Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The radical scavenging activity of monocaffeoylquinic acids: the role of neighboring hydroxyl groups and pH levels†
Quan V. Vo *a,
Duong Thi Thuy Hoab,
Nguyen Thi Hoaa,
Manh Duc Tranb and
Adam Mechler
*a,
Duong Thi Thuy Hoab,
Nguyen Thi Hoaa,
Manh Duc Tranb and
Adam Mechler c
c
aThe University of Danang – University of Technology and Education, Danang 550000, Vietnam. E-mail: vvquan@ute.udn.vn
bThe University of Danang – University of Sciences and Education, Danang 550000, Vietnam
cDepartment of Biochemistry and Chemistry, La Trobe University, Victoria 3086, Australia
First published on 30th January 2024
Abstract
Caffeoylquinic acids (CQAs) are well-known antioxidants. However, a key aspect of their radical scavenging activity – the mechanism of action – has not been addressed in detail thus far. Here we report on a computational study of the mechanism of activity of CQAs in scavenging hydroperoxyl radicals. In water at physiological pH, the CQAs demonstrated ≈ 104 times higher HOO˙ antiradical activity than in lipid medium (k(lipid) ≈ 104 M−1 s−1). The activity in the aqueous solution was determined by the hydrogen transfer mechanism of the adjacent hydroxyl group (O6′–H) of the dianion states (Γ = 93.2–95.2%), while the single electron transfer reaction of these species contributed 4.8–6.8% to the total rate constants. The kinetics estimated by the calculations are consistent with experimental findings in water (pH = 7.5), yielding a kcalculated/kexperimental = 2.4, reinforcing the reliability and precision of the computational method and demonstrating its utility for evaluating radical reactions in silico. The results also revealed the pH dependence of the HOO˙ scavenging activity of the CQAs; activity was comparable for all compounds below pH 3, however at higher pH values 5CQA reacted with the HOO˙ with lower activity than 3CQA or 4CQA. It was also found that CQAs are less active than Trolox below pH 4.7, however over pH 5.0 they showed higher activity than the reference. The CQAs had the best HOO˙ antiradical activity at pH values between 5.0 and 8.6. Therefore, in the physiological environment, the hydroperoxyl antiradical capacity of CQAs exhibits similarity to renowned natural antioxidants including resveratrol, ascorbic acid, and Trolox.
1. Introduction
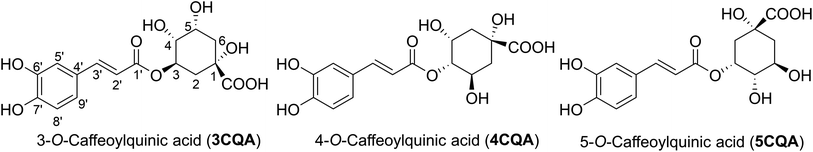
Caffeoylquinic acids (CQAs) are a class of bioactive metabolites that are synthesized through the phenylpropanoid biosynthesis pathway.1 These compounds are esters formed by the conjugation of caffeic acid and quinic acid. CQAs are frequently found in a diverse array of food sources, encompassing fruits, coffee, vegetables, spices, and an extensive variety of plant species.2,3 CQAs have a diverse array of potential therapeutic uses in humans. It has been reported in a range of studies that these compounds possess antibacterial, anticancer, antiviral, anti-Alzheimer's, neuroprotective, and antioxidant properties.1,4–14 The most common CQA in the plant kingdom is 5-O-caffeoylquinic acid (5CQA), also known as chlorogenic acid. This compound is usually found in combination with 3-O-caffeoylquinic acid (3CQA), also known as neochlorogenic acid, and 4-O-caffeoylquinic acid (4CQA), also known as cryptochlorogenic acid (Fig. 1).15Chlorogenic acids have the ability to scavenge free radicals from a variety of sources, including 2,21-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radicals, 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals, superoxide anions (O2), hydroxyl radicals (OH) and peroxynitrite (ONOO).13,16,17 Kinetics of 5CQA were determined experimentally: it reacts with the superoxide, peroxynitrite, and peroxyl radical with second-order kinetics and rate constants of 3.34 × 109, 9.60 × 105, 1.6 × 105 and 1.28 × 105 M−1 s−1, respectively,17 whereas the 5CQA reacted with HO˙ radicals with rate constant of 109–1010 M−1 s−1.18,19 Computational approaches were also used to evaluate the antioxidant activity of CQAs,18–23 however the mechanism and kinetics of the HOO˙ radical scavenging activity, especially in physiological environments and at different pH values have not been thoroughly investigated. In particular, the effect of different pH levels on the kinetics and mechanism of the radical scavenging activity of phenolic acids is well known,24–26 and thus a more comprehensive examination is desirable. Furthermore, the examination of radical reactions involving HO˙ and HOO˙ is significant not only in the advanced oxidation processes,27–29 but also in the radical scavenging activity of antioxidants.30–33 Accordingly, in this study, thermodynamic and kinetic calculations were used to examine the hydroperoxyl radical scavenging activity of the CQAs in physiological conditions and at varied pH levels.
2. Computational details
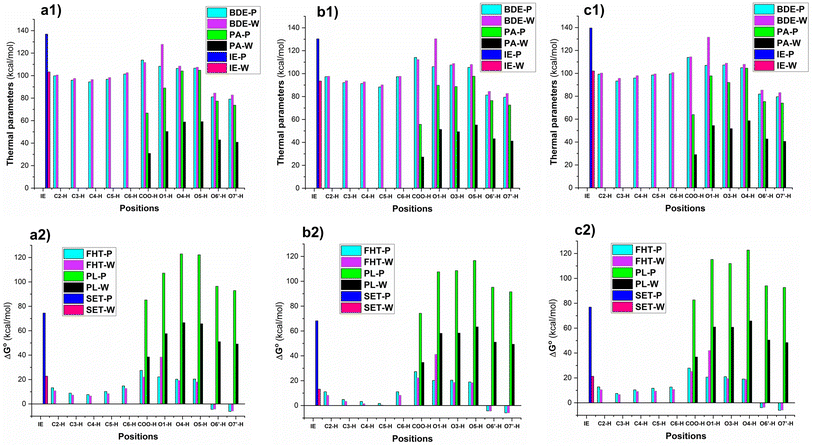
The thermochemical characteristics (bond dissociation energies (BDEs), ionization energies (IEs), and proton affinities (PAs)) of the compound were investigated at the M06-2X/6-311++G(d,p) level of theory. Additionally, the kinetic parameters of the compound, including their activation energies (ΔG≠) in kcal mol−1, tunneling corrections (κ), and rate constants (k), were computed. The activities of the compounds were modelled in the gas phase, the physiological environment (the lipid medium consisted of pentyl ethanoate). In comparison to other intricate procedures, such as G3(MP2)-RAD, and empirical data, it has been demonstrated that the M06-2X/6-311++G(d,p) method has appropriate accuracy in the computation of thermodynamic properties, with an acceptable margin of error34–38 and overall low error rates (kcalc/kexp ratio = 0.3–2.9).39–42 The kinetic calculations were conducted following the established technique for the quantum mechanics-based assay designed to evaluate the overall free radical scavenging activity (QM-ORSA) with the solvation model based on the density (SMD) method for pentyl ethanoate and water solvents. The aforementioned test has been widely employed in order to assess the antiradical properties of antioxidants.34,35,39,40,43The rate constant (k) was determined through the application of the usual transition state theory (TST) under the conditions of a 1 M standard state,44–48 and the details are shown in Table S1, ESI.†
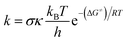 | (1) |
The computations were performed utilizing the Gaussian 16 suite of programs52 and the Eyringpy code, depending on the particular circumstance.53,54 Atom-in-molecule (AIM) analysis55 was performed by using the AIM2000 software.56
3. Results and discussion
3.1. The thermodynamic study
Based on the core structure of CQA, the hexagon rings, HO, and COOH groups can undergo rotation to yield a variety of conformers. The most likely conformer to participate in a radical scavenging reaction is the most stable one, and thus electron energy levels of all possible conformers of each CQA were evaluated in the first stage.57 Subsequently, the five conformers with the lowest electronic energy were subjected to free energy analysis using the M06-2X/6-311++G (d,p) level of theory. Details are shown in the ESI (Fig. S2).† It was found that the ΔG° value of 3CQA (i.e. the structure as drawn in Fig. S2, ESI†) was determined to be the lowest among all the 3CQA conformers (3CQA-1–4) by 2.8–5.1 kcal mol−1. Similarly, the lowest energy 4CQA and 5CQA conformers are drawn in Fig. S2, ESI.† The estimation of conformer relative populations using the Maxwell–Boltzmann distribution58,59 revealed that the conformers 3CQA, 4CQA, and 5CQA dominate the populations (>95%) under standard conditions; consequently, these conformers were used in subsequent investigations.In order to evaluate the likelihood of reacting with free radicals of all possible X–H (X = C, O) bonds including C–H (C2, C3, C4, C5 and C6), and O–H (COOH, O1, O3, O5, O6′ and O7′, Fig. 1) key thermochemical characteristics: bond dissociation energies (BDEs), proton affinities (PAs), and ionization energies (IEs) that provide a first approximation for the probability of reactions following either of the three respective mechanisms, i.e. FHT (formal hydrogen transfer), SET (single electron transfer) and PL (proton loss),41,60,61 were first computed in physiological environments (and water (W) and pentyl ethanoate (P)). The results are shown in Fig. 2.
 | ||
| Fig. 2 The computed BDE, PA, IE (kcal mol−1) of CQAs and ΔG° (kcal mol−1) of the HOO˙ + CQAs reactions following the SET, PL, and FHT mechanisms (a: 3CQA; b: 4CQA; c: 5CQA). | ||
The lowest BDE values were observed at the O6′(7′)–H bonds in all of the studied acids with BDE(O6′(7′)–H) = 79.1–81.9 kcal mol−1 for the lipid medium and BDE(O6′(7′)–H) = 82.7–85.4 kcal mol−1 for the aqueous solution (Fig. 2a1–c1). The active site can be attributed in all cases to the formation of intramolecular hydrogen bonds between the hydrogen atoms of the adjacent hydroxyl groups and the O6′(O7′) radicals.60,62 While the values for other O–H bonds ranged from 104.9 to 131.6 kcal mol−1, the BDE(C–H) values were between 88.3 and 102.6 kcal mol−1. Surprisingly the H-abstraction of the COO–H bond was less likely with the BDE(COO–H) = 112.3–114.4 kcal mol−1 (to emphasize, this refers to hydrogen abstraction; proton dissociation is much more likely, see below). The BDE values in the water were slightly higher than those in the pentyl ethanoate solvent. As expected the PA and IE values in the polar medium were lower than those of the nonpolar environment. The deprotonation was in the order of COO–H > O7′–H > O6′–H in all of the studied compounds, whereas the IE values varied from 93.5 to 139.6 kcal mol−1.
The evaluation in the Gibbs free energies (ΔG°, Fig. 2a2–c2) of the HOO˙ + CQAs reactions following either of the three pathways revealed that the HOO˙ radical trapping activity of CQAs is only spontaneous via the hydrogen transfer of the O6′(7′)-H bonds (ΔG° = −3.6 to −6.2 kcal mol−1), whereas the other FHT reactions cannot happen in the studied media due to the positive ΔG° values. The SET mechanism is not spontaneous either in any of the studied environments, thus this reaction of the neutral states of CQAs can be safely ignored in the kinetic study. It is important to notice that the PL reactions are not spontaneous either in any of the studied environments; however, the PA values were substantially lower than the corresponding BDE values, thus the deprotonation of CQAs should be considered in the aqueous solution. Previous studies indicated that the addition reaction into the α,β-unsaturated bond had no contributions to the ROO˙ radical (i.e., HOO˙ and CH3OO˙) scavenging activity, particularly in the physiological environments,19,24,63 and RAF reaction is not supported for the π system of aromatic rings.64,65 Thus, this reaction was omitted in our study. Hence, in the lipid medium, the H-abstraction of the O6′(7′)–H bonds should be used to compute rate constants, whereas, in the aqueous solution, proton dissociation should be assessed before the kinetic investigation.
3.2. The kinetics of antioxidant activity
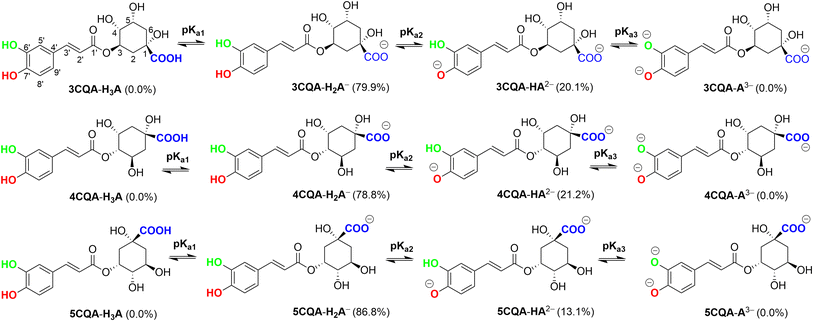
| Comp. | Group | pKa | f (pH = 7.40)c | ||
|---|---|---|---|---|---|
| a Ref. 66.b Calculated in this work.c f Molar fraction. | |||||
| 3CQA | COOH | 1 | 3.95a | H3A | 0.000 |
| O6′–H | 2 | 8.00b | H2A− | 0.799 | |
| O7′–H | 3 | 12.59b | HA2− | 0.201 | |
| A3− | 0.000 | ||||
| 4CQA | COOH | 1 | 4.14a | H3A | 0.000 |
| O6′–H | 2 | 7.97b | H2A− | 0.788 | |
| O7′–H | 3 | 12.52b | HA2− | 0.212 | |
| A3− | 0.000 | ||||
| 5CQA | COOH | 1 | 3.95a | H3A | 0.000 |
| O6′–H | 2 | 8.22b | H2A− | 0.868 | |
| O7′–H | 3 | 12.27b | HA2− | 0.131 | |
| A3− | 0.000 | ||||
The pKa1 values for 3CQA and 5CQA are 3.95, while those for 4CQA are 4.14 (Table 1). The range of pKa2 values is 7.97 to 8.22, while the range of pKa3 values is 12.27 to 12.59. The calculated pKa values of 5CQA (pKa2 = 8.22 and pKa3 = 12.27) closely align with the experimental results (pKa2 = 8.21 and pKa3 = 12.5),67 providing evidence for the accuracy and validity of the computational approach. The mole fractions f(H2A−) and f(HA2−) range between 0.788 and 0.868 and between 0.131 and 0.212, respectively, while the H3A and A3− phases are not present in water at pH = 7.40. Therefore, the CQAs exist in both anionic and dianionic states in water with a pH of 7.4. These two states were examined in the subsequent investigation.
| Comp. | Mechanisms | Pentyl ethanoate | Water | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔG≠ | kapp | Γ | States | ΔG≠ | kapp | F | kf | Γ | |||
| 3CQA | SET | H2A− | 34.3 | 15.9 | 0.799 | 3.84 × 10−13 | 0.0 | ||||
| HA2− | 6.4 | 1.20 × 108 | 0.201 | 2.41 × 107 | 4.8 | ||||||
| FHT | O6′–H | 15.3 | 6.30 × 103 | 57.8 | H2A− | 15.9 | 4.80 × 103 | 0.799 | 3.84 × 103 | 0.0 | |
| O7′–H | 15.2 | 4.60 × 103 | 42.2 | 16.6 | 1.00 × 103 | 0.799 | 7.99 × 102 | 0.0 | |||
| O6′–H (HA2−) | HA2− | 1.0 | 2.40 × 109 | 0.201 | 4.82 × 108 | 95.2 | |||||
| ktotal | 1.09 × 104 | 5.07 × 108 | |||||||||
| 4CQA | SET | H2A− | 32.3 | 17.2 | 0.788 | 1.10 × 10−8 | 0.0 | ||||
| HA2− | 6.4 | 1.30 × 108 | 0.212 | 2.76 × 107 | 5.2 | ||||||
| FHT | O6′–H | 14.9 | 8.00 × 102 | 4.8 | H2A− | 16.2 | 2.10 × 103 | 0.788 | 1.65 × 103 | 0.0 | |
| O7′–H | 14.6 | 1.60 × 104 | 95.2 | 16.1 | 4.70 × 103 | 0.788 | 3.70 × 103 | 0.0 | |||
| O6′–H (HA2−) | HA2− | 1.8 | 2.38 × 109 | 0.212 | 5.05 × 108 | 94.8 | |||||
| ktotal | 1.68 × 104 | 5.32 × 108 | |||||||||
| 5CQA | SET | H2A− | 38.2 | 15.1 | 0.869 | 5.56 × 10−16 | 0.0 | ||||
| HA2− | 6.8 | 6.60 × 107 | 0.131 | 8.65 × 106 | 6.8 | ||||||
| FHT | O6′–H | 15.3 | 9.50 × 103 | 49.2 | H2A− | 18.0 | 2.40 × 102 | 0.869 | 2.09 × 102 | 0.0 | |
| O7′–H | 15.0 | 9.80 × 103 | 50.8 | 17.5 | 3.10 × 102 | 0.869 | 2.69 × 102 | 0.0 | |||
| O6′–H (HA2−) | HA2− | 5.4 | 9.00 × 108 | 0.131 | 1.18 × 108 | 93.2 | |||||
| ktotal | 1.93 × 104 | 1.27 × 108 | |||||||||
Lipid environment:
| ktotal = kapp(FHT(O6′–H)-neutral) + kapp(FHT(O7′–H)-neutral) | (2) |
Water at physiological pH:
| ktotal = kf(SET-HA−) + kf(FHT(O6′–H)-HA−) + kf(FHT(O7′–H)-HA−) + kf(SET-A2−) + kf(FHT(O6′–H)-A2−) | (3) |
| kf = kapp·f | (4) |
As shown in Table 2, the calculations suggest that CQAs can be potent HOO˙ scavengers in the nonpolar environment, with ktotal = 1.09 × 104–1.93 × 104 M−1 s−1. The rate constant for the reaction between 5CQA and HOO˙ was found to be the highest, whereas the reaction between 3CQA and HOO˙ exhibited the lowest rate constant. Based on the calculated data, the HOO˙ radical trapping ability of CQAs in the lipid medium can be ranked as follows: 5CQA > 4CQA > 3CQA. Thus, the activity of CQAs in the nonpolar medium is comparable to reference antioxidants including ascorbic acid (k = 5.71 × 103 M−1 s−1),40 resveratrol (k = 1.31 × 104 M−1 s−1),64 and Trolox (k = 3.40 × 103 M−1 s−1).68
In water at pH = 7.40, the FHT mechanism of the neighboring hydroxyl group (O6′–H) of the dianion states determined the HOO˙ radical scavenging activity of the CQAs (Γ = 93.2–95.2%), while the SET reaction of these species contributed approximately 4.8–6.8% to the ktotal. It should be noted that the tunneling corrections (κ) had a negligible impact on the H-abstraction rate constant of the dianion state, the substantial imaginary frequencies (ν > 3000 cm−1) of these transition states notwithstanding. This suggests that the remarkably swift reaction rates are caused solely by the excessively low Gibb activation energy (ΔG≠ = 1.0–5.4 kcal mol−1) (kTST ≈ kD, where kD denotes the diffusion rate). The HOO˙ radical scavenging activity of the CQAs was not influenced by the monoanion states, despite the fact these states exist about 13.1% to 21.2% (Table 1) of the CQAs in water at pH = 7.4. All compounds exhibit outstanding HOO˙ antiradical activity with ktotal ≈ 108 M−1 s−1. 4CQA had the maximum activity with ktotal = 5.32 × 108 M−1 s−1 that is approximately 4.19 and 1.05 times faster than 5CQA and 3CQA, respectively. In water at pH = 7.4, the radical trapping activity of CQAs against HOO˙ is ranked as follows: 4CQA > 5CQA > 3CQA. In water at physiological pH, the CQAs demonstrated ≈ 104 times greater HOO˙ radical scavenging ability than in the nonpolar environment. In water, CQAs have greater HOO˙ antiradical capacity than Trolox (k = 8.96 × 104 M−1 s−1),68 resveratrol (k = 5.62 × 107 M−1 s−1),64 and ascorbic acid (k = 9.97 × 107 M−1 s−1),40 but the fairly similar activity to caffeic acid (k = 2.69 × 108 M−1 s−1), ferulic acid (k = 3.36 × 108 M−1 s−1) and dihydrocaffeic acid (k = 1.04 × 108 M−1 s−1).63 Consequently, CQAs are promising natural antioxidants.
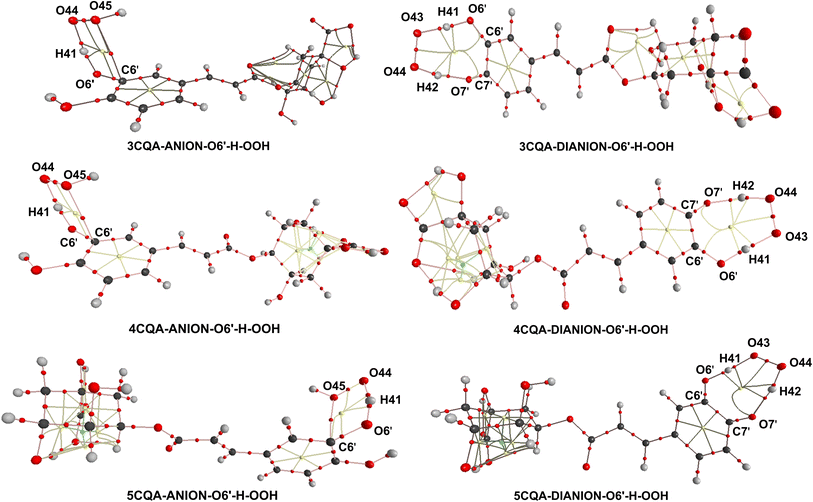
According to the information provided above, the removal of a hydrogen atom from the neighboring hydroxyl group (O6′–H bond) of the dianion states is responsible for the scavenging activity of CQAs in water at a pH level that is characteristic of physiological conditions. In this section, AIM analysis was employed to examine the structural characteristics of the transition states (TSs) pertaining to the O6′–H bond. The findings are displayed in Table S3, ESI,† and Fig. 5. The analysis of the AIM data reveals that the stability of the TSs-DIANION-O6′-H-OOH species can be attributed to intermolecular interactions occurring at certain sites, namely O6′⋯H41, O43⋯H41, O7′⋯H42, and PCR(O6′–H41–O43–O44–H42–O7′–C7′–C6′) (Fig. 5), whereas the stability of TSs-ANION-O6′–H–OOH is given by the intramolecular hydrogen bonding at H41⋯O6′, H41⋯O44 and O45⋯C6′ and at PCR(C6′–O6′–H41–O44–O45). The formation of an intramolecular hydrogen bond between O7′ and H42 in the TSs-DIANION-O6′-H-OOH results in the creation of an 8-atom ring, which includes O6′–H41–O43–O44–H42–O7′–C7′–C6′ (Fig. 5). The electron density between O7′ and H42 exhibits characteristics of partial covalent bonding, as evidenced by ∇2ρ(r) > 0, G(r)/|V(r)| ≤ 1 and H(r) < 0.69,70 This electron density significantly contributes to the stability of the transition state, as revealed by the substantial negative values of EHD (−26.9 to −27.9 kcal mol−1). Thus, the total EHB of TSs-DIANION-O6′–H–OOH (EHB = −211.0 to −212.9 kcal mol−1) is about 1.13 times lower than those of TSs-ANION-O6′–H–OOH (EHB = −184.6 to −186.7 kcal mol−1, respectively). This underpins the increased stability of TSs-DIANION-O6′–H–OOH and consequently the decreased ΔG≠ values (ΔG≠ = 1.0–5.4 kcal mol−1) in comparison with the remaining TSs.
3.3. The effect of pH values on the reactions of CQAs with HOO˙ in water
The impact of solution pH on the rate constants was also evaluated. Eqn (5)–(8) were employed in the computation of several key parameters, namely the rate constant (k), the rate constant specific to each protonation state (kstate), the total rate constant (ktotal), and the overall rate constant (koverall). The outcomes are displayed in Fig. 6 and Table 3.| k = kapp(SET) + ∑kapp(FHT) | (5) |
| kstate = k·f(CQA) | (6) |
| ktotal = ∑kstate | (7) |
| koverall = f(HOO˙)·ktotal | (8) |
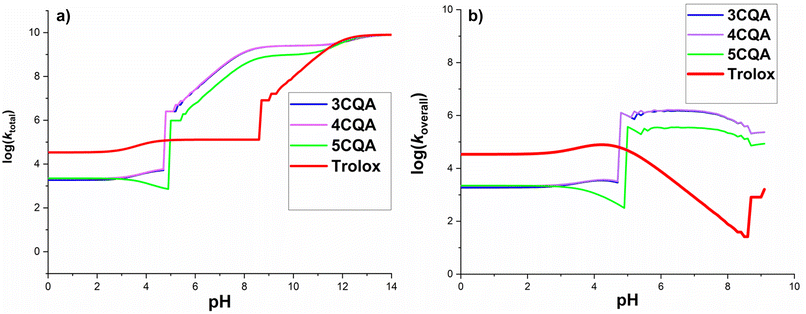 | ||
| Fig. 6 Calculated log(ktotal) (a) and log(koverall) (b) at 298.15 K, in the CQAs + HOO˙ in water as a function of pH values. | ||
| Comp. | States | Mechanisms | ΔG≠ | kapp | k | |
|---|---|---|---|---|---|---|
| 3CQA | H3A | FHT | O6′–H | 17.4 | 5.80 × 102 | 1.86 × 103 |
| O7′–H | 17.1 | 1.28 × 103 | ||||
| H2A− | SET | 34.3 | 4.80 × 10−13 | 5.80 × 103 | ||
| FHT | O6′–H | 15.9 | 4.80 × 103 | |||
| O7′–H | 16.6 | 1.00 × 103 | ||||
| HA2− | SET | 6.4 | 1.20 × 108 | 2.52 × 109 | ||
| FHT | O6′–H | 1.0 | 2.40 × 109 | |||
| A3− | SET | 0.0 | 8.10 × 109 | 8.10 × 109 | ||
| 4CQA | H3A | FHT | O6′–H | 16.3 | 5.00 × 101 | 2.15 × 103 |
| O7′–H | 16.6 | 2.01 × 103 | ||||
| H2A− | SET | 32.3 | 1.40 × 10−11 | 6.80 × 103 | ||
| FHT | O6′–H | 16.2 | 2.10 × 103 | |||
| O7′–H | 16.1 | 4.70 × 103 | ||||
| HA2− | SET | 6.4 | 1.30 × 108 | 2.51 × 109 | ||
| FHT | O6′–H | 1.8 | 2.38 × 109 | |||
| A3− | SET | 0.0 | 8.10 × 109 | 8.10 × 109 | ||
| 5CQA | H3A | FHT | O6′–H | 17.0 | 1.80 × 103 | 2.23 × 103 |
| O7′–H | 18.0 | 4.30 × 102 | ||||
| H2A− | SET | 38.2 | 6.40 × 10−16 | 5.50 × 102 | ||
| FHT | O6′–H | 18.0 | 2.40 × 102 | |||
| O7′–H | 17.5 | 3.10 × 102 | ||||
| HA2− | SET | 6.8 | 6.60 × 107 | 9.66 × 108 | ||
| FHT | O6′–H | 5.4 | 9.00 × 108 | |||
| A3− | SET | 0.0 | 8.30 × 109 | 8.30 × 109 | ||
The log(ktotal) for the total rate constant (Fig. 6a) did not change below pH 4.7, however, it increased significantly between pH = 4.8 and 8.3 by 4–6 units and afterward grew progressively until pH 14. The sudden increase in the log(ktotal) figures at pH = 4.8 and 8.3 is due to the appearance of HA2− states and thus the onset of rapid SET processes. In acidic media (pH < 4.7), the reactions between CQAs and HOO˙ are sluggish because the majority of the CQAs exist in the H3A states (neutral states), acting via a slow FHT reaction.
It was demonstrated that pKa(HOO˙) is 4.80, and thus the f(HOO˙) value is zero at pH > 9.1. Since only pH levels below 9.1 had any impact on the koverall values of reactions between CQAs and HOO˙, only these were looked at (Fig. 6b). It was found that as the pH levels rose, the koverall changed. Most of the studied acids showed a rise in log(koverall) at pH 4; after a brief fall, the log(koverall) significantly rose at pH = 4.7–6.5, before decreasing once again. For this range, koverall was 0 because f(HOO˙) = 0 at pH > 9.2 (Fig. 6b).
In terms of the CQAs, the exhibition was fairly similar to the HOO˙ antiradical activity at pH < 3, however at the rest pH values, 5CQA reacted with the HOO˙ lower than 3CQA or 4CQA. It is important to notice that the 3CQA acid had a fairly similar HOO˙ radical scavenging activity to 4CQA in all of the studied pH levels. Compared with typical antioxidant-Trolox, at pH 4.7, CQAs had less HOO˙ radical scavenging activity than Trolox; nevertheless, at pH > 5.0, these acids reacted with the HOO˙ more quickly than the standard. According to the calculated data, in the pH range of 5.0–8.6, the CQAs had the highest HOO˙ antiradical activity (log(koverall) = 5.1–6.2). It was found that the calculated rate constant for the 5CQA + HOO˙ reaction (koverall(calculation) = 3.10 × 105 M−1 s−1) exhibit a high level of consistence with the empirical observations (kexp = 1.28 × 105 M−1 s−1, pH = 7.5).17 Therefore, the computed kinetic values are fairly accurate.
4. Conclusion
DFT calculations were performed to examine the effectiveness of monocaffeoylquinic acids in scavenging hydroperoxyl radicals. In water at physiological pH, the CQAs demonstrated ≈ 104 times (k(water, pH = 7.4) = 1.27–5.32 × 108 M−1 s−1) greater HOO˙ radical-trapping activity than in the nonpolar environment (k(lipid) = 1.09–1.93 × 104 M−1 s−1). The FHT reaction of the neighboring hydroxyl group (O6′–H) of the dianion states determined the activity in the aqueous solution (Γ = 93.2–95.2%), while the SET mechanism of these states contributed 4.8–6.8% to the total rate constants. It is significant that the computed rate constant of the HOO˙ radical-trapping activity in water at pH 7.5 agrees favorably with experimental findings (kcalculated/kexperimental = 2.4), supporting the computational method. CQAs exhibited similar HOO˙ antiradical activities at pH < 3, however at higher pH values, 5CQA reaction with HOO˙ was slower than that of 3CQA or 4CQA. It was also found that CQAs had less HOO˙ radical scavenging activity than Trolox at pH 4.7 while at pH > 5.0 CQAs are better radical scavengers than the reference. The CQAs had the highest HOO˙ antiradical activity at pH = 5.0–8.6. Thus, in the physiological environments, the HOO˙ antiradical ability of CQAs is generally better than the reference antioxidants resveratrol, ascorbic acid, and Trolox.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research is funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.06-2021.14.References
- A. Alcázar Magaña, N. Kamimura, A. Soumyanath, J. F. Stevens and C. S. Maier, Plant J., 2021, 107, 1299–1319 CrossRef PubMed.
- S. Moyo, J. Serem, M. Bester, V. Mavumengwana and E. Kayitesi, Food Chem., 2020, 311, 126023 CrossRef CAS PubMed.
- C. Ma, K. Dastmalchi, B. D. Whitaker and E. J. Kennelly, J. Agric. Food Chem., 2011, 59, 9645–9651 CrossRef CAS PubMed.
- Y. Nakajima, M. Shimazawa, S. Mishima and H. Hara, Life Sci., 2007, 80, 370–377 CrossRef CAS PubMed.
- X. Li, K. Li, H. Xie, Y. Xie, Y. Li, X. Zhao, X. Jiang and D. Chen, Molecules, 2018, 23, 222 CrossRef PubMed.
- W. Liu, J. Li, X. Zhang, Y. Zu, Y. Yang, W. Liu, Z. Xu, H. Gao, X. Sun and X. Jiang, J. Agric. Food Chem., 2020, 68, 10489–10516 CrossRef CAS PubMed.
- M. Naveed, V. Hejazi, M. Abbas, A. A. Kamboh, G. J. Khan, M. Shumzaid, F. Ahmad, D. Babazadeh, X. FangFang and F. Modarresi-Ghazani, Biomed. Pharmacother., 2018, 97, 67–74 CrossRef CAS PubMed.
- O. Bender and A. Atalay, in Cancer, Elsevier, 2021, pp. 311–321, DOI:10.1016/B978-0-12-819547-5.
- Y. S. Hamed, M. Abdin, G. Chen, H. M. S. Akhtar and X. Zeng, Int. J. Food Sci. Technol., 2020, 55, 3082–3090 CrossRef CAS.
- F. K. Nzekoue, S. Angeloni, L. Navarini, C. Angeloni, M. Freschi, S. Hrelia, L. A. Vitali, G. Sagratini, S. Vittori and G. Caprioli, Food Res. Int., 2020, 133, 109128 CrossRef CAS PubMed.
- M. Carvalho, B. M. Silva, R. Silva, P. Valentao, P. B. Andrade and M. L. Bastos, J. Agric. Food Chem., 2010, 58, 3366–3370 CrossRef CAS PubMed.
- N. Benchikha, M. Messaoudi, I. Larkem, H. Ouakouak, A. Rebiai, S. Boubekeur, M. A. Ferhat, A. Benarfa, S. Begaa and M. Benmohamed, Life, 2022, 12, 1579 CrossRef CAS PubMed.
- N. Liang and D. D. Kitts, Nutrients, 2015, 8, 16 CrossRef PubMed.
- R. Socodato, C. C. Portugal, T. Canedo, I. Domith, N. A. Oliveira, R. Paes-de-Carvalho, J. B. Relvas and M. Cossenza, Free Radical Biol. Med., 2015, 79, 45–55 CrossRef CAS PubMed.
- D. Woźniak, I. Nawrot-Hadzik, W. Kozłowska, S. Ślusarczyk and A. Matkowski, Caffeoylquinic Acids, 2021 Search PubMed.
- J.-G. Xu, Q.-P. Hu and Y. Liu, J. Agric. Food Chem., 2012, 60, 11625–11630 CrossRef CAS PubMed.
- Y. Kono, K. Kobayashi, S. Tagawa, K. Adachi, A. Ueda, Y. Sawa and H. Shibata, Biochim. Biophys. Acta, Gen. Subj., 1997, 1335, 335–342 CrossRef CAS PubMed.
- J. Tošović, S. Marković, J. M. D. Marković, M. Mojović and D. Milenković, Food Chem., 2017, 237, 390–398 CrossRef PubMed.
- J. Tošović and S. Marković, Food Chem., 2019, 278, 469–475 CrossRef PubMed.
- S. Marković and J. Tošović, Food Chem., 2016, 210, 585–592 CrossRef PubMed.
- J. G. Uranga, N. S. Podio, D. A. Wunderlin and A. N. Santiago, ChemistrySelect, 2016, 1, 4113–4120 CrossRef CAS.
- S. Dobrev and S. Angelova, Bulg. Chem. Commun., 2020, 52, 48–53 Search PubMed.
- O. K. Yusuff, M. A. O. Abdul Raheem, A. A. Mukadam and R. O. Sulaimon, ACS Omega, 2019, 4, 13671–13680 CrossRef CAS PubMed.
- H. Boulebd, A. Mechler, N. T. Hoa and Q. V. Vo, New J. Chem., 2022, 46, 7403–7409 RSC.
- N. T. Hoa and Q. V. Vo, RSC Adv., 2022, 12, 15115–15122 RSC.
- I. I. Junior, M. A. Do Nascimento, R. O. M. A. de Souza, A. Dufour and R. Wojcieszak, Green Chem., 2020, 22, 5859–5880 RSC.
- D. S. Dimić, D. A. Milenković, E. H. Avdović, Đ. J. Nakarada, J. M. Dimitrić Marković and Z. S. Marković, J. Chem. Eng., 2021, 424, 130331 CrossRef.
- D. A. Milenković, D. S. Dimić, E. H. Avdović, A. D. Amić, J. M. Dimitrić Marković and Z. S. Marković, J. Chem. Eng., 2020, 395, 124971 CrossRef.
- N. T. Hoa and Q. V. Vo, Chemosphere, 2023, 314, 137682 CrossRef CAS PubMed.
- A. Galano, A. Pérez-González, R. Castañeda-Arriaga, L. Muñoz-Rugeles, G. Mendoza-Sarmiento, A. Romero-Silva, A. Ibarra-Escutia, A. M. Rebollar-Zepeda, J. R. León-Carmona, M. A. Hernández-Olivares and J. R. Alvarez-Idaboy, J. Chem. Inf. Model., 2016, 56, 1714–1724 CrossRef CAS PubMed.
- Q. V. Vo, N. T. Hoa, N. M. Thong and A. Mechler, Phytochemistry, 2021, 192, 112968 CrossRef CAS PubMed.
- A. S. M. Gonçalves, R. G. Peixe, A. Sato, M. F. Muzitano, R. O. M. A. de Souza, T. de Barros Machado, A. C. F. Amaral, M. R. L. Moura, N. K. Simas and I. C. R. Leal, Food Res. Int., 2015, 70, 110–117 CrossRef.
- M. C. Marcondes, A. C. Fernandes, I. Itabaiana Jr, R. O. de Souza, M. Sola-Penna and P. Zancan, PLoS One, 2015, 10, e0130555 CrossRef PubMed.
- M. Carreon‐Gonzalez, A. Vivier‐Bunge and J. R. Alvarez‐Idaboy, J. Comput. Chem., 2019, 40, 2103–2110 CrossRef PubMed.
- A. Galano and J. Raúl Alvarez‐Idaboy, Int. J. Quantum Chem., 2019, 119, e25665 CrossRef.
- Y. Zhao, N. E. Schultz and D. G. Truhlar, J. Chem. Theory Comput., 2006, 2, 364–382 CrossRef PubMed.
- A. Galano and J. R. Alvarez-Idaboy, J. Comput. Chem., 2014, 35, 2019–2026 CrossRef CAS PubMed.
- Y. Zhao and D. G. Truhlar, J. Phys. Chem. A, 2008, 112, 1095–1099 CrossRef CAS PubMed.
- J. R. l. Alvarez-Idaboy and A. Galano, J. Phys. Chem. B, 2012, 116, 9316–9325 CrossRef CAS PubMed.
- A. Galano and J. R. Alvarez‐Idaboy, J. Comput. Chem., 2013, 34, 2430–2445 CrossRef CAS PubMed.
- Q. V. Vo, M. V. Bay, P. C. Nam, D. T. Quang, M. Flavel, N. T. Hoa and A. Mechler, J. Org. Chem., 2020, 85, 15514–15520 CrossRef CAS PubMed.
- Q. V. Vo, N. T. Hoa and A. Mechler, Polym. Degrad. Stab., 2021, 185, 109483 CrossRef CAS.
- A. Galano, J. Mex. Chem. Soc., 2015, 59, 231–262 Search PubMed.
- M. G. Evans and M. Polanyi, Trans. Faraday Soc., 1935, 31, 875–894 RSC.
- H. Eyring, J. Chem. Phys., 1935, 3, 107–115 CrossRef CAS.
- D. G. Truhlar, W. L. Hase and J. T. Hynes, J. Phys. Chem., 1983, 87, 2664–2682 CrossRef CAS.
- T. Furuncuoglu, I. Ugur, I. Degirmenci and V. Aviyente, Macromolecules, 2010, 43, 1823–1835 CrossRef CAS.
- E. Vélez, J. Quijano, R. Notario, E. Pabón, J. Murillo, J. Leal, E. Zapata and G. Alarcón, J. Phys. Org. Chem., 2009, 22, 971–977 CrossRef.
- E. Pollak and P. Pechukas, J. Am. Chem. Soc., 1978, 100, 2984–2991 CrossRef CAS.
- A. Fernández-Ramos, B. A. Ellingson, R. Meana-Pañeda, J. M. Marques and D. G. Truhlar, Theor. Chem. Acc., 2007, 118, 813–826 Search PubMed.
- C. Eckart, Phys. Rev., 1930, 35, 1303 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, G. Z. J. Bloino, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. P. Jr., F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian, Inc., Wallingford CT, 2016.
- E. Dzib, J. L. Cabellos, F. Ortíz‐Chi, S. Pan, A. Galano and G. Merino, Int. J. Quantum Chem., 2019, 119, e25686 CrossRef.
- E. Dzib, J. L. Cabellos, F. Ortiz-Chi, S. Pan, A. Galano and G. Merino, Eyringpy 1.0.2, Cinvestav, Mérida, Yucatán, 2018 Search PubMed.
- R. F. Bader, Chem. Rev., 1991, 91, 893–928 CrossRef CAS.
- F. Biegler-König, AIM 2000, University of Applied Sciences, Bielefeld, Germany, 2000 Search PubMed.
- W. Hehre, J. Yu, P. Klunzinger and L. Lou, Spartan Software, Wavefunction Inc, Irvine, 2000 Search PubMed.
- A. Galano and J. R. Alvarez-Idaboy, RSC Adv., 2011, 1, 1763–1771 RSC.
- Q. V. Vo, N. M. Tam, M. Van Bay and A. Mechler, Chem. Phys. Lett., 2020, 739, 137004 CrossRef CAS.
- K. U. Ingold and D. A. Pratt, Chem. Rev., 2014, 114, 9022–9046 CrossRef CAS PubMed.
- A. Galano, G. Mazzone, R. Alvarez-Diduk, T. Marino, J. R. Alvarez-Idaboy and N. Russo, Annu. Rev. Food Sci. Technol., 2016, 7, 335–352 CrossRef CAS PubMed.
- D. Dimić, D. Milenković, J. D. Marković and Z. Marković, Phys. Chem. Chem. Phys., 2017, 19, 12970–12980 RSC.
- J. R. León-Carmona, J. R. Alvarez-Idaboy and A. Galano, Phys. Chem. Chem. Phys., 2012, 14, 12534–12543 RSC.
- M. Cordova-Gomez, A. Galano and J. R. Alvarez-Idaboy, RSC Adv., 2013, 3, 20209–20218 RSC.
- C. Iuga, J. R. l. Alvarez-Idaboy and N. Russo, J. Org. Chem., 2012, 77, 3868–3877 CrossRef CAS PubMed.
- J. Wang, D. Lu, Q. Sun, H. Zhao, X. Ling and P. Ouyang, Chem. Eng. Sci., 2012, 78, 53–62 CrossRef CAS.
- T. Kiss, G. Nagy, M. Pécsi, H. Kozlowski, G. Micera and L. S. Erre, Polyhedron, 1989, 8, 2345–2349 CrossRef CAS.
- M. E. Alberto, N. Russo, A. Grand and A. Galano, Phys. Chem. Chem. Phys., 2013, 15, 4642–4650 RSC.
- M. Ziółkowski, S. J. Grabowski and J. Leszczynski, J. Phys. Chem. A, 2006, 110, 6514–6521 CrossRef PubMed.
- I. Rozas, I. Alkorta and J. Elguero, J. Am. Chem. Soc., 2000, 122, 11154–11161 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra08460d |
| This journal is © The Royal Society of Chemistry 2024 |