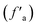Open Access Article
Open Access ArticleRetracted Article: Research on CO2/CH4/N2 competitive adsorption characteristics of anthracite coal from Shanxi Sihe coal mine
Jia Jinzhangab and
Xiao Lingyi *ab
*ab
aCollege of Safety Science and Engineering, Liaoning Technical University, Fuxin, Liaoning 123000, China. E-mail: xly010218@163.com
bKey Laboratory of Mine Thermal Power Disaster and Prevention of Ministry of Education, Liaoning Technical University, Huludao, Liaoning 125100, China
First published on 22nd January 2024
Abstract
This study aims to solve the problem of unsatisfactory development and utilization of coalbed methane and CO2 storage efficiency. It is focused on the adsorption behavior of CO2, CH4, and N2 in the macromolecular structure model of Shanxi Sihe coal mine anthracite, as well as the competitive adsorption behavior of CO2/CH4 and CH4/N2 binary gas mixtures with different ratios. Experimental analysis such as elemental analysis, solid 13C nuclear magnetic resonance (13C NMR), Fourier transform infrared (FT-IR) spectroscopy, X-ray photoelectron spectroscopy (XPS) and X-ray diffraction (XRD) analysis were used to construct the Shanxi Sihe coal mine model of the macromolecular structure of anthracite coal. The Grand Canonical Monte Carlo (GCMC) and molecular dynamics (MD) simulation methods were used to study the adsorption capacity and heat characteristics of CO2, CH4, and N2 at different temperatures using a molecular model of anthracite coal from Shanxi Sihe coal mine, as well as the competitive adsorption characteristics of CO2/CH4 and CH4/N2 binary mixtures. The mechanism of the influence of temperature and gas properties on the adsorption capacity and heat of adsorption was revealed from a microscopic perspective. The results indicated that the aromatic carbon content of anthracite in the Sihe coal mine, Shanxi is 81.19%, and the ratio Xbp of aromatic bridgehead carbon to surrounding carbon is 0.489. The aromatic structure is mainly composed of pyrene and anthracene. The molecular formula of the macromolecular structure model of anthracite in Shanxi Sihe coal mine is C233H157O13N2. The adsorption capacity and equivalent adsorption heat of the macromolecular model for adsorbing single-component gas CO2/CH4/N2 decrease with the increase in temperature. The temperature has the greatest impact on CO2 adsorption capacity and adsorption heat, followed by CH4 and N2. Under the competitive adsorption conditions of CO2/CH4 and CH4/N2 binary mixtures, the higher the partial pressure of a single-component gas in the mixture, the greater the adsorption capacity of the gas. The difference in the adsorption heat of CH4 and N2 is smaller than that of CH4 and CO2. The conclusions obtained from the study can provide technical and theoretical support for formulating reasonable drainage methods for coalbed methane wells.
1 Introduction
Anthracite coal seams have excellent ability for coalbed methane adsorption. Understanding the adsorption mechanism and revealing the adsorption laws of anthracite coalbed methane is conducive to formulating a reasonable system for the discharge and extraction of anthracite coalbed methane wells, thereby achieving the goal of maximizing the production capacity of anthracite coalbed methane wells, reducing gas disasters, promoting resource utilization, and improving economic benefits.1–5 Therefore, establishing a reasonable macromolecular structure model of anthracite and conducting gas adsorption molecular simulation research on anthracite provide critical theoretical guidance significant for the efficient utilization of underground coalbed methane in different environments. Shen et al.6 studied the adsorption and diffusion behaviors of deep coal samples and conducted high-pressure adsorption experiments on CH4 in coalbed methane using the volumetric method. The study found that the adsorption rate of CH4 showed a complex nonlinear functional relationship with the change in coal rank, while the adsorption amount of CH4 showed a linear relationship with coal rank. Meng et al.7 established a macromolecular structure model of lignite based on the experimental results of Fourier transform infrared (FT-IR) spectroscopy and X-ray photoelectron spectroscopy (XPS), and studied the low-temperature oxidation reaction of lignite. The results indicated that in addition to active aliphatic chains, the low-temperature oxidation reactants of lignite also include α carbon atoms, hydroxyl groups, and aromatic ring ethers, and the main active structures are carbonyl and carboxyl groups. The accuracy of the model was verified by analyzing the content of each functional group in lignite through infrared spectroscopy. Krooss et al.8 conducted high-pressure adsorption experiments of CH4 and CO2 gases in dry and water-containing coal samples, and explored the effects of coal rank and moisture content on gas adsorption. The results indicated that coal rank and moisture had less effects on CH4 adsorption. The adsorption amount of CO2 and CH4 has obvious inhibitory effects. Zhang et al.9 used a self-constructed coal macromolecular model to study the competitive adsorption behavior of CH4 and CO2. The results indicated that the adsorption selectivity of CH4/CO2 depends on the CO2 concentration. Kurniawan et al.10 conducted isothermal adsorption experiments on CO2/CH4 mixtures of different components in 1.5 nm graphite slit holes, and the results indicated that the adsorption capacity of CO2 significantly increased with the increase in CO2 concentration.Researchers have carried out a large number of theoretical, experimental and simulation studies on the gas adsorption and molecular model construction of coal at the macro and micro levels.11–15 However, there is relatively little research on the construction of coal macromolecular models for anthracite and the micro mechanisms of adsorption and competitive adsorption at the molecular level. According to relevant studies, the estimated reserves of smokeless coal in China are 4.74 × 108 t,16 accounting for one tenth of the total coal reserves in China, have the characteristics of low volatile matter and high degree of metamorphism. Coal mines with high content of anthracite are more suitable for the research of carbon sequestration technology. Therefore, Shanxi Sihe anthracite coal was chosen as the research object. In this study, through microscopic characterization experiments such as FT-IR spectroscopy, XPS, XRD and 13C NMR, parameters such as functional group distribution, aromatic structure, aliphatic structure, and element occurrence state of anthracite coal from the Sihe coal mine in Shanxi province were obtained. A macromolecular structure model of Shanxi Sihe coal mine anthracite coal was constructed, and its macromolecular structure model was used as the starting point. Based on the molecular dynamics (MD) and Grand Canonical Monte Carlo (GCMC) simulation methods, the characteristic laws of adsorption behavior and mixed gas competitive adsorption behavior of the macromolecular structure model of anthracite coal from Shanxi Sihe coal mine were explored. The research results contribute to a deeper understanding of the CH4, CO2, and N2 adsorption characteristics of anthracite coal, aiming to reveal the mechanism of the influence of adsorption pressure, temperature, and other factors on the gas molecule adsorption behavior at the molecular level. The conclusions obtained from this study can provide vital theoretical support and technical optimization guidance for the efficient extraction and CO2 storage of coal seam CH4.
2 Materials and methods
2.1 Coal sample preparation
The experiment selected anthracite coal from the Sihe coal mine in Shanxi, China as the research object. Coal sample preparation was carried out in strict compliance with the instructions of the “Preparation method of coal samples” (GB474-2008). The pulverized coal sample in the air-drying state was used as the raw material, which was repeatedly crushed and screened using a crusher and vibrating screen machine. The coal sample was separated by passing through a 200 mesh sieve and sealed for storage. The Elementar Unicube elemental analyzer was used to determine the C, H, O, N, and S contents in coal samples. The elemental analysis results are shown in Table 1.| Coal sample | Element distribution, wdaf/% | ||||
|---|---|---|---|---|---|
| C | H | O | N | S | |
| Anthracite coal | 91.11 | 0.64 | 6.99 | 0.76 | 0.50 |
2.2 Solid-state nuclear magnetic resonance (13C NMR) spectroscopy analysis
The Bruker Avance NEO 400WB high-resolution solid-state nuclear magnetic resonance spectrometer was used for 13C NMR spectrum measurement. The rotor was made of zirconia, with a working speed of 10 kHz and a pulse width of 4 μs. The cycle delay time was 0.5 s.2.3 Fourier transform infrared (FT-IR) spectroscopy analysis
The FT-IR spectra of pulverized coal samples were recorded using a Fourier transform infrared spectrometer model, Thermo Scientific Nicolet iS20. In a dry environment, 200 mesh pulverized coal samples and potassium bromide powder were taken and added to the mortar at a mass ratio of 1/200. They are thoroughly ground multiple times and then placed on a tablet press to make transparent sheets with a thickness of 0.2–0.5 mm. The test wavenumber range was 400–4000 cm−1, with 32 scans, a moving mirror speed of 0.475, and a resolution of 4 cm−1.2.4 X-ray photoelectron spectroscopy (XPS)
A Thermo Fisher ESCALAB 250Xi X-ray photoelectron spectrometer was used for testing, and the total acquisition time was 2 minutes and 16 seconds. The excitation source used Al Kα rays, and the spot size was 500 μm. The pass energy was set to 100.0 eV, energy step size was 1.0 eV, and the number of energy steps was 1361.2.5 X-ray diffraction (XRD) analysis
A Rigaku Ultima IV X-ray diffractometer was selected, with a voltage of 40 kV, a current of 40 mA, a test range of 5–90°, a wavelength of 1.5418, and a scanning speed of 2° min−1, and the light source was Cu-Kα rays.2.6 Construction and optimization of macromolecular models
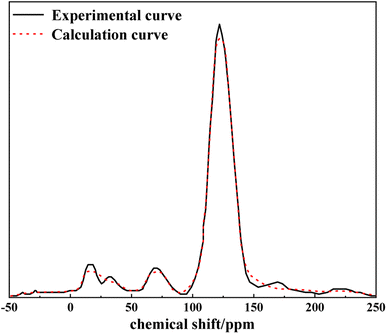
Based on the analytical data of the aromatic structure, aliphatic carbon structure and heteroatom structure of the anthracite coal from Shanxi Sihe coal mine, its molecular formula was deduced. The KingDraw software was used to build the Shanxi Sihe coal mine anthracite macromolecular model, which was then imported into the MestReNova software. By adjusting the positions and connection methods of different functional groups in the macromolecular structure model of anthracite from Shanxi Sihe coal mine, the 13C NMR spectrum of the constructed model was compared with the experimental 13C NMR test spectrum to verify its accuracy.3 Results and discussion
3.1 13C NMR analysis
The experimental results of −50–200 ppm chemical shift 13C NMR characterization of anthracite from Shanxi Sihe coal mine were processed by peak splitting (see Fig. 1). The 13C NMR peak positions and chemical shift assignments refer to the research results reported in the literature.17–19 As can be seen from Fig. 1, the 13C NMR peak fitting curve of anthracite is divided into 11 peaks, corresponding to the type and content of carbon functional groups. The peak surface of the pulverized coal sample is mainly composed of two peak groups: aliphatic carbon (fal) and aromatic carbon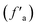 .
.
The chemical shift attribution properties of different types of carbon in 13C NMR are shown in Tables 2 and 3.
The aromatic size and type can be calculated from the ratio of bridge carbon to pericyclic carbon (Xbp) in the anthracite macromolecular structure, and the degree of condensation of the aromatic structure can be characterized. The calculation formula is shown in eqn (1).20 Through calculation, it can be concluded that the structural parameters of the anthracite coal from Shanxi Sihe coal mine are non-protonated aromatic carbon (fNa) accounting for 34.02%, carboxyl carbon and carbonyl carbon (fCa) accounting for 3.34%, protonated aromatic carbon (fHa) accounting for 47.18%, bridged aromatic carbon (fBa) accounting for 26.70%, side-branched aromatic carbon (fSa) accounting for 4.089%, oxygen-substituted aromatic carbon (fPa) accounting for 3.23%, aliphatic carbon (fal) accounting for 15.45%, and total aromatic carbon (fa) accounting for 84.54%. The aromatic carbon ratio 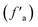 is 81.19%, and the condensation degree of the aromatic structure is 0.489.
is 81.19%, and the condensation degree of the aromatic structure is 0.489.
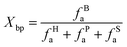 | (1) |
The above-mentioned parameters can provide vital basis for constructing a macromolecular structure model of anthracite from Shanxi Sihe coal mine.
3.2 FT-IR analysis
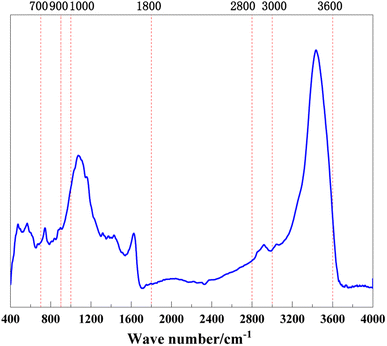
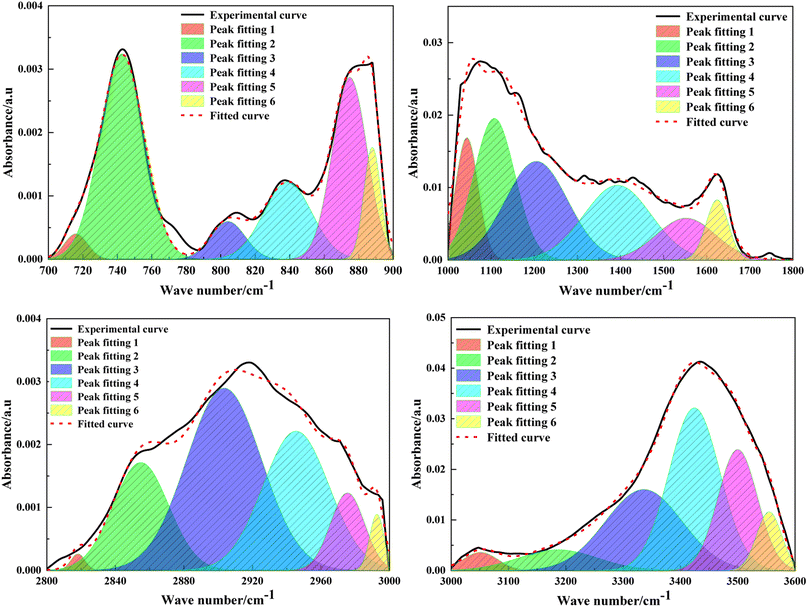
The peak fitting was performed on the spectrum in the 400–4000 cm−1 band area of anthracite coal from Shanxi Sihe coal mine.21 The fitting results are shown in Fig. 2 and 3. On the basis of peak splitting fitting, the peak splitting parameters of the four-band absorption peaks of anthracite coal from Shanxi Sihe coal mine were calculated and analyzed.The fitting results indicated that the benzene ring in the macromolecular structure of anthracite from Shanxi Sihe coal mine is mainly substituted with benzene ring disubstituted (relative area: 42.33%) and benzene ring pentasubstituted (relative area: 35.63%), with benzene ring tetrasubstituted as auxiliary (relative area: 16.33%). The relative area ratio of the absorption peaks caused by C–O vibrations of phenols, alcohols, ethers, and esters in the 1000–1800 cm−1 band is 62.26%. The absorption peaks at 1393.20 cm−1 and 1552.14 cm−1 represent CH3-, CH2-deformation vibration and aromatic hydrocarbon C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C skeleton vibration respectively, with relative area ratios of 21.42% and 10.65% respectively. The interval with a wave number of 2800–3000 cm−1 belongs to the absorption range of –CHx in aliphatic chains and aliphatic rings. It is dominated by the asymmetric CH3 stretching vibration (relative area: 38.68%), supplemented by the CH stretching vibration (relative area: 33.17%). It is demonstrated that the aliphatic chain in the molecular structure of anthracite is dominated by short-chain structures. The total hydroxyl groups are mainly provided by OH–OH hydrogen bonds (relative area 63.18%) and OH–π hydrogen bonds (27.11%), followed by cyclic hydrogen bonds (7.11%); OH–N hydrogen bonds (2.61%) have the least content.
C skeleton vibration respectively, with relative area ratios of 21.42% and 10.65% respectively. The interval with a wave number of 2800–3000 cm−1 belongs to the absorption range of –CHx in aliphatic chains and aliphatic rings. It is dominated by the asymmetric CH3 stretching vibration (relative area: 38.68%), supplemented by the CH stretching vibration (relative area: 33.17%). It is demonstrated that the aliphatic chain in the molecular structure of anthracite is dominated by short-chain structures. The total hydroxyl groups are mainly provided by OH–OH hydrogen bonds (relative area 63.18%) and OH–π hydrogen bonds (27.11%), followed by cyclic hydrogen bonds (7.11%); OH–N hydrogen bonds (2.61%) have the least content.
The basic parameters required to construct the anthracite macromolecular structure model are shown in Table 4, and the calculation formula is shown in eqn (2).22 The length of the aliphatic hydrocarbon chain can reveal the length of the aliphatic chain and the degree of branching in the anthracite coal from Shanxi Sihe coal mine, expressed by the ratio of –CH3 to –CH2. The larger the parameter, the longer the aliphatic chain of the anthracite.
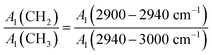 | (2) |
| Coal sample | Fat chain length | Aromaticity | Degree of condensation of aromatic rings | Aromatization index | Aromatic carbon ratio |
|---|---|---|---|---|---|
| Anthracite | 1.068 | 0.68 | 0.486 | 4.01 | 0.66 |
The aromaticity can represent the richness of aromatic functional groups to aliphatic functional groups in the anthracite coal, which is expressed by formula (3):
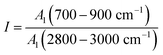 | (3) |
The degree of condensation (DOC) of aromatic rings can characterize the degree of condensation of aromatic rings in the anthracite structure of Shanxi Sihe coal mine. It is the ratio of the out-of-plane deformation vibration of aromatic ring CH in the peak position 700–900 cm−1 area to the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C skeleton vibration in the peak position 1600 cm−1 area, which can be expressed as follows:
C skeleton vibration in the peak position 1600 cm−1 area, which can be expressed as follows:
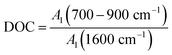 | (4) |
The aromatization index (Har/Hal) is the ratio of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibration of aromatic hydrocarbons at peak positions 1520–1650 cm−1 and the vibration of aliphatic hydrocarbons at peak positions 2800–3000 cm−1 in the anthracite coal of Shanxi Sihe coal mine. It can be represented by eqn (5):
C vibration of aromatic hydrocarbons at peak positions 1520–1650 cm−1 and the vibration of aliphatic hydrocarbons at peak positions 2800–3000 cm−1 in the anthracite coal of Shanxi Sihe coal mine. It can be represented by eqn (5):
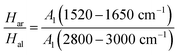 | (5) |
The aromatic carbon ratio (fCar) refers to the ratio of the number of carbon atoms in the molecular structure of anthracite coal in Shanxi Sihe coal mine to the total number of carbon atoms. The aromatic carbon ratio of anthracite coal can be calculated using formula (6):
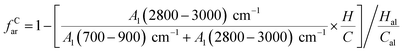 | (6) |
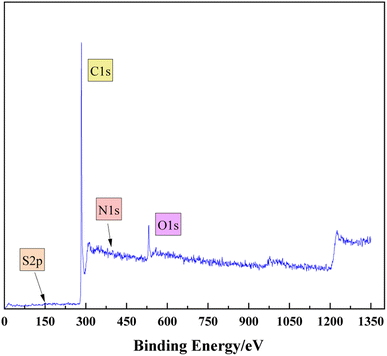
3.3 XPS analysis
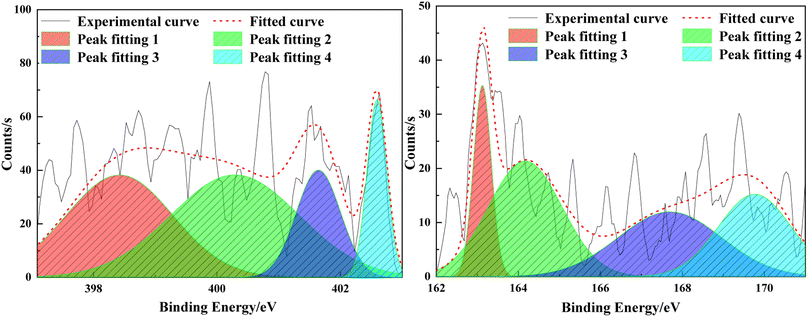
The composition of heteroatoms in coal molecules, as well as the presence and relative content of different elements, can be determined through XPS.23 C 1s (284.8 eV) is used as the standard for calibration. Fig. 4 and 5 respectively represent the XPS spectra and peak fitting spectra of nitrogen and sulfur elements in the anthracite of Shanxi Sihe coal mine. The peak fitting results of the existing forms and contents of nitrogen and sulfur elements are obtained, further clarifying the occurrence characteristics of nitrogen and sulfur in the anthracite.The fitting results indicated that the main forms of nitrogen in the anthracite of Shanxi Sihe coal mine are pyridine nitrogen C5H5N and pyrrole nitrogen C4H5N, accounting for approximately 32.59% and 41.26% of the total nitrogen elements, respectively. Since pyridine nitrogen and pyrrole nitrogen have stable aromatic conjugated structures, they are largely preserved during the evolution of anthracite. Quaternary nitrogen accounts for approximately 15.01% of the total nitrogen element. The proportion of nitrogen oxides (NxOy) is relatively small, and they are mainly nitrogen oxides generated by the oxidation of C5H5N and C4H5N, accounting for approximately 11.15% of the total nitrogen element. The main occurrence form of sulfur in anthracite is thiophenes, which account for about 35.01% of the total sulfur element. In anthracite, thiophenes have the characteristic of aromatic structure and are one of the products of unstable side chain sulfur after aromatization. Therefore, thiophenes have gradually become the main organic sulfur structure in the anthracite. Sulfoxides account for approximately 27.74% of the total sulfur content, while mercaptans/thioethers and inorganic sulfur contents are relatively low, accounting for 14.23% and 23.01% of the total sulfur content, respectively.
3.4 XRD analysis
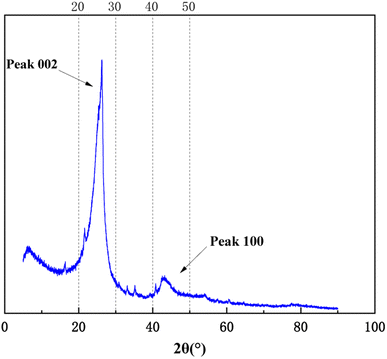
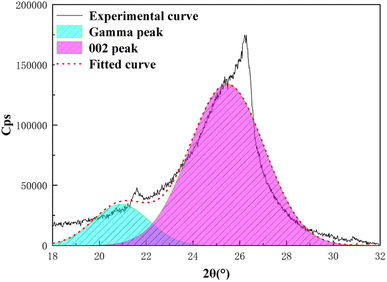
X-ray diffraction analysis is a technical means to study the structural characteristics of microcrystals, revealing the arrangement rules of carbon atoms, and to characterize the coal aggregation structure.24–26 As shown in Fig. 6, the XRD pattern of anthracite coal has two broad peaks in the range of diffraction angles 2θ 20°–30° and 40°–50°, which respectively represent the 002 peak and 100 peak of the anthracite coal microcrystalline structure. The 002 peak is formed by the superposition of the γ band and the 002 band (as shown in Fig. 7), which represents the spatial arrangement of aromatic ring carbons in anthracite and the distance between the aromatic ring carbons and the aromatic ring layer. The γ band indicated the aliphatic carbon structure in anthracite. The value 2θ = 40°–50° is the 100 peak of the microcrystalline structure, which represents the degree of aromatic ring condensation of anthracite.Formula (7) can be used to calculate the layer spacing (d), stacking degree (Lc), extensibility of aromatic lamellae (La), number of aromatic lamella stacking layers (Nave) and aromaticity (fa) of the anthracite coal. The calculation results of the microcrystalline structure parameters of anthracite coal from Shanxi Sihe coal mine are shown in Table 5. The above-mentioned parameters are all key parameters for constructing the anthracite macromolecule model.27
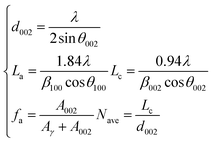 | (7) |
| Coal sample | θ002/° | θ100/° | d002/nm | La/nm | Lc/nm | fa | Nave |
|---|---|---|---|---|---|---|---|
| Anthracite | 12.73 | 21.78 | 0.35 | 2.99 | 2.25 | 0.841 | 6.426 |
3.5 Construction and optimization of the macromolecular structure model of anthracite coal in Shanxi Sihe coal mine
The ratio of bridgehead carbon to surrounding carbon in the anthracite from the Sihe coal mine in Shanxi is 0.489, indicating that the aromatic structures in the model are mainly pyrene and anthracene, supplemented by naphthalene. Through the MATLAB programming calculations, the type and number of aromatic structures closest to the bridge-circumference ratio of the anthracite macromolecular structure of Shanxi Sihe coal mine were obtained, as shown in Table 6. The total number of aromatic carbons in the anthracite coal molecules is 189. According to 13C NMR analysis, aromatic carbon accounts for 81.19%. Therefore, the total number of carbons in the macromolecular structure of anthracite coal in Shanxi Sihe coal mine is 233. According to the elemental analysis results, the carbon content in the anthracite sample is 91.11%, the oxygen content is 6.99%, the nitrogen content is 0.76%, and the sulfur content is 0.50%. From the above-mentioned values, it can be calculated that there are 13 oxygen atoms and 2 nitrogen atoms in the macromolecular structure of anthracite coal. Due to the low sulfur content, the quantity is less than one. Therefore, the macromolecular structure of Shanxi Sihe coal mine constructed in this article does not contain sulfur. According to XPS analysis, it can be concluded that the main forms of N element in anthracite from the Sihe coal mine in Shanxi province are pyridine nitrogen and pyrrole nitrogen, with a quantity ratio of approximately 1/1. Therefore, there is one pyridine nitrogen and one pyrrole nitrogen.| Existing form | Pyridine | Pyrrole | Pyrene | Anthracene | Naphthalene |
|---|---|---|---|---|---|
| Number | 1 | 1 | 8 | 3 | 1 |
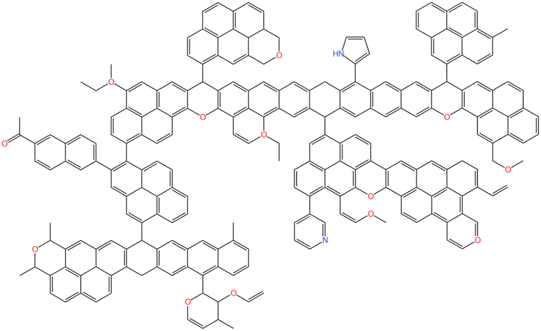
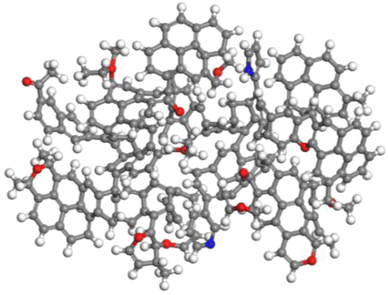
According to the analysis results, the KingDraw software was used to build a macromolecular model of anthracite from Shanxi Sihe coal mine,28–30 as shown in Fig. 8. Then, the Shanxi Sihe coal mine anthracite macromolecular model was imported into the MestReNova software, and the positions and connection methods of different functional groups in the anthracite macromolecular model were adjusted. Finally, the constructed model 13C NMR spectra were compared with the experimental 13C NMR spectra, as shown in Fig. 9. The results indicated that the 13C NMR spectra of the anthracite macromolecular model established in this study are in good agreement with the experimental spectra. The molecular formula of anthracite from Shanxi Sihe coal mine was ultimately determined to be C233H157O13N2.
The two-dimensional planar molecular model of anthracite was imported into the Materials Studio molecular simulation software, and the Forcite module and COMPASS force field were used to perform geometric optimization and molecular dynamics optimization on the anthracite macromolecular structure model of Shanxi Sihe coal mine. The iteration steps, calculation accuracy, charges term, dynamic ensemble, and time step are set to 3000, medium, forcefield assigned charge, NVT option, and 1.00 fs, respectively.28 After multiple optimization processes, Shanxi Sihe coal mine anthracite was finally obtained. The lowest energy configuration of the macromolecule is shown in Fig. 10.
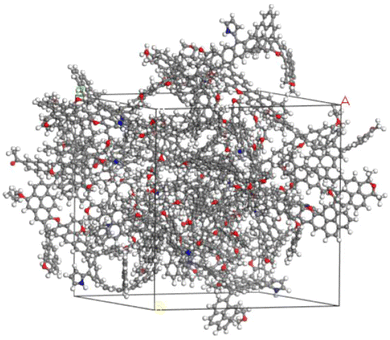
Fifteen macromolecular structural models of anthracite were selected using the amorphous cell module, with a calculation accuracy of fine and a force field of COMPASS. Fifteen single-molecule structures were placed into the crystal cells and subjected to three-dimensional periodic boundary conditions, with densities set at 1.32 g cm−3. The geometry optimization module was used to optimize the structure of the crystal cell model of anthracite. The COMPASS force field was used, with a calculation accuracy of fine and smart methods, and a maximum iteration step of 5000.29 Further dynamic optimization processing was carried out using the Anne module and dynamics module, resulting in a structure model size of A = B = C = 3.89034 nm and a molecular formula of C3495H2355N30O195 for anthracite. The constructed molecular model is shown in Fig. 11.
3.6 Adsorption capacity and adsorption heat of CO2/CH4/N2 single-component gas in anthracite
The combined method of GCMC and MD simulation was used to simulate and analyze the adsorption characteristics of single-component gas molecules CO2, CH4, and N2 in the macromolecular structure model of smokeless coal in Sihe coal mine, Shanxi. The competitive adsorption characteristics of smokeless coal on CO2/CH4 and CH4/N2 binary mixtures were studied. Gas adsorption simulation was performed using the fixed pressure task in the sorption module, with the customized calculation accuracy. The steps is set to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, the calculation accuracy is set to customized, the COMPASS option is selected as the force field option, and the electrical and van der Waals are set to Ewald and atom based, respectively.30–32
000, the calculation accuracy is set to customized, the COMPASS option is selected as the force field option, and the electrical and van der Waals are set to Ewald and atom based, respectively.30–32
The molecular radius of the probe is the molecular dynamics radius of CO2, CH4 and N2, which are 0.165 nm, 0.19 nm and 0.182 nm respectively. In the molecular structure of anthracite, the micropore volumes detected by CO2, CH4 and N2 are 2967.13 Å3, 2087.29 Å3 and 2389.81 Å3 respectively, and the specific surface areas are 3699.57 Å2, 2689.82 Å2 and 3011.34 Å2 respectively. The micropore volume and specific surface area detected by CO2 molecules are higher than those of CH4 and N2, which indicate that some micropores in anthracite can be detected by CO2 gas molecules, but not by CH4 and N2 gas molecules. Therefore, CO2 gas is more easily adsorbed by anthracite than CH4 and N2 gas.
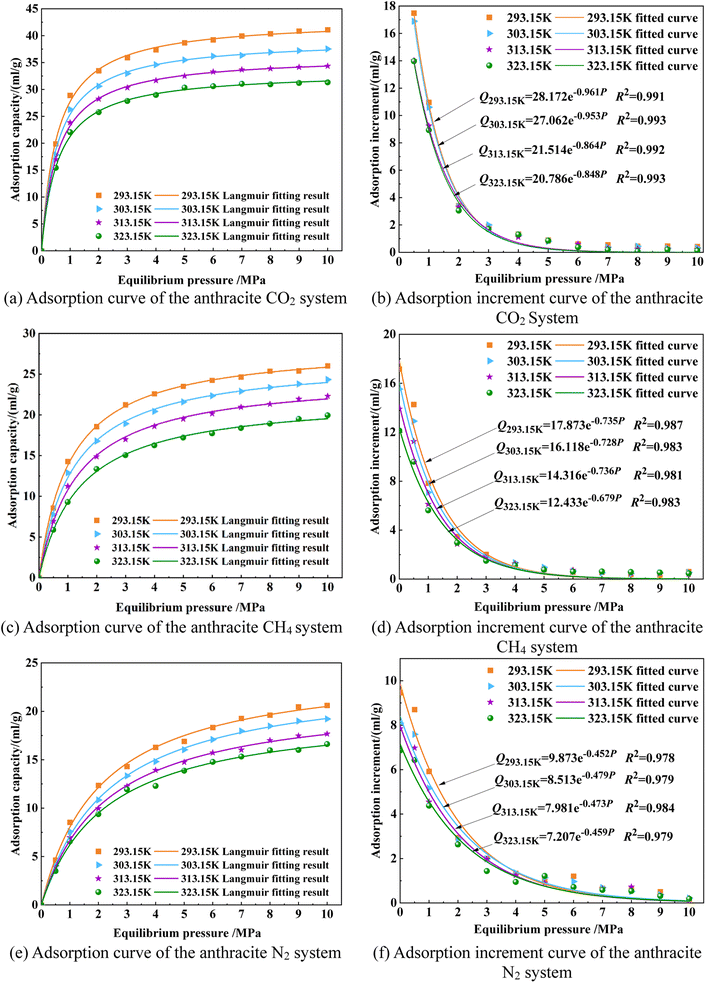
The adsorption kinetics curves of CO2/CH4/N2 gas small molecules in the large molecule model of smokeless coal in Shanxi Sihe coal mine at different temperatures are shown in Fig. 12. By analyzing the adsorption capacity of smokeless coal in Shanxi Sihe coal mine at different temperatures (293.15 K, 303.15 K, 313.15 K, and 323.15 K), it can be concluded that at any temperature, the adsorption capacity of CO2/CH4/N2 gas small molecules in the smokeless coal macromolecular model increases with the increase in adsorption pressure, while the gas adsorption increment of smokeless coal shows the opposite trend. The entire adsorption process can be divided into the initial adsorption stage (P < 3 MPa) and the gradual adsorption stage (P > 3 MPa). During the initial adsorption stage, gas molecules can fully enter the micropores of the coal molecular model, resulting in a rapid increase in adsorption capacity. In the macromolecular model of anthracite coal, the adsorption capacity of CO2 gas molecules is the largest, followed by CH4, and the adsorption capacity of N2 is the smallest.
The increase in temperature is not conducive to the adsorption of CO2/CH4/N2 gas molecules by the smokeless coal macromolecular model. The reason is that the increase in adsorption temperature can promote the increase in energy, activity, and kinetic energy of CO2/CH4/N2 gas molecules, which is not conducive to the “capture” of CO2/CH4/N2 gas molecules on the surface of smokeless coal molecules during the adsorption process. Moreover, high temperatures can inhibit the transformation of CO2/CH4/N2 gas molecules from the free state to the adsorbed state. Some stable adsorbed gases will also desorb and transform into free gas due to high temperatures, and hence, the adsorption capacity of anthracite for CO2/CH4/N2 gases will decrease with the increase in temperature. To sum up, in the anthracite molecular model of Shanxi Sihe coal mine, the increase in temperature has the greatest impact on the adsorption capacity of CO2, followed by CH4 and N2.
In the study of the single-component gas adsorption behavior of anthracite coal from Shanxi Sihe coal mine, in addition to studying the amount of gas adsorption by anthracite, the isosteric heat of adsorption is also another key parameter characterizing the adsorption behavior of anthracite.33 The isosteric heat of adsorption can indicate the heat in the system, and the change information is of great significance for explaining the adsorption law and adsorption mechanism. According to the energy particle fluctuation calculation in the giant canonical ensemble, the isosteric adsorption heat Qst during the adsorption process of anthracite and gas can be obtained. The formula is as follows:
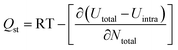 | (8) |
Within the range of simulation temperature, the isosteric adsorption heat of CO2/CH4/N2 gas molecules adsorbed by the anthracite macromolecular model of Shanxi Sihe coal mine decreases with the increase in temperature, as shown in Fig. 13. In the macromolecular structure of anthracite, the average isobaric adsorption heat of CO2 is the largest, followed by CH4, and N2 is the smallest, which is also consistent with the change in adsorption amount. The reason for the different adsorption heat of the three gases is that CO2 not only has the highest polarizability, but also has the highest of four dipole moments and the smallest molecular dynamics diameter. Compared to CH4 and N2, there is a strong interaction force between CO2 and the surface of smokeless coal in Shanxi Sihe coal mine, resulting in the maximum heat released by the adsorption of CO2 by smokeless coal in Shanxi Sihe coal mine, followed by the polarization rate of CH4, and the molecular dynamics diameter greater than CO2. The isobaric adsorption capacity of CH4 is less than that of CO2, the polarizability of N2 is smaller than that of CH4, and although the molecular dynamic diameter is smaller than that of CH4, the isobaric adsorption heat is the smallest. Therefore, the isobaric adsorption heat of CO2/CH4/N2 is affected by the dynamic diameter, quadruple dipole moment and polarizability of the gas molecules. The increase in temperature has the greatest impact on the adsorption heat of CO2, followed by CH4, and N2 is the smallest. That is, the greater the adsorption heat of the gas itself, the greater the impact of temperature increase on it. This is consistent with the sequence of the effects of temperature increase on the adsorption amount, indicating that the adsorption heat can be used to characterize the adsorption characteristics of anthracite coal in Shanxi Sihe coal mine.
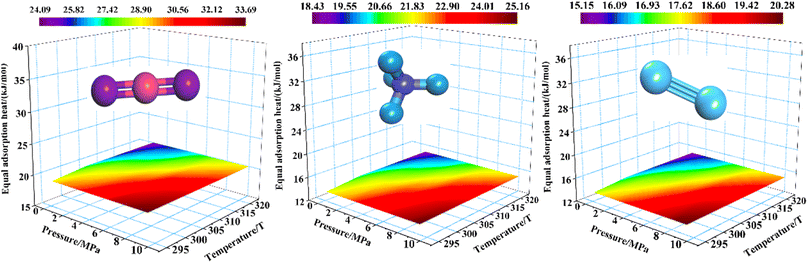 | ||
| Fig. 13 Equivalent adsorption heat of the CO2/CH4/N2 single-component gas adsorbed by anthracite coal molecules at different temperatures. | ||
3.7 Adsorption characteristics of the CO2/CH4 mixed gas and CH4/N2 mixed gas in anthracite coal
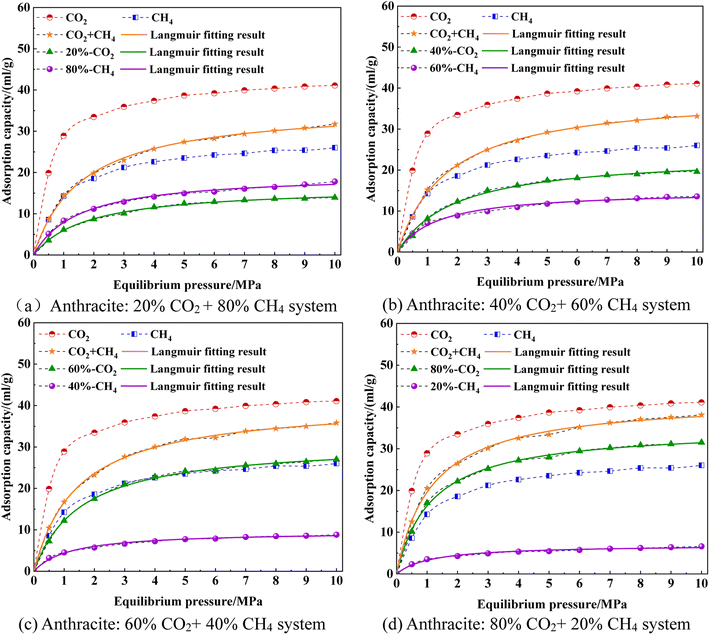
The competitive adsorption characteristics of CO2/CH4 and CH4/N2 binary mixtures in the macromolecular model of smokeless coal from the Sihe coal mine in Shanxi province were studied using molecular dynamics (MD) and Grand Canonical Monte Carlo (GCMC) simulation methods.34 The ratios of CO2/CH4 and CH4/N2 binary mixtures are set to 0.8/0.2, 0.6/0.4, 0.4/0.6, and 0.2/0.8, and the temperature is set to 293.15 K. The isothermal adsorption curves of CO2/CH4 and CH4/N2 binary mixtures on the macromolecular structure model of smokeless coal from the Sihe coal mine, Shanxi province are shown in Fig. 14 and 15.As shown in Fig. 14, the variation pattern of the total adsorption amount of CO2/CH4 mixed gas was similar to that of pure-component gas adsorption. As the pressure increased, the total adsorption amount also increased. The total adsorption amount of CO2/CH4 mixed gas was between that of CH4 and pure CO2 components, while the adsorption amount of single-component gas in the CO2/CH4 mixed gas was reduced compared to pure components. As the proportion of CO2 in the CO2/CH4 mixture increased and the proportion of CH4 decreased, the total adsorption amount of the CO2/CH4 mixture increased. The higher the proportion of CO2, the higher the total adsorption amount. This indicated that in the CO2/CH4 mixture, the molecular surface of smokeless coal has a stronger adsorption capacity for CO2 than CH4, making it easier to adsorb CO2 onto the surface of smokeless coal, thereby reducing the interaction between CH4 and the surface of smokeless coal. It was also indicating from a microscopic perspective that CO2 injection improves the extraction rate of coalbed methane, and the feasibility of sealing CO2 in coal seams.
The difference between the adsorption capacity of single-component CH4 in a CO2/CH4 mixed gas and the adsorption capacity of a pure CH4 component was the recovery capacity of gas injection and extraction. The higher the proportion of CO2 injected, the greater the recovery capacity of CH4. As for four different ratios of single-component gases in anthracite, the adsorption capacity gradually increases with the increase in pressure, and the increase in adsorption capacity was more significant in the low-pressure stage (0–3 MPa), while the increase in adsorption capacity in the high-pressure stage (3–10 MPa) gradually slowed down. When the proportion of CH4 reached 80%, the adsorption capacity of CH4 was greater than that of CO2. This was because although the adsorption capacity of CO2 in anthracite was greater than that of CH4, the partial pressure of CH4 was greater than that of CO2. When the CO2/CH4 mixture gas competed for adsorption on the surface of anthracite, the adsorption capacity of anthracite for each single-component gas not only depends on the adsorption capacity of the gas itself, but also on the partial pressure of the single-component gas. Under these environmental conditions, the partial pressure of a single-component gas played a dominant role in the adsorption of CH4 throughout the entire adsorption process. As the adsorption behavior progressed, the adsorption capacity of CH4 in anthracite coal quickly approached saturation, while the adsorption capacity of CO2 had not yet reached saturation. With the increase in pressure, the competitive adsorption advantage of CO2 for CH4 in CO2/CH4 mixed gas was fully reflected. Therefore, the adsorption capacity of CO2 in the CO2/CH4 mixed gas gradually increased and exceeded that of CH4.
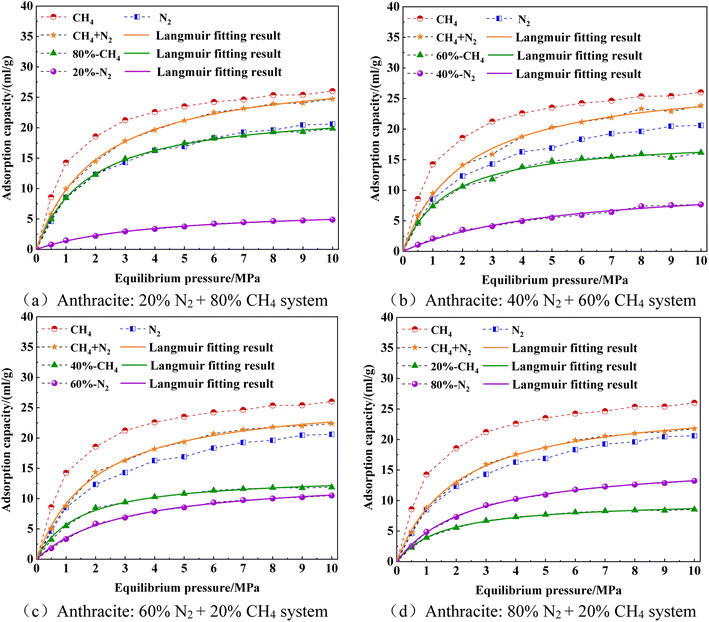
As shown in Fig. 15, the adsorption capacity of both CH4/N2 mixed gas and pure-component gas increased with the increase in adsorption pressure, and the total adsorption capacity of CH4/N2 mixed gas was between that of pure CH4 and N2. The higher the proportion of CH4 in the CH4/N2 mixture, the more the CH4 molecules adsorbed, and the adsorption curve of the CH4/N2 mixture was closer to the pure CH4 adsorption curve. The higher the N2 ratio, the more the N2 molecules adsorbed, and the adsorption curve of the CH4/N2 mixed gas was closer to the pure N2 adsorption curve.
The difference in adsorption capacity between single-component CH4 and pure-component CH4 was the increased recovery of CH4. The higher the proportion of injected N2, the greater the recovery of CH4. In addition, when the proportion of N2 in the CH4/N2 mixture reached 60%, the adsorption capacity of N2 was already close to that of CH4. When the proportion of N2 reaches 80%, the phenomenon of N2 adsorption exceeding CH4 adsorption occurs within the simulated pressure range. This is because when the proportion coefficient of N2 in the CH4/N2 mixed gas was high, it can greatly promote the probability of collision between N2 molecules and anthracite surface molecules during thermal motion, which will help N2 molecules occupy more space on the anthracite surface. The adsorption sites fully compete with CH4 molecules for adsorption, thus reducing the adsorption sites for CH4 and the adsorption amount was less than the adsorption amount of N2. This proved the feasibility of injecting N2 to improve CH4 recovery from the molecular level.
Overall, the adsorption capacity changes of CH4/N2 mixtures with different ratios on the molecular structure model of anthracite are similar to those of CO2/CH4 mixtures. However, due to the differences in molecular diameter, boiling point, pressure, and other factors of CO2/CH4/N2 single-component gases in the mixed system, the total adsorption amount and adsorption capacity of each component of the two mixtures are different. In addition, the difference in total adsorption amount between single-component CH4 and pure-component CH4 after CO2 injection is greater than the difference in total adsorption amount between single-component CH4 and pure-component CH4 after N2 injection. This indicates that injecting CO2 into anthracite coal seams can more effectively improve the extraction rate of CH4 in anthracite coal seams than injecting N2.
Fig. 16 shows the isometric adsorption heat of CO2/CH4 and CH4/N2 mixed gases on the macromolecular structure of anthracite coal in Shanxi Sihe coal mine. It can be seen from Fig. 16 that under competitive adsorption conditions, as the gas adsorption pressure continued to increase, the isobaric adsorption heat of single-component CO2 and CH4 gas molecules in the CO2/CH4 mixed gas slowly increased on the anthracite macromolecular structure model. Compared with CO2, the isosteric adsorption heat of CH4 on the anthracite macromolecular structure model was smaller, which indicated that the interaction force of anthracite on CO2 gas was stronger than that of CH4. In the high-pressure stage of CO2/CH4 mixed gas, the magnitude of the change in the equivalent adsorption heat of CO2 and CH4 was relatively small. This was due to the combined effect of the decrease in the active adsorption sites of anthracite molecules and the increase in gas adsorption capacity. As the proportion coefficient of CO2 in the CO2/CH4 mixture gas increased, the adsorption heat of CO2 and CH4 gas in a single component decreased. In the CO2/CH4 mixture gas, the adsorption heat of CO2 in an equal amount was greatly affected by the change in CH4 proportion, while the adsorption heat of CH4 in an equal amount was less affected by the change in CO2 proportion.
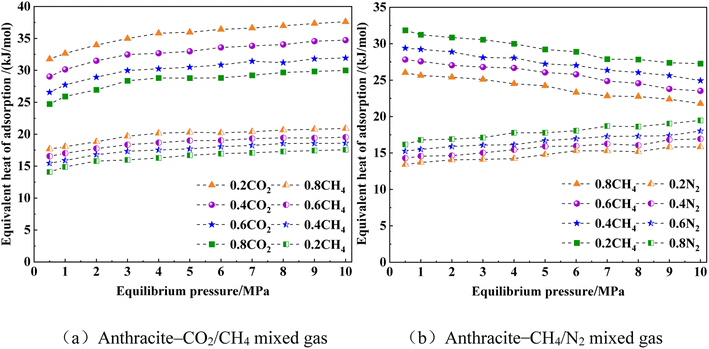 | ||
| Fig. 16 Heat curve of equivalent adsorption of CO2/CH4 and CH4/N2 mixed gas in anthracite. (a) Anthracite–CO2/CH4 mixed gas. (b) Anthracite–CH4/N2 mixed gas. | ||
As the pressure increased, t, the adsorption heat of CH4 molecules in the CH4/N2 mixture gas, slowly decreased, while the adsorption heat of N2 gas molecules slowly increased. The trend of equal adsorption heat of CH4 gas molecules in the CH4/N2 mixture gas was opposite to that in the CO2/CH4 mixture gas, indicating that the addition of different gases had different effects on the adsorption heat of CH4. This was because the competitive adsorption behavior of mixed gases on the surface of anthracite was related to the properties and composition of each individual component gas in the system. The increasing in adsorption pressure had little effect on the adsorption heat of two single-component gases in the CH4/N2 mixture. Under competitive adsorption conditions, the equivalent adsorption heat of CH4 was greater than that of N2. This indicated that the interaction force between anthracite molecules and CH4 was stronger, resulting in a greater adsorption capacity of CH4 than that of N2. As the CH4 content in the mixture increased, the isosteric adsorption heat of CH4 and N2 gas molecules on the surface of anthracite decreased. The isosteric adsorption heat of CH4 was greatly affected by changes in the N2 component, indicating the presence of strong adsorbates in the competitive adsorption process of the mixture. The increase in proportion caused the isobaric adsorption heat of both strong adsorbates and weak adsorbates to decrease, and the increase in the proportion of weak adsorbates had a greater impact on the isobaric adsorption heat of strong adsorbates. The average isobaric adsorption heat of CH4 in the CH4/N2 mixed gas was greater than the isobaric adsorption heat in the CO2/CH4 mixed gas, indicating that the inhibitory effect of CO2 injection on CH4 adsorption in anthracite coal seams was stronger than the inhibitory effect of N2 injection. The difference in the isosteric heat of CH4 and N2 was smaller than the difference in the isosteric heat of CH4 and CO2.
4 Conclusions
This study constructed a three-dimensional macromolecular structure model of anthracite coal from the Sihe coal mine in Shanxi province through elemental analysis, nuclear magnetic resonance (13C NMR), Fourier transform infrared (FT-IR) spectroscopy, X-ray photoelectron spectroscopy (XPS), and X-ray diffraction (XRD) characterization experiments. On this basis, molecular simulation studies were conducted on the adsorption capacity, adsorption heat, and competitive adsorption of single-component and binary-component mixed gases by anthracite coal from Shanxi Sihe coal mine, revealing the micro mechanism of anthracite coal adsorption of gases in Shanxi Sihe coal mine. The following main conclusions were obtained:(1) The main forms of nitrogen in the anthracite of Shanxi Sihe coal mine are pyridine nitrogen and pyrrole nitrogen. The ratio Xbp of aromatic bridgehead carbon to surrounding carbon is 0.489, the aromatic carbon rate is 81.19%, and the aromatic carbon structures are mainly pyrene and anthracene. The molecular formula of the anthracite molecular structure model of Shanxi Sihe coal mine is C233H157O13N2.
(2) In the macromolecular structure model of anthracite coal in Shanxi Sihe coal mine, the adsorption capacity of single-component gas molecules CO2/CH4/N2 decreases with the increase in temperature. The increase in temperature has the greatest impact on the adsorption capacity of CO2, followed by CH4, and N2 is the smallest. The isosteric adsorption heat of CO2 adsorbed by anthracite molecules is the largest, followed by CH4, and N2 is the smallest. The isosteric adsorption heat decreases with the increase in temperature. The temperature increase has the greatest impact on the CO2 adsorption heat, followed by CH4, and N2 is the smallest. The greater the heat and adsorption capacity of gas adsorption, the greater the effect of temperature increase on it.
(3) In the competitive adsorption process, when the proportion of CO2 reaches 40%, the adsorption capacity of CO2 is greater than the adsorption capacity of CH4. When the proportion of N2 reaches 60%, the adsorption capacity of N2 is close to that of CH4. When the proportion of N2 reaches 80%, the adsorption capacity of N2 is greater than the adsorption capacity of CH4. Injecting CO2 into anthracite coal in Shanxi Sihe coal mine is more conducive to increasing CH4 recovery than injecting N2.
(4) The isobaric adsorption heat of CO2 and CH4 in the CO2/CH4 mixture gas increases slowly with the increase in pressure in the anthracite macromolecular structure of Shanxi Sihe coal mine. The molecular structure decreases slowly as the pressure increases, and the N2 adsorption heat increases slowly as the pressure increases. The difference in isosteric heat of CH4 and N2 is smaller than the difference in isosteric heat of CH4 and CO2.
Author contributions
Jia Jinzhang: formal analysis and contributed to the conception of the study and the data analysis. Xiao Lingyi: performed the experiment, manuscript preparation and writing original manuscript.Conflicts of interest
The authors declare that they have no potential conflict of interests to influence the work reported in this paper.Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (grant number 52174183).References
- F. Sun, D. Liu and Y. Cai, et al., Surface jump mechanism of gas molecules in strong adsorption field of coalbed methane reservoirs, Appl. Energy, 2023, 349, 121605 CrossRef CAS.
- S. Li, Y. Qin and D. Tang, et al., A comprehensive review of deep coalbed methane and recent developments in China, Int. J. Coal Geol., 2023, 104369 CrossRef CAS.
- C. Li, Y. Qin and T. Guo, et al., Supercritical methane adsorption in coal and implications for the occurrence of deep coalbed methane based on dual adsorption modes, Chem. Eng. J., 2023, 474, 145931 CrossRef CAS.
- F. Sun, D. Liu and Y. Cai, et al., Coal rank-pressure coupling control mechanism on gas adsorption/desorption in coalbed methane reservoirs, Energy, 2023, 270, 126849 CrossRef CAS.
- B. Nie and S. Sun, Thermal recovery of coalbed methane: Modeling of heat and mass transfer in wellbores, Energy, 2023, 263, 125899 CrossRef CAS.
- J. Shen, Y. Qin and X. Fu, et al., Study of high–pressure sorption of methane on Chinese coals of different rank, Arabian J. Geosci., 2015, 8(6), 3451–3460 CrossRef CAS.
- Y. Meng and Z. P. Li, Triaxial experiments on adsorption deformation and permeability of different sorbing gases in anthracite coal, J. Nat. Gas Sci. Eng., 2017, 46, 59–70 CrossRef CAS.
- B. M. Krooss, F. van Bergen and Y. Gensterblum, et al., High–pressure methane and carbon dioxide adsorption on dry and moisture–equilibrated Pennsylvanian coals, Int. J. Coal Geol., 2002, 51(2), 69–92 CrossRef CAS.
- J. Zhang, K. Liu and M. B. Clennell, et al., Molecular simulation of CO2-CH4 competitive adsorption and induced coal swelling, Fuel, 2015, 160, 309–317 CrossRef CAS.
- Y. Kurniawan, S. K. Bhatia and V. Rudolph, Simulation of binary mixture adsorption of methane and CO2 at supercritical conditions in carbons, AIChE J., 2006, 52(3), 957–967 CrossRef CAS.
- Z. Bai, C. Wang and J. Deng, et al., Experimental investigation on using ionic liquid to control spontaneous combustion of lignite, Process Saf. Environ. Prot., 2020, 142, 138–149 CrossRef CAS.
- B. Chen, Z. Diao and H. Lu, Using the ReaxFF reactive force field for molecular dynamics simulations of the spontaneous combustion of lignite with the Hatcher lignite model, Fuel, 2014, 116, 7–13 CrossRef CAS.
- J. H. Lv, X. Y. Wei and Y. Y. Zhang, et al., Structural characterization of Baiyinhua lignite via direct and thermal decomposition methods, Fuel, 2019, 253, 1042–1047 CrossRef CAS.
- S. Bhoi, T. Banerjee and K. Mohanty, Molecular dynamic simulation of spontaneous combustion and pyrolysis of brown coal using ReaxFF, Fuel, 2014, 136, 326–333 CrossRef CAS.
- W. Fuchs and A. G. Sandhoff, Theory of coal pyrolysis, Ind. amp; Eng. Chem., 1942, 34(5), 567–571 CrossRef CAS.
- P. H. Given, The distribution of hydrogen in coals and its relation to coalStructure, Fuel, 1960, 39(2), 147–153 CAS.
- W. H. Wiser, Reported in division of fuel Chemistry, Preprints, 1975, 20(1), 122 Search PubMed.
- J. H. Shinn, From coal to single-stage and two stage products: A reactive model of coal structure, Fuel, 1984, 63(9), 1187–1196 CrossRef CAS.
- L. Lian, Z. Qin and C. Li, et al., Molecular model construction of the dense medium component scaffold in coal for molecular aggregate simulation, ACS Omega, 2020, 5(22), 13375–13383 CrossRef CAS PubMed.
- Z. Zhang, Q. Kang and S. Wei, et al., Large scale molecular model construction of Xishan bituminous coal, Energy Fuels, 2017, 31(2), 1310–1317 CrossRef CAS.
- S. B. Singh, N. Haskin and A. Seyed, Dastgheib. Coal-Based Graphene Oxide-like Materials: A Comprehensive Review, Carbon, 2023, 118447 CrossRef CAS.
- (a) S. B. Singh and A. Seyed, Dastgheib. Physicochemical transformation of graphene oxide during heat treatment at 110–200 °C, Carbon Trends, 2023, 100251 CrossRef CAS; (b) X. Cui, H. Yan and P. Zhao, et al., Modeling of molecular and properties of anthracite base on structural accuracy identification methods, J. Mol. Struct., 2019, 1183, 313–323 CrossRef CAS.
- M. J. Roberts, R. C. Everson and H. W. J. P. Neomagus, et al., Influence of maceral composition on the structure, properties and behaviour of chars derived from South African coals, Fuel, 2015, 142, 9–20 CrossRef CAS.
- L. Feng, G. Zhao and Y. Zhao, et al., Construction of the molecular structure model of the Shengli lignite using TG-GC/MS and FTIR spectrometry data, Fuel, 2017, 203, 924–931 CrossRef CAS.
- S. Li, Y. Zhu and Y. Wang, et al., The chemical and alignment structural properties of coal: insights from Raman, solid-state 13C NMR, XRD, and HRTEM techniques, ACS Omega, 2021, 6(17), 11266–11279 CrossRef CAS PubMed.
- J. Wang, Y. He and H. Li, et al., The molecular structure of Inner Mongolia lignite utilizing XRD, solid state 13C NMR, HRTEM and XPS techniques, Fuel, 2017, 203, 764–773 CrossRef CAS.
- S. T. Perry, E. M. Hambly and T. H. Fletcher, et al., Solid-state 13C NMR characterization of matched tars and chars from rapid coal devolatilization, Proc. Combust. Inst., 2000, 28(2), 2313–2319 CrossRef CAS.
- G. N. Okolo, H. W. J. P. Neomagus and R. C. Everson, et al., Chemical–structural properties of South African bituminous coals: Insights from wide angle XRD–carbon fraction analysis, ATR–FTIR, solid state 13C NMR, and HRTEM techniques, Fuel, 2015, 158, 779–792 CrossRef CAS.
- X. He, X. Liu and B. Nie, et al., FTIR and Raman spectroscopy characterization of functional groups in various rank coals, Fuel, 2017, 206, 555–563 CrossRef CAS.
- Y. Chen, M. Mastalerz and A. Schimmelmann, Characterization of chemical functional groups in macerals across different coal ranks via micro-FTIR spectroscopy, Int. J. Coal Geol., 2012, 104, 22–33 CrossRef CAS.
- H. Rahmania and A. Rohman, The employment of FTIR spectroscopy in combination with chemometrics for analysis of rat meat in meatball formulation, Meat Sci., 2015, 100, 301–305 CrossRef CAS PubMed.
- M. Kozłowski, XPS study of reductively and non-reductively modified coals, Fuel, 2004, 83(3), 259–265 CrossRef.
- I. E. HAJJ, L. SPEYER and S. CAHEN, et al., Crystal structure of first stage strontium-graphite intercalation compound, Carbon, 2020, 168(1), 1–12 Search PubMed.
- C. Y. Chang, L. Zuo and H. L. Yip, et al., A Versatile Fluoro-Containing Low-Bandgap Polymer for Efficient Semitransparent and Tandem Polymer Solar Cells, Adv. Funct. Mater., 2013, 23(40), 5084–5090 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |