Open Access Article
Open Access ArticleA new plant-esterase inhibition based electrochemical sensor with signal amplification by MoS2@N-CDs for chlorpyrifos detection†
Jiayu Chena,
Chun Jib,
Xiao Wanga,
Yunxia Tiana and
Han Tao *a
*a
aSchool of Liquor and Food Engineering, Guizhou Province Key Laboratory of Fermentation Engineering and Biopharmacy, Guizhou University, Huaxi District, Guiyang 550025, China. E-mail: taohanedu@126.com
bSchool of Pharmaceutical Sciences, Guizhou University, Huaxi District, Guiyang 550025, China
First published on 2nd April 2024
Abstract
Chlorpyrifos (CPF) is the most common pesticide entering the food chain and posing a threat to human health. This study presents a new electrochemical biosensor based on molybdenum disulfide nanosheets and nitrogen-doped carbon dot nanocomposite (MoS2@N-CDs) and kidney bean esterase (KdBE), and it is shown to achieve accurate detection of CPF. MoS2@N-CDs were prepared by a facile solvothermal method and characterized by electron microscopy, X-ray diffraction and X-ray photoelectron spectroscopy. Electrochemical characterization confirmed that MoS2@N-CDs facilitated electron transfer and increased the electroactive surface area of the electrode, thereby improved the sensing performance of the electrode. The oxidation peak current of 1-naphthol, which was produced by the hydrolysis of 1-naphthyl acetate catalyzed by KdBE, was adopted as the signal of the sensor. CPF can suppress KdBE activity and consequently cause a decrease in the sensing signal. The experimental results show that the variation of sensing signal is a reliable index to evaluate the CPF level. Under the optimized conditions, the developed enzyme sensor showed superior CPF assay performance with a linear detection range as wide as 0.01–500 μg L−1 and LOD as low as 3.5 × 10−3 μg L−1 (S/N = 3). The inter- and intra-batch RSDs for electrode testing were 4.02% and 2.69%, respectively. Moreover, the developed biosensor also showed good stability and anti-interference. The spiked recoveries of CPF in oilseed rape and cabbage ranged from 98.09% to 106.01% with low relative standard deviation (RSD) (<5.23%), suggesting that the sensor is a promising tool to enable simple, low-cost but highly sensitive large-scale screening of CPF residues in food.
1. Introduction
CPF is an organophosphorus pesticide that has been widely used for pest control in vegetables, fruits, grains and other crops since 1965.1 CPF is already used in nearly 100 countries, and is likely to be even more widely used in the next 30 years due to food production needs.2 However, the continued widespread use and abuse of CPF over the years has resulted in it being the most common pesticide entering the food chain and affecting human health.3 Therefore, there is a huge demand for CPF monitoring, which leads to the urgent need for a simple, low-cost and sensitive CPF detection method.Currently, multiple quantitative approaches including HPLC,4 GC-MS/MS,5 surface-enhanced Raman spectroscopy,6 fluorescence,7 magnetic relaxation switching biosensors,8 enzyme-linked immunosorbent assay9 and electrochemistry10 have been developed for CPF detection. Among them, electrochemical biosensing techniques utilizing the inhibitory effect of CPF on acetylcholinesterase (AChE) activity have emerged as an important tool for trace CPF residual detection.11,12 Tun et al.13 prepared an AChE-based electrochemical sensor modified with cellulose nanofibers and graphene oxide to implement highly sensitive CPF detection based on the inhibition of CPF on AChE activity. Suwannachat et al.14 constructed an electrochemical biosensor based on AChE and utilized CuNWs and rGO to modify the screen-printed electrode for highly sensitive detection of chlorpyrifos. These methods are undoubtedly simple, fast and highly sensitive. However, AChE is usually acquired from animal blood or tissues with complex extraction and purification steps, which leads to the high cost of AChE, thus greatly limiting its wider application. But, it is gratifying that studies have shown that organophosphorus pesticides can also inhibit the activity of plant esterases.15,16 More attractively, plant esterases are derived from plants, which are far more accessible, ethical and economical than animals, and the extraction of plant esterases is also easier than that of AChE. Therefore, plant esterases can be utilized as a promising and low-cost alternative enzyme source of AChE for large-scale detection of pesticides detection, but electrochemical studies on plant esterases are currently very scarce.
Moreover, to achieve accurate and reliable assay of trace CPF residues in food, the detection technique needs to be highly sensitive. Employing nanomaterials to enhance the sensitivity of electrochemical sensing platforms is a simple and effective strategy. When nanomaterials are introduced to the electrode surface, their special electronic structure and crystal defects can influence the kinetic process of the electrochemical reaction, resulting in a higher analytical sensitivity. Molybdenum disulfide (MoS2) nanosheets is another rapidly developing 2D nanomaterial after graphene, which has the advantages of strong adsorption capacity, high reactivity and catalytic performance.17,18 Moreover, the electron–electron interactions between molybdenum atoms contribute to the enhancement of electron transfer. Hence, MoS2 nanosheets are particularly suitable for the development of advanced sensors. Further, compositing MoS2 with other functional materials often results in better performance through the advantages of materials complementing each other. Nitrogen-doped carbon dots (N-CDs) is quasi-spherical zero-dimensional carbon nanoparticles with sizes within 10 nm. Studies have demonstrated that N-CDs can improve electrode adsorption capacity, electrocatalytic activity and electrode surface stability.19 Also, N-CDs is simple to prepare and easy to combine with other materials. As a result, we considered compositing MoS2 nanosheets with N-CDs and then using them for functionalized modification of the electrodes, with a view to improving the electrode performance so as to effectively increase pesticide detection sensitivity.
Herein, a new electrochemical sensor was fabricated for the low-cost and high-sensitive detection of CPF. In this sensor, KdBE, which was derived from bean crops widely cultivated worldwide, was used as a susceptible biomolecule for the recognition of CPF. To improve detection sensitivity, as-prepared MoS2@N-CDs nanocomposite was modified on a bare glassy carbon electrode as an electrocatalyst to amplify the electrochemical signals. Subsequently, gold nanoparticles (GNPs) were further decorated on the MoS2@N-CDs-functionalized electrode as a substrate for immobilizing KdBE, and then the outer layer was coated with chitosan, which has good film-forming properties and biocompatibility20 to prevent enzyme leakage. CPF can hinder the bioactivity of KdBE and decrease the oxidative current signal of 1-naphthol, a product of enzymatic hydrolysis. By monitoring the fluctuation of the electrochemical signal produced by 1-naphthol, simple, low-cost but highly sensitive detection for CPF can be achieved readily. To the best of our knowledge, no study of MoS2@N-CDs in electrochemical sensing has been reported, nor has the detection of CPF with plant esterases been reported.
2. Experimental
2.1. Materials and reagents
Polyethylene glycol (PEG1000) (AR) was purchased from Shanghai Bioengineering Co., Ltd. Ethylene glycol (99.8%) and thiourea (99%) were purchased from Chongqing Chuandong Chemical Co., Ltd. 1-Naphthyl acetate (1-NA) (≥99.5%) and CPF (99%) were obtained from Sigma-Aldrich Co. Ethylenediamine (≥99.5%), sodium molybdate (≥99%), chlortetracycline (≥99.9%), solid blue B (Dye content∼95%), K4[Fe(CN)6] (99%), K3[Fe(CN)6] (≥99.5%), NaH2PO4 (99%), lindane (Analytical Standard) and deltamethrin (Analytical Standard) were purchased from Aladdin Reagents Co., Ltd. Water used throughout all experiments was ultrapure water (18.2 MΩ cm).2.2. Apparatus
The electrochemical tests were completed by a CHI660E electrochemical analyser (CHI, China), using glassy carbon electrode (GCE) as working electrode, Ag/AgCl electrode as reference electrode and platinum wire as counter electrode. Freeze-drying of KdBE was carried out using a VaCo5 freeze-dryer (Zirbus, Germany). X-ray photoelectron spectroscopy (XPS) was collected on ESCALAB 250Xi (Thermo Fisher Scientific, USA) with an Al Kα radiation source (hν = 1486.6 eV) by pass energy settings of 200 eV (full spectrum) and 50 eV (fine spectrum). X-Ray diffraction (XRD) measurements were performed using an 8 Advance diffractometer (Bruker, Germany) with a voltage of 40 kV, a current of 40 mA, a test range of 5–90°, and a scanning speed of 10° min−1. Scanning electron microscope (SEM) images were acquired using a cold field emission scanning electron microscope SU8010 (Hitachi, Japan) with an accelerating voltage of 10 kV. Transmission electron microscope (TEM) images were obtained from JEM 2100F (JEOL, Japan) with an accelerating voltage of 200 kV. Atomic force microscopy (AFM) was performed by Dimension icon (Bruker, Germany). AFM images of film surface were taken with a scan rate of 1.00 Hz under Tapping mode. The model of probe was Tap300A with k = 40 N m−1.2.3. Preparation of nanomaterials
Preparation of N-CDs: 3.2 mL of ethylenediamine and 2.52 g of citric acid were dissolved in 60 mL of water under magnetic stirring at room temperature. The solution was transferred to a Teflon autoclave and reacted at 180 °C for 5 h. The resulting solution was dialyzed for 24 h and then vacuum dried at 60 °C to obtain N-CDs.Preparation of MoS2@N-CDs nanocomposite: 0.726 g of sodium molybdate was ultrasonically dispersed in 40 mL of ethylene glycol, then 1.1418 g of thiourea and 0.05 g of N-CDs were added and dispersed uniformly. The resulting solution was transferred to an autoclave and reacted at 180 °C for 12 h. The black product was washed several times with ethanol and water and then dried under vacuum at 70 °C to obtain black MoS2@N-CDs powder. MoS2 was prepared with the same method as that of MoS2@N-CDs, except that no N-CDs was added. The preparation process of MoS2@N-CDs is illustrated in Scheme 1A.
Gold colloid were prepared by referring to the method reported previously.21
2.4. Extraction of KdBE
20 g of kidney bean was ground into powder, then 100 mL of water was poured into and stirred for 30 min. The extract was centrifuged and the supernatant was taken as the crude enzyme solution, which was further purified via a two-step bi-aqueous extraction method.22 Briefly, the first step of extraction is carried out in a two-phase aqueous system consisting of 27% PEG1000 and 13% NaH2PO4, and the upper phase was collected after equilibrium of partitioning was reached. Subsequently, (NH4)2SO4 (6%) was added to form the second bi-aqueous system, and the lower phase was collected after partitioning. Afterwards, the lower phase was dialyzed in 0.05 mol L−1 PBS (pH 6.5) for 48 h and then freeze-dried to powder. The image of obtain KdBE is shown in Fig S1.†2.5. Sensor's construction
The surface of GCE was polished with 0.05 μm Al2O3, followed by ultrasonic cleaning in acetone and water sequentially, and then the cleaned GCE was placed in PBS solution for cyclic voltammetry scanning 10 cycles from 0 V–1 V. After dried in air, 20 μL MoS2@N-CDs solution (1.0 mg mL−1) was drop-coated on the GCE surface and dried to obtain MoS2@N-CDs/GCE. Then, 12 μL gold colloid was further coated on and dried to get Au/MoS2@N-CDs/GCE. Subsequently, 10 μL KdBE solution (19.03 U mL−1) was dropped onto Au/MoS2@N-CDs/GCE surface and dried at 4 °C. Finally, 10 μL 0.25% chitosan solution was covered on the outer layer of the modified electrode to obtain CS/KdBE/Au/MoS2@N-CDs/GCE.2.6. Electrochemical measurements
Electrochemical characterization of the electrodes was achieved by cyclic voltammetry (CV) and AC impedance technique in 5.0 mmol L−1 K4[Fe(CN)6]/K3[Fe(CN)6] probe solution. The CV experiments were performed in the potential range of −0.2 ∼ 0.7 V with a sweep rate of 0.1 V s−1. The frequency range of the AC impedance experiment is 1 Hz–105 Hz and the amplitude is 5 mV. To detect CPF, the square wave voltammetry (SWV) technique was used in 0.1 M PBS (pH 7.0) in the range of 0 V–0.8 V. The procedures were as follows: first, the CS/KdBE/Au/MoS2@N-CDs/GCE was placed in 0.1 mol L−1 PBS containing 0.8 mmol L−1 1-NA for SWV testing, corresponding peak current was defined as I0. After incubation with CPF solutions for 15 minutes, the electrode underwent another SWV test in the same test solution, and the peak current was recorded as I1. The inhibition rate of peak current was calculated by eqn (1). The schematic diagram of pesticide detection is depicted in Scheme 1B.
ΔI(%) = (I0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I1)/I0 × 100% I1)/I0 × 100%
| (1) |
3. Results and discussion
3.1. Characterization of nanomaterials
The morphologies of the prepared nanomaterials were characterized by SEM and TEM. As seen in Fig. 1A, MoS2 is assembled by many nano-layers, and the layers are stacked with each other to form a flower-like structure, which can increase the specific surface area and facilitate the loading of other materials. Fig. 1B displays the TEM image of N-CDs, revealing spherical particles with an average size of 3.11 nm that are evenly dispersed. Fig. 1C demonstrates that GNPs in gold colloid are also spherical particles with an average size of 9 nm. The inset of Fig. 1C is the absorption spectrum of GNPs, which shows that the absorbance value at 450 nm was 1.06. According to the method reported by Haiss et al.23 the concentration of GNPs can be estimated to be 23.92 nM. Fig. 1D is the SEM image of MoS2@N-CDs nanocomposite. It is apparent that the morphology of MoS2 remained unchanged almost despite complexation with N-CDs. The inset of Fig. 1E shows the selected area electron diffraction pattern (SAED) of MoS2@N-CDs, and three rings, representing the (002), (100) and (110) crystal planes of MoS2, are clearly visible. The TEM image of MoS2@N-CDs (Fig. 1E) clearly depicts that MoS2 exhibits a muslin-like nanosheet layer structure, while N-CDs are uniformly incorporated in MoS2 nanosheets, thus verifying the successful preparation of the MoS2@N-CDs. The introduction of N-CDs is conducive to the enhancement of electrocatalytic performance of the material, which in turn can improve the detection sensitivity.24Further, the XPS and XRD spectra of MoS2@N-CDs are displayed in Fig. 2. In the full spectra of MoS2@N-CDs (Fig. 2A), five peaks appeared, corresponding to Mo 3d, S 2p, C 1s, N 1s and O 1s, respectively, confirming the existence of C, N, O, Mo and S elements in MoS2@N-CDs. Fig. 2B shows the high-resolution spectrum of Mo 3d, the peaks appeared at 228.7 eV and 232.1 eV are ascribed to Mo(IV)3d5/2 and Mo(IV)3d3/2 of 1T-MoS2, respectively, while the peaks at 229.6 eV and 233.0 eV are assigned to 2H-MoS2. According to the proportion of peak area, the content of 1T phase is estimated to be about 76%. In Fig. 2C, the S 2p3/2 and S 2p1/2 for 1T-MoS2 were located at 161.9 and 163.0 eV, respectively. In C 1s spectrum (Fig. 2D), the peaks at 284.8 eV, 286.0 eV and 288.4 eV correspond to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N and C
C, C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively. Among them, the strongest peak at 284.8 eV indicates the existence of graphite C, while the C–N bond suggests the formation of N-CDs. The XPS spectrum of N 1s shows three peaks (Fig. 2E) corresponding to pyridine-N (398.7 eV), pyrrole-N (402.5 eV) and graphite-N (403.9 eV), verifying that the N elements were doped into the carbon dots. Graphite-N can improve the conductivity of MoS2@N-CD, and pyridine-N is beneficial for enhancing the electrochemical sensing of organic compounds.25 Fig. 2F shows the XRD spectras of CDs, MoS2, MoS2@N-CDs. A broad and relatively intense diffraction peak of N-CDs at 2θ = 27.3° is assigned to the (002) plane that is slightly shifted from that of graphite (2θ = 26.62°). This is due to the decrease in lattice spacing resulting in a larger angle of the diffraction peaks, which proves that the CDs are doped with nitrogen atoms between the crystal planes.26 In the XRD pattern of MoS2, the three diffraction peaks appearing at 14.6°, 31.6°, and 56.1° correspond to the (002), (100), and (110) diffraction crystal planes of MoS2, respectively. And in the XRD pattern of MoS2@N-CDs, three diffraction peaks also appeared, which belonged to the (002), (100) and (110) diffraction crystal planes of MoS2. While the diffraction peak appearing at 8.9° corresponded to CDs, which is in accordance with previous report27 The experimental results of XPS and XRD confirm that MoS2@N-CDs were successfully prepared.
O, respectively. Among them, the strongest peak at 284.8 eV indicates the existence of graphite C, while the C–N bond suggests the formation of N-CDs. The XPS spectrum of N 1s shows three peaks (Fig. 2E) corresponding to pyridine-N (398.7 eV), pyrrole-N (402.5 eV) and graphite-N (403.9 eV), verifying that the N elements were doped into the carbon dots. Graphite-N can improve the conductivity of MoS2@N-CD, and pyridine-N is beneficial for enhancing the electrochemical sensing of organic compounds.25 Fig. 2F shows the XRD spectras of CDs, MoS2, MoS2@N-CDs. A broad and relatively intense diffraction peak of N-CDs at 2θ = 27.3° is assigned to the (002) plane that is slightly shifted from that of graphite (2θ = 26.62°). This is due to the decrease in lattice spacing resulting in a larger angle of the diffraction peaks, which proves that the CDs are doped with nitrogen atoms between the crystal planes.26 In the XRD pattern of MoS2, the three diffraction peaks appearing at 14.6°, 31.6°, and 56.1° correspond to the (002), (100), and (110) diffraction crystal planes of MoS2, respectively. And in the XRD pattern of MoS2@N-CDs, three diffraction peaks also appeared, which belonged to the (002), (100) and (110) diffraction crystal planes of MoS2. While the diffraction peak appearing at 8.9° corresponded to CDs, which is in accordance with previous report27 The experimental results of XPS and XRD confirm that MoS2@N-CDs were successfully prepared.
 | ||
| Fig. 2 XPS spectra of (A) MoS2@N-CDs, (B) Mo 3d, (C) S 2p, (D) C 1s and (E) N 1s, XRD spectras of N-CDs, MoS2 and MoS2@N-CDs (F). | ||
3.2. Electrochemical characterization of electrodes
CV curves of various electrodes in 5.0 mmol L−1 K4[Fe(CN)6]/K3[Fe(CN)6] solution are displayed in Fig. 3 A. In the scanning range, all electrodes showed a pair of Fe3+/Fe2+ redox peaks. Compared with unmodified GCE (curve a), the anodic peak current (Ipa) and cathodic peak current (Ipc) on MoS2/GCE were increased by 1.43-fold and 1.38-fold, respectively, confirming the electrocatalytic effect of MoS2. After doped with N-CDs, the peak current values on MoS2@N-CDs/GCE continued to increase, indicating N-CDs further improves the electrode performance. On Au/MoS2@N-CDs/GCE (curve d), as a result of the high conductivity of GNPs, Ipa and Ipc continuously increased to 2.17 and 1.95 times of the bare GCE. However, when KdBE and CS were decorated onto the electrode, the current value decreased dramatically (curve e), which was attributed to the non-conducting KdBE and CS hindering the electron transfer.For more details, the CV curves of different electrodes at various scan rates were recorded in Fig. S2,† and it can be observed that the peak current increased with increment of scan rate (v). The effective electroactive surface area (A) for different electrodes was calculated according to Randles–Sevcik equation (eqn (2)).
| Ipa = 2.69 × 105n3/2AD1/2Cv1/2 | (2) |
| Ipa(GCE) = 174.77v1/2 + 6.59 R2 = 0.9957 | (3) |
| Ipa(MoS2/GCE) = 240.73v1/2 +14.02 R2 = 0.9996 | (4) |
| Ipa(MoS2@N-CDs/GCE) = 290.13v1/2 + 18.44 R2 = 0.9956 | (5) |
| Ipa(Au/MoS2@N-CDs/GCE) = 369.96v1/2 + 20.13 R2 = 0.9996 | (6) |
The calculated A for GCE, MoS2/GCE, MoS2@N-CDs/GCE, Au/MoS2@N-CDs/GCE is 0.051 cm2, 0.070 cm2, 0.085 cm2, 0.108 cm2, respectively. It can be seen that the electroactive surface area of electrodes was significantly increased by modifying MoS2@N-CDs and GNPs, which allowed more reactions to occur simultaneously and then resulted in higher electrochemical signals, which undoubtedly contributed to improving the sensitivity of electrochemical analysis.
The influence of material modifications on charge transfer resistance (Rct) was also studied by AC impedance technique, the corresponding Nyquist curves was presented in Fig. 3B and fitted by Randles equivalent circuit and the results were listed in Table S1.† The Rct of unmodified GCE was 1079 Ω, but MoS2/GCE showed a smaller semicircle, the corresponding Rct was reduced to 730.8 Ω, indicating that MoS2 facilitated the electron transport. After modified with MoS2@N-CDs on the electrode surface, the semicircle diameter decreased even more with Rct reduced to 482.6 Ω (curve c), demonstrating that the doping of N-CDs effectively improved the conductivity of the nanomaterial. Then, the Rct decreased rapidly to 95.6 Ω after continued modification with GNPs (curve d), which was attributed to the high conductivity of GNPs. However, on CS/KbPE/Au/MoS2@N-CDs/GCE, the semicircle diameter increased dramatically and Rct also increased significantly to 966.9 Ω, which was due to the weak conductivity of enzyme and CS hindering the electron transfer. The above experimental results show that MoS2@N-CDs nanocomposites and GNPs can effectively promote the electron transfer efficiency, which enables the electrodes to have a faster current response and thus better sensitivity for electrochemical analysis.
3.3. AFM characterization of KdBE loading
The morphology and roughness of electrode surface before and after KdBE loading were inspected using AFM. Images were processed using a Nanoscope Analysis v 1.50 software and were subjected to second-order flattening. Use root mean square roughness (Rq) to evaluate the surface roughness of the sample, as defined below.
 | (7) |
As can be seen from Fig. 4A–D, the maximum surface height of the electrode was 51.5 nm and the roughness was 18.6 nm before KdBE loading, whereas after KdBE loading, the surface morphology of the electrode was obviously changed, while the maximum surface height increased to 133.9 nm and the surface roughness increased to 27.9 nm, implying that KdBE was loaded onto the electrode.
 | ||
| Fig. 4 AFM image of electrode surface: height difference map (A) and surface morphology (B) of Au/MoS2@N-CDs/GCE, height difference map (C) and surface morphology (D) of KdBE/Au/MoS2@N-CDs/GCE. | ||
3.4. Electrochemical response of different electrodes to 1-naphthol
Fig. 5A shows the CV curve of CS/KdBE/Au/MoS2@N-CDs/GCE in 0.1 mol L−1 PBS with or without 1-NA. In the PBS solution without 1-NA, no oxidation or reduction peak appeared in the CV curve. However, in PBS solutions with 0.8 mM 1-NA, a distinct oxidation peak appeared around 0.48 V, which derived from the electrochemical oxidation of 1-naphthol produced by KdBE-catalyzed hydrolysis of 1-NA. To confirm the production of 1-naphthol, we collected fluorescence spectra of different reaction systems (Fig. 5B). It can be noticed that no fluorescence emission peak appeared in the PBS system only containing 1-NA, but in the PBS system containing KdBE and 1-NA, a fluorescence emission peak appeared at 470 nm, and the location of the peak was consistent with that of the fluorescence emission peak of 1-naphthol, which proved that KdBE did catalyze the hydrolysis of 1-NA to produce 1-naphthol. Fig. 5A and B also show that KdBE was successfully loaded to the electrode and still had good enzymatic activity.To examine the effect of the prepared nanomaterials on 1-naphthol sensing signals, the CV responses of various modified electrodes in 0.1 M PBS containing the same concentration of 1-NA were tested (Fig. 5C). It is observed that although the oxidation peak of 1-naphthol appeared on all electrodes, the smallest peak current (Ip) was obtained on the electrode without nanomaterials modification (curve a), whereas the Ip on CS/KdBE/MoS2/GCE (curve b) was significantly increased to 1.99 folds of that on CS/KdBE/GCE. When modified with MoS2@N-CDs, the Ip increased to 2.74 times that of CS/KdBE/GCE (curve c), which reconfirmed that MoS2@N-CDs is a good electrocatalyst to effectively amplify the electrochemical oxidation signal of 1-naphthol. When modified with GNPs, the Ip of CS/KdBE/Au/GCE was 2.79 times higher than that of CS/KdBE/GCE, suggesting that GNPs can also enhance the electrical signals. Under the synergistic effect of MoS2@N-CDs and GNPs, the highest Ip was obtained on CS/KdBE/Au/MoS2@N-CDs/GCE (curve d), which was 3.43 times higher than that on CS/KdBE/GCE. The above experimental results indicate that the constructed electrochemical sensing platform has good response sensitivity and promises to achieve highly sensitive detection of pesticide.
3.5. Optimization of experimental parameters
The influence of solution pH upon current response was firstly investigated. The results in Fig. 6 A showed that Ip reached the maximum at pH 7.0, indicating the enzyme catalyzed by KdBE is most effective at pH 7.0. Then, the effect of MoS2@N-CDs amount was optimized (Fig. 6B). With the increase of MoS2@N-CDs loading on the electrode, the Ip firstly showed an upward trend, reaching the maximum at 20 μL, and then decreased. As a result, 20 μL of 1 mg mL−1 MoS2@N-CDs was chosen as the optimal amount.Fig. 6C shows the influence of GNPs loading on Ip, with the increase of GNPs loading, the Ip rose accordingly until it reached the maximum at 12 μL. Later on, with the increase of GNPs loading, the Ip decreased instead. This is because that GNPs has high conductivity and catalytic activity.28 Within a certain range, more GNPs is favorable for the oxidation of 1-naphthol. However, too much GNPs may agglomerate and form a thick film, which is rather unfavorable to the electron transfer at the electrode interface and consequently leads to a decrease in the current response. So, 12 μL was selected as the optimal loading volume of GNPs.
The optimization results of KdBE dosage are shown in Fig. 6D. Ip increased with increasing KdBE dosage, but decreased above 0.19 U. This is because more KdBE can generate more 1-naphthol and then produce a larger electrochemical signal. However, as non-conducting protein, enzyme loading can enlarge the resistance of the modified electrode and hinder electron transfer,29 which will result in a decrease in current response. The combination effect of the two aspects gave 0.19 U as the optimum enzyme dosage.
The influence of the enzyme–substrate interaction time is shown in Fig. 6E. As the reaction time prolonged, more 1-naphthol was generated and the Ip increased accordingly, but Ip value did not change much after 5 min, indicating that the enzymatic reaction reached equilibrium and then an optimal reaction time of 5 min was chosen.
Finally, the incubation time of CPF with KdBE was optimized (Fig. 6F). It was observed that Ip decreased with the increase of incubation time, but almost kept the same after 15 min, indicating that the effect of CPF on KdBE basically reached equilibrium. Therefore, 15 min was chosen as the optimal incubation time.
3.6. CPF detection
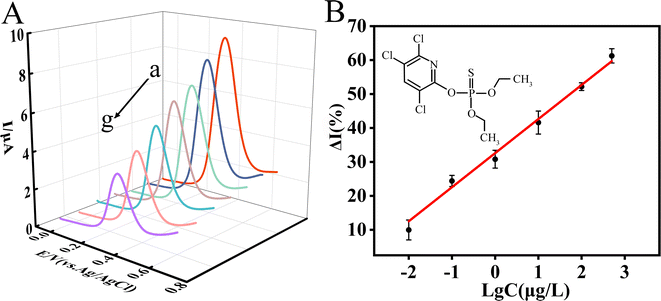
Under optimal experimental conditions, the detection performance of the developed sensor on CPF was examined by SWV. It can be observed that the oxidation peak current obtained in the absence of CPF was the highest, which gradually decreased with the increase of CPF concentration (Fig. 7A). The experiments in Fig. 5 confirmed that KdBE can catalyze the hydrolysis of 1-NA to produce 1-naphthol (Fig. 5B), which is an electrically active substance that can be electrocatalytically oxidized to generate an oxidation peak (Fig. 5A). The more 1-naphthol is present, the higher the peak current is generated. Thus, the experimental results shown in Fig. 7A indicate that the bioactivity of KdBE was inhibited when CPF existed, resulting in less 1-naphthol production. And the more CPF there is, the stronger the inhibition of KdBE activity, and then the less 1-naphthol was produced, causing a lower oxidation peak current.Taking the inhibition rate (ΔI%) of CPF on current signals as the quantitative index, a linear correlation was observed between ΔI% and the logarithm of CPF concentration (lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C) in the range of 0.01–500 μg L−1. The linear equation was ΔI(%) = 10.03
C) in the range of 0.01–500 μg L−1. The linear equation was ΔI(%) = 10.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg
lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C + 32.48 (R2 = 0.9921) (eqn (7)), and the limit of detection (LOD) was 3.5 × 10−3 μg L−1 (S/N = 3).
C + 32.48 (R2 = 0.9921) (eqn (7)), and the limit of detection (LOD) was 3.5 × 10−3 μg L−1 (S/N = 3).
Table 1 compares the method in this work with previously reported CPF detection methods. It can be noticed that the proposed method has a wide linear range and the LOD value is comparable or even lower. More advantageously, the detection is based on the changes in the electrical signal caused by CPF, thus effectively overcoming the interference of the food matrix's color on the determination.
| Method | Linear rang (mol L−1) | LOD (mol L−1) | Ref. |
|---|---|---|---|
| MSPE/GC-MS | 1.99 × 10−10–1.4 × 10−6 | 5.71 × 10−11 | 30 |
| MSPE-GC-μECD | 8.56 × 10−10–5.70 × 10−7 | 2.28 × 10−10 | 31 |
| SPME-HPLC-UV | 7.13 × 10−10–5.70 × 10−7 | 2.14 × 10−10 | 32 |
| HPLC-UV | 1.14 × 10−8–7.13 × 10−7 | 2.28 × 10−9 | 33 |
| Colorimetry | 1.43 × 10−9–2.28 × 10−5 | 5.99 × 10−8 | 34 |
| Spectrophotometry | 2.50 × 10−8–2.50 × 10−6 | 6.50 × 10−9 | 35 |
| Immunosensor | 2.85 × 10−11–1.43 × 10−7 | 2.11 × 10−11 | 36 |
| ECL | 1.00 × 10−14–1.00 × 10−9 | 6.23 × 10−17 | 37 |
| CNTf-AgNPs/PGE | 2.50 × 10−7–5.00 × 10−5 | 5.33 × 10−7 | 38 |
| MnFe-MOF/SPE | 1.00 × 10−9–1.00 × 10−7 | 8.50 × 10−10 | 39 |
| Au@CeO2/ITO | 1.00 × 10−11–5.00 × 10−7 | 1.20 × 10−13 | 40 |
| Rod-CeO2/CA/GCE(PAM-Cl) | 5.00 × 10−11–1.00 × 10−7 | 3.40 × 10−12 | 41 |
| CNFs/GO/CS-GO/AChE/SPCE | 2.50 × 10−9–1.00 × 10−6 | 2.20 × 10−9 | 13 |
| CuNWs/rGO/SPCE | 2.85 × 10−8–5.70 × 10−7 | 8.84 × 10−9 | 14 |
| CS/KdBE/GNPs/MoS2@N-CDs/GCE | 2.85 × 10−11–1.43 × 10−6 | 9.98 × 10−12 | This work |
3.7. Reproducibility, stability and anti-interference capability
Under the same test conditions, RSD of the peak current values obtained from five individual CS/KdBE/Au/MoS2@N-CDs/GCE measured in PBS containing 0.8 mM 1-NA was 4.02%. Meanwhile, the same CS/KdBE/Au/MoS2@N-CDs/GCE was repeatedly measured in PBS containing 0.8 mM 1-NA for five times, and the RSD of the peak currents was 2.69%. The above results show that the proposed sensor has a satisfactory reproducibility.The storage stability of CS/KdBE/Au/MoS2@N-CDs/GCE was investigated (Fig. 8A). The results showed that for the same concentration of 1-NA, the current response of the sensors decreased to 91.2% of the initial peak current after 15 days of storage and to 79.7% after 30 days, indicating that the sensors have good storage stability. The influence of possible co-existing substances and other types of pesticides on CPF assay was studied (Fig. 8B). Taking the peak current obtained only in the presence of 30 μg L−1 CPF as 100%, 300 μg L−1 ascorbic acid, citric acid, glucose, oxalic acid, urea, NaCl, MgCl2, FeCl3, Na2SO4, NaNO2, and 30 μg L−1 of lindane and deltamethrin did not have significant influence on the peak current signals, which indicated that the constructed sensor had acceptable selectivity.
3.8. Actual sample assay
To verify the practicability of the proposed method, oilseed rape and cabbage bought from local farmers' markets were used for actual tests. First, 10 g of sample was cut into small pieces and extracted with 100 mL water for 5 min, followed by centrifugation for 10 min at 8000 rpm, the supernatant was collected and diluted 100 times with PBS 7.0 as actual samples for detection and no CPF was found. Subsequently, the recoveries of CPF spiked in the samples were measured, and each spiked concentration was tested three times in parallel, the results are presented in Table 2. As shown, the spiked recoveries of CPF were in the range of 98.09% to 106.01%, with RSDs in the range of 2.20% to 5.23%. Table S2† compares the assay performance of different CPF detection methods for real samples. It was found that the recoveries and RSD values of this work were within reasonable ranges. This indicates that the proposed sensor is suitable for detecting CPF in real samples.| Samples | Added (μg L−1) | Found (μg L−1) | Recoveries (%) | RSD% (n = 3) |
|---|---|---|---|---|
| Oilseed rape | 10 | 10.60 | 106.01 | 3.91 |
| 50 | 49.23 | 98.47 | 2.31 | |
| 250 | 255.15 | 102.06 | 3.15 | |
| Cabbage | 10 | 10.57 | 105.69 | 5.23 |
| 50 | 51.89 | 103.79 | 3.38 | |
| 250 | 245.21 | 98.09 | 2.20 |
4. Conclusion
In this work, a new enzyme-inhibited electrochemical biosensor was developed for the highly sensitive detection of CPF using MoS2@N-CDs as the electrocatalyst and KdBE as the biorecognition element for CPF. Thanks to the sensing signal amplification effect of nanomaterials and the high susceptibility of KdBE to CPF, the fabricated enzyme electrode exhibited superior sensing performance with a LOD of 3.5 × 10−3 μg L−1. Since the response mechanism of the sensor is based on the change of current signal caused by the inhibition of KdBE activity by CPF, the interference of sample color on the assay can be eliminated effectively. What's more, the proposed sensing method has the advantages of simplicity of operation and affordability, enabling its application for widespread screening of CPF residue in diverse various agri-food samples.Author contributions
Jiayu Chen: methodology, data curation, validation, writing-original draft. Chun Ji: supervision, validation. Xiao Wang: formal analysis, investigation. Yunxia Tian: investigation, visualization. Tao Han: methodology, conceptualization, writing-review & editing, supervision, funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was financially supported by Guizhou Provincial Science and Technology Project (QKHJC-ZK [2022] Key015), National Natural Science Foundation of China (32160603).References
- X. Wang, S. Ai, A. Xiong, W. Zhou, L. He, J. Teng, X. Geng and R. Wu, Anal. Methods, 2023, 15, 6266–6274 RSC.
- R. Lyu, Y. Lei, C. Zhang, G. Li, R. Han and L. Zou, Anal. Chim. Acta, 2023, 1275, 341579 CrossRef CAS PubMed.
- P. Krishna Perumal, C.-w. Chen, B. S. Giri, R. R. Singhania, A. K. Patel and C.-D. Dong, J. Food Sci. Technol., 2023, 61, 631–641 CrossRef PubMed.
- M. R. Moghadam, B. Zargar and S. Rastegarzadeh, Analyst, 2018, 143, 2174–2182 RSC.
- V.-T. Nguyen, L.-H. Tran, T.-K. Van and D.-V. Le, J. Anal. Chem., 2022, 77, 604–610 CrossRef CAS.
- Y. Xiong, J. Huang, R. Wu, X. Geng, H. Zuo, X. Wang, L. Xu and S. Ai, Appl. Spectrosc., 2023, 77, 160–169 CrossRef CAS PubMed.
- S. Ghosh, A. R. Gul, C. Y. Park, M. W. Kim, P. Xu, S. H. Baek, J. R. Bhamore, S. K. Kailasa and T. J. Park, Chemosphere, 2021, 279, 130515 CrossRef CAS PubMed.
- Y. Dong, R. Chen, L. Wu, X. Wang, F. Jiang, Z. Fan, C. Huang and Y. Chen, Biosens. Bioelectron., 2022, 207, 114127 CrossRef CAS PubMed.
- Z. Chen, L. Zhao, Z. Zhang, J. Wu, L. Zhang, X. Jing and X. Wang, Talanta, 2023, 265, 124802 CrossRef CAS PubMed.
- M. A. Kamyabi and M. Moharramnezhad, Anal. Methods, 2022, 14, 750–762 RSC.
- R. Ding, W. Jiang, Y. Ma, Q. Yang, X. Han and X. Hou, Microchem. J., 2023, 187, 108425 CrossRef CAS.
- W. Guo, L. Liang, Y. Zhao, C. Zhao, X. Lu, Y. Cao and F. Gao, Colloids Surf., B, 2023, 224, 113238 CrossRef CAS PubMed.
- W. S. T. Tun, A. Saenchoopa, S. Daduang, J. Daduang, S. Kulchat and R. Patramanon, RSC Adv., 2023, 13, 9603–9614 RSC.
- J. Suwannachat, A. Saenchoopa, W. S. T. Tun, R. Patramanon, S. Daduang, J. Daduang and S. Kulchat, Food Chem., 2024, 434, 137431 CrossRef CAS PubMed.
- H. Tao, F. Liu, C. Ji, Y. Wu, X. Wang and Q. Shi, RSC Adv., 2022, 12, 5265–5274 RSC.
- J. Bao, C. Hou, M. Chen, J. Li, D. Huo, M. Yang, X. Luo and Y. Lei, J. Agric. Food Chem., 2015, 63, 10319–10326 CrossRef CAS PubMed.
- Q. Luo, L. Sun, Y. Zhao, C. Wang, H. Xin, D. Li and F. Ma, J. Mater. Sci. Technol., 2023, 145, 165–173 CrossRef CAS.
- L. Wang, Z. Fan, F. Yue, S. Zhang, S. Qin, C. Luo, L. Pang, J. Zhao, J. Du, B. Jin and H. Zhang, Food Chem., 2024, 430, 137027 CrossRef CAS PubMed.
- X. Ma, J. Yu, L. Wei, Q. Zhao, L. Ren and Z. Hu, Talanta, 2023, 253, 123959 CrossRef CAS PubMed.
- S. Senapati, R. Naik, S. Dash, A. Mohanty, K. Braeckmans, S. C. De Smedt and S. K. Samal, ACS Sustainable Chem. Eng., 2023, 11, 13535–13544 CrossRef CAS.
- K. Das, A. Uppal and R. K. Saini, Spectrochim. Acta, Part A, 2016, 152, 378–383 CrossRef CAS PubMed.
- L. Yang, D. Huo, C. Hou, K. He, F. Lv, H. Fa and X. Luo, Process Biochem., 2010, 45, 1664–1671 CrossRef CAS.
- W. Haiss, N. T. K. Thanh, J. Aveyard and D. G. Fernig, Anal. Chem., 2007, 79, 4215–4221 CrossRef CAS PubMed.
- F. Shalali, S. Cheraghi and M. A. Taher, Mater. Chem. Phys., 2022, 278, 125658 CrossRef CAS.
- M. Nanthagopal, P. Santhoshkumar, N. Shaji, S. Praveen, H. S. Kang, C. Senthil and C. W. Lee, Appl. Surf. Sci., 2019, 492, 871–878 CrossRef CAS.
- H. Afshary, M. Amiri, A. Bezaatpour and M. Wark, J. Electrochem. Soc., 2022, 169, 026523 CrossRef CAS.
- H. M. El Sharkawy, A. S. Dhmees, A. R. Tamman, S. M. El Sabagh, R. M. Aboushahba and N. K. Allam, J. Energy Storage, 2020, 27, 101078 CrossRef.
- D. Orenli, C. K. Selvi, F. Ozturk, P. E. Erden and E. Kilic, Anal. Biochem., 2023, 662, 115002 CrossRef CAS PubMed.
- Y. Li, L. Shi, G. Han, Y. Xiao and W. Zhou, Sens. Actuators, B, 2017, 238, 945–953 CrossRef CAS.
- M. Samadifar, Y. Yamini and M. M. Khataei, J. Food Compos. Anal., 2023, 118, 105158 CrossRef CAS.
- H. Sereshti, A. Amirafshar, A. Kadi, H. Rashidi Nodeh, S. Rezania, H. Y. Hoang, A. Barghi and Y. Vasseghian, Chemosphere, 2023, 320, 138065 CrossRef CAS PubMed.
- X. Wang, J. Yang, J. Zhao, Z. Zhou, X. Du and X. Lu, Chin. J. Chromatogr., 2022, 40, 910–920 CrossRef CAS PubMed.
- Z. Bahiraee, S. Fathi and M. Safari, Environ. Prog. Sustainable Energy, 2023, 42, e13971 CrossRef CAS.
- W. Jiang, Z. Yang, F. Tong, S. Zhang, L. Zhu, L. Wang, L. Huang, K. Liu, M. Zheng, Y. Zhou, R. Hou and Y. Liu, Biosens. Bioelectron., 2023, 224, 115074 CrossRef CAS PubMed.
- N. B. Tran, Q. K. Nguyen, T. V. Vu, A. Q. Hoang, T. D. Pham, D. T. Pham, T. A. H. Nguyen and T. N. M. Pham, Colloid Polym. Sci., 2023, 301, 239–250 CrossRef CAS.
- Y. Wang, A. M. Abd El-Aty, S. Wang, X. Cui, J. Zhao, X. Lei, L. Xu, Y. She, F. Jin, J.-B. Eun, J.-H. Shim, J. Wang, M. Jin and B. D. Hammock, Food Chem., 2023, 413, 135607 CrossRef CAS PubMed.
- Z. Sun, J. Lu, X. Zhang, X. Shan, Q. Wu, C. Li, H. Li, S. Yang and L. Tian, Microchim. Acta, 2022, 189, 473 CrossRef CAS PubMed.
- L. S. Porto, L. F. Ferreira, W. T. Pio dos Santos and A. C. Pereira, Talanta, 2022, 246, 123477 CrossRef CAS PubMed.
- P. Janjani, U. Bhardwaj, R. Gupta and H. S. Kushwaha, Anal. Chim. Acta, 2022, 1202, 339676 CrossRef CAS PubMed.
- G. B. V. S. Lakshmi, M. Poddar, T. K. Dhiman, A. K. Singh and P. R. Solanki, Colloids Surf., A, 2022, 653, 129819 CrossRef CAS.
- X. Zhang, W. Zhang, J. Du, Q. Sun, W. Yuan, H. Wang and J. Wu, Microchem. J., 2023, 191, 108891 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra00009a |
| This journal is © The Royal Society of Chemistry 2024 |