Open Access Article
Open Access ArticleInnovative snail-mucus-extract (SME)-coated nanoparticles exhibit anti-inflammatory and anti-proliferative effects for potential skin cancer prevention and treatment†
Consoli Valeriaab,
Petralia Salvatore ac,
Vanella Lucaab,
Gulisano Mariaa,
Maugeri Ludovicaa,
Satriano Cristinad,
Montenegro Luciaab,
Castellano Angelae and
Sorrenti Valeria
ac,
Vanella Lucaab,
Gulisano Mariaa,
Maugeri Ludovicaa,
Satriano Cristinad,
Montenegro Luciaab,
Castellano Angelae and
Sorrenti Valeria *ab
*ab
aDepartment of Drug and Health Sciences, University of Catania, Via Santa Sofia 64, 95125 Catania, Italy. E-mail: sorrenti@unict.it
bCERNUT-Research Centre for Nutraceuticals and Health Products, University of Catania, 95125 Catania, Italy
cCNR-Institute of Biomolecular Chemistry, Via Paolo Gaifami 18, 95126 Catania, Italy
dNanoHybrid Biointerfaces Laboratory (NHBIL), Department of Chemical Sciences, University of Catania, Viale Andrea Doria, 6, 95125 Catania, Italy
eMediterranean Nutraceutical Extracts (Medinutrex), Via Vincenzo Giuffrida 202, 95128 Catania, Italy
First published on 4th March 2024
Abstract
Nowadays, several studies have highlighted the ability of snail mucus in maintaining healthy skin conditions due to its emollient, regenerative and protective properties. In particular, mucus derived from H. aspersa muller has been reported to have beneficial effects such as antioxidant, antimicrobial activity and wound repair capacity. To enhance antioxidant activity of snail mucus, it was extracted in a hydroalcoholic solution and consequently freeze-dried. The obtained snail mucus extract (SME) was indeed endowed with higher antioxidant activity observed in cell-free models, however it was not possible to test its effects in cellular models as it creates a thick film on the cell surface. Therefore, in order to enhance beneficial effects of snail mucus and extend its potential use, SME was used to develop snail mucus extract-coated gold nanoparticles (AuNPs-SME) which exhibited anti-inflammatory properties on non-tumorigenic cells. LPS-induced inflammation in human NCTC keratinocytes was used as model to investigate the in vitro cytoprotective effects of nanoparticles. Co-treatment with LPS and AuNPs-SME significantly reduced pro-inflammatory cytokine transcription. Moreover, we demonstrated that AuNPs-SME not only can be used for anti-inflammatory treatments, but also as a sunscreen and antioxidant for potential cosmetic applications. Furthermore, AuNPs-SME's ability to selectively inhibit the growth of two human melanoma cell lines without affecting immortalized human keratinocyte viability in the same conditions was assessed. Thus, we demonstrated that snail mucus is suitable for creating innovative formulations and it can be considered a valid candidate for cosmeceutical applications to enrich the snail mucus based anti-age and sunscreen products already present on the market. Moreover, innovative formulations containing snail mucus can be potentially used for the treatment of specific skin neoplasms.
Introduction
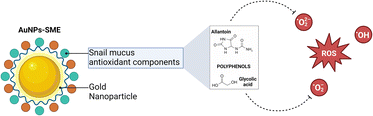
Land helix mucus use in medicine has been described by several studies highlighting the ability of snail mucus in maintaining healthy skin conditions thanks to its emollient, regenerative and protective properties. In particular, mucus derived from H. aspersa muller has been reported to have beneficial properties such as antimicrobial activity and wound repair capacity.1–3 It has been reported that snail mucus is able to repair the signs of photoaging by stimulating the formation of collagen, elastin and dermal components. Moreover, it is able to counteract the damage generated by free radicals thanks to the presence of hyaluronic acid and antioxidants.4 Helix mucus is rich in bioactive compounds such as allantoin, helicidine and helix pomatia agglutinin. Allantoin, also called 5-ureidohydantoin, is used as active ingredient in cosmetics, thanks to its keratolytic effects, the ability to increase the water content of the extracellular matrix and the capacity to enhance wound healing. Helicidine is a mucoglycoprotein able to induce broncho-relaxant effect which is associated with the release of E2 prostaglandins.5 Helix pomatia agglutinin (HPA) is a N-acetylgalactosamine (GalNAc) used as a prognostic indicator for some types of cancer, such as colon and breast tumors, since HPA-associated glycoproteins are linked to metastasis of cancers.6–8However, despite the multiple beneficial effects, poor pharmacokinetics of the main bioactive compounds found in snail mucus limit their therapeutic applications. Thus, nanotechnological formulations can be useful for improving their bioavailability.9 To date, only few studies have reported the development of nanotechnological formulations containing snail slime.10–14 Moreover, recent works reported snail slime extracted from Helix Aspersa snails employment as bio-reducing agent to obtain silver and biogenic gold nanoparticles by green, fast and economical synthesis.
It has been reported that green synthesized gold nanoparticles can exhibit antimicrobial and antioxidant activities, together with skin-lightening, anti-inflammatory and sunscreen properties,10,11 while green synthesized silver nanoparticles were proved to be effective in inhibiting bacterial growth as well as cancer cells proliferation.13,14
In this context, snail mucus among others, has gained interest for the development of novel naturally derived nanomaterials for different applications, due to improved biocompatibility and biodegradability. In particular, innovative nanomaterials as carbon dots and biopolymers have arisen as interesting alternatives to conventional synthetic materials. Interesting examples have been reported by Das and colleagues who exploited various wastes as sources of biopolymers for the development of UV-resistant polymeric thin films applicable to food and textile industry together with biomedical field.15,16 Additionally, chitin-enriched biopolymers derived from seafood wastes have recently been reported to be a useful alternative for encapsulation of poorly bioavailable compounds as curcumin. Moreover, Md. Asad Khan et al.17 reported that the encapsulation of curcumin within chitosan nanoparticles improved its bioavailability and suggested that curcumin loaded nanoformulations, might be promising candidates in cancer therapy. Fedaa Adaileh et al.18 demonstrated that curcumin-loaded γ-cyclodextrin-grafted hyaluronic acid nanoassimblies can be a promising nanocarrier for clinically useful hydrophobic molecules.
Thus, exploiting innovative nanotechnologies can be the key for the development of more effective cancer treatments, indeed nanostructures offer the benefit of targeted anticancer therapy together with the possibility of specific accumulation in cancer tissues due to leaky nature of tumor vasculature.19,20
Hybrid nanostructures combining the physical and chemical properties of more classes of nanomaterials as metal nanoparticles, graphene-oxide nanosheet, thermos-responsive polymers and calix-macrocycles hold great promise for biomedical application.21–23
In this context, equipping gold-nanoparticles (AuNPs) with various nanostructures offer good biocompatibility, effective photothermal conversion upon light absorption due to the localized surface plasmon resonance (LSPR) phenomenon and other typical properties of combined nanostructures.
Aim of the present study was to investigate the potential anti-inflammatory and sunscreen properties of gold nanoparticles in immortalized human keratinocyte NCTC cells. In addition, we evaluated the ability of AuNPs-SME to inhibit the growth of two human melanoma cell lines. The novelty of the present work lies on the use of an innovative snail mucus extract obtained by collecting snail mucus through a cruelty-free system and processing it to achieve an extract with higher concentrations of polyphenols compared to snail mucus as it stands, for the development of innovative systems for cancer prevention and treatment.
Results and discussion
Antioxidant activity of snail mucus extract (SME) in cell-free models
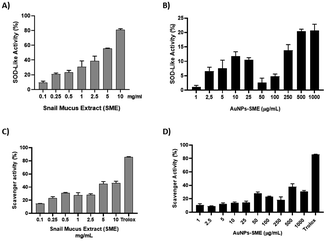
Helix mucus is rich in bioactive compounds, however its composition depends on different factors, such as the snail species, feeding and behaviour of the animals. It has been reported that snail mucus is able to counteract the damage generated by free radicals thanks to the presence of hyaluronic acid and antioxidants (Conte et al.). As previously reported,24 low levels of polyphenols justified the poor antioxidant activity of SEM provided by “La lumaca Madonita”. To enhance antioxidant activity of snail mucus, it was extracted in a hydroalcoholic solution and subsequently freeze-dried. The extraction method process has allowed to obtain a much higher polyphenol content compared to snail slime. The total phenolic content (TPC) was evaluated spectrophotometrically. The powdered Snail Mucus Extract (SME) was standardized to contain ≥0.3% TPC. SME showed significant antioxidant activity in a concentration-dependent manner, with respect to the scavenger activity of DPPH and inhibition of superoxide anion, as shown in Fig. 6. However, although this extract was endowed with high antioxidant activity in cell-free models, it was not possible to test its effects in cellular models as it created an insoluble film on cell surface. These data are in agreement with Conte et al.9 who reports that the poor pharmacokinetics of the main bioactive compounds of snail mucus, limit their therapeutic applications. Nanotechnological formulations can be useful in improving their bioavailability. Therefore, in order to enhance beneficial effects of snail mucus its extract was then used to develop snail mucus-coated gold nanoparticles (AuNPs-SME).Gold-nanoparticles-snail-mucus-extract (AuNPs-SME) preparation
In order to obtain nanostructured AuNPs-SME the chemical synthesis was carried out by direct reaction of gold precursor (HAuCl4) with an aqueous dispersion of SME. The known reducing properties of SME induces the reduction process from AuIII to Au0 to form SME capped AuNPs nanostructures. The good stability and the water dispersion of the as prepared gold nanostructures were guaranteed by the SME residue at gold surface. In details, an amount of 150 mg of dried snail-mucus-extract was dispersed in a volume of 100 mL of MilliQ water. Under continuously stirring an aliquot of 750 μL of HAuCl4 5 × 10−2 M was added and the mixture heated at 70 °C for 90 minutes. The formation of Au-nanostructures was indicated by the reddish-purple colour mixture. The dispersion was separated by centrifugation (13.000 rpm 5 min) and the reddish-purple pellet was washed three times with 1 mL of MilliQ water. Then, the AuNPs-SME was purified by dialysis using MilliQ water through a dialysis membrane (10 kDa cut off) for 12 hours. Finally, the AuNPs-SME was lyophilized and stored at room temperature. UV-vis optical absorption spectra of AuNPs-SME exhibited the typical LSPR absorption bands for Au-nanostructures at 548 nm and the absorption band at around 272 nm to indicate the presence of snail mucus as capping agent (Fig. 1).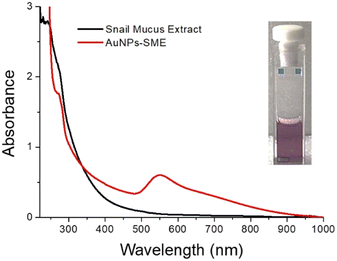 | ||
| Fig. 1 Optical absorption spectrum of AuNPs-SME and snail-mucus in water. Insets the water dispersion of AuNPs-SME. | ||
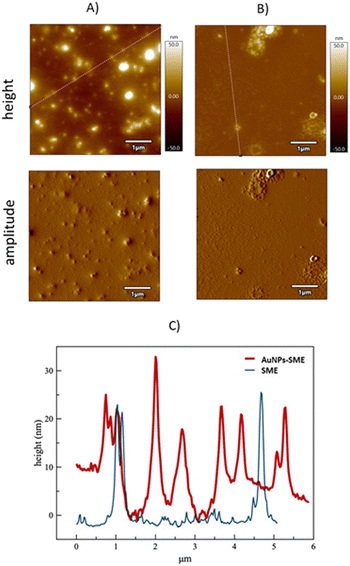
Morphology of AuNPs-SME was investigated by Atomic Force Microscopy investigation (AFM). AFM was used to visualize individual nanoparticles and groups of nanoparticles, indeed, unlike other techniques, it can offer visualization in three dimensions. AFM images for AuNPs-SME sample showed the presence of sphere-like nanostructures (Fig. 2A), the section analysis report a nanostructure size ranging from 5 to 30 nm (Fig. 2C red line). At contrary, AFM images for untreated snail mucus sample showed the presence of sporadic large aggregates with size ranging from 20 to 30 nm (Fig. 2B and C blue line).
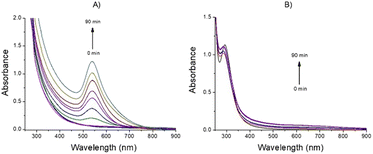
The AFM data are in line with the hydrodynamic size obtained by DLS investigations. Indeed, DLS measurements in water evidenced for AuNPs-SME the presence of small nanostructures with hydrodynamic diameter of 6.7 ± 1.5 nm and larger nanostructures sized 100.5 ± 10.1 nm, and a Z-potential value of −21.1 ± 2.9 mV. The AuNPs-SME formation was confirmed by FTIR investigation, the Fig. S1† illustrates the FTIR-ATR absorption spectra for SME and for AuNPs-SME samples. In particular, the FTIR signal at about 1613.9 cm−1 and in the region 1015–858.2 cm−1 and 1555–1530 cm−1 observed for the AuNPs-SME sample are diagnostic of the presence of the SME in the Au nanostructures. The AuNPs-SME process formation is mainly based on the reduction properties of the snail mucus, as widely reported for similar natural source in literature.25 In presence of the snail mucus the Au3+ species was rapidly reduced to Au0 nanostructures as suggested by the UV-vis optical absorption measurements recorded at various reaction times (0, 10 min, 20 min, 30 min 40 min, 50 min, 60 min, 70 min, 80 min and 90 min) whereas the appearance of the typical LSPR band at around 548 nm confirm the Au-nanostructures formation (Fig. 3A). At contrary, the optical absorption spectra recorded for the blank reaction (same experimental conditions but without the snail mucus) did not show the LSPR band, to indicate that the Au-nanostructures formation does not occur in absence of snail mucus (Fig. 3B).
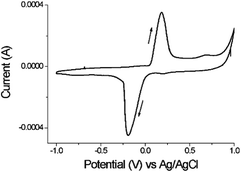
The reductant properties of snail mucus were confirmed by cyclic voltammetry (CV) investigations. The cyclic voltammograms in KCl 0.1 M at pH 7.0 showed the presence of the reduction peak at Ea = − 0.19 V and oxidation peak, at Ec = + 0.18 V (Fig. 4). The reduction potential value of −0.19 V observed (closer to the value of standard reductant used for AuNPs formation sodium citrate −0.18 V) is enough to induce the reduction process from Au3+ to Au0.
AuNPs-SME photothermal properties
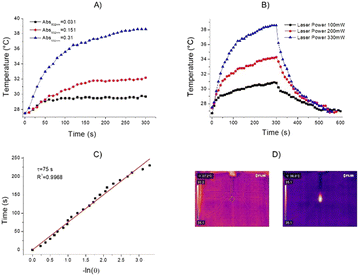
Metallic nanomaterials, as gold, have gained great scientific and technological interest for their key ability to interact with light in the visible to NIR region. When interacting with light at the appropriate wavelengths, free electrons on the metallic nanoparticle surface are excited and conduction-band electrons collectively oscillate at the same frequency. This phenomenon is identified as localized surface plasmon resonance (LSPR). Indeed, gold exhibits photothermal effects arising from their LSPR. To investigate the photothermal properties of AuNPs-SME, an aqueous dispersion (200 μL, Abs532nm = 0.31) was continuously exposed to a 532 nm laser light source. The temperature changes were monitored by a thermal camera. When the temperature of the system reached the maximum, the laser was switched off and the temperature changes during cooling was recorded to confirm the heat transfer of the system. The laser power was measured by a power meter model H410D (ScienTech). A photothermal conversion efficiency (η) value of 40.2% with a time constant of 75 s were calculated. To better investigate the correlation between the photothermal activity and the AuNPs-SME amount, experiments using AuNPs-SME dispersions with different absorbance value were performed. The results reported in Fig. 5A indicated temperature increases of about 11.1 °C, 4.6 °C and 2.5 °C for Absorbance (532 nm) values of 0.31, 0.15 and 0.03, respectively. A volume of 200 μL of sample was irradiated with a CW laser 532 nm (power 330 mW) for 5 minutes (Fig. 5A). The power-dependent behaviour was confirmed by the experiments performed with different laser power of 100 mW, 200 mW, and 330 mW, the temperature values increase was about 4.2 °C, 7.6 °C, 8 °C, and 12 °C, respectively (Fig. 5B). Fig. 5C report the time constant calculation. Fig. 5D report the representative thermos-photographs of AuNPs-SME dispersion in water during the photothermal experiments.Comparison of SME and AuNPs-SME antioxidant activity in cell-free models
In order to evaluate the potential higher antioxidant activity of SME and Au-NPs-SME (due to higher polyphenols content) compared to snail mucus (previously reported) we performed different cell-free assays. Due to the nature of the bio-organic layer on NPs given by snail mucus extract surrounding Au-core, DPPH stabilization and antioxidant activities were observed. Indeed, the presence of amino acids, proteins and different peptides may be able to exert scavenging activity. In particular, SME abundance in polyphenols and the high content of allantoin and glycolic acid, which have already been reported as strong antioxidants,26,27 are able to confer scavenging activity to AuNPs-SME (Fig. 7). SME showed significant antioxidant activity in a concentration-dependent manner, with respect to the inhibition of superoxide anion formation, as shown in Fig. 6A, however AuNPs-SME showed a slight SOD-like activity compared to SME (Fig. 6B). In addition, antioxidant activity of SME or gold nanoparticles coated with SME was evaluated also using the DPPH test. The stable free radical DPPH˙ produced is purple, absorbing at 517 nm. The solution colour changes from purple to yellow when DPPH is all in reduced form, with consequent bleaching of the absorption band of DPPH˙ at 517 nm. In this case, the reaction was slower than the SOD-Like activity, however, the obtained results occurred dose-response dependent and confirmed the antioxidant activity of the SME (Fig. 6C). AuNPs-SME maintained a similar activity for concentrations down to 50 μg mL−1, which is a very low concentration and therefore their antioxidant capacity is noteworthy (Fig. 6D). This may be due to the high antioxidants concentration of SME which is exploited to synthesize nanoparticles and stabilize them.Sun protection factor (SPF) of AuNPs-SME
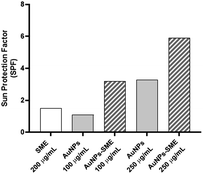
It has been reported that a sunscreen product is able to protect human skin against UV-induced damages thanks to the presence of UV filters as active ingredients. In addition, the use of antioxidants ingredients as additional protection for visible and infrared radiation has also been proposed. As reported by Xian et al. the UV-induced skin photodamage is associated with increased ROS (Reactive Oxygen Species) formation and the inactivation of NF-E2-related factor 2 (Nrf2) with consequent impairing the antioxidant defence system inducing cutaneous disorders.28 Thus, there is growing interest in combining UV-filters properties of antioxidant organic and inorganic compounds to develop hybrid systems as more effective sunscreen formulations.29,30 As SME coating resulted in AuNPs-SME with high absorption values at wavelength <400 nm, the ability of AuNPs-SME to act as UV filter was explored. The calculation of the sun protection factor (SPF) is commonly used to evaluate the efficacy of sunscreen formulations.11 As in vivo standard methods to determine SPF values in humans are quite expensive and time-consuming, alternative in vitro tests that allow obtaining reliable results have been developed.31–34 In this work, we used the spectrophotometric method based on Mansur equation to determine in vitro SPF values as this type of test has already been used to evaluate the photo-protective effects of active ingredients incorporated into nanoparticles.35 The results of SPF determination are illustrated in Fig. 8. SME at the concentration used in in vitro SPF test (200 μg mL−1) did not provide a significant photo-protective activity (SPF = 1.5). Similarly, uncoated AuNPs at different concentrations, 100 and 250 μg mL−1, showed low SPF values (1.1 and 3.3, respectively). However, it is interesting to note that increasing AuNPs concentration raised the SPF value, thus suggesting that the metallic core of AuNPs may act as inorganic sunscreen. The ability of NPs to behave as UV-blockers has already been reported by others studying the effects of lipid nanoparticles as photo-protective agents.36,37 Coating AuNPs with SME provided a notable increase of SPF value compared with uncoated AuNPs at the same concentration. In particular, about a three-folds increase was observed for AuNPs at 100 μg mL−1 (SPF = 3.2) while about a two-folds increase was obtained for AuNPs at 250 μg mL−1 (SPF = 5.9). These data suggest a synergic effect between SME coating and NP metallic core that could be useful to design topical formulations with reduced content of synthetic sunscreen agents whose safety for both humans and environment is under debate.Biological evaluation in cellular models
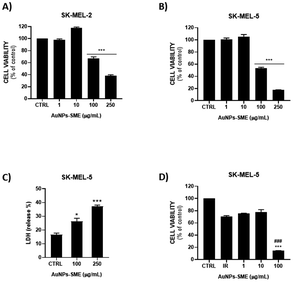
In order to evaluate potential cytotoxicity of AuNPs-SME, NCTC 2544 cells were treated with different concentrations of gold nanoparticles coated with SME (0.1, 0.5, 1, 2.5, 5, 10, 25, 50, 100, 250 μg mL−1 AuNPs-SME) for 48 h and subsequently MTT assay was performed (Fig. 9).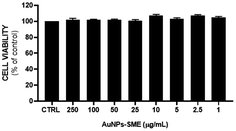 | ||
| Fig. 9 AuNPs-SME effect on keratinocytes viability after 48 h treatment. Results are expressed as mean ± SEM. | ||
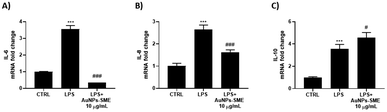
No significant cytotoxic effect was observed. To further assess the possible effects of the AuNPs-SME on the biological inflammatory response, keratinocytes were challenged with LPS and the day after treated with 10 μg mL−1 AuNPs-SME. Results showed AuNPs-SME capacity to reduce IL-6 and IL-8 pro-inflammatory cytokines transcription (Fig. 10A and B) and increase anti-inflammatory IL-10 even at low concentrations (10 μg mL−1) (Fig. 10C).
These results suggest that AuNPs-SME may have modulatory effects that could be beneficial in reducing inflammatory or pathogenic responses. Moreover, we investigated the possible therapeutic effect of AuNPs-SME against skin cancer. Indeed, ability of AuNPs-SME to inhibit the growth of two human melanoma cell lines, SK-MEL-2 and SK-MEL-5 cells, was evaluated. MTT assay was performed with different concentrations of AuNPs-SME in order to assess its effect on cell viability. Our results showed that after 48 h treatment, AuNPs-SME was not cytotoxic for NCTC 2544 cells non tumorigenic cells whereas it decreased the SK-MEL-2 and SK-MEL-5 human melanoma cell lines viability in a concentration-dependent manner (Fig. 11A and B). A potential explanation may lie behind melanogenesis process involved in tumorigenesis. Indeed, tyrosinase, which is found to be overexpressed in melanoma cells, represents a vital and rate-limiting copper-containing enzyme for melanin biosynthesis.38,39 Thus, tyrosinase has gained interest as a specific in situ weapon which can be potentially used to improve therapeutic efficacy and specificity of melanoma treatment. In this context, it is known that both snail mucus and Au-NPs can affect tyrosinase activity and subsequently act as anticancer agents, indeed it is reasonable to correlate their inhibitory activity towards tyrosinases and selectivity towards melanoma cells instead of keratinocytes, which on the other hand show lower tyrosinase levels and activity.11,40,41 The highest antiproliferative effect of AuNPs-SME was reached at the concentration of 250 μg mL−1. This effect was more evident for SK-MEL-5 (38% residual vitality) compared to SK-MEL-2 cells (18% residual vitality) (EC50 values reported in Table S1†). Moreover, the standard chemotherapeutic agent 5-FU was tested on both NCTC 2544 and SK-MEL-5 cell lines to verify its cytotoxic effect and compare it to AuNPs-SME. As reported in Fig. S2,† 5-FU treatment resulted in a reduction in both cell lines viability and unlike AuNPs-SME 5-FU did not spare keratinocytes. To investigate molecular mechanisms involved in reduction of SK-MEL-5 cell proliferation, we evaluated the percentage of LDH released into the culture medium as marker of necrosis which results in a disruption of cytoplasmic membrane with the consequent release of cytoplasmic LDH into the medium. As reported in Fig. 11C the presence of 100 and 250 μg mL−1 of AuNPs-SME caused a significant increase of LDH release in SK-MEL-5 cells. The highest AuNPs-SME concentration (250 μg mL−1) induced necrosis with about 40% LDH release in the medium. In addition, caspase and apoptosis activation were investigated by using pan-caspase inhibitor (ZVAD-FMK) in combination with AuNPs-SME to assess cell viability, however no significant caspase involvement was observed (data not shown).
Moreover, in order to validate the possible application for photothermal therapy (PTT) in more sensitive human melanoma line (SK-MEL-5), cells were exposed to irradiation treatment alone and in combination with AuNPs-SME at different concentrations (Fig. 11D). Results showed a synergistic effect of AuNPs-SME and photothermia as the combination treatment was able to drastically reduce melanoma cell viability, suggesting AuNPs-SME as potential candidate for PTT in some specific skin cancers.
Materials and methods
General
All chemicals and reagents were used as purchased, without further purification. The cell lines utilized in this study were obtained from Interlab Cell Line Collection Genoa and the American Type Culture Collection (ATCC).
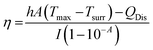 | (1) |
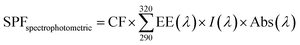 | (2) |
| % inhibition of pyrogallol autoxidation = [1 − (ΔA/ΔAmax)] × 100 |
Statistical analysis
At least three independent experiments were performed for each analysis. The statistical significance (p < 0.05) of the differences between the experimental groups was determined by Fisher's method for analyses of multiple comparisons. For comparison between treatment groups, the null hypothesis was tested via either a single-factor analysis of variance (ANOVA) for multiple groups or an unpaired t-test for two groups. Data are presented as means ± SEM.Conclusions
The integration of nanotechnology with health, beauty, and anti-aging products is anticipated to shape the future landscape significantly.45–48 Consequently, while the use of nanotechnology holds great promise in a wide spectrum of fields, it is imperative to address potential negative consequences associated with nanomaterials beforehand. Comprehensive toxicity studies are essential, along with thorough investigations into the impacts of various nanomaterials used in the industry on human health and the environment.49 Studies on skin cancer have demonstrated that encapsulating therapeutics in nanoparticles enhances efficacy compared to free agents, resulting in a notable reduction in systemic and local toxicity, however obtaining good loading capacity and controlled release of most nanoparticles are the main challenges to be overcome. In this context, nanoparticles like AuNPs-SME, where the coating with snail mucus extract provides additional benefits and functions can be advantageous. In applications aimed at preventing skin cancer, our work is in line with evidence suggesting that the addition of antioxidants enhances the stability of sun-protective agents.50 Indeed, this combined approach can be particularly significant in the context of skin cancer prevention. Despite the substantial expansion of preclinical research on nanoparticle use, its clinical application remains limited. Further studies are required to evaluate patient response to nanoparticle delivery in clinical settings. Challenges persist in manufacturing nanoparticles at a commercial scale, necessitating special attention to nanoparticle reproducibility and stability for clinical use. Nevertheless, despite these challenges, nanoparticle-based therapeutic delivery for skin cancer prevention and treatment holds significant promise in reducing skin cancer incidence and improving survivability rates, thereby benefiting populations that may have previously had limited access to optimized therapeutics.51Herein, we demonstrated that snail mucus is suitable for creating innovative formulations and it can be considered valid candidate for cosmeceutical applications to enrich the snail mucus based anti-age and sunscreen products already present in the market. Innovative formulations based on nanostructured materials can be useful in improving snail mucus bioavailability. In particular SME-capped gold nanoparticles prepared by a reagent-free green one-pot synthesis represents a mild approach to integrate the properties of natural compounds with the exclusive behaviours of metal nanostructured materials.
In our experimental conditions we demonstrated that AuNPs-SME were able to modulate the inflammatory response induced by LPS in human keratinocyte cell line NCTC 2544 by significantly down-regulating the inflammatory process and selectively targeting interleukin pathway. In addition, we found that AuNPs-SME inhibited the growth of two human melanoma cell lines and did not affect immortalized human keratinocyte NCTC 2544 cells taken as non-tumorigenic cells. So AuNPs-SS can be considered an interesting cancer cells proliferation and/or metastasis inhibitor. Therefore, innovative nanotechnological formulations containing snail mucus can be used not only as anti-age and sunscreen products but also to obtain cream/gel for specific topical application in cancer treatment.
Author contributions
Conceptualization: Sorrenti V., Vanella L., Petralia S.; data curation: Montenegro L., Consoli V., Maugeri L., Satriano C.; formal analysis: Montenegro L., Consoli V., Maugeri L., Satriano C., Gulisano M.; Castellano A. Investigation: Montenegro L., Consoli V., Maugeri L., Satriano C., Gulisano M.; Petralia S.; supervision: Sorrenti V., Petralia S., Vanella L.; writing – original draft: Consoli V., Maugeri L.; writing – review & editing: Sorrenti V., Petralia S., Montenegro L.; funding acquisition: Sorrenti V.; project administration: Sorrenti V.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the “SMART UP Project (PSR SICILIA 2014/20 Sottomisura 16.1—Bando 2018—D. D. S. 3092/2020 del 15/10/2020—CUP G66D20000610009)”.Notes and references
- G. Cilia and F. Fratini, J. Complementary Integr. Med., 2018, 15(3), 20170168 CrossRef PubMed.
- C. Trapella, R. Rizzo, S. Gallo, A. Alogna, D. Bortolotti, F. Casciano, G. Zauli, P. Secchiero and R. Voltan, Sci. Rep., 2018, 8, 17665 CrossRef PubMed.
- D. E. López Angulo and P. J. do Amaral Sobral, Int. J. Biol. Macromol., 2016, 92, 645–653 CrossRef PubMed.
- M. A. El Mubarak, F. N. Lamari and C. Kontoyannis, J. Chromatogr. A, 2013, 1322, 49–53 CrossRef CAS PubMed.
- F. Pons, M. Koenig, R. Michelot, M. Mayer and N. Frossard, Pathol. Biol., 1999, 47, 73–80 CAS.
- N. D. Rambaruth, P. Greenwell and M. V. Dwek, Glycobiology, 2012, 22, 839–848 CrossRef CAS PubMed.
- M. V. Dwek, H. A. Ross, A. J. Streets, S. A. Brooks, E. Adam, A. Titcomb, J. V. Woodside, U. Schumacher and A. J. Leathem, Int. J. Cancer, 2001, 95, 79–85 CrossRef CAS PubMed.
- S. A. Brooks and A. J. Leathem, Lancet, 1991, 338, 71–74 CrossRef CAS PubMed.
- R. Conte, Int. J. Nano Dimens., 2016, 7(3), 181–185 CAS.
- J. Gubitosa, V. Rizzi, P. Fini, A. Laurenzana, G. Fibbi, C. Veiga-Villauriz, F. Fanelli, F. Fracassi, A. Onzo, G. Bianco, C. Gaeta, A. Guerrieri and P. Cosma, Soft Matter, 2020, 16, 10876–10888 RSC.
- V. Rizzi, J. Gubitosa, P. Fini, S. Nuzzo, A. Agostiano and P. Cosma, J. Photochem. Photobiol., B, 2021, 224, 112309 CrossRef CAS PubMed.
- M. F. Di Filippo, V. Di Matteo, L. S. Dolci, B. Albertini, B. Ballarin, M. C. Cassani, N. Passerini, G. A. Gentilomi, F. Bonvicini and S. Panzavolta, Nanomaterials, 2022, 12, 3447 CrossRef CAS PubMed.
- A. A. El-Attar, H. B. El-Wakil, A. H. Hassanin, B. A. Bakr, T. M. Almutairi, M. Hagar, B. H. Elwakil and Z. A. Olama, Membranes, 2022, 12, 536 CrossRef CAS PubMed.
- P. C. Mane, S. A. R. Sayyed, D. D. Kadam, M. D Shinde, A. Fatehmulla, A. M. Aldhafiri, E. A. Alghamdi, D. P. Amalnerkar and R. D. Chaudhari, Sci. Rep., 2021, 11, 13068 CrossRef CAS PubMed.
- P. Das, M. Sherazee, P. K. Marvi, S. R. Ahmed, A. Gedanken, S. Srinivasan and A. R. Rajabzadeh, ACS Appl. Mater. Interfaces, 2023, 15, 29425–29439 CrossRef CAS PubMed.
- P. Das, S. Ganguly, S. R. Ahmed, M. Sherazee, S. Margel, A. Gedanken, S. Srinivasan and A. R. Rajabzadeh, ACS Appl. Polym. Mater., 2022, 4, 9323–9340 CrossRef CAS.
- M. A. Khan, M. Zafaryab, S. H. Mehdi, I. Ahmad and M. M. Rizvi, Int. J. Biol. Macromol., 2016, 93, 242–253 CrossRef CAS PubMed.
- F. Adaileh, W. Alshaer, H. Nsairat, D. A. Alqudah, S. Wehaibi, F. Daoud, R. Al-Buqain, S. Alsotari, A. Al Bawab and F. Odeh, J. Drug Delivery Sci. Technol., 2023, 87, 104886 CrossRef CAS.
- F. Rodríguez, P. Caruana, N. De la Fuente, P. Español, M. Gámez, J. Balart, E. Llurba, R. Rovira, R. Ruiz, C. Martín-Lorente, J. L. Corchero and M. V. Céspedes, Biomolecules, 2022, 12, 784 CrossRef PubMed.
- E. Pérez-Herrero and A. Fernández-Medarde, Eur. J. Pharm. Biopharm., 2015, 93, 52–79 CrossRef PubMed.
- G. M. L. Consoli, M. L. Giuffrida, C. Satriano, T. Musumeci, G. Forte and S. Petralia, Chem. Commun., 2022, 58, 3126–3129 RSC.
- G. M. L. Consoli, L. Maugeri, G. Forte, G. Buscarino, A. Gulino, L. Lanzanò, P. Bonacci, N. Musso and S. Petralia, J. Mater. Chem. B, 2024, 12, 952–961 RSC.
- G. M. L. Consoli, G. Forte, L. Maugeri, V. Consoli, V. Sorrenti, L. Vanella, G. Buscarino, S. Agnello, M. Camarda, G. Granata, L. Ferreri and S. Petralia, ACS Appl. Nano Mater., 2023, 6, 358–369 CrossRef CAS.
- L. Vanella, V. Consoli, I. Burò, M. Gulisano, M. S. Giglio, L. Maugeri, S. Petralia, A. Castellano and V. Sorrenti, Int. J. Mol. Sci., 2023, 24, 10185 CrossRef CAS PubMed.
- C. E. A. Botteon, L. B. Silva, G. V. Ccana-Ccapatinta, T. S. Silva, S. R. Ambrosio, R. C. S. Veneziani, J. K. Bastos and P. D. Marcato, Sci. Rep., 2021, 11, 1974 CrossRef CAS PubMed.
- E. B. Houshmand, J. Cosmet., Dermatol., 2021, 20, 776–780 CrossRef PubMed.
- Z. Selamoglu, C. Dusgun, H. Akgul and M. F. Gulhan, Iran. J. Pharm. Res., 2017, 16, 92–98 Search PubMed.
- D. Xian, X. Gao, X. Xiong, J. Xu, L. Yang, L. Pan and J. Zhong, J. Photochem. Photobiol., B, 2017, 175, 73–82 CrossRef CAS PubMed.
- V. H. P. Infante, P. M. B. G. Maia Campos, L. S. Calixto, M. E. Darvin, M. Kröger, S. Schanzer, S. B. Lohan, J. Lademann and M. C. Meinke, Int. J. Pharm., 2021, 598, 120262 CrossRef CAS PubMed.
- C. Jennifer, V. R. Gubitosa, V. Rizzi, P. Fini and Pinalysa, in Nanocosmetics, ed. A. Nanda, S. Nanda, T. A. Nguyen, S. Rajendran and Y. Slimani, Elsevier, 2020, pp. 349–373 Search PubMed.
- A. Dimitrovska Cvetkovska, S. Manfredini, P. Ziosi, S. Molesini, V. Dissette, I. Magri, C. Scapoli, A. Carrieri, E. Durini and S. Vertuani, Int. J. Cosmet. Sci., 2017, 39, 310–319 CrossRef CAS PubMed.
- M. Pissavini, C. Tricaud, G. Wiener, A. Lauer, M. Contier, L. Kolbe, C. Trullás Cabanas, F. Boyer, E. Meredith, J. de Lapuente, E. Dietrich and P. J. Matts, Int. J. Cosmet. Sci., 2020, 42, 421–428 CrossRef CAS PubMed.
- E. P. Santos, Z. M. Freitas, K. R. Souza, S. Garcia and A. Vergnanini, Int. J. Cosmet. Sci., 1999, 21, 1–5 CrossRef CAS PubMed.
- C. D. Kaur and S. Saraf, Pharmacogn. Res., 2010, 2, 22–25 CrossRef CAS PubMed.
- G. Netto MPharm and J. Jose, J. Cosmet., Dermatol., 2018, 17, 1073–1083 CrossRef PubMed.
- J. Jose and G. Netto, J. Cosmet., Dermatol., 2019, 18, 315–321 CrossRef PubMed.
- S. A. Wissing and R. H. Müller, Pharmazie, 2001, 56, 783–786 CAS.
- M. Goldfeder, M. Kanteev, S. Isaschar-Ovdat, N. Adir and A. Fishman, Nat. Commun., 2014, 5, 4505 CrossRef CAS PubMed.
- Y. Pu, B. Zhou, H. Xiang, W. Wu, H. Yin, W. Yue, Y. Yin, H. Li, Y. Chen and H. Xu, Biomaterials, 2020, 259, 120329 CrossRef CAS PubMed.
- C. Ellijimi, M. Ben Hammouda, H. Othman, W. Moslah, J. Jebali, H. B. Mabrouk, M. Morjen, M. Haoues, J. Luis, N. Marrakchi, K. Essafi-Benkhadir and N. Srairi-Abid, Biomed. Pharmacother., 2018, 101, 871–880 CrossRef CAS PubMed.
- N. Noothuan, K. Apitanyasai, S. Panha and A. Tassanakajon, BMC Res. Notes, 2021, 14, 138 CrossRef CAS PubMed.
- D. K. Roper, W. Ahn and M. Hoepfner, J. Phys. Chem. C, 2007, 111, 3636–3641 CrossRef CAS PubMed.
- E. Abreu Dutra, D. A. Gonçalves da Costa e Oliveira, E. R. M. Kedor-Hackmann and M. I. R. Miritello Santoro, Rev. Bras. Cienc. Farm., 2004, 40(3) DOI:10.1590/S1516-93322004000300014.
- R. M. Sayre, P. P. Agin, G. J. LeVee and E. Marlowe, Photochem. Photobiol., 1979, 29, 559–566 CrossRef CAS PubMed.
- K. Mitri, R. Shegokar, S. Gohla, C. Anselmi and R. H. Müller, Int. J. Pharm., 2011, 420, 141–146 CrossRef CAS PubMed.
- E. Zingale, A. Bonaccorso, C. Carbone, T. Musumeci and R. Pignatello, Pharmaceutics, 2022, 14, 691 CrossRef CAS PubMed.
- S. Kaul, N. Gulati, D. Verma, S. Mukherjee and U. Nagaich, J. Pharm., 2018, 2018, 3420204 Search PubMed.
- S. Shanbhag, A. Nayak, R. Narayan and U. Y. Nayak, Adv. Pharm. Bull., 2019, 9, 348–359 CrossRef CAS PubMed.
- C. Cardoza, V. Nagtode, A. Pratap and S. N. Mali, Health Sci. Rev., 2022, 4, 100051 Search PubMed.
- J. F. Nash, Dermatol. Clin., 2006, 24, 35–51 CrossRef CAS PubMed.
- J. Chang, B. Yu, W. M. Saltzman and M. Girardi, JID Innov., 2023, 3, 100197 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra00291a |
| This journal is © The Royal Society of Chemistry 2024 |