeability observed in graphene oxide membranes is consistently correlated with lower selectivity. This phenomenon can be attributed to several factors. Firstly, in thicker gas separation membranes, the interaction between gas molecules and the channel walls tends to impede gas permeation. Additionally, longer permeation channels lead to increased collisions between mixed gas molecules, resulting in the transfer of linear momentum from lighter molecules to heavier molecules. This phenomenon generates collective flow, significantly reducing the separation efficiency of the membrane. However, by employing porous graphene thin films with atomic-level thickness, it became possible to enhance the selectivity and permeability by adjusting the pore structure (size), thereby greatly improving the efficiency of gas separation.37
Cho et al.49 reported the preparation of a modified γ-Al2O3 membrane by sol–gel coating with boehmite (AIOOH) sol. CaO was impregnated on the γ-Al2O3 membrane to improve the separation factor by introducing interactions between CO2 gas molecules and the pore wall, but a high separation factor was not obtained. When the pressure ratio was 0.26, the separation factor was 1.72 at 298 K and 1.50 at 673 K. Kang et al.50 synthesised γ-Al2O3 composite membranes modified with microporous silica layers to improve the separation factor of CO2 to N2, and the CO2/N2 separation factors through the silica-modified γ-Al2O3 membranes by dip-coating and pressurized coating from outside the support were about 2.4 and 1.45, respectively.
Isobe et al.51 prepared an AlOOH/Al2O3 porous ceramic membrane, with the porous Al2O3 bodies having an average pore size of approximately 74 nm and porosity of 40.4%, and the CO2/N2 gas selectivity was approximately 0.8 at 0.04 MPa. Carbon dioxide separation using an α-alumina ceramic tube-supported cellulose triacetate–tributyl phosphate composite membrane was reported by Shankar et al.,52 and the CO2 permeability coefficient could reach 4248 barrer, with a CO2/N2 selectivity of 0.84.
Sharma et al.53 prepared an asymmetric graded membrane substrate comprised of a macroporous industrial alumina-based ceramic support with a systematic graded assembly of sol–gel derived γ-alumina intermediate and silica–CTAB sublayer-based multilayered interface. The ceramic membrane exhibited the optimum CO2 permeance of 599 barrer with CO2/N2 selectivity of 12.5 at 80 °C under a trans-membrane pressure drop of 0.8 bar. The CO2 separation performances of different alumina-based ceramic membranes are summarized in Table 4.
| Membrane | T (°C) | P (atm) | Permeability (barrer) | Selectivity |
|---|---|---|---|---|
| γ-Al2O3 composite membranes50 | 25 | 2.96 | CO2: 28.56 | CO2/N2: 2.4 |
| AlOOH/Al2O3 porous ceramics membrane51 | 79 | 0.39 | — | CO2/N2: 0.8 |
| α-Alumina ceramic tube-supported cellulose triacetate–tributyl phosphate membrane52 | 30 | 1.97 | CO2: 4248 | CO2/N2: 0.84 |
| Industrial alumina-based ceramic substrate amino silicate membrane53 | 80 | 0.79 | CO2: 599 | CO2/N2: 12.5 |
It can be seen that the gas selectivity of alumina membranes is low in Table 4 because the mesoporous structure of alumina determines that the transport in the membrane occurs through the Knudsen diffusion mechanism. Due to the limited selectivity under this mechanism and the diffusion rate controlled by molecular weight, the application of alumina membranes in gas separation is restricted. Although some alumina membranes have high gas permeability, the development of alumina membranes should still aim to improve their selectivity for gas separation.
Considering their narrow and controllable pore size distribution, excellent gas selectivity and permeability, high temperature resistance and high pressure capabilities, inorganic membranes are well suited for the separation of CO2. However, due to certain characteristics of inorganic materials such as high brittleness and low elasticity, the fabrication of inorganic membranes is challenging, and thus their application in the field of gas separation is limited.54
3.2 Organic membranes
In addition to inorganic membranes, organic membranes are currently receiving significant research attention. Furthermore, numerous organic membranes have been applied, demonstrating good gas separation performances and mechanical properties. The commonly used organic materials include cellulose, polyamide, polysulfone, and polyether.In 1982, Dow Chemical Company developed a separation membrane based on cellulose triacetate and industrially applied it in 1983. Its industrial operation effect was obvious. This membrane material was discovered to have strong plasticization resistance, and simultaneously poor temperature resistance. Hao et al.56 prepared a cellulose acetate separation membrane by the wet phase conversion method, which could achieve a high CO2 transmission rate without heat treatment. Wu et al.57 prepared a cellulose membrane by directly dissolving natural cellulose, and the wet cellulose membrane exhibited strong permeability to acidic gases such as CO2. At 25 °C, the permeability of the membrane to CO2 was 120 barrer. The ideal separation factors of mixed gas systems containing CO2/H2 and CO2/N2 were 15 and 50, respectively, showing a good selective separation performance. Jie et al.58 chose cellulose/NMMO (N-methylmorpholine-N-oxide)/water ternary spinning as a membrane system to obtain a dense cellulose hollow fiber membrane. At 25 °C and 0.5 MPa, the membrane had a CO2 permeation rate of 750 barrer and the ideal separation factors of the mixed gas systems containing CO2/N2 and CO2/CH4 were 45 and 30, respectively.
Ansaloni et al.59 studied a cellulose nanofiber membrane and found that it had good selectivity for CO2/N2 and CO2/CH4 mixed gases, but the flux of CO2 was low. Subsequently, a composite membrane was obtained by combining the cellulose nanofiber membrane with polyvinyl amine, and the membrane flux of CO2 was improved to a certain extent. When monoglycol and triethylene glycol were combined with a cellulose triacetate (CTA) membrane, Lu et al.60 discovered that the permeability of the membrane to CH4 and CO2 increased. The reason for this may be that the free volume of the cellulose membrane increased after treatment with an alcohol solution, which was more conducive to gas diffusion. Shang et al.61 investigated the gas separation performance of a ethyl cellulose homogeneous membrane by structural modification and solvent optimization. It was found that the separation factors of the ethyl cellulose homogeneous membrane for O2/N2 and CO2/N2 reached 6.2 and 33.0, respectively, demonstrating significantly high CO2 selectivity. The CO2 separation performances of different cellulose membranes are summarized in Table 5.
| Membrane | T (°C) | P (atm) | Permeability (barrer) | Selectivity |
|---|---|---|---|---|
| Cellulose acetate separation membrane56 | 25 | 4.93 | CO2: 520 | CO2/CH4: 12 |
| Cellulose membrane57 | 25 | 5.92 | CO2: 120 | CO2/N2: 50 |
| Cellulose/NMMO/water membrane58 | 25 | 4.93 | CO2: 750 | CO2/N2: 45 |
| Cellulose nanofiber membrane59 | 35 | 0.99 | CO2: 340 | CO2/N2: 35 |
| Cellulose triacetate membrane60 | 35 | 7.4 | CO2: 7.2 | CO2/CH4: 28 |
| Ethyl cellulose homogeneous membrane61 | 30 | 0.99 | — | CO2/N2: 33 |
It is notable that the cellulose/NMMO/water membrane exhibited remarkable selectivity and permeability, as shown Table 5. This can be attributed to several factors. Firstly, the wet method employed for the preparation of the homogeneous and non-porous cellulose hollow gas separation membrane ensured a dense structure after natural drying, which initially did not exhibit significant gas permeability. However, upon humidification, water infiltrated the amorphous regions of cellulose, forming a “water channel”. The gas molecules dissolved and diffused within this “water channel”, facilitating their permeation and separation. Consequently, the water content played a crucial role in determining the gas permeation rate. Notably, CO2 demonstrated high solubility in water, leading to enhanced permeability in the membrane post-water swelling. This phenomenon resulted in high ideal separation factors for N2, CH4, and even H2.58
Although cellulose and its derivatives have been employed as membrane materials for the longest time and demonstrated a strong CO2 separation performance in the actual usage process, the current utilization of the maximum value of cellulose materials remains on the first-order derivatization. Thus, future research on cellulose gas separation membranes should focus on further modifying and preparing secondary, tertiary and other multi-level derivatives.
Physical aging of polymer materials refers to the alteration of their appearance, physical properties, mechanical properties, and electrical properties when exposed to environmental conditions such as light, oxygen, and heat. Physical aging directly impacts the gas separation performance of polymer membranes. Following physical aging, the permeability of the membrane to CO2 typically increases significantly, while the selectivity for CO2 over other gases in a mixed gas decreases, resulting in a diminished gas separation effect.62
Thus, to enhance the CO2 permeability of polyimide membranes, researchers typically modify their manufacturing procedures. It was found that the introduction of sterically hindered groups or twisted structures in the polymer backbone can weaken or eliminate the accumulation of polymer chains and effectively improve the permeability of the polymer.63
Sysel et al.64 introduced the –(CF3)2– group in polyimide to prepare modified polyimide membranes for the separation of CO2/CH4 mixed gas. It was found that the separation coefficient of the modified polyimide membrane for the CO2/CH4 system reached 51. Fang et al.65 prepared a 6FDA-type polyimide separation membrane. At 35 °C and 101.3 kPa, the CO2 permeability coefficient of the membrane was 65 barrer and its ideal separation factor was 30. Maya et al.66 introduced ethylene oxide in the polyimide framework by copolymerization. After heating to 200 °C, the CO2 permeation rate of the modified polyimide membrane increased from 23.87 barrer to 57.25 barrer, but as the permeation rate increased, the selectivity of the membrane to gas was reduced by 10%. Zhang et al.67 prepared two types of polyimide membranes without –CF3– groups and with –CF3– groups, respectively. Following a performance comparison, it was found that the introduction of the –CF3– group enhanced the permeation rate of CO2 from 18 barrer to 1858 barrer, but the selectivity coefficient for CO2/CH4 decreased from 31.3 to 18.9.
Zhu68 prepared 9FDA (fluorinated dianhydride aromatic) polyamide by aniline and 9-fluorenone. Under the condition of 25 °C and 101.3 kPa, the separation coefficient of CO2/N2 was 26.65, and the separation coefficient of CO2/CH4 was 22.70, showing good selectivity. Si et al.69 synthesized polyimide membranes with 4,4′-diamino diphenyl ether, 4,4′-diamino diphenylmethane and 3,3′,4,4′-benzophenone tetracarboxylic dianhydride as monomers. CO2/CH4 exhibited a good gas separation performance and selectivity of 11.9. Eguchi et al.70 used glycidyl to crosslink and modify polyimide to obtain a polyimide membrane. There was a good balance between CO2/CH4 selectivity and CO2 permeability. After crosslinking and modification, the CO2 permeability of the polyimide membrane decreased from 150.5 barrer to 94.6 barrer, but its selectivity increased from 27.5 to 32.7. The CO2 separation performances of different polyamide membranes are summarized in Table 6.
| Membrane | T (°C) | P (atm) | Permeability (barrer) | Selectivity |
|---|---|---|---|---|
| Modified polyimide membrane64 | 25 | 0.99 | CO2: 8.06 | CO2/CH4: 51 |
| 6FDA-type polyimide membrane65 | 35 | 1 | CO2: 65 | CO2/N2: 30 |
| Polyimide framework membrane66 | 30 | 3 | CO2: 57.25 | CO2/N2: 54.52 |
| Polyimide membrane67 | 35 | 2 | CO2: 1858 | CO2/CH4: 18.9 |
| 9FDA polyamide membrane68 | 25 | 1 | CO2: 12.26 | CO2/N2: 26.65 |
| Polyimide membrane69 | 25 | 1 | CO2: 1.19 | CO2/CH4: 11.9 |
| Polyimide membrane70 | 35 | 4.42 | CO2: 150.5 | CO2/CH4: 27.5 |
The data presented in Table 6 reveals that the polyethylene oxide-containing copolymer membrane exhibited superior selectivity. This can be attributed to its composition, which included flexible polyethylene oxide (PEO) segments and rigid polyimide segments. PEO possesses strong affinity for CO2, thereby enhancing the gas selectivity of the membrane.66 Additionally, decarboxylation cross-linking of polyimide membranes improved their permeability. This process involved a thermally induced reaction that increases the polymer spacing of the polyamide membrane, nearing the estimated distance between cross-linked polymer chains, and consequently enhancing the membrane permeability.67 Notably, polyimides show no CO2-induced plasticization phenomenon even at pressures of up to 30 atmospheres.
Despite the fact the current research shows that polyimide and its derivative membrane materials have the benefits of high permeability and good separation performance and good application prospects in the field of CO2 gas separation, the further application of polyamide membranes is hindered by challenges such as their susceptibility to physical aging and complex preparation process.
Zhang71 synthesized a fluorine-containing polysulfone membrane by polycondensation reaction and compared its performance with that of a commercial polysulfone membrane. It was discovered that the polysulfone membranes containing fluorine had a greater gas permeability coefficient. Additionally, a hybrid membrane was created by dispersing multi-walled nanotubes in a matrix made of fluorine-containing polysulfone. The permeability coefficient for CO2 gas was 12.73 barrer at a pressure of 1 atm and temperature of 25 °C, and the selectivity coefficient was 28.9, which are 19% and 5.5% higher than that of the undoped fluorine-containing polysulfone membrane, respectively.
He72 synthesized a PSF (polysulfone)/PDMS (polydimethylsiloxane) copolymer membrane. Under the conditions of 1 atm and 55 °C, the permeability coefficients of the PSF–PDMS 10% membrane for CO2 and CH4 were 73.7 barrer and 17.6 barrer, respectively, while its selectivity coefficient for a CO2/CH4 mixture was 4.2, showing a good gas separation performance. Shahid et al.73 prepared a mixed matrix membrane using PI, PSF, and ZIF-8. When 30 wt% ZIF-8 was added, the CO2 permeability of the mixed matrix membrane was 136% higher than that of the PI (polyimide)/PSF (polysulfone) membrane. The selectivity coefficient for CO2/CH4 was not much different from that of the PI/PSF membrane. Mannan et al.74 studied the CO2/CH4 separation performance of a blend membrane based on polysulfone/polyethersulfone and found that the addition of polyethersulfone (PES) significantly improved the separation performance of the blend membrane for the CO2/CH4 mixed system.
Meng et al.75 prepared a series of mixed matrix membranes employing graphene oxide, carbon nanotubes, and polysulfone. Compared with the pure polysulfone membranes, the CO2 permeability coefficient of the mixed matrix membranes increased by as much as 422 barrer, indicating that the presence of carbon nanotubes significantly improved the CO2 permeability. Meng et al.76 selected PAF-56P (porous aromatic framework) as the separation medium to prepare a PAF-56P/PSF composite hollow fiber membrane, which showed good CO2 permeability in the process of CO2/N2 separation. The separation coefficient of the CO2/N2 mixed system reached 38.9 and the membrane permeability reached 93–141 barrer, with strong gas separation ability. The CO2 separation performances of different polysulfone membranes are summarized in Table 7.
| Membrane | T (°C) | P (atm) | Permeability (barrer) | Selectivity |
|---|---|---|---|---|
| Polysulfone membrane71 | 25 | 1 | CO2: 12.73 | CO2/CH4: 28.9 |
| PSF/PDMS membrane72 | 55 | 1 | CO2: 73.7 | CO2/CH4: 4.2 |
| PI/PSF membrane73 | 35 | 4.93 | CO2: 20 | CO2/CH4: 42 |
| PSF/PES membrane74 | 25 | 5.92 | CO2: 22.5 | CO2/CH4: 6.5 |
| Mixed matrix membrane75 | 25 | 0.99 | CO2: 975 | CO2/N2: 1.94 |
| PAF-56P/PSF membrane76 | 25 | 1 | CO2: 141 | CO2/N2: 38.9 |
The data in Table 7 demonstrate that the PI/PSF/ZIF-8 membrane exhibited the highest gas selectivity. This can be attributed to the porous structure, high specific surface area, and molecular sieving characteristics of ZIF-8. These features are expected to enhance the separation performance of the blend polymer membrane, while the presence of PI/PSF would increase the resistance of the system to plasticization.73 Furthermore, polysulfone mixed matrix membranes demonstrated improved gas permeability. The use of N,N-dimethylformamide as a solvent for polysulfone and a dispersant for inorganic filler particles proved to be effective. Additionally, the incorporation of graphene oxide and carbon nanotubes significantly enhanced the hydrophilicity of the membrane. This facilitated the permeation of CO2, given its high solubility in water, thereby improving the membrane permeability.75
Polysulfone membranes usually have good film forming properties, outstanding processing properties, excellent mechanical strength and good thermal stability. Furthermore, the separation membranes prepared using polysulfone and its derivatives have the advantages of uniform micropores and high porosity, and are popular for fluid separation. Polysulfone separation membranes will play a crucial role in gas separation for the foreseeable future.
Zhou et al.77 prepared a polyionic liquid-reinforced block polyether (F127/PIL) semi-interpenetrating network membrane. It was found that when the mass ratio of F127 to PIL was 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, the tensile strength of the F127/PIL semi-interpenetrating network membrane reached the maximum of 4.569 MPa. Wang et al.78 selected polyether copolyamide Pebax1074 as the main membrane material to prepare a PSF/PDMS/Pebax1074 gas separation membrane with an ultrathin separation layer. It was discovered that the gas penetration flow in the membrane decreased significantly with an increase in the concentration of Pebax1074 on the membrane material, but the gas selectivity gradually increased.
70, the tensile strength of the F127/PIL semi-interpenetrating network membrane reached the maximum of 4.569 MPa. Wang et al.78 selected polyether copolyamide Pebax1074 as the main membrane material to prepare a PSF/PDMS/Pebax1074 gas separation membrane with an ultrathin separation layer. It was discovered that the gas penetration flow in the membrane decreased significantly with an increase in the concentration of Pebax1074 on the membrane material, but the gas selectivity gradually increased.
Zhao et al.79 used two different polyether copolyamides (highly selective Pebax1657 and highly permeable Pebax2533) to prepare gas separation membranes. It was found that the higher the polyether content in the blend membrane, the greater the CO2 and N2 permeability coefficients; conversely, the CO2/N2 selectivity decreased as the polar ether bond concentration increased. Car et al.80 prepared a mixed membrane by blending polyethylene glycol (PEG) and Pebax1657. Compared with the pure Pebax1657 membrane, the cross section of the mixed membrane was more regular and orderly. When the performance of gas separation was examined, it was discovered that the CO2/N2 selectivity of the mixed membrane remained unchanged, while its CO2 permeability coefficient doubled.
Further, Reijerkerk et al.81 blended PEG, Pebax1657 and polydimethylsiloxane (PDMS) to prepare a mixed membrane. The CO2/N2 selectivity of the combined membrane was lower than that of the pure Pebax1657 membrane, but its CO2 permeability was five times higher. Xiao et al.82 prepared a polyimide film containing PPO segments by polymerization and studied its gas separation performance. It was found that with an increase in PPO content, the separation coefficient for CO2/N2 increased from 18.77 to 30.12, which indicated that the introduction to PPO segments had a significant effect on the gas separation performance of the membrane material. The CO2 separation performances of different polyether membranes are summarized in Table 8.
| Membrane | T (°C) | P (atm) | Permeability (barrer) | Selectivity |
|---|---|---|---|---|
| F127/PIL membrane77 | 30 | 1.18 | CO2: 155.59 | CO2/N2: 24.16 |
| PSF/PDMS/Pebax1074 membrane78 | 25 | 2.96 | CO2: 424 | CO2/N2: 41.3 |
| Polyether copolyamide membrane79 | 25 | 1.97 | CO2: 100 | CO2/N2: 50 |
| PEG/Pebax1657 membrane80 | 30 | 0.59 | CO2: 151 | CO2/N2: 47 |
| PEG/Pebax1657/PDMS membrane81 | 35 | 3.95 | CO2: 532 | CO2/N2: 36.1 |
| Polyether membrane82 | 35 | 0.99 | CO2: 131.61 | CO2/N2: 30.12 |
It was evident that the polyethylene glycol (PEG)/Pebax1657/polydimethylsiloxane (PDMS) membrane exhibited high gas permeability according to Table 8. This can be primarily attributed to the presence of PDMS in the membrane structure, which enhanced the permeability to CO2 due to its large free volume.81 Additionally, polyether copolymer membranes demonstrated relatively high gas selectivity. This was mainly due to the low concentration of polar ether bonds present in the polymer membrane. The selectivity of polyether-based CO2/N2 separation membranes predominantly relied on their dissolution selectivity. Thus, as the concentration of polar groups in the polymer matrix decreased, the CO2/N2 selectivity of the membrane generally decreased, while it increased with a decrease in the concentration of polar ether bonds.79
Studies indicated that the incorporation of polyether or block polyether in other polymers is an effective method for enhancing the gas separation capabilities of polyether membranes. This approach can effectively regulate the microstructure and separation performance of the membranes. Nonetheless, the widespread adoption of polyether membrane materials has been impeded by their complex preparation process and susceptibility to fouling, thus restricting their current utilization primarily to indoor research.
The organic membranes prepared using cellulose, polyamide, polysulfone, polyether and other polymer materials have good separation ability and good application prospect for CO2. However, the majority of organic membranes exhibit the problems of poor anti-pollution and poor mechanical properties, which have a direct impact on their service life and CO2 separation effect,83 further restricting the application of organic membranes in the field of CO2 gas separation.
3.3 Emerging membranes
As research into separating membrane materials is progressively becoming more in-depth, an increasing number of innovative membrane materials have been developed, among which, the most well-known are composite membranes, MOF membranes, ZIF membranes, CMS membranes, PIM membranes and facilitated transport membranes.The inorganic materials that are frequently utilized for the preparation of composite membranes include zeolite molecular sieves,85 metal–organic frameworks86 and carbon nanotubes.87 Jiang et al.88 introduced a zeolite thin layer into a PSF/Matrimid hollow fiber membrane and found that the selectivity for CO2 in a CO2/CH4 mixed system was enhanced by 50%. Analogously, Wu et al.89 prepared an improved mixed-matrix membrane (MMMs) by incorporating SAPO-34 zeolites as an inorganic material in the 6FDA–TrMPD (PI) polymer, and the CO2 permeability of the 40 wt% SAPO-34 crystal-loaded MMMs increased from 751 to 1663 barrer, which corresponded to an increase of 121% compared with the neat 6FDA–TrMPD membrane (Fig. 4).
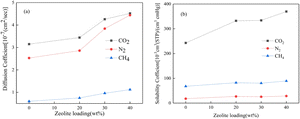 | ||
| Fig. 4 (a) Diffusivity and (b) solubility of CO2, N2, and CH4 in 6FDA–TrMPD/SAPO-34 MMMs with different zeolite loadings.89 | ||
Muhammad et al.90 explored the modification of NH2–MIL-53, and then added it to CA (cellulose acetate) to prepare a composite membrane. The composite membrane had a CO2 permeability of 52.6 barrer and CO2/N2 mixed system selectivity of 23.4, according to the gas separation performance test. Liu91 used a PI/UiO-66–PEI–pSBMA membrane as the selective layer and polydimethylsiloxane (PDMS) as the intermediate layer to obtain a polyimide multilayer composite membrane. The addition of UiO-66–PEI–pSBMA increased the CO2 permeation rate of the polyimide composite membrane by 129.34% and the selectivity for CO2/CH4 by 55.58%.
Li92 prepared a TB (Troger's base)/NH2–MIL-53(Al) hybrid membrane with a good gas separation performance successfully. During the mixed gas test, the CO2 gas permeability of the membrane reached 308 barrer and the selectivity for CO2/N2 and CO2/CH4 mixed gas was 25.4 and 23.6, respectively. Gao93 prepared a composite membrane by chemically adding amino siloxane GO to polyimide through chemical modification. The findings demonstrated that the solubility and thermal stability of the composite membrane were higher than that of the pure PI membrane and the CO2 permeability increased from 8.93 barrer to 17.33 barrer, 20.10 barrer and 24.29 barrer, respectively. The CO2 separation performances of different composite membranes are summarized in Table 9.
| Membrane | T (°C) | P (atm) | Permeability (barrer) | Selectivity |
|---|---|---|---|---|
| Improved mixed-matrix membrane89 | 35 | 2.96 | CO2: 1663 | CO2/N2: 14.8 |
| NH2–MIL-53(Al)/CA mixed matrix membrane90 | 25 | 2.96 | CO2: 52.6 | CO2/N2: 23.4 |
| PI/UiO-66–PEI–pSBMA membrane91 | 35 | 0.99 | CO2: 28 | CO2/CH4: 56 |
| TB/NH2–MIL-53(Al) mixed matrix membrane92 | 35 | 3.95 | CO2: 308 | CO2/N2: 25.4 |
| Composite membrane93 | 30 | 0.99 | CO2: 17.33 | CO2/N2: 30.58 |
The data in Table 9 suggests that the 6FDA–TrMPD mixed matrix membrane demonstrated the highest permeability. This is attributed to the incorporation of the SAPO-34 molecular sieve inorganic material during the synthesis of the membrane. SAPO-34 molecular sieves serve as an effective material for inclusion in the polymer matrix, enabling the simultaneous screening of CO2 from CH4 and N2, and thus enhancing the gas permeability. Additionally, in the aromatic polyimide containing 4,4′-(hexafluoroisopropylidene)diphthalic anhydride (6FDA), the introduction of a –C(CF3)2– group restricted the torsional movement of the adjacent benzene rings and limited the dense packing of the polymer segments to some extent. This increased the free volume of the polymer and enhanced its gas permeation properties.89
The above-mentioned inorganic–organic composite membranes had obvious advantages in CO2 selective separation; however, there are still some issues to be addressed. The interaction mechanism between the polymer and inorganic filler interface is not clear, inorganic fillers are difficult to disperse in the polymer matrix, they lack the foundation for large-scale manufacturing and application, and the CO2 gas separation membranes still have a lot of room for improvement.
Rui et al.96 reported the preparation of a metal–organic framework membrane, MOF-1, and investigated its CO2 separation performance. At feed pressures of 505 kPa and 298 K, the CO2/CH4 and CO2/N2 separation factors for the MOF-1 membrane were 328 and 410, respectively. The CO2 permeability was measured at 255 barrer, demonstrating an excellent separation performance. Additionally, Chiou et al.97 developed highly CO2-selective metal–organic framework membranes, exhibiting outstanding CO2 selectivity (ideal CO2/N2 selectivity of 42 and CO2/CH4 selectivity of 95). Among the available pure MOF membranes, this membrane also achieved the highest CO2 permeability of approximately 500 barrer, with a CO2/CH4 selectivity exceeding 30.
Numerous studies explored the fabrication of mixed matrix membranes incorporating MOFs and other materials. Perez et al.98 synthesized metal–organic framework 5 (MOF-5) nanocrystals with a high specific surface area and excellent thermal stability. These nanocrystals were added to Matrimid® to create a mixed matrix membrane for gas separation. Residual gas analysis of the permeate from mixed gases with varying ratios demonstrated an increase in CH4 selectivity. Wang et al.99 utilized porous ceramic alumina as a support and employed a solvothermal method to synthesize ZIF-62 polycrystalline MOF membranes in situ. They further prepared MOF glass membranes through a melt-quenching method, enhancing the molecular sieve separation capability through a glass transition treatment. The separation factors of the MOF glass membrane for H2/CH4, CO2/N2, and CO2/CH4 mixtures were measured to be 50.7, 34.5, and 36.6, respectively, significantly surpassing the Robeson upper limit.
Fu et al.100 presented a novel approach by growing a metal–organic framework (MOF) on a covalent organic framework (COF) membrane to fabricate a COF–MOF composite membrane. The separation selectivity of this composite membrane for H2/CO2 mixed gas surpassed that of the individual COF and MOF membranes. Strong evidence supporting the synergistic effect between these two porous materials was observed in the COF–MOF composite membrane, which surpassed the Robeson upper limit for polymer membranes in H2/CO2 mixed gas separation. Similarly, in the pursuit of enhancing the gas permeability of ultra-microporous MOF membranes, Bu et al.101 developed a COF/MOF all-nanoporous composite (ANC) membrane. This innovative approach involved doping a stable covalent organic framework (COF) with large and uniform pores (Fig. 5). In comparison to the original MOF membrane, the gas permeability of the COF/MOF membrane increased from 22 barrer to 551 barrer, with a slight decrease in selectivity. The CO2 separation performances of different MOF membranes are summarized in Table 10.
 | ||
| Fig. 5 Structure of COF-TpPa-1/ZIF-9 ANC membranes.101 | ||
Based on the data provided in Table 10, it is evident that the polycrystalline MOF membrane exhibited the highest permeability. This can be attributed to the preparation method involving the formation of a MOF glass membrane by melting and quenching the in situ solvothermal-synthesized polycrystalline ZIF-62 MOF membrane on a porous ceramic alumina carrier. During this process, the molten ZIF-62 phase infiltrated the nanopores of the carrier, thereby eliminating the intergranular defects formed in the resulting glass film. Consequently, the vitrification treatment significantly enhanced the gas permeability of the MOF membranes.99
In comparison to inorganic materials such as zeolites, MOFs exhibit distinct characteristics including high porosity, low density, ultra-high specific surface area, strong functionality, and precise size controllability, with pore sizes ranging from ultra-micropores to mesopores. Crucially, MOF materials offer facile functionalization and pore regulation through the alteration of metal ions and organic ligands, enabling tailored structural designs. The inherent attributes of high porosity, ultra-high specific surface area, and precise size controllability endow MOF materials with significant advantages over traditional inorganic porous materials, making them highly applicable in the field of gas separation membranes.102
The separation factor described in the MOF membrane section appeared to be comparable to that of the previously described inorganic membrane. This similarity is primarily due to certain shortcomings still present in MOF membranes, such as poor adhesion between the matrix and MOF layer, as well as defects at the filler–matrix interface, which ultimately reduce the selectivity of the membrane.
Although numerous MOF materials have been extensively studied, it is imperative to explore the untapped potential of MOFs in membrane gas separation applications.103 The interplay between adsorption and diffusion, a topic seldom discussed, deserves attention. Additionally, it is necessary to underscore the significance of characterizing the gas distribution within MOFs. This understanding is crucial for unraveling the structure–property relationship governing gas adsorption and diffusion in MOF membranes.
Li et al.104 successfully fabricated a zeolitic imidazolate framework (ZIF-7) molecular sieve membrane, exhibiting a promising mixture separation factor of 13.6 for H2/CO2 at 220 °C. This membrane demonstrated a commendable separation performance coupled with remarkable thermal stability. In the pursuit of enhanced membrane gas separation, Chang et al.105 introduced amino-functionalized ZIF-7 (ZIF-7–NH2), garnering attention for its pore-expanding structure post-complete removal of guest molecules (DMF). The ZIF-7–NH2 membranes, synthesized with superior H2 permeation flux and H2/CO2 selectivity compared to reported single-ligand ZIF-7 membranes, represent a significant advancement. The ZIF-7–NH2 membrane effectively enhanced the separation performance of H2/CO2, closely approaching the upper limit of inorganic microporous membranes.
The synthesis of well-developed Co-based zeolitic imidazolate framework (ZIF) membranes on porous α-Al2O3 tubes presents considerable challenges. In a notable study by Liu et al.,106 high-quality ZIF-9 membranes were successfully prepared, exhibiting remarkable H2/CO2 selectivity and thermal stability. The incorporation of 3-aminopropyltriethoxysilane (APTES) as a covalent linker for α-Al2O3 tube modification contributed to the outstanding performance of the ZIF-9 membrane. The H2/CO2 mixture separation factor of the ZIF-9 membrane reached 21.5, surpassing the corresponding Knudsen coefficients significantly. In a related approach, Chang et al.107 devised a hybrid metal zeolitic imidazolate framework membrane by introducing zinc ions and cobalt ions in the ZIF structure. The research findings highlighted the superior H2/CO2 separation factor of the hybrid metal ZIF membrane, which is attributed to its exceptionally high specific surface area.
To facilitate the widespread industrial implementation of ZIF membranes, significant efforts have been devoted to their preparation and support. A continuous ZIF-8 membrane was successfully synthesized on the outer surface of silicon nitride hollow fibers using a one-step hydrothermal method.108 This approach achieved an H2/CO2 separation factor of 11.67, which was attributed to the robust CO2 adsorption capabilities of the ZIF-8 structure. In a related study, Jia et al.109 developed ZIF-8@cellulose nanofiber (ZIF-8@CNF) composite membranes. These membranes were created through the in situ growth of ZIF-8 crystals on cellulose nanofibers (CNFs) using a vacuum filtration process. The resulting membrane exhibited a CO2 permeability of 550 barrer, with the ideal selectivity for CO2/N2 and CO2/CH4 reaching 45.5 and 36.2, respectively.
Taking inspiration from the adhesive properties of marine mussels, Nguyen110 investigated the gas separation performance of a ZIF-8 membrane synthesized on a polydopamine-functionalized stainless-steel mesh (SSNs). In single-component gas permeation tests (Fig. 6), the PDA-functionalized ZIF-8 membrane demonstrated separation abilities for CO2/N2, H2/N2, and H2/CO2 with selectivity values of 1.25, 4.30, and 3.44, respectively. Under the optimal conditions, the efficiency of the 0.2 M material solution for H2/CO2 was maximized at 11.3.
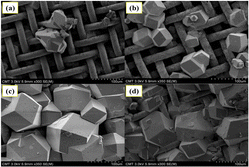 | ||
| Fig. 6 FESEM images of ZIF-8 layers prepared on PDA-functionalized SSNs with different synthesis times: 8 h (a), 16 h (b), 24 h (c), and 48 h (d).110 | ||
Building on the preparation of ZIF-8 membranes, researchers have enhanced the membrane separation performance through structural regulation.111 In a study by Lang,112 the replacement of methylimidazole (MeIM) with 5,6-dimethylbenzimidazole (DBIM) through membrane surface ligand exchange (MSLE) was explored, assessing its impact on the gas separation performance of ZIF-8 membranes with different intergranular defects (perfect and imperfect membranes). The results revealed a significant improvement in membrane selectivity, which was primarily attributed to the reduction in intercrystallite pore diffusion rate resulting from the short-term ligand exchange. This reduction was more pronounced for larger molecules than smaller molecules, leading to a decrease in gas permeability and increase in separation factor. The CO2 separation performances of different ZIF membranes are summarized in Table 11.
| Membrane | T (°C) | P (atm) | Permeability (barrer) | Selectivity |
|---|---|---|---|---|
| ZIF-7–NH2 membrane105 | 25 | 0.99 | CO2: 60 | CO2/H2: 19 |
| ZIF-9 membrane106 | 25 | 0.99 | CO2: 52.3 | CO2/H2: 21.5 |
| Hybrid metal ZIF membrane107 | 55 | 0.99 | CO2: 54 | CO2/H2: 14.1 |
| ZIF-8@CNF membrane109 | 25 | 2.96 | CO2: 550 | CO2/N2: 45.5 |
| ZIF-8 membrane110 | 35 | 0.99 | — | CO2/H2: 11.32 |
As indicated in Table 10, the ZIF-8@CNF membranes exhibited the highest selectivity and permeability. This enhancement can be attributed to the preparation method involving the synthesis of ZIF-8@cellulose nanofiber (ZIF-8@CNF) composite membranes through the in situ growth of ZIF-8 crystals on CNFs via vacuum filtration. The electrostatic forces between –COO– of CNFs and Zn2+ of ZIF-8 facilitated the homogeneous anchoring of ZIF-8 crystals on CNFs. As the loading of ZIF-8 increased sufficiently, the intrinsic selectivity of ZIF-8 significantly contributed to the gas separation.109
However, despite their advantages of easy synthesis and good stability, ZIF membranes are associated with challenges in the competitive separation of H2 and CO2, together with concerns about their mechanical strength under high pressure.113 Future research on ZIF membranes should prioritize enhancing the separation selectivity of H2 and CO2, while addressing the mechanical strength challenges associated with high-pressure applications.
Numerous studies have explored the incorporation of polyether or polyimide in membrane precursors to develop CMS membranes. Wang et al.115 synthesized a CMS membrane using carboxylated polyimides with various 6FpDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DABA molar ratios. The resulting CMS membrane, which was pyrolyzed at 576 °C, exhibited CO2 and C2H4 permeabilities of 3573 and 244.6 barrer, with CO2/CH4 and C2H4/C2H6 ideal selectivity of 51.5 and 4.80, respectively. Similarly, Hou et al.116 produced CMS membranes with a high CO2 separation performance through the pyrolysis of hydroxy-containing polyetherimide (BAHPPE–6FDA-type HPEI) precursors. The structural properties were found to significantly influence the carbon and pore structures, as well as the CO2 separation performance (Fig. 7). The CMS membrane demonstrated a relatively high CO2 permeability of approximately 10
DABA molar ratios. The resulting CMS membrane, which was pyrolyzed at 576 °C, exhibited CO2 and C2H4 permeabilities of 3573 and 244.6 barrer, with CO2/CH4 and C2H4/C2H6 ideal selectivity of 51.5 and 4.80, respectively. Similarly, Hou et al.116 produced CMS membranes with a high CO2 separation performance through the pyrolysis of hydroxy-containing polyetherimide (BAHPPE–6FDA-type HPEI) precursors. The structural properties were found to significantly influence the carbon and pore structures, as well as the CO2 separation performance (Fig. 7). The CMS membrane demonstrated a relatively high CO2 permeability of approximately 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 barrer and enhanced CO2/N2 (or CH4) selectivity was achieved after 60 days of aging under vacuum.
000 barrer and enhanced CO2/N2 (or CH4) selectivity was achieved after 60 days of aging under vacuum.
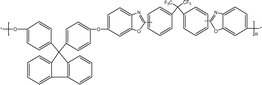 | ||
| Fig. 7 Structure of the HPEI TR-polymer.116 | ||
Pérez-Francisco et al.117 presented a CMS membrane derived from dense membranes composed of a blend of a rigid polyimide (PI) DDPD–IMM and polybenzimidazole (PBI). The CMS membrane derived from the pure PI DPPD–IMM membrane exhibited the highest permeability coefficients (PCO2 = 503 barrer) and the highest separation factors (αCO2/CH4 = 56.5). The results suggested that increasing the concentration of PI in the membrane blend precursors positively influenced the permeability, diffusion coefficients, and selectivity of the CMS membranes. Similarly, Shin et al.118 introduced a novel methodology for achieving a high separation performance in CMS fiber membranes by uniformly integrating double-stranded polysilsesquioxanes in the polyimide matrix. This innovative CMS membrane exhibited a substantially improved CO2 permeability by up to 546% compared to its precursor fiber analogues. Furthermore, a poly(dimethylsiloxane) coating delayed physical aging, maintaining a high CO2 permeability of 354 barrer with a CO2/CH4 selectivity of 56.
Supported CMS membranes are typically prepared by applying precursors on porous carriers for subsequent pyrolysis, and the choice of suitable porous materials is crucial. Nie et al.119 developed CMS membranes supported on a novel porous carbon fiber (PCF) using wood tar as the precursor. These membranes exhibited moderate H2/N2 and H2/CH4 selectivity of 155 and 340, respectively, with an H2 permeability of 86 barrer. In the pursuit of an economically viable coating and pyrolysis of porous materials, Cao et al.120 fabricated multi-layer asymmetric CMS membranes with outstanding gas separation properties. The membrane performance revealed an appealing CO2/CH4 selectivity of 58.8 and CO2 permeability of 310 barrer at 35 °C. Additionally, Lee et al.121 investigated the introduction of an alumina layer to prepare CMS membranes, highlighting the crucial role of the precursor solution viscosity in determining the membrane performance in gas separation. The CO2 separation performances of different CMS membranes are summarized in Table 12.
| Membrane | T (°C) | P (atm) | Permeability (barrer) | Selectivity |
|---|---|---|---|---|
| 6F-DABA-75-CM576 membrane115 | 35 | 3.95 | CO2: 3573 | CO2/CH4: 51.5 |
| CMS-600 membrane116 | 30 | 1.08 | CO2: 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 060 |
CO2/N2: 26.8 |
| PI100-600 membrane117 | 35 | 2 | CO2: 503 | CO2/CH4: 56.5 |
| Polysilsesquioxane CMS membrane118 | 35 | 1 | CO2: 354 | CO2/CH4: 56 |
| CMS70/PCF membrane119 | 35 | 2 | CO2: 14 | CO2/CH4: 101 |
| Multi-layer asymmetric CMS membrane120 | 35 | 1 | CO2: 310 | CO2/N2: 58.8 |
It is evident that the CMS-600 membrane exhibited the highest permeability according to Table 12. This can be attributed to its fabrication process, involving the pyrolysis of a novel hydroxyl-containing polyetherimide (BAHPPF–6FDA-type HPEI) precursor. Upon heat treatment at 450 °C, the thermally reactive ortho OH groups in HPEI underwent a structural transformation, increasing the chain stiffness of the precursor membrane. Consequently, the derived CMS membrane possessed a more open pore structure, thereby enhancing its CO2 permeability.116
The CMS membrane, known for its excellent chemical and thermal resistance, possessed a molecular size similar to polymer membranes and demonstrated exceptional selectivity in gas mixture separation with higher permeability. Despite these advantages, the current permeability of CMS membranes cannot satisfy commercial requirements. The pivotal challenge is enhancing the gas permeation, while preserving effective gas separation, a crucial aspect for advancing the development and application of CMS membranes.
By incorporating methanesulfonic acid (MSA), Han et al.123 synthesized a PIM-1 membrane featuring carboxylic acid and triazinyl crosslinking (cPIM-1) (Fig. 8). The results indicated that the cPIM-1 membrane demonstrated the optimal overall CO2 separation performance, with a permeability close to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 511 barrer. The ideal selectivity for CO2/N2 and CO2/CH4 was 24.3 and 22.2, respectively. Additionally, PIM-1 mixed matrix membranes (MMMs) were created using polyhedral oligomeric silsesquioxane (POSS) and graphene oxide (GO) functionalized with POSS (GO–POSS) by Mohsenpour et al.124 The MMM membrane containing 0.05 wt% GO–POSS exhibited a superior performance, with a CO2 permeability of 12
511 barrer. The ideal selectivity for CO2/N2 and CO2/CH4 was 24.3 and 22.2, respectively. Additionally, PIM-1 mixed matrix membranes (MMMs) were created using polyhedral oligomeric silsesquioxane (POSS) and graphene oxide (GO) functionalized with POSS (GO–POSS) by Mohsenpour et al.124 The MMM membrane containing 0.05 wt% GO–POSS exhibited a superior performance, with a CO2 permeability of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 barrer, representing a 69% increase compared to the pure PIM-1 membrane, while maintaining a similar selectivity (CO2/CH4 selectivity of 12 and CO2/N2 selectivity of 20.6).
000 barrer, representing a 69% increase compared to the pure PIM-1 membrane, while maintaining a similar selectivity (CO2/CH4 selectivity of 12 and CO2/N2 selectivity of 20.6).
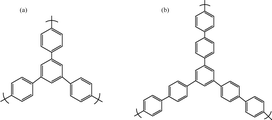 | ||
| Fig. 8 Structure of (a) PPN1 and (b) PPN2.123 | ||
Chen et al.125 introduced a straightforward approach to concurrently enhance the permeability and CO2 selectivity of PIM membranes by incorporating graphene oxide (GO) nanosheets, forming mixed matrix membranes. The GO nanosheets enhanced the hydrophilicity and surface roughness of the PIM-1 membrane, contributing to the porous nature of the mixed matrix membrane with a pore size of approximately 0.78 nm. This combination of properties significantly enhanced the gas separation performance of the PIM-1 membrane. The resulting membrane demonstrated an ultra-high CO2 permeability of up to 6169 barrer and high CO2/N2 selectivity of 123.5, which is more than 7 times that of the pure PIM-1 membrane.
Similarly, Jeong et al.126 developed an innovative CO2 separation membrane by utilizing a polymer of intrinsic microporosity (PIM) and polyimide (PIM–PI) with high permeability as the hard segment and CO2-philic PIM–polyethylene glycol/polypropylene glycol or PIM–PEG (poly(ethylene glycol))/PPG (poly(propylene glycol)) (as the soft segment) as a physical mixture. The resulting membrane exhibited high CO2 permeability (1552.6 barrer) and CO2/N2 selectivity (29.3), approaching the upper bound defined by Robeson (2008) for gas separation performance.
To enhance the gas separation performance of the PIM-1 membrane, extensive research has been conducted to manipulate its microporous structure. Recent advancements in PIM-based membranes were investigated by Shamsabadi et al.127 Their study explored polymer synthesis strategies to modify the PIM structure, aiming to achieve an improved CO2 separation performance and reduced physical aging. These strategies involved the utilization of monomers with suitable side chains, kinked segments, and stable structures.
In a separate effort to enhance the film durability, Sun et al.128 hydrolyzed PIM-1 to produce a carboxyl PIM–COOH polymer. Subsequently, they employed propylene glycol to create mono-esterified PIM and conducted thermal ester crosslinking at various temperatures and durations. The resulting membrane treated at 300 °C for 8 h exhibited remarkable CO2 permeability, CO2/CH4, and CO2/N2 selectivity values of 7421 barrer, 11.5, and 19.2, respectively, significantly surpassing the 2008 Robeson limit. The CO2 separation performances of different PIM membranes are summarized in Table 13.
| Membrane | T (°C) | P (atm) | Permeability (barrer) | Selectivity |
|---|---|---|---|---|
| cPIM-1/PPN2 membrane123 | 25 | 1.97 | CO2: 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 511 511 |
CO2/N2: 24.3 |
| GO–POSS72 membrane124 | 25 | 0.99 | CO2: 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
CO2/N2: 20.6 |
| PIM-1/GO mixed matrix membrane125 | 30 | 3.95 | CO2: 6169 | CO2/N2: 123.5 |
| PIM–PEG/PPG membrane126 | 30 | 1.97 | CO2: 1552.6 | CO2/N2: 29.3 |
| Mono-esterified PIM membrane128 | 35 | 1.97 | CO2: 7421 | CO2/N2: 19.2 |
In Table 13, the GO–POSS72 membrane exhibited the highest permeability, which is attributed to the inclusion of porous nanoparticle (NP)-modified GO nanosheets within the membrane material. These nanoparticles created additional gas transport channels, enhancing the permeability. Moreover, the high aspect ratio of GO–POSS facilitated increased interactions with the polymer chains, aiding in suppressing physical aging phenomena and maintaining a high gas separation performance.124
In terms of enhancing the gas separation performance, the efficacy of CO2 separation surpasses that of H2. Notably, many hybrid membranes comprised of organic materials and PIM-1 demonstrated heightened CO2 permeability and selectivity towards CO2.129 However, despite the efforts to mitigate physical aging through various strategies, it remains the primary challenge for industrial applications.
Currently, the amine group is the most widely utilized carrier. It is weakly alkaline and CO2 is mildly acidic, and thus they can interact chemically in a way that is reversible. Liu130 introduced a quaternary ammonium-based agglomerated zwitterionic material (pSBMA) in a CO2 separation membrane, which significantly improved its CO2 separation performance. According to the research, adding pSBMA increased the CO2 permeability coefficient from 6.2 barrer to 49.8 barrer. Matsuyama et al.131 prepared a CO2 facilitated transport membrane by blending polyethyleneimine (PEI) and polyvinyl alcohol (PVA). At low pressure, the membrane had a CO2/N2 selectivity of 230.
Wang et al.132 synthesized a macromolecule PETEDA (pentaerythrityl tetraethylenediamine) containing primary and secondary amine groups by the grafting method, and then blended it with PVA to prepare a CO2-promoted transfer membrane. The performance tests showed that the optimal CO2 permeability of the membrane was 81.4 barrer and the CO2/CH4 selectivity was 52. Taniguchi et al.133 developed a crosslink agent, 4GMAP, by using a dendritic polymer and glycidyl methacrylate, and used 4GMAP to participate in the photopolymer reaction of polyethylene glycol dimethacrylate (PEGDMA) to prepare a carbon dioxide-promoting transfer membrane with a small membrane thickness. The membrane thickness was reduced from 640 μm to 9.5 μm. At a CO2 partial pressure of 0.56 MPa at 313 K, the selectivity of the membrane for CO2 reached 10 and its permeability to CO2 was also greatly improved.
Winston et al.134 synthesized a new type of facilitated transport membrane by coating a 170 nm selective layer on a polyether sulfone nanoporous substrate. In the selection layer, poly(N-vinyl formamide-vinylamine) covalently bonded to the polymer backbone was used as a fixed-site carrier, and an amino acid salt synthesized by deprotonating sarcosine with 2-(1-piperazinyl)ethylamine was mixed as a flow carrier. The membrane showed 975 barrer CO2 permeability and over 140 CO2/N2 selectivity at 57 °C, 1 atm feed pressure and permeation pressure.
In addition to synthesizing new amine-containing carriers, researchers are also working on adjusting the structural orientation of polymers. Janakiram et al.135 dispersed surface-modified nanocellulose in PVA to enhance the retention of water clusters in the polymer matrix. The thin composite film was synthesized by using sterically hindered polyamine as the fixed carrier and PZEA-Sar as the mobile carrier. At 35 °C, the thin film composite film had a CO2 permeability of 652 barrer and CO2/N2 mixed gas selectivity of 41.
Researchers have also created various amine-free assisted transport membranes, in contrast to the usage of amine groups as carriers. Wang et al.136 found that carboxyl groups can also be used as CO2 transfer carriers through experiments. A CO2 facilitated transfer membrane was prepared with P(AAS-co-AAM) as the separation layer and PSF as the support layer. When the operating pressure was 1 bar, the membrane had a CO2 permeability of 98 barrer and CO2/N2 mixed gas selectivity of 70.
Yao et al.137 prepared a membrane for promoting transfer that is similar to carbonic anhydrase (CA), which is used by organisms to effectively catalyze the conversion of CO2 into water. The results showed that the CO2 permeability exceeded 1000 barrer and the CO2/N2 selectivity was 83, showing good gas permeability and selectivity. The CO2 separation performances of different facilitated transport membranes are summarized in Table 14.
| Membrane | T (°C) | P (atm) | Permeability (barrer) | Selectivity |
|---|---|---|---|---|
| pSBMA membrane130 | 35 | 1.97 | CO2: 104 | CO2/CH4: 82 |
| Macromolecule PETEDA membrane132 | 27 | 1.89 | CO2: 81.4 | CO2/CH4: 82 |
| Poly(amidoamine) dendrimer-containing polymeric membrane134 | 57 | 1 | CO2: 975 | CO2/N2: 140 |
| Thin composite membrane135 | 35 | 1.68 | CO2: 652 | CO2/N2: 41 |
| CO2-facilitated transfer membrane136 | 30 | 4.93 | CO2: 98 | CO2/N2: 70 |
| Poly(N-vinylimidazole)–zinc membrane137 | 30 | 0.99 | CO2: 1000 | CO2/N2: 83 |
Based on the data presented in Table 14, the poly(amidoamine) dendritic-containing polymer membrane exhibited the highest permeability and selectivity. This membrane was prepared by physically immobilizing polyamide amide (PAMAM) dendrimers within cross-linked polyethylene glycol (PEG) through the photopolymerization of PEG dimethacrylate (PEGDMA) in the presence of dendrimers in ethanol. The immiscibility between the PEG matrix and dendritic macromolecules resulted in the formation of a bicontinuous phase separation structure on the micrometer scale. This structure inhibited a reduction in the membrane thickness, thereby enhancing the CO2 permeability and selectivity.133
When comparing transfer membranes with traditional organic membranes for carbon dioxide separation, it is essential to note that the former not only relies on the carrier for facilitating the transfer process but also involves a dissolution–penetration–diffusion process similar to that of traditional organic membranes. Transfer membranes theoretically exhibit a higher gas separation efficiency than traditional organic membranes.
However, the permeability and selectivity values mentioned appeared to be comparable to that of previously described membranes. This is primarily because the water content and pressure of the feed gas significantly influence the carbon dioxide transfer through the membrane, consequently impacting its performance. Therefore, the permeability and selectivity of transfer membranes may not demonstrate significant advantages in certain scenarios.
In the case of gas permeability, facilitated transport membranes theoretically have a better separation performance than conventional organic membranes. Between them, functional carrier membranes had the advantages of good stability and simple operation. However, a clear description and mechanism of the CO2 promoting transfer process within functional carrier membranes is lacking. If the mechanism of the CO2 promoting transfer in these membranes can be clarified, it would be beneficial to address any shortcomings and further enhance their gas separation and permeation performance. This aspect is expected to become the focus in research and development of gas separation membranes in the future.
3.4 Application scenario of membranes
Membranes with different structures have different gas separation characteristics and Fig. 9–11 and Table S2, ESI† outline the gas separation performances and typical application scenarios of diverse membranes, including CO2/N2 separation, CO2/H2 separation, and CO2/CH4 separation. These application scenarios correspond to post-combustion CO2 capture, pre-combustion CO2 capture, and natural gas desulfurization, respectively.It can be seen in Fig. 9–11 that for inorganic membranes, silica membranes are mainly used to investigate the application of CO2/H2 and CO2/CH4 separation, zeolite membranes tend to be used in CO2/N2 and CO2/CH4 separation, and graphene membranes focus more on CO2/N2 separation. Cellulose membranes, polyamide membranes, and polysulfone membranes tend to be used for CO2/N2 and CO2/CH4 separation, while polyether membranes are also partially used for CO2/H2 separation.
Among the emerging membranes, composite membranes and MOF membranes are more used for CO2/N2 and CO2/CH4 separation. ZIF membranes are mainly biased towards CO2/H2 separation. CMS is mainly used for CO2/CH4 separation, and some used for CO2/N2 separation. PIM is mostly used for CO2/CH4 separation, while facilitated transport membranes are mainly used for CO2/N2 separation, and a small part used for CO2/CH4 separation.
4. Challenges and perspectives
The membrane materials serve as the foundation of membrane separation technology, given that their performance directly impacts the potential applications of this technology. Fig. S1 in the ESI† outlines the gas separation performances of diverse membranes and the gas separation membranes with a Robeson upper limit for permeability and selectivity, indicating that higher permeability resulted in lower selectivity, and vice versa. Therefore, in practical applications, membrane materials are selected based on the separation requirements, operating conditions and other relevant factors. Gas separation membranes can be classified into three groups including inorganic membranes, organic membranes, and emerging membranes.Currently, the commonly used organic and inorganic membranes have unique merits and drawbacks. Advanced materials such as composite membranes, MOF membranes, ZIF membranes, CMS membranes, PIM membranes, and facilitated transport membranes have been developed to overcome existing challenges and present new opportunities. The introduction of novel materials has the potential to address multiple issues simultaneously but also poses new challenges. Thus, further research is essential to advance the development of CO2 separation membranes with high permeability and selectivity, as follows:
(1) An optimal inorganic membrane should exhibit consistent permeability and selectivity for CO2, while overcoming processing challenges.
(2) The development of new organic membranes should prioritize enhanced resistance to high temperatures and pressures, superior thermal stability, and mechanical strength. Simultaneously, the membranes should be easy to process, improving their anti-plasticization capabilities.
(3) Due to the presence of organic and inorganic phases in composite membranes, defects at the phase interface lead to poor compatibility between the two phases and the poor dispersion of inorganic nanoparticles on organic membranes is also a problem to be solved.
(4) Further investigations are required to delve into the adsorption, diffusion, and distribution mechanisms of gases in MOF membranes. In the case of ZIF membranes, the primary focus should be on enhancing their separation selectivity for H2 and CO2, while concurrently addressing challenges related to their mechanical strength, particularly in high-pressure applications. The paramount challenge for CMS membranes is augmenting the gas permeation without compromising effective gas separation. Lastly, the industrial application of PIM membranes is primarily hindered by physical aging, signifying a crucial hurdle that warrants attention.
(5) The supported liquid membrane in facilitated transport membranes exhibits poor stability, necessitating consideration for carrier loss mitigation to enhance its stability. Additionally, carrier saturation should be considered in functional carrier membranes to ensure their optimal performance.
5. Conclusions
The issue of global warming, stemming from the escalating carbon dioxide levels, poses a severe environmental challenge. Accordingly, it is urgent to develop carbon dioxide treatment technology that is not only environmentally friendly but also highly efficient with low energy consumption. In this case, membrane separation technology, characterized by its simplicity and high effectiveness, has attracted increasing attention from researchers. Membrane separation technology is based on the acquisition of membranes with high permeability and selectivity. Herein, we provided an overview of various CO2 separation membranes, including inorganic membranes, organic membranes, and emerging membranes. Additionally, we introduced the characteristics and progress of typical membranes. The primary findings were summarized as follows.Inorganic membranes exhibit a narrow and controllable pore size distribution, excellent gas selectivity and permeability, as well as high temperature and pressure resistance. However, the fabrication of inorganic membranes is challenging, limiting their application in the field of gas separation. Organic membranes, fabricated from materials such as cellulose, polyamide, polysulfone, and polyether demonstrate effective CO2 separation capabilities. Nevertheless, many organic membranes are associated with challenges such as poor anti-pollution and poor mechanical properties, thereby limiting their broader application in CO2 gas separation.
Emerging CO2 membranes, such as composite membranes and MOF membranes, exhibit superior CO2 separation performances compared to conventional membrane materials. However, the primary challenge is maintaining effective gas separation, while enhancing gas permeability for their industrial application. Additionally, there is still a long way to go to transform their theoretical high gas permeability and selectivity into reality.
In conclusion, a multitude of advanced membranes have been investigated, significantly promoting the progress of membrane technology. To actualize the ultimate application of CO2 membrane separation in the future, a collaborative advancement in membrane technology, chemical science, and engineering applications should be developed.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the National Natural Science Foundation of China (52274029).Notes and references
- S. Yuan, D. Ma, J. Li, T. Zhou, Z. Ji and H. Han, Petrol. Explor. Dev., 2022, 49, 828–834 CrossRef CAS.
- Z. Zhao, S. Yao, S. Yang and X. Wang, Environ. Sci., 2022, 44, 1128–1138 Search PubMed.
- S. Feng, J. Ren, X. Ren, H. Li, K. Hua and M. Deng, Membr. Sci. Technol., 2012, 32, 27–33 CAS.
- X. Bu, Clean Coal Technol., 2014, 20, 9–13 CAS.
- H. B. Park, J. Kamcev, L. M. Robeson, M. Elimelech and B. D. Freeman, Science, 2017, 356, eaab0530 CrossRef PubMed.
- M. Robeson, J. Membr. Sci., 2010, 320, 390–400 CrossRef.
- L. Zhu, Masteral thesis, Dalian University of Technology, 2010.
- D. Yang and M. Xu, Inn. Mong. Petrochem. Ind., 2004, 30, 28–31 Search PubMed.
- X. Ruan, X. Yan, Y. Dai and G. He, Petrochem. Technol., 2015, 44, 785–790 CAS.
- Y. Zhao and W. S. W. Ho, Ind. Eng. Chem. Res., 2012, 52, 8774–8782 CrossRef.
- R. J. R. Uhlhorn, M. H. B. J. Huisin'tveld, K. Keizer and A. J. Burggraaf, J. Mater. Sci. Lett., 1989, 8, 1135–1138 CrossRef CAS.
- R. Igi, T. Yoshioka, Y. H. Ikuhara and Y. Iwamoto, J. Am. Ceram. Soc., 2008, 91, 2975–2981 CrossRef CAS.
- J. Yan, Q. Wei, X. Duan, J. He, Q. Li and Z. Nie, Chin. J. Inorg. Chem., 2011, 27, 1334–1340 CAS.
- W. Li, D. Li, L. Zheng, J. Fan and Z. Zhang, J. Chin. Ceram. Soc., 2014, 42, 416–422 CAS.
- Y. Gu, P. Hacarlioglu and S. T. Oyama, J. Membr. Sci., 2008, 310, 28–37 CrossRef CAS.
- Z. Zhang, L. Zheng, D. Li and W. Li, J. Shenyang Univ. Chem. Technol., 2014, 28, 308–313 CAS.
- Z. Hong, Q. Wei, G. Li, X. Wang, Z. Nie and Q. Li, Chin. J. Inorg. Chem., 2013, 29, 941–947 CAS.
- Y. Ding, Q. Wei, X. Liu, Q. Li and Z. Nie, Membr. Sci. Technol., 2014, 34, 22–27 CAS.
- D. Uhlmann, S. Liu, B. P. Ladewig and J. C. D. da Costa, J. Membr. Sci., 2009, 326, 316–321 CrossRef CAS.
- N. Xu, Chem. Ind. Eng. Prog., 2000, 4, 5–9 Search PubMed.
- J. Wang, J. Yang, Z. Chen and D. Yin, Membr. Sci. Technol., 2011, 31, 118–126 CAS.
- E. E. Mcleary, J. C. Jansen and F. Kapteijn, Microporous Mesoporous Mater., 2006, 90, 198–220 CrossRef CAS.
- J. Wang, J. Yang, H. Li and L. Li, Membr. Sci. Technol., 2014, 34, 1–7 Search PubMed.
- S. Himeno, T. Tomita, K. Suzuki, K. Nakayama and K. Yajima, Ind. Eng. Chem. Res., 2007, 46, 6989–6997 CrossRef CAS.
- Y. Cui, H. Kita and K.-i. Okamoto, Chem. Commun., 2003, 17, 2154–2155 RSC.
- H. Gong, Masteral thesis, Northeastern University, 2018.
- E. Jang, S. Hong, E. Kim, N. Choi, S. J. Cho and J. Choi, J. Membr. Sci., 2018, 549, 46–59 CrossRef CAS.
- K. Kida, Y. Maeta and K. Yogo, Sep. Purif. Technol., 2018, 197, 116–121 CrossRef CAS.
- J. Zhou, F. Gao, K. Sun, X. Jin, Y. Zhang, B. Liu and R. Zhou, Energy Fuels, 2020, 34, 11307–11314 CrossRef CAS.
- L. Yu, A. Holmgren, M. Zhou and J. Hedlund, J. Mater. Chem. A, 2018, 6, 6847–6853 RSC.
- J. C. White, P. K. Dutta, K. Shqau and H. Verweij, Langmuir, 2010, 26, 10287–10293 CrossRef CAS PubMed.
- L. Li and J. Yang, Membr. Sci. Technol., 2022, 42, 138–145 CAS.
- Z. Cao, N. Anjikar and S. Yang, Separations, 2022, 9, 9020047 CrossRef.
- L. Yang, W. Luo, C. Wang and C. Xu, J. Inorg. Mater., 2020, 35, 959–971 CrossRef.
- D.-e. Jiang, V. R. Cooper and D. Sheng, Nano Lett., 2009, 9, 4019–4024 CrossRef CAS PubMed.
- S. Wang, S. Dai and D. E. Jiang, ACS Appl. Nano Mater., 2018, 2, 379–384 CrossRef.
- K. Celebi, J. Buchheim, R. M. Wyss, A. Droudian, P. Gasser, I. Shorubalko, J.-I. Kye, C. Lee and H. G. Park, Science, 2014, 344, 289–292 CrossRef CAS PubMed.
- C. Ma, M. Wang, Z. Wang, M. Gao and J. Wang, J. CO2 Util., 2020, 42, 101296 CrossRef CAS.
- H. W. Kim, H. W. Yoon, S.-M. Yoon, B. M. Yoo, B. K. Ahn and Y. H. Cho, Science, 2013, 342, 91–95 CrossRef CAS PubMed.
- H. Li, Z. Song, X. Zhang, Y. Huang, S. Li and Y. Mao, Science, 2013, 342, 95–98 CrossRef CAS PubMed.
- C. Chi, X. Wang, Y. Peng, Y. Qian and Z. Hu, Chem. Mater., 2016, 28, 2921–2927 CrossRef CAS.
- Y. Zhang, S. Zhang and T. S. Chung, Environ. Sci. Technol., 2015, 49, 10235–10242 CrossRef CAS.
- J. W. Burress, S. Gadipelli and J. Ford, Angew. Chem., 2010, 49, 8902–8904 CrossRef CAS PubMed.
- M. Karunakaran, L. F. Villalobos, M. Kumar, R. Shevate, F. H. Akhtar and K.-V. Peinemann, J. Mater. Chem. A, 2017, 5, 649–656 RSC.
- Q. Xin, F. Ma, L. Zhang, S. Wang, Y. Li, H. Ye, X. Ding, L. Lin, Y. Zhang and X. Cao, J. Membr. Sci., 2019, 586, 23–33 CrossRef CAS.
- J. Shen, M. Zhang, G. Liu and W. Jin, RSC Adv., 2016, 6, 54281–54285 RSC.
- J. Shen, G. Liu, K. Huang, Z. Chu, W. Jin and N. Xu, ACS Nano, 2016, 10, 3398–3409 CrossRef CAS PubMed.
- B. Shimekit, H. Mukhtar, F. Ahmad and S. Maitra, Trans. Indian Ceram. Soc., 2009, 68, 115–138 CrossRef CAS.
- Y.-K. Cho, K. Han and K.-H. Lee, J. Mater. Sci., 1995, 104, 219–230 CAS.
- B. Kang and H. Sang, J. Mater. Sci., 1999, 34, 1391–1398 CrossRef CAS.
- T. Isobe, Y. Takada, S. Matsushita and A. Nakajima, Ceram. Int., 2015, 41, 7759–7765 CrossRef CAS.
- K. Shankar and P. Kandasamy, Greenhouse Gases: Sci. Technol., 2019, 9, 287–305 CrossRef CAS.
- S. K. Sharma, B. K. Sanfui, A. Katare and B. Mandal, ACS Appl. Mater. Interfaces, 2020, 12, 40269–40284 CrossRef CAS PubMed.
- J. Yang, J. Li, N. Cui, N. Cheng and M. Guo, Coal Chem. Ind., 2019, 42, 119–125 Search PubMed.
- C. Gao, L. Cai, F. Dong, H. Xie, S. Qin and Q. Zheng, J. Chem. Eng. Chin. Univ., 2019, 33, 1037–1047 CAS.
- J. Hao, Z. Wang and S. Wang, Polym. Mater. Sci. Eng., 1997, 13, 65–69 Search PubMed.
- J. Wu, PhD thesis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 2002.
- X. Jie, PhD thesis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 2005.
- L. Ansaloni, J. Salas-Gay, S. Ligi and M. G. Baschetti, J. Membr. Sci., 2017, 522, 216–225 CrossRef CAS.
- H. T. Lu, S. Kanehashi, C. A. Scholes and S. E. Kentish, J. Membr. Sci., 2017, 539, 432–440 CrossRef CAS.
- X. Shang, S. Chen, X. Shi and Z. Zhu, Chin. J. Chem. Phys., 1997, 10, 92–97 Search PubMed.
- C. Zhang, Y. Weng and W. Han, Sci. Sin.: Chim., 2020, 50, 655–668 Search PubMed.
- Y. Zhuang, J. G. Seong, Y. S. Do, H. J. Jo, M. J. Lee, G. Wang, M. D. Guiver and Y. M. Lee, Macromolecules, 2014, 47, 7947–7957 CrossRef CAS.
- P. Sysel, D. Maly, J. Vysohlid, K. Friess, K. Pilnacek, M. Lanc and O. Vopicka, Polym. Eng. Sci., 2017, 57, 1367–1373 CrossRef CAS.
- F. Jianhua, K. Hidetoshi and O. Ken-ichi, J. Membr. Sci., 2001, 182, 245–256 CrossRef.
- E. M. Maya, D. M. Muñoz, J. G. Campa, J. D. Abajo and Á. E. Lozano, Desalination, 2006, 199, 188–190 CrossRef CAS.
- C. Zhang, L. Pei and C. Bing, J. Membr. Sci., 2017, 528, 206–216 CrossRef CAS.
- T. Zhu, Masteral thesis, Taiyuan University of Technology, 2018.
- H. Si, H. Jia, P. Jiang, S. Zhao and S. Zhang, Journal of Qiqihar University (Natural Science Edition), 2021, 37, 77–80 Search PubMed.
- H. Eguchi, D. J. Kim and W. J. Koros, Polymer, 2015, 58, 121–129 CrossRef CAS.
- M. Zhang, Masteral thesis, Taiyuan University of Technology, 2015.
- X. He, Masteral thesis, Taiyuan University of Technology, 2018.
- S. Shahid and K. Nijmeijer, Sep. Purif. Technol., 2017, S1383586617307852 Search PubMed.
- H. A. Mannan, H. Mukhtar, M. S. Shaharun, M. R. Othman and T. Murugesan, J. Appl. Polym. Sci., 2016, 133, 42496 Search PubMed.
- Y. Meng, Masteral thesis, Lanzhou University of Technology, 2020.
- L. Meng, Masteral thesis, Tiangong University, 2016.
- X. Zhou, J. Yan, H. Gao, A. Wang and Y. Bai, N. Chem. Mater., 2018, 46, 195–199 Search PubMed.
- Y. Wang, J. Ren, C. Xu, H. Li, S. Feng and M. Deng, Membr. Sci. Technol., 2014, 34, 27–32 CAS.
- Q. Zhao, S. He, J. Lin and Q. Lin, China Plast., 2019, 33, 13–20 Search PubMed.
- A. Car, C. Stropnik, W. Yave and K.-V. Peinemann, J. Membr. Sci., 2008, 307, 88–95 CrossRef CAS.
- S. R. Reijerkerk, M. H. Knoef, K. Nijmeijer and M. Wessling, J. Membr. Sci., 2010, 352, 126–135 CrossRef CAS.
- X. Xiao, X. Xu, X. Li, J. Dong, X. Zhao and Q. Zhang, Acta Polym. Sin., 2022, 53, 505–513 CAS.
- Z. Wang, X. Liu, X. Li, Z. Tao, P. Yu, Q. Zhang and H. Shi, Shandong Chem. Ind., 2021, 50, 74–75+79 CAS.
- Y. Cao, C. Yang and W. Wang, Spec. Petrochem., 2015, 32, 53–60 CAS.
- W. Wang, X. Jiang, Y. Li, L. Su, Y. Zou and Z. Tong, CIESC J., 2020, 71, 3807–3818 CAS.
- Y. Cheng, Y. Ying, S. Japip, S.-D. Jiang and T.-S. Chung, Adv. Mater., 2018, 30, e1802401 CrossRef PubMed.
- X. Gong, L. Zhu, Y. Xu and B. Zhu, Membr. Sci. Technol., 2011, 31, 89–93 CAS.
- L. Y. Jiang, T. S. Chung and S. Kulprathipanja, AIChE J., 2010, 52, 2898–2908 CrossRef.
- T. Wu, Y. Shi, Y. Liu, I. Kumakiri, K. Tanaka, X. Chen and H. Kita, Energy Fuels, 2021, 35, 10680–10688 CrossRef CAS.
- M. Mubashir, Y. Y. Fong, L. K. Keong, C. T. Leng and N. Jusoh, Sep. Purif. Technol., 2018, 199, 140–151 CrossRef CAS.
- B. Liu, PhD thesis, Harbin Institute of Technology, 2021.
- C. Li, Masteral thesis, China University of Petroleum, Beijing, 2019.
- Y. Gao, Masteral thesis, Shenyang University of Technology, 2021.
- E. Adatoz, A. K. Avci and S. Keskin, Sep. Purif. Technol., 2015, 152, 207–237 CrossRef CAS.
- Q. Qian, P. A. Asinger, M. J. Lee, G. Han, K. M. Rodriguez, S. Lin, F. M. Benedetti, A. X. Wu, W. S. Chi and Z. P. Smith, Chem. Rev., 2020, 120, 8161–8266 CrossRef CAS PubMed.
- Z. Rui, J. B. James, A. Kasik and Y. S. Lin, AIChE J., 2016, 62, 3836–3841 CrossRef CAS.
- D. S. Chiou, H. J. Yu, T. H. Hung, Q. Lyu, C. K. Chang, J. S. Lee, L. C. Lin and D. Y. Kang, Adv. Funct. Mater., 2020, 31, 2006924 CrossRef.
- E. V. Perez, K. J. Balkus, J. P. Ferraris and I. H. Musselman, J. Membr. Sci., 2009, 328, 165–173 CrossRef CAS.
- Y. Wang, H. Jin, Q. Ma, K. Mo, H. Mao, A. Feldhoff, X. Cao, Y. Li, F. Pan and Z. Jiang, Angew. Chem., Int. Ed., 2020, 59, 4365–4369 CrossRef CAS PubMed.
- J. Fu, S. Das, G. Xing, T. Ben, V. Valtchev and S. Qiu, J. Am. Chem. Soc., 2016, 138, 7673–7680 CrossRef CAS PubMed.
- M. Bu, Y. Feng, Q. Li, Y. Wang, S. Feng, K. Zhang, Y. Jiang, L. Fan, Z. Kang and D. Sun, Inorg. Chem. Front., 2021, 8, 5016–5023 RSC.
- R. Hardian, J. Jia, A. Diaz-Marquez, S. Naskar, D. Fan, O. Shekhah, G. Maurin, M. Eddaoudi and G. Szekely, Adv. Mater., 2024, 36, 2314206 CrossRef CAS.
- D.-Y. Kang and J. S. Lee, Langmuir, 2023, 39, 2871–2880 CrossRef CAS PubMed.
- Y. Li, F. Liang, H. Bux, W. Yang and J. Caro, J. Membr. Sci., 2010, 354, 48–54 CrossRef CAS.
- H. Chang, Y. Wang, L. Xiang, D. Liu, C. Wang and Y. Pan, Chem. Eng. Sci., 2018, 192, 85–93 CrossRef CAS.
- J. Liu, C. Liu and A. Huang, Int. J. Hydrogen Energy, 2020, 45, 703–711 CrossRef CAS.
- P.-H. Chang, Y.-T. Lee and C.-H. Peng, Materials, 2020, 13, 5009 CrossRef CAS PubMed.
- J.-W. Wang, N.-X. Li, Z.-R. Li, J.-R. Wang, X. Xu and C.-S. Chen, Ceram. Int., 2016, 42, 8949–8954 CrossRef CAS.
- M. Jia, X.-F. Zhang, Y. Feng, Y. Zhou and J. Yao, J. Membr. Sci., 2020, 595, 117579 CrossRef CAS.
- T.-M. T. Nguyen, J.-W. Chen, M.-T. Pham, H. M. Bui, C.-C. Hu, S.-J. You and Y.-F. Wang, Environ. Technol. Innov., 2023, 31, 103169 CrossRef CAS.
- Y. Song, M. He, J. Zhao and W. Jin, Sep. Purif. Technol., 2021, 270, 118722 CrossRef CAS.
- L. Lang, F. Banihashemi, J. B. James, J. Miao and J. Y. S. Lin, J. Membr. Sci., 2021, 619, 118743 CrossRef CAS.
- Z. Lai, Curr. Opin. Chem. Eng., 2018, 20, 78–85 CrossRef.
- L. Li, R. Xu, C. Song, B. Zhang, Q. Liu and T. Wang, Membranes, 2018, 8, 134 CrossRef PubMed.
- Q. Wang, F. Huang, C. J. Cornelius and Y. Fan, J. Membr. Sci., 2021, 621, 118785 CrossRef CAS.
- M. Hou, L. Li, Z. He, R. Xu, Y. Lu, J. Zhang, Z. Pan, C. Song and T. Wang, J. Membr. Sci., 2022, 656, 120639 CrossRef CAS.
- J. M. Pérez-Francisco, J. L. Santiago-García, M. I. Loría-Bastarrachea, D. R. Paul, B. D. Freeman and M. Aguilar-Vega, J. Membr. Sci., 2020, 597, 117703 CrossRef.
- J. H. Shin, H. J. Yu, H. An, A. S. Lee, S. S. Hwang, S. Y. Lee and J. S. Lee, J. Membr. Sci., 2019, 570–571, 504–512 CrossRef CAS.
- J. Nie, F. Okada, H. Kita, K. Tanaka, T. Mihara, D. Kondo, Y. Yamashita and N. Yahagi, Energy Fuels, 2022, 36, 7147–7157 CrossRef CAS.
- Y. Cao, K. Zhang, O. Sanyal and W. J. Koros, Angew. Chem., Int. Ed., 2019, 58, 12149–12153 CrossRef CAS.
- P.-S. Lee, D. Kim, S.-E. Nam and R. R. Bhave, Microporous Mesoporous Mater., 2016, 224, 332–338 CrossRef CAS.
- Y. Wang, B. S. Ghanem, Z. Ali, K. Hazazi, Y. Han and I. Pinnau, Small Struct., 2021, 2, 210049 Search PubMed.
- W. Han, C. Zhang, M. Zhao, F. Yang, Y. Yang and Y. Weng, J. Membr. Sci., 2021, 636, 119544 CrossRef CAS.
- S. Mohsenpour, A. W. Ameen, S. Leaper, C. Skuse, F. Almansour, P. M. Budd and P. Gorgojo, Sep. Purif. Technol., 2022, 298, 121447 CrossRef CAS.
- M. Chen, F. Soyekwo, Q. Zhang, C. Hu, A. Zhu and Q. Liu, J. Ind. Eng. Chem., 2018, 63, 296–302 CrossRef CAS.
- I. Jeong, I. Hossain, A. Husna and T. H. Kim, Macromol. Mater. Eng., 2022, 308, 2200596 CrossRef.
- A. A. Shamsabadi, M. Rezakazemi, F. Seidi, H. Riazi, T. Aminabhavi and M. Soroush, Prog. Energy Combust. Sci., 2021, 84, 100903 CrossRef.
- Y. Sun, J. Zhang, H. Li, F. Fan, Q. Zhao, G. He and C. Ma, Sep. Purif. Technol., 2023, 314, 123623 CrossRef CAS.
- S. He, B. Zhu, S. Li, Y. Zhang, X. Jiang, C. H. Lau and L. Shao, Sep. Purif. Technol., 2022, 284, 120277 CrossRef CAS.
- Y. Liu, Masteral thesis, Tianjin University, 2015.
- M. Hideto, T. Akihiro, N. Tadashi and K. Yoshiiro, J. Membr. Sci., 1999, 163, 221–227 CrossRef.
- Z. Wang, M. Li, Y. Cai, J. Wang and S. Wang, J. Membr. Sci., 2007, 290, 250–258 CrossRef CAS.
- I. Taniguchi, T. Kai, S. Duan, S. Kazama and H. Jinnai, J. Membr. Sci., 2015, 475, 175–183 CrossRef CAS.
- Z. Tong and W. S. W. Ho, Sep. Sci. Technol., 2016, 52, 156–167 CrossRef.
- S. Janakiram, X. Yu, L. Ansaloni, Z. Dai and L. Deng, ACS Appl. Mater. Interfaces, 2019, 11, 33302–33313 CrossRef CAS.
- M. Wang, Z. Wang, J. Wang, Y. Zhu and S. Wang, Energy Environ. Sci., 2011, 4, 3955–3959 RSC.
- Y. Kai, W. Zhi, W. Jixiao and W. Shichang, Chem. Commun., 2012, 48, 1766–1768 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra00444b |
| This journal is © The Royal Society of Chemistry 2024 |